Abstract
Background and Aims
Bacterial infections are common and severe in cirrhosis, but their pathogenesis is poorly understood. Dysfunction of liver macrophages may play a role, but information about their function in cirrhosis is limited. Our aims were to investigate the specific profile and function of liver macrophages in cirrhosis and their contribution to infections. Macrophages from human cirrhotic livers were characterized phenotypically by transcriptome analysis and flow cytometry; function was assessed in vivo by single photon emission computerized tomography in patients with cirrhosis. Serum levels of specific proteins and expression in peripheral monocytes were determined by ELISA and flow cytometry. In vivo phagocytic activity of liver macrophages was measured by spinning disk intravital microscopy in a mouse model of chronic liver injury.
Approach and Results
Liver macrophages from patients with cirrhosis overexpressed proteins related to immune exhaustion, such as programmed death ligand 1 (PD‐L1), macrophage receptor with collagenous structure (MARCO), and CD163. In vivo phagocytic activity of liver macrophages in patients with cirrhosis was markedly impaired. Monocytes from patients with cirrhosis showed overexpression of PD‐L1 that paralleled disease severity, correlated with its serum levels, and was associated with increased risk of infections. Blockade of PD‐L1 with anti‐PD‐L1 antibody caused a shift in macrophage phenotype toward a less immunosuppressive profile, restored liver macrophage in vivo phagocytic activity, and reduced bacterial dissemination.
Conclusion
Liver cirrhosis is characterized by a remarkable impairment of phagocytic function of macrophages associated with an immunosuppressive transcriptome profile. The programmed cell death receptor 1/PD‐L1 axis plays a major role in the impaired activity of liver macrophages. PD‐L1 blockade reverses the immune suppressive profile and increases antimicrobial activity of liver macrophages in cirrhosis.
Abbreviations
- 99mTc
Technetium‐99m
- CCl4
carbon tetrachloryde
- DDC
3,5‐diethoxycarbonyl‐1,4‐dihydrocollidine
- IFN
interferon
- LPS
lipopolysaccharide
- MARCO
macrophage receptor with collagenous structure
- NES
normalized enrichment score
- PD‐1
programmed cell death receptor 1
- PBMCs
peripheral blood mononuclear cells
- PD‐L1
programmed death ligand 1
- SD‐IVM
spinning disk intravital microscopy
- SPECT
single photon emission computerized tomography
- TREM2
triggering receptor expressed on myeloid cells 2
Liver cirrhosis is the final stage of chronic liver disease and one of the leading causes of death in adults worldwide.( 1 ) Cirrhosis causes a marked impairment of the immune system, which is particularly relevant from a clinical perspective because it is responsible for the high risk of bacterial infections of patients with advanced cirrhosis.( 2, 3, 4 ) Bacterial infections account for a large number of hospital admissions and high cost for health care systems and are one of the leading causes of death in patients with cirrhosis.( 2 )
The current approach to preventing bacterial infections in cirrhosis is based on antibiotic prophylaxis in patients who have high risk of infection.( 5 ) This approach does not correct the alterations of the immune system and may cause an increased colonization of the enteric flora by multidrug‐resistant bacteria.( 6, 7 ) Therefore, there is need for more effective strategies targeting key factors in the pathogenesis of infections. One of the key factors in the defense against bacterial infections is liver macrophages, which are the resident macrophages within the liver that have high phagocytic capacity to remove bacteria from the circulation.( 8 ) It is currently believed that phagocytic activity of liver macrophages decreases with the progression of cirrhosis and contributes to the increased risk of bacterial infections, particularly those from enteric origin.( 9 ) However, information is very limited, and there are no studies assessing the molecular mediators responsible for the impairment of macrophage activity.( 10, 11 )
Programmed cell death receptor 1 (PD‐1) and programmed death ligand 1 (PD‐L1) are checkpoint molecules that suppress immune response. Although PD‐1 is mainly produced by T and B lymphocytes and natural killer cells in response to antigen activation, PD‐L1 is expressed by different hematopoietic and nonhematopoietic cell types as tumor cells, dendritic cells, B lymphocytes, monocytes, and macrophages,( 12 ) and its production has been shown to be inducible in a transient manner by inflammatory cytokines as interferon (IFN)‐γ.( 13 ) The PD‐1/PD‐L1 pathway exerts inhibitory effects by regulating T‐cell activation, tolerance, and immunopathology. Moreover, PD‐L1 activation delivers constitutive negative signals to macrophages, resulting in an immune suppressive cell phenotype.( 14 ) Sustained hyperexpression of PD‐1/PD‐L1 by immune cells has been shown to promote immune exhaustion, leading to persistent infections in sepsis and tumor evasion in cancer.( 15, 16 ) Moreover, PD‐1 expression by peripheral lymphocytes has been associated with a higher risk of infections in alcohol‐associated hepatitis.( 17 ) Importantly, blocking of the PD‐1/PD‐L1 pathway has been shown to induce immunological response to cancer cells and has carried a change of paradigm for the treatment of different cancers.( 16, 17 )
With this background, the current study was designed to evaluate the liver macrophages’ number and function in experimental models of chronic liver diseases as well as in human cirrhosis. Additionally, we sought to determine whether the activation of the PD‐1/PD‐L1 pathway could account for altered functionality of liver macrophages in cirrhosis and if targeting this axis could be used as an immunotherapeutic target to treat infections in patients with cirrhosis.
Materials and Methods
Experiments With Human Liver Biopsies
Informed consent in writing was obtained from each patient and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committee. For analysis of the area occupied by macrophages in liver biopsies, paraffin‐embedded cuts from patients with cirrhosis and controls were analyzed.
Characteristics of the patients included in this experiment are described in detail in the Supporting Information.
Technique
Paraffin‐embedded liver sections were incubated with mouse CD68 antibody. Detailed protocol is described in the Supporting Information.
Hepatic Single Photon Emission Computerized Tomography With Technetium‐99m Phytate Scintigraphy
Technique
Quantitation of relative distribution of radiocolloid by the liver and spleen has been reported using planar and single photon emission computerized tomography (SPECT; Infinia, Hawkeye, GE Healthcare, Milwaukee, WI).( 18 )
Details on image acquisition and analysis and also characteristics of the patients included in this experiment are provided in the Supporting Information.
Experiments With Human Liver Macrophages
Human Liver Macrophages Isolation Protocol
For isolation of liver macrophages, liver tissue specimens obtained from patients with cirrhosis and healthy individuals were processed following a described protocol.( 19 ) Details on the isolation protocol are provided in the Supporting Information. Further experiments with human liver macrophages with RNA extraction, sequencing analysis, principal component analysis, gene set enrichment analysis, gene ontology enrichment analysis, protein network interactions, and flow cytometry analysis are described in detail in the Supporting Information.
Characteristics of the patients included in this experiment also described in detail in the Supporting Information.
Experiments With Human Peripheral Monocytes
Peripheral Monocytes Isolation Protocol
Details on peripheral blood mononuclear cells isolation protocol, flow cytometry analysis, in vitro stimulation of peripheral monocytes and liver macrophages, and ELISA experiments are described in detail in the Supporting Information.
Characteristics of the patients included in this experiment are described in detail in the Supporting Information.
Experimental Mouse Model
For experiments performed in the model of chronic liver injury, mice treated with 3,5‐diethoxycarbonyl‐1,4‐dihydrocollidine (DDC) for 1 or 3 weeks were used. This model has been previously reported to mimic histological and pathophysiological characteristics of chronic liver diseases in humans. All animal studies were approved by the Ethics Committee of the University of Barcelona. Chronic liver injury was induced in 7‐week‐old male C57BL/6 mice (Charles River) by feeding for 1 or 3 weeks with 0.1% DDC diet. An advanced model of chronic liver injury was performed by the chronic administration of carbon tetrachloride (CCl4). C57BL/6 mice were treated with carbon tetrachloride (CCl4) injected intraperitoneally at a dose of 0.5 mL/kg twice a week for a total of 10 weeks. Control mice were injected with corn oil. A more detailed description of these experimental models is represented in Supporting Fig. S1. Description of the anti‐PD‐L1 experiments, mouse liver macrophages isolation protocol, spinning disk intravital microscopy (SD‐IVM) experiment, bacterial dissemination assay, serum biochemical assays, and quantitative PCR analysis are explained in detail in the Supporting Information.
Statistical Methods
Categorical variables were summarized by counts and proportions and continuous variables by mean (standard deviation) or median (interquartile range) depending on the normality of their distribution. Chi‐square tests were used to compare categorical variables, whereas Student t tests or ANOVAs were used to compare normally distributed continuous variables. Wilcoxon test or Kruskal‐Wallis nonparametric ANOVA were used for the rest of the continuous variables.
Results
Liver Cirrhosis Is Characterized by a Reduction in Number and Phagocytic Capacity of Liver Macrophages
To assess the hypothesis that chronic liver diseases are characterized by reduction in number and function of macrophages, we investigated liver macrophages in a well‐established experimental model of chronic liver injury in mice (i.e., DDC model: mice fed with 0.1% DDC for 1 week) (Supporting Fig. S1). The number of liver macrophages (F4/80+ cells) per area of the liver as estimated by intravital microscopy was markedly lower in mice with chronic liver disease compared with control mice (Fig. 1A). We then analyzed the function of liver macrophages by assessing their ability to capture Staphylococcus aureus (S. aureus) from the bloodstream using SD‐IVM. The number of S. aureus caught by macrophages was strikingly lower in livers with chronic liver diseases compared with normal livers (Fig. 1B; Supporting Videos S1 and S2). As shown in Fig. 1C, after S. aureus administration, mice with chronic liver disease had markedly higher bacterial counts in blood compared with control mice. Moreover, S. aureus bacteremia in mice with chronic liver disease was associated with increased mortality compared with control mice (Fig. 1C). In addition, we investigated the phagocytic capacity of liver macrophages in a more advanced liver injury mouse model. For this purpose, we infected mice with advanced liver injury, induced by chronic administration of CCl4, with S. aureus or Escherichia coli (E. coli), and bacterial dissemination was assessed after 24 hours of infection. Consistently, all the organs examined (liver, spleen, lung, and kidney) exhibited higher counts of bacteria in CCl4 mice compared with control (Fig. 1D). Histological characterization of both mouse models used, DDC and CCl4, is shown in Supporting Fig. S1. Because circulating bacteria are principally eliminated by macrophages localized in hepatic sinusoids in homeostasis,( 9 ) our findings indicate that a decreased capture of circulating bacteria by macrophages in an experimental model of chronic liver disease results in increased bacteremia and high mortality rate.
FIG. 1.

Number and function of liver‐resident macrophages in cirrhosis. (A) SD‐IVM image of liver macrophages (F4/80 purple) in control (n = 6) and in 1‐week DDC‐fed mice (n = 6). Graph representing the number of liver macrophages per field of view (FOV) in control and in DDC mice. (B) SD‐IVM image of S. aureus (MW2 overexpressing green fluorescent protein; green) being captured by liver macrophages in control (n = 6) and DDC‐fed (n = 6) mice after 20 minutes of IV infection (5 × 107 colony‐forming units [CFU], IV). Quantification of staphylococcal caught by macrophages in livers of DDC or control mice 15 minutes after infection (5 × 107 CFUs) (n = 5 per group). (C) Staphylococcal bacteremia (CFUs/mL) 30 minutes after IV infection (5 × 107 CFUs) in mice fed with DDC or standard chow (control) (n = 5 per group). Survival curve in a period of 90 hours after infection in DDC and control groups (n = 5 per group). (D) Bacterial dissemination to different organs in mice subjected to an advanced liver injury. Quantification of bacterial dissemination to spleen, lung, kidney, and liver 24 hours after infection within E. coli (107) or staphylococcus (107) in mice receiving IP administration of carbon tetrachloride (CCl4) (0.5 mg/kg) or oil over 10 weeks. (E) Representative immunohistochemistry of CD68 in human liver tissue showing macrophages in liver biopsies from a subject without chronic liver injury (control) and a patient with cirrhosis. Quantification of area occupied by CD68+ cells comparing different groups: left: patients with cirrhosis (n = 37) compared with patients without chronic liver disease (n = 9), and right: patients with cirrhosis from different etiologies: alcohol (n = 21), hepatitis C (n = 10), and NASH (n = 7). (F) single photon emission computed tomography (SPECT) images of axial cuts from a control showing normal distribution of 99mTc‐phytate and from a patient with decompensated cirrhosis displaying abnormal hepatic uptake of 99mTc‐phytate after 20 minutes of administration. Phagocytic capacity of liver macrophages was evaluated by hepatic uptake of 99mTc‐phytate assessed by SPECT and expressed by liver/spleen+bone marrow ratio in patients with cirrhosis (n = 19: 5 compensated and 14 decompensated cirrhosis) and controls (n = 4). (G) SPECT images representing hepatic uptake of 99mTc‐phytate at different time points in a control and a patient with cirrhosis. Time activity curves representing normalized liver and blood activity on m99‐Tcphytate administration in the group of controls, patients with compensated cirrhosis, and patients with decompensated cirrhosis (in blue, red, and gray, respectively). (H) Liver/spleen+bone marrow ratio in patients with cirrhosis, comparing those who presented infections (n = 6) in a 3‐month follow‐up period with those who did not present infections in the follow‐up (n = 13). Data presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. See also Supporting Videos [Link], [Link], [Link]. CH, cirrhosis SPECT‐TAC.
To evaluate whether the hypothesis is also true in human chronic liver disease, we assessed the number and function of liver macrophages in patients with cirrhosis. The population of macrophages was estimated by CD68 immunohistochemistry in histology sections from liver biopsies of patients submitted to a liver biopsy for diagnostic purposes (see the Supporting Information for cohort details). The number of macrophages was estimated by the area occupied by cells stained positive by CD68. The percentage of the total area of the biopsy occupied by macrophages in liver biopsies of patients with cirrhosis (n = 36) was significantly lower compared with that of controls (n = 9) (1.4% [0.9‐1.8] vs. 2.6% [2.5‐4.9], respectively; P < 0.001) (Fig. 1E). There were no significant differences in the area occupied by macrophages when different etiologies of liver cirrhosis were compared (alcohol, hepatitis C infection, or nonalcoholic steatohepatitis) (Fig. 1E). Overall, these findings indicate that human cirrhosis is characterized by a reduced number of liver macrophages that are independent of the factor causing liver injury and are already present at early stages of cirrhosis.
We then assessed whether the impaired phagocytic activity found in the experimental model of chronic liver disease also occurs in human cirrhosis. For this purpose, we used a radio‐labeled colloid (Technetium‐99m [99mTc]‐phytate colloid) to measure the phagocytic capacity of macrophages in the liver and the disappearance rate from plasma using SPECT.( 20 ) In healthy subjects, most of the colloid is captured by macrophages in the liver with only small amounts being taken up by the spleen and bone marrow.( 18 ) Uptake capacity of 99mTc‐phytate by the liver, as assessed by the liver/spleen+bone marrow ratio, was markedly reduced in patients with cirrhosis (n = 19) compared with that of subjects without liver disease (n = 4) (liver/spleen+bone marrow ratio 0.77 vs. 3.41 in patients with cirrhosis vs controls, respectively; P = 0.007) (Fig. 1F) (see the Supporting Information for cohort details). Moreover, patients with decompensated cirrhosis had significantly lower uptake of 99mTc‐phytate colloid compared with patients with compensated cirrhosis (ratio: 0.45 [0.27‐0.59] vs. 1.29 [1.00‐1.55], respectively; P < 0.0001) (Fig 1E). Furthermore, patients with cirrhosis had a reduced plasma disappearance rate of 99mTc‐phytate colloid compared with controls that was more pronounced in patients with more advanced disease (Fig. 1G; Supporting Videos [Link], [Link], [Link], [Link]). We also aimed at investigating if the uptake capacity of 99mTc‐phytate colloid by the liver was influenced by liver perfusion. Because we did not have direct measurements of portal vein flow in the patients of this cohort, we investigated if the presence of portosystemic shunts evaluated by computed tomography (CT) scan or ultrasound could influence the uptake capacity of liver macrophages in the liver. In our cohort, 7 out of 19 patients had portosystemic shunts described in the ultrasound or CT scan. Interestingly, patients with portosystemic shunts presented a trend to lower liver/spleen ratio when compared with patients without portosystemic shunts, although differences were not significant (liver/spleen ratio of 0.38 vs. 0.77 in patients with and without portosystemic shunts, respectively; P = 0.36).
These findings confirm the existence of an impaired phagocytic activity of liver macrophages in cirrhosis compared with control subjects that correlates with disease progression.
To evaluate whether this abnormality was correlated with an increased risk of bacterial infections, we prospectively assessed the frequency of bacterial infections over 3 months. Eight of the 19 patients with cirrhosis had bacterial infections during follow‐up. Liver macrophages’ phagocytic activity was significantly lower in patients who developed infections compared with those who did not (median liver/spleen ratio 0.29 [0.18‐0.63] vs. 0.88 [0.45‐1.23], respectively; P = 0.03) (Fig. 1H). The liver/spleen ratio and history of hospitalizations for decompensations of cirrhosis in the previous 3 months were the only two factors associated with the risk of developing infections at 3 months in the univariate analysis (Supporting Table S1). No significant differences were found between survivors and nonsurvivors at 3 months, although only 2 patients died in the 3‐month follow‐up period. Thus, in an animal model of chronic liver disease and in patients with liver cirrhosis, we observed a decreased number and decreased function of liver macrophages, which appeared to be more marked with disease progression, which is associated with an increased risk of bacterial infections.
Liver Macrophages From Patients With Liver Cirrhosis Display an Immunosuppressive Phenotype
To identify the specific gene expression profile of human macrophages from cirrhotic livers, we performed a whole transcriptome sequencing of macrophages isolated from cirrhotic and control livers.
We included two groups of patients, one being patients with cirrhosis who had undergone liver transplantation (n = 4) and a control group of patients who had undergone surgical resection for liver tumors and in whom healthy liver tissue was available (n = 5) (see the Supporting Information for cohort details). In this latter group of patients, liver tissue for cell isolation was collected from the part of the normal liver obtained in the surgical resection that was more distant to the tumor.
As shown in Fig. 2A, an unsupervised clustering analysis grouped gene expression profile of macrophages from cirrhotic livers and control livers separately, suggesting that macrophages residing in a cirrhotic liver adopt a distinct and unique gene expression profile that is markedly different from that found in livers in the absence of chronic liver disease. Gene ontology enrichment analysis performed on the top 100 genes that are most significantly deregulated in macrophages isolated from cirrhotic livers compared with control livers revealed that the biological processes significantly deregulated in cirrhotic liver were related to innate immune or defense response (Fig. 2B; Supporting Table S1). To further investigate the significant changes at a transcriptional level of macrophages resident in cirrhotic livers compared with a healthy environment, we evaluated whether the expression of key markers of M1 or M2 phenotype were up‐regulated in cirrhotic liver macrophages. We performed a gene set enrichment analysis by using as gene sets the up‐regulated and down‐regulated genes from the comparison of gene expression profiles specific for M1 and M2 phenotypes previously reported (GSE5099; Supporting Table S2). Interestingly, the transcriptome of macrophages from cirrhotic livers was enriched in M1 phenotype compared with M2 phenotype (normalized enrichment score [NES] = 1.683; P < 0.001 vs. NES = −1.174; P < 0.001, for up‐regulated and down‐regulated genes of the comparison of M1 vs. M2 gene expression profiles, respectively) (Fig. 2C; Supporting Table S1). Moreover, a protein‐protein interaction network analysis with the 100 most significant genes from the transcriptome comparison of cirrhotic versus control liver macrophages revealed that nodules within the main network include genes that have been previously described as immunosuppressive genes, including PD‐L1 (CD274), macrophage receptor with collagenous structure (MARCO) (CD206), and CD163, and also M1 markers, including CD80 or CD86 (Fig. 2D). Indeed, immunosuppressive and M1 markers were up‐regulated at the transcriptional level in macrophages isolated from cirrhotic livers compared with those from control livers, suggesting that macrophages resident in cirrhotic livers have a specific proinflammatory and immunosuppressive phenotype (Fig. 2E). To further confirm at the protein level that cirrhotic liver macrophages expressed key associated inmunoexhaustion and M1 markers, we evaluated by FACS the expression of PD‐L1 (CD274), MARCO (CD206), CD163, CD80, and CD86 in a cohort of 8 patients with cirrhosis versus 6 controls. As shown in Fig. 2F, expression of immunosuppressive and M1‐associated markers was found to be increased in macrophages from cirrhotic livers compared with control livers. Overexpression of immunosuppressive genes was also observed in macrophages isolated from mice with chronic liver disease (Supporting Fig. S2).
FIG. 2.
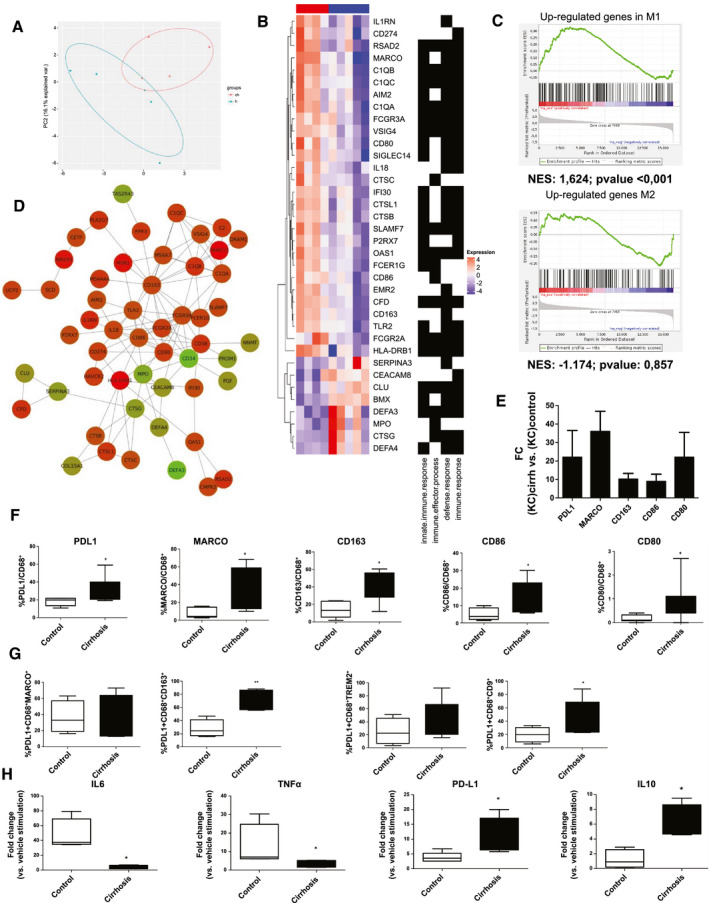
Gene expression profile of human cirrhotic liver macrophages. (A) Principal‐component (PC) analysis representing transcriptomal profile of liver macrophages isolated from cirrhotic (n = 4) and control (n = 5) livers (B) Gene ontology enrichment analysis performed on the top 100 deregulated genes from the transcriptomic comparison of cirrhotic and control liver macrophages. (C) Gene set enrichment analysis of cirrhotic liver macrophages gene expression profile with up‐regulated and down‐regulated genes from the comparison of gene expression profiles specific for M1 and M2 phenotypes (NES). (D) Protein‐protein interaction network from the top 100 significant deregulated genes in the transcriptome of cirrhotic liver macrophages. Network showing protein interactions generated using a database of known predicted interactions (www.string‐db.org). Proteins and networks that were not connected were removed. Red and green nodes indicate that the gene is up‐regulated or down‐regulated, respectively, in cirrhotic compared with healthy liver macrophages. Darker colors show higher regulation. (E) Gene expression comparison of immunosuppression markers in macrophages isolated from cirrhotic and control livers. Gene expression represented as fold change versus control. (F) Quantification of the percentage of immunosuppression markers in macrophages (CD68+) isolated from control (n = 6) and cirrhotic livers (n = 8) assessed by flow cytometry. (G) Percentage of liver‐resident and scar‐associated macrophages subsets expressing PD‐L1 in control and cirrhotic livers. FACS analysis of macrophages subpopulations expressing PD‐L1 in livers isolated from control (n = 4) and patients with cirrhosis (n = 6). The liver‐resident macrophages subset was defined as CD68+CMARCO+ or CD68+CD163+, whereas scar‐associated macrophages were defined as CD68+TREM2+ or CD68+CD9+ populations. (H) Expression of proinflammatory (IL‐6, TNF‐α) and anti‐inflammatory (PDL‐1, IL‐10) cytokines in liver macrophages from patients with cirrhosis (n = 5) as compared with a control group (n = 5) after in vitro stimulation with LPS (100 ng/mL) or vehicle. Gene expression expressed as a fold change versus vehicle. Data presented as mean ± SEM. *P < 0.05; **P < 0.01. AIM, absent in melanoma 2; C1Q, complement 1q subcomponent; CEACAM8, carcinoembryonic antigen‐related cell adhesion molecule 8; CFD, complement factor D; Cirrh, cirrhosis; CLU, clustering; CTSB, cathepsin B; CTSC, cathepsin C; CTSG, cathepsin G; CTSL1, cathepsin L1; DEFA, human α‐defensins; EMR2, EGF‐like module‐containing mucin‐like hormone receptor‐like 2; FCER1G, fc fragment of IgE receptor Ig; FCGR, fcgamma receptors; HLA‐DRB1, HLA class II histocompatibility antigen DRB1 beta chain; IFI30, gamma‐interferon‐inducible lysosomal thiol reductase; IL1RN, interleukin 1 rceptor antagonist; MPO, myeloperoxidase; NES, nestin; OAS1, 2'‐5'‐oligoadenylate synthetase 1; P2RX7, P2X purinoceptor 7; RSAD2, radical s‐adenosyl methionine domain containing 2; SERPINA3, serpin family a member 3; SIGLEC14, sialic acid binding Ig like lectin 14; SLAMF7, SLAM family member 7; TLR2, toll‐like receptor 2; TREM2, triggering receptor expressed on myeloid cells 2; VSIG4, V‐set and immunoglobulin domain containing 4.
Because liver macrophages represent a pool of different cell subpopulations,( 21 ) we aimed to evaluate the expression of PD‐L1 in liver‐resident (MARCO+CD163+) and scar‐associated (TREM2+CD9+) macrophages from cirrhotic and control livers. The scar‐associated macrophage subpopulation was found to be significantly enriched in cirrhosis, whereas no differences were found in the percentage of liver‐resident macrophages in cirrhotic versus control livers (Supporting Fig. S3). Interestingly, we observed that both liver macrophage subpopulations overexpress PD‐L1 in cirrhotic compared with control livers (Fig. 2G). Gating strategies for the FACS analysis are shown in Supporting Fig. S4.
Next, we aimed at investigating other functions of liver macrophages in cirrhosis besides their antibacterial activity. We evaluated the expression of fibrolytic enzymes, their capacity to response to inflammatory stimuli, and their effect on T‐cell polarization. No differences in the expression of fibrolytic enzymes were observed between cirrhotic and control liver macrophages (Supporting Fig. S5). To assess the inflammatory response of cirrhotic macrophages, we in vitro stimulated macrophages isolated from cirrhotic and control livers with a potent inflammatory mediator, such as lipopolysaccharide at high concentrations (100 ng/mL). Liver macrophages isolated from patients with cirrhosis displayed a lower response to lipopolysaccharide (LPS) stimulus, as estimated by a markedly lower expression of proinflammatory cytokine genes, such as TNF‐α and IL‐6, and a higher expression of anti‐inflammatory cytokines, such as PD‐L1 and IL‐10 (Fig. 2H). Moreover, to address whether cirrhotic macrophages exert an effect on adaptative immune response, we assessed the effect of cirrhotic liver macrophages on lymphocyte polarization. Secretome derived from liver macrophages isolated from cirrhotic or control livers was incubated with peripheral T cells. Interestingly, secretome of cirrhotic macrophages induced a significant up‐regulation of forkhead box P3, suggesting that liver macrophages might induce regulatory T‐cell polarization in cirrhosis (Supporting Fig. S5).
Overall, these findings suggest that macrophages from cirrhotic livers exhibit an altered transcriptome profile markedly different from that of control livers, associated with an increased immune exhaustion and inflammation.
Liver Macrophages and Peripheral Monocytes From Patients With Cirrhosis Express PD‐L1 in Response to IFN‐γ
Because liver macrophages from patients with cirrhosis express PD‐L1 at high levels and PD‐L1 has been shown to play an important role in down‐regulating the immune response in many cancers,( 22 ) we then hypothesized that PD‐L1 could play a role in the impaired phagocytic function in cirrhosis. Monocytes from peripheral blood and liver macrophages from patients with cirrhosis were used in the following experiments. As shown in Fig. 3A, the percentage of peripheral monocytes expressing PD‐L1 isolated from the blood of patients with decompensated cirrhosis (n = 50) was higher than that from patients with compensated cirrhosis (n = 12) and healthy subjects (n = 11). In order to determine which subpopulation of monocytes was expressing PD‐L1, we analyzed by FACS the percentage of classical (CD14+CD16−) and nonclassical (CD14+CD16+) monocytes expressing PD‐L1 in peripheral blood of patients with cirrhosis. We observed that the monocyte subset that predominantly expressed PD‐L1 in cirrhosis was the nonclassical subset. Likewise, lymphocytes from patients with decompensated cirrhosis exhibited a higher expression of PD‐1 as compared with lymphocytes from patients with compensated cirrhosis and healthy subjects (Fig. 3A). In patients with decompensated cirrhosis, there was a direct correlation between the percentage of monocytes expressing PD‐L1 and that of lymphocytes expressing PD‐1 (Fig. 3B). Interestingly, the percentage of monocytes expressing PD‐L1 correlated with severity of cirrhosis, as estimated by Model for End‐Stage Liver Disease score, in such a way that patients with more advanced cirrhosis had higher percentages of monocytes expressing PD‐L1 compared with patients with less advanced cirrhosis (Fig. 3C), with no significant differences in the total number of monocytes, lymphocytes, or neutrophils between patients with infections at 3 months and those without infections at 3 months (Supporting Table S4). We further evaluated the PD‐1–PD‐L1 axis by measuring the circulating levels of PD‐L1 in serum from the same population of patients as previously mentioned. PD‐L1 is thought to be released from cells to the circulation, and serum levels are believed to reflect the expression of the system.( 23 ) Patients with decompensated cirrhosis had serum levels of PD‐L1 that were remarkably higher than those of patients with compensated cirrhosis and healthy subjects (Fig. 3D). Moreover, serum levels of PD‐L1 correlated directly with the percentage of cells expressing PD‐L1, which suggests that peripheral monocytes may be a source, at least partly, of increased serum levels of PD‐L1 (Fig. 3D). The percentage of peripheral monocytes expressing PD‐L1 and the serum levels of PD‐L1 were evaluated for their association with the occurrence of bacterial infections within a 3‐month follow‐up period. Patients who developed infections had a significantly greater percentage of monocytes expressing PD‐L1 and higher serum PD‐L1 levels compared with those of patients who did not develop bacterial infections (Fig. 3E).
FIG. 3.

Expression of PD‐L1 in liver macrophages and peripheral monocytes in response to IFN‐γ. (A) Quantification of percentage of peripheral monocytes (CD14+) expressing PD‐L1 and peripheral lymphocytes (CD3+) expressing PD1 as assessed by flow cytometry in patients with compensated (n = 11) and decompensated cirrhosis (n = 47) compared with healthy subjects (n = 11). In addition, percentage of classical (CD14+CD16‐) and nonclassical (CD14+CD16+) monocytes expressing PD‐L1 in patients with cirrhosis (n = 7). (B) Correlation between PD‐L1 expression in monocytes and PD‐1 expression in lymphocytes in peripheral blood mononuclear cells (PBMCs) from the group of patients with decompensated cirrhosis (n = 50). (C) Correlation between percentage of expression of PD‐L1 in peripheral monocytes and Model for End‐Stage Liver Disease score in PBMCs from patients with cirrhosis (n = 62). (D) Serum levels of PD‐L1 in patients with compensated and decompensated cirrhosis as compared with healthy subjects. Correlation between percentage of expression of PD‐L1 in peripheral monocytes and serum levels of PD‐L1 in patients with cirrhosis. (E) Quantification of percentage of peripheral monocytes expressing PD‐L1 in patients with cirrhosis who presented infections in 3 months of follow‐up versus those who did not. Serum levels of PD‐L1 in patients with cirrhosis who presented infections in 3 months of follow‐up versus those who did not. (F) IFN‐γ levels in patients with compensated and decompensated cirrhosis as compared with healthy subjects. (G) Gene expression of PD‐L1 in liver macrophages (n = 7) and peripheral monocytes (n = 6) from patients with cirrhosis after stimulation with IFN‐γ (50 ng/mL) and LPS (100 ng/mL) for 24 hours as assessed by quantitative PCR. Gene expressions levels are expressed as a fold change with respect to vehicle stimulation. Data presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Comp CH, compensated cirrhosis; Decomp CH, decompen sated cirrhosis; FC, fold change.
Because IFN‐γ is a strong inducer of PD‐L1 expression in immune cells and tumor cells,( 24 ) we then assessed serum levels of IFNγ in our patient population and also whether “in vitro” IFN‐γ stimulation increases the expression of PD‐L1 in monocytes and liver macrophages from patients with cirrhosis. Serum levels of IFN‐γ were markedly increased in patients with decompensated cirrhosis compared with those in patients with compensated cirrhosis and in healthy subjects (Fig. 3F). Stimulation of monocytes or liver macrophages of patients with cirrhosis with IFN‐γ caused a marked increase in PD‐L1 expression compared with that caused by LPS, which is also known to stimulate PD‐L1 expression (Fig. 3G). Altogether, these findings suggest a role for chronic inflammation with high IFN‐γ levels in increasing PD‐L1 expression in peripheral monocytes and liver macrophages.
Anti‐PD‐L1 Antibody Treatment Improves Liver Macrophage Function in a Mouse Model of Chronic Liver Injury
Given the high levels of expression of PD‐L1 in immune cells, the increased plasma levels in human cirrhosis, and PD‐L1’s potential association with bacterial infections, we then investigated whether blockade of PD‐L1 with anti‐PD‐L1 antibodies improves liver macrophages function and bacterial clearance in an experimental model of chronic liver injury in mice. An experimental model of chronic liver disease with superimposed infection with mice fed with DDC during 3 weeks and infected with E. coli was used, and 24 hours after infection, the organs were harvested for bacterial count (Supporting Fig. S6). Interestingly, anti‐PD‐L1 antibody administration reduced both bacteremia and bacterial dissemination to extrahepatic organs such as spleen, lung, and kidneys (Fig. 4A). Moreover, anti‐PD‐L1 antibody treatment prevented the impairment in liver injury tests related to the infection, which was characterized by increased aminotransferases, alkaline phosphatase, and bilirubin levels (Fig. 4B).
FIG. 4.
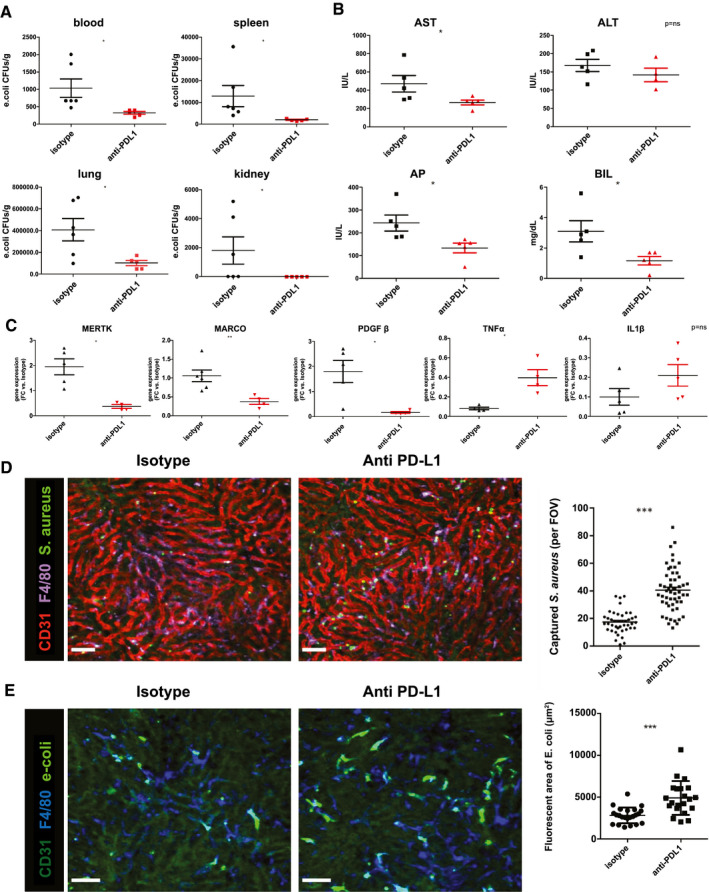
Anti‐PD‐L1 antibody administration induces a therapeutic effect in DDC‐fed mice infected with E. coli. (A) Bacterial load assessed as colony‐forming unit (CFU) levels in blood, spleen, lung, and kidney at 24 hours after PD‐L1 antibody or isotype administration in mice infected with E. coli. (B) Enzymatic biochemical parameters (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [AP], bilirubin [BIL]) of anti‐PD‐L1 and isotype‐treated mice. (C) Gene expression levels of immunosuppression (MERTK, MARCO, platelet‐derived growth factor [PDGF] β) and proinflammatory markers (TNF‐α, IL‐1β) in isolated liver macrophages of DDC‐fed mice treated with anti‐PD‐L1 antibody or isotype. (D) SD‐IVM image of S. aureus (S. aureus: green fluorescent protein) being captured by liver macrophages (F4/80, purple) in DDC‐fed mice treated with anti‐PDL‐1 antibody or isotype 24 hours prior infection. Bacteria quantification performed in 6‐10 liver intravital images within 15 minutes on infection by counting number of captured S. aureus per field of view (FOV). Anti‐CD31 (red) was administrated to better visualize hepatic sinusoid. (E) SD‐IVM image of E. coli (labeled with Syto13) being sequestered by liver macrophages cells (F4/80, blue) in DDC‐fed mice and treated with anti‐PDL‐1 antibody or isotype 24 hours before infection. Captured bacteria quantification was assessed in 6‐10 liver intravital images within 15 minutes on infection by counting total green fluorescent areas stained per FOV. Scale bar = 50 μm. n = 3 mice per group. Gene expression levels represented as fold change versus isotype. Data presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. FC, fold change; IU, international units; MERTK, proto‐oncogene tyrosine‐protein kinase MER; MARCO, macrophage receptor with collagenous structure.
To examine whether PD‐L1 blockade affects KC phenotype, we isolated KC from livers of mice with chronic liver injury that received anti‐PD‐L1 antibody or isotype 24 hours before (Supporting Fig. S6). Interestingly, PD‐L1 blockade was associated with markedly lower expression of immunosuppressive markers such as proto‐oncogene tyrosine‐protein kinase MER (MERTK), MARCO, and platelet‐derived growth factor β and a higher TNF‐α expression compared with that in mice treated with the isotype, findings suggestive of a switch of liver macrophage to a less immune‐exhausted phenotype. IL‐1β expression was not modified by anti‐PD‐L1 treatment. (Fig. 4C).
Finally, we investigated whether anti‐PD‐L1 blockade was associated with an improvement of KC phagocytic activity in vivo in the mice model of chronic liver disease. We evaluated the capacity of liver macrophage to capture either methicillin‐resistant S. aureus or E. coli from circulation in mice treated with anti‐PD‐L1 antibody or isotype. Using SD‐IVM, we examined the in vivo phagocytic ability of macrophages 24 hours after the administration of anti‐PD‐L1 antibody or isotype and 15 minutes after IV injection of bacteria. Anti‐PD‐L1 antibody treatment caused a consistent improvement of the phagocytic capacity of KC compared with isotype in mice with chronic liver injury (Fig. 4D). These results demonstrate that the impaired phagocytic capacity of liver macrophages in a mouse model of chronic liver injury is improved with anti‐PD‐L1 antibody administration.
Discussion
Liver cirrhosis is one of the human diseases that is associated with the highest risk of infections, similar to that of cancer and hematologic diseases.( 6, 7 ) It is currently believed that the main mechanisms of this increased rate of infections are related to gut dysbiosis together with marked impairment of defensive mechanisms, particularly those existing in the liver.( 25 ) Nevertheless, despite the major clinical relevance of infections in human cirrhosis, the function of the liver macrophages in chronic liver diseases has barely been investigated. It has been shown that clearance of intestinal microbes from the systemic circulation is defective in mouse models of liver diseases,( 9 ) but the direct phagocytic function of liver macrophages and the cause of its likely impaired function have not been specifically assessed. Here, using intravital microscopy, we show that the phagocytic function of liver macrophages is markedly impaired in two mouse models of chronic liver disease, a DDC model and a CCl4 model with advanced fibrosis This impairment in phagocytic function is associated with systemic infection, as estimated by increased bacterial counts in several organs. Moreover, we also showed that in patients with cirrhosis, there is reduced phagocytic activity of liver macrophages, as assessed by uptake of Tc‐phytate colloid from the circulation that correlated with the risk of bacterial infections during patient follow‐up, thus linking impaired liver macrophage activity with infections in human disease.
Studies performed in cancer and sepsis indicate that antigen‐presenting cells, including dendritic cells, monocytes, and macrophages, overexpress a number of immunosuppressive antigens that are believed to play a role in the immunosuppression associated with these conditions.( 26, 27, 28 ) One of the systems that has been studied more extensively is the PD‐1/PD‐L1 axis. Expression of PD‐L1 in antigen‐presenting cells is markedly increased by IFN‐γ released in the context of local and systemic inflammation in cancer and sepsis.( 24, 29 ) Stimulation of PD‐1 then causes a number of inhibitory effects on lymphocytes that lead to immunosuppression.( 30 ) On the other hand, PD‐L1 signaling also regulates macrophages proliferation and activation.( 14 )
PD‐L1 is constitutively expressed in liver macrophages from healthy animals, and overexpression of PD‐L1 in macrophages and peripheral monocytes has been shown in experimental models of sepsis and chronic viral infections in the liver.( 31, 32, 33 ) Moreover, antagonism of PD‐L1 is associated with an increased systemic bacterial clearance and improved survival in experimental models of sepsis.( 34, 35 ) However, whether PD‐L1 antagonism specifically improves the phagocytic activity of liver macrophages is not known. The results of the current study demonstrate that chronic liver diseases are associated with a dramatic change in the expression of immunosuppressive genes in liver macrophages. A wide array of immunosuppressive molecules was found to be overexpressed at a gene and protein level in macrophages from human cirrhotic livers obtained from patients submitted to liver transplantation, including PD‐L1, MARCO, and CD163. PD‐L1 overexpression was also found in peripheral monocytes from patients with cirrhosis and correlated with disease severity and PD‐1 expression in peripheral lymphocytes. Moreover, circulating levels of PD‐L1 were increased in patients with cirrhosis, particularly in those with more advanced disease. Interestingly, both serum PD‐L1 levels and PD‐L1 expression in monocytes correlated directly with the risk of infections of these patients during follow‐up, suggesting that serum PD‐L1 levels can be useful as a biomarker that links disease severity and risk of infections in cirrhosis and possibly predicts the risk of infections in patients with cirrhosis, although these findings need to be confirmed in large prospective studies.
Of interest, IFN‐γ caused a marked overexpression of PD‐L1 gene in both liver macrophages as well as peripheral monocytes, and plasma levels of IFN‐γ were increased in patients with advanced cirrhosis, suggesting a role for inflammation in the increased PD‐1/PD‐L1 axis in cirrhosis, as suggested for other conditions.( 24, 36 ) Overall, these findings clearly indicate increased activity of the PD‐1/PD‐L1 axis in liver macrophages and peripheral monocytes in cirrhosis that parallels disease progression and is associated with systemic inflammation.
The clinical experiments of this study have some limitations that should be acknowledged. First, although the total number of patients included in the clinical experiments is relatively high, the number of patients per experiment is limited, and different cohorts of patients were included for each experiment, which could cause some heterogeneity and difficulty for interpretation of results. However, as the experiments were designed as proof‐of‐concept analysis, the number of patients included per experiment was considered sufficient for demonstration of the specific hypotheses.
The most convincing demonstration of the role of the PD‐1/PD‐L1 axis in the impaired phagocytic activity of liver macrophages in chronic liver diseases was derived from experiments assessing the effects of blockade of PD‐L1 in experimental models of chronic liver diseases. PD‐L1 blockade reduced bacteremia and markedly reduced dissemination of bacteria to extrahepatic organs. It is remarkable that this effect occurred without antibiotic treatment. Moreover, the important alterations of liver function that usually occur as a consequence of sepsis were also reduced by PD‐L1 blockade; similar findings have been reported with PD‐L1 blockade in animal models of sepsis.( 34 ) We also evaluated the effects of PD‐L1 blockade on gene expression of mouse liver macrophages and showed a partial reversal of the increased expression of immunosuppressive genes. However, the most interesting result was that anti‐PD‐L1 blockade markedly increased the phagocytic activity of liver macrophage cells, as assessed by intravital microscopy, in an animal model of chronic liver disease. These results suggest that PD‐L1 overexpression in liver macrophages not only exerts a paracrine action on lymphocytes by inhibiting their function but also caused a reduced phagocytic capacity to macrophages itself.
Taken together, all these findings support a major role of the PD‐1/PD‐L1 system as a mechanism of immunosuppression and increased risk of bacterial infections in chronic liver diseases in humans in a way that is similar to that in cancer or sepsis.
The PD‐1/PD‐L1 axis may represent a target to improve the immune system in human cirrhosis, preventing the high morbidity and mortality associated with infections in this condition.
Author Contributions
E.P. participated in the design of this study, performed experiments, and drafted the manuscript; M.C. participated in the conceptual design of the study, supervised the experiments, and critically reviewed the manuscript; C.M.‐S. performed FACS analysis; C.C. and M.V.‐D. participated in bacterial dissemination assays; Z.Z. and B.G.J.S. performed spinning disk intravital experiments; J.J.L. performed bioinformatics analysis; S.A. helped with in vitro assays; A.N.‐B., D.F. and J.P. performed hepatic SPECT scintigraphy to patients and interpreted the images; I.G. recruited patients for the study and contributed to the critical review of the manuscript; É.M. participated in performing experiments; F.L. participated in critically reviewing immunological assays; P.S.‐B. interpreted data and contributed to the critical review of the manuscript; P.K. and P.G. conceived and designed the study, critically reviewed the manuscript and supervised the study.
Supporting information
Video S1
Video S2
Video S3A
Video S3B
Video S3c
Video S3d
Supplementary Material
Acknowledgment
This work was performed in part at the Centre Esther Koplowitz. We thank Pau Bosch for his technical support. We are grateful to the Citomics and Genomic Units of the Institut d’Investigacions Biomèdiques August Pi i Sunyer.
Supported by public grants from Instituto de Salud Carlos III through the PlanEstatal de Investigación Científica y Técnica y de Innovación 2013‐2016 (project reference PI16/00043) and by the European Regional Development Fund (FEDER), Agencia de Gestió d’Ajuts Universitaris I de Recerca (AGAUR) Grant/Award Number: 2017‐SGR 1281. P. G. is a recipient of an ICREA Academia Award. Research of P. G. is also supported by a grant from the EU Horizon 20/20 European program (H20/20 SC1‐2016‐RTD; LIVERHOPE grant number 731875) related to new therapies on chronic diseases (program SC1‐PM‐09‐2016). M. C. and I. G. are funded by a grant from Instituto de Salud Carlos III (FIS PI18/00862); F. L. is funded by Spanish Ministerio de Economía y Competitividad (MINECO; SAF‐2016‐80535‐R and PID2019‐106658RB‐I00) and Agència de Gestiò d’Ajuts Universitaris i de Recerca (AGAUR; 2017/SGR/1582). C. C. and M. V‐D. are funded by the Spanish Ministerio de Economía y Competitividad (MINECO) (BES‐2017‐082107 and BES‐2014‐069237, respectively).
Potential conflict of interest: Dr. Ginès advises and received grants from Grifols, Gilead, and Mallinckrodt. He advises Martin and Novartis.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 2014;60:1310‐1324. [DOI] [PubMed] [Google Scholar]
- 3.Clària J, Arroyo V, Moreau R. The acute‐on‐chronic liver failure syndrome, or when the innate immune system goes astray. J Immunol 2016;197:3755‐3761. [DOI] [PubMed] [Google Scholar]
- 4.Albillos A, Lario M, Álvarez‐Mon M. Cirrhosis‐associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385‐1396. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406‐460. 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, et al. Multidrug‐resistant bacterial infections in patients with decompensated cirrhosis and with acute‐on‐chronic liver failure in Europe. J Hepatol 2018;70:398‐411. 10.1016/j.jhep.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology 2019;156:1368‐1380.e10. [DOI] [PubMed] [Google Scholar]
- 8.Surewaard BG, Deniset JF, Zemp FJ, Amrein M, Otto M, Conly J, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo . J Exp Med 2016;213:1141‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balmer ML, Slack E, De Gottardi A, Lawson MA, Hapfelmeier S, Miele L, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med 2014;6:237ra66. [DOI] [PubMed] [Google Scholar]
- 10.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol 2016;13:316‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heymann F, Tacke F. Immunology in the liver—from homeostasis to disease. Nat Rev Gastroenterol Hepatol 2016;13:88‐110. [DOI] [PubMed] [Google Scholar]
- 12.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor‐associated B7‐H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793‐800. [DOI] [PubMed] [Google Scholar]
- 13.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov 2015;5:915‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartley GP, Chow L, Ammons DT, Wheat WH, Dow SW. Programmed cell death ligand 1 (PD‐L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res 2018;6:1260‐1273. [DOI] [PubMed] [Google Scholar]
- 15.Fallon EA, Biron‐Girard BM, Chung CS, Lomas‐Neira J, Heffernan DS, Monaghan SF, et al. A novel role for coinhibitory receptors/checkpoint proteins in the immunopathology of sepsis. J Leukoc Biol 2018;103:1151‐1164. 10.1002/JLB.2MIR0917-377R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen DS, Mellman I. Elements of cancer immunity and the cancer‐immune set point. Nature 2017;541:321‐330. [DOI] [PubMed] [Google Scholar]
- 17.Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology 2015;148:590‐602.e10. [DOI] [PubMed] [Google Scholar]
- 18.Zuckerman E, Slobodin G, Sabo E, Yeshurun D, Naschitz JE, Groshar D. Quantitative liver‐spleen scan using single photon emission computerized tomography (SPECT) for assessment of hepatic function in cirrhotic patients. J Hepatol 2003;39:326‐332. [DOI] [PubMed] [Google Scholar]
- 19.Perea L, Coll M, Sanjurjo L, Blaya D, Taghdouini AE, Rodrigo‐Torres D, et al. Pentraxin‐3 modulates lipopolysaccharide‐induced inflammatory response and attenuates liver injury. Hepatology 2017;66:953‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groshar D, Slobodin G, Zuckerman E. Quantitation of liver and spleen uptake of (99m)Tc‐phytate colloid using SPECT: detection of liver cirrhosis. J Nucl Med 2002;43:312‐317. [PubMed] [Google Scholar]
- 21.Ramachandran P, Dobie R, Wilson‐Kanamori JR, Dora EF, Henderson BE, Luu NT, et al. Resolving the fibrotic niche of human liver cirrhosis at single‐cell level. Nature 2019;575:512‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou W, Wolchok JD, Chen L. PD‐L1 (B7‐H1) and PD‐1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Y, Sun C, Li J, Hu L, Li M, Liu J, et al. The prognostic significance of soluble programmed death ligand 1 expression in cancers: a systematic review and meta‐analysis. Scand J Immunol 2017;86:361‐367. [DOI] [PubMed] [Google Scholar]
- 24.Garcia‐Diaz A, Shin DS, Moreno BH, Saco J, Escuin‐Ordinas H, Rodriguez GA, et al. Interferon receptor signaling pathways regulating PD‐L1 and PD‐L2 expression. Cell Rep 2017;19:1189‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strnad P, Tacke F, Koch A, Trautwein C. Liver—guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol 2017;14:55‐66. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons Johnson RM, Dong H. Functional expression of programmed death‐ligand 1 (B7‐H1) by immune cells and tumor cells. Front Immunol 2017;8:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour‐associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, et al. Blockade of programmed death‐1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003;170:1257‐1266. [DOI] [PubMed] [Google Scholar]
- 29.Langereis JD, Pickkers P, de Kleijn S, Gerretsen J, de Jonge MI, Kox M. Spleen‐derived IFN‐γ induces generation of PD‐L1+‐suppressive neutrophils during endotoxemia. J Leukoc Biol 2017;102:1401‐1409. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3—potential mechanisms of action. Nat Rev Immunol 2015;15:45‐56. [DOI] [PubMed] [Google Scholar]
- 31.Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, et al. PD‐L1 is induced in hepatocytes by viral infection and by interferon‐α and ‐γ and mediates T cell apoptosis. J. Hepatol 2006;45:520‐528. [DOI] [PubMed] [Google Scholar]
- 32.Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS, et al. Chronically inflamed livers up‐regulate expression of inhibitory B7 family members. Hepatology 2009;50:1625‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline science: defects in immune function in patients with sepsis are associated with PD‐1 or PD‐L1 expression and can be restored by antibodies targeting PD‐1 or PD‐L1. J Leukoc Biol 2016;100:1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu W, Bao R, Fan X, Tao T, Zhu J, Wang J, et al. PD‐L1 blockade attenuated sepsis‐induced liver injury in a mouse cecal ligation and puncture model. Mediators Inflamm 2013;2013:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patil NK, Luan L, Bohannon JK, Hernandez A, Guo Y, Sherwood ER. Frontline science: anti‐PD‐L1 protects against infection with common bacterial pathogens after burn injury. J Leukoc Biol 2018;103:23‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartley G, Regan D, Guth A, Dow S. Regulation of PD‐L1 expression on murine tumor‐associated monocytes and macrophages by locally produced TNF‐α. Cancer Immunol Immunother 2017;66:523‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Video S2
Video S3A
Video S3B
Video S3c
Video S3d
Supplementary Material


