Abstract
Background
Children born with esophageal atresia experience long‐term neurodevelopmental deficits, with unknown origin.
Aims
To find associations between perioperative variables during primary esophageal atresia repair and motor function at age 5 years.
Methods
This ambidirectional cohort study included children born with esophageal atresia who consecutively had been operated on in the Erasmus MC‐Sophia Children's Hospital, University Medical Center, Rotterdam, from January 2007 through June 2013. The perioperative data of this cohort were collected retrospectively; the motor function data prospectively.
Results
After exclusion of patients with syndromal congenital diseases (n = 8) and lost to follow‐up (n = 10), the data of 53 children were included. The mean (SD) total motor function impairment z‐score at 5 years of age was −0.66 (0.99), significantly below normal (p < .001). In multivariable linear regression analysis, number of postoperative days endotracheal intubation (B = −0.211, 95% CI: −0.389 to −0.033, p = .021) was negatively associated with motor outcome, whereas high blood pressure (B = 0.022, 95% CI 0.001 to 0.042, p = .038) was positively associated. Preoperative nasal oxygen supplementation versus room air (B = 0.706, 95% CI: 0.132 to 1.280, p = .016) was positively associated with motor outcome, which we cannot explain.
Conclusions
Motor function in 5‐year‐old esophageal atresia patients was impaired and negatively associated with the number of postoperative days of endotracheal intubation and positively associated with high blood pressure. Prospective studies with critical perioperative monitoring and monitoring during stay at the intensive care unit are recommended.
Keywords: congenital anomalies, esophageal atresia, follow‐up, neurodevelopment, outcome, perioperative monitoring
1. INTRODUCTION
Thanks to improved intraoperative and perioperative intensive care, survival rates of infants born with esophageal atresia nowadays exceed 90%.1 This major congenital malformation, which occurs in 2.43 per 10 000 live births, consists of a discontinuity of the esophagus, in over 90% of cases co‐occurring with a tracheoesophageal fistula.2, 3 Postnatal surgical intervention is needed to enable enteral feeding and to prevent respiratory complications. Primary repair involves closing the tracheoesophageal fistula and constructing an end‐to‐end anastomosis of the esophagus.2, 4 As mortality rates of esophageal atresia have decreased, attention has shifted toward the survivors' long‐term morbidity. Follow‐up studies have mainly focused on physical impairments such as gastroesophageal reflux, pulmonary infections, and dysphagia.1, 5, 6
Neurodevelopmental outcome has been less well studied; the outcomes point at impaired cognitive performance and motor function in children born with esophageal atresia, compared to the normal population.7, 8, 9, 10 A previous study from our institution in children born with esophageal atresia between 1999 and 2006 found that the cumulative duration of anesthesia within the first 24 months was negatively associated with motor outcome in that cohort,8 whereas most other studies did not examine possible causes of impaired neurodevelopment. Various perioperative factors have been suggested to contribute to impaired neurodevelopment, including comorbidities, intraoperative factors (surgical and anesthetic techniques, acidosis, hypercapnia, hypoxia, and anesthetic neurotoxicity), severity of disease, postoperative factors (airway infections, and growth), and fluctuations in children's respiratory, hemodynamic, and metabolic status.11, 12 However, associations of specific perioperative variables, such as blood pressure, heart rate, and blood pH, with long‐term outcome have not been investigated thus far. In a previous study, we showed severe derangement in blood pressure and pH in the operative phase during esophageal atresia surgery, and we hypothesized that these factors might be causative with impaired brain function.13 Therefore, in the present cohort of children born between 2007 and 2013, we evaluated associations between pre‐, intra‐, and postoperative variables with esophageal atresia patients' motor development at age 5 years.
2. METHODS
2.1. Participants
This prospective, single‐center cohort study included consecutive patients operated on for all types of esophageal atresia from January 2007 through June 2013, in a tertiary referral specialized pediatric academic hospital (the Erasmus MC‐Sophia Children's Hospital, the Netherlands). The study was authorized by the Medical Ethics Review Board of Erasmus University Medical Center, Rotterdam, the Netherlands. Written consent was obtained from all participants' parents, and the study complied with the standards set by the Declaration of Helsinki. This manuscript adheres to the applicable STROBE guidelines.
Since 1999, all esophageal atresia patients are offered a standardized follow‐up program with motor function assessments at 5, 8, and 12 years.8 Electronic anesthesia records before 2007 are not available; therefore, inclusion and analysis of patients born before 2007 were not possible.
2.2. Perioperative variables
The present study is an analysis of the perioperative data of esophageal atresia patients born between 2007 and 2017 who underwent primary surgery in the Erasmus MC‐Sophia Children's Hospital, and which results have been published previously.13 Additional perioperative data in the present study were retrieved from the electronic pre‐, intra‐, and postoperative hospital charts. All heart rate and blood pressure measurements had been stored electronically, every second for invasive measurements and every 5 min for noninvasive blood pressure measurements. A specific protocol for the choice of noninvasive or invasive blood pressure measurements during surgery was not available. We preferred to include invasive blood pressures, but if these were not available, we included noninvasive blood pressures. Blood pressure was considered persistently low, or high, on the basis of at least a 5‐min period for invasive blood pressure measurement, or two consecutive measurements with a 5‐min interval for noninvasive blood pressure measurement. Cardiac comorbidities and anomalies at birth were classified as major and minor cardiac heart disease according to the Hoffman criteria.14 Only major cardiac anomalies that were of clinical relevance on the child's cardiac performance and circulation were classified as cardiac abnormalities; minor cardiac anomalies were not included for statistical analysis. The described preoperatively diagnosed pulmonary problems are the problems that caused respiratory problems and had been preoperatively confirmed by X‐ray. The lowest pH‐value of all blood gas measurements during the anesthetic period was included in the analysis, irrespective of the method of sampling (arterial, capillary, and/or venous).15, 16
2.3. Follow‐up data
The follow‐up patient data had been prospectively collected within the framework of our standardized follow‐up program.11 We excluded children who could not be tested with the standardized assessment instrument as well as children with motor impairment as a result of a genetic syndrome or a congenital skeletal malformation (Figure 1).17
FIGURE 1.
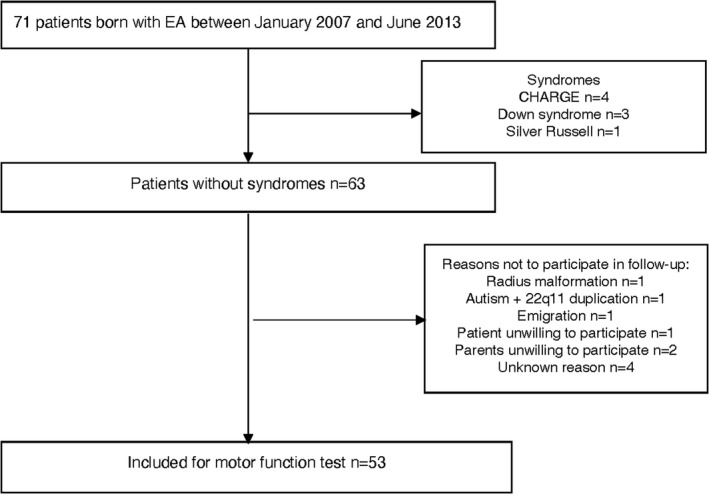
Inclusion flowchart. This is a subgroup of patients reported previously13
As standard of care, all children had been assessed with the Movement Assessment Battery for Children (MABC) band 1 by an experienced pediatric physical therapist.8, 11 The MABC 1 had been used until October 2012 (n = 6), the MABC 2 from November 2012 onwards (n = 47). The content of the two editions is the same, and both have been validated and standardized for healthy Dutch children.18 The outcome scores of the elements of the MABC are combined in the Total Impairment Score, which was compared to age‐related normative scores of Dutch children.
2.4. Statistical analysis
To combine the outcome scores of MABC 1 and 2, percentile scores based on Dutch age‐specific norms were transformed into z‐scores using inverse normal transformations. The z‐scores were compared to the Dutch population mean (z = 0) using the one‐sample t test.18 Normally distributed data are presented as mean (SD); continuous variables as median with interquartile range (IQR).
To investigate whether preoperative and perioperative characteristics were associated with long‐term motor outcome, we defined in advance the 11 variables that we considered clinically most relevant, and included those in the univariable and multivariable regression models. These variables related to three phases:
preoperative phase: weight at time of surgery (kg), major cardiac/pulmonary problem (yes/no), mode of respiration (three categories: spontaneous breathing with room air; spontaneous breathing nasal oxygen supplementation; and preoperative endotracheal intubation);
operative phase: duration of surgery (min), highest intraoperative heart rate during surgery (heart rate), highest and lowest intraoperative mean arterial pressure during surgery (MAP) for a minimum duration of at least 5 min (mmHg), lowest intraoperative pH during surgery and duration of surgery; and
postoperative phase: number of postoperative endotracheal intubation days (days), intensive care unit (ICU) length of stay after surgery (days), and total number of surgeries performed in the first 3 years of life.13
Associations between each variable and motor outcome at 5 years of age were explored using univariable and multivariable linear regression analysis. All above‐mentioned 11 variables were included in the multivariable model. Preoperative nasal oxygen supplementation and preoperative endotracheal intubation were considered categorical values, each with room air as reference value. To account for missing pH values in the operative phase, missing data were imputed using multiple imputation with fully conditional specification.19, 20 Fifty imputed data sets were created, and the results of these 50 data sets were pooled using Rubin's rules.
For the multivariable linear regression analysis, multicollinearity was assessed using variance inflation factors (VIF). VIF levels lower than 3.0 were considered acceptable. The authors had selected the variables which would be included into the statistical analysis before the start of analysis.
All analyses were performed with SPSS Statistics Version 24 (IBM Corporation).
3. RESULTS
3.1. Participants
In total, 71 patients had undergone esophageal atresia repair surgery in the study period, and follow‐up data were available for 53 of them. Follow‐up data were missing for 18 patients because for eight patients, the MABC was not a suitable test to evaluate their motor function because of associated congenital disease (CHARGE, Down and Silver Russell), and for ten patients for other reasons (radius malformation, autism, 22q11 duplication, emigration, and refusal to participate; Figure 1).
3.2. Perioperative phase
Surgery had been performed with either an open approach (n = 15), a thoracoscopic (n = 36) approach, or a thoracoscopic approach converted to an open approach (n = 2). Most patients had esophageal atresia type C (Table 1).4 Twenty‐one patients had received preoperative respiratory support, of whom six had preoperatively been intubated (29%) and fifteen (71%) had received nasal oxygen supplementation (Table 1). Reasons for preoperative intubation are reported in Table S1. Thirty patients had a preoperatively diagnosed cardiac malformation, of which two were major cardiac anomalies that required surgery after the primary esophageal atresia repair.
TABLE 1.
Perioperative patient characteristicsd
| n = 53 | |
|---|---|
| Preoperative variables | |
| Boys n (%) | 33 (62) |
| Gestational age at birth, median [IQR], week | 38.0 [36.4–39.4] |
| Born premature (<37 weeks) | 17 (32) |
| Birth weight, median [IQR], kg | 2.9 [2.3–3.1] |
| Esophageal atresia type A, n (%) | 2 (4) |
| Esophageal atresia type C n (%) | 47 (89) |
| Esophageal atresia type D n (%) | 1 (2) |
| Esophageal atresia type E n (%) | 3 (6) |
| Surgical approach | |
| Open surgical approach n (%) | 15 (28) |
| Thoracoscopic surgical approach n (%) | 36 (68) |
| Converted thoracoscopic to open n (%) | 2 (4) |
| Minor/non hemodynamic cardiac anomaly n (%)a | 33 (62) |
| Major cardiac anomaly n (%)b | 2 (3.8) |
| Preoperative lung problems n (%)c | 21 (40) |
| Atelectasis n (%) | 9 (17) |
| Pneumothorax n (%) | 4 (8) |
| Lung hypoplasia n (%) | 2 (4) |
| IRDS n (%) | 2 (4) |
| Other n (%) | 4 (8) |
| Preoperative respiratory support n (%) | 21 (39) |
| Preoperative endotracheal intubation n (%) | 6 (11) |
| Preoperative oxygen n (%) | 15 (28) |
| Weight at time of surgery, median [IQR], kg | 3.0 [2.4–3.3] |
| Patients from another hospital, n (%) | 6 (11%) |
| Intraoperative variables | |
| Duration of surgery, median [IQR], min | 150 [126–191] |
| Highest heart rate, median [IQR], bpm | 153 [140–165] |
| Highest MAP during surgery, median [IQR], mmHg | 61 [49–72] |
| Lowest MAP during surgery, median [IQR], mmHg | 29 [25–33] |
| Lowest pH, median [IQR], (n = 35) | 7.20 [7.09–7.29] |
| PaO2 [IQR] n = 35 | 12.5 [8.15–17.0] |
| Highest paCO2 [IQR], n = 35 | 8.50 [6.38–12.70] |
| Postoperative variables | |
| Time in ICU, median [IQR], days | 7.0 [3–13] |
| Time in hospital, median [IQR], days | 16.0 [11–32] |
| Postoperative endotracheal intubation, median [IQR], days | 1.0 [1–2] |
| Number of esophageal dilatations, median [IQR] | 0 [0–3] |
| Number of surgeries first 3 years, median [IQR] | 3.0 [2.0–6.0] |
Minor cardiac anomalies/cardiac anomalies without hemodynamic consequences: open foramen ovale (OFO) 7 patients; open ductus Botalli (ODB) 3 patients; OFO + ODB 13 patients; ventricular septum defect (VSD) 5 patients; pulmonary artery stenosis + atrial septum defect type 2 (ASD2) + OFO + right atrial and right ventricular dilatation 1 patient; mitral valve insufficiency + OFO + ODB 1 patient; dextroposition of the heart 1 patient; OFO + ASD2 1 patient; VSD + OFO + ODB 1 patient. Minor cardiac anomalies are not included in the univariable or multivariable linear regression analysis.
Major cardiac anomalies were defined according to Hoffman. One patient with dextroposition of the heart with atrium septum defect (ASD) and open ductus (ODB). One patient with dextroposition of the heart with pulmonary veins ending in the sinus coronaries, ASD, ODB, ventricular septum defect.
Diagnosed with x‐thorax. Others: atelectasis + pneumothorax 1 patient; wet lung 2 patients; hyperinflation of the lung 1 patient.
This is a subgroup of patients reported previously.13
Blood gas was measured in arterial blood (n = 31) or capillary blood (n = 2) during surgery; measurements were missing for 20 patients. Thirty‐five patients had an arterial line for invasive blood pressure measurements; only noninvasive blood pressure measurements were available for 18 patients. The median for the lowest MAP was 29 mmHg (IQR 25–33); the median for the highest MAP was 61 mmHg (IQR 49–72), median lowest pH was 7.20 (IQR 7.09–7.29; Table 1).
The main reasons for prolonged postoperative intubation (n = 5) were respiratory failure and infections, leading to 5–8 days postoperative intubation (Table S2). The median overall duration of intubation was 1 day; the overall length of ICU stay was 7 days (IQR 3–13) (Table 1). Reasons for ICU stay longer than the median 7 days (n = 17) were infections and sepsis, pneumothorax, and esophageal atresia complications such as anastomotic leakage (Table S3). The number of surgeries in the first 3 years of life was median 3 (Table 1).
3.3. Motor outcome
The children's median age at the time of assessment with the MABC test was 5.1 (IQR 5.1–5.2) years. The z‐score for the motor outcome was −0.66 (SD 0.99), which is significantly lower than the score in the age‐related reference population (p<0.001, Figure 2). These scores indicate that 17% of our study population scored below the 5th percentile, which percentage is higher than the expected 5% from the norm scores.
FIGURE 2.
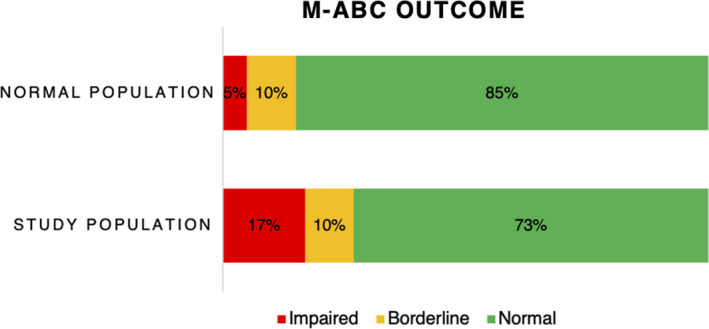
MABC outcome in 5‐year‐old children after esophageal atresia repair in the neonatal phase14 [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. Association of perioperative factors with motor outcome
Univariable analysis showed that the number of postoperative days with endotracheal intubation (B = −0.198, 95% CI: −0.356 to −0.039), ICU length of stay from surgery (days) (B = −0.018, 95% CI: −0.032 to −0.005) and the total number of surgeries in the first 3 years of life (B = −0.117, 95%CI −0.180 to −0.055) were negatively associated with motor outcome. Preoperative nasal oxygen supplementation, compared to room air, was positively associated with motor outcome (B = 0.824, 95% CI: 0.260 to 1.389); (Table 2).
TABLE 2.
Predictive factors (univariable and multivariable linear regression model)
| Variable | Univariable linear regression analysis | Multivariable linear regression analysis | ||
|---|---|---|---|---|
| Estimated coefficient (95% CI) n = 53 | p‐value | Estimated coefficient (95% CI) n = 53 | p‐value | |
| Weight at surgery (kilogram) | −0.082 (0.421 to 0.258) | .631 | 0.002 (−0.344 to 0.348) | .991 |
| Mode of ventilation | .019 | — | ||
| Room air | Reference | Reference | ||
| Preoperative nasal oxygen supplementation (yes/no) | 0.738 (0.221 to 1.254) | .006 | 0.706 (0.132 to 1.280) | .016 |
| Preoperative endotracheal intubation (yes/no) | 0.489 (−0.369 to 1.347) | .257 | 0.553 (−0.384 to 1.489) | .247 |
| Major cardiac/Pulmonary problem (yes/no)a | −0.155 (−0.705 to 0.396) | .576 | −0.070 (−0.614 to 0.473) | .799 |
| Duration of surgery (min) | 0.000 (−0.004 to 0.004) | .924 | −0.002 (−0.008 to 0.003) | .432 |
| Highest heart rate (frequency) | 0.006 (−0.010 to 0.022) | .467 | 0.009 (−0.007 to 0.025) | .263 |
| Highest MAP (mmHg) | 0.012 (−0.008 to 0.033) | .229 | 0.019 (−0.003 to 0.040) | .091 |
| Lowest MAP (mmHg) | 0.003 (−0.042 to 0.047) | .909 | −0.008 (−0.051 to 0.035) | .706 |
| Intraoperative lowest pH | −1.784 (−4.337 to 0.768) | .164 | −0.317 (−2.433 to 1.799) | .769 |
| Postoperative days endotracheal intubation (days) | −0.198 (−0.356 to −0.039) | .015 | −0.173 (−0.361 to 0.015) | .071 |
| ICU LOS from surgery (days) | −0.018 (−0.032 to −0.005) | .01 | −0.004 (−0.022 to 0.014) | .645 |
| Number of surgeries first 3 years | −0.117 (−0.180 to −0.055) | <.001 | −0.075 (−0.161 to 0.011) | .089 |
Abbreviations: CI, confidence interval; LOS, length of stay.
Only major cardiac anomalies were included. The described preoperatively diagnosed pulmonary problems are the causes of respiratory problems and had been preoperatively confirmed by X‐ray.
The multivariable linear regression analysis indicated that the number of postoperative days endotracheal intubation (B = −0.211, 95% CI: −0.389 to −0.033) was negatively associated with motor outcome. A persistently high blood pressure (B = 0.022, 95% CI 0.001 to 0.042) was positively associated with motor outcome. Preoperative nasal oxygen supplementation compared to room air (B = 0.706, 95% CI: 0.132 to 1.280) was positively associated with long‐term motor outcome (Table 2). The multivariable analyses did not show signs of multicollinearity (all VIFs < 3.0).
4. DISCUSSION
This study analyzes associations between perioperative variables and long‐term motor outcome of esophageal atresia patients at age 5 years, and found that motor outcome at this age was negatively associated with the duration of endotracheal intubation postoperatively, and positively associated with an episode of high blood pressure. Preoperative nasal oxygen supplementation was found to be positively associated with motor outcome compared to room air. Intraoperative factors such as heart rate, low blood pressure, and pH were not associated with motor outcome at age 5 years.
There is an ongoing discussion about the possible effects of perioperative surgical and anesthesiologic events on the development of children's brain in the long term.21 This study did not find associations between intraoperative variables and long‐term motor outcome; still, various pre‐ and postoperative variables were associated with long‐term outcome.
Up to now, few studies have investigated the long‐term neurodevelopmental outcome of children born with esophageal atresia. Most of these considered somatic, medical, and psychosocial development (respiratory performance, tracheomalacia, and mental health).5, 22, 23 As far as we know, associations between intraoperative variables and long‐term motor outcome have not yet been studied. Our group previously reported normal cognitive performance and impaired psychomotor outcome in children with esophageal atresia born in 1999–2003 and assessed at ages 6–24 months.11 In a cohort born between 1999 and 2006, we found significantly decreased z‐scores on motor performance at ages 5 and 8 years.8 Duration of anesthesia within the first 24 months of life of patients in that cohort born with esophageal atresia between 1999 and 2003 was negatively associated with motor function at the age of 8 years.8 The cohort in the present study, born between 2007 and 2013, showed impaired motor outcome at 5 years of age, similar to the previous cohort born between 1999 and 2006 (z‐score MABC −0.66 vs −0.75).8 The findings indicate that 17% of our study population scored below the 5th percentile. A score ≤ p5 on the MABC is indicative of serious motor problems, and is an indication for early intervention, such as pediatric physical therapy or targeted advice for parents and other caregivers. A lower motor performance score has impact on different facets in the child's life. Sufficient motor skills provide children with the opportunity to interact with their social environment and their physical surroundings. If motor skills are impaired, a child is likely to have impaired social functioning, resulting in difficulty creating relations with peers and, additionally, academic performance could be negatively affected.24, 25
As reported previously, several severe perioperative metabolic derangements may occur during primary esophageal atresia repair (Table 1).13, 26, 27
Hypercapnia (PaCO2 > 6.4 kPa) has proven relevant to the long‐term neurodevelopmental outcome because it can affect the cerebral metabolism and may cause apoptosis in the neonatal brain.28, 29, 30 In a previous study, we found that the majority of esophageal atresia patients had hypercapnia during the surgical procedure: median 7.6 kPa (IQR 5.8–9.3 kPa).13 High PaCO2 levels lead to acidosis, which may affect children's neurologic development.31 However, in the present study, we did not find a significant association of intraoperative pH with long‐term motor outcome in the univariable regression analysis. Since the missing pH data mainly concerned the years 2007 and 2008, we may reasonably assume that these data are missing at random.20, 32 Associations were neither found after imputing missing pH values.
Furthermore, hemodynamic instability could potentially lead to cerebral hypoxia, which might be associated with impaired outcome.33, 34 The present results show that the lowest blood pressure and highest heart rate were not associated with impaired motor outcome, whereas the highest blood pressure was associated with better long‐term outcome. The median highest and lowest MAP in our study is 61 and 29 mmHg, respectively, which values fall within the +2SD and −2SD margin for blood pressure in neonates; still, value for some patients were above and below these thresholds.35 The highest MAP was not significantly different between patients with and without supplementation of vasopressors.
The high number of surgeries suggests that these patients are suffering critical illness or had complications or concomitant (congenital) diseases. The high numbers of surgeries for the patients in the present study led to many hospital admissions, repeated anesthesia exposure, and more complications. The univariable analysis of this study showed a negative association between the number of surgeries performed within 3 years after esophageal atresia repair and the motor outcome at age 5 years. We did not find an association between the motor outcome and the duration of the primary esophageal atresia repair. In a previous study, the total anesthesia time (of repeated anesthesia) before the age of 3 years was negatively associated with long‐term outcome.36 Another study found an association between the number of surgeries in the first year of life and the motor function at age 12 months.37
As we did not find an association between the duration of the primary surgery and the motor outcome at age 5 years, we hypothesize that impaired motor function might be related to repeated anesthesia rather than to one lengthy anesthesia exposure for primary esophageal atresia repair.8, 36, 38 The association between number of surgeries in the first 3 years of life and motor function in the univariate regression analysis might indicate that the surgeries, but also the hospital stays as such, may negatively affect the long‐term neurodevelopment.
The univariable regression and multivariable regression analyses showed a positive association of preoperative nasal oxygen supplementation, compared to room air, with motor outcome at age 5 years. As this was unexpected and we could not explain it, we had a closer look at the data and found a few high outliers on the MABC score in the patients who had received preoperative nasal oxygen supplementation (Figure S1). Due to the retrospective nature of this study, we could not identify why nasal oxygen supplementation had been started in these patients. In our hospital, there are no protocols for the use of nasal oxygen supplementation in this patient population prior to surgery. Therefore, it is highly dependent on the health professional whether a patient receives nasal oxygen supplementation or not. The potential role of preoperative nasal oxygen suppletion should be further investigated.
Furthermore, preoperatively endotracheally intubated patients in this study had been primarily intubated in a less well‐specialized center without prenatal diagnosis of the esophageal atresia. Previous reports have shown the benefits of in‐born versus out‐born neonates for the management of complex neonatal conditions.39, 40
The results of the present and other studies37, 41 show an association between the duration of endotracheal intubation postoperatively and long‐term cognitive and motor outcomes after surgery for noncardiac malformations. Prolonged postoperative intubation might be required on account of comorbidities and other factors that might be associated with impaired long‐term motor outcome.
Due to the retrospective nature of the data collection, only few perioperative variables could be included for analysis. Therefore, we predefined those variables which we considered most clinically relevant for the long‐term outcome and could be retrieved from the patient files: duration of surgery, highest heart rate, highest and lowest MAP, and lowest pH. We did not find significant associations between each of those variables and motor outcome. This might indicate that we are overlooking potential critical perioperative parameters. Thus, protocolized treatment of esophageal atresia patients and prospective registration of perioperative events are recommended for future studies.
Unfortunately, the number of variables to be analyzed was hampered by the limited number of patients since we are working with a rare congenital anomaly, leading to a small number of patients. Therefore, we chose 11 variables to be included in the univariable and multivariable regression models. We may have missed other variables possibly associated with long‐term motor outcome, such as surgical approach, sex, gestational age, SpO2, prematurity, and open or thoracoscopic surgical approach. Due to the limited sample size and the relatively large number of potentially relevant predictors, we preferred to prespecify our statistical analysis plan as much as possible. Adding more independent variables in a post hoc analysis would have been problematic due to the risk of overfitting. Furthermore, many other confounders may affect outcome but have not been incorporated in the present analyses. It might have been that many other physiologic insults and major life events in their childhood may affect outcome. Yet, a previous study on the perioperative management of esophageal atresia/tracheoesophageal fistula did not find any differences in perioperative variables between open, thoracoscopic, and converted thoracoscopic to open surgical approach.13 Research on long‐term motor outcome of extremely premature children shows impaired motor function on the long‐term.42 As the number of extremely premature children in the present cohort was one (32 weeks of gestation), we decided to include weight at birth in the regression analysis, as a proxy of many other risk factors including prematurity and dysmaturity.
Our data do not allow for speculations regarding a change in treatment protocols but emphasize the need for re‐evaluation of treatment protocols and more advanced perioperative monitoring. Prospective registration of perioperative data and prolonged follow‐up may be useful to detect and reduce long‐term motor function impairment, since early interventions could be advantageous for this population.
5. CONCLUSIONS
In conclusion, the present study shows that the included children born with esophageal atresia have more motor problems than is to be expected from norm values. Long‐term motor outcome at 5 years of age was negatively associated with the duration of endotracheal intubation postoperatively, and positively associated with high blood pressure. We cannot explain the positive association we found with preoperative nasal oxygen supplementation as compared to room air. We found associations between perioperative data and long‐term outcome in patients with esophageal atresia, in which specific intraoperative variables were not found to be associated with long‐term motor outcome. Prospective, detailed perioperative monitoring to prove the effects on long‐term neurodevelopmental outcome should be our goal.
CONFLICT OF INTEREST
Dr Jurgen de Graaff is a member of the editorial board at Pediatric Anesthesia.
Supporting information
Figure S1
Table S1‐S3
ACKNOWLEDGEMENTS
We thank Ko Hagoort of the department of pediatric surgery at the Erasmus MC‐Sophia Children's Hospital University Medical Center, Rotterdam, The Netherlands for editorial assistance.
van Hoorn CE, van der Cammen‐van Zijp MHM, Stolker RJ, van Rosmalen J, Wijnen RMH, de Graaff JC. Associations of perioperative characteristics with motor function in preschool children born with esophageal atresia. Pediatr Anesth. 2021;31:854–862. 10.1111/pan.14204
Funding information
What is already known about the topic?
- Children born with esophageal atresia suffer long‐term neurodevelopmental impairments, of unknown origin.
What new information does this study add?
- Motor function of children born with esophageal atresia is impaired at 5 years.
- Motor outcome of children born with esophageal atresia is negatively associated with the number of postoperative days of endotracheal intubation.
- Motor function in these children is positively associated with a high blood pressure.
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Sulkowski JP, Cooper JN, Lopez JJ, et al. Morbidity and mortality in patients with esophageal atresia. Surgery. 2014;156(2):483‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spitz L. Oesophageal atresia. Orphanet J Rare Dis. 2007;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen RN, Calzolari E, Husby S, Garne E; EUROCAT Working Group . Oesophageal atresia: prevalence, prenatal diagnosis and associated anomalies in 23 European regions. Arch Dis Child. 2012;97(3):227‐232. [DOI] [PubMed] [Google Scholar]
- 4.Gross RE. The Surgery of Infancy and Childhood: Its Principles and Techniques. Philadelphia: Saunders; 1953. [Google Scholar]
- 5.Kovesi T. Long‐term respiratory complications of congenital esophageal atresia with or without tracheoesophageal fistula: an update. Dis Esophagus. 2013;26(4):413‐416. [DOI] [PubMed] [Google Scholar]
- 6.Toussaint‐Duyster LCC, van der Cammen‐van Zijp MHM, Spoel M, et al. Determinants of exercise capacity in school‐aged esophageal atresia patients. Pediatr Pulmonol. 2017;52(9):1198‐1205. [DOI] [PubMed] [Google Scholar]
- 7.Deurloo JA, Ekkelkamp S, Hartman EE, Sprangers MA, Aronson DC. Quality of life in adult survivors of correction of esophageal atresia. Arch Surg. 2005;140(10):976‐980. [DOI] [PubMed] [Google Scholar]
- 8.Harmsen WJ, Aarsen FJ, van der Cammen‐van Zijp MH, et al. Developmental problems in patients with oesophageal atresia: a longitudinal follow‐up study. Arch Dis Child Fetal Neonatal Ed. 2017;102(3):F214–f9. [DOI] [PubMed] [Google Scholar]
- 9.IJsselstijn H, Gischler SJ, Toussaint L, et al. Growth and development after oesophageal atresia surgery: need for long‐term multidisciplinary follow‐up. Paediatr Respir Rev. 2016;19:34‐38. [DOI] [PubMed] [Google Scholar]
- 10.van der Cammen‐van Zijp MH, Gischler SJ, Mazer P, van Dijk M, Tibboel D, Ijsselstijn H. Motor‐function and exercise capacity in children with major anatomical congenital anomalies: an evaluation at 5 years of age. Early Hum Dev. 2010;86(8):523‐528. [DOI] [PubMed] [Google Scholar]
- 11.Gischler SJ, Mazer P, Duivenvoorden HJ, et al. Interdisciplinary structural follow‐up of surgical newborns: a prospective evaluation. J Pediatr Surg. 2009;44(7):1382‐1389. [DOI] [PubMed] [Google Scholar]
- 12.Sistonen SJ, Pakarinen MP, Rintala RJ. Long‐term results of esophageal atresia: Helsinki experience and review of literature. Pediatr Surg Int. 2011;27(11):1141‐1149. [DOI] [PubMed] [Google Scholar]
- 13.van Hoorn CE, Costerus SA, Lau J, et al. Perioperative management of esophageal atresia/tracheo‐esophageal fistula: an analysis of data of 101 consecutive patients. Paediatr Anaesth. 2019;29(10):1024‐1032. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890‐1900. [DOI] [PubMed] [Google Scholar]
- 15.Higgins C. Capillary blood gases: to arterialize or not. MLO Med Lab Obs. 2008;40(11):42, 44‐47. [PubMed] [Google Scholar]
- 16.Zavorsky GS, Cao J, Mayo NE, Gabbay R, Murias JM. Arterial versus capillary blood gases: a meta‐analysis. Respir Physiol Neurobiol. 2007;155(3):268‐279. [DOI] [PubMed] [Google Scholar]
- 17.Dammeyer J. Development and characteristics of children with Usher syndrome and CHARGE syndrome. Int J Pediatr Otorhinolaryngol. 2012;76(9):1292‐1296. [DOI] [PubMed] [Google Scholar]
- 18.Smits‐Engelsman B. Dutch Manual Movement Assessment Battery for Children. Lisse, The Netherlands: Swets en Zeitlinger; 1998. [Google Scholar]
- 19.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087‐1091. [DOI] [PubMed] [Google Scholar]
- 20.Groenwold RH, Donders AR, Roes KC, Harrell FE Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol. 2012;175(3):210‐217. [DOI] [PubMed] [Google Scholar]
- 21.McCann ME, Soriano SG. Does general anesthesia affect neurodevelopment in infants and children? BMJ. 2019;367:l6459. [DOI] [PubMed] [Google Scholar]
- 22.Dingemann C, Meyer A, Kircher G, et al. Long‐term health‐related quality of life after complex and/or complicated esophageal atresia in adults and children registered in a German patient support group. J Pediatr Surg. 2014;49(4):631‐638. [DOI] [PubMed] [Google Scholar]
- 23.Faugli A, Bjornland K, Emblem R, Novik TS, Diseth TH. Mental health and psychosocial functioning in adolescents with esophageal atresia. J Pediatr Surg. 2009;44(4):729‐737. [DOI] [PubMed] [Google Scholar]
- 24.Leonard HC. The impact of poor motor skills on perceptual, social and cognitive development: the case of developmental coordination disorder. Front Psychol. 2016;7:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt M, Egger F, Benzing V, et al. Disentangling the relationship between children's motor ability, executive function and academic achievement. PLoS One. 2017;12(8):e0182845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishay M, Giacomello L, Retrosi G, et al. Hypercapnia and acidosis during open and thoracoscopic repair of congenital diaphragmatic hernia and esophageal atresia: results of a pilot randomized controlled trial. Ann Surg. 2013;258(6):895‐900. [DOI] [PubMed] [Google Scholar]
- 27.Pierro A. Hypercapnia and acidosis during the thoracoscopic repair of oesophageal atresia and congenital diaphragmatic hernia. J Pediatr Surg. 2015;50(2):247‐249. [DOI] [PubMed] [Google Scholar]
- 28.Fritz KI, Zubrow A, Mishra OP, Delivoria‐Papadopoulos M. Hypercapnia‐induced modifications of neuronal function in the cerebral cortex of newborn piglets. Pediatr Res. 2005;57(2):299‐304. [DOI] [PubMed] [Google Scholar]
- 29.Zhou W, Liu W. Hypercapnia and hypocapnia in neonates. World J Pediatr. 2008;4(3):192‐196. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser JR, Gauss CH, Williams DK. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr Res. 2005;58(5):931‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giffard RG, Monyer H, Christine CW, Choi DW. Acidosis reduces NMDA receptor activation, glutamate neurotoxicity, and oxygen‐glucose deprivation neuronal injury in cortical cultures. Brain Res. 1990;506(2):339‐342. [DOI] [PubMed] [Google Scholar]
- 32.Janssen KJ, Donders AR, Harrell FE Jr, et al. Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol. 2010;63(7):721‐727. [DOI] [PubMed] [Google Scholar]
- 33.McCann ME, Schouten AN. Beyond survival; influences of blood pressure, cerebral perfusion and anesthesia on neurodevelopment. Paediatr Anaesth. 2014;24(1):68‐73. [DOI] [PubMed] [Google Scholar]
- 34.McCann ME, Schouten AN, Dobija N, et al. Infantile postoperative encephalopathy: perioperative factors as a cause for concern. Pediatrics. 2014;133(3):e751‐757. [DOI] [PubMed] [Google Scholar]
- 35.de Graaff JC, Pasma W, van Buuren S, et al. Reference values for noninvasive blood pressure in children during anesthesia: a multicentered retrospective observational cohort study. Anesthesiology. 2016;125(5):904‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaccariello MJ, Frank RD, Lee M, et al. Patterns of neuropsychological changes after general anaesthesia in young children: secondary analysis of the Mayo Anesthesia Safety in Kids study. Br J Anaesth. 2019;122(5):671‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bevilacqua F, Rava L, Valfre L, et al. Factors affecting short‐term neurodevelopmental outcome in children operated on for major congenital anomalies. J Pediatr Surg. 2015;50(7):1125‐1129. [DOI] [PubMed] [Google Scholar]
- 38.Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi‐institutional study. Crit Care Med. 2000;28(4):1173‐1179. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki Y, Ishikawa K, Yokoi A, et al. Short‐ and long‐term outcomes of extremely preterm infants in Japan according to outborn/inborn birth status*. Pediatr Crit Care Med. 2019;20(10):963‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amer R, Moddemann D, Seshia M, et al. Neurodevelopmental outcomes of infants born at <29 weeks of gestation admitted to Canadian Neonatal Intensive Care units based on location of birth. J Pediatr. 2018;196:31‐37.e31. [DOI] [PubMed] [Google Scholar]
- 41.Peters RT, Ragab H, Columb MO, Bruce J, MacKinnon RJ, Craigie RJ. Mortality and morbidity in oesophageal atresia. Pediatr Surg Int. 2017;33(9):989‐994. [DOI] [PubMed] [Google Scholar]
- 42.Burnett AC, Anderson PJ, Joseph RM, et al. Hand preference and cognitive, motor, and behavioral functioning in 10‐year‐old extremely preterm children. J Pediatr. 2018;195:279‐282.e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1‐S3
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


