Abstract
Background
In traumatic bleeding, transfusion practice has shifted toward higher doses of platelets and plasma transfusion. The aim of this systematic review was to investigate whether a higher platelet‐to‐red blood cell (RBC) transfusion ratio improves mortality without worsening organ failure when compared with a lower ratio of platelet‐to‐RBC.
Methods
Pubmed, Medline, and Embase were screened for randomized controlled trials (RCTs) in bleeding trauma patients (age ≥16 years) receiving platelet transfusion between 1946 until October 2020. High platelet:RBC ratio was defined as being the highest ratio within an included study. Primary outcome was 24 hour mortality. Secondary outcomes were 30‐day mortality, thromboembolic events, organ failure, and correction of coagulopathy.
Results
In total five RCTs (n = 1757 patients) were included. A high platelet:RBC compared with a low platelet:RBC ratio significantly improved 24 hour mortality (odds ratio [OR] 0.69 [0.53–0.89]) and 30‐ day mortality (OR 0.78 [0.63–0.98]). There was no difference between platelet:RBC ratio groups in thromboembolic events and organ failure. Correction of coagulopathy was reported in five studies, in which platelet dose had no impact on trauma‐induced coagulopathy.
Conclusions
In traumatic bleeding, a high platelet:RBC improves mortality as compared to low platelet:RBC ratio. The high platelet:RBC ratio does not influence thromboembolic or organ failure event rates.
Keywords: coagulopathy, organ failure, platelet, transfusion, trauma
Abbreviations
- CCT
conventional coagulation test
- ISS
injury severity score
- MODS
multiple organ dysfunction syndrome
- RBC
red blood cell
- RCT
randomized controlled trial
- TEE
thromboembolic events
- TIC
trauma‐induced coagulopathy
- TXA
tranexamic acid
- VHA
viscoelastic hemostatic assay
1. INTRODUCTION
Uncontrolled hemorrhage remains one of the major causes of preventable death after injury.1 Trauma‐induced coagulopathy (TIC) develops in 30%–40% of these patients.2, 3 TIC is associated with both early and late mortality,2, 4, 5 with rates as high as 40%.6, 7 Furthermore, TIC is associated with an increased incidence of acute lung injury, multiple organ failure, and infections.8, 9, 10
Platelets play an important role in the pathophysiology of TIC.11 Although platelet count typically does not decrease early after traumatic injury,12, 13 several studies report severe platelet dysfunction after injury, associated with increased mortality.14 Interestingly, higher platelet counts later during intensive care stay are associated with improved survival,15, 16, 17 suggesting that maintaining high levels of platelets may prevent, at least partially, the loss of functionality. Alternatively, increasing platelet counts through platelet transfusion might contribute to earlier or improved hemostasis, possibly by limiting hyperfibrinolysis.18
Observational studies in civilian and military trauma patients suggest a survival benefit for patients receiving higher platelet ratios, which is underlined by results of a randomized controlled trial (RCT).8, 19, 20, 21 Observational studies however, remain subject to bias. Also, it is unclear what the effect is of higher platelet ratios on organ failure and thromboembolic events. Therefore, the aim of this review was to investigate the effect of a high platelet:RBC ratio on mortality when compared with a lower platelet:RBC ratio as reported in RCTs. Furthermore, the effect of high platelet:RBC ratios on the correction of coagulopathy and on the development of thromboembolic events or organ failure was assessed. The hypothesis was that high platelet:RBC ratios are associated with lower early mortality when compared with lower platelet:RBC ratios, but have similar effects on the occurrence of organ failure and thrombosis.
2. METHODS
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta‐Analyses guidelines (PRISMA).22
Medline, PubMed, and Embase were searched with the assistance of a clinical librarian. The search strategy was initially developed for Medline and adapted for the other data sources (Appendix S1). In addition, we searched for ongoing trials on www.controlled-trials.com and www.clinicaltrials.gov. All articles until October 2020 were screened for inclusion.
2.1. Study selection
All RCTs encompassing hemorrhaging trauma patients of at least 16 years old. Only English, German, and Dutch language articles were selected. Cohort studies, Case–control studies, reviews, correspondences, experimental studies, and case reports/series were not eligible for inclusion. Studies were included if the dose of platelet transfusion was reported, as well as mortality rates or organ failure. Studies reporting on other patients than trauma were excluded.
Burn victims were excluded, because transfusion practices are markedly different in this population.23, 24 Bibliographies of systematic reviews and all included studies were screened for relevant publications. Selection of articles was performed by two reviewers (D.K. and R.v.A.). Discrepancies in the inclusion of articles were discussed and, if needed, a third independent reviewer was consulted.
2.2. Data extraction
The design of the included studies was determined using the Cochrane group checklist.25 The following patient characteristics were extracted from the studies: type of trauma (military or civilian), number of patients, sex, age, trauma mechanism (blunt or penetrating), and injury severity score. Furthermore, the total number of blood products administered to the patients was collected. Ratios of units of platelet:RBC and plasma:RBC were calculated using the means. If studies did not disclose means, they were estimated using the median and interquartile range.26 The type of platelet product or the amount of patients receiving platelet transfusion was often not detailed. Thereby, a quantitative definition of a ratio was not possible. To enable performing our primary analysis, which is the comparison between high and low platelet:RBC ratios, the platelet dose was evaluated separately for every study, in which both pooled and apheresis platelet products were treated the same in the calculation of the platelet:RBC ratio in the study arms.26 A high ratio was defined as being the highest comparator arm, whereas the low platelet:RBC ratio was defined as the lowest comparator arm. The platelet:RBC ratio for each study was calculated within 24 hours (h) after admission.
2.3. Outcome measures
Primary outcome was mortality, defined as early mortality occurring within 24 h after admission to the hospital. Secondary outcomes were late mortality occurring within 30 days, thromboembolic events, organ failure, and correction of coagulopathy, defined as a normalization of coagulation test results within 24 h after transfusion.
2.4. Quality assessment
A quality assessment of the RCTs included in this systematic review was performed using the Cochrane Collaboration tool for assessing risk of bias. The cohort was representative when it consisted of a majority of blunt trauma for civilian studies and penetrating trauma for military studies. Comparability was defined adequate when there was a correction for injury severity scores and blunt trauma. The follow‐up was defined as satisfactory when it was at least as long as hospital stay.
2.5. Statistical analysis
Review Manager (RevMan 5, The Nordic Cochrane Centre, Copenhagen, Denmark) was used for the meta‐analysis. Odds ratio (OR) with 95% confidence interval (CI) was used as a measure for dichotomous variables. Fixed‐effects model was used for the analysis of RCTs because of the expected small number of studies and because only bleeding trauma patients in the civilian setting were included and no other causes of blood loss. Thereby, it can be postulated that the effect size of platelet dose on TIC is similar between studies.27 A p value of .05 was considered to be statistically significant.
3. RESULTS
3.1. Study selection and data extraction
After removal of duplicates, 2620 articles were screened. After title and abstract screening, 11 full texts were evaluated. From the 11 studies, 6 articles were excluded (Figure 1). Reasons for exclusions after full‐text screening were as follows: no platelet transfusion given (n = 4), or reported (n = 1) and no outcome parameters (n = 1).
FIGURE 1.
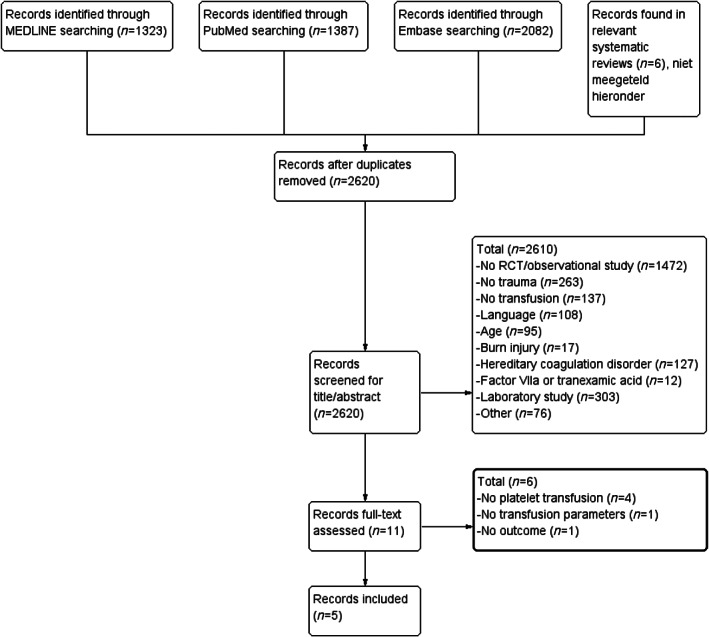
Flowchart of selection process
In total, this review included five studies,21, 28, 29, 30, 31 encompassing 1757 patients. The baseline characteristics of the patients in the RCTs are shown in Table 1. The calculated differences of platelet:RBC ratios are shown in Table 2.
TABLE 1.
Characteristics of trauma patients included in randomized controlled trials
| Study | Year | Randomization arm | Group (n) | Male (%) | Blunt (%) | Lactate (mmol/L) | Base excess (mEq/L) | ISS | RBC (units) | Plasma (units) | PLT (units) | PLT product | Cryoprecipitate (units) | PLT:RBC | Plasma:RBC | TXA (%) | MODS (%) | TEE (%) | ICU free days | 24 h mortality (%) | 30 days mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baksaas‐Aasen et al.31 | 2020 | CCT | 195 | 82.0 | 67.0 | 5.0* | −7.9* | 26* | 6.5* | 7.3* | 4* | P | N.R. | 0.62:1 | 1.12:1 | 98.4 | 84.3 | 13.8 | 13* | 17 | 28 |
| VHA | 201 | 73.2 | 67.2 | 5.0* | −8.4* | 27* | 6.3* | 6.3* | 8* | P | N.R. | 1.27:1 | 1:1 | 93.5 | 86.0 | 8.5 | 12* | 14 | 25 | ||
| Sperry et al.30 | 2018 | Standard care | 271 | 73.8 | 83.4 | 3.9* | N.R. | 21* | 6.5* | 1* | 0.3* | aA/P | N.R. | 0.04:1 | 0.15:1 | N.R. | 57.6 | N.R. | N.R. | 22 | 33 |
| Prehospital plasma | 230 | 71.3 | 81.3 | 3.5* | N.R. | 23* | 4* | 0.8* | 0.3* | aA/P | N.R. | 0.06:1 | 0.19:1 | N.R. | 63 | N.R. | N.R. | 14 | 22 | ||
| Gonzalez et al.29 | 2016 | CCT | 55 | 74.5 | 65.5 | 5.7* | −13.6* | 34* | 11* | 8* | 1* | A | 1* | 0.09:1 | 0.73:1 | 16.4 | 5.5 | 10.9 | 9* | 22 | 15 |
| VHA | 56 | 66.0 | 69.6 | 6.3* | −11.8* | 31* | 10* | 5.5* | 1* | A | 0.5* | 0.10:1 | 0.55:1 | 7.1 | 3.6 | 16.1 | 14* | 7 | 13 | ||
| Holcomb et al.21 | 2015 | 1:1:1 | 338 | 77.8 | 54.7 | N.R. | −8.1* | 28* | 9.5* | 7.5* | 12* | P | 0* | 1.26:1 | 0.79:1 | 18.9 | 5.9 | 11.5 | 5* | 13 | 22 |
| 1:1:2 | 342 | 82.7 | 50.6 | N.R | −8.6* | 27* | 10* | 5.5* | 6* | P | 2.3* | 0.60:1 | 0.55:1 | 19.9 | 4.4 | 10.8 | 5* | 17 | 26 | ||
| Nascimento et al.28 | 2013 | Fixed ratio | 37 | 64.9 | 64.9 | N.R. | N.R. | 35 | 7.5* | 6* | 7* | P | 0* | 0.93:1 | 0.80:1 | 13.5 | 2.7 | N.R. | 21* | 22 | 30 |
| Lab guided ratio | 32 | 71.9 | 59.4 | N.R. | N.R. | 35 | 8.5* | 4.8* | 4* | P | 2.5* | 0.47:1 | 0.56:1 | 18.7 | 0 | N.R. | 17* | 9 | 9 |
Note: Baseline characteristics of included randomized controlled trials. All units are reported in mean or *calculated mean. All ratios are calculated with the mean. 1:1:1 = ratio platelets:plasma:red blood cell. Standard care in Sperry et al. 2018 received both prehospital red blood cells and crystalloids.
Abbreviations: A, apheresis platelet product; CCT, conventional coagulation test; ISS, injury severity score; MODS, multiple organ dysfunction syndrome; N, number of patients; N.R., not reported; P, pooled platelet product; PLT, platelet; RBC, red blood cell; TEE, thromboembolic events; TXA, tranexamic acid; VHA, viscoelastic hemostatic assay.
Personal communication with Prof. dr. J. Sperry: site dependent use of pooled or apheresis platelet product.
TABLE 2.
Calculated transfusion ratios for all meta‐analyses
| Outcome | Mean (SD) ratio in high group | Mean (SD) ratio in low group | Median (IQR) ratio in high group | Median (IQR) ratio in low group |
|---|---|---|---|---|
| 24 h and 30 day mortality Platelet:RBC ratio | 0.85 (0.56) | 0.40 (0.27) | 1.26 (0.06–1.26) | 0.60 (0.04–0.60) |
| 24 h and 30 day mortality Platelet:plasma ratio | 1.08 (0.56) | 0.65 (0.38) | 1.28 (0.33–1.60) | 0.55 (0.25–1.09) |
| 24 h and 30 day mortality Plasma:RBC ratio | 0.70 (0.34) | 0.53 (0.30) | 0.79 (0.19–0.80) | 0.55 (0.15–0.56) |
| TEE Platelet:RBC ratio | 1.15 (0.34) | 0.56 (0.15) | 1.26 (1.26–1.27) | 0.60 (0.60–0.62) |
| MODS Platelet:RBC ratio | 0.83 (0.56) | 0.39 (0.27) | 1.26 (0.06–1.26) | 0.60 (0.04–0.60) |
Note: Mean and median ratios of blood products with either SD or interquartile range (IQR), respectively. Groups in studies are divided into having a low or high blood product ratio and based on the units of transfusion products used in that specific study.
Abbreviations: TEE, thromboembolic events; MODS, multiple organ dysfunction syndrome.
The five RCTs consisted of 856 patients transfused with a high platelet:RBC ratio and 901 patients with a low platelet:RBC ratio. Injury severity scores were similar in both groups. The majority of the patients were male and had sustained a blunt trauma.
3.2. Quality assessments
The overall quality of the studies was moderate, as shown in Figure 2. All but one RCT scored high risk of bias, due to the impossibility of blinding of personnel to the allocation of treatment strategy.
FIGURE 2.
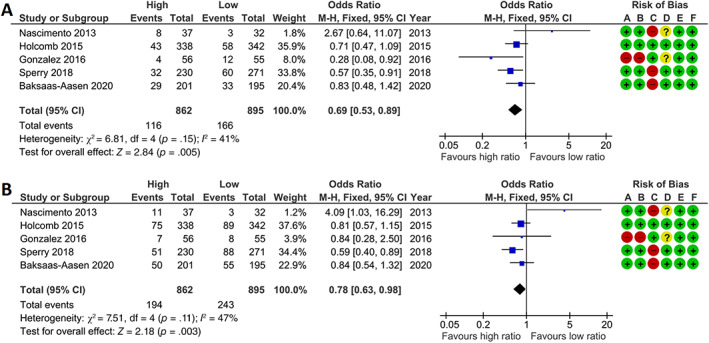
High platelet:RBC ratio improves 24‐h and 30‐day mortality in randomized controlled trials compared with low platelet:RBC ratio. Meta‐analyses of (A) 24‐h and (B) 30‐day mortality represented in a forest plot. Weighted mean platelet:red blood cell ratio in the high ratio group was 0.85 (SD 0.56) and in the low platelet dose group 0.40 (0.27). Events = number of deceased, total = total number of patients, high = group with highest platelet:RBC ratio, low = group with lowest platelet:RBC ratio, M‐H = Mantel–Haenszel. Risk of bias was assessed for (A) random sequence generation, (B) allocation concealment, (C) blinding of participants and personnel, (D) blinding of outcome assessment, (E) incomplete outcome data, (F) selective reporting. + = low risk, − = high risk, ? = unknown risk
3.3. The effect of PLT:RBC ratio on mortality
Transfusion of a high platelet:RBC ratio was associated with lower 24‐h mortality compared with a low platelet:RBC ratio (Figure 2, OR 0.69 [95% CI: 0.53–0.89]). Furthermore, a high platelet:RBC ratio was associated with lower 30‐day mortality compared with a low platelet:RBC ratio (OR 0.78 [95% CI: 0.63–0.98]). To determine whether these results were influenced by a concomitant higher dose of plasma transfusions, additional analyses were performed by comparing high plasma:RBC ratio versus low plasma:RBC ratio and high platelet:plasma ratio versus low platelet:plasma ratio (Figure 3A–D). A high platelet:plasma ratio remained associated with a lower mortality compared with a low platelet:plasma ratio (Figure 3A,B). Contrastingly, a high plasma:RBC ratio was not associated with improvements in 24‐h mortality and 30‐day mortality compared with a low plasma:RBC ratio in these included studies (Figure 3C,D). This suggests that the observed benefit of a high platelet:RBC ratio in this analysis is independent of the amount of plasma transfusions employed.
FIGURE 3.
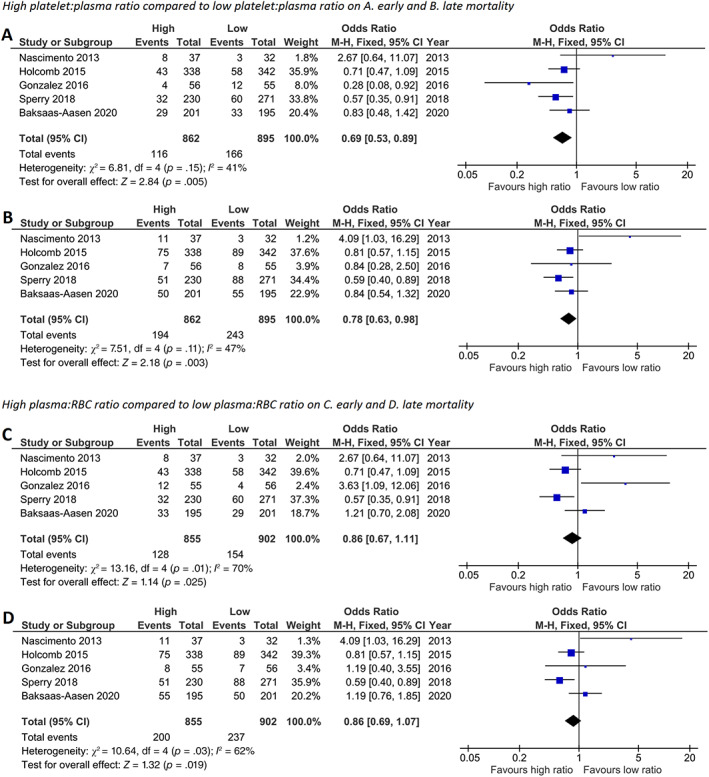
High platelet:plasma, but not high plasma:RBC ratio, improves 24‐h mortality and 30‐day mortality in randomized controlled trials. Meta‐analyses of high and low platelet:plasma ratio on (A) 24‐h and (B) 30‐day mortality and high and low plasma:RBC ratio on (C) 24‐h and (D) 30‐day mortality represented in a forest plot. Weighted mean platelet:plasma ratio in the high ratio group was 1.08 (SD 0.56) and in the low ratio group 0.65 (0.38). Weighted mean plasma:RBC ratio in the high ratio group was 0.70 (0.34) and in the low ratio group 0.53 (0.30). Events = number of deceased, total = total number of patients, high = group with highest platelet:plasma or plasma:RBC ratio, low = group with lowest platelet:plasma or plasma:RBC ratio, M‐H = Mantel–Haenszel
3.4. The effect of PLT:RBC ratio on thromboembolic events and organ failure
Three out of five RCTs described thromboembolic events (Figure 4A). No difference in thromboembolic events was observed in high compared with low platelet:RBC ratio groups (OR 0.91 [95% CI: 0.64–1.31]). All RCTs reported organ failure outcomes (Figure 4B). No difference in organ failure was seen in high compared with low platelet:RBC ratio (OR 1.24 [95% CI: 0.94–1.64]).
FIGURE 4.
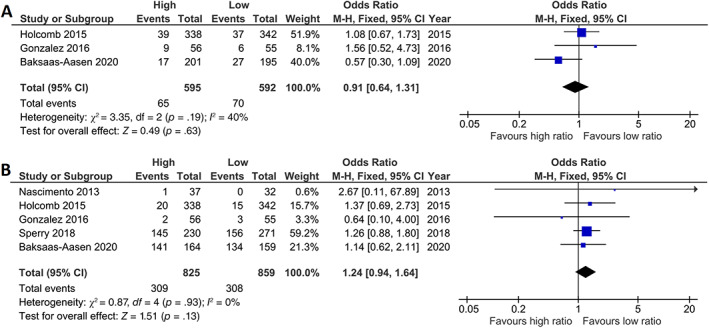
High platelet:red blood cell ratio has no effect on thromboembolic events or organ failure in trauma patients included in randomized controlled trials. Meta‐analyses of high and low platelet:RBC ratio on (A) thromboembolic events (TEE) and (B) multiple organ dysfunction syndrome (MODS) represented in a forest plot. Weighted mean platelet:RBC ratio in the high ratio group was 1.15 (SD 0.34) and in the low ratio group 0.56 (0.15) for TEE and 0.83 (0.56) and 0.39 (0.27) for MODS. Events = number of patients affected, total = total number of patients, high = group with highest platelet:RBC ratio, low = group with lowest platelet:RBC ratio, M‐H = Mantel–Haenszel
3.5. The effect of PLT:RBC ratio on correction of coagulopathy
Correction of coagulopathy after transfusion was reported in four studies.21, 28, 29, 31 In these studies, no significant differences were observed among patients transfused with high or low platelet:RBC ratios in terms of correction of deranged international normalized ratio, platelet counts, and fibrinogen levels within 24 h posttransfusion.28, 29, 32 For example, in the high ratio group, platelet count dropped from 215 [165–279] × 109/L to 97 [72–128] × 109/L, and in the low ratio group, platelet count dropped from 144 [117–157] × 109/L to 109 [91–130] × 109/L in the first 24 h.29 However, there were no different effects of high and low platelet dose on platelet counts.29 In the most recent RCT, the percentage of patients with a prothrombin time ratio more than 1.2 at time of hemostasis was similar.31 Thromboelastography results 24 h after resuscitation were reported in another study, and no significant differences in these values were observed between both groups.29
4. DISCUSSION
In this systematic review of RCTs on the impact of platelet dose on outcome of bleeding trauma patients, a high compared to a low platelet:RBC ratio was associated with improved early and late mortality. With regard to thromboembolic events and organ failure, no difference was found between platelet:RBC dose groups. Also, no differences were observed on correction of coagulopathy, although most studies only reported conventional coagulation test results.
Patients receiving higher platelet:RBC ratios had lower mortality rates than patients receiving a lower ratio of platelet:RBC. As patients who received a high platelet dose concomitantly received a high dose of plasma, additional analyses were done by comparing a high plasma:platelet or plasma:RBC to a low plasma:platelet or plasma:RBC ratio, respectively. In these analyses, no differences were observed between high and low plasma:RBC ratios. This suggests that the effect on mortality in these analyses could, at least in part, be attributed to platelet dose.33
The effect of a high platelet dose was greater for 24‐h mortality than for 30‐day mortality. This may not be surprising, as the high platelet dose may improve TIC and thereby prevent death due to exsanguination. However, correction of TIC was not different between groups, although often not assessed with viscoelastic tests. Thereby, conclusions as to how platelet transfusion would improve TIC cannot be drawn. Observational data suggest that replenishment of platelets may improve platelet function as well as mitigating hyperfibrinolysis, possibly due to the release of platelet plasminogen activator inhibitor‐1 or antiplasmin.18, 34 The optimal dose of platelets remains to be determined. Preclinical data from our research group suggest that a platelet:RBC ratio of 2:1 resulted in improved correction of coagulopathy as determined by viscoelastic tests, without the occurrence of thrombi in organs.35 In future trials, besides platelet count, evaluation of platelet function in the trauma population after resuscitation therapies is crucial as it may reveal new therapeutic targets to enhance platelet function early in trauma.
No differences in thromboembolic events and organ failure scores were observed in RCTs comparing high and low platelet:RBC ratios, underlining that a high platelet:RBC ratio was also associated with lower 30 thinsp;days mortality.
Taken together, the implications of this review may be that a median platelet:RBC ratio of higher than 0.6 should be targeted in clinical practice, however the exact ratio remains to be determined.
There are several limitations in this systematic review. The approach of calculating platelet:RBC ratio resulted in possible overlap in transfusion ratio for high and low ratio groups, which could have influenced the analyses. However, in determining ratios, not all studies reported on the type of platelet product (apheresis or pooled buffy coat platelets) and the amount of patients receiving platelet transfusions, rendering it impossible to calculate the absolute platelet:RBC ratio. Furthermore, if overlap in transfusion ratios is present, the effect of a high platelet dose on mortality could potentially be even larger.
5. CONCLUSION
Resuscitation with a high compared to low platelet:RBC ratio improves early and late mortality in patients with traumatic bleeding. The high platelet:RBC ratio did not influence the occurrence of organ failure. The optimal ratio for platelet:RBC and its effect on platelet function in traumatic bleeding remains to be determined.
CONFLICTS OF INTEREST
Dr Hollmann is Executive Section Editor Pharmacology with Anesthesiology and Section Editor Anesthesiology with the Journal of Clinical Medicine. He has received research funding from ZonMW, STW, SCA, ESA, Eurocept BV, Edwards Life Sciences. Dr Hollmann served as consultant for Eurocept BV and ECHO BV and received speakers fees from CSL Behring and BBraun. All other authors have disclosed no conflicts of interest.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
The authors would like to thank the clinical librarian Faridi S. van Etten‐Jamaludin for the help with the search strategy. Funding support was provided solely from institutional and/or departmental sources.
Kleinveld DJB, van Amstel RBE, Wirtz MR, et al. Platelet‐to‐red blood cell ratio and mortality in bleeding trauma patients: A systematic review and meta‐analysis. Transfusion. 2021;61:S243–S251. 10.1111/trf.16455
REFERENCES
- 1.Koh EY, Oyeniyi BT, Fox EE, Scerbo M, Tomasek JS, Wade CE, et al. Trends in potentially preventable trauma deaths between 2005‐2006 and 2012‐2013. Am J Surg. 2019;218(3):501–6. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–7. discussion 7. [DOI] [PubMed] [Google Scholar]
- 3.Brohi K, Gruen RL, Holcomb JB. Why are bleeding trauma patients still dying? Intensive Care Med. 2019;45(5):709–11. [DOI] [PubMed] [Google Scholar]
- 4.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13(6):680–5. [DOI] [PubMed] [Google Scholar]
- 5.Frith D, Goslings JC, Gaarder C, Maegele M, Cohen MJ, Allard S, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8(9):1919–25. [DOI] [PubMed] [Google Scholar]
- 6.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, et al. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261(3):586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93. [DOI] [PubMed] [Google Scholar]
- 8.Borgman MA, Spinella PC, Holcomb JB, Blackbourne LH, Wade CE, Lefering R, et al. The effect of FFP:RBC ratio on morbidity and mortality in trauma patients based on transfusion prediction score. Vox Sang. 2011;101(1):44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40(9):912–8. [DOI] [PubMed] [Google Scholar]
- 10.MacLeod JB, Winkler AM, McCoy CC, Hillyer CD, Shaz BH. Early trauma induced coagulopathy (ETIC): prevalence across the injury spectrum. Injury. 2014;45(5):910–5. [DOI] [PubMed] [Google Scholar]
- 11.Vulliamy P, Gillespie S, Armstrong PC, Allan HE, Warner TD, Brohi K. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc Natl Acad Sci U S A. 2019;116(35):17444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown LM, Call MS, Margaret Knudson M, Cohen MJ, Holcomb JB, Wade CE, et al. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma. 2011;71(2 Suppl 3):S337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stansbury LG, Hess AS, Thompson K, Kramer B, Scalea TM, Hess JR. The clinical significance of platelet counts in the first 24 hours after severe injury. Transfusion. 2013;53(4):783–9. [DOI] [PubMed] [Google Scholar]
- 14.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valade N, Decailliot F, Rébufat Y, Heurtematte Y, Duvaldestin P, Stéphan F. Thrombocytosis after trauma: incidence, aetiology, and clinical significance. Br J Anaesth. 2004;94(1):18–23. [DOI] [PubMed] [Google Scholar]
- 16.Salim A, Hadjizacharia P, DuBose J, Kobayashi L, Inaba K, Chan LS, et al. What is the significance of thrombocytosis in patients with trauma? J Trauma Acute Care Surg. 2009;66(5):1349–54. [DOI] [PubMed] [Google Scholar]
- 17.Nydam TL, Kashuk JL, Moore EE, Johnson JL, Burlew CC, Biffl WL, et al. Refractory postinjury thrombocytopenia is associated with multiple organ failure and adverse outcomes. J Trauma Acute Care Surg. 2011;70(2):401–7. [DOI] [PubMed] [Google Scholar]
- 18.Vulliamy P, Gillespie S, Gall LS, Green L, Brohi K, Davenport RA. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. J Trauma Acute Care Surg. 2017;83(3):388–97. [DOI] [PubMed] [Google Scholar]
- 19.Spinella PC, Wade CE, Blackbourne LH, Borgman MA, Zarzabal LA, Du F, et al. The association of blood component use ratios with the survival of massively transfused trauma patients with and without severe brain injury. J Trauma. 2011;71(2 Suppl 3):S343–52. [DOI] [PubMed] [Google Scholar]
- 20.Maegele M, Spinella PC, Schochl H. The acute coagulopathy of trauma: mechanisms and tools for risk stratification. Shock. 2012;38(5):450–8. [DOI] [PubMed] [Google Scholar]
- 21.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tejiram S, Romanowski KS, Palmieri TL. Initial management of severe burn injury. Curr Opin Crit Care. 2019;25(6):647–52. [DOI] [PubMed] [Google Scholar]
- 24.Palmieri TL. Burn injury and blood transfusion. Curr Opin Anaesthesiol. 2019;32:247–51. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions; Chichester, UK: John Wiley & Sons Ltd; 2008. [Google Scholar]
- 26.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta‐analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207. [DOI] [PubMed] [Google Scholar]
- 28.Nascimento B, Callum J, Tien H, Rubenfeld G, Pinto R, Lin Y, et al. Effect of a fixed‐ratio (1:1:1) transfusion protocol versus laboratory‐results‐guided transfusion in patients with severe trauma: a randomized feasibility trial. Can Med Assoc J. 2013;185(12):E583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, et al. Goal‐directed hemostatic resuscitation of trauma‐induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early‐Young BJ, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315–26. [DOI] [PubMed] [Google Scholar]
- 31.Baksaas‐Aasen K, Gall LS, Stensballe J, Juffermans NP, Curry N, Maegele M, et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med. 2021;47:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardinale M, Cungi PJ, Esnault P, Nguyen C, Cotte J, Montcriol A, et al. Impact of high‐dose norepinephrine during intra‐hospital damage control resuscitation of traumatic haemorrhagic shock: a propensity‐score analysis. Injury. 2020;51:1164–71. [DOI] [PubMed] [Google Scholar]
- 33.Cardenas JC, Zhang X, Fox EE, Cotton BA, Hess JR, Schreiber MA, et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv. 2018;2(14):1696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gall LS, Vulliamy P, Gillespie S, Jones TF, Pierre RSJ, Breukers SE, et al. Targeted action for curing trauma‐induced coagulopathy p. The S100A10 pathway mediates an occult hyperfibrinolytic subtype in trauma patients. Ann Surg. 2019;269(6):1184–91. [DOI] [PubMed] [Google Scholar]
- 35.Kleinveld DJB, Wirtz MR, van den Brink DP, Maas MAW, Roelofs J, Goslings JC, et al. Use of a high platelet‐to‐RBC ratio of 2:1 is more effective in correcting trauma‐induced coagulopathy than a ratio of 1:1 in a rat multiple trauma transfusion model. Intensive Care Med Exp. 2019;7(Suppl 1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


