Abstract
Ghrelin and its endogenous antagonist liver‐expressed antimicrobial peptide‐2 (LEAP‐2) are involved in GH secretion and glucose/lipids metabolism. LEAP‐2 expression in conditions of metabolic impairment may be upregulated, usually pairing with a concomitant reduction in ghrelin secretion. Adult growth hormone deficiency (aGHD) is characterized by insulin resistance, weight gain, and increased fat mass. Therefore, the primary endpoint of this cross‐sectional observational pilot study was to compare circulating LEAP‐2 and ghrelin levels in aGHD and healthy controls. Thirty patients were included in the study. Group A included adult GHD: 15 patients, 8 females, and 7 males. Median and interquartile range age of the group was 53 (41–57) years, while BMI was 27.1 (25–35) kg/m2. Group B was formed by 15 healthy controls (10 females and 5 males). Median and interquartile range age was 47 (36–57) years, while BMI 22.9 (20.8–33.1) kg/m2. They were evaluated for serum glucose and insulin, HOMA‐index, QUICKI‐index, total/LDL/HDL cholesterol, triglycerides, IGF‐1, ghrelin, and LEAP‐2. Ghrelin levels in the aGHD group were significantly lower than in healthy controls. In contrast, LEAP‐2 showed a trend toward higher levels, although the differences were not significant. However, the LEAP‐2/Ghrelin ratio was significantly higher in aGHD. No significant correlations between ghrelin and LEAP‐2 with BMI and HOMA index were found in aGHD population. However, a significant inverse correlation (r 2 = 0.15, p = .047) between BMI and ghrelin was evidenced when considering the whole population. Taken together, these results may suggest a body adaptation to a metabolic scenario typical of aGHD. The decrease in ghrelin production could prevent further weight gain and fat mass increase, although losing its secretagogue effect.
Keywords: biomarkers, GHD, ghrelin, insulin resistance, LEAP‐2
Abbreviations
- aGHD
adult growth hormone deficiency
- GHRH
Growth Hormone‐Releasing Hormone
- GHS‐R
Growth hormone secretagogue receptor
- HDL
High‐density lipoprotein
- IGF‐1
Insulin‐like growth factor‐1
- LEAP‐2
Liver expressed antimicrobial peptide
- LDL
Low‐density lipoprotein
- r‐hGH
Recombinant human GH
1. INTRODUCTION
Adult growth hormone deficiency (aGHD) is defined as the clinical expression of a reduced GH secretion caused by congenital or acquired diseases affecting the hypothalamus or pituitary gland.1 Metabolic impairments are among GHD most known features. An increase in visceral fat and reduction in lean mass closely related to worse muscle strength and reduced exercise tolerance has been described.2 Adult GHD patients, with higher or normal BMI, are often affected by impaired glucose tolerance, insulin resistance, and a high risk of developing type 2 diabetes mellitus.3 Finally, as regards the lipid profile, GHD is characterized by an increase in total cholesterol, low‐density lipoprotein (LDL) cholesterol, triglycerides, and a reduction in high‐density lipoprotein (HDL) cholesterol.4 All these features concur to the well‐known cardiovascular complications of this disease.5
GH secretion is modulated by several stimuli, among which ghrelin plays a pivotal role. Ghrelin is a peptide hormone produced by the oxyntic glands of the gastric fundus which acts as endogenous ligand for growth hormone secretagogue receptor (GHS‐R) 1a, stimulating GH release from the anterior pituitary gland.6 Among its main functions: food intake and satiety control7, 8, 9; body weight, adiposity, and glucose metabolism regulation10; stimulation of gut motility and gastric acid secretion11, 12; modulation of stress, anxiety, taste, and reward seeking behavior,13, 14 and anti‐sarcopenic action.15, 16 In GHD ghrelin levels tend to be lower than healthy controls with no change after GH replacement therapy.17
Ghrelin physiological regulation has been enriched with the recent discovery of liver‐expressed antimicrobial peptide‐2 (LEAP‐2),18 released in circulation after gastrointestinal secretion (with highest expression in the jejunum, followed by duodenum, ileum and liver). LEAP‐2 has been originally described as antimicrobial peptide with hydrolyzing properties.19 Its action on ghrelin axis has been recently defined: LEAP‐2 binds GHS‐R1a and acts as a competitive antagonist with slow dissociation from the receptor. It blunts the magnitude of ghrelin activation binding GSH‐R1a, acting as a brake for all the ghrelin‐related functions above cited.20, 21 It could also exert inverse agonist effects.22
As a result of the extensive metabolic activities of the two hormones, ghrelin/LEAP‐2 interplay may be altered under conditions of metabolic impairment.23, 24, 25 Moreover, there is a growing interest in the use of GSH‐R agonist or antagonist in several pathologies such as obesity, anorexia, and sarcopenia to counteract the respective complications.26
Since the identification of an altered ghrelin/LEAP‐2 relationship may offer a possible therapeutic target for counteracting metabolic deterioration in adult GHD and as no studies are available in the literature that have analyzed the behavior of LEAP‐2 in this syndrome, we conducted a cross‐sectional observational pilot study, whose primary objective has been the evaluation of circulating levels of ghrelin and LEAP‐2 in adults with GHD compared to healthy controls, whose secondary objective has been to define the possible correlations between these hormones and the metabolic alterations in this clinical setting.
2. MATERIALS AND METHODS
Subjects involved in this study were admitted to the University Hospital “Policlinico Gemelli” and were enrolled after being given an explanation of purposes and nature of the study, conducted in accordance with the Declaration of Helsinki, as revised in 2013. The study protocol was approved by the Institutional Board of our Hospital. After being given a written consent, GHD untreated patients and healthy controls, age‐matched, of both sexes were included in the study.
We included 30 patients divided in two groups:
Group A, Adult GHD (15 patients, 8 females and 7 males). GHD was diagnosed with dynamic test using Growth Hormone‐Releasing Hormone (GHRH 50 μg i.v. + arginine 0,5 g/Kg), with a peak GH response <11 μg/L when BMI was <25 kg/m2, peak GH response <8 μg/L when BMI was between 25 and 30 kg/m2 or < 4 μg/L when BMI was >30 kg/m2. 1 Patients were tested accordingly to current guidelines1, 27 or for a strong clinical suspicion as previously reported.28 Etiologies of GHD were: 5 idiopathic empty sellas, 1 pituitary cyst, 1 post‐surgical hypopituitarism, 8 idiopathic with negative MRI pictures. GHRH+arginine stimulation tests were repeated twice in idiopathic aGHD to confirm if they truly had aGHD. Median and interquartile range age of the group was 53 (41–57) years, while BMI was 27.125, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 kg/m2, as expected due to the features of the syndrome.
Group B, Healthy Controls (15 patients, 10 females and 5 males). Median and interquartile range age was 47 (36–57) years, while BMI 22.9 (20.8–33.1) kg/m2.
Exclusion criteria were: age under 18 or over 70; obesity of genetic origin or related to other endocrinopathies; history of cranial hypertension or active cranial hypertension; decompensated type 1 or 2 diabetes mellitus; autoimmune diseases under immunosuppressive treatment; corticosteroid treatment (except for topic, inhaled and oral hydrocortisone as replacement regimen); other diseases characterized by low insulin‐like growth factor‐1 (IGF‐1) such as liver disease, malabsorption, and malnutrition; active malignancy. No patients received GH therapy at the study enrolment.
Blood samples were collected after an overnight fasting into pyrogen‐free tubes with heparin as anticoagulant. In all patients, the following metabolic parameters were determined in plasma: glucose and insulin, total/HDL/LDL cholesterol, triglycerides and uric acid, and IGF‐1.
HOMA index was calculated according to the formula {[fasting insulin (U/ml)] × [fasting glucose (mmol/l)]}/22.5.29 Insulin resistance was defined as a HOMA‐index higher than 2.5.
QUICKI index was calculated according to the formula 1/log [fasting insulin (μUI/ml)] + log [fasting glucose (mg/dl)].30
For plasma evaluation, the blood samples collected into pyrogen‐free tubes containing heparin were centrifuged within 1 hr at 4,000 rpm for 15 min; separate plasma aliquots were stored at −80°C until assayed. Plasma concentrations of glucose, total cholesterol, HDL‐cholesterol, triglycerides, and uric acid were measured by using enzymatic assays and on Olympus AU2700 chemistry analyzer (Olympus America Inc., Center Valley, PA). The intra‐and inter‐assay coefficients of variation (CV) for total cholesterol, triglycerides, and uric acid were <1.5%, and <2.5%, respectively. The intra‐ and inter‐assay CV for HDL‐cholesterol were <2.5%, and <3.0%, respectively. LDL cholesterol was calculated by the Friedewald's equation: LDL cholesterol = total cholesterol − (HDL cholesterol + triglycerides/5).
For serum evaluation, the blood samples collected into vacutainer tube(s) containing no anticoagulant were incubated in upright position at room temperature for 30–45 min (no longer than 60 min) to allow clotting and then centrifuged at 2,500 rpm for 15′ at 4°C without brake. Supernatant (serum) was collected and stored at −80°C until use.
Insulin (normal values 3–20 μUI/ml) and IGF‐1 (normal values 80–330 ng/ml) were measured using ChemoLuminescent Immunoassay (CLIA).
Plasma concentrations of ghrelin were measured by an Elisa kit (Invitrogen, Thermo Fisher Scientific, Inc., USA; Cat. N. BMS2192) following the protocol provided by the manufacturer. The anti‐human ghrelin coating antibody adsorbed onto microwells recognizes both acylated and unacylated forms of ghrelin. The Elisa kit limit of detection of human ghrelin is 11.8 pg/ml and the calculated overall intra‐assay and inter‐assay coefficients of variation are 6.0 and 8.5%, respectively. Plasma concentrations of LEAP‐2 were measured by an EIA kit (Phoenix Pharmaceuticals, Inc., USA; Cat. N. EK‐075‐40) according to a slightly modified version of the manufacturer's protocol.31 The samples were diluted 1:25 in the Elisa kit buffer. The EIA kit intra‐assay coefficient of variation is 6.4%.25
The statistical analysis was performed using GraphPad Prism 7. The planned sample size of minimum 15 cases for each group was not based on formal power estimates, due to the lack of any information on the size of the minimal difference worth detecting for each of the study parameters. Instead, the sample size was established based on considerations of statistical practicality (with 30 + 30 subjects a normal distribution of the mean can be usually assumed) and feasibility, due to the rarity of GHD disease. With this sample size, the study has power >80% to detect any difference between cases and controls that is as large or greater than 75% of the standard deviation of that specific parameter. Considering that the comparison concerns differences between diseased subjects and healthy controls in physiologic parameters of molecules such as ghrelin and LEAP‐2, smaller differences are of little, if any, interest.
Data are expressed as medians and interquartile ranges. According to Shapiro–Wilk test, the variables did not follow normal distribution. The statistical analysis was carried out using the Mann Whitney test to study the differences between groups. Linear regression analysis was also performed, with the calculation of the Spearman coefficient. Linear correlation analyses were performed to evaluate the correlation between variables. The level of significance has been set at .05.
3. RESULTS
Table 1 shows median and interquartile range of metabolic and hormonal parameters in patients with GH deficiency and controls. Regarding the metabolic parameters, significantly increased insulin levels and HOMA‐index were observed in aGHD patients compared to healthy controls. A trend to worse lipidic pattern (LDL cholesterol and triglycerides) was observed in aGHD patients, with significantly lower HDL cholesterol. Regarding hormonal parameters, IGF‐1 levels were not significantly different in the two groups.
TABLE 1.
Median and interquartile range of metabolic parameters and hormonal parameters in subjects enrolled: Adult GHD versus controls
| aGHD | Controls | |
|---|---|---|
| Age (years) | 53.0 (41–57) | 47.0 (36–57) |
| BMI (kg/m2) | 27.1 (25–35) | 22.9 (20.8–33.1) |
| Glucose (mg/dl) | 84.0 (79–95) | 84.0 (76.5–88.5) |
| Insulin (μUI/ml) | 10.1 (7.7–27.9)* | 4.8 (4.0–7.8) |
| HOMA‐IR | 2.2 (1.5–3.2)* | 1.0 (0.7–1.5) |
| QUICKI | 0.34 (0.32–0.37) | 0.38 (0.36–0.40) |
| Total cholesterol (mg/dl) | 168 (157–192) | 182 (154–209) |
| LDL cholesterol (mg/dl) | 106 (94.0–123.0) | 108 (75–125) |
| HDL cholesterol (mg/dl) | 47.0 (41.0–53.0)* | 54 (47–65) |
| Triglycerides (mg/dl) | 111 (94.0–121.0) | 107 (65–152) |
| Uric acid (mg/dl) | 5.4 (4.2–7.3) | 4.1 (3.5–4.7) |
| IGF‐1 (ng/ml) | 123.5 (97.0–141.0) | 127 (81–142) |
p < .05.
Figure 1 depicts ghrelin and LEAP‐2 plasma concentrations and LEAP‐2/ghrelin ratio in aGHD patients compared to healthy controls. Ghrelin levels in the aGHD group were significantly lower than in controls (0.9 nM [0.54–1.1 nM], n = 15, and 1.1 nM [0.95–1.2 nM], n = 15, respectively; p < .01). In contrast, LEAP‐2 plasma concentrations in aGHD patients and in healthy controls were not significantly different (4.4 nM [3.9–5.7 nM], n = 15, and 4.1 nM [3.5–5.0 nM], n = 15, respectively; p = .32). However, the LEAP‐2/ghrelin ratio was significantly higher in aGHD patients than in the healthy controls (6.2 [3.8–8.7], n = 15, and 3.9 [3.1–4.8], n = 15, respectively; p < .05). In both groups, the linear correlation analyses did not find any significant correlation between ghrelin or LEAP‐2 with BMI or HOMA index. However, a significant inverse correlation (r 2 = 0.15, p = .047) between BMI and ghrelin was evidenced when considering the whole population (Figure 2).
FIGURE 1.
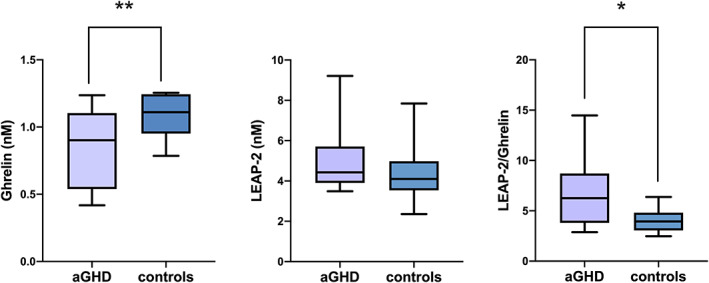
Box plot of ghrelin, LEAP‐2 levels and ratio in aGHD population compared to controls. *p <.05
FIGURE 2.
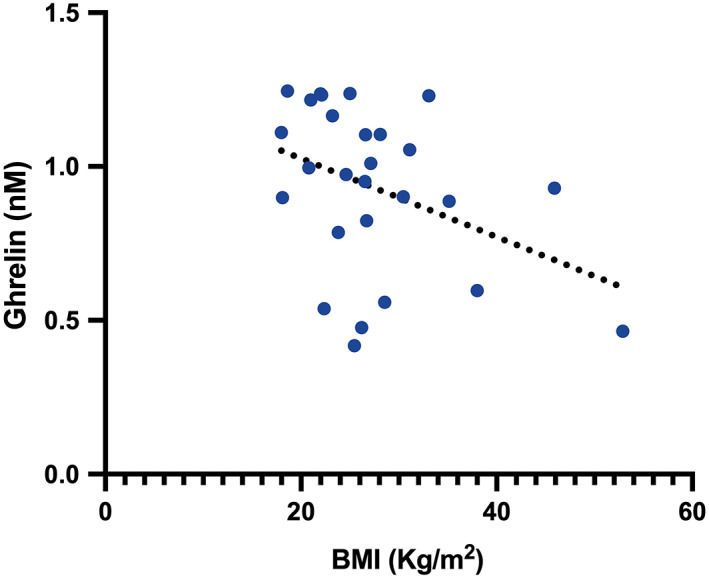
BMI and ghrelin inverse correlation in the whole population. p < .05, (R 2 = 0.15)
4. DISCUSSION
To the best knowledge of the authors, this is the first description of LEAP‐2 levels in adult GHD subjects, while reports on ghrelin behavior in such condition are scarce. The first dealing with this problem was the study of Janssen et al.,17 who evidenced that aGHD patients naïve to treatment tend to have lower circulating ghrelin levels in comparison to healthy subjects. In the same work, 1 year of GH replacement therapy did not alter ghrelin levels, which remained lower than in controls.17 Engstrom et al.32 showed a 29% decrease of ghrelin circulating levels after the administration of recombinant human GH (r‐hGH) for 9 months in aGHD patients, suggesting a possible role of GH as an inhibitor of ghrelin production through its effects on lipid mobilization and glucose production. An opposite hypothesis was suggested by Giavoli's group,33 which studied GHD patients before and after short‐ and long‐term (1 year) administration of r‐hGH. Ghrelin plasma levels were lower in GHD patients at baseline, probably due to the higher body fat percentage in these patients. Ghrelin increased after long‐term treatment, probably for a significant decrease in BMI, body fat, and insulin levels. Conversely short‐term r‐hGH administration determined a decrease in ghrelin production, suggesting a feedback inhibitory role for GH and/or IGF‐I on ghrelin release.33 The hypothesis of a negative feedback of GH on ghrelin production has then been disowned by Tarantini et al.34 and nowadays GH is not considered as a regulator of ghrelin secretion.24 Our data confirmed lower ghrelin levels in aGHD, probably as a consequence of higher BMI and insulin resistance related to GH deficiency condition, even though no correlation between ghrelin and BMI and ghrelin and insulin‐resistance indexes has been found in our aGHD cohort. Nevertheless, in the entire population evaluated a significant inverse correlation between ghrelin and BMI was detected. The absence of this inverse correlation in aGHD group may suggest other factors, related to the syndrome, influencing ghrelin plasmatic levels, as also supported by the above cited in treatment studies. This phenomenon may act as an adaptation to condition of unfavorable metabolic profile, where ghrelin may worsen complications due to its effects on energy balance, overcoming the benefits related to GH stimulation. Differently, in childhood GHD, where hypostaturalism is predominant in comparison to cardio‐metabolic impairment, ghrelin circulating levels tend to be higher than in controls,35, 36, 37 suggesting a prominent secretagogue boost to unlock the blunted GH secretion.
Ghrelin action is influenced by LEAP‐2, an endogenous GHS‐R1a antagonist.23 In rodents, the functions of this peptide are well described: decrease of adiposity, food intake, and body weight, decrease of GH production and gluconeogenesis through the inhibiting effect on ghrelin action. Its levels are higher in obese models and streptozotocin‐induced type 1 diabetes mellitus with a direct correlation with fat mass and body weight. Hyperglycemia and oral glucose administration induce LEAP‐2 plasmatic increase, whether fasting and weight loss exert the opposite effect.25 In humans, studies are still scarce and controversial,23 yet no data are reported on aGHD. Mani et al.25 showed that plasma LEAP‐2 levels are regulated by metabolic status, with a direct correlation with BMI, fat mass, HOMA index, triglycerides, and glycaemia, while they decreased with fasting and bariatric surgery. LEAP‐2 role on innate immune response has arisen its interest even in other fields, such as rheumatoid arthritis where LEAP‐2 levels were significantly increased, correlating with inflammatory parameters.38 Notably, patients enrolled in the last cited study exhibited overweight trend. In our cohort, aGHD patients showed similar plasma LEAP‐2 levels with controls, with no correlation with BMI and HOMA index. While the lack of any correlations with BMI and HOMA index may be due to the numerosity of the cohort here represented, another element could be the different pattern of low‐grade inflammation expressed in aGHD in comparison with metabolic syndrome, according to our previous reports.39, 40, 41
Another novelty presented in this study is the evaluation of LEAP‐2/total ghrelin molar ratio. Mani et al.25 recently reported LEAP‐2/acyl ghrelin molar ratio as higher in obese mice than in lean ones. Moreover, the ratio appeared to be lower after fasting.25 It is known that ghrelin can be found in circulation as acyl ghrelin, as a result of post‐translational acylation mediated by ghrelin‐O‐acyltransferase and capable of high‐affinity bind to GSH‐R1a, and desacyl ghrelin with GHS‐R1a independent biological activity.42, 43, 44, 45, 46 However, the group of Blatnik hypothesized that des‐acyl ghrelin in human plasma could be a mere artefact of sample handling and acyl ghrelin could correspond to total ghrelin.47 According to this evidence, we sought to analyze LEAP‐2 and its molar ratio with total ghrelin levels. Patients affected by aGHD presented higher LEAP‐2/ghrelin molar ratio than healthy controls, thus indicating a reduced receptor activation by ghrelin. In ligand‐binding assays, acylated ghrelin and LEAP‐2 displace bioluminescent ghrelin or LEAP‐2 from GHS‐R1a with IC50s of approximately 3.5–3.7 nM and 5.2–5.4 nM, respectively.21 Thus, both peptides bind competitively to the orthosteric site of GSH‐R1a with similar affinities, but LEAP‐2 dissociates much more slowly from receptors than ghrelin (dissociation half‐lives of ~15 and 1 min, respectively).21 In cAMP response‐element‐controlled reporter assay and calcium mobilization assay, ghrelin EC50s were approximately 0.78–0.98 nM.21 We measured median ghrelin and LEAP‐2 plasma concentrations of 1.1 and 4.1 nM, respectively, with a median LEAP‐2/ghrelin concentration ratio of 3.9 in healthy controls. Thus, median plasma concentrations of LEAP‐2 are close to the reported IC50s and the median plasma concentrations of ghrelin are close to the reported EC50s. From a pharmacological point of view, this is an ideal condition in which small variations in the plasma concentrations of one or the other ligand can finely regulate the activation of GSH‐R1a. In GHD patients, the LEAP‐2/ghrelin concentration ratio is approximately 1.6 times that observed in healthy controls. Therefore, considering also the much lower receptor dissociation rate of LEAP‐2 compared to ghrelin, a significant reduction of ghrelin‐induced effects can be expected in these patients.
Taken all data together, while therapeutic perspectives about LEAP‐2 employment in obesity and metabolic disorders have been hypothesized,23 our data do not support any eventual use in aGHD.
In spite of all precautions, our study may be still subject to certain biases, and some main potential restrictions should be considered. The number of subjects in the two groups is slightly small, due to the rarity of this condition, so the statistical power of the study is limited; consequently, our findings will need to be confirmed in a larger population and will help to delineate the impact of ghrelin/LEAP‐2 in GHD. Therefore, the study design and the power analysis cannot draw a cause‐effect relationship. Moreover, the moment of the disease history in which the patient is evaluated may affect ghrelin and LEAP‐2 production. Another possible bias may be due to the adoption of HOMA index, commonly used as index of insulin resistance in large epidemiological studies, in a small population. Anyhow, the role of ghrelin and LEAP‐2 in adult GHD merit further studies to develop targeted personalized therapy.
CONFLICT OF INTEREST
There are no conflict of interest to declare.
ACKNOWLEDGMENTS
Funding was supported by Fondi di Ateneo Linea D1 (N. R4124500530) to Diego Currò and partially supported by a non‐profit grant (MS200104_0016) released by Merck KGaA, Darmstadt, Germany to Antonio Mancini (the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript).
Vergani E, Bruno C, Gavotti C, et al. LEAP‐2/ghrelin interplay in adult growth hormone deficiency: Cause or consequence? A pilot study. IUBMB Life. 2021;73:978–984. 10.1002/iub.2504
Funding information Merck KGaA, Grant/Award Number: MS200104_0016; Fondi di Ateneo Linea D1, Grant/Award Number: R4124500530
REFERENCES
- 1.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine Society . Evaluation and treatment of adult growth hormone deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–1609. [DOI] [PubMed] [Google Scholar]
- 2.Johannsson G, Sverrisdóttir YB, Ellegård L, Lundberg PA, Herlitz H. GH increases extracellular volume by stimulating sodium reabsorption in the distal nephron and preventing pressure natriuresis. J Clin Endocrinol Metabol. 2002;87:1743–1749. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen JOL, Krag M, Jessen N, et al. Growth hormone and glucose homeostasis. Horm Res. 2004;62:51–55. [DOI] [PubMed] [Google Scholar]
- 4.Christ ER, Cummings MH, Albany E, Umpleby AM, Lumb PJ, et al. Effects of growth hormone (GH) replacement therapy on very low density lipoprotein apolipoprotein B100 kinetics in patients with adult GH deficiency: A stable isotope study. J Clin Endocrinol Metabol. 1999;84:307–316. [DOI] [PubMed] [Google Scholar]
- 5.Lombardi G, Di Somma C, Grasso LFS, Savanelli MC, Colao A, et al. The cardiovascular system in growth hormone excess and growth hormone deficiency. J Endocrinol Invest. 2012;35:1021–1029. 10.3275/8717. [DOI] [PubMed] [Google Scholar]
- 6.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature. 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 7.Guan XM, Yu H, Palyha OC, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Mol Brain Res. 1997;48:23–29. [DOI] [PubMed] [Google Scholar]
- 8.Willesen MG, Kristensen P, Rømer J. Co‐localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. [DOI] [PubMed] [Google Scholar]
- 9.Dickson SL, Luckman SM. Induction of c‐fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)‐releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH‐releasing peptide‐6. Endocrinology. 1997;138:771–777. [DOI] [PubMed] [Google Scholar]
- 10.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. [DOI] [PubMed] [Google Scholar]
- 11.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, et al. Ghrelin is an appetite‐stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. [DOI] [PubMed] [Google Scholar]
- 12.Masuda Y, Tanaka T, Inomata N, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. [DOI] [PubMed] [Google Scholar]
- 13.Lutter M, Sakata I, Osborne‐Lawrence S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai H, Cong W, Daimon CM, Wang R, Tschöp MH, et al. Altered lipid and salt taste responsivity in ghrelin and GOAT null mice. PLoS One. 2013;8:e76553. 10.1371/journal.pone.0076553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filigheddu N, Gnocchi VF, Coscia M, et al. Ghrelin and des‐acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol Biol Cell. 2007;18:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porporato PE, Filigheddu N, Reano S, et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J Clin Investig. 2013;123:611–622. 10.1172/JCI39920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen JAMJL, Van Der Toorn FM, Hofland LJ, Van Koetsveld P, Broglio F, et al. Systemic ghrelin levels in subjects with growth hormone deficiency are not modified by one year of growth hormone replacement therapy. Eur J Endocrinol. 2001;145:711–716. [DOI] [PubMed] [Google Scholar]
- 18.Krause A. Isolation and biochemical characterization of LEAP‐2, a novel blood peptide expressed in the liver. Protein Sci. 2003;12:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townes CL, Michailidis G, Hall J. The interaction of the antimicrobial peptide cLEAP‐2 and the bacterial membrane. Biochem Biophys Res Commun. 2009;387:500–503. [DOI] [PubMed] [Google Scholar]
- 20.Ge X, Yang H, Bednarek MA, et al. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metab. 2018;27:461–469.e6. [DOI] [PubMed] [Google Scholar]
- 21.Wang JH, Li HZ, Shao XX, et al. Identifying the binding mechanism of LEAP2 to receptor GHSR1a. FEBS J. 2019;286:1332–1345. [DOI] [PubMed] [Google Scholar]
- 22.Gupta D, Ogden SB, Shankar K, Varshney S, Zigman JM. A LEAP 2 conclusions? Targeting the ghrelin system to treat obesity and diabetes. Mol Metab. 2020;46:101128. 10.1016/j.molmet.2020.101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al‐Massadi O, Müller T, Tschöp M, Diéguez C, Nogueiras R. Ghrelin and LEAP‐2: Rivals in energy metabolism. Trends Pharmacol Sci. 2018;39:685–694. [DOI] [PubMed] [Google Scholar]
- 24.Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, et al. Ghrelin. Mol Metab. 2015;4:437–460. 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mani BK, Puzziferri N, He Z, et al. LEAP2 changes with body mass and food intake in humans and mice. J Clin Investig. 2019;129:3909–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nass R, Gaylinn BD, Thorner MO. The role of ghrelin in GH secretion and GH disorders. Mol Cell Endocrinol. 2011;340:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuen KCJ, Biller BMK, Radovick S, et al. American Association of Clinical endocrinologists and American College of Endocrinology guidelines for management of growth hormone deficiency in adults and patients transitioning from pediatric to adult care. Endocr Pract. 2019;25:1191–1232. [DOI] [PubMed] [Google Scholar]
- 28.Mancini A, Bruno C, Vergani E, Brunetti A, Palmisano G, et al. “Non‐classical” indication for provocative testing of growth hormone: A retrospective cohort study in adult patients under replacement therapy. Endocr Metab Immune Disord Drug Targets. 2020;20:1–7. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and β‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 30.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metabol. 2000;85:2402–2410. [DOI] [PubMed] [Google Scholar]
- 31.Fittipaldi AS, Hernández J, Castrogiovanni D, Lufrano D, de Francesco PN, et al. Plasma levels of ghrelin, des‐acyl ghrelin and LEAP2 in children with obesity: Correlation with age and insulin resistance. Eur J Endocrinol. 2020;182:165–175. 10.1530/EJE-19-0684. [DOI] [PubMed] [Google Scholar]
- 32.Engström BE, Burman P, Holdstock C, Karlsson FA. Effects of growth hormone (GH) on ghrelin, leptin, and adiponectin in GH‐deficient patients. J Clin Endocrinol Metabol. 2003;88:5193–5198. [DOI] [PubMed] [Google Scholar]
- 33.Giavoli C, Cappiello V, Corbetta S, et al. Different effects of short‐ and long‐term recombinant hGH administration on ghrelin and adiponectin levels in GH‐deficient adults. Clin Endocrinol (Oxf). 2004;61:81–87. [DOI] [PubMed] [Google Scholar]
- 34.Tarantini B, Ciuoli C, Checchi S, et al. Serum ghrelin levels in growth hormone‐sufficient and growth hormone‐deficient patients during growth hormone‐releasing hormone plus arginine test. J Endocrinol Invest. 2009;32:335–337. [DOI] [PubMed] [Google Scholar]
- 35.Stawerska R, Kolasa‐Kicińska M, Łupińska A, Hilczer M, Lewiński A. Comparison of nocturnal and morning ghrelin concentration in children with growth hormone deficiency and with idiopathic short stature. Chronobiol Int. 2020;37:1629–1635. [DOI] [PubMed] [Google Scholar]
- 36.Stawerska R, Smyczyńska J, Czkwianianc E, Hilczer M, Lewiński A. High concentration of ghrelin in children with growth hormone deficiency and neurosecretory dysfunction. Neuroendocrinol Lett. 2012;33:331–339. [PubMed] [Google Scholar]
- 37.Zhou G, Du R. Dynamic ghrelin and GH serum levels during combined simultaneous arginine clonidine stimulation test in children with dwarfism. Ital J Pediatr. 2019;45:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francisco V, Tovar S, Conde J, Pino J, Mera A, et al. Levels of the novel endogenous antagonist of ghrelin receptor, liver‐enriched antimicrobial peptide‐2, in patients with rheumatoid arthritis. Nutrients. 2020;12:1006. 10.3390/nu12041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Currò D, Vergani E, Bruno C, Comi S, D'Abate C, Mancini A. Plasmatic lipocalin‐2 levels in chronic low‐grade inflammation syndromes: Comparison between metabolic syndrome, total and partial adult growth hormone deficiency. Biofactors. 2020;46:629–636. [DOI] [PubMed] [Google Scholar]
- 40.Basile U, Bruno C, Napodano C, et al. Plasmatic free light chains as inflammatory marker in insulin resistance: Comparison of metabolic syndrome with adult growth hormone deficiency. Biofactors. 2018;44:480–484. [DOI] [PubMed] [Google Scholar]
- 41.Mancini A, Di Segni C, Bruno C, Olivieri G, Guidi F, et al. Oxidative stress in adult growth hormone deficiency: Different plasma antioxidant patterns in comparison with metabolic syndrome. Endocrine. 2017;59:130–136. 10.1007/s12020-017-1468-1. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that Octanoylates ghrelin, an appetite‐stimulating peptide hormone. Cell. 2008;132:387–396. [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105:6320–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inhoff T, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Is desacyl ghrelin a modulator of food intake? Peptides. 2009;30:991–994. [DOI] [PubMed] [Google Scholar]
- 45.Inhoff T, Mönnikes H, Noetzel S, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29:2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, et al. Non‐acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004;89:3062–3065. 10.1210/jc.2003-031964. [DOI] [PubMed] [Google Scholar]
- 47.Blatnik M, Soderstrom CI, Dysinger M, Fraser SA. Prandial ghrelin attenuation provides evidence that des‐acyl ghrelin may be an artifact of sample handling in human plasma. Bioanalysis. 2012;4:2447–2455. 10.4155/bio.12.248. [DOI] [PubMed] [Google Scholar]


