Abstract
There are over 50 SARS‐CoV‐2 candidate vaccines undergoing Phase II and III clinical trials. Several vaccines have been approved by regulatory authorities and rolled out for use in different countries. Due to concerns of potential teratogenicity or adverse effect on maternal physiology, pregnancy has been a specific exclusion criterion for most vaccine trials with only two trials not excluding pregnant women. Thus, other than limited animal studies, gradually emerging development and reproductive toxicity data, and observational data from vaccine registries, there is a paucity of reliable information to guide recommendations for the safe vaccination of pregnant women. Pregnancy is a risk factor for severe COVID‐19, especially in women with comorbidities, resulting in increased rates of preterm birth and maternal morbidity. We discuss the major SARS‐CoV‐2 vaccines, their mechanisms of action, efficacy, safety profile and possible benefits to the maternal‐fetal dyad to create a rational approach towards maternal vaccination while anticipating and mitigating vaccine‐related complications. Pregnant women with high exposure risks or co‐morbidities predisposing to severe COVID‐19 infection should be prioritised for vaccination. Those with risk factors for adverse effects should be counselled accordingly. It is essential to support patient autonomy by shared decision‐making involving a risk‐benefit discussion with the pregnant woman.
Key points
What is already known about this topic?
COVID‐19 infection in pregnancy leads to an increase in adverse maternal outcomes.
Owing to paucity of data regarding SARS‐CoV‐2 vaccine use in pregnancy there is uncertainty regarding safety of use and subsequent pregnancy outcomes.
What does this study add?
Provides an overview of the available SARS‐CoV‐2 vaccines, their mechanisms of action and feasibility of use in pregnancy.
Summarises recommendations regarding vaccination of pregnant or lactating women.
1. INTRODUCTION
On 8th December, 2020, a 90‐year‐old woman in the United Kingdom was the first clinical recipient of a SARS‐CoV‐2 vaccine.1 At the time of writing this article, almost 1.5 billion doses of SARS‐CoV‐2 vaccine have been administered. As much as globalisation has contributed to the rapid spread of COVID‐19, with almost 130 million infected and three million lives lost, it has also promoted a high degree of international cooperation and collaboration in therapeutics and vaccine development. Emergency‐use authorisation (EUA) and federal level investments in the United States of America (USA), European Union (EU), China and India have expedited development of the SARS‐CoV‐2 vaccine and implementation of global vaccination programs.2
2. PATHOPHYSIOLOGY AND IMMUNOLOGY OF SARS‐COV‐2 IN PREGNANCY
SARS‐CoV‐2, like other human coronaviruses, consists of a single‐stranded positive sense RNA genome encased in a helical nucleocapsid, with an outer envelope of four structural proteins: envelope (E), spike (S), membrane (M) and nucleocapsid (N) proteins. The S1 subunit of the S protein contains the receptor‐binding domain (RBD) responsible for binding to host cell angiotensin converting enzyme‐2 (ACE2), after being primed by the serine protease TMPRSS.3, 4, 5 High levels of neutralising antibody response against RBD‐located epitopes6, 7 have been observed in convalescent individuals, correlating with CD4+ T cell response.8 Convalescent plasma has, thus, been used for treatment9 with variable results. Additionally, earlier work on SARS‐CoV demonstrated the suitability of the spike protein as a target for vaccine development.10 Like other vaccines, it is postulated that SARS‐CoV‐2 vaccine needs to elicit both antibody‐mediated and T cell‐mediated immunity for effective protection.5, 10
The second trimester of pregnancy is characterised by an anti‐inflammatory, TH2‐biased microenvironment with increased immunoglobulin synthesis and decreased cell‐mediated response to infection. This switches to a pro‐inflammatory TH1‐type response in the third trimester.11 While the relationship between these immunological shifts in pregnancy and SARS‐CoV‐2 remains unclear, it may potentially exaggerate the ‘cytokine storm’ due to the strong TH1‐polarised response to virus‐linked programmed lytic cell death of infected cells that characterises severe SARS‐CoV‐2 infection.5 This may explain the increased rates of intensive care admission, mechanical ventilation, and death among pregnant women with symptomatic COVID‐19 infection,12, 13 especially during the third trimester. Pro‐inflammatory cytokines IL‐1β, IL‐2, IL‐6, TNF and IFNγ are released by cell‐mediated pathways and the Toll‐like receptors activation14, 15 at the maternal‐fetal interface. These disrupt the protective anti‐inflammatory milieu maintained at the maternal‐fetal interface by decidual natural killer cells and Treg cells11, 16, 17 and may contribute to the observed increased stillbirth and preterm delivery rates.18, 19, 20, 21
Vertical transmission of SARS‐CoV‐2 has been extensively discussed.22, 23, 24, 25 While large observational studies report that 2.6%–5% of infants born to mothers with SARS‐CoV‐2 test positive,19, 26, 27 only a few case reports28, 29, 30, 31 have provided evidence of congenital infection through isolating viral particles in fetal tissue not exposed to maternal fluids or tissue.32 Both the ACE‐2 receptor and TMPRSS2 serine protease are required for infection but placental expression of these is highly variable with few placental cells consistently co‐expressing both throughout pregnancy.33, 34, 35, 36, 37 Thus, it is biologically not surprising that vertical transmission rates are low.
Finally, SARS‐CoV‐2 IgG has been identified in offspring of serology positive mothers38, 39 and, together with IgM and IgA, has been found in breastmilk of mothers in active or convalescent phase of SARS‐CoV‐2 infection40, 41, 42, 43 with proof of specificity against the RBD.43, 44 However, the efficacy and duration of this protection remains to be assessed by longitudinal cohort studies.
3. COVID‐19 VACCINES
Vaccination in pregnancy has dual‐fold benefit, protecting the mother through the induction of cell‐mediated and humoral immunity, and passive protection of the offspring via transplacental transfer of maternal IgG. Recommendations regarding vaccines in pregnancy are summarised in Table 1. Most of the information regarding safety of inactivated vaccines in pregnancy comes from observational studies and historical data.45 While live attenuated vaccines (LAV) are generally more immunogenic than inactivated vaccines,46 they retain replication potential, may be trafficked transplacentally and are therefore relatively contraindicated in pregnancy. Reassuringly though, observational studies of women inadvertently vaccinated during early pregnancy with rubella, plio, yellow‐fever and dengue LAV have not demonstrated an increased incidence of malformations, prematurity, stillbirth, neonatal death or miscarriage.47, 48, 49, 50 Smallpox51 and anthrax52 LAVs, however, have been associated with a small increase in malformations.
TABLE 1.
Various vaccines and recommendations for use in pregnancy
| Type | Examples | Recommendation for use in pregnancy |
|---|---|---|
| Inactivated | Inactivated influenza | Recommended in pregnancy. |
| Inactivated polio | ||
| Hepatitis A | Use if high‐risk of exposure and benefits outweigh risks of vaccination. | |
| Rabiesa | ||
| Peptide/toxoid | Diphtheria | Recommended in pregnancy. |
| Tetanus | ||
| Acellular pertussis | ||
| Haemophilus influenza B |
|
|
| Hepatitis B | ||
| Pneumococcal | ||
| Meningococcal | ||
| Typhoid vi capsular polysaccharide | ||
| Live attenuated | Mumps, measles and rubella |
|
| Rotavirus | ||
| Varicella zoster | ||
| Smallpoxa | ||
| Anthrax | ||
| Influenza | ||
| Oral polio | ||
| Live typhoid | ||
| Yellow fever | Consider if travel to endemic regions is unavoidable. | |
| Nucleic acid vaccines (DNA plasmid, mRNA) | Zika, HIV, SARS‐CoV‐1 | Under investigation. |
| SARS‐CoV‐2 |
|
|
| Viral vector | SARS‐CoV‐2 | Under investigation. |
| Ebola rVSV‐ZEBOV |
Should be given post‐exposure.
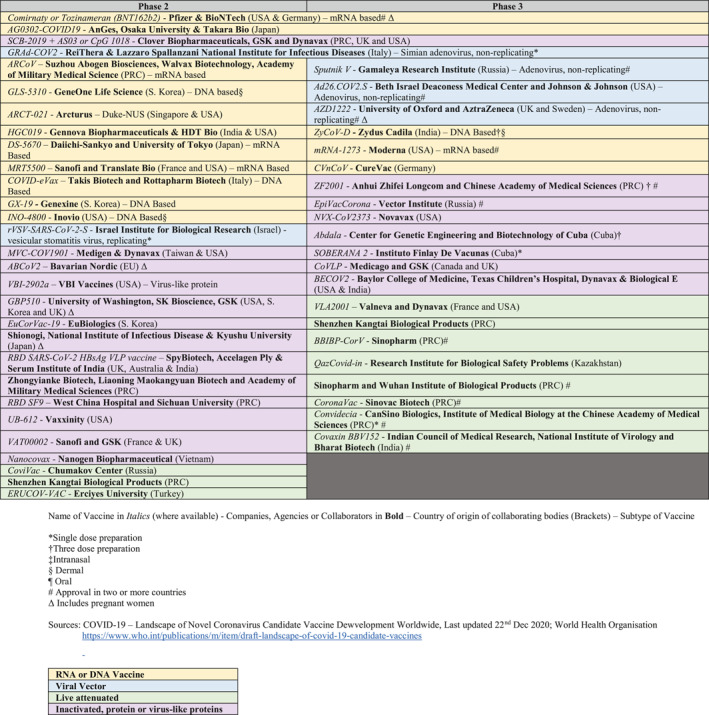
At the time of writing this paper, 50 SARS‐CoV‐2 vaccines, utilising both familiar and novel mechanisms, are either in Phase 2 or 3 clinical trials53, 54 (Table 2) or in clinical use. While it normally takes about 3 years for vaccines to complete Phase 3 trials, in the face of a pandemic, the USA's Food and Drug Administration (FDA) grants EUA following evaluation of Phase 3 safety data for at least 3000 vaccine recipients followed up for 2 months.55 Table 3 summarises the characteristics of the five most widely used vaccines that have been approved in at least two or more countries.
TABLE 2.
SARS‐CoV‐2 vaccines which have been approved for use or are in Phase 2 or 3 Trials (Updated 15th May 2021)
|
TABLE 3.
SARS‐CoV‐2 vaccines approved for use in two or more countries (updated 15th May 2021)
| Vaccine candidate (Manufacturer – Name) | Type | No. of doses | Storage temperature (°C) | Vaccine efficacy against symptomatic, laboratory‐confirmed COVID‐19 in Phase III trials (%) | Significant adverse effects | DART studies & pregnancy data |
|---|---|---|---|---|---|---|
| BioNTech–Pfizer – BNT162b2 or Tozinameran/Comirnaty | mRNA vaccine | 2 | −70 | 95 |
|
|
| Moderna – mRNA‐1273 | mRNA vaccine | 2 |
2–8 up to 30 days −20 for long‐term storage |
94.1 |
|
|
| AstraZeneca & University of Oxford – AZD1222/Vaxzevria | ChADOx1 non‐replicating chimpanzee adenoviral vector | 2 | 2–8 | 70.4 |
|
• DART in progress |
| Johnson & Johnson – Janssen – Ad26.COV2.S | Ad26 non‐replicating human adenoviral vector | 1 |
2–8 up to 3 months −4 for long‐term storage |
65.5–66.3a |
|
|
| Gamelaya research Institute – Sputnik V | Ad5 and Ad26 non‐replicating human adenoviral vector | 2 | 2–8 | 91.6 |
|
• No data |
| Sinopharm – BBIBP‐CorV | Inactivated SARS‐CoV‐2 (vero cells) | 2 | 2–8 | 79–86a | Injection site reactions 35%b | • No data |
| Sinovac – CoronaVac | Inactivated SARS‐CoV‐2 | 2 | 2–8 | 50–91.25a | Injection site reactions 27.5%b | • No data |
Abbreviations: DART, Developmental and Reproductive Toxicity studies; TTS, Thrombosis with thrombocytopenia syndrome.
Self‐reported from interim analysis published in press, but not in a peer‐reviewed journal.
Based on phase 1/2 studies.
The use of DNA‐ or RNA‐based technology has contributed to the speed of vaccine production by being both quick to design and easy to manufacture.56, 57 Moderna and Pfizer‐BioNTech mRNA‐based vaccines were the first two COVID‐19 vaccines to attain FDA EUA for administration in the USA and represent the first ever mRNA‐based vaccines provisionally approved for clinical use. These novel vaccines with efficacies of almost 95% in Phase 3 trials58, 59 and above 85% in real‐world cohort studies60, 61, 62 and work by injecting mRNAs nucleosides coding for S Protein peptides, encased in transfection reagents such as lipid nanoparticles, that induce host production of spike proteins peptides using the native cellular translation machinery. These spike proteins are expressed on the cellular surface, triggering the activation of cell‐mediated and humoral immune systems and the creation of B memory cells which produce SARS‐CoV‐2‐spike protein specific antibodies. The vaccine mRNA does not enter the nucleus or change host DNA,63 are replication‐deficient and have short lifespan characteristics, thus the likelihood of transplacental transfer of mRNA vaccine active compounds is low.57, 64, 65
Pfizer‐BioNTech’s BNT162b2’s Phase 3 clinical trial data reports side‐effects such as local injection‐site reactions (66%–88%), and mild to moderate systemic events including fever, fatigue, headache, and musculoskeletal pain (less than 60%). Two deaths were reported in the intervention arm but were deemed unrelated.58 The CDC has determined that anaphylaxis with the first dose of BNT162b2 and Moderna’s mRNA‐1273 is uncommon at 11.166 , 67 and 2.568 cases per million doses, respectively. While this rate is higher than the seasonal influenza vaccine (1.3 cases per million doses),69 it is similar to penicillin (1.9–27.2 per million).70
Developmental and reproductive toxicity (DART) studies for the mRNA‐1273 in pregnant and lactating female Sprague Dawley rats did not demonstrate adverse effects on the female reproductive system or fetal development following administration of the therapeutic dose.71 DART animal data for BNT162b2 vaccine have not been published although preliminary reports do not reveal any safety concerns.65 A review of 35,691 pregnant vaccine‐recipients who submitted data to the CDC's ‘v‐safe’ smartphone‐based post COVID‐19 vaccination health checker reported more frequent injection‐site pain but less frequent systemic side‐effects compared to non‐pregnant women. The v‐safe pregnancy registry with 3958 pregnant women reported similar miscarriage, stillbirth, congenital anomaly, intrauterine growth restriction and other antenatal complications compared to background rates,72 however robust long term data spanning the entire gestational length of pregnancy and beyond is required.
Two prospective cohort studies73, 74 of mRNA‐1273 or BNT162b2 vaccine recipients comparing non‐pregnant and pregnant or lactating women did not reveal serious adverse effects. Comparable vaccine‐induced neutralising antibody titres, functional antibody responses and cell‐mediated immune responses were seen, and these were higher than in the infected and unvaccinated. Neutralising IgG antibodies titres were present in the umbilical cord and breastmilk but were lower compared to maternal sera.73, 74 Breastmilk IgG, but not IgA, was boosted by a second vaccine dose.73
University of Oxford and AstraZeneca's AZD1222 is a double‐dose replication‐deficient chimpanzee adenoviral vector vaccine.75 Interim analysis reports an overall vaccine efficacy of 70.4%.76 This vaccine is comparatively more affordable than mRNA vaccines75 and does not require ultra‐low temperature storage.77 The AZD1222 vaccine trial is one of the few which did not exclude pregnant women.78 Johnson & Johnson–Janssen Pharmaceutical's Ad26.COV2.S is a single‐dose non‐replicating human adenoviral vector vaccine that has been recently approved by the FDA and European Union with interim efficacy reported at 65.5%–66.3%.79 DART studies for Ad26.COV.S vaccine in pregnant and lactating female rabbits did not demonstrate adverse effects on the female reproductive system or fetal development. While in Phase 3 trials, 8 participants had inadvertent pregnancies during vaccination but no adverse effects were reported except for one spontaneous miscarriage and one ectopic pregnancy in the study arm.80
Vaccine‐induced thrombosis with thrombocytopenia syndrome (TTS) is autoimmune‐mediated and guidelines have been formulated for its effective management.81 Reports of TTS have emerged82, 83 in association with AZD122284, 85 and Ad26.COV2.S86 with a higher predisposition for the latter amongst women less than 50‐years‐age (7 per million vaccine doses in women less than 50‐years‐age vs. 0.9 for older women and none amongst males for Ad26.COV2.S).86 A maternal death due to stroke at 23‐weeks‐gestation in an AZD1222 recipient in Brazil attracted further attention87 and several countries have started restricting their usage in younger persons with varying upper age limits.88 Regulatory bodies, currently, maintain that there remains an overall positive benefit‐risk profile with absolute rates remaining very low89, 90 when seen in the context of COVID‐19's mortality rate of 116 deaths per million infections. Further investigation and long‐term monitoring for these events are required.91 mRNA vaccines have not been implicated in TTS86, 92 although a pre‐print has reported a higher incidence of cerebral venous sinus thrombosis (CVST), the hallmark of TTS, in individuals vaccinated with the Pfizer and Moderna vaccines. This incidence is higher than that following influenza infection but much lower than following natural COVID‐19 infection.93 While CVST is rare, it has been associated with pregnancy and larger observational studies are required to ascertain the modification of CSVT risk in pregnant vaccines.
Russia's Gamaleya Research Institute's Sputnik V vaccine utilises Ad5 and Ad26 adenoviruses and has obtained early approval within Russia, Hungary, Belarus and Argentina53 with Phase 3 trials reporting 91.6% efficacy and no significant increase in serious adverse effects, the finalised clinical trial results are awaited. A potential limitation of viral vector vaccines is the possibility of pre‐existing or vaccine‐induced anti‐vector immunity, which could reduce the efficacy of the starting or booster vaccines, respectively.94
The Peoples Republic of China (PRC) has developed two inactivated vaccines: Sinovac's CoronaVac95 and Sinopharm's BBIBP‐CorV.96 The latter has received EUA within the PRC, Indonesia, Bahrain, and United Arab Emirates. Unpublished Phase 3 interim analysis show vaccine efficacies of 50%–91.3%97, 98 and 79%–86%99, 100, respectively. Favourable safety profiles in Phase 1 and 2 trials have been published, with febrile symptoms reported in <5% of subjects.95, 96, 101
Novavax is a recombinant Spike protein peptide vaccine102 that is undergoing Phase 3 trials and is likely to receive FDA EUA. Virus‐like particles have been used in human papillomavirus vaccines, which involves particles mimicking viral structures that can present antigenic proteins to antigen‐presenting cells.
SARS‐CoV‐2 variants of concern (VOC) B.1.1.7, B.1.351, P.1, B.1.427, B.1.429, B.1.617 with S protein mutations have emerged from England, South Africa, Brazil and India. These VOCs have slightly higher transmissibility,85 increased disease severity85 along with concerns of reduced vaccine efficacy103 and immune escape.104 The B.1.617 variant, in particular, is thought to be responsible for India's second wave.105 Analysis of B.1.351's neutralization activity titres for AZD1222 and BNT162b2 show reductions of 9‐fold and 7.6‐fold, respectively.85 One cohort study amongst pregnant and lactating BNT162b2 and mRNA‐1273 vaccine recipients showed reduced neutralising antibody titres against B.1.1.17 and B.1.351 but preserved T‐cell response.74 Amongst vaccinated healthcare workers, BNT162b2 has shown efficacy against B.1.1.17.62 Real world data corroborates that the currently licensed vaccines are effective against the major VOCs.106 The role of additional booster doses and pre‐clinical candidate variant‐specific boosters are being evaluated.107
Figure 1 illustrates the mechanism of action of various types of SARS‐CoV‐2 vaccines.
FIGURE 1.
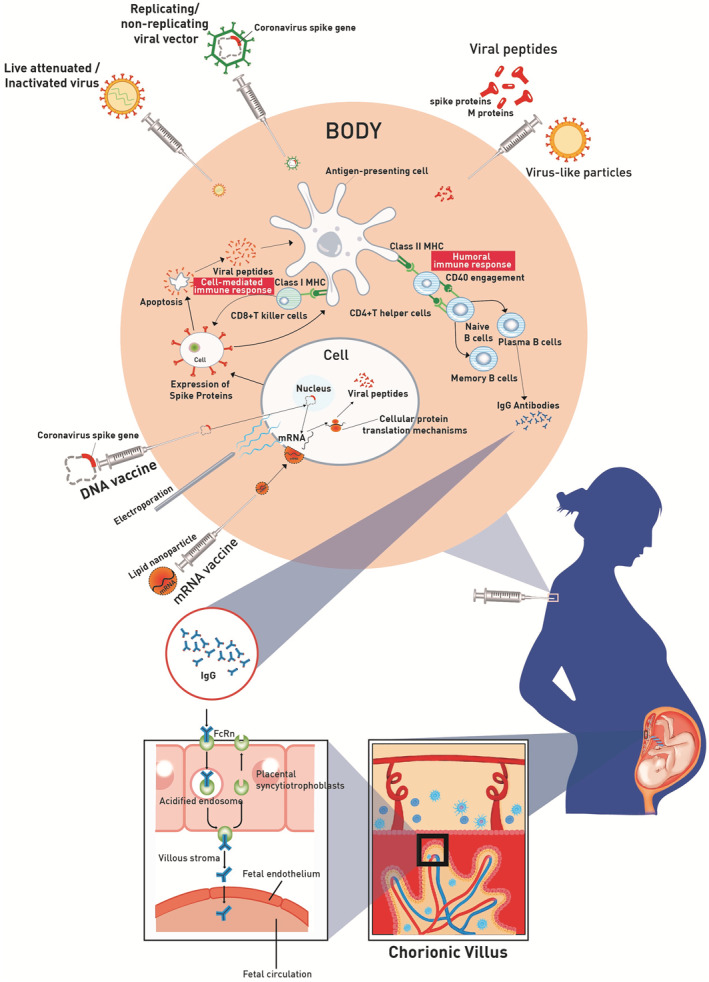
Mechanism of action of various types of SARS‐CoV‐2 vaccines
As of 15th May 2021, of the 50 candidate vaccines in Phase 2 or 3 trials, 40.0% (20) are protein or virus‐like protein vaccines, 28.0% (14) are DNA or RNA‐based, 10.0% (5) are viral vector vaccines, 22.0% (11) are live attenuated. Of these, 42.0% (21) are undergoing Phase 3 clinical trials and 12 have been approved in at least 2 countries (Table 2). Almost all the trials exclude pregnancy except for AZD122278 and BNT162b2.108
As vaccination campaigns progress, the herculean tasks of large‐scale manufacture, cold‐chain transportation, and equitable distribution of vaccines requires prioritisation of high‐risk and vulnerable groups such as the elderly and healthcare workers.75, 109, 110, 111 The WHO's Strategic Advisory of Experts (SAGE) recommends a three‐staged prioritisation process depending on each country's vaccine availability. Where vaccine availability exists for 1%–10% of a population, the highest Stage 1 targets healthcare workers and seniors.112 Uniquely, pregnant healthcare workers who care for a largely unvaccinated pregnant population may find themselves at a higher risk of exposure. While there has been conflicting evidence on whether COVID‐19 in pregnancy is associated with adverse effects113, 114, 115, 116 larger studies demonstrate a higher risk of death, intensive care unit admission, invasive ventilation and extracorporeal membrane oxygenation117 as well as increased preterm delivery and stillbirth rates.18, 19 Despite this, pregnant women have been placed in the lowest prioritisation Stage 3. With over 130 million births per year globally, increases in maternal mortality will have significant societal impacts. Awaiting long‐term vaccine safety and efficacy data may lead to pregnant women and their offspring bearing a disproportionate burden of disease. It is thus critical, to address the issue of vaccination of pregnant women.
Currently, a clear global consensus on vaccination of pregnant women against COVID‐19 is yet to be formulated (Table 4). The American College of Obstetrics and Gynecology (ACOG) and the Society for Maternal Fetal Medicine (SMFM)65, 120 endorse the inclusion of pregnant and lactating women in vaccination campaigns. The UK's Joint Committee of Vaccination and Immunisation (JCVI)75 and Royal College of Obstetricians and Gynaecologists121 opine that pregnant women should be offered vaccination based on their age and clinical risks and preferable vaccines would be Pfizer‐BioNTech's BNT162b2 or Moderna's mRNA‐1273. Those at risk of SARS‐CoV‐2 exposure (healthcare or social workers, residential carers, drivers, cleaners) or COVID‐19 complications with infection (diabetes, solid organ transplant, immunosuppression, chronic respiratory, heart, or kidney disease, BMI > 40) should be offered vaccination.75 Royal Australian and New Zealand College of Obstetricians and Gynecologists (RANZCOG)122 recommend risk based discussion of vaccination with women who are susceptible to complicated COVID‐19 infection. Additionally, RANZCOG recommends that pregnant women with high SARS‐CoV‐2 exposure risk should have their duties adjusted to reduce exposure or, if this is not possible, consider vaccination. The European Medicines Agency states that BNT162b2123, 124 and mRNA‐1273125 can be considered on a case‐by‐case basis after close consultation on the risks and benefits but more studies are needed. The Society of Obstetricians and Gynaecologists of Canada126 additionally recommends against counselling for termination of pregnancy in the event of inadvertent pregnancy during a vaccination series and to discuss the pros and cons of continuing or delaying of subsequent dose(s) depending on the woman's vulnerability factors. Determining the local rate of community transmission and individualised exposure risk would facilitate a risk‐benefit based discussion tailoring the recommendations to the individual.119
TABLE 4.
Recommendations by international professional and regulatory bodies regarding SARS‐CoV‐2 vaccination in pregnancy (updated 15th May 2021)
| Country/region | Professional or regulatory body | Key recommendations |
|---|---|---|
| United States of America | American College of Obstetricians and Gynecologists |
|
| Food and Drug Administration |
|
|
| Centre for Disease Control and Prevention |
|
|
| American Society for Reproductive Medicine | Patients who are undergoing fertility treatment, are pregnant or breastfeeding should be encouraged to receive vaccination, based on eligibility criteria | |
| Europe | European Society of Human Reproduction & Embryology |
|
| European Medicines Agency | Vaccinating pregnant women with BNT162b2 or mRNA‐1273 can be considered on a case‐by‐case basis and after close consultation with a healthcare professional while considering the benefits and risks. | |
| United Kingdom | Joint Commission on Vaccination & Immunisation |
|
| Royal College of Obstetricians & Gynaecologists | ||
| Canada | Society of Obstetricians & Gynaecologists of Canada |
|
| National Advisory Committee on Vaccination | ||
| Australia & New Zealand | Royal Australian and New Zealand College of Obstetrics & Gynaecology |
|
| Australian Technical Advisory Group on Immunisation (ATAGI) | ||
| Global | World Health Organisation118 |
|
| International Federation of Obstetrics and Gynecology119 |
|
While emerging data from observational studies73 and post‐vaccination surveillance data72 suggest minimal harm with vaccinating pregnant or lactating women, there is still insufficient data to be certain about the safety of COVID‐19 vaccines in this population until longer‐term studies can be conducted. Current guidelines recommend risk‐based vaccination of breastfeeding women and those vaccinated can continue breastfeeding.86, 98 Women planning pregnancy can be vaccinated and there is no evidence that vaccines affect fertility.
While endorsement of universal vaccination in pregnancy is not feasible at present, these organisations recognise that pregnancy or lactation is not be an absolute contraindication for vaccination, and individualisation of risk and informed decision making is crucial.
4. ETHICAL CONSIDERATIONS
In the absence of conclusive evidence of harm, there are reasonable ethical arguments to support vaccinating pregnant women (Table 5). Following the debacle surrounding thalidomide and diethylstilbestrol, various research‐related legislations and guidelines protect pregnant women and their fetuses from unintended harm.127 This unfortunately, has also led to pregnant women being frequently excluded from trials to avoid exposure to ‘greater than minimal harm’.128, 129 This exclusion may be a result of anticipated socio‐cultural complications if the vaccination program reports adverse pregnancy outcomes which could potentially jeopardise the entire program. However, during a pandemic, the balance between ‘benefit’ and ‘minimal harm’ might vary significantly when compared to a non‐pandemic situation.130 For example, during the West African Ebola pandemic of 2013–2016, pregnant women faced a case fatality rate of 89%–93%.131 While inclusion of pregnant women in drug and vaccine trials under such circumstances could have been justified, they were excluded despite the World Health Organisation Ethics Review Committee132 recommending protocol amendments when there were no objective reasons to exclude pregnancy. A review of 927 SARS‐CoV‐2 clinical trials found that only 3 (1.7%) were pregnancy‐specific and 52% excluded pregnancy.133 Towards the goal of harmonising safety data collection in clinical trials, the Global Alignment of Immunization Safety Assessment in Pregnancy (GAIA project) has formulated guidance on data collection and reporting in vaccine trials involving pregnant women allowing applicability in diverse settings.134
TABLE 5.
Ethical principles guiding SARS‐CoV‐2 vaccination in pregnancy
| Ethical principle | Application |
|---|---|
| Justice | Pregnant women, just as any other human subject, should have the right to be included in research trials and participate in vaccination campaigns where there are no compelling scientific or ethical reasons to exclude them. |
| Non‐maleficence | The risks of mRNA vaccine in pregnancy are unknown with little precedent amongst the non‐pregnant population. As with any new drug class, the potential for teratogenicity should be balanced with the untapped potential for benefit to the mother, fetus and neonate. Limited animal Developmental and reproductive toxicity studies and observations in Phase 3 participants with inadvertent pregnancy suggest no harm. |
| Beneficence | COVID‐19 in pregnancy has a greater risk of severe complications and there exists a potential to reduce morbidity and mortality amongst pregnant women by including them in vaccination. There is also a possibility of passive immunisation of the fetus or neonates through transplacental IgG transfer or through breastfeeding. |
| Autonomy | Adult pregnant women regularly exercise autonomy by providing informed consent for clinical decision‐making. Similarly, they have the capacity to weigh the risks and benefits of vaccination and provide informed consent. |
With no precedent for mRNA vaccines and accelerated vaccine‐development programs lacking long‐term Phase 3 safety data, it is right for investigators, funding bodies and the public to be concerned about the risk of harm in pregnancy. However, as with any new drug category, the potential for teratogenicity must be balanced against the potential for significant benefit, especially given that COVID‐19 has significantly higher morbidity during pregnancy.19, 117 To address non‐maleficence, the pharmacokinetics of mRNA vaccines (i.e., short‐lived and having very local effect), DART studies,65, 71 cohort studies73, 74 and post‐vaccination registry reviews72 do not suggest significant risk of harm while demonstrating comparable levels of vaccine efficacy. Additionally, cohort studies suggest that vaccinating pregnant women may benefit the offspring through the passive transfer of antibodies. The safety of adenoviral vector vaccines in pregnant women, however, remains an unanswered concern given the predisposition of young women for TTS and reports of cerebral venous thrombosis. More data from prospective cohort studies and passive registries are needed.
The classification of pregnant women as vulnerable individuals has been challenged by various bodies arguing that pregnant women routinely exercise autonomy in complex clinical decisions to protect both their own and fetuses' interests.135, 136 It is unreasonable to expect a pregnant woman not to have capacity to weigh the risks and benefits of vaccination even in the face of limited evidence and provide informed consent for or against vaccination.
5. RECOMMENDATIONS
A personalised risk‐benefit analysis‐based discussion is required for each pregnant woman to balance the paucity of pregnancy specific and long term data with the risks of non‐vaccination and potentially severe SARS‐CoV‐2 infection,137 with an aim of supporting her decision regardless of whether she is in favour of, or against Covid 19 vaccination.
Pregnant women with risk factors (advanced maternal age, obesity, diabetes, hypertension, solid organ transplant, immunosuppression, sickle cell disease, chronic lung, kidney or heart disease27, 117, 138, 139, 140) or high exposure risks (healthcare workers, drivers, cleaners or residential home carers)121, 122, 126, 141 may be prioritised for early vaccination. Healthcare workers are within the highest WHO SAGE Stage 1 vaccine priority112 and women comprise about 70% of that workforce,142 a large proportion being of reproductive age. Other high risk groups, are those who are socially disadvantaged, including African American and Hispanic women in USA,65, 143 as they have higher rates of COVID‐19 infection144 and death.117 These groups could also be considered for preconception vaccination. Studies determining vaccine acceptability and factors influencing decisions in pregnant women are required.145
As concerns surface regarding vaccine‐related anaphylaxis66, 146 clinicians need to be cognizant that severe anaphylaxis in pregnancy poses additional risks of preterm labour, fetal hypoxic brain injury or intrauterine fetal death.147, 148, 149, 150, 151, 152, 153 The CDC reports an anaphylaxis rate of 11.167 (Pfizer‐BioNTech's BNT162b2) and 2.568 (Moderna's mRNA's‐1273) cases per million doses after the first dose, compared to 1.3 cases per million following inactivated influenza vaccine.69 Majority of the cases occurred within 30 min of vaccination, mostly in women, and/or in persons with history of allergies. Reasons for female predisposition are unclear and has also been seen with the 2009H1N1 influenza vaccine.68, 154 While a history of allergy to the mRNA vaccine, polyethylene glycol or polysorbate are strict contraindications to vaccination,146, 155 drug or food allergies and previous anaphylaxis should be declared for closer post‐vaccination anaphylaxis monitoring. Despite low absolute risks of TTS in adenoviral‐vector vaccines, women of reproductive age or those pregnant or breastfeeding should be allowed the autonomy to select alternate vaccines156 pending more conclusive data.
Maternal vaccination should be done in units with capacity for emergency obstetric resuscitation and management.121 Equipment and medication for managing anaphylaxis should be within easy access with at least three doses of epinephrine on hand, preferably in prefilled syringes or autoinjectors.146 Currently, post‐vaccination observation of 30 min is recommended.118, 146 Additionally, all healthcare workers at vaccination centres, first‐responders, frontline medical services such as emergency departments and primary care providers should be educated on early recognition and management of anaphylaxis in pregnancy,152, 153, 157 which may present atypically with delayed features of shock, preterm labour, low back pain and uterine cramps. Additionally, they should be proficient in the principles of maternal resuscitation.158
A personalised vaccine card stating pregnancy status, vaccine name, dosage and schedule should be provided along with patient information leaflets outlining expected symptoms and red flags requiring urgent medical review. As a cautionary measure, a two‐week gap is recommended between COVID‐19 and influenza or pertussis vaccination. However, with availability of more safety data, currently COVID‐19 vaccines may be co‐administered along with other vaccines.119
As Phase 3 mRNA vaccines trials58, 59 report a 15% rate of moderate to severe fever (38.5 ≥°C) after the second dose and potential complications of pyrexia in the first trimester include oral clefts, neural tube or congenital heart defects in the fetus159 adequate and appropriate anti‐pyrexial treatment is advisable.
Currently, women with positive SARS‐CoV‐2 IgG serology should still be offered vaccination, as although naturally acquired antibodies and T‐cell responses appear to be protective,160 the nature and duration of protection from reinfection is presently unknown. Similar to other coronavirus infections, SARS‐Cov‐2 neutralising antibody titres demonstrate a decline, especially in individuals with less severe infection.161 As circulating antibody titres may not indicate B‐memory cell efficacy and cell‐mediated TH1 response, it is unclear if this reduction in titres is associated with increased re‐infection risk7, 8, 40, 162, 163, 164 although convalescent individuals demonstrate strong CD4+ & CD8+ T cell memory responses against S40 and N165 proteins. Additionally, there have been concerns of antibody‐dependent enhancement due to the waning of neutralising antibodies,166 although clinical evidence from vaccine trials, treatment with passive antibodies and infections in patients with previous human coronavirus infections have not conclusively demonstrated this.10, 167 Furthermore, Phase 1 studies for Moderna's mRNA‐1273 vaccine168 suggest superior vaccine‐mediated immunity when compared to natural infection,169, 170, 171 though this requires validation through the larger Phases 3 and 4 cohorts.
Caution is necessary and more vaccine safety data in pregnancy is vital. Safety surveillance systems should be maintained by public health bodies, professional medical organisations or healthcare facilities at a local, regional or national level with attention to pregnant women172, 173 such as the UK Obstetric Surveillance System (UKOSS)/UK Teratology Information Service (UKTIS) and the CDC's ‘v‐safe’ COVID‐19 Vaccine Pregnancy Registry.66 Maternity services may take the lead in registering pregnant women under their jurisdiction either by administering these vaccines or coordinating notification of vaccination.121 While awaiting results from prospective trials,108 recommendations on vaccinating pregnant women should be constantly reviewed based on accumulated observational evidence. Interim reports should be regularly released indicating vaccine‐related adverse effects and efficacy, and rates of miscarriages, terminations of pregnancy, fetal anomalies, preterm births and perinatal mortality.174
Contemporaneously updated guidelines and frequently asked questions featured in clinic posters, patient information leaflets, public health websites and maternity forums could serve as an adjunct to professional counselling prior to informed consent. Improved patient education through easily understood and reliable information would complement pre‐vaccination counselling and alleviate burdens on busy healthcare services.
These recommendations have been summarized in Box 1.
Box 1. Proposed recommendations regarding vaccination of pregnant women.
Vaccination in pregnant women should be individualised based on risks and intended benefits. Informed consent should be obtained. Relevant information and frequently asked questions regarding maternal vaccination should be posted on easily accessible media (websites, posters at vaccination sites, clinics and maternity units, patient information leaflets).
Priority for pre‐conception or antenatal vaccination could be given to women who are at high risk for
-
•
COVID‐19 exposure: Healthcare workers, social workers, residential home carers.
-
•
Complicated COVID‐19: Advanced maternal age or co‐morbidities such as obesity, diabetes, solid organ transplant, sickle cell disease and chronic cardiac, lung, or kidney diseases.
Priority for pre‐conception or antenatal vaccination could be given to women who are at high risk for
Women with positive SARS‐CoV‐2 IgG serology could still be offered vaccination as the degree and duration of immunity conferred by previous infection is unknown and vaccine‐mediated immunity is superior to naturally acquired immunity.
Maternal vaccination should be performed in facilities with access to services competent in managing anaphylaxis and maternal resuscitation. Healthcare workers at vaccination centres, first responders and frontline health services should be educated on recognition of anaphylaxis in pregnancy and the principles of maternal resuscitation.
The following should be made available in all centres conducting maternal vaccination.
-
•
Medical equipment, emergency medications, personnel competent in managing anaphylaxis and resuscitation in pregnancy.
-
•
Clear written guidelines.
-
•
Facility for post vaccination observation.
-
•
Post‐vaccination leaflet, tailored to pregnancy detailing common side effects, red flag symptoms for adverse effects and anaphylaxis, emergency contact information.
Anti‐pyretic management should be pre‐emptively provided, especially with mRNA vaccines.
Passive registries should be maintained to observe pregnancy outcomes of women who undergo vaccination. Data should be analysed to formulate regular updates to guide future vaccination strategies in pregnancy.
6. CONCLUSION
COVID‐19 in pregnancy is associated with higher rates of pregnancy‐related complications. With a likely future of intermittent outbreaks, exclusion of pregnant women from vaccination may lead to a disproportionately higher burden being borne by this vulnerable cohort and their offspring. This must be balanced against the risks related to vaccination. Given the rapidity of new information emerging, it is vital that healthcare providers keep abreast of recent developments and guidelines.
While it was initially postulated that ‘herd immunity’ for SARS‐CoV‐2 could be achieved by attaining at least 70% population immunity,175 logistical challenges in vaccine distribution,176 the economic desire to open borders and the emergence of VOCs which may reduce vaccine‐efficacy makes passive protection of pregnant women through herd immunity a long term objective which may take years. While we await high vaccination rates, similar to that of measles, rubella and polio, periodic outbreaks may continue due to importation from low vaccination areas or vaccine non‐response, albeit with lower severity.62, 177, 178, 179 Mask wearing, hand hygiene and social distancing will remain important methods for pregnant women who remain unvaccinated either due to medical reasons, personal preference or exclusion from vaccination policies.
Informed consent and patient autonomy should be the cornerstones for decision‐making regarding vaccination. Individualised discussion covering each woman's specific risks and possible benefits of vaccination should be conducted. Women at high priority for vaccination include those at high exposure risk or who have co‐morbidities that place them at high risk for severe COVID‐19.
Pregnant women are a significant part of the spectrum of every population. We hope that future clinical trials will consider including pregnant women, whenever pre‐clinical studies show no objective reason to exclude them.
CONFLICT OF INTEREST
The authors have no conflicts of interest in connection with this article.
ACKNOWLEDGEMENTS
We would like to acknowledge Mr Mohesh K Mohan for his assistance in the illustration, Ms Cecille Laureano Asibal for her administrative support.
Pramanick A, Kanneganti A, Wong JLJ, et al. A reasoned approach towards administering COVID‐19 vaccines to pregnant women. Prenatal Diagnosis. 2021;41(8):1018–1035. 10.1002/pd.5985
Angsumita Pramanick and Abhiram Kanneganti are co‐first authors.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Haynes S. COVID‐19 Vaccines Are Now Being Rolled Out in the U.K. Here's Who Got Them First. TIME; 2020. https://time.com/5918842/first‐covid‐19‐vaccine‐patients/. Accessed 27 January 2021. [Google Scholar]
- 2.Singh JA, Upshur REG. The granting of emergency use designation to COVID‐19 candidate vaccines: implications for COVID‐19 vaccine trials. Lancet Infect Dis. 2020;21(4):E103‐E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noy‐Porat T, Makdasi E, Alcalay R, et al. A panel of human neutralizing mAbs targeting SARS‐CoV‐2 spike at multiple epitopes. Nat Commun. 2020;11(1):4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183(1):158‐168e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T Cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell. 2020;181(7):1489‐1501.e1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. J Am Med Assoc. 2020;323(16):1582‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17(8):469‐482. [DOI] [PubMed] [Google Scholar]
- 12.Delahoy MJ, Whitaker M, O'Halloran A, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory‐confirmed COVID‐19 – COVID‐NET, 13 states, March 1‐august 22, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1347‐1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;224(5):510e1‐510e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koga K, Cardenas I, Aldo P, et al. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61(3):196‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga K, Izumi G, Mor G, Fujii T, Osuga Y. Toll‐like receptors at the maternal‐fetal interface in normal pregnancy and pregnancy complications. Am J Reprod Immunol. 2014;72(2):192‐205. [DOI] [PubMed] [Google Scholar]
- 16.Alberca RW, Pereira NZ, Oliveira L, Gozzi‐Silva SC, Sato MN. Pregnancy, viral infection, and COVID‐19. Front Immunol. 2020;11:1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherer ML, Lei J, Creisher P, et al. Dysregulated immunity in SARS‐CoV‐2 infected pregnant women. medRxiv. 2020:2020.2011.2013.20231373. [Google Scholar]
- 18.Khalil A, von Dadelszen P, Draycott T, et al. Change in the incidence of stillbirth and preterm delivery during the COVID‐19 pandemic. J Am Med Assoc. 2020;324(7):705‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodworth KR, Olsen EO, Neelam V, et al. Birth and infant outcomes following laboratory‐confirmed SARS‐CoV‐2 infection in pregnancy – SET‐NET, 16 jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1635‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID‐19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM. 2020;2(2).100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin‐1beta and tumor necrosis factor‐alpha but not by interleukin‐6 or interleukin‐8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195(6):1578‐1589. [DOI] [PubMed] [Google Scholar]
- 22.Mahyuddin AP, Kanneganti A, Wong JJL, et al. Mechanisms and evidence of vertical transmission of infections in pregnancy including SARS‐CoV‐2s. Prenat Diagn. 2020;40:1655‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh XL, Low YF, Ng CH, Amin Z, Ng YPM. Incidence of SARS‐CoV‐2 vertical transmission: a meta‐analysis. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):112‐113. [DOI] [PubMed] [Google Scholar]
- 24.Lamouroux A, Attie‐Bitach T, Martinovic J, et al. Evidence for and against vertical transmission for severe acute respiratory syndrome coronavirus 2. Am J Obstet Gynecol. 2020;223(1):91e91‐91e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimberlin DW, Stagno S. Can SARS‐CoV‐2 infection Be acquired in utero? More definitive evidence is needed. J Am Med Assoc. 2020;323(18):1788‐1789. [DOI] [PubMed] [Google Scholar]
- 26.Ferrazzi E, Frigerio L, Savasi V, et al. Vaginal delivery in SARS‐CoV‐2‐infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020;127(9):1116‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS‐CoV‐2 infection in UK: national population based cohort study. BMJ. 2020;369.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baud D, Greub G, Favre G, et al. Second‐trimester miscarriage in a pregnant woman with SARS‐CoV‐2 infection. J Am Med Assoc. 2020;323(21):2198‐2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenizia C, Biasin M, Cetin I, et al. Analysis of SARS‐CoV‐2 vertical transmission during pregnancy. Nat Commun. 2020;11(1):5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivanti AJ, Vauloup‐Fellous C, Prevot S, et al. Transplacental transmission of SARS‐CoV‐2 infection. Nat Commun. 2020;11(1):3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID‐19 infection. Prenat Diagn. 2020;40(13):1759‐1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS‐CoV‐2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99(5):565‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valdes G, Neves LA, Anton L, et al. Distribution of angiotensin‐(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27(2–3):200‐207. [DOI] [PubMed] [Google Scholar]
- 34.Pringle KG, Tadros MA, Callister RJ, Lumbers ER. The expression and localization of the human placental prorenin/renin‐angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta. 2011;32(12):956‐962. [DOI] [PubMed] [Google Scholar]
- 35.Pavlicev M, Wagner GP, Chavan AR, et al. Single‐cell transcriptomics of the human placenta: inferring the cell communication network of the maternal‐fetal interface. Genome Res 2017;27(3):349‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Chen L, Zhang J, et al. The SARS‐CoV‐2 receptor ACE2 expression of maternal‐fetal interface and fetal organs by single‐cell transcriptome study. PLoS One. 2020;15(4).e0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hecht JL, Quade B, Deshpande V, et al. SARS‐CoV‐2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID‐19‐positive mothers. Mod Pathol. 2020;33(11):2092‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID‐19 pneumonia. J Am Med Assoc. 2020;323(18):1848‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vendola N, Stampini V, Amadori R, Gerbino M, Curatolo A, surico D. Vertical transmission of antibodies in infants born from mothers with positive serology to COVID‐19 pneumonia. Eur J Obstet Gynecol Reprod Biol. 2020;253:331‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS‐CoV‐2 in UK convalescent individuals following COVID‐19. Nat Immunol. 2020;21(11):1336‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebrao CW, Cruz MN, Silva MHD, et al. Early identification of IgA anti‐SARSCoV‐2 in milk of mother with COVID‐19 infection. J Hum Lactation. 2020;36(4):609‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao X, Wang S, Zeng W, et al. Clinical and immunologic features among COVID‐19‐affected mother‐infant pairs: antibodies to SARS‐CoV‐2 detected in breast milk. New Microbes New Infect. 2020;37.100752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox A, Marino J, Amanat F, et al. Robust and specific secretory IgA against SARS‐CoV‐2 detected in human milk. iScience. 2020;23(11).101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pace RM, Williams JE, Jarvinen KM, et al. COVID‐19 and Human Milk: SARS‐CoV‐2, Antibodies, and Neutralizing Capacity. medRxiv; 2020. [Google Scholar]
- 45.Muller‐Schulte E, Gartner BC. Vaccinations during pregnancy: a call to sting into action. Future Microbiol 2019;14:995‐1006. [DOI] [PubMed] [Google Scholar]
- 46.Lauring AS, Jones JO, Andino R. Rationalizing the development of live attenuated virus vaccines. Nat Biotechnol. 2010;28(6):573‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harjulehto‐Mervaala T, Aro T, Hiilesmaa VK, Saxén H, Hovi T, Saxén L. Oral polio vaccination during pregnancy‐ No increase in the occurrence of congenital malformations. Am J Epidemiol. 1993;138(6):407‐414. [DOI] [PubMed] [Google Scholar]
- 48.Suzano CE, Amaral E, Sato HK, Papaiordanou PM. Campinas Group on Yellow Fever Immunization during P. The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine. 2006;24(9):1421‐1426. [DOI] [PubMed] [Google Scholar]
- 49.Laris‐Gonzalez A, Bernal‐Serrano D, Jarde A, Kampmann B. Safety of administering live vaccines during pregnancy: a systematic review and meta‐analysis of pregnancy outcomes. Vaccines (Basel). 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organisation Global Advisory Committee on Vaccine Safety . Safety of immunization during pregnancy ‐ a review of the evidence. World Health Organisation; 2014. https://www.who.int/vaccine_safety/publications/safety_pregnancy_nov2014.pdf. Accessed January 27, 2021. [Google Scholar]
- 51.Badell ML, Meaney‐Delman D, Tuuli MG, et al. Risks associated with smallpox vaccination in pregnancy: a systematic review and meta‐analysis. Obstet Gynecol. 2015;125(6):1439‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan MA, Smith TC, Sevick CJ, et al. Birth defects among infants born to women who received anthrax vaccine in pregnancy. Am J Epidemiol. 2008;168(4):434‐442. [DOI] [PubMed] [Google Scholar]
- 53.Zimmer C, Corum J, Wee S‐L. Coronavirus Vaccine Tracker. The New York Times; 2021. https://www.nytimes.com/interactive/2020/science/coronavirus‐vaccine‐tracker.html. Accessed January 27, 2021. [Google Scholar]
- 54.World Health Organization . Draft Landscape and Tracker of COVID‐19 Candidate Vaccines. World Health Organization; 2021. https://www.who.int/publications/m/item/draft‐landscape‐of‐covid‐19‐candidate‐vaccines. Accessed January 26, 2021. [Google Scholar]
- 55.ADMINISTRATION USFD . Emergency Use Authorization for Vaccines Explained. 2020. https://www.fda.gov/vaccines‐blood‐biologics/vaccines/emergency‐use‐authorization‐vaccines‐explained#:∼:text=An%20Emergency%20Use%20Authorization%20(EUA)%20is%20a%20mechanism%20to%20facilitate. Accessed January 7, 2021. [Google Scholar]
- 56.Brisse M, Vrba SM, Kirk N, Liang Y, Ly H. Emerging concepts and technologies in vaccine development. Front Immunol. 2020;11:583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2020;384:403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall VJ, Foulkes S, Saei A, et al. COVID‐19 vaccine coverage in health‐care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalafat E, O'Brien P, Heath PT, et al. Benefits and potential harms of COVID‐19 vaccination during pregnancy: evidence summary for patient counseling. Ultrasound Obstet Gynecol. 2021;57(5):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Food and Drug Administration . Interim Clinical Considerations for Use of mRNA COVID‐19 Vaccines Currently Authorized in the United States; 2021. https://www.cdc.gov/vaccines/covid‐19/info‐by‐product/clinical‐considerations.html. Accessed February 3, 2021. [Google Scholar]
- 65.American College of Obstetricians and Gynecologists . Vaccinating Pregnant and Lactating Patients against COVID‐19 American College of Obstetricians and Gynecologists; 2020. https://www.acog.org/clinical/clinical‐guidance/practice‐advisory/articles/2020/12/vaccinating‐pregnant‐and‐lactating‐patients‐against‐covid‐19. Accessed January 27, 2021. [Google Scholar]
- 66.Castells MC, Phillips EJ. Maintaining safety with SARS‐CoV‐2 vaccines. N Engl J Med. 2020;384:643‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention COVID‐19 Response Team, Food and Drug Administration . Allergic Reactions Including Anaphylaxis after Receipt of the First Dose of Pfizer‐BioNTech COVID‐19 Vaccine – United States, December 14–23. MMWR Morb Mortal Wkly Rep Web site; 2020. Accessed January 27, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention COVID‐19 Response Team, Food and Drug Administration . Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID‐19 vaccine—United States. December. 2020;21. January 10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: an investigation into its epidemiology. Archives Intern Med. 2001;161(1):15‐21. [DOI] [PubMed] [Google Scholar]
- 71.Food and Drug Administration . Vaccines and Related Biological Products Advisory Committee December 17, 2020 Meeting Briefing Document – Sponsor Food and Drug Administration; 2020. https://www.fda.gov/media/144452/download. Accessed January 27, 2021. [Google Scholar]
- 72.Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA covid‐19 vaccine safety in pregnant persons. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gray KJ, Bordt EA, Atyeo C, et al. COVID‐19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Collier A‐rY, McMahan K, Yu J, et al. Immunogenicity of COVID‐19 mRNA vaccines in pregnant and lactating women. J Am Med Assoc. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joint Committee on Vaccination and Immunisation . Priority Groups for Coronavirus (COVID‐19) Vaccination: Advice From the JCVI. Gov.UK; 2020. https://www.gov.uk/government/publications/priority‐groups‐for‐coronavirus‐covid‐19‐vaccination‐advice‐from‐the‐jcvi‐30‐december‐2020. Accessed January 8, 2021. [Google Scholar]
- 76.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knoll MD, Wonodi C. Oxford‐AstraZeneca COVID‐19 vaccine efficacy. Lancet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.ClinicalTrials.gov . Phase III Double‐Blind, Placebo‐Controlled Study of AZD1222 for the Prevention of COVID‐19 in Adults; 2020. https://clinicaltrials.gov/ct2/show/NCT04516746. Accessed February 2, 2021. [Google Scholar]
- 79.Oliver SE, Gargano JW, Scobie H, et al. The advisory committee on immunization practices' interim recommendation for use of Janssen COVID‐19 vaccine ‐ United States, february 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Food and Drug Administration . Vaccines and Related Biological Products Advisory Committee February 26, 2021 ‐ Janssen Ad26.COV2.S Vaccine for the Prevention of COVID‐19. Food and Drug Administration; 2021. https://www.fda.gov/media/146217/download. Accessed March 21, 2021. [Google Scholar]
- 81.James BB, Douglas BC, Cynthia ED, et al. Thrombosis with Thrombocytopenia Syndrome (also termed Vaccine‐induced Thrombotic Thrombocytopenia). Version 1.4; 2021. https://www.hematology.org/covid‐19/vaccine‐induced‐immune‐thrombotic‐thrombocytopenia. Accessed May 17, 2021. [Google Scholar]
- 82.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to april 21, 2021. J Am Med Assoc. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Global Advisory Committee on Vaccine Safety WHO . Global Advisory Committee on Vaccine Safety (GACVS) Review of Latest Evidence of Rare Adverse Blood Coagulation Events with AstraZeneca COVID‐19 Vaccine (Vaxzevria and Covishield); 2021. https://www.who.int/news/item/16‐04‐2021‐global‐advisory‐committee‐on‐vaccine‐safety‐(gacvs)‐review‐of‐latest‐evidence‐of‐rare‐adverse‐blood‐coagulation‐events‐with‐astrazeneca‐covid‐19‐vaccine‐(vaxzevria‐and‐covishield). Accessed April 16, 2021. [Google Scholar]
- 84.Østergaard SD, Schmidt M, Horváth‐Puhó E, Thomsen RW, Sørensen HT. Thromboembolism and the Oxford–AstraZeneca COVID‐19 vaccine: side‐effect or coincidence? Lancet. 2021;397(10283):1441‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MHpR Agency. Coronavirus Vaccine – Weekly Summary of Yellow Card Reporting; 2021. https://www.gov.uk/government/publications/coronavirus‐covid‐19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting. Accessed May 13, 2021. [Google Scholar]
- 86.Shimabukuro T. Thrombosis With Thrombocytopenia Syndrome (TTS) Following Janssen COVID‐19 Vaccine; 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides‐2021‐04‐23/03‐COVID‐Shimabukuro‐508.pdf2021/23/04 [Google Scholar]
- 87.Fonseca P, Brito R. Brazil Suspends Use of AstraZeneca Vaccine in Pregnant Women Nationally after Death. Reuters. 2021. https://www.reuters.com/business/healthcare‐pharmaceuticals/brazil‐health‐agency‐calls‐halt‐astrazeneca‐vaccine‐pregnant‐women‐2021‐05‐11/. Accessed May 15, 2021. [Google Scholar]
- 88.Reuters . Factbox: Details of Use of AstraZeneca, J&J COVID Vaccines. Reuters; 2021. https://www.reuters.com/business/healthcare‐pharmaceuticals/some‐countries‐limit‐astrazeneca‐vaccine‐use‐eu‐findings‐jj‐shot‐expected‐2021‐04‐20/. Accessed May 15, 2021. [Google Scholar]
- 89.Agency EM . Signal Assessment Report on Embolic and Thrombotic Events (SMQ) With COVID‐19 Vaccine (ChAdOx1‐S [recombinant]) – COVID‐19 Vaccine AstraZeneca. (Other viral vaccines). https://www.ema.europa.eu/en/documents/prac‐recommendation/signal‐assessment‐report‐embolic‐thrombotic‐events‐smq‐covid‐19‐vaccine‐chadox1‐s‐recombinant‐covid_en.pdf2021 [Google Scholar]
- 90.Global Advisory Committee on Vaccine Safety WHO . Interim Statement of the COVID‐19 Subcommittee of the WHO Global Advisory Committee on Vaccine Safety on AstraZeneca COVID‐19 Vaccine; 2021. https://www.who.int/news/item/07‐04‐2021‐interim‐statement‐of‐the‐covid‐19‐subcommittee‐of‐the‐who‐global‐advisory‐committee‐on‐vaccine‐safety. Accessed April 7, 2021. [PMC free article] [PubMed] [Google Scholar]
- 91.Subcommittee WGC . Statement of the WHO Global Advisory Committee on Vaccine Safety (GACVS) COVID‐19 Subcommittee on Safety Signals Related to the AstraZeneca COVID‐19 Vaccine. WHO; 2021. https://www.who.int/news/item/19‐03‐2021‐statement‐of‐the‐who‐global‐advisory‐committee‐on‐vaccine‐safety‐(gacvs)‐covid‐19‐subcommittee‐on‐safety‐signals‐related‐to‐the‐astrazeneca‐covid‐19‐vaccine. Accessed March 28, 2021. [Google Scholar]
- 92.ASo Haematology. Thrombosis with Thrombocytopenia Syndrome (Also Termed Vaccine‐Induced Thrombotic Thrombocytopenia); 2021. https://www.hematology.org/covid‐19/vaccine‐induced‐immune‐thrombotic‐thrombocytopenia#:∼:text=Estimates%20to%20date%20suggest%20that,with%20platelet%20transfusions%20if%20bleeding. Accessed May 15, 2021. [Google Scholar]
- 93.Maxime Taquet MH, Geddes JR, Luciano S, Harrison PJ. Cerebral Venous Thrombosis and Portal Vein Thrombosis: A Retrospective Cohort Study of 537,913 COVID‐19 Cases; 2021. https://osf.io/a9jdq/. Accessed May 19, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fonseca P. Brazil institute says CoronaVac efficacy above 50%, but delays full results. Reuters; 2020. [Google Scholar]
- 98.The Associated Press . Turkish Official Says CoronaVac Vaccine 91.25% Effective. The Associated Press; 2020. https://abcnews.go.com/Health/wireStory/turkish‐official‐coronavac‐vaccine‐9125‐effective‐74899577. Accessed December 28, 2021. [Google Scholar]
- 99.Reuters . UAE Says Sinopharm Vaccine Has 86% Efficacy against COVID‐19. Reuters, 2021. https://www.reuters.com/article/health‐coronavirus‐emirates‐idUSKBN28J0G4. Accessed January 27, 2021. [Google Scholar]
- 100.Reuters . Sinopharm's COVID‐19 Vaccine 79% Effective, Seeks Approval in China. Reuters; 2020. https://www.reuters.com/article/us‐health‐coronavirus‐china‐vaccine/sinopharms‐covid‐19‐vaccine‐79‐effective‐seeks‐approval‐in‐china‐idUSKBN2940C8. Accessed January 27, 2021. [Google Scholar]
- 101.Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID‐19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS‐CoV‐2. Lancet Infect Dis. 2021;21(2):e26‐e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Callaway E. Mallapaty S. Novavax offers first evidence that COVID vaccines protect people against variants. Nature; 2021. https://www.nature.com/articles/d41586‐021‐00268‐9. Accessed February 2, 2021. [DOI] [PubMed] [Google Scholar]
- 103.Abdool Karim SS, de Oliveira T. New SARS‐CoV‐2 variants — clinical, public health, and vaccine implications. N Engl J Med. 2021;384:1866‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS‐CoV‐2 variant B.1.351 from natural and vaccine‐induced sera. Cell. 2021;184(9):2348‐2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vaidyanathan G. Coronavirus variants are spreading in India — what scientists know so far. Nature; 2021. [DOI] [PubMed] [Google Scholar]
- 106.Abu‐Raddad LJ, Chemaitelly H, Butt AA, National Study Group for C‐V . Effectiveness of the BNT162b2 covid‐19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moderna Inc . Moderna COVID‐19 Vaccine Retains Neutralizing Activity against Emerging Variants First Identified in the U.K. and the Republic of South Africa; 2021. https://investors.modernatx.com/news‐releases/news‐release‐details/moderna‐covid‐19‐vaccine‐retains‐neutralizing‐activity‐against. Accessed January 27, 2021. [Google Scholar]
- 108.Pfizer . Pfizer and Biontech Commence Global Clinical Trial to Evaluate COVID‐19 Vaccine in Pregnant Women; 2021. https://www.pfizer.com/news/press‐release/press‐release‐detail/pfizer‐and‐biontech‐commence‐global‐clinical‐trial‐evaluate. Accessed March 28, 2021. [Google Scholar]
- 109.Government of Canada National Advisory Committee on Immunization . Preliminary Guidance on Key Populations for Early COVID‐19 Immunization Government of Canada; 2021. https://www.canada.ca/en/public‐health/services/immunization/national‐advisory‐committee‐on‐immunization‐naci/guidance‐key‐populations‐early‐covid‐19‐immunization.html#a7. Accessed January 27, 2021. [Google Scholar]
- 110.European Commission . Coronavirus: Commission Lists Key Steps for Effective Vaccination Strategies and Vaccines Deployment. European Commission; 2020. https://ec.europa.eu/commission/presscorner/detail/en/ip_20_1903. Accessed January 3, 2021. [Google Scholar]
- 111.Government of Singapore Ministry of Health Expert Committee on COVID‐19 Vaccination . Expert Committee on COVID‐19 Vaccination Endorses Use of Pfizer‐BioNTech COVID‐19 Vaccine Government of Singapore. Ministry of Health; 2020. https://www.moh.gov.sg/news‐highlights/details/expert‐committee‐on‐covid‐19‐vaccination‐endorses‐use‐of‐pfizer‐biontech‐covid‐19‐vaccine. Accessed January 3, 2021. [Google Scholar]
- 112.World Health Organization Strategic Advisory Group of Experts . Roadmap for Prioritizing Uses of COVID‐19 Vaccines in the Context of Limited Supply. World Health Organization; 2020. https://www.who.int/docs/default‐source/immunization/sage/covid/sage‐prioritization‐roadmap‐covid19‐vaccines.pdf?Status=Temp&sfvrsn=bf227443_2. Accessed January 27, 2021. [Google Scholar]
- 113.Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3(11).e2029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blitz MJ, Grünebaum A, Tekbali A, et al. Intensive care unit admissions for pregnant and non‐pregnant women with COVID‐19. Am J Obstet Gynecol. 2020;223(2):290‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID‐19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222(6):521‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ashokka B, Loh M‐H, Tan CH, et al. Care of the pregnant woman with coronavirus disease 2019 in labor and delivery: anesthesia, emergency cesarean delivery, differential diagnosis in the acutely ill parturient, care of the newborn, and protection of the healthcare personnel. Am J Obstet Gynecol. 2020;223(1):66‐74e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status ‐ United States, January 22‐October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.World Health Organization Strategic Advisory Group of Experts . Interim Recommendations for Use of the Pfizer–BioNTech COVID‐19 Vaccine, BNT162b2, under Emergency Use Listing. World Health Organization; 2021. https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐vaccines‐SAGE_recommendation‐BNT162b2‐2021.1. Accessed January 27, 2021. [Google Scholar]
- 119.FIGO . COVID‐19 Vaccination for Pregnant and Breastfeeding Women. FIGO; 2021. https://www.figo.org/covid‐19‐vaccination‐pregnant‐and‐breastfeeding‐women. Accessed March 28, 2021. [Google Scholar]
- 120.Society for Maternal Fetal Medicine . SARS‐CoV‐2 Vaccination in Pregnancy. Society for Maternal Fetal Medicine; 2021. https://s3.amazonaws.com/cdn.smfm.org/media/2591/SMFM_Vaccine_Statement_12‐1‐20_(final).pdfUpdated2020/01/12. Accessed January 27, 2021. [Google Scholar]
- 121.Royal College of Obstetricians and Gynaecologists . Updated Advice on COVID‐19 Vaccination in Pregnancy and Women Who Are Breastfeeding. Royal College of Obstetricians and Gynaecologists; 2020. https://www.rcog.org.uk/en/news/updated‐advice‐on‐covid‐19‐vaccination‐in‐pregnancy‐and‐women‐who‐are‐breastfeeding/. Accessed December 30, 2020. [Google Scholar]
- 122.Royal Australian and New Zealand College of Obstetricians and Gynaecologists . COVID‐19 Vaccination in Pregnant and Breastfeeding Women; 2021. https://ranzcog.edu.au/statements‐guidelines/covid‐19‐statement/covid‐19‐vaccination‐information. Accessed January 27, 2021. [Google Scholar]
- 123.Europe Medicines Agency . Comirnaty. COVID‐19 mRNA Vaccine (Nucleoside‐modified). Europe Medicines Agency; 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty. Accessed January 27, 2021. [Google Scholar]
- 124.Reuters . EU Regulator Says Pfizer Vaccine Should Be Considered Case by Case for Pregnant Women. Reuters; 2020. https://www.reuters.com/article/us‐health‐coronavirus‐vaccine‐ema‐pregna/eu‐regulator‐says‐pfizer‐vaccine‐should‐be‐considered‐case‐by‐case‐for‐pregnant‐women‐idUSKBN28V1VV. Accessed January 27, 2021. [Google Scholar]
- 125.Europe Medicines Agency . COVID‐19 Vaccine Moderna. European Medicines Agency; 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/covid‐19‐vaccine‐moderna. Accessed January 27, 2021. [Google Scholar]
- 126.Society of Obstetricians and Gynaecologists of Canada . SOGC Statement on COVID‐19 Vaccination in Pregnancy. Society of Obstetricians and Gynaecologists of Canada; 2021. https://sogc.org/common/Uploaded%20files/Latest%20News/SOGC_Statement_COVID‐19_Vaccination_in_Pregnancy.pdf. Accessed January 27, 2021. [Google Scholar]
- 127.The United States Department of Health and Human Services. Code of Federal Regulations, Title 45, Public Welfare, Part 46, Protection of Human Subjects. The United States Department of Health and Human Services; 2009. https://www.hhs.gov/ohrp/sites/default/files/ohrp/policy/ohrpregulations.pdf. Accessed January 27, 2021. [Google Scholar]
- 128.Heyrana K, Byers HM, Stratton P. Increasing the participation of pregnant women in clinical trials. J Am Med Assoc. 2018;320(20):2077‐2078. [DOI] [PubMed] [Google Scholar]
- 129.van der Graaf R, van der Zande ISE, den Ruijter HM, et al. Fair inclusion of pregnant women in clinical trials: an integrated scientific and ethical approach. Trials. 2018;19(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Graham AL. When is it acceptable to vaccinate pregnant women? Risk, ethics, and politics of governance in epidemic crises. Current Trop Med Rep. 2019;6(4):205‐212. [Google Scholar]
- 131.Bebell LM, Riley LE. Ebola virus disease and Marburg disease in pregnancy: a review and management considerations for filovirus infection. Obstet Gynecol. 2015;125(6):1293‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alirol E, Kuesel AC, Guraiib MM, de la Fuente‐Núñez V, Saxena A, Gomes MF. Ethics review of studies during public health emergencies ‐ the experience of the WHO ethics review committee during the Ebola virus disease epidemic. BMC Med Ethics. 2017;18(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith DD, Pippen JL, Adesomo AA, et al. Exclusion of pregnant women from clinical trials during the coronavirus disease 2019 pandemic: a review of international registries. Am J Perinatol. 2020;37(8):792‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jones CE, Munoz FM, Spiegel HML, et al. Guideline for collection, analysis and presentation of safety data in clinical trials of vaccines in pregnant women. Vaccine. 2016;34(49):5998‐6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blehar MC, Spong C, Grady C, Goldkind SF, Sahin L, Clayton JA. Enrolling pregnant women: issues in clinical research. Womens Health Issues. 2013;23(1):e39‐e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.American College of Obstetricians and Gynecologists. Committee Opinion No. 646 . Ethical considerations for including women as research participants. Obstet Gynecol. 2016;127(5):e100‐107. [DOI] [PubMed] [Google Scholar]
- 137.Chervenak FA, McCullough LB, Bornstein E, et al. Professionally responsible COVID‐19 vaccination counseling of obstetric/gynecologic patients: counseling patients about COVID‐19 vaccination. Am J Obstet Gynecol. 2021;224(5):470‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Panagiotakopoulos L, Myers TR, Gee J, et al. SARS‐CoV‐2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics – eight U.S. Health care centers, March 1–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1355‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mattar CN, Kalimuddin S, Sadarangani SP, et al. Pregnancy outcomes in COVID‐19: a prospective cohort study in Singapore. Ann Acad Med Singapore. 2020;49(11):857‐869. [PubMed] [Google Scholar]
- 140.Turan O, Hakim A, Dashraath P, Jeslyn WJL, Wright A, Abdul‐Kadir R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS‐CoV‐2 infection among hospitalized pregnant women: a systematic review. Int J Gynaecol Obstet. 2020;151(1):7‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chan GMF, Kanneganti A, Yasin N, Ismail‐Pratt I, Logan SJS. Well‐being, obstetrics and gynaecology and COVID‐19: leaving no trainee behind. Aust N. Z J Obstet Gynaecol. 2020;60(6):983‐986. [DOI] [PubMed] [Google Scholar]
- 142.World Health Organization Health Workforce Department Gender equity in the health workforce: Analysis of 104 countries (Health Workforce Working Paper 1). World Health Organization; 2019. https://apps.who.int/iris/bitstream/handle/10665/311314/WHO‐HIS‐HWF‐Gender‐WP1‐2019.1‐eng.pdf?ua=1. Accessed January 27, 2021. [Google Scholar]
- 143.Blitz MJ, Rochelson B, Minkoff H, et al. Maternal mortality among women with coronavirus disease 2019 admitted to the intensive care unit. Am J Obstet Gynecol. 2020;223(4):595‐599e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Flannery DD, Gouma S, Dhudasia MB, et al. SARS‐CoV‐2 seroprevalence among parturient women in Philadelphia. Sci Immunol. 2020;5(49).eabd5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hoque ABM, Hoque M, Hoque ME, Van Hal G. COVID‐19 vaccine acceptability among pregnant women at a primary health care facility in Durban, South Africa. Eur J Med Health Sci. 2020;2. [Google Scholar]
- 146.Centers for Disease Control and Prevention . mRNA COVID‐19 Vaccines: Interim Considerations: Preparing for the Potential Management of Anaphylaxis After COVID‐19 Vaccination. Centers for Disease Control and Prevention; 2021. https://www.cdc.gov/vaccines/covid‐19/info‐by‐product/clinical‐considerations.html. Accessed January 27, 2021. [Google Scholar]
- 147.Jensen‐Jarolim E, Reider N, Fritsch R, Breiteneder H. Fatal outcome of anaphylaxis to camomile‐containing enema during labor: a case study. J Allergy Clin Immunol. 1998;102(6 Pt 1):1041‐1042. [DOI] [PubMed] [Google Scholar]
- 148.Mulla ZD, Ebrahim MS, Gonzalez JL. Anaphylaxis in the obstetric patient: analysis of a statewide hospital discharge database. Ann Allergy Asthma Immunol. 2010;104(1):55‐59. [DOI] [PubMed] [Google Scholar]
- 149.Porter BJ, Acharya U, Ormerod AD, Herriot R. Latex/chlorhexidine‐induced anaphylaxis in pregnancy. Allergy. 1998;53(4):455‐457. [DOI] [PubMed] [Google Scholar]
- 150.Sheikh J. Intrapartum anaphylaxis to penicillin in a woman with rheumatoid arthritis who had no prior penicillin allergy. Ann Allergy Asthma Immunol. 2007;99(3):287‐289. [DOI] [PubMed] [Google Scholar]
- 151.Lieberman TLHaP. Anaphylaxis in pregnancy. Immunol Allergy Clin. 2000;20(4):831‐838. [Google Scholar]
- 152.Chaudhuri K, Gonzales J, Jesurun CA, Ambat MT, Mandal‐Chaudhuri S. Anaphylactic shock in pregnancy: a case study and review of the literature. Int J Obstet Anesth. 2008;17(4):350‐357. [DOI] [PubMed] [Google Scholar]
- 153.Simons FE, Schatz M. Anaphylaxis during pregnancy. J Allergy Clin Immunol. 2012;130(3):597‐606. [DOI] [PubMed] [Google Scholar]
- 154.Halsey NA, Griffioen M, Dreskin SC, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine. 2013;31(51):6107‐6112. [DOI] [PubMed] [Google Scholar]
- 155.Glover RE, Urquhart R, Lukawska J, Blumenthal KG. Vaccinating against covid‐19 in people who report allergies. BMJ. 2021;372:n120. [DOI] [PubMed] [Google Scholar]
- 156.Gallagher J. Under 40s to Be Offered Alternative to AZ Vaccine. BBC News; 2021. https://www.bbc.com/news/health‐57021738. Accessed May 15, 2021. [Google Scholar]
- 157.Heinly T, Lieberman P. Anaphylaxis in pregnancy. Immunol Allergy Clin. 2000;20(4):831‐838. [Google Scholar]
- 158.Jeejeebhoy FM, Zelop CM, Lipman S, et al. Cardiac arrest in pregnancy: a scientific statement from the American heart association. Circulation. 2015;132(18):1747‐1773. [DOI] [PubMed] [Google Scholar]
- 159.Dreier JW, Andersen AM, Berg‐Beckhoff G. Systematic review and meta‐analyses: fever in pregnancy and health impacts in the offspring. Pediatrics. 2014;133(3):e674‐e688. [DOI] [PubMed] [Google Scholar]
- 160.Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS‐CoV‐2 infection in health care workers. N Engl J Med. 2020;384:533‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Callow KA, Parry HF, Sergeant M, Tyrrell DA. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105(2):435‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wilk AJ, Rustagi A, Zhao NQ, et al. A single‐cell atlas of the peripheral immune response in patients with severe COVID‐19. Nat Med. 2020;26(7):1070‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun J, Zhuang Z, Zheng J, et al. Generation of a broadly useful model for COVID‐19 pathogenesis, vaccination, and treatment. Cell. 2020;182(3):734‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Juno JA, Tan HX, Lee WS, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID‐19. Nat Med. 2020;26(9):1428‐1434. [DOI] [PubMed] [Google Scholar]
- 165.Le Bert N, Tan AT, Kunasegaran K, et al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature. 2020;584(7821):457‐462. [DOI] [PubMed] [Google Scholar]
- 166.Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody‐dependent enhancement and SARS‐CoV‐2 vaccines and therapies. Nat Microbiol. 2020;5(10):1185‐1191. [DOI] [PubMed] [Google Scholar]
- 167.Arvin AM, Fink K, Schmid MA, et al. A perspective on potential antibody‐dependent enhancement of SARS‐CoV‐2. Nature. 2020;584(7821):353‐363. [DOI] [PubMed] [Google Scholar]
- 168.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2 ‐ preliminary report. N Engl J Med. 2020;383(20):1920‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS‐CoV‐2 in Iceland. N Engl J Med. 2020;383(18):1724‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS‐CoV‐2 mRNA‐1273 vaccination. N Engl J Med. 2021;384(1):80‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jennifer M , Dan JM , Yu K , et al. Immunological memory to SARS‐CoV‐2 assessed for greater than six months after infection. Biorxiv; 2020. [Google Scholar]
- 172.European Medicines Agency . Consideration on Core Requirements for RMPs of COVID19 Vaccines; 2020. https://www.ema.europa.eu/en/documents/other/consideration‐core‐requirements‐rmps‐covid‐19‐vaccines_en.pdf. Accessed December 12, 2021. [Google Scholar]
- 173.Lee GM, Romero JR, Bell BP. Postapproval vaccine safety surveillance for COVID‐19 vaccines in the US. J Am Med Assoc. 2020;324(19):1937‐1938. [DOI] [PubMed] [Google Scholar]
- 174.Shimabukuro T. ACIP Presentation Slides: February 28 – March 1, 2021 Meeting – COVID‐19 Vaccine Safety Update. Centers for Disease Control and Prevention Advisory Committee on Immunization Practices; 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides‐2021‐02/28‐03‐01/05‐covid‐Shimabukuro.pdf. Accessed March 1, 2021. [Google Scholar]
- 175.Fontanet A, Cauchemez S. COVID‐19 herd immunity: where are we? Nat Rev Immunol. 2020;20(10):583‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.NPR . D S. Transporting and Distributing Vaccines Will Be ‘Unprecedented’ Logistical Challenge; 2020. https://www.npr.org/2020/12/11/945670606/transporting‐and‐distributing‐vaccines‐will‐be‐unprecedented‐logistical‐operation. Accessed December 14, 2020. [Google Scholar]
- 177.Cavanaugh AM, Fortier S, Lewis P, et al. COVID‐19 outbreak associated with a SARS‐CoV‐2 R.1 lineage variant in a skilled nursing facility after vaccination program – Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Keehner J, Horton LE, Pfeffer MA, et al. SARS‐CoV‐2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384(18):1774‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Amit S, Beni SA, Biber A, Grinberg A, Leshem E, Regev‐Yochay G. Postvaccination COVID‐19 among healthcare workers, Israel. Emerg Infect Dis. 2021;27(4):1220‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


