Abstract
Background
Osseodensification (OD) has shown to improve implant stability; however, the influences of implant design, dimensions, and surgical site characteristics are unknown.
Purpose
To compare the insertion torque (IT) and temporal implant stability quotients (ISQ) of implants placed via OD or subtractive drilling (SD).
Materials and Methods
This multicenter controlled clinical trial enrolled 56 patients, whom were in need of at least 2 implants (n = 150 implants). Patients were treated with narrow, regular, or wide implants and short, regular, or long implants in the anterior or posterior region of the maxilla or in the posterior region of the mandible. Osteotomies were performed following manufacturers recommendation. IT was recorded with a torque indicator. ISQ was recorded with resonance frequency analysis immediately after surgery, 3 and 6 weeks.
Results
Data complied as a function of osteotomy indicated significantly higher IT for OD relative to SD. OD outperformed conventional SD for all pairwise comparisons of arches (maxilla and mandible) and areas operated (anterior and posterior), diameters and lengths of the implants, except for short implants. Overall, ISQ data also demonstrated significantly higher values for OD compared to SD regardless of the healing period. Relative to immediate readings, ISQ values significantly decreased at 3 weeks, returning to immediate levels at 6 weeks; however, ISQ values strictly remained above 68 throughout healing time for OD. Data as a function of arch operated and osteotomy, area operated and osteotomy, implant dimensions and osteotomy, also exhibited higher ISQ values for OD relative to SD on pairwise comparisons, except for short implants.
Conclusions
OD demonstrated higher IT and temporal ISQ values relative to SD, irrespective of arch and area operated as well as implant design and dimension, with an exception for short implants. Future studies should focus on biomechanical parameters and bone level change evaluation after loading.
Keywords: clinical trial, dental implants, osseodensification, osseointegration, osteotomy
What is known:
Osseodensification (OD), a nonsubtractive instrumentation method, has been shown to increase the bone density and biomechanical engagement at the bone‐implant interface; however, its short‐term temporal benefits on clinical parameters are scarcely addressed in the evidence‐based literature.
What this study adds:
OD has demonstrated higher primary and secondary stability relative to more traditional subtractive drilling techniques for all implant dimensions, with the exception of short implants. This supports the concept of a rapid and stable onset of secondary stability with no measurable detrimental effect on the remodeling process.
1. INTRODUCTION
Osseointegration reflects the formation of a structural and functional bone‐to‐implant interface, without the interposition of soft tissue, where bone metabolism is challenged by the placement of a foreign body that induces stress/strain in the peri‐implant tissue and triggers highly integrated and complex immunomodulated inflammatory reactions, eventually leading to new bone formation.1, 2 The dental literature supports evidence‐based long‐term prospective reports which have demonstrated approximately 95% implant survival rates after 10 years of observational follow‐up.3
Achieving an optimal degree of primary stability at the time of surgery, which can be defined as bone‐to‐implant biomechanical engagement with a micromotion lower than 150 μm, has been considered essential to a successful osseointegration and to predict loading time.4, 5 Clinically, the degree of implant stability can be objectively estimated by the insertion torque (IT) values using surgical hand pieces (IT) or subjectively obtained implant stability quotients (ISQ) using resonance frequency analysis; whereby IT values above 35 N.cm and/or ISQ above 68 have been considered reasonable values for a predictable osseointegration and earlier loading6, suggesting that such values should not only be achieved after implant placement, but also ideally maintained over the initial course of osseointegration.
Given bone elastic properties, the interfacial stress distribution during implant installation and the respective peri‐implant tissue strain due to frictional forces, present a linear relationship. Thus, bone density in the peri‐implant vicinity along with implant macrogeometry and its related surgical instrumentation have been assumed as key morphometric predictors of IT, as well as healing kinetics.7, 8, 9, 10, 11 A robust body of research has investigated the interplay between such factors, where the mismatch between the implant and instrumented bone walls dictates the course of osseointegration around the metallic device through a predominantly interfacial bone remodeling, predominantly intramembranous‐like healing or hybrid healing pathways, which affects the rate at which secondary stability occurs.8, 9, 10, 11, 12 Certain adjustments in the osteotomy preparation protocols have recently been proposed to achieve atemporally stable devices. These include osseodensification (OD) drilling.8, 9, 10, 11, 13, 14
The relatively novel osteotomy preparatory technique of OD has prompted a paradigm shift in implant site preparation through a multistepped drilling concept using uniquely designed burs that promotes lateralization of autogenous bone into the surrounding cancellous structure and expands the surrounding osseous environment by a rolling and sliding contact with controlled bone deformation all with minimal heat elevation.13, 14 Such an OD14 technique has been based on the bone elastic and plastic properties which facilitate bone bulk preservation and compaction, resulting in the autografting of osseous material into the trabecular space and enhancing its density. This may prove to be particularly helpful in clinical scenarios of poor bone quality.13, 14, 15 OD osteotomies have shown ~90% reduction in the objective osteotomy diameter when left empty, this due to residual strain and bone spring‐back effect.14, 16 Such an effect produces gentle compressive forces against the implant, thereby enhancing the initial biomechanical bone‐to‐implant interlocking and thus an increase in primary stability; all of which have exhibited osteogenic activity through a mechanobiological healing process without the excessive strain that would lead to extensive remodeling and subsequently a significant stability dip.14, 16, 17, 18, 19, 20, 21 Moreover, the bone fragments compacted during OD drilling function as nucleating agents which promote an accelerated osteogenesis in the implant bed and accelerate de novo bone formation.14, 16, 17, 18, 19, 20, 21
Highly translational preclinical biomechanical and histological data have demonstrated significantly higher IT and temporal removal torque for OD relative to the conventional subtractive drilling (SD).14, 17, 20 Given that removal torque was comparable throughout the healing period which was evaluated in this body of evidence, OD drilling has been suggested to provide atemporal biomechanical competence.17 Clinical outcomes of OD technique have shown short‐ and long‐term efficacy in several clinical scenarios, thereby enhancing implant primary and secondary stability.22, 23, 24 Therefore, this multicenter controlled clinical trial aimed to compare the IT values and temporal ISQ values (immediate, 3 and 6 weeks) of paired osteotomy sites prepared with standard SD and OD surgical instrumentation. The postulated null hypothesis was that osteotomy surgical technique would not influence clinical parameters of implant primary stability measured by IT and implant secondary stability measured by ISQ values up to 6 weeks after implant placement.
2. MATERIALS AND METHODS
2.1. Study design
This study was designed as a multicenter prospective evaluation, investigating the influence of osteotomy technique on the first 6 weeks of clinical implant osseointegration parameters, according to STROBE guidelines. The interventions were performed in accordance with the ethical standards of the revised Helsinki Declaration for biomedical research involving human subjects. The study was performed in different treatment centers and approved by their respective local ethical committees (protocol numbers #10295719.1.0000.5417 and #SH004 Integ Review). The study was registered in the Clinical Trial Register (NCT04779203). Each patient received a detailed description of the study protocol, signed the inform consent form and gave written approval to be included in the study population.
At the beginning of the investigation, a sample size calculation was performed based upon the preliminary data of IT, which was considered the primary dependent variable of the current study. Considering that arch operated (maxilla and mandible); area operated (anterior and posterior); implant diameter (narrow, regular, and wide); implant length (short, regular, and long); and osteotomy (SD, and OD) were the independent variables evaluated, the minimum sample size calculated to obtain a statistical test power of 80% and a 5% alpha error within an effect size of 11.75 was 8 implants and an extra increase by 20% to account for potential losses and refusals, approximately 10 implants were required per factor (G*Power 3.1, HHU University, Germany), totaling the need of at least 120 implants.
Patients in need to receive at least two dental implants in single‐unit or larger span edentulous spaces were recruited from September 2019 to August 2020. All patients were subjected to a preliminary evaluation that included careful review of their medical and dental histories, detailed clinical examination, and evaluation of oral hygiene. Patients included in the study should present at least 18 years of age; sufficient residual bone volume for implant placement without the need for bone augmentation, where the minimum ridge height and width should be ≥9 and ≥6 mm, respectively; healed bone sites with at least 4 months postextraction period. The exclusion criteria included alcoholism, smoking, use of illicit drugs, heart diseases, diabetes, previous bone regenerative or augmentation procedures, bleeding disorders, compromised immune system, irradiated patients, previous or active treatment with steroids or bisphosphonates.
All patients underwent radiographic evaluation including both periapical radiographs and cone‐beam computerized tomography scans prior to implant placement for surgical planning and assessment of bone dimensions around the site of implantation. Each implant system utilized had an internal conical connection, tapered macrogeometry, and a sandblasted acid‐etched surface: Strong SW Plus (S.I.N Implant System, Sao Paulo, SP, Brazil), Zimmer Biomet (Warsaw, IN), and IS‐III Active (NeoBiotech, Pasadena, CA).
The implants placed in the current study were categorized based on their dimensions into the following categories: (i) narrow (≥3 mm to <3.75 mm), (ii) regular (≥3.75 mm to <5 mm), and (iii) wide (≥5 mm) diameters, and (i) short (>6 mm to <10 mm), (ii) regular (≥10 mm to <13 mm), and (iii) long (≥13 mm) lengths.25 The patients were treated with narrow‐, regular‐, or wide‐diameter implants and short‐, regular‐, or long‐length implants in the posterior region of the mandible or in the anterior or posterior region of the maxilla as described in the criteria detailed below:
If split‐mouth, implants were installed to replace the same tooth/teeth bilaterally where one side received OD instrumentation and the other respective side standard SD with matched dimension implants to osteotomy diameter. Right and left sides for both techniques were alternated in the subsequent patients to avoid allocation bias.
If adjacent teeth in the same side, the first site received OD drilling on the more mesial osteotomy and SD on the distal with same‐dimension implants. This prescribed implant/site order was established to avoid allocation and implant dimension bias.
Therefore, implants were placed in an equal distribution of arch and area operated, as well as implant dimensions. Comparable osteotomy diameters within the specific implant recommended drilling protocols as well as the OD recommended protocols were followed. This resulted in balanced surgical procedures that allowed the investigation of the effect of such factors and drilling techniques on clinical parameters. Patients were not informed of the area to be operated with either the OD or SD drillings protocols. Additionally, the operator whom performed the IT and ISQ readings was blinded as to which drilling protocol was followed. At the end of the study, all patients were informed of the results obtained for both drilling techniques.
2.2. Surgical technique
Preoperatively, patients' blood pressure was taken and noted. Subsequently, patients were instructed to rinse with 0.2% chlorhexidine solution for 1 min and expectorate. After these preoperative procedures were completed, sterile surgical drapes were used to cover the patient's chest to minimize the potential contamination from extraoral sources. The surgical procedure was performed under local anesthesia (mepivacaine or articaine with epinephrine 1:100 000). After local anesthesia was achieved, full‐thickness surgical flaps were elevated and implant osteotomies were performed with the assistance of saline irrigation. The osteotomies were performed at 1100 rpm with the use of sequential burs of similar diameter for both surgical techniques (SD conventional burs or OD drilling burs) and the instrumentation was performed according to the recommended drilling protocols for each implant system; either by standard drilling, as recommended by specific implant company protocols, or by OD as recommended by the densifying reference guide for each the specific implant systems. The insertion of the implants was initiated with the motor handpiece, without irrigation at 20–50 rpm, and installation was completed with a manual surgical torque wrench indicator. IT values were recorded as the maximum torque value (N.cm) reached at the termination of implant insertion.
Subsequent to final seating of the implant, a Smartpeg specific for the implant system and restorative platform diameter was used for each implant and a resonance frequency analysis was performed using an OsstellMentor device (Ostell/Integration Diagnostics, Gothenburg, Sweden) to record ISQ values in all implant surfaces. New, sterile healing abutments were inserted after the implant installation, and the incision was sutured to close the wounds. These sutures were removed 1‐week postoperatively (Figure 1).
FIGURE 1.
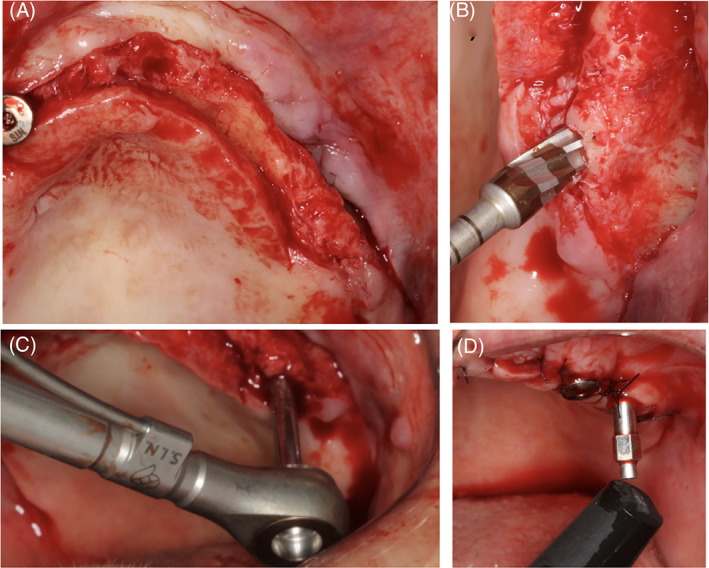
Representative images of the sequence of the surgical procedure through flap elevation (A), osteotomy (B), insertion torque reading (C), and implant stability quotients (ISQ) reading
All patients were instructed to follow a soft and tepid diet in the first 3 days after surgery, along with instructions for oral hygiene. They received a prescription for Amoxicillin 500 mg, one tablet every 8 h for 7 days, starting 1 h presurgery. Additional prescriptions included anti‐inflammatory and analgesic drugs for 3 days, Nimesulide 100 mg every 12 h and Paracetamol every 8 h.
ISQ values were also recorded at 3 and 6 weeks of healing during follow‐up visits. The rationale for this sequencing of measurements comes from derived curves of primary versus secondary stability development, suggesting that at 2–4 weeks after implantation, a stability dip is generally expected.26 Healing abutments were placed at the day of surgery so subsequent ISQ readings could be readily obtained. After healing, an impression of the implants spatial positioning and orientation was made, and a final restoration fabricated according to the respective clinical scenario.
2.3. Statistical analysis
Descriptive statistics including mean values and the corresponding 95% confidence interval (CI) were calculated for each variable. Analyses of the data have demonstrated normal distribution (Shapiro–Wilk test, all p > 0.05) and homogeneity of variance across groups (Levene test, all p > 0.25). For each center, all readings were performed by only one examiner and intraclass correlation coefficients scores have indicate a strong reliability (0.84 1‐day difference between readings). The linear mixed model test and least significant difference for multiple comparisons were performed to compare IT values. Repeated‐measure analysis of variance and Tukey tests for multiple comparisons were used for ISQ data analysis. The Pearson correlation test was applied to investigate the relationship between the IT and immediate ISQ values for all variable studied. The analyses were accomplished using SPSS with a significance level of 5% (IBM SPSS 23, IBM Corp., Armonk, NY).
3. RESULTS
The study population were comprised of 56 patients, 30 (53.6%) were female and 26 (46.4%) were male with mean age of 54.2 years (±3.5 years—95%CI). A total of 150 implants were placed by different dental surgeons in various institutions following an identical protocol, where two osteotomies, either SD or OD drilling, were evaluated as a function of different factors. These variables included arch operated, area operated, and implant dimensions (diameter and length). Overall, the clinical findings demonstrated an eventual implant healing process, with no signs and symptoms of peri‐implant tissue inflammation and/or infection and implant mobility at the time of implant reopening surgery.
The statistical analysis of IT as a function osteotomy indicated that OD drilling (60 ± 3.4 N.cm) presented higher IT compared to SD (35 ± 3.4 N.cm), approximately 41% difference (p < 0.001), OD outperformed conventional SD irrespective of arch operated, maxilla and mandible, and area operated anterior and posterior (p < 0.001) (Figure 2(A,B)). While there was no significant difference on IT values for implants placed using SD in the maxilla (35 ± 3.3 N.cm) relative to the mandible (34 ± 3.2 N.cm) (p = 0.964), implants placed using OD showed higher IT values in the mandible (73 ± 7.7 N.cm) than in the maxilla (54 ± 7.7 N.cm) (p < 0.001), approximately 27% difference (Figure 2(A)). Implants installed in the anterior region of the maxilla (40 ± 4.7 N.cm) demonstrated higher IT values when compared to the posterior region of the arch (32 ± 4.5 N.cm) when using SD technique, approximately 23% difference (p = 0.002), whereas no significant difference was observed between both regions for implants placed using OD drilling technique (anterior: 55 ± 6.1 N.cm/posterior: 58 ± 4.4 N.cm) (p = 0.402) (Figure 2(B)).
FIGURE 2.
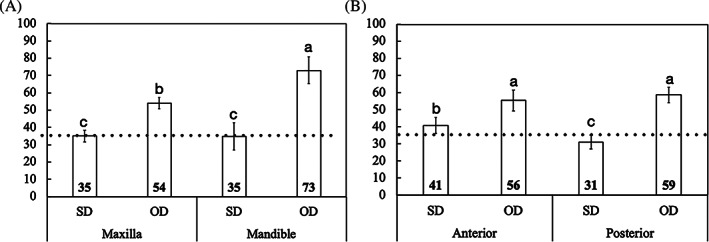
(A) Mean insertion torque (IT) values and the corresponding 95% confidence interval as a function of osteotomy and arch operated, maxilla and mandible, (B) and area operated, anterior and posterior regions of the maxilla. Gray line: IT reference for immediate loading (ISQ ≥ 35). Different letters indicate statistically significant difference
Similarly, IT data as a function of implant dimensions, either diameter or length, and drilling technique utilized exhibited higher values for OD relative to SD for all pairwise comparisons (p < 0.004), 19–63% difference, except for short implants (p = 0.119) (Figure 3(A,B)). While the implants of different diameters placed using conventional SD presented similar IT (narrow: 34 ± 7.3 N.cm/regular: 32 ± 5.6 N.cm/wide: 30 ± 10.6 N.cm) (p > 0.507), wide implants installed using OD showed higher IT (82 ± 12.4 N.cm) relative to regular (57 ± 5.6 N.cm) and narrow (49 ± 8.4 N.cm) implants (p < 0.001), up to 40% difference, both without statistically significant difference (p = 0.140) (Figure 3(A)). Also, implants placed with SD of different lengths demonstrated no significant difference on the IT values (short: 37 ± 7.3 N.cm/regular: 33 ± 4.2 N.cm/long: 28 ± 10.8 N.cm) (p > 0.150), whereas for the OD regular‐length implants exhibited higher IT (64 ± 4.7 N.cm) compared to short implants (46 ± 9.6 N.cm), 28% difference (p = 0.001).
FIGURE 3.
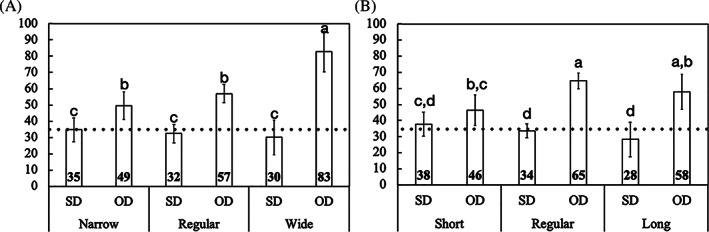
(A) Mean insertion torque (IT) values and the corresponding 95% confidence interval as a function of osteotomy and implant diameter, narrow, regular, and wide; (B) and implant length, short, regular, and long. Gray line: IT reference for immediate loading (ISQ≥35). Different letters indicate statistically significant difference
The overall ISQ data demonstrated higher values for OD group (I: 73 ± 2.0, 3W: 70 ± 2.0, and 3W: 74 ± 1.5) compared to conventional SD technique (I: 62 ± 2.0, 3W: 59 ± 2.0, and 3W: 66 ± 1.5), regardless of period evaluated. ISQ values significantly decreased from day of placement to 3 weeks for the SD group and increased at 6 weeks (p < 0.001); however, the values remained above the reference value of 68 throughout the follow‐up period only for OD technique. In fact, the ISQ values for the OD instrumentation started high and remained consistently elevated for all implant geometries; diameter and length (with less significance in short implant group), as well as arch (maxilla and mandible) and area (anterior, posterior) operated. The ISQ values for all OD experimental parameters remained above the threshold value of 68; in comparison, the SD instrumentation group started with lower ISQ values and usually reached its lowest values at 3 weeks and gradually increased to reach its initial values at 6 weeks (Figures 4, 5, 6, 7).
FIGURE 4.
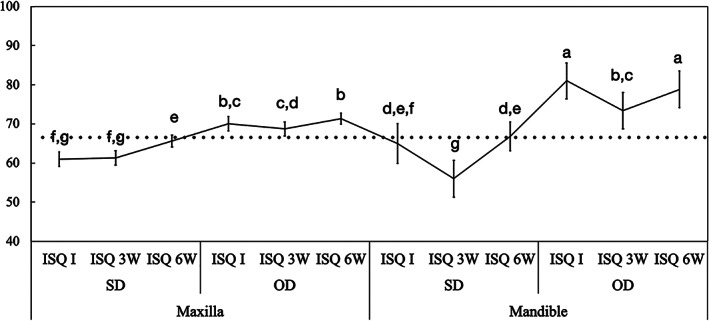
Mean implant stability quotient (ISQ) values and the corresponding 95% confidence interval as a function of osteotomy and arch operated, maxilla and mandible. Gray line: ISQ reference for immediate loading (ISQ≥68). Different letters indicate statistically significant difference
FIGURE 5.
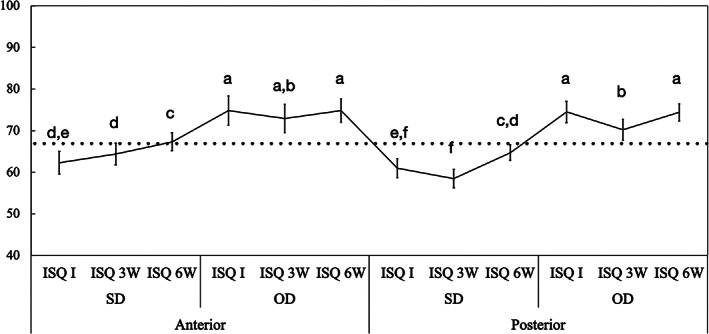
Mean implant stability quotient (ISQ) values and the corresponding 95% confidence interval as a function of osteotomy and area operated, anterior and posterior regions of the maxilla. Gray line: ISQ reference for immediate loading (ISQ ≥ 68). Different letters indicate statistically significant difference
FIGURE 6.
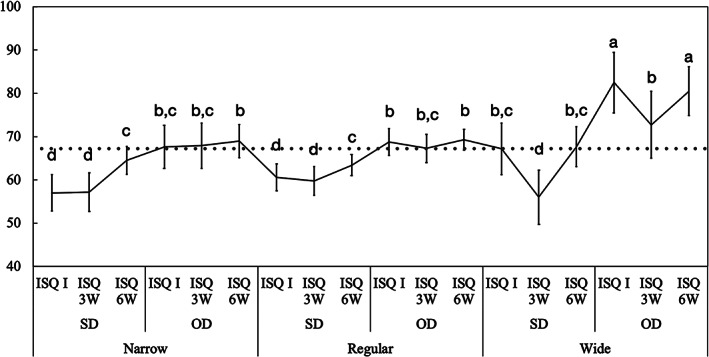
Mean implant stability quotient (ISQ) values and the corresponding 95% confidence interval as a function of osteotomy and implant diameter: narrow, regular, and wide. Gray line: ISQ reference for immediate loading (ISQ ≥ 68). Different letters indicate statistically significant difference
FIGURE 7.
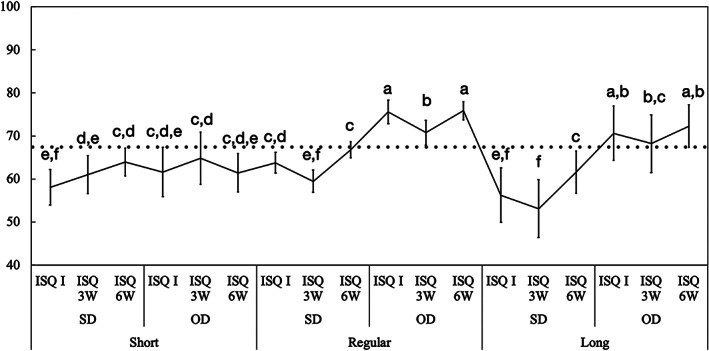
Mean implant stability quotient (ISQ) values and the corresponding 95% confidence interval as a function of osteotomy implant length: short, regular, and long. Gray line: ISQ reference for immediate loading (ISQ ≥ 68). Different letters indicate statistically significant difference
Data as a function of arch operated and osteotomy confirmed the higher ISQ values of OD relative to SD for implants placed in both maxilla and mandible arches (p < 0.001). While for OD surgical technique the highest ISQ values were observed for implants placed in the mandible (I: 81 ± 4.6, 3W: 73 ± 4.6, and 3W: 80 ± 3.7) relative to maxilla (I: 70 ± 1.7, 3W: 68 ± 1.8, and 3W: 71 ± 1.4) (p < 0.001), ISQ values were similar between arches for implants placed using the SD technique (Maxilla I: 60 ± 1.8, 3W: 61 ± 1.8, and 3W: 65 ± 1.4; Mandible I: 66 ± 4.6, 3W: 56 ± 4.6, and 3W: 67 ± 3.7) (p > 0.073) (Figure 4).
The statistical analysis of ISQ data as a function of area operated and osteotomy also demonstrated higher ISQ values for OD relative to SD osteotomies for implants placed in both anterior (OD ‐ I: 70 ± 2.6, 3W: 71 ± 2.7, and 3W: 72 ± 2.1/SD ‐ I: 62 ± 2.7, 3W: 64 ± 2.7, and 3W: 67 ± 2.2) and posterior (I: 75 ± 2.6, 3W: 69 ± 2.6, and 3W: 75 ± 2.0) SD (I: 60 ± 2.6, 3W: 57 ± 12.6, and 3W: 65 ± 12.0) regions of the maxilla (p < 0.001). Irrespective of surgical technique, the ISQ values observed for implants placed to replace the incisors and canines areas followed the same general pattern for premolars and molars sites for all time points (p > 0.071), except for SD at 3 weeks (p = 0.002) (Figure 5).
Data as a function of implant diameter and method of osteotomy development corroborated as well with the higher ISQ values for OD relative to SD techniques for all implant diameters pairwise comparisons (p < 0.002). For the SD group, the ISQ values were similar between narrow‐ (I: 57 ± 4.1, 3W: 57 ± 4.4, and 3W: 64 ± 3.2); regular‐ (I: 60 ± 3.1, 3W: 59 ± 3.3, and 3W: 63 ± 2.4); and wide‐diameter (I: 67 ± 7.0, 3W: 56 ± 7.7, and 3W: 67 ± 4.6) implants for all time points (p > 0.110), whereas for the OD group the ISQ values of wide (I: 82 ± 7.0, 3W: 72 ± 7.7, and 3W: 80 ± 5.6) implants were higher than regular (I: 68 ± 3.0, 3W: 67 ± 3.2, and 3W: 69 ± 2.4) and narrow (I: 67 ± 4.9, 3W: 67 ± 5.2, and 3W: 68 ± 3.8) implants for all time points (p < 0.001), except at 3 weeks (p > 0.199) (Figure 6).
ISQ data analyzed as a function of implant length followed the same pattern and demonstrated higher ISQ values for OD group relative to SD group for long‐and regular‐length implants for all time points (p < 0.003). SD group ISQ values for long (I: 56 ± 6.2, 3W: 53 ± 6.6, and 3W: 61 ± 24.8) and regular (I: 63 ± 2.4, 3W: 59 ± 2.5, and 3W: 66 ± 1.8) implants were significantly lower than the OD group values for long (I: 70 ± 6.2, 3W: 68 ± 6.7, and 3W: 72 ± 4.9) and regular (I: 75 ± 2.7, 3W: 70 ± 2.9, and 3W: 75 ± 2.1) implants throughout the healing time (p < 0.038). Relatively, ISQ values were not significantly different for short implants using SD (I: 60 ± 4.4, 3W: 58 ± 4.1, and 3W: 61 ± 4.4) and OD group (I: 61 ± 5.7, 3W: 64 ± 6.0, and 3W: 63 ± 3.2) (p > 0.316). However, for short implants, the ISQ values using OD technique started relatively higher and remained constant over 6 weeks (p > 0.073), whereas for the SD group, it started low and gradually increased at 6 weeks (p < 0.032) (Figure 7).
The IT values demonstrated a moderate positive correlation with immediate ISQ values when all data were collapsed (ρ: 0.58; p < 0.001). Similarly, the correlation between ISQ and IT data for the different variables studied ranged chiefly from a moderate to a strong positive correlation (Table 1).
TABLE 1.
Pearson correlation results of the relationship between the studied variables and IT and ISQ values
| Maxilla ISQ | Mandible ISQ | Anterior ISQ | Posterior ISQ | Narrow ISQ | Regular ISQ | Wide ISQ | Short ISQ | Regular ISQ | Long ISQ | SD ISQ | OD ISQ | Total ISQ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maxilla IT | ρ: 0.53; p < 0.001 | — | — | — | — | — | — | — | — | — | — | — | — |
| Mandible IT | — | ρ: 0.79; p < 0.001 | — | — | — | — | — | — | — | — | — | — | — |
| Anterior IT | — | — | ρ: 0.79; p < 0.001 | — | — | — | — | — | — | — | — | — | — |
| Posterior IT | — | — | — | ρ: 0.58; p < 0.001 | — | — | — | — | — | — | — | — | — |
| Narrow IT | — | — | — | — | ρ: 0.68; p < 0.001 | — | — | — | — | — | — | — | — |
| Regular IT | — | — | — | — | — | ρ: 0.51; p < 0.001 | — | — | — | — | — | — | — |
| Wide IT | — | — | — | — | — | — | ρ: 0.89; p < 0.001 | — | — | — | — | — | — |
| Short IT | — | — | — | — | — | — | — | ρ: 0.49; p = 0.016 | — | — | — | — | — |
| Regular IT | — | — | — | — | — | — | — | — | ρ: 0.62; p < 0.001 | — | — | — | — |
| Long IT | — | — | — | — | — | — | — | — | — | ρ: 0.65; p = 0.023 | — | — | — |
| SD IT | — | — | — | — | — | — | — | — | — | — | ρ: 0.24; p = 0.038 | — | — |
| ISQ IT | — | — | — | — | — | — | — | — | — | — | — | ρ: 0.49; p < 0.001 | — |
| Total IT | — | — | — | — | — | — | — | — | — | — | — | — | ρ: 0.58; p < 0.001 |
Abbreviations: ISQ, implant stability quotients; IT, insertion torque; SD, subtractive drilling.
4. DISCUSSION
Selecting a surgical instrumentation which improves upon implant stability in the native alveolar bone is critical to the achievement of successful osseointegration, specifically, in scenarios where bone availability and quality are not optimal.8, 9, 10, 11 Historically, derived curves of primary versus secondary stability development have suggested that at 2–4 weeks after implantation, a stability dip would generally be present.26 To address the potential limitations of conventional SD, an alternative approach has been proposed and developed. Rather than removing bone particles in the more conventional SD techniques, it has been proposed that an OD drilling sequence will maintain bone by compacting the particles into the osteotomy wall.13, 14 Biomechanical and preclinical studies have indicated significantly higher biomechanical and histomorphometric parameters for OD relative to conventional SD in temporal investigations.14, 17, 18, 19, 20, 21 This clinical trial investigated the influence of both surgical instrumentation techniques, SD and OD, on IT and ISQ during the first 6 weeks of healing of implants with different diameters and lengths placed in the anterior and posterior regions of the maxilla and in the mandible. Implant IT and ISQ are two clinically accepted parameters to determine implant primary stability, where both higher IT and ISQ values are positive indicators for implant stability as well as diminished micromotion which can be critical for immediate loading and subsequently enhanced osseointegration.4, 5, 27, 28, 29 ISQ is an efficient indicator that compares subsequent measurements over prescribed time intervals. This objective measure is independent and incomparable to IT value obtained at the time of implant placement.30 The analyses of the overall data demonstrated significantly higher IT and ISQ values, irrespective of evaluation period for OD instrumentation group relative to SD instrumentation group. Therefore, the postulated null hypothesis that osteotomy surgical technique would not influence clinical parameters of implant primary stability measured by IT and implant secondary stability measured by ISQ values up to 6 weeks after implant placement was rejected.
A successful osteotomy for dental rehabilitation allows the implant to be inserted in a biologically and restoratively driven three‐dimensional position with adequate biomechanical stability, where the implant bed is adequately prepared to a precise size using a progressive series of drills, avoiding overheating‐induced tissue damage.11 Achieving high levels of biomechanical stability have been increasingly required in clinical practice to accommodate the current tendency toward early loading protocols, even for bone types with low density.31, 32 Therefore, the OD instrumentation has demonstrated to improve bone quality as osteotomy size is expanded and guarantee greater levels of physical interlocking at the implant interface, especially in such challenging scenarios.14, 17, 18, 19, 20, 21 Histomorphometric analyses of OD in preclinical animal models have exhibited an increased bone mineral density zone of approximately 1 mm circumferentially and apically to the osteotomy wall produced by compacted autograft particles that act as new bone formation nucleating sites, hastening osseointegration.14, 17, 18, 19, 20, 21 The maximized biomechanical behavior of the OD technique has been possible due to the creation of novel osteotomy burs which take advantage of bone elastic and plastic properties while applying a time‐dependent stress (force) to create a time‐dependent strain (deformation), compacting bone particles into the trabecular space instead of removing them.13, 14 Moreover, such a technique has shown a simultaneous sealing/bridging into the intrathread spaces as a result of the reversed compression exerted by the bone spring‐back effect created by the residual elastic strain generated during the osteotomy, without the excessive strain that would lead to an extensive remodeling and stability dip of conventional press‐fit undersized preparations.14, 17, 18, 19, 20, 21, 33
The interfacial stress distribution and the respective peri‐implant tissue strain due to frictional forces resulting from the interplay between osteotomy and macrogeometry, during implant placement, have shown to control the mechanical interlocking necessary for increased primary stability, as well as enhanced bone healing response.8, 9, 10, 11 Bone tissue tolerates certain levels of compressive strain, even beyond the yield point without affecting the osseointegration progression, which through the elastic behavior improves the physical engagement at the bone‐implant interface, resulting in higher IT and ISQ values.34 Nonetheless, when the strain level generated is markedly higher than the yield point, the plastic deformation and the presence of microcracks may trigger an extensive interfacial bone remodeling healing, decreasing initial stability and shifting the period from primary to secondary stability.8, 9, 10, 11, 34 The statistical analysis of this multicenter clinical data indicated significantly higher IT, by 41%, and ‐ ISQ (immediate), by 15%, values for OD drilling relative to SD, denoting a higher biomechanical engagement for OD prepared sites due to the increased bone mineral density due to compaction‐autografting and the elastic spring‐back effect which generates a higher bone‐to‐implant contact, as previously mentioned.15, 16, 17, 18, 19, 20, 21 Nonetheless, for both drilling techniques, the ISQ values showed reduction at 3 weeks. This reduction was more significant in the SD group and increased at 6 weeks, whereas OD groups ISQ values started high and remained relatively unchanged, above 68, throughout the follow‐up period at 0, 3, and 6 weeks. Therefore, it can be assumed that the strain generated for both SD and OD techniques have the potential to induce different interfacial bone remodeling, where, even at a higher level of physical interlocking, no meaningful negative bone response could be observed for the OD instrumentation, which showed ISQ values above the minimum requirements for load‐bearing capacity over time.6 This fact has been associated with elastic reverse compression of the bone tissue toward the implant due to the spring‐back effect created by the OD drilling protocol which may be the main responsible factor for the improved secondary stability.14, 16 So rather the implant compresses the bone, the bone reverse‐compresses the implant and this is vastly different than undersizing drilling to create a “misfit” between the prepared osteotomy and the inserted implant; which creates an excessive strain that would lead to excessive deformation, microcracking, and extensive remodeling, potentially compromising the biomechanical competence and healing.8, 9, 10, 11 To the best of the authors' knowledge, this is the first clinical comparative study investigating the benefits of OD on IT and ISQ relative to conventional SD. Nonetheless, highly translational preclinical animal models have also demonstrated 30–40% higher biomechanical and histometric parameters values for OD when compared to conventional SD protocols.14, 16, 17, 18, 19, 20, 21
It is noteworthy that the availability of cortical and trabecular bone at the implant interface may influence the biomechanical implant stability and bone healing response.8, 9, 10, 11 The current study investigated the influence of the different osteotomy techniques in various clinical scenarios of different bone mineral densities, where SD and OD drillings were evenly assigned according to arch operated, maxilla (bone density 2, 3, and 4 ‐D2/D3/D4 bone) or mandible (predominantly D2 bone in the posterior region), and region of the maxilla operated, anterior (incisors and canines—D2‐D3 bone) or posterior (premolars and molars—D2/D3/D4 bone).35, 36 While there was no significant difference in the IT and ISQ values for implants placed in the maxilla relative to the mandible using SD, higher IT and ISQ values were observed for implants placed in the mandible than in the maxilla using OD. The absence of significant difference in the SD for both arches has been associated with the preparation dimensions and subtractive nature of the conventional drilling, which tend to undersize the osteotomy in maxillary bone to deliver similar frictional forces during implant placement, without major changes in the biomechanical interlocking and strain in the prepared bone.8, 9, 10, 11, 37 In contrast, OD drilling require no significant undersizing of the osteotomies both in the maxilla or in the mandible but still induced increased level of both primary and secondary stability over 6 weeks of healing. In fact, for both osteotomies, ISQ values of implants installed in the mandible decreased at 3 weeks and returned to the baseline levels at 6 weeks, but the decrease was more significant at 3 weeks for the SD group; the ISQ values in the OD group started high and remained high throughout 6 weeks regardless of implants geometry. Despite OD technique has shown favorable results in the mandible in the current study, caution has been advised in one systematic review, regarding mandibular regions with denser bone types. These anatomical areas are densely corticated and due to the sequellae of an inadequate downsizing osteotomy technique, necrosis and extensive remodeling may result.38 OD has also been indicated in the mandible as a favorable technique for alveolar ridge expansion.23, 39 Additionally, for implants placed in different‐density bone types in the anterior and posterior region of the maxilla, OD increased the bone density to such an extent that there was no significant difference between both areas in both IT values and ISQ values; while for SD, implants placed in the anterior region, predominantly D2 bone, presented higher IT values than in the posterior region, predominantly D3/D4 bone. Moreover, a similar remodeling rate was observed between both regions for both drilling techniques, with OD outperforming SD for all pairwise comparisons. Therefore, OD may be particularly useful during implant placement in sites with adequate trabecular bone volume in both the maxilla and the mandible and provides the possibilities of enhancing both bone mineral density, due to its nonsubtractive nature, and subsequently the implant primary stability, due to the spring back effect, eventually hastening osseointegration, with more significant effect in the maxilla due to the relatively higher amount of trabecular bone.38 Additionally, OD has provided high initial stability in the maxilla, a rapid and stable onset of secondary stability could be observed, with no detrimental effect of the remodeling process.17
Implant design has also substantially evolved over the years, and implant dimensions, in terms of length and diameter, changed and further increased the spectrum of clinical indications, decreasing the complexity of the treatment and reducing time, costs and morbidity by avoiding the need for minor horizontal and vertical augmentative procedures.25, 40, 41, 42 Previous studies have shown a direct relationship between primary stability and diameter and length of the implant so that wider and longer implants were preferable.43, 44, 45 In the current study, IT and ISQ data as a function of implant dimensions, either diameter or length, and drilling technique exhibited higher values for OD relative to SD, except for short implants pairwise comparisons, which may lie on the reduced contact area available for the benefits provided by the OD drilling in increasing the bone mineral density and the biomechanical engagement to reach a clinically significant effect on the clinical parameters.43, 46 This confirms previous findings that ISQ values are lower in short implants.47 Furthermore, factors combination indicated similar IT and ISQ values for implants of different diameters placed using conventional SD, whereas wide implants installed using OD showed higher IT relative to regular and narrow implants. A similar result has been previously demonstrated in clinical studies, where a trend toward higher ISQ values for implants with larger diameters was observed, while implant diameters lower than 4.2 mm showed no significant influence on the stabilization levels of tapered implants.48 Nonetheless, with the placement of a wider implant diameter specially in a tight‐fit undersized osteotomy with standard SD, a higher rate of remodeling was noted. Whereas for narrow and regular diameter implants, the remodeling process is less frequently observed due to a decrease in the amount of SD required. Historically, utilizing the SD methodologies, the wider the osteotomy, the greater is the amount of extracted bone, which creates an increased strain level generated by the interplay between osteotomy‐implant dimensions. This may result in a more evident bone remodeling healing phenomenon.11, 34, 37 Therefore, OD provides the balance between preserving bone bulk and producing higher implant stability (due to the spring back effect) without the need to create severely downsized “misfit” osteotomies.
Multicenter studies have several advantages over single center studies including more rigorous study protocols to ensure uniform data collection and higher rates of patient enrolment; however, such studies require strong efforts to maintain the clinical practice homogeneity, as well as conduct long‐term follow‐up visits,49 which is acknowledged as a limitation of such a study design. Despite the fact that current data have demonstrated that OD outperformed conventional SD in hastening osseointegration, improving primary and secondary stability, future long‐term prospective investigations should be performed to evaluate implant stability in different clinical scenarios of bone type and implant macro‐ and microgeometries, along with implant stability after loading and bone level changes over time.
5. CONCLUSION
OD drilling presented significantly higher primary implant stability depicted by IT and higher implant secondary stability depicted by quotients (ISQ) during the first 6 weeks of healing relative to conventional SD for both arches and area operated, maxilla and mandible and anterior and posterior region of the maxilla, as well as for all implant dimensions evaluated, diameter (narrow, regular, and wide) and length (regular and long), except for short implants.
CONFLICT OF INTERESTS
Dr S. H. developed the novel osseousdensification technique and invented the patented multifluted densifying burs which were utilized. None of the remaining authors have any conflicts to declare related to this study.
AUTHOR CONTRIBUTIONS
Edmara T. P. Bergamo: Data curation, formal analysis, investigations, methodology, writing. Abbas Zahoui: Data curation, investigations, methodology, writing. Raúl Bravo Barrera: Data curation, investigations, methodology, writing. Salah Huwais: Conceptualization, data curation, investigations, methodology, writing – review and editing. Paulo G. Coelho: Conceptualization, methodology, writing – review and editing. Edward Dwayne Karateew: Conceptualization, methodology, writing – review and editing. Estevam A. Bonfante: Conceptualization, data curation, investigations, methodology, writing – review and editing.
Supporting information
AppendixS1: Supplementary Information
ACKNOWLEDGMENTS
To Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) Young Investigators Award grant 2012/19078‐7, FAPESP 2019/08693‐1, to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grants 304589/2017‐9 and 434487/2018‐0, and to CAPES Finance Code 001.
Bergamo ETP, Zahoui A, Barrera RB, et al. Osseodensification effect on implants primary and secondary stability: Multicenter controlled clinical trial. Clin Implant Dent Relat Res. 2021;23(3):317–328. 10.1111/cid.13007
Funding information CAPES Finance Code 001; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grant/Award Numbers: 434487/2018‐0, 304589/2017‐9; Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) Young Investigators Award: 2012/19078‐7 and scholarship 2019/06893‐1
DATA AVAILABILITY STATEMENT
Data will be provided upon request.
REFERENCES
- 1.Branemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10‐year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1‐132. [PubMed] [Google Scholar]
- 2.Trindade R, Albrektsson T, Tengvall P, Wennerberg A. Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clin Implant Dent Relat Res. 2016;18(1):192‐203. 10.1111/cid.12274. [DOI] [PubMed] [Google Scholar]
- 3.Hjalmarsson L, Gheisarifar M, Jemt T. A systematic review of survival of single implants as presented in longitudinal studies with a follow‐up of at least 10 years. Eur J Oral Implantol. 2016;9(suppl 1):S155‐S162. [PubMed] [Google Scholar]
- 4.Faot F, Bielemann AM, Schuster AJ, et al. Influence of insertion torque on clinical and biological outcomes before and after loading of mandibular implant‐retained overdentures in atrophic edentulous mandibles. Biomed Res Int. 2019;2019:8132520. 10.1155/2019/8132520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker LR, Morris GA, Novotny PJ. Implant insertional torque values predict outcomes. J Oral Maxillofac Surg. 2011;69(5):1344‐1349. 10.1016/j.joms.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Gallucci GO, Benic GI, Eckert SE, et al. Consensus statements and clinical recommendations for implant loading protocols. Int J Oral Maxillofac Implants. 2014;29(suppl):287‐290. 10.11607/jomi.2013.g4. [DOI] [PubMed] [Google Scholar]
- 7.Albrektsson T, Chrcanovic B, Ostman PO, Sennerby L. Initial and long‐term crestal bone responses to modern dental implants. Periodontol 2000. 2017;73(1):41‐50. 10.1111/prd.12176. [DOI] [PubMed] [Google Scholar]
- 8.Bonfante EA, Jimbo R, Witek L, et al. Biomaterial and biomechanical considerations to prevent risks in implant therapy. Periodontol 2000. 2019;81(1):139‐151. 10.1111/prd.12288. [DOI] [PubMed] [Google Scholar]
- 9.Coelho PG, Jimbo R. Osseointegration of metallic devices: current trends based on implant hardware design. Arch Biochem Biophys. 2014;561:99‐108. 10.1016/j.abb.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Coelho PG, Jimbo R, Tovar N, Bonfante EA. Osseointegration: hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent Mater. 2015;31(1):37‐52. 10.1016/j.dental.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Stocchero M, Toia M, Cecchinato D, Becktor JP, Coelho PG, Jimbo R. Biomechanical, biologic, and clinical outcomes of undersized implant surgical preparation: a systematic review. Int J Oral Maxillofac Implants. 2016;31(6):1247‐1263. 10.11607/jomi.5340. [DOI] [PubMed] [Google Scholar]
- 12.Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003;14(3):251‐262. [DOI] [PubMed] [Google Scholar]
- 13.Huwais S. Fluted osteotome and surgical method for use. 2013.
- 14.Huwais S, Meyer EG. A novel osseous densification approach in implant osteotomy preparation to increase biomechanical primary stability, bone mineral density, and bone‐to‐implant contact. Int J Oral Maxillofac Implants. 2017;32(1):27‐36. 10.11607/jomi.4817. [DOI] [PubMed] [Google Scholar]
- 15.Wu D, Isaksson P, Ferguson SJ, Persson C. Young's modulus of trabecular bone at the tissue level: a review. Acta Biomater. 2018;78:1‐12. 10.1016/j.actbio.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Kold S, Bechtold JE, Ding M, Chareancholvanich K, Rahbek O, Soballe K. Compacted cancellous bone has a spring‐back effect. Acta Orthop Scand. 2003;74(5):591‐595. 10.1080/00016470310018018. [DOI] [PubMed] [Google Scholar]
- 17.Alifarag AM, Lopez CD, Neiva RF, Tovar N, Witek L, Coelho PG. Atemporal osseointegration: early biomechanical stability through osseodensification. J Orthop Res. 2018;36(9):2516‐2523. 10.1002/jor.23893. [DOI] [PubMed] [Google Scholar]
- 18.Lahens B, Lopez CD, Neiva RF, et al. The effect of osseodensification drilling for endosteal implants with different surface treatments: a study in sheep. J Biomed Mater Res B Appl Biomater. 2019;107(3):615‐623. 10.1002/jbm.b.34154. [DOI] [PubMed] [Google Scholar]
- 19.Lahens B, Neiva R, Tovar N, et al. Biomechanical and histologic basis of osseodensification drilling for endosteal implant placement in low density bone. An experimental study in sheep. J Mech Behav Biomed Mater. 2016;63:56‐65. 10.1016/j.jmbbm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira P, Bergamo ETP, Neiva R, et al. Osseodensification outperforms conventional implant subtractive instrumentation: a study in sheep. Korean J Couns Psychother. 2018;90:300‐307. 10.1016/j.msec.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 21.Trisi P, Berardini M, Falco A, Podaliri VM. New osseodensification implant site preparation method to increase bone density in low‐density bone: in vivo evaluation in sheep. Implant Dent. 2016;25(1):24‐31. 10.1097/ID.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huwais S, Mazor Z, Ioannou AL, Gluckman H, Neiva R. A multicenter retrospective clinical study with up‐to‐5‐year follow‐up utilizing a method that enhances bone density and allows for Transcrestal sinus augmentation through compaction grafting. Int J Oral Maxillofac Implants. 2018;33(6):1305‐1311. 10.11607/jomi.6770. [DOI] [PubMed] [Google Scholar]
- 23.Koutouzis T, Huwais S, Hasan F, Trahan W, Waldrop T, Neiva R. Alveolar ridge expansion by osseodensification‐mediated plastic deformation and compaction autografting: a multicenter retrospective study. Implant Dent. 2019;28(4):349‐355. 10.1097/ID.0000000000000898. [DOI] [PubMed] [Google Scholar]
- 24.Tanello B, Huwais S, Tawil I, Rosen P, Neiva R. Osseodensification protocols for enhancement of primary and secondary implant stability – A retrospective 5‐year follow‐up multi‐center study. Clinical Oral Implants Research, 2019;30(S19):414. 10.1111/clr.370_13509. [DOI] [Google Scholar]
- 25.Al‐Johany SS, Al Amri MD, Alsaeed S, Alalola B. Dental implant length and diameter: a proposed classification scheme. J Prosthodont. 2017;26(3):252‐260. 10.1111/jopr.12517. [DOI] [PubMed] [Google Scholar]
- 26.Raghavendra S, Wood MC, Taylor TD. Early wound healing around endosseous implants: a review of the literature. Int J Oral Maxillofac Implants. 2005;20(3):425‐431. [PubMed] [Google Scholar]
- 27.Nedir R, Bischof M, Szmukler‐Moncler S, Bernard JP, Samson J. Predicting osseointegration by means of implant primary stability: a resonance‐frequency analysis study with delayed and immediately loaded ITI SLA implants. Clin Oral Implants Res. 2004;15(5):520‐528. [DOI] [PubMed] [Google Scholar]
- 28.Trisi P, Perfetti G, Baldoni E, Berardi D, Colagiovanni M, Scogna G. Implant micromotion is related to peak insertion torque and bone density. Clin Oral Implants Res. 2009;20(5):467‐471. [DOI] [PubMed] [Google Scholar]
- 29.Trisi P, Berardini M, Falco A, Podaliri VM. Validation of value of actual micromotion as a direct measure of implant micromobility after healing (secondary implant stability). An in vivo histologic and biomechanical study. Clin Oral Implants Res. 2016;27(11):1423‐1430. [DOI] [PubMed] [Google Scholar]
- 30.Lages FS, Douglas‐de Oliveira DW, Costa FO. Relationship between implant stability measurements obtained by insertion torque and resonance frequency analysis: a systematic review. Clin Implant Dent Relat Res. 2018;20(1):26‐33. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Q, Su YY, Wang X, Chen S. Clinical outcomes following immediate loading of single‐tooth implants in the esthetic zone: a systematic review and meta‐analysis. Int J Oral Maxillofac Implants. 2020;35(1):167‐177. 10.11607/jomi.7548. [DOI] [PubMed] [Google Scholar]
- 32.Gallardo YNR, da Silva‐Olivio IR, Gonzaga L, Sesma N, Martin W. A systematic review of clinical outcomes on patients rehabilitated with complete‐arch fixed implant‐supported prostheses according to the time of loading. J Prosthodont. 2019;28(9):958‐968. 10.1111/jopr.13104. [DOI] [PubMed] [Google Scholar]
- 33.Witek L, Neiva R, Alifarag A, et al. Absence of healing impairment in osteotomies prepared via osseodensification drilling. Int J Periodontics Restorative Dent. 2019;39(1):65‐71. 10.11607/prd.3504. [DOI] [PubMed] [Google Scholar]
- 34.Halldin A, Jimbo R, Johansson CB, et al. The effect of static bone strain on implant stability and bone remodeling. Bone. 2011;49(4):783‐789. 10.1016/j.bone.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Alghamdi HS. Methods to improve Osseointegration of dental implants in low quality (type‐IV) bone: an overview. J Funct Biomater. 2018;9(1):1‐8. 10.3390/jfb9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuh LJ, Huang HL, Chen CS, et al. Variations in bone density at dental implant sites in different regions of the jawbone. J Oral Rehabil. 2010;37(5):346‐351. 10.1111/j.1365-2842.2010.02061.x. [DOI] [PubMed] [Google Scholar]
- 37.Beutel BG, Danna NR, Granato R, et al. Implant design and its effects on osseointegration over time within cortical and trabecular bone. J Biomed Mater Res B Appl Biomater. 2016;104(6):1091‐1097. 10.1002/jbm.b.33463. [DOI] [PubMed] [Google Scholar]
- 38.Padhye NM, Padhye AM, Bhatavadekar NB. Osseodensification—a systematic review and qualitative analysis of published literature. J Oral Biol Craniofac Res. 2020;10(1):375‐380. 10.1016/j.jobcr.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian JH, Neiva R, Coelho PG, et al. Alveolar ridge expansion: comparison of osseodensification and conventional osteotome techniques. J Craniofac Surg. 2019;30(2):607‐610. 10.1097/SCS.0000000000004956. [DOI] [PubMed] [Google Scholar]
- 40.Thoma DS, Haas R, Sporniak‐Tutak K, Garcia A, Taylor TD, Hammerle CHF. Randomized controlled multicentre study comparing short dental implants (6 mm) versus longer dental implants (11‐15 mm) in combination with sinus floor elevation procedures: 5‐year data. J Clin Periodontol. 2018;45(12):1465‐1474. 10.1111/jcpe.13025. [DOI] [PubMed] [Google Scholar]
- 41.Walton JN, MacEntee MI. Choosing or refusing oral implants: a prospective study of edentulous volunteers for a clinical trial. Int J Prosthodont. 2005;18(6):483‐488. [PubMed] [Google Scholar]
- 42.Zinsli B, Sagesser T, Mericske E, Mericske‐Stern R. Clinical evaluation of small‐diameter ITI implants: a prospective study. Int J Oral Maxillofac Implants. 2004;19(1):92‐99. [PubMed] [Google Scholar]
- 43.Lemos CA, Ferro‐Alves ML, Okamoto R, Mendonca MR, Pellizzer EP. Short dental implants versus standard dental implants placed in the posterior jaws: a systematic review and meta‐analysis. J Dent. 2016;47:8‐17. 10.1016/j.jdent.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Sohrabi K, Mushantat A, Esfandiari S, Feine J. How successful are small‐diameter implants? A literature review. Clin Oral Implants Res. 2012;23(5):515‐525. 10.1111/j.1600-0501.2011.02410.x. [DOI] [PubMed] [Google Scholar]
- 45.Schiegnitz E, Al‐Nawas B. Narrow‐diameter implants: a systematic review and meta‐analysis. Clin Oral Implants Res. 2018;29(suppl 16):21‐40. 10.1111/clr.13272. [DOI] [PubMed] [Google Scholar]
- 46.Tawil G, Aboujaoude N, Younan R. Influence of prosthetic parameters on the survival and complication rates of short implants. Int J Oral Maxillofac Implants. 2006;21(2):275‐282. [PubMed] [Google Scholar]
- 47.Sim CP, Lang NP. Factors influencing resonance frequency analysis assessed by Osstell mentor during implant tissue integration: I. instrument positioning, bone structure, implant length. Clin Oral Implants Res. 2010;21(6):598‐604. 10.1111/j.1600-0501.2009.01878.x. [DOI] [PubMed] [Google Scholar]
- 48.Torroella‐Saura G, Mareque‐Bueno J, Cabratosa‐Termes J, Hernandez‐Alfaro F, Ferres‐Padro E, Calvo‐Guirado JL. Effect of implant design in immediate loading. A randomized, controlled, split‐mouth, prospective clinical trial. Clin Oral Implants Res. 2015;26(3):240‐244. 10.1111/clr.12506. [DOI] [PubMed] [Google Scholar]
- 49.Chung KC, Song JW, Group WS . A guide to organizing a multicenter clinical trial. Plast Reconstr Surg. 2010;126(2):515‐523. 10.1097/PRS.0b013e3181df64fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1: Supplementary Information
Data Availability Statement
Data will be provided upon request.


