Abstract
Cases of unusual thrombosis and thrombocytopenia after administration of the ChAdOx1 nCoV‐19 vaccine (AstraZeneca) have been reported. The term vaccine‐induced prothrombotic immune thrombocytopenia (VIPIT) was coined to reflect this new phenomenon. In vitro experiments with VIPIT patient sera indicated that high‐dose intravenous immunoglobulins (IVIG) competitively inhibit the platelet‐activating properties of ChAdOx1 nCoV‐19 vaccine induced antibodies. Here, we report a case of a 62‐year‐old woman who had received this vaccine and developed VIPIT. She visited the emergency ward because of petechiae and hematomas. In the laboratory work‐up, thrombocytopenia, low fibrinogen, elevated D‐dimer, and positivity in the platelet factor 4/heparin‐enzyme‐immunoassay were present. Signs and symptoms of thrombosis were absent. Upon immediate therapy with non‐heparin anticoagulation, high‐dose IVIG, and prednisolone, laboratory parameters steadily improved and the patient was discharged from hospital without thrombotic complications. We conclude that early initiation of VIPIT treatment results in a swift response without thrombotic complications.
Keywords: COVID‐19, ChAdOx1 vaccine, vaccine‐induced prothrombotic immune thrombocytopenia, VIPIT, high‐dose intravenous immunoglobulins
Essentials.
Cases of thrombosis and thrombocytopenia following the ChAdOx1 nCoV‐19 vaccination have been reported.
The term vaccine‐induced prothrombotic immune thrombocytopenia (VIPIT) was coined to describe this phenomenon.
High‐dose intravenous immunoglobulins and non‐heparin anticoagulation were recommended to treat VIPIT based on in vitro data.
We describe a case of early VIPIT treatment, resulting in swift response without thrombotic complications.
1. INTRODUCTION
In March 2021, cases of thrombosis, including thrombosis at unusual sites (cerebral vein thrombosis or splanchnic vein thrombosis), and thrombocytopenia were reported after administration of the ChAdOx1 nCOV‐19 vaccine (AstraZeneca) in several countries.1, 2, 3 A potential pathomechanism was suggested and the term vaccine‐induced prothrombotic immune thrombocytopenia (VIPIT) was coined to describe the phenomenon.3 The ChAdOx1 nCOV‐19 vaccine seems to induce the production of antibodies causing massive activation of platelets via the Fc receptor, resembling heparin‐induced thrombocytopenia (HIT), but without previous contact with heparin (HIT mimicry). These antibodies and clinical symptoms seem to occur 4 to 16 days after vaccination. In vitro experiments with sera from VIPIT patients indicate that high‐dose intravenous immunoglobulins (IVIG) competitively inhibit the platelet activating properties of ChAdOx1 nCOV‐19–induced antibodies.3 Based on these observations, recent guidance was published that recommends considering the administration of IVIG in case of severe thromboembolic complications after VIPIT confirmation by heparin induced platelet activation (HIPA) assay/modified HIPA assay or serotonin release assay (SRA).1 Practical restrictions of this recommendation are the limited availability of HIPA/SRA assays in non‐specialized coagulation laboratories and the lack of guidance on the preemptive use of IVIG for prevention of thrombosis in patients with VIPIT.
Two recent studies report on VIPIT patients who had developed unusual thrombosis.4, 5 A substantial proportion of these patients died (3/5 patients and 6/11 patients, respectively). One of these studies provides information on VIPIT treatment, indicating that administration of high‐dose IVIG is indeed effective.4
Here, we describe the first clinical case of a patient with early VIPIT diagnosis and its management, resulting in swift normalization of laboratory parameters and subsequent hospital discharge without thrombotic complications.
2. INITIAL PRESENTATION OF CASE
A 62‐year‐old woman in good health condition received the ChAdOx1 nCOV‐19 vaccine (day 0). The following day she developed flu‐like symptoms including aching joints, moderate headache, and moderate dizziness. She self‐medicated 1 g paracetamol, was afebrile, but stayed the whole day at home, most of the time in bed (day 1). The next day, she felt significantly better but self‐medicated 400 mg aspirin (day 2). On days 3 and 4, she felt completely recovered and on day 4 she drove herself 100 miles by car to the lower Austrian alpine foothills for vacation. On day 5, she was cross‐country skiing for several hours without complaints. The same evening, she developed chills and high fever (39.8°C/103.6°F) and took 400 mg aspirin. The following morning (day 6) again she took 400 mg aspirin, she was afebrile, felt much better, and drove back home by car. On days 7 and 8, she had no complaints and returned to work as a psychotherapist. The evening of day 8, she slightly bit her lip and developed an unusually large hematoma. She also noticed bleedings at the gums, which she never had before. The morning of day 9, she recognized an atraumatic hematoma at the right ankle. She decided to visit the nearby emergency ward of the Vienna General Hospital of the Medical University of Vienna.
At the emergency ward, she had no general health complaints. Her medical history revealed substituted hypothyroidism of unresolved genesis since age 20, two vaginal deliveries without complications, no other prior diseases, and no prior surgery. Body mass index was 23.4 kg/m2. Asking for bleedings prior to the current medical condition, she scored 0 in the ISTH bleeding assessment tool (BAT)6. She was afebrile, slightly hypertensive (RR 150/90), and had normal heart and respiration rates. Venous blood gas analysis was completely normal. The SARS‐CoV‐2 real time reverse‐transcriptase polymerase chain reaction assay of a nasopharyngeal swab was negative.
Small hematomas and petechiae of the limbs were evident in the clinical examination. The quantitative rapid D‐dimer test was positive. The hematologist on duty was consulted. VIPIT was suspected, a computed tomography (CT) scan (cerebral, chest, and abdominal) was performed, and she was transferred to the hematological ward for further management.
3. DIAGNOSIS
The CT scan showed no pathologies, especially no signs of venous or arterial thrombosis. The hemogram revealed isolated thrombocytopenia (normal differential blood count, no schistocytes). D‐dimer was highly elevated and fibrinogen was low (Figure 1; Table S1 in supporting information). Global coagulation tests were within the normal range. The anti‐platelet factor 4 (PF4)/heparin IgG enzyme‐immunoassay (Zymutest HIA IgG, HYPHEN Biomed), which has been recommended for VIPIT screening,1 was highly positive and supported the suspected diagnosis of VIPIT.
FIGURE 1.
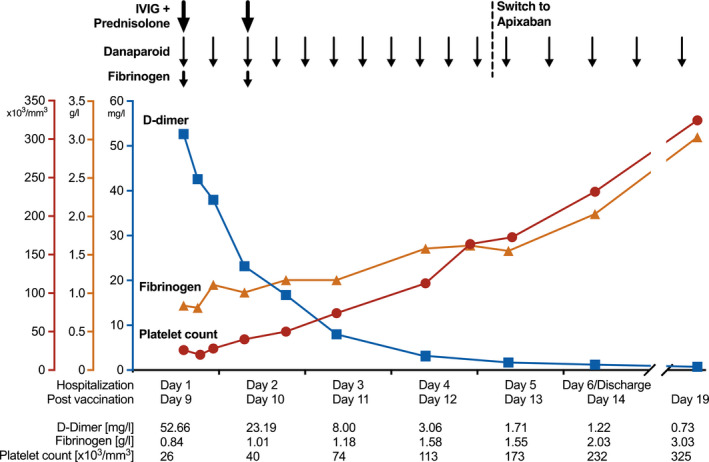
Time course of platelet count (red), fibrinogen concentration (yellow), and D‐dimer (blue) during treatment of vaccine‐induced prothrombotic immune thrombocytopenia
4. MANAGEMENT AND DISCUSSION
Due to the clinically obvious signs of bleeding, which could be attributed to thrombocytopenia and hypofibrinogenemia, and intake of aspirin, we substituted a low dose (1 g) of a fibrinogen concentrate (Fibryga, Octapharma) on day 1 of hospitalization (repeated on day 2) prior to HIT‐compatible anticoagulation (i.e., avoiding heparins) with short‐acting danaparoid‐sodium (Orgaran, Mylan; 750 IE intravenous bolus plus 1500 IE subcutaneously, followed by 1500 IE subcutaneously every 8 h). The anticoagulation with danaparoid was regularly monitored to obtain anti‐Xa target trough levels (measured with danaparoid standard curve) of approximately 0.5 IU/ml. In accordance with a recent guidance statement, we also started high‐dose IVIG (Intratect, Biotest; 1 g/kg) and prednisolone (0.75 mg/kg; for immunosuppression and to reduce possible side effects of IVIG) for two consecutive days. Laboratory parameters and clinical bleeding signs were closely monitored.
D‐dimer levels had already dropped significantly 4 h after initiation of treatment and continued to decrease the following days (Figure 1). Conversely, platelet count and fibrinogen steadily rose and reached normal values at day 4 of hospitalization (i.e., day 12 after vaccination). At this time‐point, anticoagulation was switched to oral apixaban 5 mg twice daily.
The only clinical events our patient experienced during the management of VIPIT were headaches after the second administration of high‐dose IVIG (day 10 after vaccination, day 2 of hospitalization). In cerebral magnetic resonance imaging, performed on the same day, no signs of thrombosis and bleeding were found. Headaches resolved during the following days. On day 6 of hospitalization (day 14 after vaccination), we were able to discharge our patient in very good overall health condition and a joyful mood, and invited her to an outpatient visit 5 days later. During this visit, all lab values were within normal ranges and her condition was normal.
The concept of autoimmune HIT (aHIT) indicates the presence of anti‐PF4‐polyanion IgG antibodies that are able to activate platelets even in the absence of heparin exposure. This may have important implications for the treatment of VIPIT, because good/excellent response was achieved in aHIT patients receiving high‐dose IVIG7 Consistently, in a recent case series of five patients with VIPIT and thrombosis four patients were treated with high‐dose IVIG. Three of these patients responded to IVIG and platelet counts steadily rose. Still, two patients receiving IVIG deteriorated and died due to cerebral hemorrhage (receiving 17 platelet concentrates before administration of IVIG) and massive cerebral vein thrombosis.
Distinct pathomechanisms have been identified in aHIT that may also play a role in VIPIT including antibody‐mediated platelet activation in the absence of heparin via PF4/chondroitin sulfate complexes on platelet surfaces,8 release of polyphosphates from platelet‐densegranules,9 and PF4/IgG‐complex‐‐mediated platelet activation.10 Similar to aHIT, arterial and/or venous thrombosis, especially thrombosis at unusual sites, is observed in VIPIT. The exact pathogenesis of VIPIT, however, remains to be elucidated, as well as the unusual appearing coagulopathy (with almost normal global coagulation tests, but low fibrinogen and very high D‐dimers).
Certain factors that may have played an important role in the very favorable outcome of our patient warrant discussion. First, our patient self‐medicated 1200 mg aspirin after vaccination. Inhibition of platelet aggregation due to aspirin intake may had prevented occurrence of thrombosis prior to hospitalization. Second, we immediately administered high‐dose IVIG, which seem to readily inhibit platelet‐activating antibodies. Third, non‐heparin anticoagulation with short‐acting danaparoid‐sodium (monitoring anti‐Xa target trough levels) may have prevented thrombosis and administration of fibrinogen concentrates at low endogenous fibrinogen levels may have prevented hemorrhage.
In conclusion, our case is the first report that suggests that early non‐heparin anticoagulation in conjunction with early administration of high‐dose IVIG can interrupt the prothrombotic process in patients with suspected VIPIT and may be life saving.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
J.T., K.V.G., A.W.H., F.C., J.G., and P.K. treated the patient; J.T., C.A., I.P., K.V.G., A.W.H., F.C., J.G., and P.K. analyzed and interpreted the data; J.T., C.A., I.P., K.V.G., A.W.H., F.C., J.G., and P.K. wrote, reviewed, edited, and finally approved the article
Supporting information
Table S1
Manuscript Handled by: David Lillicrap
Final decision: David Lillicrap, 18 April 2021
REFERENCES
- 1.Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and mamagement of vaccine‐related thrombosis following AstraZeneca COVID‐19 vaccination: guidance statement from the GTH. Hämostaseologie (ahead of print) 10.1055/a-1469-7481 [DOI] [PubMed] [Google Scholar]
- 2.Vogel G, Kupferschmidt K. New problems erode confidence in AstraZeneca's vaccine. Science. 2021;371:1294–1295. 10.1126/science.371.6536.1294 [DOI] [PubMed] [Google Scholar]
- 3.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle P, Eichinger S. A prothrombotic thrombocytopenic disorder resembling heparin‐induced thrombocytopenia following Coronavirus‐19 vaccination. Research Square [Peprint] 28 Mar, 2021 [cited 4 Apr, 2021]. Avaiable from 10.21203/rs.3.rs-362354/v1 [DOI] [Google Scholar]
- 4.Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodeghiero F, Tosetto A, Abshire T, et al. Perinatal/Pediatric Hemostasis Subcommittees Working G. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8:2063–2065. 10.1111/j.1538-7836.2010.03975.x [DOI] [PubMed] [Google Scholar]
- 7.Warkentin TE. High‐dose intravenous immunoglobulin for the treatment and prevention of heparin‐induced thrombocytopenia: a review. Expert Rev Hematol. 2019;12:685–698. 10.1080/17474086.2019.1636645 [DOI] [PubMed] [Google Scholar]
- 8.Padmanabhan A, Jones CG, Bougie DW, et al. Heparin‐independent, PF4‐dependent binding of HIT antibodies to platelets: implications for HIT pathogenesis. Blood. 2015;125:155–161. 10.1182/blood-2014-06-580894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cines DB, Yarovoi SV, Zaitsev SV, et al. Polyphosphate/platelet factor 4 complexes can mediate heparin‐independent platelet activation in heparin‐induced thrombocytopenia. Blood Adv. 2016;1:62–74. 10.1182/bloodadvances.2016000877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TH, Medvedev N, Delcea M, Greinacher A. Anti‐platelet factor 4/polyanion antibodies mediate a new mechanism of autoimmunity. Nat Commun. 2017;8:14945. 10.1038/ncomms14945 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


