Abstract
Objective
To study the association between insulin receptors (isoforms α and β), insulin growth factor‐1 (IGF1) and serine/arginine splicing factor 1 (SRSF‐1) in patients with prostate cancer (PC) and diabetes.
Materials and Methods
We retrospectively analyzed data from 368 patients who underwent surgery for PC or benign prostatic hyperplasia (BPH) between 2010 and 2020 at the Department of Urology, University of Catania. Tissue microarray slides were constructed and they were stained for androgen receptor (AR), insulin receptor‐α and ‐β, IGF1 (IGF1‐R), Ki‐67, and prostate specific membrane antigen (PSMA) expression using validated score.
Results
The final cohort was represented by 100 patients with BPH and 268 with PC, with a median age of 68 years. We found that SRSF‐1 expression was associated with AR (odds ratio [OR]: 1.66), PSMA (OR: 2.13), Ki‐67 (OR: 5.99), insulin receptor (IR)‐α (OR: 2.38), IR‐β (OR: 3.48), IGF1‐R (OR: 1.53), and microvascular density (MVD) was associated with PSMA (OR: 3.44), Ki‐67 (OR: 2.23), IR‐α (OR: 2.91), IR‐β (OR: 3.02), IGF1‐R (OR: 2.95), and SRSF‐1 (OR: 2.21). In the sub cohort of PC patients, we found that SRSF‐1 expression was associated with AR (OR: 2.34), Ki‐67 (OR: 6.77), IR‐α (OR: 2.7), and MVD (OR: 1.98). At the Kaplan–Meier analysis, SRSF‐1+ patients had worse 5‐ and 9‐year biochemical recurrence (36% and 6%) respect to SRSF‐1− (67% and 7%; p < .01) and similarly MVD+ patients (44% and 7%) respect to MVD− (64% and 8%; p < .01). Restricting the analysis only in patients with PC and diabetes, we found that SRSF‐1+ was associated with Ki‐67+ (OR: 8.75; p < .05) and MVD+ (OR: 7.5; p < .05).
Conclusions
PC exhibits widespread heterogeneity in protein expression. In particular, the expressions of the SRSF‐1 protein and of the MVD are associated with a worse prognosis and in particular with a greater cell proliferation. These results, although preliminary, may offer new future scientific insights with the aim of highlighting possible genetic alterations linked to a greater expression of SRSF‐1 and associated with a worse prognosis.
Keywords: androgen receptor, diabetes, hormonal therapy, proliferation, risk factors, srsf1
1. INTRODUCTION
Prostate cancer (PC) still represents the first cancer diagnosed in the male population in 2020.1 Many risk factors have been identified over the years including hypertension, metabolic syndrome, obesity, but diabetes mellitus is certainly considered that risk factor capable of determining consistent structural changes in tumor cells.2 For example, previous authors have showed that insulin growth factor‐1 (IGF‐1) may promote cancer aggressiveness in diabetic patients3 and two different mechanisms have been identified including the upregulation of the androgen receptor, presumably via alteration in the insulin/IGF‐1 signaling cascade and the disinhibition of androgen signaling due to decreased levels of protective estrogen receptor ligands.4 Other identified pathways that have associated diabetes to PC are represented by the alteration of insulin and insulin‐like growth factor‐I (IGF‐I),5 the modifications of sex steroid pathways, such as increased serum 17ß‐estradiol levels, sex hormone‐binding globulin concentration and decreased free testosterone level.6, 7
In recent years, a particular interest has emerged in alternative splicing of vascular endothelial growth factor ‐alpha (VEGFA), which encodes vascular endothelial growth factor (VEGF). The alternative junction of exons 5–7 of the VEGFA transcript may determine its different activity and bioavailability.
The VEGFA transcript consists of eight exons and the alternative junction of exons 5–7 results in the expression of junction isoforms with different activities and bioavailability. Serine/arginine splicing factor 1 (SRSF1) (Ser/Arg (SR)‐rich splicing factor) was the first splice factor to be described as a proto‐oncogene8, 9 and its activity is modified by splice factor kinases such as SRPK1 which exhibits oncogenic properties.
Interestingly Malakar et al.10 found that insulin signaling, through activation of the Ras‐MAPK pathway, upregulates the splicing factor SRSF1 and induces inclusion of insulin receptor (IR) exon 11 to generate elevated levels of the IR‐β isoform in pancreatic cells.
However, the relationship between IR isoforms and SFRS1 in PC has been not well studied.
Based on all these premises, current research aims to study the association between insulin receptors (isoforms α and β), IGF1, and SRSF1 in patients with PC and diabetes.
2. MATERIALS AND METHODS
We retrospectively analyzed data from 368 patients who underwent surgery for PC or benign prostatic hyperplasia (BPH) between 2010 and 2020 at the Department of Urology, University of Catania. Patients who were diagnosed with PC underwent radical prostatectomy and patients with BPH underwent transurethral resection of the prostate. The presence of diabetes was evaluated from hospital records or fasting serum blood glucose.
This study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Ethics Committee of the Policlinic Hospital of Catania (#131/2015).
2.1. Tissue microarray construction
All specimens were stained by hematoxylin and eosin to find the representative cores for the tissue microarray construction. For the tissue micro array (TMA) construction the Galileo TMA CK3500 (Integrated System Engineering, Milan, Italy) was used. It is a semiautomatic and computer‐assisted tissue microarrayer with a dedicated software that assists the user throughout the operating phases from tissue microarray design to final reporting. This instrument is associated with an X‐Y‐Z automated stage that allows one to directly place selected tissue cores in the recipient TMA block containing premade holes, ensuring not only a significant reduction in the array construction time but also an extreme alignment accuracy.11
2.2. Immunohistochemistry (IHC)
Immunohistochemical slides were evaluated by three pathologists (G.B., E.P. and R.C.) with no information on patient clinical data. The presence of brown chromogen within the cell nuclei was interpreted as positive SRSF1 staining as previously described.12 Intensity of staining (IS) was graded on a 0–3 scale (0 = absent staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining). Five categories (0–4) of percentage of SRSF1 immunopositive cells (Extent Score [ES]) were identified: <5%; 5–30%; 31–50%; 51–75%; >75%. IS was multiplied by ES to obtain the immunoreactivity score (IRS); low (L‐IRS) and high (H‐IRS) expression of SRSF1 were defined as IRS <6 and IRS ≥6, respectively.13, 14, 15 Assessment of Blood Vascular (microvascular density [MVD]) was evaluated as previously described.16, 17 Briefly, vascular hotspots were identified on tissue sections stained with anti‐CD31 immunohistochemical antibody (JC70A; working dilution 1:40; DAKO) by a light microscope at ×4 and ×10 magnifications. MVD represented the total amount of vessels per mm2 (conversion factor: 1 mm2 = 4 high power fields‐ HPFs‐). Areas with ≥50 of viable tumor tissue were counted; extensive necrosis, hemorrhage and desmoplasia were considered as exclusion factors. Each single stained endothelial cell and every lumen for long branched vessels and glomeruloid tufts were counted. Finally, small clusters of ≥2 staining endothelial cells within the same vascular structure were counted as a single vessel.
For the Ki‐67 nuclear positivity the total number of Ki‐67 positive tumor nuclei were counted in malignant cells on each individual TMA spot, regardless of the intensity of immunostaining or the Gleason score. This count was reported on the total number of malignant cells, calculated by using a 100‐cell template moved over the whole tissue core. A TMA spot was rejected when neoplastic glands covered less than 30% of the tissue. Moreover, a minimum number of 500 tumor cells per patient was require keeping the sample for analysis. The mean percentage of Ki‐67 positive cells for each patient was calculated and used in further analysis as the Ki‐67 labeling index.18
For the AR, IR‐α, IR‐β, IGF1‐R, and prostate specific membrane antigen (PSMA) expression, the scoring system included an analysis of IS as previously described.13, 14
Sections of unaffected gallbladder mucosa were used as positive control for SRSF1, while negative control slides were obtained by incubating them with phosphate‐buffered saline instead of the primary antibody.
2.3. Statistical analysis
Continuous variables are presented as median and interquartile range (IQR) and were compared by the Student independent t test or the Mann–Whitney U test based on their normal or not‐normal distribution, respectively (normality of variables' distribution was tested by the Kolmogorov–Smirnov test). Categorical variables were tested with the χ 2 test. Univariate and multivariate logistic regression has been used to test independent variables associated with IHC scores. PC was classified into low, intermediate and high according to EAU guidelines.19
Kaplan–Meier curve were used to verify biochemical recurrence. For all statistical comparisons, a significance level of p < .05 was considered to show differences between the groups. Data analysis was performed under the guidance of our statistics expert, using SPSS version 17 (Statistical Package for Social Science. SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0.) (SPSS Inc.).
3. RESULTS
The final cohort was represented by 100 patients with BPH and 268 with PC, with a median age of 68 years (IQR: 63.0–75.0), median fasting blood glucose of 97.0 (IQR: 87.0–109.0), median total cholesterol of 188.0 (IQR: 161.0–216.0) and median triglycerides was 100.0 (IQR: 69.0–137.0) (Table 1).
Table 1.
Characteristics of the population (N = 368)
| Variables | |
|---|---|
| Age (years) median (IQR) | 68.0 (63.0–73.0) |
| BPH, n (%) | 99 (27.17) |
| Prostate cancer, n (%) | 268 (73.83) |
| Diabetes, n (%) | 65 (17.66%) |
| Fasting blood glucose (mg/dl), median (IQR) | 97.0 (87.0–109.0) |
| Total cholesterol (mg/dl), median (IQR) | 188.0 (161.0–216.0) |
| Triglycerides (mg/dl), median (IQR) | 100.0 (69.0–137.0) |
| PSA (ng/ml), median (IQR) | 7.0 (4.85–10.63) |
| BCR, n (%) | 42 (16.28) |
Abbreviations: BCR, biochemical recurrence; BPH, benign prostatic hyperplasia; IQR, interquartile range.
Figure 1 shows SRSF‐1 and MVD expression in PC patients. We found that rate of SRSF‐1 positive expression was greater in patients with PC respect to BPH (81.38% vs. 64.44%; p < .01). Furthermore, high‐risk PC had slightly higher rate of positive SRSF‐1 (24.34 vs. 16.38%; p = .054) and it was significantly associated with higher expression of Ki‐67 positive score (21.61% vs. 4.44%; p < .01), AR positive score (52.66% vs. 40.0; p < .05); PSMA positive score (48.40% vs. 30.56; p < .01), IR‐α positive score (76.06% vs. 57.22%; p < .01) and IR‐β positive score (9.04% vs. 2.78%; p < .05) (Table 2).
Figure 1.
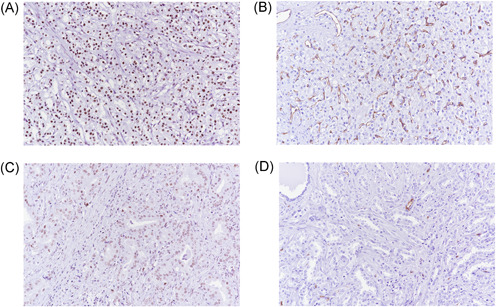
High (A and B) and Low (C and D) expression of SRSF1 and MVD. MVD, microvascular density [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
SRSF‐1 expression according to IHC score
| SRSF‐1 | |||
|---|---|---|---|
| Negative (n = 180) | Positive (n = 188) | p Value | |
| Age (years), median (IQR) | 76.0 (70.0–79.0) | 67.0 (63.0–72.0) | .82 |
| PSA (ng/ml), median IQR) | 2.05 (0.9–4.27) | 7.8 (5.7–11.6) | <.01 |
| Fasting glucose (mg/dl), median (IQR) | 95.0 (84.0–116.0) | 97.0 (88.0–107.5) | .51 |
| Total cholesterol (mg/dl), median (IQR) | 172.0 (150.0–194.0) | 191.5 (168.0–224.0) | .60 |
| Triglycerides (mg/dl), median (IQR) | 110.0 (69.0–164.0) | 97.0 (66.0–130.0) | .85 |
| Diabetes, n (%) | 38 (21.1) | 27 (14.36) | .09 |
| Group, n (%) | <.01 | ||
| BPH | 64 (35.56) | 35 (18.62) | |
| PC | 116 (64.44) | 153 (81.38) | |
| ISUP Gleason score, n (%) | .04 | ||
| 1 | 42 (36.21) | 34 (22.37) | |
| 2 | 44 (37.93) | 58 (38.16) | |
| 3 | 21 (18.10) | 44 (28.95) | |
| 4 | 3 (2.59) | 10 (6.58) | |
| 5 | 6 (5.17) | 6 (3.95) | |
| Pathological stage, n (%) | .46 | ||
| T2 | 64 (72.41) | 99 (65.79) | |
| T3 | 18 (15.52) | 32 (21.05) | |
| T4 | 14 (12.07) | 20 (13.16) | |
| Classification risk of PC, n (%) | .054 | ||
| Low risk | 50 (43.10) | 45 (29.61) | |
| Intermediate risk | 47 (40.52) | 70 (46.05) | |
| High risk | 19 (16.38) | 27 (24.34) | |
| Ki‐67 positive score, n (%) | 8 (4.44) | 41 (21.81) | <.01 |
| AR positive score, n (%) | 72 (40.0) | 99 (52.66) | <.05 |
| PSMA positive score, n (%) | 55 (30.56) | 91 (48.40) | <.01 |
| PSA positive score, n (%) | 17 (34.0) | 154 (48.43) | .057 |
| IR‐α positive score, n (%) | 103 (57.22) | 143 (76.06) | <.01 |
| IR‐β positive score, n (%) | 5 (2.78) | 17 (9.04) | <.05 |
| IGF‐1R positive score, n (%) | 24 (13.33) | 36 (19.15) | 0.13 |
| MVD, median (IQR) | 32.0 (27.0–38.0) | 88.0 (44.0–111.0) | |
Abbreviations: AR, androgenic receptor; BPH, benign prostatic hyperplasia; IGF‐1R, insulin‐like growth factor‐1 receptor; IQR, interquartile range; IR, insulin receptor; PCa, prostate cancer; PSA, prostate‐specific antigen; PSMA, prostate specific membrane antigen.
When assessing MVD positive expression at IHC, we found that PC patients had greater positivity respect to BPH (97.75% vs. 49.47%; p < .01) and that it was significantly associated with Ki‐67 positive score (92.7% vs. 80.53%; p < .01), PSMA positive score (54.49% vs. 49.47%; p < .01), IR‐α positive score (76.85% vs. 55.79%; p < .01), IR‐β positive score (8.99% vs. 3.16%; p < .05), IGF‐1R (23.6% vs. 9.47%; p < .01) and SRSF‐1 (61.24% vs. 9.47%; p < .01) (Table 3).
Table 3.
MVD expression according to IHC score
| MVD | |||
|---|---|---|---|
| Negative (n = 190) | Positive (n = 178) | p Value | |
| Age (years), median (IQR) | 71.0 (64.0–76.0) | 68.0 (63.0–72.0) | <.01 |
| PSA (ng/ml), median IQR) | 5.25 (2.08–8.625) | 7.85 (5.7–11.4) | <.01 |
| Fasting glucose (mg/dl), median (IQR) | 96.0 (87.0–115.0) | 96.0 (87.5–106.0) | .33 |
| Total cholesterol (mg/dl), median (IQR) | 176.0 (151.0–200.0) | 198.0 (168.0–225.0) | <.01 |
| Triglycerides (mg/dl), median (IQR) | 103.0 (65.0–150.0) | 97.0 (73.0–133.0) | .72 |
| Diabetes, n (%) | 39 (20.53) | 26 (14.61) | .14 |
| Group, n (%) | <.01 | ||
| BPH | 96 (50.53) | 4 (2.25) | |
| PC | 94 (49.47) | 174 (97.75) | |
| ISUP Gleason score, n (%) | .30 | ||
| 1 | 31 (32.98) | 45 (25.86) | |
| 2 | 29 (30.85) | 73 (41.95) | |
| 3 | 27 (28.72) | 38 (21.84) | |
| 4 | 3 (3.19) | 10 (5.75) | |
| 5 | 4 (4.26) | 8 (4.6) | |
| Pathological stage, n (%) | <.01 | ||
| T2 | 60 (63.83) | 124 (71.26) | |
| T3 | 27 (28.72) | 23 (13.22) | |
| T4 | 7 (7.45) | 27 (15.52) | |
| Classification risk of PC, n (%) | .29 | ||
| Low risk | 39 (41.49) | 56 (32.18) | |
| Intermediate risk | 36 (38.30) | 81 (46.55) | |
| High risk | 19 (20.21) | 37 (21.26) | |
| Ki‐67 positive score, n (%) | 153 (80.53) | 165 (92.70) | <.01 |
| AR positive score, n (%) | 94 (49.47) | 77 (43.26) | .23 |
| PSMA positive score, n (%) | 49 (25.79) | 97 (54.49) | <.01 |
| PSA positive score, n (%) | 153 (80.53) | 165 (92.70) | <.01 |
| IR‐α positive score, n (%) | 106 (55.79) | 140 (78.65) | <.01 |
| IR‐β positive score, n (%) | 6 (3.16) | 16 (8.99) | <.05 |
| IGF‐1R positive score, n (%) | 18 (9.47) | 42 (23.60) | <.01 |
| SRSF‐1 positive score, n (%) | 79 (41.58) | 109 (61.24) | <.01 |
Abbreviations: AR, androgenic receptor; BPH, benign prostatic hyperplasia; IGF‐1R, insulin‐like growth factor‐1 receptor; IHC, immunohistochemistry; IQR, interquartile range; IR, insulin receptor; MVD, microvascular density; PCa, prostate cancer; PSA, prostate‐specific antigen; PSMA, prostate specific membrane antigen; SRSF1, serine/arginine splicing factor 1.
We performed the univariate logistic regression between IHC and clinical and pathological variables in our patients and we found that SRSF‐1 expression was associated with AR (odds ratio [OR]: 1.66), PSMA (OR: 2.13), Ki‐67 (OR: 5.99), IR‐α (OR: 2.38), IR‐β (OR: 3.48), IGF1‐R (OR: 1.53), and MVD was associated with PSMA (OR: 3.44), Ki‐67 (OR: 2.23), IR‐α (OR: 2.91), IR‐β (OR: 3.02), IGF1‐R (OR: 2.95), and SRSF‐1 (OR: 2.21) (Table 4).
Table 4.
Univariate logistic regression between immunohistochemistry results and clinical and pathological variables in the whole cohort patients
| AR + vs. − | PSMA + vs. − | Ki67 + vs. – | IR‐α + vs. – | IR‐β + vs. – | IGF‐1R + vs. – | SRSF‐1 + vs. – | MVD + vs − | |
|---|---|---|---|---|---|---|---|---|
| PSA (ng/ml), continuous | 0.99 (0.9.8–1.01) | 1.03 (1.01–1.06)a | 0.99 (0.97–1.02) | 1.01 (1.00–1.08) | 0.98 (0.92–1.03) | 1.02 (1.00–1.04) | 1.03 (1.00–1.05) | 1.05 (1.02–1.09) |
| Group, PC vs. BPH | 0.73 (0.46–1.16) | 17.13 (7.25–40.46)a | 10.42 (2.48–43.76)a | 7.35 (4.42–12.21)a | 1.0 | 1.0 | 2.32 (1.44–3.74)a | 44.42 (15.86–124.58)a |
| Fasting blood glucose (mg/dl), continuous | 1.00 (0.99–1.01) | 0.99 (0.98–1.00) | 1.00 (0.98–1.01) | 0.98 (0.97–0.99) | 0.99 (0.98–1.01) | 0.99 (0.98–1.00) | 0.99 (0.99–1.00) | 0.99 (0.98–1.00) |
| Total cholesterol (mg/dl), continuous | 0.99 (0.99–1.00) | 1.00 (1.00–1.01)a | 1.00 (0.99–1.01) | 1.01 (1.00–1.02) | 1.01 (0.99–1.02) | 1.00 (0.99–1.01) | 1.00 (0.99–1.00) | 1.01 (1.00–1.02) |
| Triglycerides (mg/dl), continuous | 0.99 (0.99–1.00) | 0.99 (0.99–1.00) | 0.00 (0.99–1.00) | 0.99 (0.99–1.00) | 0.99 (0.98–1.01) | 1.00 (0.99–1.00) | 0.99 (0.99−1.00) | 0.99 (0.99–1.00) |
| Diabetes, yes vs. no | 0.91 (0.53–1.56) | 0.62 (0.35–1.10) | 0.90 (0.40–2.01) | 0.48 (0.28–0.81) | 0.20 (0.0–1.58) | 0.92 (0.44–1.93) | 0.62 (0.36–1.07) | 0.66 (0.38–1.14) |
| Pathological stage, pT3/4 vs. pT2 | 1.29 (0.77–2.17) | 1.69 (0.98–2.80) | 2.02 (1.06–3.86)a | 0.86 (0.46–1.61) | 0.80 (0.30–2.15) | 1.36 (0.75–2.50) | 1.36 (0.80–2.31) | 0.71 (0.42–1.21) |
| ISUP Gleason, ≥4 vs. <4 | 1.17 (0.51–2.67) | 1.18 (0.51–2.70) | 3.03 (1.25–7.36)a | 1.06 (0.38–2.96) | 0.44 (0.06–3.42) | 2.11 (0.88–5.07) | 1.40 (0.59–3.28) | 1.43 (0.58–3.56) |
| AR, + vs. – | – | 0.70 (0.46–1.06) | 4.25 (1.14–8.47)a | 2.33 (1.48–3.67)a | 1.71 (0.72–4.12) | 0.67 (0.38–1.18) | 1.66 (1.10–2.52)a | 0.77 (0.52–1.17) |
| PSMA, + vs. − | 0.70 (0.46‐1.06) | – | 3.75 (1.98–7.12)a | 4.01 (2.40–6.70)a | 1.89 (0.80–4.51) | 4.2 (2.3–7.54)a | 2.13 (1.40–3.26)a | 3.44 (2.22–5.34)a |
| Ki‐67, + vs. – | 4.25 (1.14–8.47)a | 3.75 (1.98–7.12)a | – | 9.12 (2.77–29.98)a | 4.25 (1.70–10.75)a | 3.82 (1.97–7.44)a | 5.99 (2.72–13.19)a | 2.23 (1.19–4.18)a |
| IR‐α, + vs. – | 2.33 (1.48–3.67)a | 4.01 (2.40–6.70)a | 9.12 (2.77–29.98)a | – | 1.0 | 2.85 (1.40–5.85)a | 2.38 (1.52–3.71)a | 2.91 (1.85–4.62)a |
| IR‐β, + vs. – | 1.71 (0.72–4.12) | 1.89 (0.80–4.51) | 4.25 (1.70–10.75)a | 1.0 | – | 3.23 (1.29–8.08)a | 3.48 (1.25–9.64)a | 3.02 (1.15–7.92)a |
| IGF‐1R, + vs. – | 0.67 (0.38–1.18) | 4.2 (2.3–7.54)a | 3.82 (1.97–7.44)a | 2.85 (1.40–5.85)a | 3.23 (1.29–8.08)a | – | 1.53 (0.88–2.70) | 2.95 (1.62–5.35)a |
| SRSF‐1, + vs. − | 1.66 (1.10–2.52)a | 2.13 (1.40–3.26)a | 5.99 (2.72–13.19)a | 2.38 (1.52–3.71)a | 3.48 (1.25–9.64)a | 1.53 (0.88–2.70) | – | 2.21 (1.46–3.36)a |
| MVD, + vs. − | 0.77 (0.52–1.17) | 3.44 (2.22–5.34)a | 2.23 (1.19–4.18)a | 2.91 (1.85–4.62)a | 3.02 (1.15–7.92)a | 2.95 (1.62–5.35)a | 2.21 (1.46–3.36)a | – |
Note: Data are expressed as odds ratio (interquartile range).
Abbreviations: AR, androgenic receptor; BPH, benign prostatic hyperplasia; IGF‐1R, insulin‐like growth factor‐1 receptor; IQR, interquartile range; IR, insulin receptor; MVD, microvascular density; PCa, prostate cancer; PSA, prostate‐specific antigen; PSMA, prostate specific membrane antigen; SRSF1, serine/arginine splicing factor 1.
p < .05.
We performed the univariate logistic regression in the sub cohort of PC patients and we found that SRSF‐1 expression was associated with AR (OR: 2.34), Ki‐67 (OR: 6.77), IR‐α (OR: 2.7), and MVD (OR: 1.98) (Table 5).
Table 5.
Univariate logistic regression between immunohistochemistry results and clinical and pathological variables in PC patients
| AR + vs. − | PSMA + vs. − | Ki67 + vs. – | IR‐α + vs. – | IR‐β + vs. – | IGF‐1R + vs. – | SRSF‐1 + vs. – | MVD + vs − | |
|---|---|---|---|---|---|---|---|---|
| PSA (ng/ml), continuous | 1.00 (0.98–1.02) | 1.00 (0.98–1.03) | 0.97 (0.93–1.01) | 0.99 (0.97–1.02) | 0.91 (0.80–1.01) | 1.00 (0.98–1.02) | 1.01 (0.99–1.03) | 1.01 (0.98–1.03) |
| Fasting blood glucose (mg/dl), continuous | 0.99 (0.98–1.01) | 0.99 (0.98–1.00) | 1.00 (0.99–1.02) | 0.99 (0.98–1.00) | 1.00 (0.98–1.02) | 0.99 (0.98–1.01) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) |
| Total cholesterol (mg/dl), continuous | 0.99 (0.98–1.00) | 1.00 (0.99–1.00) | 0.99 (0.98–1.00) | 1.00 (0.99–1.01) | 1.00 (0.98–1.02) | 0.99 (0.98–1.0) | 0.99 (0.98–1.00) | 1.00 (0.99–1.01) |
| Triglycerides (mg/dl), continuous | 1.00 (0.99–1.00) | 0.99 (0.99–1.00) | 1.00 (0.99–1.00) | 1.00 (0.99–1.01) | 0.99 (0.98–1.01) | 1.23 (0.56–2.70) | 0.99 (0.99–1.00) | 1.00 (0.99–1.00) |
| Diabetes, yes vs. no | 0.96 (0.48–1.90) | 0.59 (0.29–1.17) | 0.83 (0.33–2.11) | 0.62 (0.29–1.34) | 0.26 (0.03–1.99) | 1.23 (0.56–2.70) | 0.53 (0.27–1.06) | 1.09 (0.53–2.24) |
| Pathological stage, pT3/4 vs. pT2 | 1.30 (0.77–2.17) | 1.64 (0.98–2.79) | 2.02 (1.06–3.86)a | 0.86 (0.46–1.60) | 0.44 (0.05–3.42) | 1.36 (0.75–2.49) | 1.36 (0.80–2.31) | 0.71 (0.42–1.21) |
| ISUP Gleason, ≥4 vs. <4 | 1.17 (0.51–2.67) | 1.18 (0.51–2.70) | 3.03 (1.24–736)a | 1.06 (0.38–2.96) | 0.80 (0.30–2.14) | 2.11 (0.88–5.07) | 1.39 (0.59–3.28) | 1.43 (0.58–3.56) |
| AR, + vs. – | – | 0.77 (0.48–1.26) | 4.75 (2.33–9.67)a | 2.94 (1.52–5.70)a | 1.90 (0.79–4.63) | 0.72 (0.40–1.30) | 2.34 (1.42–3.87)a | 0.98 (0.59–1.62) |
| PSMA, + vs. − | 0.77 (0.48–1.26) | – | 2.51 (1‐27–4.94)a | 1.94 (1.06–3.53)a | 1.10 (0.46–2.65) | 2.37 (1.29–4.36)a | 1.59 (0.98–2.59) | 1.49 (0.90–2.47) |
| Ki‐67, + vs. – | 4.75 (2.33–9.67)a | 2.51 (1.27–4.94)a | – | 4.62 (1.38–15.51)a | 3.03 (1.29–7.71)a | 2.64 (1.34–5.20)a | 6.77 (2.76–16.59)a | 2.02 (1.06–3.86)a |
| IR‐α, + vs. – | 2.94 (1.52–5.70)a | 1.94 (1.06–3.53)a | 4.62 (1.38–15.51)a | – | 1.0 | 1.42 (0.66–3.01) | 2.70 (1.46–4.94)a | 1.25 (0.68–2.30) |
| IR‐β, + vs. – | 1.90 (0.79–4.63) | 1.10 (0.46–2.65) | 3.03 (1.29–7.71)a | 1.0 | – | 2.13 (0.85–5.35) | 2.80 (0.99–7.81) | 1.48 (0.56–3.93) |
| IGF‐1R, + vs. – | 0.72 (0.40–1.30) | 2.37 (1.29–4.36)a | 2.64 (1.34–5.20)a | 1.42 (0.66–3.01) | 2.13 (0.85–5.35) | – | 1.18 (0.66–2.13) | 1.34 (0.72–2.49) |
| SRSF‐1, + vs. − | 2.34 (1.42–3.87)a | 1.59 (0.98–2.59) | 6.77 (2.76–16.59)a | 2.70 (1.46–4.94)a | 2.80 (0.99–7.81) | 1.18 (0.66–2.13) | – | 1.98 (1.19–3.30)a |
| MVD, + vs. − | 0.98 (0.59–1.62) | 1.49 (0.90–2.47) | 2.02 (1.06–3.86)a | 1.25 (0.68–2.30)a | 1.48 (0.56–3.93) | 1.34 (0.72–2.49) | 1.98 (1.19–3.30)a | – |
Note: Data are expressed as odds ratio (interquartile range).
Abbreviations: AR, androgenic receptor; BPH, benign prostatic hyperplasia; IGF‐1R, insulin‐like growth factor‐1 receptor; IQR, interquartile range; IR, insulin receptor; MVD, microvascular density; PCa, prostate cancer; PSA, prostate‐specific antigen; PSMA, prostate specific membrane antigen; SRSF1, serine/arginine splicing factor 1.s
p < .05.
At the Kaplan–Meier analysis, SRSF‐1+ patients had worse 5‐ and 9‐year biochemical recurrence (36% and 6%) respect to SRSF‐1− (67% and 7%; p < .01) and similarly MVD+ patients (44% and 7%) respect to MVD− (64% and 8%) (p < .01) (Figure 2).
Figure 2.
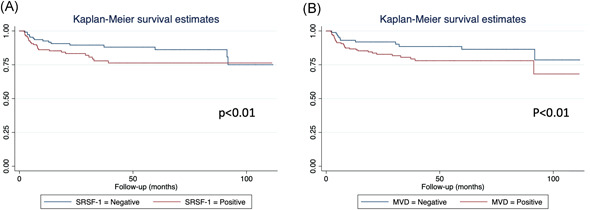
Kaplan–Meier survival in according to SRSF‐1 (A) and MVD (B) expression. MVD, microvascular density; SRSF1, serine/arginine splicing factor 1 [Color figure can be viewed at wileyonlinelibrary.com]
Restricting the analysis only in patients with PC and diabetes, we found that SRSF‐1+ was associated with Ki‐67+ (OR: 8.75; p < .05) and MVD+ (OR: 7.5; p < .05).
When analyzing data of patients with PC and without diabetes, SRSF‐1+ was associated with IR‐α (OR: 2.61; p < .01), AR (OR: 2.43; p < .01), and Ki‐67 (OR: 6.5; p < .01).
3.1. In silico analysis of SFRS‐1 expression from TCGA
Using linear regression analysis, messenger RNA (mRNA) expressions of SFRS‐1 was not associated with AR (p = .26), Ki‐67 (p = .32), IR (p = .51), and IGF‐1R (p = .15) but it was negatively associated with PSMA (p = .03) (Figure 3).20
Figure 3.
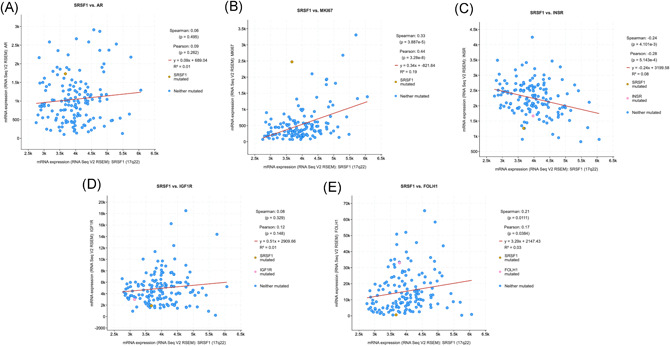
Regression analysis between RNA expression of SFRS‐1 and androgen receptor (A), Ki‐67 (B), insulin receptor (C), insulin growth factor 1 (D) and PSMA (E). PSMA, prostate specific membrane antigen; SRSF1, serine/arginine splicing factor 1 [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In the present work we demonstrated how the expression of the SRSF‐1 protein is associated with a greater expression of the androgen receptor, PSMA, both isoforms of the insulin receptor as well as with greater cellular (Ki‐67) and vascular (MVD) proliferation in PC patients. Furthermore, even in patients with PC, this expression was more associated with the androgen receptor, the insulin receptor alpha, and vascular proliferation. When depicting differences on the basis of diabetes, SRSF‐1 positivity was greater related to proliferation (Ki‐67 and CD‐31+) in diabetic patients but with IR‐α and AR in those without diabetes. Finally, SRSF‐1 and CD‐31 positive patients have a greater risk of developing biochemical recurrence (Figure 2).
The mechanisms underlying the development and progression of PC in the general population are not yet fully known and certainly are decidedly complex when we must also take into consideration the role of the patient's clinic from a metabolic point of view. Previous evidences gave partly focused on the role of the metabolic syndrome of diabetes and the greater aggressiveness of PC. However, there are conflicting findings about the influence of serum levels for example of IGF‐1 and it is the risk of developing PC, precisely because some studies have shown an increased risk while others have shown no correlation.21 Pandini and colleagues in 2005, however, highlighted how androgens in PC cells are able to over‐regulate the expression of the IGF‐1 receptor and as a consequence in determining an increase in the same proliferation and invasion when stimulated with IGF‐1.22 These results in fact show how there is a synergy of functionality between the androgen receptor and IGF‐1.23
In PC cell lines, insulin and IGF‐1 increase cell proliferation and glucose uptake while IR‐β is not associated with cancer cell proliferation but it induces differentiation in noncancerous prostate cells. However, IGF‐1R and IR‐α overexpression enhances angiogenesis and proliferation.24
Furthermore, one of the most crucial connection between diabetes and PC is secondary to the connection among AR and glucose due the downregulation of AR mRNA by the activation of nuclear factor kappa B. To this regard, an altered composition of insulin receptor isoforms has been found in many cancers but also in PC3, 25 and, in particular, IR isoform A is higher expressed compared to isoform B. Heni et al. found reduced levels of the cell cycle inhibitor p27Kip1 in samples with higher IR isoform A and interestingly, both insulin and IGF‐II can activate this isoform25 with consequent effect on proliferation.3
Lutz et al.26 showed that at least two distinct mechanisms may contribute to the poor prognosis of PCa in patients with diabetes: (i) upregulation of the AR, presumably via alteration in the insulin/IGF‐1 signaling cascade; and (ii) disinhibition of androgen signaling due to decreased levels of protective estrogen receptor ligands.
In this context, the docking proteins downstream of IR and other receptor tyrosine kinases (e.g., IGF‐1 receptor), insulin receptor α and β are crucial to further communicate signals from these receptors.27
However, there are no confirmed and corroborated clinical data on the role of SRSF‐1 in PC and how this can be affected by the insulin receptor downstream. What we know today is that SRSF1 in turn promotes the expression of splice isoforms that favors tumor growth, including proangiogenic VEGF promoting.
Previous data have demonstrated that that VEGFA pre‐mRNA can be alternatively spliced generating both proangiogenic isoforms (VEGF‐Axxxa) and antiangiogenic isoforms (VEGF‐Axxxb), depending on the recognition of a proximal splicing site (PSS) within the eighth exon of VEGFA pre‐mRNA by SRSF1: specifically, the higher the amount of SRSF1 binding this PSS, the higher the retention of full‐length eighth exon is, improving the synthesis of VEGF‐Axxxa isoforms.16, 28
In particular, Belali et al showed that in PC3 cells, WT1 transcription factor is able to activate SRPK1 transcription and contributing to oncogenic processes through the activity of oncogenic splice factors such as SRSF1, and consequently by the expression of proangiogenic VEGF.29
Interestingly, an interplay between SRSF‐1 and insulin receptor in pancreatic cells have been demonstrated. Malakar et al.10 found that that insulin signaling, through activation of the Ras‐MAPK pathway, upregulates the splicing factor SRSF1 and induces inclusion of IR exon 11 to generate elevated levels of the IR‐β isoform.10
How this mechanism can be present in PC cells is not well known but what we determined is that SRSF1 expression is associated with worse prognosis and higher proliferation, as determined by the increase expression of AR, KI‐67 and vascularization (MVD+).
This latter IHC finding is a is a quantitative measurement of angiogenesis. Although previous studies reported conflicting results as predictive value in PC,30, 31 recently Yuri et al.32 demonstrated that MVD number significantly influence response to systemic therapy in metastatic PC.
Despite we could not speculate a potential a downstream role of SRSF‐1 and PC aggressiveness in diabetic patients, we demonstrated that in this sub‐cohort of patients, SRSF‐1 positivity was related to greater cell (Ki‐67+) and vascular (MVD+) proliferation while in nondiabetic patients SRSF‐1 positivity is associated with IR‐α. Although we are not able to verify if this latter finding may be related to a condition of prediabetes.
Before concluding we wish to underline some limitations, including the low number of patients that was able to complete survival including patients with/without diabetes and positivity at SFRS‐1 and MVD; the lack of information about drugs used for diabetes. Finally, we did not assess mRNA expression to better understand signaling of SRSF‐1 and PC related proteins.
5. CONCLUSION
PC exhibits widespread heterogeneity in protein expression. In particular, the expression of the SRSF‐1 protein and of the MVD are associated with a worse prognosis and in particular with a greater cell proliferation. These results, although preliminary, may offer new future scientific insights with the aim of highlighting possible genetic alterations linked to a greater expression of SRSF‐1 and associated with a worse prognosis.
CONFLICT OF INTEREST
The authors declare that there are no conflict of interests.
Broggi G, Lo Giudice A, Di Mauro M, et al. SRSF‐1 and microvessel density immunohistochemical analysis by semi‐automated tissue microarray in prostate cancer patients with diabetes (DIAMOND study) The Prostate. 2021;81:882‐892. 10.1002/pros.24185
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Gacci M, Russo GI, De Nunzio C, et al. Meta‐analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis. 2017;20(2):146‐155. 10.1038/pcan.2017.1 [DOI] [PubMed] [Google Scholar]
- 3.Heni M, Hennenlotter J, Scharpf M, et al. Insulin receptor isoforms A and B as well as insulin receptor substrates‐1 and −2 are differentially expressed in prostate cancer. PLoS One. 2012;7(12):50953. 10.1371/journal.pone.0050953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutz SZ, Todenhöfer T, Wagner R, et al. Higher prevalence of lymph node metastasis in prostate cancer in patients with diabetes. Endocr Relat Cancer. 2018;25(3):L19‐L22. 10.1530/ERC-17-0465 [DOI] [PubMed] [Google Scholar]
- 5.Barnard RJ, Aronson WJ, Tymchuk CN, Ngo TH. Prostate cancer: another aspect of the insulin‐resistance syndrome? Obes Rev. 2002;3(4):303‐308. 10.1046/j.1467-789X.2002.00081.x [DOI] [PubMed] [Google Scholar]
- 6.Han JH, Choi NY, Bang SH, et al. Relationship between serum prostate‐specific antigen levels and components of metabolic syndrome in healthy men. Urology. 2008;72(4):749‐754. 10.1016/j.urology.2008.01.084 [DOI] [PubMed] [Google Scholar]
- 7.Zhang PL, Rosen S, Veeramachaneni R, Kao J, DeWolf WC, Bubley G. Association between prostate cancer and serum testosterone levels. Prostate. 2002;53(3):179‐182. 10.1002/pros.10140 [DOI] [PubMed] [Google Scholar]
- 8.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto‐oncogene. Nat Struct Mol Biol. 2007;14(3):185‐193. 10.1038/nsmb1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anczuków O, Rosenberg AZ, Akerman M, et al. The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat Struct Mol Biol. 2012;19(2):220‐228. 10.1038/nsmb.2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malakar P, Chartarifsky L, Hija A, et al. Insulin receptor alternative splicing is regulated by insulin signaling and modulates beta cell survival. Sci Rep. 2016;6(1):31222. 10.1038/srep31222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardano M, Diaferia GR, Falavigna M, et al. Cell and tissue microarray technologies for protein and nucleic acid expression profiling. J Histochem Cytochem. 2013;61(2):116‐124. 10.1369/0022155412470455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbagallo D, Caponnetto A, Barbagallo C, et al. The GAUGAA motif is responsible for the binding between circSMARCA5 and SRSF1 and related downstream effects on glioblastoma multiforme cell migration and angiogenic potential. Int J Mol Sci. 2021;22(4):1678. 10.3390/ijms22041678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cammarata FP, Forte GI, Broggi G, et al. Molecular investigation on a triple negative breast cancer xenograft model exposed to proton beams. Int J Mol Sci. 2020;21(17):6337. 10.3390/ijms21176337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broggi G, Filetti V, Ieni A, et al. MacroH2A1 immunoexpression in breast cancer. Front Oncol. 2020;10:10. 10.3389/fonc.2020.01519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broggi G, Musumeci G, Puzzo L, et al. Immunohistochemical Expression of ABCB5 as a potential prognostic factor in uveal melanoma. Appl Sci. 2019;9(7):1316. 10.3390/app9071316 [DOI] [Google Scholar]
- 16.Barbagallo D, Caponnetto A, Brex D, et al. CircSMARCA5 regulates VEGFA mRNA splicing and angiogenesis in glioblastoma multiforme through the binding of SRSF1. Cancers (Basel). 2019;11(2):194. 10.3390/cancers11020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broggi G, Ieni A, Russo D, et al. The macro‐autophagy‐related protein Beclin‐1 immunohistochemical expression correlates with tumor cell type and clinical behavior of uveal melanoma. Front Oncol. 2020;10:10. 10.3389/fonc.2020.589849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmeules P, Hovington H, Nguilé‐Makao M, et al. Comparison of digital image analysis and visual scoring of KI‐67 in prostate cancer prognosis after prostatectomy. Diagn Pathol. 2015;10(1):67. 10.1186/s13000-015-0294-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mottet N, van den Bergh RCN, Briers E, et al. EAU‐EANM‐ESTRO‐ESUR‐SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243‐262. 10.1016/j.eururo.2020.09.042 [DOI] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research Network . The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011‐1025. 10.1016/j.cell.2015.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan JM. Insulin‐like growth factor‐I (IGF‐I) and IGF binding protein‐3 as predictors of advanced‐stage prostate cancer. CancerSpectrum Knowl Environ. 2002;94(14):1099‐1106. 10.1093/jnci/94.14.1099 [DOI] [PubMed] [Google Scholar]
- 22.Pandini G, Mineo R, Frasca F, et al. Androgens up‐regulate the insulin‐like growth factor‐I receptor in prostate cancer cells. Cancer Res. 2005;65(5):1849‐1857. 10.1158/0008-5472.CAN-04-1837 [DOI] [PubMed] [Google Scholar]
- 23.Bleach R, Sherlock M, O'Reilly MW, McIlroy M. Growth hormone/insulin growth factor axis in sex steroid associated disorders and related cancers. Front Cell Dev Biol. 2021;9:9. 10.3389/fcell.2021.630503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belfiore A, Malaguarnera R, Vella V, et al. Insulin receptor isoforms in physiology and disease: an updated view. Endocr Rev. 2017;38(5):379‐431. 10.1210/er.2017-00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin‐like growth factors in cancer. Trends Endocrinol Metab. 2010;21(10):610‐618. 10.1016/j.tem.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz SZ, Hennenlotter J, Scharpf MO, et al. Androgen receptor overexpression in prostate cancer in type 2 diabetes. Mol Metab. 2018;8:158‐166. 10.1016/j.molmet.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85‐96. 10.1038/nrm1837 [DOI] [PubMed] [Google Scholar]
- 28.Amin EM, Oltean S, Hua J, et al. WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing. Cancer Cell. 2011;20(6):768‐780. 10.1016/j.ccr.2011.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belali T, Wodi C, Clark B, et al. WT1 activates transcription of the splice factor kinase SRPK1 gene in PC3 and K562 cancer cells in the absence of corepressor BASP1. Biochim Biophys Acta—Gene Regul Mech. 2020;1863(12):194642. 10.1016/j.bbagrm.2020.194642 [DOI] [PubMed] [Google Scholar]
- 30.Hu W, Qian Y, Yu F, et al. Alternatively activated macrophages are associated with metastasis and poor prognosis in prostate adenocarcinoma. Oncol Lett. 2015;10(3):1390‐1396. 10.3892/ol.2015.3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanciotti M, Masieri L, Raspollini MR, et al. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. BioMed Res Int. 2014;2014:1‐6. 10.1155/2014/486798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuri P, Shigemura K, Kitagawa K, et al. Increased tumor‐associated macrophages in the prostate cancer microenvironment predicted patients' survival and responses to androgen deprivation therapies in Indonesian patients cohort. Prostate Int. 2020;8(2):62‐69. 10.1016/j.prnil.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.


