Abstract
Patients with cirrhosis frequently have complex alterations in their hemostatic system. Although routine diagnostic tests of hemostasis in cirrhosis (platelet count, prothrombin time, fibrinogen level) are suggestive of a bleeding tendency, it is now widely accepted that these tests do not reflect hemostatic competence in this population. Rather, patients with cirrhosis appear to have a rebalanced hemostatic system with hypercoagulable elements. Therefore, routine correction of hemostasis laboratory values, for example by fresh frozen plasma or platelet concentrates, with the aim to avoid spontaneous or procedure‐related bleeding is not indicated as is outlined in recent clinical guidance documents. However, little guidance on how to manage patients with cirrhosis that are actively bleeding is available. Here we present three common bleeding scenarios, variceal bleeding, post‐procedural bleeding and bleeding in a critically ill cirrhosis patient, with specific management suggestions. As patients with cirrhosis generally have adequate hemostatic competence and as bleeding complications may be unrelated to hemostatic failure, prohemostatic therapy is not the first line of management in bleeding patients with cirrhosis, even in the presence of markedly abnormal platelet counts and/or prothrombin times. We provide a rationale for the restrictive approach to prohemostatic therapy in bleeding patients with cirrhosis.
Keywords: portal hypertension, prothrombin time, platelet transfusion, blood transfusion, antifibrinolytic agents
1. INTRODUCTION
Prevention and treatment of bleeding are common clinical scenarios encountered across a broad range of medical specialties for which hematologists are often consulted. Although for specific scenarios such as treatment of bleeding in patients with trauma,1 post‐partum bleeding,2 and prevention of bleeding in patients using antithrombotic therapy3 evidence‐based guidelines exist, it is unclear what the value of these protocols is in other settings. For example, the CRASH‐24 and WOMAN5 trials on the use of tranexamic acid (TXA) in massive bleeding in trauma or post‐partum hemorrhage may have led to the assumption that TXA should be part of (massive) bleeding protocols in general. However, TXA was ineffective and caused harm in the HALT‐IT trial on gastrointestinal (GI) bleeding6 and was ineffective in the ULTRA trial on patients with subarachnoid hemorrhage.7 Also, although blood components such as fresh frozen plasma (FFP), platelet concentrates, and coagulation factor concentrates are frequently used in prevention and treatment of bleeding, there is very little evidence these treatments are effective.8, 9, 10 Hematologists are frequently consulted for prevention or treatment of bleeding in patients with cirrhosis. Importantly, many bleeding complications are unrelated to hemostatic failure, but are a consequence of portal hypertension or related to inadvertent vessel lacerations, for example during invasive procedures (Figure 1). Nevertheless, hemostatic interventions to prevent or treat bleeding complications that are unrelated to hemostatic failure are commonly administered, for example when using a general massive bleeding protocol in variceal bleeding.11 Also, prophylactic prohemostatic therapy is frequently administered in patients with liver disease prior to procedures with a very low bleeding risk such as paracentesis, diagnostic endoscopy, or placement of a central venous catheter.12 In clinical practice, the clinician performing the procedure will often mandate prohemostatic therapy because of abnormalities in routine diagnostic tests of hemostasis.
FIGURE 1.
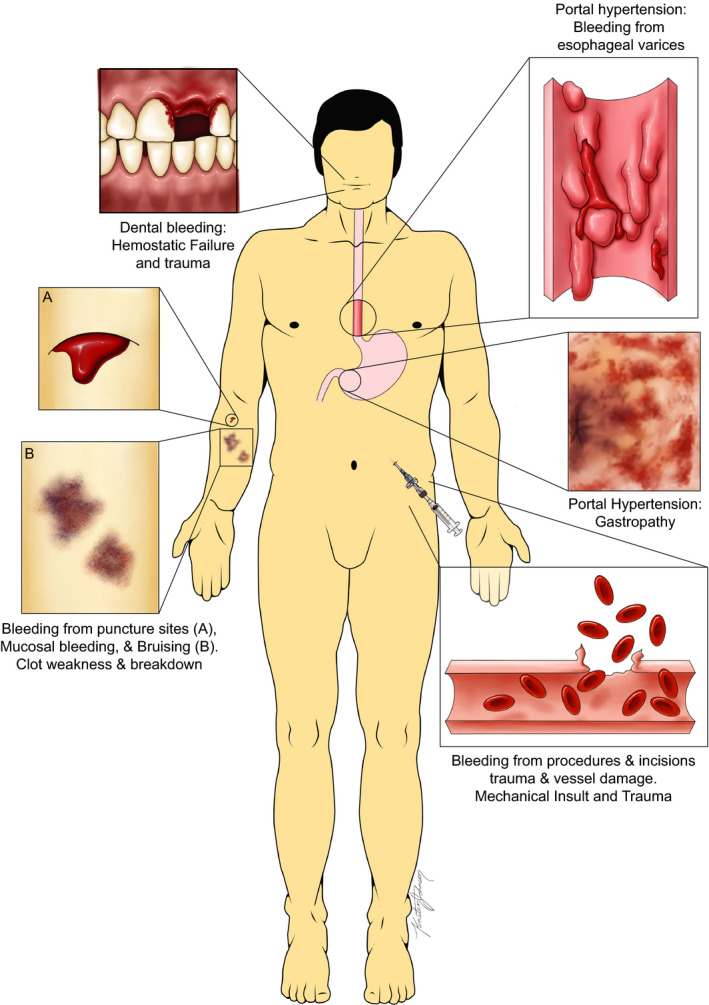
Common sources of bleeding in patients with cirrhosis. Many bleeding events are due to spontaneous mechanical sources such as ruptured esophageal varices while others are related to related to trauma to blood vessels and tissues, often related to medical interventions. The minority of bleeding events are purely due to the hemostatic failure of end stage liver disease. Figure adapted from Northup, et al.17 Used with permission.
The common practice to administer prohemostatic therapy in prophylactic or therapeutic settings in patients with cirrhosis is driven by the combination of frequent clinical bleeding and frequent abnormalities in routine diagnostic tests of hemostasis. These laboratory abnormalities include thrombocytopenia, prolongations in the prothrombin time (PT) and activated partial thromboplastin time (APTT), and, in the sickest patients, low fibrinogen levels. These laboratory abnormalities are frequently interpreted as indicative of a bleeding tendency, but extensive laboratory studies have demonstrated that a hemostatic balance is generally maintained in patients with liver disease.13, 14 Maintenance of the hemostatic balance in patients with liver disease is a consequence of simultaneous changes in pro‐ and antihemostatic pathways, which are not reflected in routine diagnostic tests of hemostasis. The hemostatic profile of patients with liver disease even has prothrombotic components, which may explain why patients with liver disease are at increased risk for development of thrombotic events.15
The concept of rebalanced hemostasis in patients with liver disease has, to some extent, led to changes in prophylactic hemostatic management. For example, in patients undergoing liver transplant surgery many centers have changed from a protocol driven preoperative correction of thrombocytopenia and a prolonged PT, to an on‐demand strategy in which abnormal laboratory values are accepted and prohemostatic therapy is only given when intraoperative bleeding complications occur.16 Similarly, prophylactic correction of abnormal laboratory values is discouraged for other invasive procedures by societal guidance documents.17 Nevertheless, implementation of a much more restrictive approach to prophylactic prohemostatic therapy is not universal due to the lack of randomized trial evidence, and a continued belief that abnormal laboratory tests require correction.
Although there is extensive literature describing expert opinion on best practices for prophylactic prohemostatic therapy in patients with cirrhosis, there is very little literature on how to manage active bleeding in these patients. Given that bleeding in patients with cirrhosis may not be directly related to hemostatic failure and hemostatic balance in patients with cirrhosis is generally maintained despite thrombocytopenia and prolongations in the PT and APTT, strategies to manage active bleeding in these patients are not straightforward and may not necessarily involve prohemostatic interventions. Here, we will present three cases of bleeding complications in patients with cirrhosis with considerations on how to best manage these bleeds.
2. CASE 1: ACUTE UPPER GI BLEEDING
A 56‐year‐old man with a history of non‐alcoholic fatty liver disease‐related cirrhosis presents to the emergency department with acute onset hematemesis and melena which began earlier in the day. He has never had bleeding before but routine upper endoscopy 3 months ago showed large esophageal varices (EV) which were electively band ligated endoscopically but the varices were not completely eradicated. There is no history of ascites or hepatic encephalopathy and the patient is not on anticoagulant or antiplatelet agents. On presentation, he is hypotensive but awake. Initial laboratory testing shows hemoglobin 6.2 g/dl, total bilirubin 2.8 mg/dl, albumin 3.0 g/dl, PT 28 s, international normalized ratio (INR) 2.4, creatinine 1.9 mg/dl, platelet count 39 000/µl, fibrinogen 100 mg/dl. The patient is Child Turcotte Pugh (CTP) class B and the model for end stage liver disease (MELD) score is 26.
This patient presents with a common complication of cirrhosis, acute large volume upper GI bleeding. The most likely source of the bleeding in this patient is from ruptured EV although peptic ulcer disease and other non‐portal hypertension related sources are not uncommon in this population. Acute esophageal variceal bleeding (EVB) occurs at a rate of 10%–15% per year in patients with portal hypertension and cirrhosis18 and is often the initial decompensating event in the course of progressive portal hypertension. Multiple factors are associated with increased risk for rupture of EV including measures of progressive liver disease, increasing size of the varices, and the appearance of wall thinning of the vessel when viewed endoscopically (“red wale marks”) but fundamentally they all relate to the effects of increased pressure in the portal venous system.19 It is this increased pressure within the maximally distended varices that leads to rupture and hemorrhage, thus EVB is primarily a mechanical or pressure‐related hemorrhagic event and not directly based on hemostatic failure. Data showing that patients with cirrhosis actively taking anticoagulants at the time of EVB have similar adjusted rebleeding and survival outcomes compared to those not taking these medications20 and the lack of benefit of recombinant factor VIIa (rVIIa) infusion in EVB21, 22 both support the argument that hemostatic failure is not a major contributor to variceal bleeding or outcome. ‘If an alternate source of bleeding is discovered, the contribution of hemostatic failure remains uncertain; the principles further discussed below remain relevant.’
Urgent diagnostic and potentially therapeutic endoscopic evaluation is needed in this patient and improved outcomes are demonstrated when performed in less than 12 h after presentation. There are extensive evidence‐based management recommendations for acute variceal bleeding that are beyond the scope of this document.19, 23, 24 Endoscopic and pharmacologic therapies are the mainstays of treatment for this life‐threatening disorder and are well described elsewhere.19 However, this patient will be admitted to an intensive care unit and requires resuscitation prior to endoscopic or other procedural therapy. This stage of patient care suffers from less evidence‐based recommendations. During this stage, the requirement for packed red blood cells (PRBC) raises the question whether addition of FFP and platelet concentrates are also required given the elevated INR and decreased platelet count in this patient.
Data from randomized controlled trials support a lower threshold of 7 g/dl for hemoglobin values when transfusing patients with cirrhosis and acute GI bleeding with PRBC. In a prospective randomized controlled trial of 921 patients (271 with cirrhosis) with severe GI bleeding, those assigned to transfusion using a lower threshold of 7 g/dl versus those assigned to 9 g/dl had improved 6‐week survival and less recurrent bleeding.25 The group assigned to the higher threshold also had significantly increased portal pressure within the first 5 days of hospitalization compared to the group with the lower threshold. This increase in portal pressures is contradictory to the aim of reducing overall portal pressures in the resuscitation of these patients and was likely a significant contributor to rebleeding rates in the patients undergoing more transfusions. This phenomenon of increasing portal hypertension with volume expansion has also been shown in experimental models with blood transfusion and crystalloid infusion.26 Care should be taken in this population to avoid aggressive volume overexpansion not only with PRBC but also with crystalloid and FFP in order to avoid paradoxical increase in portal hypertension and rebleeding risk.
Once hemodynamic and oxygen carrying capacity is stabilized, correction of components of the hemostatic system can be considered. As mentioned above, at steady state in cirrhosis, there is a rebalance in all phases of hemostasis which is marked by compensatory changes in both the prohemostatic and antihemostatic systems. However, this hemostatic rebalance may be unpredictably disturbed during critical illness especially in patients with acute‐on‐chronic liver failure (ACLF).27 Excluding liver transplantation, there are very sparse data on specific changes in hemostasis in non‐steady state circumstances such as acute bleeding. One study published only in abstract form demonstrated stable thrombin generating potential in patients with acute variceal bleeding. 28
Some authors recommend the use of viscoelastic testing to help determine the need for factor and platelet replacement therapy in patients with cirrhosis. There are two published randomized controlled studies comparing the use of thromboelastography (TEG) measurements to routine diagnostic tests (platelet count, PT, fibrinogen) as a guide to transfusion in patients with cirrhosis and acute bleeding, one in variceal bleeding29 and a similar study in non‐variceal upper GI bleeding.30 In both studies, the authors found that TEG findings were within the normal range in the majority of patients, which led to a significant decrease in the use of both platelet and FFP transfusions in the TEG arm of both studies. These findings suggest that hemostatic competence is maintained even in the bleeding cirrhosis patient. Importantly, whereas TEG better reflects hemostatic status than routine diagnostic tests, it likely still underestimates hemostatic potential because of the lack of sensitivity for VWF levels and the protein C system.31
There is no definitive evidence that correction of thrombocytopenia, a prolonged PT, or abnormal TEG or ROTEM tests by infusion of FFP, platelet concentrates, or low volume coagulation factor concentrates is effective at achieving a more rapid hemostasis. There are no clinical studies evaluating adequate platelet thresholds for the purpose of enhancing hemostasis in the bleeding cirrhosis patient. Furthermore, transfusions with platelet concentrates are frequently complicated by febrile reactions and less commonly by potentially life‐threatening transfusion related acute lung injury (TRALI) and transfusion associated circulatory overload (TACO).32, 33 Importantly, the risk of TRALI appears to be increased in patients with underlying liver disease.34 Platelet concentrates have been dose‐dependently associated with increased mortality in the setting of liver transplantation.35 Similarly, there is no evidence of benefit of FFP, but transfusion‐related side effects have significant potential to harm the patient with cirrhosis. In addition, the large volumes of FFP realistically needed to improve the INR makes transfusion of FFP very likely more harmful than beneficial in the treatment of variceal bleeding. Therefore, in the cirrhosis population, the use of FFP during acute variceal bleeding cannot be recommended. Lower volume factor replacements like the prothrombin complex concentrates (PCC) and rVIIa are more effective than FFP in decreasing INR values in cirrhosis36 and do not carry the risk of volume overload. However, their use in acute variceal bleeding has not been adequately studied and could theoretically increase thrombotic risk.
This patient's fibrinogen level is low and this is a common finding in critically ill patients with cirrhosis. In one study of 211 patients with cirrhosis admitted to an intensive care unit, 47% had a fibrinogen <200 mg/dl and 16% < 100 mg/dl. In this study low values of fibrinogen were independently associated with increased bleeding risk but most bleeding events were portal hypertension related.37 The low fibrinogen in this case is most likely a reflection of severity of liver disease and protein synthetic dysfunction and may not be causally related to bleeding. A retrospective study of 237 critically ill patients with cirrhosis showed no independent association of low fibrinogen levels with mortality or bleeding events and concluded that low fibrinogen levels were a reflection of disease severity not independent of other cirrhosis severity measures such as MELD or ACLF scores.38 That study also showed no bleeding or survival benefit with cryoprecipitate transfusion administered either prophylactically or in actively bleeding patients. As mentioned above, in a randomized controlled trial, TXA in the treatment of acute GI bleeding in patients with cirrhosis was associated with an increased risk of venous thromboembolic events and showed no efficacy in the treatment of bleeding. Thus, its use in this setting cannot be recommended.6
3. CASE 2: DENTAL EXTRACTIONS AND RISK OF BLEEDING
A 60‐year‐old woman with decompensated cirrhosis related to chronic hepatitis C infection is under evaluation for liver transplantation. She has extensive dental caries and prior to transplantation needs multiple tooth extractions. She has no history of excessive spontaneous or surgical bleeding and tolerated dental work prior to onset of her cirrhosis without complications. She has CTP C cirrhosis, MELD 24, with ascites and intermittent hepatic encephalopathy. Her PT is 23 s, INR is 1.9, and platelet count 41 000/µl. Following multidisciplinary discussion, the dental extractions are performed without hemostatic support. There is no intra‐operative excess bleeding. However, she returns the following day with persistent oozing from an open socket.
Dental decay is a potential source of infection post liver transplantation. Formal dental evaluation (and extractions where necessary) are therefore recommended as part of the liver transplantation work up.39 The prevalence of periodontal disease in this group is reported to be as high as 75%.40
Procedural bleeding risk can be classified as high or low based on the estimated incidence of major bleeding and its potential consequence. By definition, a low bleeding risk procedure carries a risk of major bleeding of <1.5% or where bleeding can be easily controlled with local measures. Conversely, high risk is defined as major bleeding risk of >1.5% and/or where bleeding (in its event) would be difficult to control (e.g. non compressible site) and/or of significant consequence (e.g. intracerebral bleeding).41 Observational cohorts report bleeding rates following simple dental extraction of 2.9%–6% in patients with chronic liver disease.42, 43, 44 All bleeds were successfully controlled with local interventions with no patients requiring hospitalization. Hemoglobin values were not reported in this study but it is unlikely these bleeds met criteria for major bleeding. Dental extractions should therefore be considered low bleeding risk procedures. Similarly, whilst the incidence of bleeding post dental extraction is higher than that reported in patients without liver disease (estimated at 1%), it is comparable to that reported in patients on anticoagulation, where a higher bleeding risk of 4%–9% is accepted.45, 46, 47
As a personal history of previous bleeding may be associated with a higher bleeding risk, further details should be sought to establish context. For example, prior procedural bleeding will be more relevant than a prior history of variceal bleed (as this is secondary to portal hypertension rather than hemostatic impairment as discussed above). Renal impairment is associated with higher post paracentesis bleeding but not specifically reported in relation to dental extractions.48 The use of anticoagulants and antiplatelet agents are associated with increased risk of bleeding but are not relevant to this case.49
Whilst INR and thrombocytopenia may indicate an increased bleeding risk in the absence of cirrhosis, as discussed above, neither have been consistently demonstrated to predict procedural bleeding in patients with cirrhosis. One series desribed a small observational cohort (n = 23 patients, 84 dental extractions over 35 procedures), including patients with platelet count >30 000/µl (range 31–160) and INR < 3 (range 0.98–2.5). One postoperative bleed (2.9%) was observed despite a platelet count of <50 000/µl in 34% of procedures and INR > 2 in 9%. All patients were managed with post procedural gauze compression.44
Dental procedures should be performed in settings with access to local hemostatic measures including socket packing and suturing.49 Topical TXA (soaked gauze/mouthwash) are advocated by some but there is no specific evidence of reduced bleeding.45, 49 All patients should be given general advice to minimize delayed bleeding and when to seek further dental /medical attention.
There are few studies investigating systemic therapy to reduce bleeding in dental extractions. A small study randomized patients with cirrhosis and platelet count 30–50 000/µl and/or INR 2–3, to either intranasal desmopressin vs FFP and/or platelet transfusion prior to dental extraction.50 Of 36 patients randomized and receiving the planned intervention, there was no significant difference in bleeding between groups (with a single bleed in the transfusion arm) but the study was underpowered to show a difference between treatments and should thus be interpreted cautiously. A further study investigating laboratory parameters in patients with cirrhosis who received DDAVP infusion found no effect on platelet adhesion, further questioning this approach.51
Thrombopoietin receptor agonists (TPO‐RA), avatrombopag and lusutrombopag are now licensed for use with chronic liver disease and thrombocytopenia in the periprocedural setting. The study design of the seminal trials in preprocedural thrombocytopenia in patients with cirrhosis (ADAPT, L‐PLUS‐2) focused on achieving a prespecified platelet threshold rather than direct impact on bleeding incidence. Therefore, their role in reducing periprocedural bleeding remains uncertain.52, 53 A subsequent meta‐analysis reported a 35% relative risk reduction in periprocedural bleeding.54 These agents may thus be useful in patients with marked thrombocytopenia (<30 000/µl) or with additional bleeding risk factors.
The low bleeding rate, limited consequence of delayed bleeding and uncertain benefit of the above treatments in prevention of bleeding currently favors not utilizing these interventions in routine practice. At presentation with bleeding in our case, the initial management should focus on local hemostatic control with gauze compression, packing and/or sutures. This is usually successful in controlling bleeding. Antifibrinolytics (topical soaked gauze, mouthwash or oral) may be considered, although specific evidence of efficacy in this setting is lacking.45, 49, 55
4. CASE 3: DIFFUSE BLEEDING IN A CRITICALLY ILL CIRRHOSIS PATIENT
A 68‐year‐old woman with primary biliary cholangitis and decompensated cirrhosis is admitted to the intensive care unit with gram‐negative septic shock related to spontaneous bacterial peritonitis. She is intubated and on mechanical ventilation and delirious. She has rapidly progressive acute kidney injury and was recently placed on continuous renal replacement therapy. The right internal jugular dialysis line has been steadily oozing and soaking through the dressings with blood. She also has a large subcutaneous hematoma in the left lower quadrant at the site of a paracentesis performed 48 h ago. Her hemoglobin levels have been steadily dropping each day and she has needed several blood transfusions over the past week. Her platelet count is 36 000/µl, fibrinogen 77 mg/dl, PT 28 s, and INR 3.2. The bleeding has not responded to platelet or cryoprecipitate transfusion administered by the critical care team prior to our consultation. The critical care team is requesting advice on the potential source of her continuous low level bleeding and any therapies that might offer benefit.
The transition from an acutely decompensated state to ACLF requiring organ support, is accompanied by a further decline in platelet count and fibrinogen levels, and a further increase in the PT/INR.56 Whilst global hemostasis assays demonstrate rebalanced hemostasis with hypercoagulable features in patients with acute decompensation, in ACLF there is broad individual variation with evidence of both hypo‐ and hypercoagulable characteristics and variable fibrinolytic activity.56, 57 Sepsis, acute kidney injury and procedural intervention predispose to bleeding and are common in patients with cirrhosis.58, 59, 60 Importantly, over a third of admissions to critical care units are precipitated by sepsis.37 AKI is present in up to 20% of those hospitalized with decompensation and 75% in those requiring critical care support.37, 61 The incidence of major bleeding in ACLF was reported as 17% in a single center cohort (n = 211).37 Comprehensive laboratory evaluation of 80 patients with decompensated liver disease with/without AKI (predominantly ward based care) demonstrated both hypo and hypercoagulable features, with greater platelet dysfunction, lower factor XIII and increased fibrinolysis, along with further hypercoagulable features and hypofibrinolytic features.59, 62 These factors likely contribute to instability of an already delicately rebalanced coagulation status in this patient. Renal replacement therapy may contribute to rebalancing hemostasis by optimization of fluid balance and improving platelet function, although there are no studies investigating this.
Further derangement of routine coagulation parameters seen in ACLF resembles that of disseminated intravascular coagulation (DIC). However, the defining characteristics of DIC are of systemic coagulation activation with intravascular fibrin formation leading to small/medium vessel thrombosis and organ dysfunction63 rather than hepatic synthetic failure and endothelial activation of ACLF. The diagnosis of DIC relies on the use of a scoring system in the presence of a recognized associated condition. This is problematic in liver disease as all components of the DIC score are frequently abnormal in ACLF (Table 1). In the aforementioned critically ill cohort of patients with cirrhosis (n = 211), 63% of patients met the laboratory diagnostic criteria for DIC.37 Whilst the DIC score was significantly associated with an increased risk of major bleeding, marked thrombocytopenia (platelets < 30 000/µl) and hypofibrinogenemia (<60 mg/dl) were stronger predictors of bleeding (along with aPTT > 100 s). Hypofibrinogenemia is the least common feature of DIC,64 but is prevalent in critically ill patients with advanced liver disease. The need and/or benefit of correcting it is not well established. Hyperfibrinolysis is a further diagnosis to consider; this is extensively described during liver transplantation secondary to endothelial release of tissue plasminogen activator (t‐PA).65 Whether it is a defined feature of ACLF remains uncertain; recent studies utilizing plasma‐based clot lysis assays have demonstrated shortened clot lysis times with increasing disease severity, but with wide inter‐individual variation.57 In this cohort, sepsis appeared to be associated with hypofibrinolysis, and bleeding events were not associated with shortened clot lysis times.
TABLE 1.
Routine laboratory findings in ACLF, acquired hyperfibrinolysis and disseminated intravascular coagulation
| ACLF | Acquired hyperfibrinolysis | Disseminated intravascular coagulation | |
|---|---|---|---|
| Blood film | Nonspecific may include macrocytosis, thrombocytopenia | No specific features |
Red cell fragments, thrombocytopenia |
| Platelet count | ↓↓‐↓ | ↔ | ↓↓‐↓ |
| Prothrombin time | ↑‐↑↑ | ↑↑ | ↔‐↑↑ |
| APTT | ↔‐↑ | ↑↑ | ↔‐↑↑ |
| Fibrinogen | ↓↓‐↓ | ↓↓‐↓ | ↓↓‐↔ |
| D‐dimer | ↑‐↑↑ | ↑↑ | ↑↑ |
| Viscoelastic tests (not routinely performed) |
Often normal May have prolonged clotting times, reduced amplitude (particularly on functional fibrinogen assay) |
Increased clot lysis – but often normal | Prolonged clotting times and reduced amplitude |
Abbreviations: ↑, mildly increased; ↑↑, markedly increased. Bold indicates most frequent finding; ↓, mildly reduced; ↓↓ markedly reduced; ↔, normal; ACLF, acute on chronic liver failure; APTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation.
The overlapping features of these diagnoses are highlighted in (Table 1). There are no specific established therapies for bleeding in ACLF and the cornerstone of treatment is to address the underlying cause, in this case sepsis and associated AKI. The role of continued hemostatic support is uncertain, conventional supportive targets for bleeding patients with DIC are unlikely to be appropriate in this patient group given the specified thresholds for platelet count (<50 000/µl), PT (<1.5× normal) and fibrinogen (<1.5 g/L) may be ‘supranormal’ compared to the individual's baseline coagulation parameters. Alternate replacement therapy such as PCC and fibrinogen concentrates should be reserved for those with volume overload where FFP/cryoprecipitate transfusion is not possible (although there is no evidence to support their use in this setting).63 Outside GI bleeding (HALT‐IT), there are no specific studies of antifibrinolytics in ACLF and bleeding. However, antifibrinolytics are suggested as a rescue therapy for controlling bleeding post procedurally.55, 66
For this patient, the mainstay of therapy is management of sepsis and AKI. Whilst there is no evidence, a trial of antifibrinolytics is warranted (with prompt cessation when bleeding stops). If this fails, low dose fibrinogen concentrate (for example 1 g as a single dose) may be appropriate, particularly if fluid balance is challenging. This should not be continued to achieve a specific fibrinogen target but emphasis should be on bleeding control. Given the low yield and questionable efficacy of platelet concentrates in this setting, platelet concentrates are not recommended. Similarly, as large volumes of FFP are required for a meaningful reduction of the PT/INR, its questionable efficacy, and the risk of volume overload resulting in a paradoxical increased bleeding risk, FFP is not recommended.
5. SUMMARY
Here we have highlighted three common scenarios of active bleeding in patients with cirrhosis. In variceal bleeding, a common complication of advanced cirrhosis, prohemostatic therapy is not indicated and control of bleeding should be achieved by local measures combined with pharmacological intervention to reduce portal pressure. FFP and platelet concentrates may do harm and may exacerbate bleeding by increasing portal pressure. TXA should not be given based on the HALT‐IT trial. Post‐procedural bleeding is uncommon and frequently unrelated to hemostatic failure. Therefore, recent guidance statements have argued against prophylactic correction of hemostasis parameters. In those cases with postprocedural bleeding, local measures may be sufficient and again prohemostatic therapy is not indicated despite thrombocytopenia and a prolonged PT/INR as illustrated in our case of bleeding subsequent to dental extraction. Finally, active bleeding in critically ill patients with cirrhosis may be managed by addressing underlying causes for bleeding such as infection and renal failure. The role of prohemostatic therapy in this setting is uncertain, and we favor an individualized approach in patients with intractable bleeding.
In summary, in patients with cirrhosis who are actively bleeding, strategies to improve the platelet count or a prolonged PT/INR are frequently not indicated (Figure 2). This seemingly counterintuitive strategy of restrictive use of blood components in patients with cirrhosis does require formal evaluation in clinical studies.
FIGURE 2.
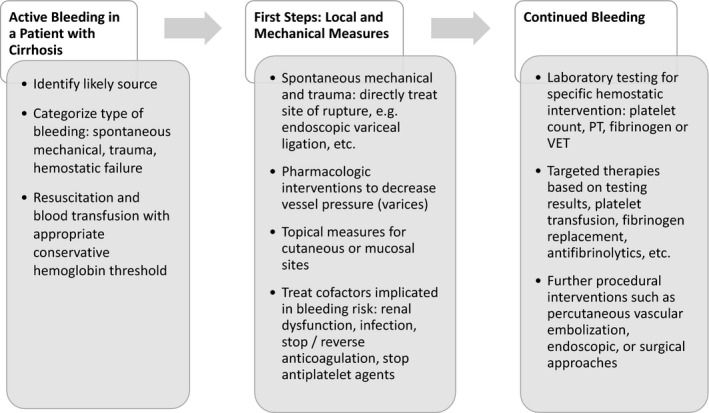
Evaluation and treatment schema for bleeding in a patient with cirrhosis. Abbreviations: VET: viscoelastic testing.
CONFLICT OF INTEREST
The authors have no financial conflict of interests with the material covered in this manuscript.
AUTHOR CONTRIBUTIONS
PGN – wrote all case descriptions and case 1, and co‐wrote the remainder of the manuscript; TL – wrote introduction and summary and co‐wrote the remainder of the manuscript; LR – wrote case 2 and 3 and co‐wrote the remainder of the manuscript.
Manuscript handled by: Jean Connors
REFERENCES
- 1.Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23(1):98. 10.1186/s13054-019-2347-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muñoz M, Stensballe J, Ducloy‐Bouthors AS, et al. Patient blood management in obstetrics: prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood transfus. 2019;17:112‐136. 10.2450/2019.0245-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spyropoulos AC, Brohi K, Caprini J, et al. Scientific and Standardization Committee Communication: Guidance document on the periprocedural management of patients on chronic oral anticoagulant therapy: Recommendations for standardized reporting of procedural/surgical bleed risk and patient‐specific thromboembolic risk. J Thromb Haemost. 2019;17:1966‐1972. 10.1111/jth.14598 [DOI] [PubMed] [Google Scholar]
- 4.Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death. vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet. 2010;376:23‐32. 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 5.Shakur H, Roberts I, Fawole B, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post‐partum haemorrhage (WOMAN): an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2017;389:2105‐2116. 10.1016/S0140-6736(17)30638-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts I, Shakur‐Still H, Afolabi A, et al. Effects of a high‐dose 24‐h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT‐IT): an international randomised, double‐blind, placebo‐controlled trial. Lancet. 2020;395:1927‐1936. 10.1016/S0140-6736(20)30848-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Post R, Germans MR, Tjerkstra MA, et al. Ultra‐early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet. 2021;397:112‐118. 10.1016/S0140-6736(20)32518-6 [DOI] [PubMed] [Google Scholar]
- 8.Huber J, Stanworth SJ, Doree C, et al. Prophylactic plasma transfusion for patients without inherited bleeding disorders or anticoagulant use undergoing non‐cardiac surgery or invasive procedures. Cochrane Database Syst Rev. 2019;11:Cd012745. 10.1002/14651858.CD012745.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newland A, Bentley R, Jakubowska A, et al. A systematic literature review on the use of platelet transfusions in patients with thrombocytopenia. Hematology. 2019;24:679‐719. 10.1080/16078454.2019.1662200 [DOI] [PubMed] [Google Scholar]
- 10.Fabes J, Brunskill SJ, Curry N, Doree C, Stanworth SJ. Pro‐coagulant haemostatic factors for the prevention and treatment of bleeding in people without haemophilia. Cochrane Database Syst Rev. 2018;12:Cd010649. 10.1002/14651858.CD010649.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jairath V, Rehal S, Logan R, et al. Acute variceal haemorrhage in the United Kingdom: Patient characteristics, management and outcomes in a nationwide audit. Dig Liver Dis. 2014;46:419‐426. 10.1016/j.dld.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 12.Desborough MJ, Hockley B, Sekhar M, Burroughs AK, Stanworth SJ, Jairath V. Patterns of blood component use in cirrhosis: a nationwide study. Liver Int. 2016;36:522‐529. 10.1111/liv.12999. [DOI] [PubMed] [Google Scholar]
- 13.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878‐885. 10.1182/blood-2010-02-261891 [DOI] [PubMed] [Google Scholar]
- 14.Lisman T, Hernandez‐Gea V, Magnusson M, et al. The concept of rebalanced hemostasis in patients with liver disease: communication from the ISTH SSC Working Group on Hemostatic Management of Patients with Liver Disease. J Thromb Haemost. 2021;19(4):1116‐1122. [In Press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosino P, Tarantino L, Di Minno G, et al. The risk of venous thromboembolism in patients with cirrhosis. A systematic review and meta‐analysis. Thromb Haemost. 2017;117:139‐148. 10.1160/th16-06-0450 [DOI] [PubMed] [Google Scholar]
- 16.de Boer MT, Molenaar IQ, Hendriks HGD, Slooff MJH, Porte RJ. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22:265‐275. 10.1159/000088056 [DOI] [PubMed] [Google Scholar]
- 17.Northup PG, Garcia‐Pagan JC, Garcia‐Tsao G, et al. Practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73(1):366‐413. 10.1002/hep.31646 [DOI] [PubMed] [Google Scholar]
- 18.North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices . Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983‐989. 10.1056/nejm198810133191505 [DOI] [PubMed] [Google Scholar]
- 19.Garcia‐Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310‐335. 10.1002/hep.28906 [DOI] [PubMed] [Google Scholar]
- 20.Cerini F, Gonzalez JM, Torres F, et al. Impact of anticoagulation on upper‐gastrointestinal bleeding in irrhosis. A retrospective multicenter study. Hepatology. 2015;62:575‐583. 10.1002/hep.27783 [DOI] [PubMed] [Google Scholar]
- 21.Bosch J, Thabut D, Bendtsen F, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double‐blind trial. Gastroenterology. 2004;127:1123‐1130. 10.1053/j.gastro.2004.07.015 [DOI] [PubMed] [Google Scholar]
- 22.Bosch J, Thabut D, Albillos A, et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: A randomized, controlled trial. Hepatology. 2008;47:1604‐1614. 10.1002/hep.22216 [DOI] [PubMed] [Google Scholar]
- 23.de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743‐752. 10.1016/j.jhep.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 24.European Association for the Study of the Liver . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406‐460. 10.1016/j.jhep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 25.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11‐21. 10.1056/NEJMoa1211801 [DOI] [PubMed] [Google Scholar]
- 26.Zimmon DS, Kessler RE. The portal pressure‐blood volume relationship in cirrhosis. Gut. 1974;15:99‐101. 10.1136/gut.15.2.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blasi A, Calvo A, Prado V, et al. Coagulation failure in patients with acute‐on‐chronic liver failure and decompensated cirrhosis: beyond the international normalized ratio. Hepatology. 2018;68:2325‐2337. 10.1002/hep.30103 [DOI] [PubMed] [Google Scholar]
- 28.Jairath V, Harrison P, Stanworth S, Collier J, Murphy M, Barnes E. PWE‐295 Thrombin generation is normal in cirrhotics with acute variceal haemorrhage: results from a prospective study. Gut. 2012;61:A418. 10.1136/gutjnl-2012-302514d.295 [DOI] [Google Scholar]
- 29.Rout G, Shalimar GD, Mahapatra SJ, Kedia S, Garg PK, Nayak B. Thromboelastography‐guided blood product transfusion in cirrhosis patients with variceal bleeding: a randomized controlled trial. J Clin Gastroenterol. 2019;54(3):255‐262. 10.1097/MCG.0000000000001214 [DOI] [PubMed] [Google Scholar]
- 30.Kumar M, Ahmad J, Maiwall R, et al. Thromboelastography‐guided blood component use in patients with cirrhosis with nonvariceal bleeding: a randomized controlled trial. Hepatology. 2020;71:235‐246. 10.1002/hep.30794 [DOI] [PubMed] [Google Scholar]
- 31.Lisman T. Interpreting hemostatic profiles assessed with viscoelastic tests in patients with cirrhosis. J Clin Gastroenterol. 2020;54:389‐391. 10.1097/mcg.0000000000001327 [DOI] [PubMed] [Google Scholar]
- 32.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion‐related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774‐1789. 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 33.Vossoughi S, Gorlin J, Kessler DA, et al. Ten years of TRALI mitigation: measuring our progress. Transfusion. 2019;59:2567‐2574. 10.1111/trf.15387 [DOI] [PubMed] [Google Scholar]
- 34.Benson AB, Austin GL, Berg M, et al. Transfusion‐related acute lung injury in ICU patients admitted with gastrointestinal bleeding. Intensive Care Med. 2010;36:1710‐1717. 10.1007/s00134-010-1954-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereboom IT, de Boer MT, Haagsma EB, Hendriks HG, Lisman T, Porte RJ. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108:1083‐1091. 10.1213/ane.0b013e3181948a59 [DOI] [PubMed] [Google Scholar]
- 36.Kwon JO, MacLaren R. Comparison of fresh‐frozen plasma, four‐factor prothrombin complex concentrates, and recombinant factor VIIa to facilitate procedures in critically Ill patients with coagulopathy from liver disease: a retrospective cohort study. Pharmacotherapy. 2016;36:1047‐1054. 10.1002/phar.1827 [DOI] [PubMed] [Google Scholar]
- 37.Drolz A, Horvatits T, Roedl K, et al. Coagulation parameters and major bleeding in critically ill patients with cirrhosis. Hepatology. 2016;64:556‐568. 10.1002/hep.28628 [DOI] [PubMed] [Google Scholar]
- 38.Budnick I, Davis J, Sundararaghavan A, et al. Transfusion with cryoprecipitate for very low fibrinogen levels does not affect bleeding or survival in critically Ill cirrhosis patients. Thromb Haemost. 2021. 10.1055/a-1355-3716 [DOI] [PubMed] [Google Scholar]
- 39.Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144‐1165. 10.1002/hep.26972 [DOI] [PubMed] [Google Scholar]
- 40.Oliveira CS, Galdino TM, Limeira FIR, Moreira AN, de Magalhaes CS, Abreu LG. Is dental caries associated with liver transplantation? A systematic review and meta‐analysis. Oral Dis. 2020. 10.1111/odi.13439 [DOI] [PubMed] [Google Scholar]
- 41.Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image‐guided Interventions ‐ Part II: Recommendations. J Vasc Interv Radiol. 2019;30:1168‐1184. [DOI] [PubMed] [Google Scholar]
- 42.Cocero N, Bezzi M, Martini S, Carossa S. Oral surgical treatment of patients with chronic liver disease: assessments of bleeding and its relationship with thrombocytopenia and blood coagulation parameters. J Oral Maxillofac Surg. 2017;75(1):28–34. 10.1016/j.joms.2016.08.033 [DOI] [PubMed] [Google Scholar]
- 43.Medina JB, Andrade NS, de Paula EF, et al. Bleeding during and after dental extractions in patients with liver cirrhosis. Int J Oral Maxillofac Surg. 2018;47:1543‐1549. 10.1016/j.ijom.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 44.Perdigao JP, de Almeida PC, Rocha TD, et al. Postoperative bleeding after dental extraction in liver pretransplant patients. J Oral Maxillofac Surg. 2012;70(3):e177‐e184. 10.1016/j.joms.2011.10.033 [DOI] [PubMed] [Google Scholar]
- 45.Randall C. Surgical management of the primary care dental patient on warfarin. Dent Update. 2005;32(7):414–426. 10.12968/denu.2005.32.7.414 [DOI] [PubMed] [Google Scholar]
- 46.Patel JP, Woolscombe S, Patel RK, et al. Managing direct oral anticoagulants in patients undergoing dentoalveolar surgery. Br Dent J. 2017;222:245‐249. [DOI] [PubMed] [Google Scholar]
- 47.Zanon E, Martinelli F, Bacci C, Cordioli GP, Girolami A. Safety of dental extraction among consecutive patients on oral anticoagulant treatment managed using a specific dental management protocol. Blood Coag Fibrinol. 2003;14:27‐30. [DOI] [PubMed] [Google Scholar]
- 48.Hung A, Garcia‐Tsao G. Acute kidney injury, but not sepsis, is associated with higher procedure‐related bleeding in patients with decompensated cirrhosis. Liver Int. 2018;38:1437‐1441. 10.1111/liv.13712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scottish Dental Clinical Effectiveness Programme . Management of Dental Patients Taking Anticoagulants or Antiplatelets. 2015.
- 50.Stanca CM, Montazem AH, Lawal A, Zhang JX, Schiano TD. Intranasal desmopressin versus blood transfusion in cirrhotic patients with coagulopathy undergoing dental extraction: a randomized controlled trial. J Oral Maxillofac Surg. 2010;68:138‐143. 10.1016/j.joms.2009.07.081 [DOI] [PubMed] [Google Scholar]
- 51.Arshad F, Stoof SC, Leebeek FW, et al. Infusion of DDAVP does not improve primary hemostasis in patients with cirrhosis. Liver Int. 2015;35:1809‐1815. 10.1111/liv.12765 [DOI] [PubMed] [Google Scholar]
- 52.Terrault N, Chen Y‐C, Izumi N, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology. 2018;155:705‐718. 10.1053/j.gastro.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 53.Peck‐Radosavljevic M, Simon K, Iacobellis A, et al. Lusutrombopag for the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures (L‐PLUS 2). Hepatology. 2019;70:1336‐1348. 10.1002/hep.30561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindquist I, Olson SR, Li A, et al. The efficacy and safety of thrombopoietin receptor agonists in patients with chronic liver disease undergoing elective procedures: a systematic review and meta‐analysis. Platelets. 2021;1‐7. 10.1080/09537104.2020.1859102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157:34‐43. [DOI] [PubMed] [Google Scholar]
- 56.Fisher C, Patel V, Stoy SH, et al. Balanced haemostasis with both hypo‐ and hyper‐coagulable features in critically ill patients with acute‐on‐chronic‐liver failure. J Crit Care. 2018;43:54‐60. [DOI] [PubMed] [Google Scholar]
- 57.Blasi A, Patel VC, Adelmeijer J, et al. Mixed fibrinolytic phenotypes in decompensated cirrhosis and acute‐on‐chronic liver failure with hypofibrinolysis in those with complications and poor survival. Hepatology. 2020;71:1381‐1390. 10.1002/hep.30915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts LN, Bernal W. Incidence of bleeding and thrombosis in patients with liver disease. Semin Thromb Hemost. 2020;46:656‐664. [DOI] [PubMed] [Google Scholar]
- 59.Zanetto A, Rinder HM, Campello E, et al. Acute kidney injury in decompensated cirrhosis is associated with both hypo‐ and hyper‐coagulable features. Hepatology. 2020;72(4):1327‐1340. 10.1002/hep.31443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montalto P, Vlachogiannakos J, Cox DJ, Pastacaldi S, Patch D, Burroughs AK. Bacterial infection in cirrhosis impairs coagulation by a heparin effect: a prospective study. J Hepatol. 2002;37:463‐470. [DOI] [PubMed] [Google Scholar]
- 61.Garcia‐Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064‐2077. 10.1002/hep.22605 [DOI] [PubMed] [Google Scholar]
- 62.Intagliata NM, Davis JPE, Lafond J, et al. Acute kidney injury is associated with low factor XIII in decompensated cirrhosis. Dig Liver Dis. 2019;51:1409‐1415. 10.1016/j.dld.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 63.Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11:761‐767. 10.1111/jth.12155 [DOI] [PubMed] [Google Scholar]
- 64.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br J Haematol. 2009;145:24‐33. [DOI] [PubMed] [Google Scholar]
- 65.Porte RJ, Bontempo FA, Knot EAR, Lewis JH, Kang YG, Starzl TE. Systenic effects of tissue plasminogen activator‐associated fibrinolysis and its relation to thrombin generation in orthotopic liver transplantation. Transplantation. 1989;47:978‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunawan B, Runyon B. The efficacy and safety of epsilon‐aminocaproic acid treatment in patients with cirrhosis and hyperfibrinolysis. Aliment Pharmacol Ther. 2006;23:115‐120. 10.1111/j.1365-2036.2006.02730.x [DOI] [PubMed] [Google Scholar]


