Abstract
Alpine large herbivores have developed physiological and behavioural mechanisms to cope with fluctuations in climate and resource availability that may become maladaptive under climate warming. We tested this hypothesis in female Alpine ibex (Capra ibex) by modelling annual and daily movement and activity patterns in relation to temperature, vegetation productivity and reproductive status based on bio‐logging data and climate change projections. In summer, ibex moved upslope, tracking the green wave. Ibex decreased diel activity sharply above a threshold temperature of 13–14°C, indicating thermal stress, but compensated behaviourally by foraging both earlier and later in the day, and by moving further upslope than on cooler days, especially reproductive females. This critical temperature will be exceeded three times as often under climate change projections. Under such scenarios, the altitudinal extent of the area will limit the available habitat providing thermal shelter, potentially impacting performance and population distribution of this emblematic mountain ungulate.
Keywords: accelerometer, activity budget, altitudinal migration, Alpine ibex, behavioural responses, Capra ibex, climate change, foraging, GPS telemetry, thermoregulation
We assessed the behavioral mechanisms of female Alpine ibex (Capra ibex) to cope with fluctuations in climate and resource availability that may become maladaptive under climate warming. Ibex optimized the use of resources along the Alpine altitudinal gradient by tracking the seasonal green wave. Further, females responded to temperatures exceeding 13–14°C by redistributing the foraging time throughout the day and moving upslope, especially reproductive individuals. Under climate change scenarios, increasingly hotter conditions will limit the possibility for behavioural compensation, especially in Alpine ranges with a limited altitudinal extent.
INTRODUCTION
The effects of climate warming are accentuated across the marked environmental and climatic gradients of Alpine environments (Ernakovich et al., 2014). Higher temperatures alter the altitudinal distribution of plants (Parolo & Rossi, 2008; Rammig et al., 2010) and increase energy expenditure for thermoregulation in animals (Arnold, 1988; Huey et al., 2012). Moreover temperature effects on vegetation green‐up and senescence (Ernakovich et al., 2014; Wipf et al., 2009) may cascade to primary consumers’ demographic performance, as observed in mountain ungulates (Pettorelli et al., 2007; Mason et al., 2014a; Lovari et al., 2020).
In addition to physiological adaptations (e.g., Arnold et al., 2004; Heldmaier et al., 2004; Signer et al., 2011), mountain ungulates have developed numerous behavioural mechanisms to cope with climatic constraints and variation in resource availability. Across seasons, some species adjust their activity budget to buffer the effect of temperature variation for homeothermy (Arnold et al., 2006; Bourgoin et al., 2008; Green & Bear, 1990), or modulate habitat selection to seek thermal shelter (Brivio et al., 2019; van Beest et al., 2012). In turn, resource acquisition must be adjusted to maintain thermo‐neutrality, while ensuring sufficient energy for survival, maintenance and reproduction. Mountainous environments harbour high spatio‐temporal heterogeneity in resource distribution. As a result, seasonal movements are common in mountain ungulates (e.g., Grignolio et al., 2004; Herfindal et al., 2019; Rice, 2008; Zeng et al., 2008), such as altitudinal shifts to avoid winter harshness (Nicholson et al., 1997; Peters et al., 2019) and enhance access to high‐quality food in relation to vegetation green‐up (forage maturation hypothesis FMH, e.g., Hebblewhite & Merrill, 2009, and green wave hypothesis GWH, e.g., Merkle et al., 2016). Alternatively, a behavioural trade‐off between thermoregulation and foraging has been suggested in relation to these seasonal movements, with high altitudes being cooler, but less productive than lower areas (Mason et al., 2017).
Some of these behavioural adaptations are also expressed at the diel scale, mainly in response to the marked day/night contrast in temperature. Mountain ungulates are known to exploit the altitudinal thermal gradient to compensate for daily temperature fluctuations, because, in summer, high altitude areas may provide thermal cover. Additionally, foraging activity in summer has been observed to vary with the diel cycle and in relation to temperature, generally decreasing in the hottest hours of the hottest days (male Alpine ibex Capra ibex: Aublet et al., 2009; mouflon Ovis sp.: Bourgoin et al., 2011; male chamois Rupicapra rupicapra: Mason et al., 2014b).
As behavioural adjustments are more rapid than physiological adaptation (Wong & Candolin, 2015), the aforementioned compensatory mechanisms might buffer direct (i.e., heat‐stress; Huey et al., 2012) or indirect (e.g., shifts in the phenology of vegetation green‐up; Aikens et al., 2017) consequences of climate change. If not, acute heat‐stress might force ungulates to decrease daytime foraging activity, incurring an energetic debt unless they can compensate at night, or to select sub‐optimal forage habitats when seeking thermal protection (van Beest & Milner, 2013). Similarly, earlier and shorter green‐up periods may inhibit their capacity to synchronise movement with the phenology of high‐quality resources (Aikens et al., 2017). Current evidence on Alpine ungulates’ behavioural responses provides a mixed‐picture. Mouflon (Bourgoin et al., 2011), and male chamois (Grignolio et al., 2018), but not male Alpine ibex to date (Aublet et al., 2009; Mason et al., 2017), have been shown to increase foraging activity at night on hot days. In turn, the utilisation of thermal cover, through altitudinal shifts, has been indicated as maladaptive, because of diminished access to high quality forage habitats (chamois: Mason et al., 2014b; male Alpine ibex: Mason et al., 2017, Brivio et al., 2019). However, the quality of high‐altitude habitats has been indexed by vegetation productivity (as indicated by NDVI), rather than by its rate of change, or ‘green‐up’, as assessed in other systems (Aikens et al., 2017; Bischof et al., 2012). Finally, to date, we know little about how the behavioural responses of Alpine ungulates to environmental variability might depend on fitness‐related traits, particularly reproductive status (Hamel & Côté, 2008).
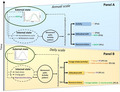
We addressed these knowledge gaps by investigating the behavioural compensation mechanisms for coping with heat‐stress and fluctuating energy needs in Alpine ibex, a high altitude‐ranging and temperature‐sensitive Alpine ungulate (Signer et al., 2011; Toïgo et al., 2002), using a multi‐scale movement ecology framework (Figure 1; Nathan et al., 2008). First, at the annual scale, we evaluated whether female ibex adjusted their activity and movement to variations in temperature and resource availability (‘external state’, via hidden internal state processes; Figure 1A; Q1). We predicted that ibex would increase the total active time per day mainly in response to the seasonal increase in temperature, in accordance with physiological cycles (Signer et al., 2011; P1a), and that they would shift altitude in summer, influenced by both temperature and vegetation productivity, to exploit thermal refuges and maximise access to high quality food (Aublet et al., 2009; P1b). Therefore, we also predicted that these movements would provide individuals with access to areas with better resource quality during summer compared to their respective winter ranges, that is, ibex would exploit the change in vegetation productivity (‘green‐up’, GWH; Aikens et al., 2017; P1c).
FIGURE 1.

Conceptual framework, based on the movement ecology paradigm (Nathan et al., 2008), of the female Alpine ibex behavioural compensatory responses to external abiotic (temperature) and biotic (vegetation productivity, as indicated by Normalized Difference Vegetation Index ‐ NDVI, and vegetation green‐up, as indicated by NDVI relative 8‐day period variation ‐ ΔNDVI) constraints. The relations are assessed both at the seasonal (panel A) and summer daily (panel B) temporal scales. For the summer analysis, we accounted for the impact of reproductive status on behaviour, as a component of individual internal state. Dashed lines: hidden relationships; continuous lines: emergent relationships tested in this study
Second, at the daily scale, we assessed whether female ibex could compensate for heat‐stress by adjusting their diel patterns of activity and altitudinal movements, given their reproductive status (Figure 1b; Q2.1). We predicted that ibex would decrease activity during the hottest hours of the day, especially on the hottest days (Aublet et al., 2009), to maintain thermo‐neutrality (Signer et al., 2011) (P2.1a), while increasing activity at dawn, dusk, or night in order to maintain a globally constant diel activity budget (Bourgoin et al., 2011) (P2.1b). We predicted that reproductive females would have higher activity levels due to the energetic constraints of rearing kids, potentially limiting their compensatory behaviour. Furthermore, we predicted that female ibex would use high altitude areas as thermal refuges (Aublet et al., 2009) in relation to hourly temperature (P2.1c), with reproductive females remaining at higher altitudes due to the lower mobility of kids. Assuming that these behaviours were compensatory mechanisms to meet resource needs while minimising heat‐stress, we also evaluated whether habitat selection varied over the day in relation to activity rhythms (Q2.2), with the most pronounced selection for forage habitats during activity peaks (P2.2). Finally, in order to explore how these behavioural adjustments could be influenced by climate change, we generated predictions of activity rhythms and altitudinal shifts under future scenarios of forecasted climate warming in the Alps (Bucchignani et al., 2015).
Our results indicate that female ibex behaviourally responded to annual temperature and resource availability variations, while adopting compensatory mechanisms to cope with heat‐stress and foraging requirements in relation to reproductive needs at the daily scale during summer. However, we suggest that the efficiency of these mechanisms will likely be impaired under the predicted warming scenarios, notably due to lack of suitable thermal habitat, potentially resulting in detrimental effects on individual fitness and population dynamics of this inhabitant of extreme and threatened Alpine environments.
MATERIALS AND METHODS
Study area
The study area (45 km2 ca) is situated on the Southern slopes of the Marmolada massif (1,700–2,900 m a.s.l.), in the Italian Dolomites (46°26’ 13” N, 11°51’ 54” E). These are isolated, wild, and narrow valleys, with steep slopes and rocky ridges dominated by abrupt vegetation shifts along the elevation gradient, from dense and sparse conifer forest, to alpine shrubs and grassland. Steep screes and rocky cliffs dominate the landscape above 2,100 m where pioneer organisms include lichens and cushion plants (e.g., Androsacae sp.). Roe deer (Capreolus capreolus), red deer (Cervus elaphus) and Alpine chamois, hunted by stalking in autumn/winter, are the only large herbivores potentially overlapping the ibex range. No paved roads reach the area. Hiking trails and cattle grazing pastures are restricted to valley bottoms and are used only in July–August.
The climate is currently Alpine (snowy and humid climate with cool summers/polar tundra; Köppen‐Geiger climate classification, Rubel et al., 2017). Total yearly precipitation is 1,227 mm (IC 95%: 1,114–1,340 mm), with snowfalls starting in mid‐October, and mean annual temperature is 4.4°C (IC 95%: 3.7–5.1°C] (see Appendix S1 in Supporting Information).
Marked animal data
During the study period, the local Alpine ibex colony comprised ca 200 individuals (minimum number alive recorded from block counts: 161–233; adult females only, i.e., 2 yrs. or older: 63–99; see more information on Alpine ibex conservation status and the studied colony in Appendix S2).
From 2010 to 2015, we caught (July–October) 24 adult female ibex by dart‐gunning with sedation, and equipped them with GPS‐GSM sensors (Vectronic Aerospace GmbH), collecting and transmitting locations, and with 2‐axes accelerometers, storing data which were downloadable only at collar retrieval. At capture, we recorded the reproductive status (active lactation), that we confirmed via direct observation (female feeding the kid) during the same season, and re‐assessed during the following year (except two females that could not be observed in the second season; Appendix S2).
GPS collars were programmed to attempt a location every hour, and to drop‐off after 54 weeks. We obtained locations for 24 individuals (fix success rate: 95%; outliers excluded as in Urbano & Cagnacci, 2014; median location error <10 m, Párraga Aguado et al., 2017). We derived activity status from 18 of 24 collars that could be retrieved after drop‐off (days with incomplete accelerometer data <1%, excluded from subsequent analysis). We classified accelerometer data with a binomial activity index based on a threshold value (Gervasi et al., 2006: ‘active’ or ‘inactive’ status, with 5‐minute resolution according to sensor output; Appendix S3). We validated the automatic classification with direct observations between July and September, choosing coarse behavioural categories that would hold throughout the year (Appendix S3). From the activity index values, we computed ‘total‐active‐time‐per‐day’ (in minutes per day) for the annual analysis, and ‘total‐active‐time‐per‐hour’ (in minutes per hour) for the summer diel analysis.
Spatio‐temporal variables
We analysed ibex behaviour in relation to environmental covariates year‐round and at the diel scale in summer only (Figure 1). We ordered the weeks starting from the first day of winter solstice (21st of December) and we defined ‘summer’ as the six‐month period preceding the approximate date of early snowfall, that is, from mid‐April to mid‐October (17th–42nd week; Appendix S1).
Static spatial covariates
For modelling female ibex altitude and habitat use, we used a high resolution Digital Elevation Model (5 m) (https://www.regione.veneto.it/web/ambiente‐e‐territorio/ctr; http://www.territorio.provincia.tn.it) and local land‐cover map (Scillitani et al., 2013; 50 m), to account for the pronounced topography of the study area. Habitats were categorised in five classes: ‘forest’ (coniferous and mixed forest, and shrubs), ‘grassland’ (Alpine pastures and natural grasslands), ‘scree’, ‘grassland‐mixed‐rock’ (grassland interspersed with rock and scree), and ‘rock’ (bare rocks).
Time‐varying spatial covariates
At the annual scale, we first averaged the daily temperatures of each weather station (Appendix S1) to obtain the weekly mean, and then averaged again across all stations to obtain the temporal series of the ‘mean‐weekly‐temperature’ for the study area. Similarly, we extracted the values of the Normalized Difference Vegetation Index (NDVI; MODIS‐NASA processed as in Klisch & Atzberger, 2016; Appendix S4) for 5,000 points within the study area (resolution: 250 m, 8 days) and averaged them to obtain the temporal series of mean vegetation productivity for the study area. We computed raster layers for the change in vegetation productivity (∆NDVI, commonly referred to as ‘green‐up’) by computing the standardised difference between values of subsequent NDVI layers. For these ∆NDVI time series, we finally evaluated the altitudinal gradient in plant phenology, or timing of green‐up, regressing ∆NDVI against altitude (Appendix S4).
At the diel scale, and for ‘summer’ only, we used the highest weather station (Appendix S1) to obtain records of ‘hourly‐temperature’ and ‘mean‐daily‐temperature’. We also reclassified the ‘mean‐daily‐temperature’ values in three levels based on quantiles, obtaining the categorical variable ‘daily‐summer‐temperature‐class’ (‘low’, 0–25%: ‘mean‐daily‐temperature’ ≤3.80°C; ‘intermediate’, 25–75%: >3.80°C and <10.2°C; and ‘high’, 75–100%: ≥10.2°C). To evaluate the effect of climate change on ibex behavioural responses, we used climate projections from low–medium emission and high emission rate scenarios (RCP 4.5 and RCP 8.5, respectively; Riahi et al., 2011; Thomson et al., 2011) to predict variation in the ‘mean‐daily‐temperature’ between 2006 and 2070 at the same weather station. We then derived the three levels of the ‘daily‐summer‐temperature‐class’ according to the quantiles of these distributions between 2051 and 2070 (Bucchignani et al., 2015; Appendix S5).
Statistical analysis
We analysed data in R 4.0.2 (R Core Team, 2016) using the package mgcv 1.8‐33 (Wood, 2018) to fit Generalized Additive Mixed Models (GAMM) to our empirical dataset. In all sets of models, we included individual identity as a random factor on the intercept and used Akaike Information Criterion (AIC; Burnham et al. 2002) for model selection. The sample size was 24 and 18 animals for analyses based on GPS and activity data, respectively, minus two animals for analyses including reproductive status.
Annual behavioural responses to temperature, vegetation productivity and its rate of change (Q1)
To evaluate whether activity and altitudinal movement of female ibex varied over the year in relation to thermal shelter and access to resources (Q1, Figure 1A), we first modelled the pattern of annual variation in ‘total‐active‐time‐per‐day’ (P1a) and ‘mean‐altitude‐used‐per‐day’ (P1b) by fitting GAMM models to the week as linear effect or cyclic cubic smoother (Table S6.1a), with year as a random effect on the intercept. Then, because vegetation productivity is dependent on temperature, we modelled NDVI as a linear function of ‘mean‐weekly‐temperature’, and extracted the residuals of NDVI not explained by temperature. Finally, we analysed both ‘total‐active‐time‐per‐day’ and ‘mean‐altitude‐used‐per‐day’ by fitting GAMM models to the smoothers of ‘mean‐weekly‐temperature’ and the residuals of the relation between NDVI and temperature, to disentangle their respective effects on the annual pattern of the response variables (Table S6.1c).
To further evaluate whether altitudinal movement allowed female ibex to exploit the change in vegetation productivity (‘green‐up’), we compared ∆NDVI values experienced by ibex at locations actually used in summer, with those of locations used by females in the latest part of winter (13th‐16th week; P1c). To this end, we built a binomial variable ‘summer_loc’ expressing whether ∆NDVI values referred to summer locations (‘summer_loc’ =1), or late winter ones (‘summer_loc’ =0). We fitted a GAMM to ∆NDVI with the cyclic cubic smoother of the week, ‘summer_loc’ as a fixed factor, and year as a random intercept (Table S6.2a).
Diel behavioural responses to heat‐stress during summer in relation to current conditions and climate change scenarios (Q2)
To evaluate whether female ibex compensated for thermal stress during summer by adjusting their diel pattern of activity and altitudinal movements (Q2.1), while maintaining access to feeding habitats (Q2.2) (Figure 1b), we computed ‘total‐active‐time‐per‐hour’ (in minutes per hour; P2.1a) and ‘mean‐altitude‐used‐per‐hour’ (P2.1c). We then modelled these two response variables by fitting GAMM models with linear or non‐linear effects of the hour of the day, with ‘hourly‐temperature’ or ‘daily‐summer‐temperature‐class’, and ‘reproductive‐status’ as fixed effects (Tables S6.3a‐S6.7a). We included daylength in all models to account for seasonality (Bonnot et al., 2016; Ensing et al., 2014; Krop‐Benesch et al., 2013). We also computed the ‘mean‐total‐diel‐activity‐budget’ for groups of days with different ‘daily‐summer‐temperature‐class’ values (P2.1b).
To forecast diel activity patterns of female ibex in relation to climate change scenarios, we re‐ran the model selection as for P2.1a (total‐active‐time‐per‐hour) using the covariate ‘daily‐summer‐temperature‐class’ computed under climate warming scenarios (Appendix S5; Tables S6.5a‐b). We then estimated ‘mean‐total‐diel‐activity‐budget’ for these predicted temperature classes (P2.1b). To predict altitudinal shifts due to climate warming (P2.1c), we modelled ‘maximum‐altitude‐used‐per‐day’ as a function of ‘mean‐daily‐temperature’ in interaction with ‘reproductive‐status’ (Table S6.9), and multiplied this rate by the expected temperature increase under climate warming scenarios.
Finally, we assessed summer habitat selection for each hour with respect to the rest of the day by means of hourly matched‐case Resource Selection Functions (RSF, Boyce & McDonald, 1999; P2.2). Specifically, for each day with at least 20 locations, we extracted 10 points per used location at a distance that was randomly extracted from the empirical distribution of the hourly step lengths across all locations and all individuals (Fig. S6.1), and at a random absolute angle drawn from a uniform distribution, thus obtaining a set of between 200 and 240 available points/individual/day. We matched each hourly location with its corresponding daily set of available points to fit two‐step conditional logistic regression models for each hour of the day, obtaining the relative probability of selecting a given habitat in relation to the observed daily trajectories of each female ibex (R package TwoStepCLogit, Craiu et al., 2011, 2016).
RESULTS
Annual behavioural responses to temperature, vegetation productivity and its rate of change (Q1)
At the annual scale, the total time that female ibex were active per day varied over the year (Table S6.1b, Figure 2a; adjusted R 2 = 0.46), with very low activity levels in core winter months, a sharp increase from late winter to the beginning of May (12th‐20th week), then a gradual decrease until early October (42nd week), when activity fell to winter levels. The annual trend of activity was mostly explained by ‘mean‐weekly‐temperature’, although NDVI residuals featured in the best model (Tables S6.1c‐d, Figure 2c; adjusted R 2 = 0.45; P1a). Activity was at minimum values for temperatures lower than −5°C, increased steeply above this threshold, before flattening out between 13 and 15°C. NDVI also explained some variation in annual activity when residuals were higher than 0.15 (Figure 2e), that is, in late summer (approximately 40th‐42nd week; see also Fig. S6.2).
FIGURE 2.

Panel (a): predictive annual pattern of ‘total‐active‐time‐per‐day’ for female ibex, monitored in the Marmolada massif from 2010 to 2016, fitted with a smoothed effect of the week, with individual identity and year as random effects on the intercept. Panel (b): predictive annual pattern of ‘mean‐altitude‐used‐per‐day’ by female ibex, monitored in the Marmolada massif from 2010 to 2016, fitted with a smoothed effect of the week, with individual identity and year as random effects on the intercept. In both panels, we superimposed the smoothed splines of mean NDVI (dashed green line) and 'mean‐daily‐temperature' (dotted tan line) of the study area, both modelled with a smoothed effect of the week and year as random effect on the intercept (Appendix S1, S4). The dashed vertical lines delimit summer and winter (17th–42nd week). Panel (c) and panel (d): predictive plots of the ‘total‐active‐time‐per‐day’ (panel c) and ‘mean‐altitude‐used‐per‐day’ (panel d) by female ibex monitored in the Marmolada massif from 2010 to 2016, fitted with a smoothed effect of the 'mean‐weekly‐temperature' of the study area with individual identity as random effect. Panel (e) and panel (f): predictive plots of the ‘total‐active‐time‐per‐day’ (panel e) and ‘mean‐altitude‐used‐per‐day’ (panel f) by female ibex monitored in the Marmolada massif from 2010 to 2016, fitted with a smoothed effect of the residuals of the linear regression of the 8‐day period NDVI in relation to the 'mean‐weekly‐temperature' of the study area, with individual identity as random effect. For panels (a, c and e): N = 18 (i.e., minus the six females for which collars could not be retrieved, hence activity data could not be downloaded); for panels (b, d and f): N = 24. In each panel, the shaded areas indicate 95% confidence intervals
Similarly, female ibex showed a seasonal shift towards higher altitudes (‘mean‐altitude‐used‐per‐day’, Table S6.1b, Figure 2b; adjusted R 2 = 0.59) that began at approximately the seasonal activity peak (20th week), increased at a rate that was notably synchronised with the increase in vegetation productivity (NDVI) over the study area, peaked in mid‐July (30th week), before subsequently declining, more sharply after early October (42nd week). The annual trend of the altitude used increased almost linearly with both ‘mean‐weekly‐temperature’ and NDVI residuals (P1b and P1c; Table S6.1d, Figure 2d–2f; adjusted R 2 = 0.61).
The change in vegetation productivity was positive (ΔNDVI>0, green‐up) and higher for locations actually used by ibex between mid‐April and late July (approximately 17th‐30th week; P1c; Table S6.2b, Figure 3; adjusted R 2 = 0.46), when female ibex moved towards higher altitudes (Figure 2b; Fig. S4.2 for the altitudinal gradient in the timing of vegetation green‐up), than for their late winter locations. From late July, vegetation productivity at ibex locations stabilised at high values for a few weeks (ΔNDVI~0, plateau of NDVI at high values, or delayed senescence; see also Fig. S4.1c vs S4.1a: NDVI curve of ibex locations vs whole study area is skewed towards later weeks, with delayed green‐up of habitats at high altitudes). Conversely, productivity started to decrease earlier at winter range locations (ΔNDVI<0, senescence, 29th week). Vegetation productivity at ibex locations dropped sharply during October (after 40th week), when ibex moved to lower altitudes.
FIGURE 3.

Predictive variation in the 8‐day relative values of the Normalized Difference Vegetation Index (ΔNDVI) for average locations used by 24 female ibex, monitored in the Marmolada massif from 2010 to 2016, fitted with a smoothed effect of the week (solid line, green shade for the 95% confidence intervals), compared with the ΔNDVI of locations used by the females during the last 4 weeks of winter (dashed line, blue shade for the 95% confidence intervals). The dashed vertical lines delimit summer and winter (17th–42nd week)
Diel behavioural responses to heat‐stress during summer in relation to current conditions and climate change scenarios (Q2)
During summer, female ibex followed a very strong bimodal pattern of diel activity that varied in intensity in relation to ‘mean‐daily‐temperature‐class’ (Table S6.3b, Figure 4a; only two classes shown for clarity). Activity peaked in the early morning and in late afternoon or evening, and was lowest at mid‐morning (Figure 4a). During hot days, this daily low was much more pronounced compared to cooler days (‘active‐time‐per‐hour’ almost 50% lower). As a result, ibex were active earlier (30’‐40’ ca), and the evening peak occurred later (about an hour, or more) and lasted longer on hot days. Reproductive females behaved somewhat differently during hot days, being more active at dawn and mid‐morning (by about 15%), but compensating at night with a lower activity level, comparable to that of cool days. In all cases, these behavioural adjustments generated a total activity budget per day that was practically constant (reproductive and non‐reproductive females, respectively, for cool days: 12 h 03’±16’ and 11 h 59’±13’; for hot days: 12 h 15’±10’ and 12 h 14’±9’; Table S6.4).
FIGURE 4.

Predictive plots for daily activity and altitude used in summer by female ibex, monitored in the Marmolada massif from 2010 to 2016. Panel (a): ‘total‐active‐time‐per‐hour’ (in minutes) fitted with a cyclic cubic smoother of the hour (local time) as a function of ‘mean‐daily‐temperature‐class’, and ‘reproductive‐status’ (high temperature class: ≥10.2°C, orange shade; low: ≤3.7°C, blue shade; continuous and dashed lines: reproductive and non‐reproductive females, respectively). Panel (b): same model with ‘mean‐daily‐temperature‐classes’ estimated under the projections under the climate warming scenario IPCC RCP 8.5 (high: ≥14.5°C; low: ≤6.5°C). In both panels (a) and (b), the intermediate ‘mean‐daily‐temperature‐class’ was omitted for clarity. Panel (c): ‘total‐active‐time‐per‐hour’ fitted with a spline smoother of hourly temperature (as continuous variable) as a function of ‘reproductive‐status’. Panel (d): ‘mean‐altitude‐used‐per‐hour’ fitted with a spline smoother of hourly temperature as a function of ‘reproductive‐status’. Panel (e): daily pattern of ‘mean‐altitude‐used‐per‐hour’ fitted with a spline smoother of the hour as a function of ‘reproductive‐status’. Panel (f): ‘maximum‐daily‐altitude‐used’ as a linear regression of ‘mean‐daily‐temperature’ in interaction with ‘reproductive‐status’. For panels (a, b and c): N = 16 (i.e., minus six females for which collars could not be retrieved, hence activity data could not be downloaded, and two females for which the ‘reproductive‐status’ could not be assessed); for panels (d, e and f): N = 22 (i.e., minus two females for which the ‘reproductive‐status’ could not be assessed). Shaded areas denote the 95% confidence intervals. The panels marked with an asterisk represent predictions of the most parsimonious models (Tables S6.3b for panel a; S6.5c for panel b; S6.8 for panel d)
The analysis performed on the forecasted temperature classes for the two climate warming scenarios produced similar patterns (Tables S6.5c), but with more pronounced lows during the hottest hours, higher peaks at twilight (especially at dusk, and dawn for reproductive females), and higher nighttime activity, especially for non‐reproductive females (RCP 8.5: Figure 4b; RCP 4.5: Fig. S6.3). Under these forecasts, ‘daily‐activity‐budget’ of ibex was projected to be almost identical to that under current conditions (Table S6.4). However, the activity peaks were further displaced towards nighttime, even with respect to the current hottest days (ca half an hour earlier in the mornings or later in the evenings for non‐reproductive and reproductive females, respectively; RCP 8.5). For visualisation purposes, we also plotted the ‘active‐time‐per‐hour’ predicted by ‘hourly‐temperature’ as a continuous variable (Table S6.6): the activity level of female ibex steadily increased with temperature up to 14°C and 13°C for reproductive and non‐reproductive females, respectively, before dropping sharply beyond this threshold (Figure 4c). During the study period (2010–2016), female ibex actually experienced a ‘mean‐daily‐temperature’ >13°C (16 days in median) on less than 10% of summer days. From climate forecasts, more than 25% of summer days would exceed this threshold under both climate warming scenarios (median of 46.5 days and 55.5 days for RCP 4.5 and RCP 8.5, respectively; Appendix S5, Fig. S5.2).
Altitude used by female ibex increased almost linearly as a function of ‘hourly‐temperature’ for reproductive females, while non‐reproductive females exhibited a lower rate of increase above 15°C (Table S6.7b; Figure 4d). As a result, females moved daily over an altitudinal gradient of about 50 to 80 m (reproductive and non‐reproductive, respectively; Figure 4e), with an upslope shift after dawn to reach the highest altitudes around mid‐morning, and a gradual return to lower altitudes during the day (Figure 4e; Table S6.8). The maximum altitude used by ibex increased by 29.7 m and 25.2 m for each °C increase in ‘mean‐daily‐temperature’ for reproductive and non‐reproductive females, respectively (Figure 4f; Table S6.9). This would result in an average projected upward shift of around 89.2 m and 75.5 m respectively under RCP 8.5 (62.4 m and 52.9 m under RCP 4.5), for the period 2051–2070. The spatial projections of these forecasts onto our study area (Figure 5 – S6.4 – S6.5) revealed that these distributional upslope shifts would correspond to a reduction of available suitable habitats (RCP 8.5: 33% and 27% less available surface due to the conic mountain shape for reproductive and non‐reproductive females, respectively, consisting in +16% and +13% ‘rock’; −1% and +4% ‘scree’; −37% and −33% ‘grassland‐mixed‐rock’; −57% and −52% ‘grassland’; and no more ‘forest’, based on the current landcover).
FIGURE 5.

Spatial projection of the ‘maximum‐daily‐altitude‐used’ (25%–75% of the distribution) by reproductive female ibex in the Marmolada massif in summer, according to IPCC RCP 8.5 warming scenario. Blue line‐delimited polygons: projection for 2006–2025; red line‐delimited polygons: projection for 2051–2070; shaded area: overlap between the two projections. See Fig. S6.4 for non‐reproductive females, scenario RCP 8.5; Fig. S6.5 for both reproductive and non‐reproductive females, scenario RCP 4.5. The image was generated with QGIS 3.16 (QGIS.org, 2021. QGIS Geographic Information System. QGIS Association)
During summer, the relative probability of use of land‐cover types by female ibex varied over the day in concert with variations in activity and altitude used (Figure 6). ‘Rock’ and ‘scree’ were selected more than ‘forest’ during daylight. ‘Grassland’ was positively selected during the two activity peaks (early morning and late evening‐early night). ‘Grassland‐mixed‐rock’ was selected more than ‘forest’ during daylight. With the exception of ‘grassland‐mixed‐rock’, all land cover types were negatively selected with respect to ‘forest’ at night.
FIGURE 6.

Plot of the coefficients of the relative probability of use of land‐cover types (‘grassland’: green filled circles, ‘grassland‐mixed‐rock’: turquoise squares, ‘rock’: blue triangles, ‘scree’: red open circles) by 24 female ibex in summer, monitored in the Marmolada massif from 2010 to 2016, as estimated by hourly Resource Selection Functions. Used locations refer to each hour, and are conditionally matched to all available locations on the same day. The coefficients are relative to ‘forest’ as the reference category
DISCUSSION
In this work, we evaluated the hypothesis that behavioural plasticity in space use promotes resilience to current climate change (Boyles et al., 2011; Huey et al., 2012). We showed that female ibex used compensatory behavioural mechanisms to cope with heat‐stress, while satisfying foraging needs, at both annual (Q1; Figure 1a) and daily (Q2; Figure 1b) scales. Female ibex exploited the altitudinal gradient by migrating seasonally upslope in synchrony with variation in ambient temperature and vegetation green‐up, surfing the green wave to benefit from delayed senescence of forage at high altitudes. Similarly, in summer, females also compensated for heat‐stress through a bimodal, temperature‐mediated, diel activity cycle. In particular, they foraged more intensively at dawn and dusk, when more productive habitats were selected, and rested in thermal‐refuges at high altitudes during the hottest hours of the day. These responses were modulated in relation to reproductive constraints, as females with kids limited their diel altitudinal shifts, remaining at higher altitudes, and were highly active during the day, with little‐to‐no compensation at night. Our forecasts under climate change scenarios, however, indicate that these mechanisms will likely be insufficient to behaviourally shield Alpine ibex from future thermal stress, because: (1) critical temperatures will occur much more frequently, so that physiological and behavioural thermoregulation is likely to be stretched to the limit, with resilience to high temperature then becoming a selective pressure (Huey et al., 2012); and (2) habitats that provide shelter will become rarer due to the altitudinal limits of the mountain range. As reproductive females adopted a different behavioural tactic compared to non‐reproductive individuals, remaining at higher altitudes and foraging more during daytime, the effects of increased temperature on Alpine ibex fitness needs to be further evaluated, considering the energetic and behavioural constraints of having kids at heel.
Species that are exposed to an increase in temperature beyond the thermal limits within which they evolved are expected to experience decreased fitness (Huey et al., 2012), as they pay an energetic cost through either heat dissipation, or reduced resource intake (Speakman & Krol, 2010). The temperature trends in the Alps may induce a contraction or diel time‐shift of foraging bouts (Bourgoin et al., 2011; Grignolio et al., 2018; Mason et al., 2014b). Here, we showed that female ibex drastically reduced activity above the threshold value of 13–14°C (previously reported as limits for heat‐stress: Aublet et al., 2009; Signer et al., 2011) and compensated by increasing twilight and nocturnal foraging (P2.1a), a behaviour so far not investigated in ibex. The total daily time spent foraging was, as a result, almost constant across a wide range of mean daily temperatures (P2.1b). At the same time, ibex selected forage habitats during peak activity, and refuge habitats during resting periods (P2.2). Therefore, by moving altitudinally (P2.1c) through food‐rich habitats when shifting between resting sites, female ibex optimised access to both thermal‐cover and high quality resources. In this context, reproductive females are particularly affected by environmental constraints because, as ‘follower’ ungulates (Lent, 1974), they must trade‐off their higher energetic requirements against the vulnerability and limited mobility of offspring (Barber‐Meyer & Meach, 2008). Reproductive females were shown to select specific habitats (Grignolio et al., 2007), typically remaining at high altitude, likely to limit movements, obtain thermal protection and access refuge areas. Climate models indicate a marked increase (from 8% to 25%) in the proportion of summer days leading to heat‐stress, to which ibex may respond by further shifting foraging towards the night (by one hour in addition to that currently observed on hot days) and upslope (up to 90 m). Activity budget compensation of reproductive females, that we show for the first time in an Alpine ungulate, might be limited by the lessened mobility of kids at night on steep and treacherous cliffs. Moreover the spatial projections showed that further range shifts towards higher elevations would be limited by mountain ridges, and the likely reduction in foraging habitats (up to 60% less, based on current landcover). Temperate conditions are intruding into Alpine regions, with a predictable upward shift of vegetation (Rubel et al., 2017), especially forest habitat (Leonelli et al., 2011). For grasslands, however, this shift will be likely limited in the Dolomites because of the lack of specific adaptations of plants to rocky and unstable substrates (Cannone et al., 2003).
Climate affects mountain ungulates even indirectly, via spatio‐temporal variation of forage availability (Aikens et al., 2020). In a capital breeder, like Alpine ibex (Toïgo et al., 2002), fitness depends on cumulative resource acquisition throughout summer. In our study, we showed that activity of female ibex varied across the year, driven mainly by temperature (P1a), while seasonal altitudinal migration positively depended both on temperature (see Herfindal et al., 2019) and the increase in vegetation productivity (P1b), previously shown to index availability of high quality vegetation (Hamel et al., 2009). This expands on previous studies that indicated a trade‐off between the selection of thermal refuges vs. optimal forage habitats (Brivio et al., 2019; Mason et al., 2017). Notably, ibex increased rapidly their activity in late winter and started to move upslope a few weeks later. Hence, our results suggest that ibex initially fed on new shoots of vegetation in the winter range at the snowmelt, before exploiting the altitudinal gradient in vegetation green‐up by migrating upslope. During this period, ibex were able to access areas with a more rapid green‐up compared to their winter ranges, despite lower absolute vegetation productivity (P1c). As the green‐up slowed, female ibex ceased their altitudinal migration, remaining at altitudes that experienced a delay in vegetation senescence, thus optimising resource acquisition up to the onset of winter (35th‐42nd week). Our evidence is one of the first demonstrations of the Green Wave Hypothesis for a Caprinae (but see Jesmer et al., 2018), as it was previously tested for grazers and intermediate feeders (Aikens et al., 2020; Bischof et al., 2012; Geremia et al., 2019; Merkle et al., 2016). Furthermore, our results indicate the importance for ibex of acquiring high quality resources also later in the season, exploiting delayed senescence of vegetation at high altitudes. This can be expected in a capital breeder, relying on cumulative resource acquisition more than on instantaneous high gain, opposed to income breeders (Kerby & Post, 2013). Further research on the biomass and quality of plant species consumed in these extreme environments in relation to green‐up phenology could clarify the potential impact of climate change on behavioural adaptations, and the consequences for individual fitness (Mason et al., 2014a; Lovari et al., 2020), such as the potential mismatch with the energetic demands of species (Williams et al., 2017).
The conservation ecology of this and other iconic mountain ungulates under direct and indirect threats of climate change would greatly benefit from large‐scale comparative studies across a range of geomorphological contexts. In this regard, it will be important to evaluate whether physiological traits are becoming maladaptive, and to what degree behavioural responses provide mechanisms to compensate for higher temperatures and so increase resilience, as we have shown here for the Alpine ibex.
AUTHORSHIP
FC and MR conceived and designed the study. ES, MR and PS organised and managed animal marking. PS collected and managed the bio‐telemetry and observational data. EE processed climate warming projections. PS, FC and MR designed statistical analyses. PS analysed the data. PS and FC wrote the first draft of the manuscript, with comments from NM, AJMH, FO and MR. All co‐authors revised further versions of the manuscript. MR competitively obtained funds to support the study, with contributions from FC.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
ACKNOWLEDGEMENTS
Regional Police Corp of Belluno and Forestry Police Corp of the State ‐ Veneto section and Luca Rossi (Department of Veterinary Sciences, Torino University) provided strong logistic support and essential help in capturing ibex and rescuing collars after drop‐off. The authors also thank M. Á. Párraga Aguado, and members of the Ramanzin and Cagnacci Labs for help in collar rescuing. M. Á. Párraga Aguado contributed to data collection and management during the early periods of the study. F. Urbano and J. De Groeve provided helpful suggestions on the database structure and data management procedure. Regional Agency for the Environmental Protection – Meteorological Service (Agenzia Regionale per la Prevenzione e Protezione Ambientale del Veneto ‐ Dipartimento Regionale Sicurezza del Territorio Servizio Meteorologico, ARPAV) provided the meteorological data. We also thank Paola Mercogliano (Euro‐Mediterranean Center on Climate Change, CMCC) for providing the original climate projections, using data supplied by the GEMINA project (funded by the Italian Ministry of Education, University, and Research and by the Italian Ministry of the Environment, Land, and Sea). This project was funded by University of Padova, project n. CPDA094513/09, MIUR ex 60%, projects 60A08‐2154/14 and 60A08‐2017/15, and co‐funded by Veneto region, Wildlife and Hunting Service (Regione Veneto‐Unità di Progetto Caccia e Pesca) and Fondazione Edmund Mach, ordinary funds from Autonomous Province of Trento. We are grateful to the editor and three anonymous reviewers for very insightful comments on earlier versions of the paper.
DATA AVAILABILITY STATEMENT
Data are currently stored in the Euroungulates database (www.euromammals.org) and access can be provided after log in. Datasets and scripts used for analysis are published on Zenodo, https://doi.org/10.5281/zenodo.4625629
References
- Aikens, E.O., Kauffman, M.J., Merkle, J.A., Dwinnell, S.P.H., Fralick, G.L. & Monteith, K.L. (2017) The greenscape shapes surfing of resource waves in a large migratory herbivore. Ecology Letters, 20, 741–750. [DOI] [PubMed] [Google Scholar]
- Aikens, E.O., Mysterud, A., Merkle, J.A., Cagnacci, F., Rivrud, I.M., Hebblewhite, M. et al. (2020) Wave‐like patterns of plant phenology determine ungulate movement tactics. Current Biology, 30, 3444–3449. [DOI] [PubMed] [Google Scholar]
- Arnold, W. (1988) Social thermoregulation during hibernation in alpine marmots (Marmota marmota). Journal of Comparative Physiology B, 158, 151–156. [DOI] [PubMed] [Google Scholar]
- Arnold, W., Ruf, T. & Kuntz, R. (2006) Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) II. Energy expenditure. Journal of Experimental Biology, 209, 4566–4573. [DOI] [PubMed] [Google Scholar]
- Arnold, W., Ruf, T., Reimoser, S., Tataruch, F., Onderscheka, K. & Schober, F. (2004) Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus). American Journal of Physiology ‐ Regulatory, Integrative and Comparative Physiology, 286, R174–R181. [DOI] [PubMed] [Google Scholar]
- Aublet, J.‐F., Festa‐Bianchet, M., Bergero, D. & Bassano, B. (2009) Temperature constraints on foraging behaviour of male Alpine ibex (Capra ibex) in summer. Oecologia, 159, 237–247. [DOI] [PubMed] [Google Scholar]
- Barber‐Meyer, S. & Mech, L. (2008) Factors influencing predation on juvenile ungulates and natural selection implications. Wildlife Biology in Practice, 4(1), 8–29. [Google Scholar]
- Bischof, R., Loe, L.E., Meisingset, E.L., Zimmermann, B., Van Moorter, B., Mysterud, A. et al. (2012) A migratory northern ungulate in the pursuit of spring: Jumping or surfing the green wave? American Naturalist, 180, 407–424. [DOI] [PubMed] [Google Scholar]
- Bonnot, N.C., Morellet, N., Hewison, A.J.M., Martin, J.‐L., Benhamou, S. & Chamaillé‐Jammes, S. (2016) Sitka black‐tailed deer (Odocoileus hemionus sitkensis) adjust habitat selection and activity rhythm to the absence of predators. Canadian Journal of Zoology, 94, 385–394. [Google Scholar]
- Bourgoin, G., Garel, M., Blanchard, P., Dubray, D., Maillard, D. & Gaillard, J.‐M. (2011) Daily responses of mouflon (Ovis gmelini musimon×Ovis sp.) activity to summer climatic conditions. Canadian Journal of Zoology, 89, 765–773. [Google Scholar]
- Bourgoin, G., Garel, M., Van Moorter, B., Dubray, D., Maillard, D., Marty, E. et al. (2008) Determinants of seasonal variation in activity patterns of mouflon. Canadian Journal of Zoology, 86, 1410–1418. [Google Scholar]
- Boyce, M.S. & McDonald, L.L. (1999) Relating populations to habitats using resource selection functions. Trends in Ecology & Evolution, 14, 268–272. [DOI] [PubMed] [Google Scholar]
- Boyles, J.G., Seebacher, F., Smit, B. & McKechnie, A.E. (2011) Adaptive thermoregulation in endotherms may alter responses to climate change. Integrative and Comparative Biology, 51, 676–690. [DOI] [PubMed] [Google Scholar]
- Brivio, F., Zurmühl, M., Grignolio, S., von Hardenberg, J., Apollonio, M. & Ciuti, S. (2019) Forecasting the response to global warming in a heat‐sensitive species. Scientific Reports, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucchignani, E., Montesarchio, M., Zollo, A.L. & Mercogliano, P. (2015) High‐resolution climate simulations with COSMO‐CLM over Italy: Performance evaluation and climate projections for the 21st century. International Journal of Climatology, 36, 735–756. [Google Scholar]
- Burnham, K.P. & Anderson, D.R. (2002) Model selection and multimodel inference: a practical information‐theoretic approach, 2nd edition. New York: Springer. [Google Scholar]
- Cannone, N. & Gerdol, R. (2003) Vegetation as an ecological indicator of surface instability in rock glaciers. Arctic, Antarctic, and Alpine Research, 35, 384–390. [Google Scholar]
- Craiu, R.V., Duchesne, T., Fortin, D. & Baillargeon, S. (2011) Conditional logistic regression with longitudinal follow‐up and individual‐level random coefficients: A stable and efficient two‐step estimation method. Journal of Computational and Graphical Statistics, 20, 767–784. [Google Scholar]
- Craiu, R.V., Duchesne, T., Fortin, D., Baillargeon, S. & Duchesne, M.T. (2016). Package ‘TwoStepCLogit.’
- Ensing, E.P., Ciuti, S., de Wijs, F.A.L.M., Lentferink, D.H., ten Hoedt, A., Boyce, M.S. et al. (2014) GPS based daily activity patterns in European red deer and North American elk (Cervus elaphus): indication for a weak circadian clock in ungulates. PLoS One, 9, e106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernakovich, J.G., Hopping, K.A., Berdanier, A.B., Simpson, R.T., Kachergis, E.J., Steltzer, H. et al. (2014) Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Global Change Biology, 20, 3256–3269. [DOI] [PubMed] [Google Scholar]
- Geremia, C., Merkle, J.A., Eacker, D.R., Wallen, R.L., White, P.j., Hebblewhite, M. et al. (2019) Migrating bison engineer the green wave. Proceedings of the National Academy of Sciences, 116, 25707–25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi, V., Brunberg, S. & Swenson, J.E. (2006) An individual‐based method to measure animal activity levels: A test on brown bears. Wildlife Society Bulletin, 34, 1314–1319. [Google Scholar]
- Green, R.A. & Bear, G.D. (1990) Seasonal cycles and daily activity patterns of Rocky Mountain elk. The Journal of Wildlife Management, 54, 272–279. [Google Scholar]
- Grignolio, S., Brivio, F., Apollonio, M., Frigato, E., Tettamanti, F., Filli, F. et al. (2018) Is nocturnal activity compensatory in chamois? A study of activity in a cathemeral ungulate. Mammalian Biology, 93(1), 173–181. [Google Scholar]
- Grignolio, S., Rossi, I., Bertolotto, E., Bassano, B. & Apollonio, M. (2007) Influence of the kid on space use and habitat selection of female Alpine ibex. Journal of Wildlife Management, 71, 713–719. [Google Scholar]
- Grignolio, S., Rossi, L., Bassano, B., Parrini, F. & Apollonio, M. (2004) Seasonal variations of spatial behaviour in female Alpine ibex (Capra ibex ibex) in relation to climatic conditions and age. Ethology Ecology and Evolution, 16, 255–264. [Google Scholar]
- Hamel, S. & Côté, S.D. (2008) Trade‐offs in activity budget in an alpine ungulate: contrasting lactating and nonlactating females. Animal Behavior, 75, 217–227. [Google Scholar]
- Hamel, S., Garel, M., Festa‐Bianchet, M., Gaillard, J.‐M. & Côté, S.D. (2009) Spring Normalized Difference Vegetation Index (NDVI) predicts annual variation in timing of peak faecal crude protein in mountain ungulates. Journal of Applied Ecology, 46, 582–589. [Google Scholar]
- Hebblewhite, M. & Merrill, E.H. (2009) Trade‐offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology, 90, 3445–3454. [DOI] [PubMed] [Google Scholar]
- Heldmaier, G., Ortmann, S. & Elvert, R. (2004) Natural hypometabolism during hibernation and daily torpor in mammals. Respiratory Physiology and Neurobiology Hypoxic Hypometabolism, 141, 317–329. [DOI] [PubMed] [Google Scholar]
- Herfindal, I., Anderwald, P., Filli, F., Andri, S.C. & Rempfler, T. (2019) Climate, competition and weather conditions drive vertical displacement and habitat use of an alpine ungulate in a highly topographic landscape. Landscape Ecology, 34, 2523–2539. [Google Scholar]
- Huey, R.B., Kearney, M.R., Krockenberger, A., Holtum, J.A.M., Jess, M. & Williams, S.E. (2012) Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesmer, B.R., Merkle, J.A., Goheen, J.R., Aikens, E.O., Beck, J.L., Courtemanch, A.B. et al. (2018) Is ungulate migration culturally transmitted? Evidence of social learning from translocated animals. Science, 361, 1023–1025. [DOI] [PubMed] [Google Scholar]
- Kerby, J. & Post, E. (2013) Capital and income breeding traits differentiate trophic match–mismatch dynamics in large herbivores. Philosophical Transactions of the Royal Society B: Biological Sciences, 368, 20120484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klisch, A. & Atzberger, C. (2016) Operational drought monitoring in Kenya using MODIS NDVI time series. Remote Sensing, 8, 267. [Google Scholar]
- Krop‐Benesch, A., Berger, A., Hofer, H. & Heurich, M. (2013) Long‐term measurement of roe deer (Capreolus capreolus) (Mammalia: Cervidae) activity using two‐axis accelerometers in GPS‐collars. The Italian Journal of Zoology, 80, 69–81. [Google Scholar]
- Lent, P.C. (1974). Mother‐infant relationships in ungulates. In: Geist, V. & Walther, F. (Eds.) The behaviour of ungulates and its relation to management, Morges, Switzerland: IUCN, Vol. I, pp. 14‐55. [Google Scholar]
- Leonelli, G., Pelfini, M., Morra di Cella, U. & Garavaglia, V. (2011) Climate warming and the recent treeline shift in the european alps: the role of geomorphological factors in high‐altitude sites. Ambio, 40, 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovari, S., Franceschi, S., Chiatante, G., Fattorini, L., Fattorini, N. & Ferretti, F. (2020) Climatic changes and the fate of mountain herbivores. Climatic Change, 162(4), 2319–2337. [Google Scholar]
- Mason, T.H.E., Apollonio, M., Chirichella, R., Willis, S.G. & Stephens, P.A. (2014a) Environmental change and long‐term body mass declines in an alpine mammal. Frontiers in Zoology, 11, 69. [Google Scholar]
- Mason, T.H.E., Brivio, F., Stephens, P.A., Apollonio, M. & Grignolio, S. (2017) The behavioral trade‐off between thermoregulation and foraging in a heat‐sensitive species. Behavioral Ecology, 28, 908–918. [Google Scholar]
- Mason, T.H.E., Stephens, P.A., Apollonio, M. & Willis, S.G. (2014b) Predicting potential responses to future climate in an alpine ungulate: interspecific interactions exceed climate effects. Global Change Biology, 20, 3872–3882. [DOI] [PubMed] [Google Scholar]
- Merkle, J.A., Monteith, K.L., Aikens, E.O., Hayes, M.M., Hersey, K.R., Middleton, A.D. et al. (2016) Large herbivores surf waves of green‐up during spring. Proceedings of the Royal Society B‐Biological Sciences, 283, 20160456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan, R., Getz, W.m., Revilla, E., Holyoak, M., Kadmon, R., Saltz, D. et al. (2008) A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences, 105, 19052–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, M.C., Bowyer, R.T. & Kie, J.G. (1997) Habitat selection and survival of mule deer: tradeoffs associated with migration. Journal of Mammalogy, 78, 483–504. [Google Scholar]
- Parolo, G. & Rossi, G. (2008) Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology, 9, 100–107. [Google Scholar]
- Párraga Aguado, M.Á., Sturaro, E. & Ramanzin, M. (2017) Individual activity interacts with climate and habitat features in influencing GPS telemetry performance in an Alpine herbivore. Hystrix Ital . Journal of Mammalogy, 28, 36–42. [Google Scholar]
- Peters, W., Hebblewhite, M., Mysterud, A., Eacker, D., Hewison, A.J.M., Linnell, J.D.C. et al. (2019) Large herbivore migration plasticity along environmental gradients in Europe: life‐history traits modulate forage effects. Oikos, 128, 416–429. [Google Scholar]
- Pettorelli, N., Pelletier, F., von Hardenberg, A., Festa‐Bianchet, M. & Côté, S.D. (2007) Early onset of vegetation growth Vs. rapid green‐up: Impacts on juvenile mountain ungulates. Ecology, 88, 381–390. [DOI] [PubMed] [Google Scholar]
- R Core Team (2016) R: A language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing. [Google Scholar]
- Rammig, A., Jonas, T., Zimmermann, N.E. & Rixen, C. (2010) Changes in alpine plant growth under future climate conditions. Biogeosciences, 7, 2013–2024. [Google Scholar]
- Riahi, K., Rao, S., Krey, V., Cho, C., Chirkov, V., Fischer, G. et al. (2011) RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Climatic Change, 109, 33. [Google Scholar]
- Rice, C.G. (2008) Seasonal altitudinal movements of mountain goats. The Journal of Wildlife Management, 72, 1706–1716. [Google Scholar]
- Rubel, F., Brugger, K., Haslinger, K. & Auer, I. (2017) The climate of the European Alps: Shift of very high resolution Köppen‐Geiger climate zones 1800–2100. Meteorologische Zeitschrift, 26, 115–125. [Google Scholar]
- Scillitani, L., Darmon, G., Monaco, A., Cocca, G., Sturaro, E., Rossi, L. et al. (2013) Habitat selection in translocated gregarious ungulate species: An interplay between sociality and ecological requirements. The Journal of Wildlife Management, 77, 761–769. [Google Scholar]
- Signer, C., Ruf, T. & Arnold, W. (2011) Hypometabolism and basking: The strategies of Alpine ibex to endure harsh over‐wintering conditions. Functional Ecology, 25, 537–547. [Google Scholar]
- Speakman, J.R. & Król, E. (2010) Maximal heat dissipation capacity and hyperthermia risk: Neglected key factors in the ecology of endotherms: Heat dissipation limit theory. Journal of Animal Ecology, 79, 726–746. [DOI] [PubMed] [Google Scholar]
- Thomson, A.M., Calvin, K.V., Smith, S.J., Kyle, G.P., Volke, A., Patel, P. et al. (2011) RCP4.5: A pathway for stabilization of radiative forcing by 2100. Climatic Change, 109, 77. [Google Scholar]
- Toïgo, C., Gaillard, J.‐M., Gauthier, D., Girard, I., Martinot, J.‐P. & Michallet, J. (2002) Female reproductive success and costs in an alpine capital breeder under contrasting environments. Ecoscience, 9, 427–433. [Google Scholar]
- Urbano, F. & Cagnacci, F. (2014) Spatial Database for GPS Wildlife Tracking Data. Cham: Springer International Publishing. [Google Scholar]
- van Beest, F.M. & Milner, J.M. (2013) Behavioural responses to thermal conditions affect seasonal mass change in a heat‐sensitive northern ungulate. PLoS One, 8, e65972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beest, F.M., Van Moorter, B. & Milner, J.M. (2012) Temperature‐mediated habitat use and selection by a heat‐sensitive northern ungulate. Animal Behavior, 84, 723–735. [Google Scholar]
- Williams, C.T., Klaassen, M., Barnes, B.M., Buck, C.L., Arnold, W., Giroud, S. et al. (2017) Seasonal reproductive tactics: Annual timing and the capital‐to‐income breeder continuum. Phil. Trans. R. Soc. B, 372, 20160250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipf, S., Stoeckli, V. & Bebi, P. (2009) Winter climate change in alpine tundra: Plant responses to changes in snow depth and snowmelt timing. Climatic Change, 94, 105–121. [Google Scholar]
- Wong, B.B.M. & Candolin, U. (2015) Behavioral responses to changing environments. Behavioral Ecology, 26, 665–673. [Google Scholar]
- Wood, S. (2018) Mixed GAM computation vehicle with GCV/AIC/REML smoothness estimation and GAMMs by REML/PQL. R package version, 1.8‐23.
- Zeng, Z.‐G., Skidmore, A.K., Song, Y.‐L., Wang, T.‐J. & Gong, H.‐S. (2008) Seasonal altitudinal movements of golden takin in the qinling mountains of China. The Journal of Wildlife Management, 72, 611–617. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Data Availability Statement
Data are currently stored in the Euroungulates database (www.euromammals.org) and access can be provided after log in. Datasets and scripts used for analysis are published on Zenodo, https://doi.org/10.5281/zenodo.4625629


