Abstract
Objective
We aimed to characterize the clinical profile and outcomes of new onset refractory status epilepticus (NORSE) in children, and investigated the relationship between fever onset and status epilepticus (SE).
Methods
Patients with refractory SE (RSE) between June 1, 2011 and October 1, 2016 were prospectively enrolled in the pSERG (Pediatric Status Epilepticus Research Group) cohort. Cases meeting the definition of NORSE were classified as "NORSE of known etiology" or "NORSE of unknown etiology." Subgroup analysis of NORSE of unknown etiology was completed based on the presence and time of fever occurrence relative to RSE onset: fever at onset (≤24 h), previous fever (2 weeks–24 h), and without fever.
Results
Of 279 patients with RSE, 46 patients met the criteria for NORSE. The median age was 2.4 years, and 25 (54%) were female. Forty (87%) patients had NORSE of unknown etiology. Nineteen (48%) presented with fever at SE onset, 16 (40%) had a previous fever, and five (12%) had no fever. The patients with preceding fever had more prolonged SE and worse outcomes, and 25% recovered baseline neurological function. The patients with fever at onset were younger and had shorter SE episodes, and 89% recovered baseline function.
Significance
Among pediatric patients with RSE, 16% met diagnostic criteria for NORSE, including the subcategory of febrile infection‐related epilepsy syndrome (FIRES). Pediatric NORSE cases may also overlap with refractory febrile SE (FSE). FIRES occurs more frequently in older children, the course is usually prolonged, and outcomes are worse, as compared to refractory FSE. Fever occurring more than 24 h before the onset of seizures differentiates a subgroup of NORSE patients with distinctive clinical characteristics and worse outcomes.
Keywords: clinical neurology, epilepsy, febrile infection‐related epilepsy syndrome, new onset refractory status epilepticus, pediatric, refractory status epilepticus, status epilepticus
Key Points.
We describe clinical profiles of children with NORSE, FIRES, and refractory FSE based on current NORSE definitions
Timing of fever onset may help identify separate subgroups presenting with different SE severity and outcomes
SE duration and age at onset are relevant distinctive features between FIRES and refractory FSE, which may contribute to further improvement of the current NORSE and FIRES definitions
1. INTRODUCTION
New onset refractory status epilepticus (NORSE) is a life‐threatening condition characterized by the acute onset of refractory status epilepticus (RSE) in previously healthy patients. In adults, seizures often progress to prolonged RSE, with mortality rates up to 20%. Chronic, intractable seizures with neurologic sequelae occur in 60% of these patients.1, 2, 3 In one third of patients, a mild febrile illness precedes the onset of status epilepticus (SE). The underlying etiology is identified in nearly half of the patients, and autoimmune encephalitis is the most frequently identified cause.1, 4, 5 A similar syndrome occurs in younger patients, termed febrile infection‐related epilepsy syndrome (FIRES),2 that presents without a clear etiology6, 7 and with high morbidity and mortality.6, 8, 9, 10, 11 The overall mortality rate in children with superrefractory SE including FIRES is approximately 12%,6, 12 with up to two thirds of the survivors developing cognitive impairment and functional disability.2, 8, 9, 10, 11, 13 Pediatric patients with RSE have a lower mortality rate than adult patients.14 Based on the updated ILAE definitions for research, FIRES is a subset of NORSE; specifically, RSE is preceded by a febrile illness between 2 weeks and 24 h before SE onset.2 These definitions aim to provide a common framework for clinical research, as prior descriptions of NORSE and FIRES were based on variable inclusion criteria and terms.
In this article, we aimed to evaluate a cohort of children with RSE meeting criteria for NORSE and FIRES and to assess the effect of fever occurrence on the outcome. To date, outcomes, in particular in the setting of ILAE diagnostic criteria for NORSE and FIRES, have not been well described in detail in larger pediatric populations. We, therefore, aimed to address this gap in the literature by characterizing the clinical presentation and outcomes of children with NORSE and FIRES in a prospectively collected RSE cohort. Children with refractory febrile SE (FSE) were included here, as patients with FSE may meet current criteria for NORSE.
2. MATERIALS AND METHODS
2.1. Study design
Data on a subset of RSE patients were prospectively obtained from 11 centers in the United States within the Pediatric Status Epilepticus Research Group (pSERG).15 This study was approved by the institutional review board at each institution. Written informed consent was obtained from the parents or guardians of each patient.
2.2. Inclusion and exclusion criteria
Inclusion criteria were (1) age from 1 month to 21 years; (2) admission to a pSERG institution between June 1, 2011 and October 1, 2016; and (3) focal or generalized convulsive seizures at the onset that continued after administration of at least two antiseizure medications (ASMs), including at least one nonbenzodiazepine (non‐BZD) ASM, or the use of anesthetics in continuous infusion. Exclusion criteria were (1) nonconvulsive SE (NCSE) detected on electroencephalogram (EEG) lacking convulsive seizure at onset, (2) NCSE with motor manifestations limited to infrequent myoclonic jerks, (3) history of epilepsy, and (4) readily identifiable cause of SE easily detected by routine diagnostic tests (e.g., stroke, brain lesion, acute medical condition, bacterial meningitis).2 If more than one episode of RSE occurred during the study period, only the first was included.
2.3. Definitions
NORSE: “A clinical presentation, not a specific diagnosis, in a patient without active epilepsy or other preexisting relevant neurological disorder, with new onset of refractory status epilepticus, without a clear acute or active structural, toxic or metabolic cause. The term 'NORSE of unknown etiology' applies to patients with the clinical presentation of NORSE, but in whom the cause remains unknown after extensive workup (which may take more than 72 h to complete).”2
FIRES: “A subcategory of NORSE, applicable to all ages, that requires a prior febrile infection starting between 2 weeks and 24 h prior to onset of refractory status epilepticus, with or without fever at onset of status epilepticus.”2
FSE: “Status epilepticus that also meets the definition of febrile seizure (a provoked seizure where the sole acute provocation is fever without prior history of afebrile seizures and with no evidence of an acute central nervous system [CNS] infection or insult16) in children who have onset of fever <24 h prior to onset of seizures or whose fever is recognized only after the onset of seizures.”2
RSE: “Status epilepticus persisting despite adequate administration of BZDs and at least one non‐BZD ASM.”17
2.4. Clinical and outcome variables
We acquired information from the pSERG database and extracted additional variables for patients with NORSE by chart review. The prospective pSERG data acquisition tool included demographics and clinical information such as prodromal history, medical history, family history, type of SE, time of SE onset, SE duration, laboratory, EEG, and magnetic resonance imaging (MRI) findings, treatments, and complications. We acquired information on fever (temperature ≥ 38°C), recurrence of seizures, and cognitive function at the last follow‐up. Any symptom preceding SE onset by between 24 h and 15 days was considered a prodromal symptom. We determined etiology based on available test results performed by the individual centers. Management and treatment followed best medical practices.
We classified patients into two main categories: "NORSE of known etiology" (genetic, autoimmune, CNS infections) and "NORSE of unknown etiology." The etiology of the first group was not readily identifiable based on NORSE criteria, but the diagnosis was made after extensive evaluations (infectious, genetic, and autoimmune analyses). We categorized NORSE cases of unknown etiology further into three subgroups based on the presence and timing of fever occurrence: (1) previous fever: the fever started more than 24 h and less than 15 days before SE onset (and may have been present or absent at onset of seizures), (2) fever at onset: the fever started within 24 h of SE onset, and (3) no fever. We described the two main NORSE categories but only performed statistical analysis on the three subgroups of NORSE with unknown etiology. Finally, in a supplementary analysis, we evaluated and compared two subcategories of patients according to their age, following the definition of febrile seizures: (1) 6 years or younger and (2) older than 6 years.18
We assessed short‐term outcomes during admission (complications such as hypotension) and at the time of discharge. We categorized findings at discharge into three groups: death, morbidity (development of a neurological deficit), and recovery (return to baseline, reported by the medical team and the parents). Death and morbidity defined a poor outcome, and return to baseline defined a good outcome. Additionally, we assessed long‐term outcomes at the last follow‐up evaluating seizure recurrence, need for ASM, and cognitive abilities as reported by the treating pediatric neurologist.
2.5. Statistical analysis
Demographic and clinical characteristics were summarized with descriptive statistics. We presented continuous variables as median (interquartile range [IQR]), and categorical variables as count (percent). The univariate analysis highlighted differences between the three subgroups of NORSE of unknown etiology by the presence of fever and between the two age subgroups. We used Fisher exact and Kruskal–Wallis tests to compare categorical and continuous data, respectively, to evaluate the differences between groups in terms of clinical characteristics and outcome. We applied a correction for multiple testing according to Bonferroni. We performed statistical analyses using R, a language and environment for statistical computing (R Core Team; RStudio). A p‐value of less than .05 was considered significant.
2.6. Data availability
All statistical analyses and results are available in GitHub at https://cristinabarcia.github.io/pSERG‐NORSE/.
3. RESULTS
3.1. Study population and etiologies
Of the 279 patients enrolled in pSERG, 187 (67%) were excluded because of a prior diagnosis of epilepsy (including 35 with a prior episode of SE). Forty‐three (15%) patients were excluded due to a readily identifiable cause of SE. Forty‐nine children met NORSE criteria. Of these 49 cases, three additional patients were excluded due to incomplete data. Thus, 46 cases were included in this study (Figure 1; Table 1), representing 16% of all the RSE cases. Patients fell into the following categories: 40 (87%) had NORSE of unknown etiology and six (13%) had an identified etiology of NORSE (CNS infections [n = 3], autoimmune encephalitis [n = 2], and genetic epilepsy [n = 1]). Among the patients with unknown etiology, 19 (48%) presented with fever at SE onset, 16 (40%) had a previous fever, and five (12%) had no fever (Figure 1).
FIGURE 1.
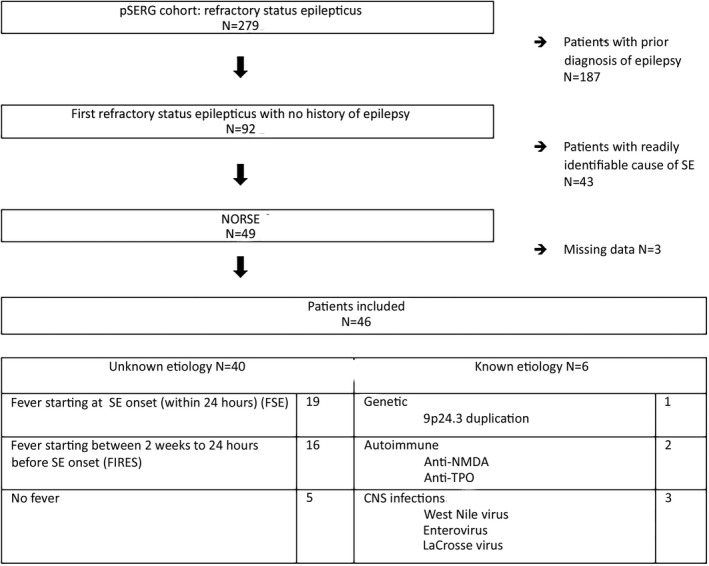
Study population selection diagram. CNS, central nervous system; FIRES, febrile infection‐related epilepsy syndrome; FSE, febrile SE; NMDA, N‐methyl‐d‐aspartate; NORSE, new onset refractory SE; pSERG, Pediatric Status Epilepticus Research Group; SE, status epilepticus; TPO, thyroid peroxidase
TABLE 1.
Characteristics of patients with new onset refractory SE based on etiology and presence of fever
| Characteristic | All cases, n = 46 | NORSE with unknown etiology, n = 40 | NORSE with known etiology, n = 6a | ||
|---|---|---|---|---|---|
| Fever ≤24 h prior, FSE, n = 19 | Fever >24 h prior, FIRES, n = 16 | No fever, n = 5 | |||
| Age, years | 2.4 [1.2–8.6] | 1.2 [.9–1.7] | 5.3 [2.4–8.9] | 10.2 [2.3–10.2] | 6.3 [3.2–11.0] |
| Sex, male | 21 (46) | 10 (52) | 8 (50) | 3 (60) | 0 (0) |
| Prodromal symptoms | 28 (61) | 6 (31) | 16 (100) | 1 (20) | 5 (83) |
| Fever still present at SE onset | 33 (75) | 19 (100) | 12 (75) | 0 (0) | 3 (50) |
| SE duration, h, n = 44 | 24 [7–128] | 10 [4–33] | 684 [103.5–1158] | 12 [8.2–17.2] | 60 [30–126] |
| Duration of ICU stay, days | 3.5 [2–24] | 2 [1–3] | 49 [24–64] | 1.5 [1–2] | 6 [4–8] |
| Epileptiform discharges, n = 42 | 20 (48) | 3/16 (19) | 11/15 (73) | 2/5 (40) | 4/6 (67) |
| Seizures | 13 | 2 | 8 | 0 | 3 |
| Periodic discharges | 12 | 1 | 10 | 0 | 1 |
| Sporadic epileptiform discharges | 12 | 2 | 5 | 2 | 3 |
| Abnormal MRI, n = 37 | 20 (46) | 1/12 (8) | 13/16 (69) | 2/5 (40) | 4 (67) |
| T2/FLAIR hyperintensity | 18 | 0 | 12 | 2 | 3 |
| DWI hyperintensity | 12 | 0 | 10 | 0 | 1 |
| Abnormal CSF, n = 41 | 14 (39) | 1/16 (6) | 10/16 (63) | 0/3 (0) | 3 (50) |
| Elevated protein level | 12 | 0 | 8 | 0 | 3 |
| Elevated white cell count | 11 | 1 | 8 | 0 | 2 |
| Non‐BZD ASM | 2 [2–2] | 2 [1–2] | 3 [2–3] | 2 [1–2] | 2 [2–2] |
| Continuous infusion | |||||
| n (%) | 25 (54) | 8 (42) | 13 (81) | 2 (40) | 2 (33) |
| Median [IQR] | 1 [0–1] | 0 [0–1] | 2 [1–3] | 1 [0–1] | 0 [0–1] |
| Ketogenic diet | 9 (19) | 0 | 9 (56) | 0 (0) | 0 (0) |
| Immunotherapies | 12 (26) | 0 | 11 (69) | 1 (20) | 0 (0) |
| IV steroids | 11 | 10 | 1 | ||
| IVIG | 9 | 9 | 0 | ||
| Plasma exchange | 3 | 3 | 0 | ||
| Complications, any | 20 (43) | 3 (16) | 13 (81) | 1 (20) | 3 (50) |
| Good outcome | 28 (61) | 17 (89) | 4 (25) | 4 (80) | 3 (50) |
Data are presented as n (%) or median [IQR].
Abbreviations: ASM, antiseizure medication; BZD, benzodiazepine; CSF, cerebrospinal fluid; DWI, diffusion‐weighted imaging; FIRES, febrile infection‐related epilepsy syndrome; FLAIR, fluid‐attenuated inversion recovery; FSE, febrile SE; ICU, intensive care unit; IQR, interquartile range; IV, intravenous; IVIG, intravenous immunoglobulin; MRI, magnetic resonance imaging; NORSE, new onset refractory SE; SE, status epilepticus.
NORSE with known etiology; the etiology was initially unknown but found after extensive investigations.
Classifying NORSE patients by age, 29 (73%) were 6 years or younger and 11 (27%) were older than 6 years. Among the youngest group, 18 (62%) presented with fever at onset, nine (31%) had a previous fever, and two (7%) had no fever. The older group presented more frequently with previous fever (64%; Table 2).
TABLE 2.
Characteristics of the new onset refractory SE group of unknown etiology divided by age (≤6 years or >6 years)
| Characteristic | All cases, n = 40 | ≤6 years, n = 29 | >6 years, n = 11 | p |
|---|---|---|---|---|
| Sex, male | 19 (48) | 13 (45) | 6 (55) | .72 |
| Prodromal symptoms | 23 (58) | 16 (55) | 7 (66) | .72 |
| Fever ≤24 h prior to SE onset | 19 (48) | 18 (62) | 1 (9) | .003 |
| Fever >24 h prior to SE onset | 16 (40) | 9 (31) | 7 (64) | .07 |
| No fever | 5 (13) | 2 (7) | 3 (27) | .11 |
| SE duration, h, n = 32 | 19 [6–96.7] | 8.5 [4.25–40] | 52 [24–1200] | .008 |
| Duration of ICU stay, days | 3 [1.5–33] | 3 [1–6.2] | 42 [3.5–62] | .01 |
| Epileptiform discharges, n = 36 | 16 (44) | 8/25 (32) | 8/11 (73) | .03 |
| Seizures | 10 (25) | 6 (21) | 4 (36) | |
| Periodic discharges | 11 (30) | 6 (21) | 5 (55) | |
| Sporadic epileptiform discharges | 9 (23) | 3 (11) | 6 (55) | |
| Abnormal MRI, n = 31 | 16 (40) | 8/21 (38) | 8/10 (80) | .05 |
| T2/FLAIR hyperintensity | 14 (35) | 8 (38) | 6 (60) | |
| DWI hyperintensity | 9 (23) | 5 (24) | 4 (40) | |
| Abnormal CSF, n = 35 | 12 (34) | 6/24 (25) | 6/11 (55) | .11 |
| Elevated protein level, n = 33 | 9 (27) | 3 (13) | 6 (55) | |
| Elevated white cell count, n = 34 | 9 (27) | 6 (25) | 3 (27) | |
| ASMs | 2 [1.7–2.2] | 2 [2–2] | 2 [1.5–3] | .69 |
| Continuous infusion | ||||
| Median [IQR] | 1 [0–1] | 0 [0–1] | 1 [1–2.5] | .05 |
| n (%) | 25 (63) | 15 (52) | 10 (91) | |
| Ketogenic diet | 9 (23) | 5 (17) | 4 (36) | .22 |
| Immunotherapies | 12 (30) | 6 (21) | 6 (55) | .05 |
| IV steroids | 11 (28) | 5 (17) | 6 (54) | |
| IVIG | 9 (26) | 5 (17) | 4 (3) | |
| Plasma exchange | 3 (8) | 2 (7) | 1 (9) | |
| Complications, any | 17 (43) | 10 (35) | 7 (64) | .15 |
| Good outcome | 25 (63) | 22 (76) | 3 (27) | .009 |
Data are presented as n (%) or median [IQR].
Abbreviations: ASM, antiseizure medication; CSF, cerebrospinal fluid; DWI, diffusion‐weighted imaging; FLAIR, fluid‐attenuated inversion recovery; ICU, intensive care unit; IQR, interquartile range; IV, intravenous; IVIG, intravenous immunoglobulin; MRI, magnetic resonance imaging; SE, status epilepticus.
3.2. Demographic and clinical characteristics
The median age was 2.4 (IQR = 1.2–8.6) years, and 25 (54%) were female. Prodromal symptoms were noted in 28 (61%) patients, including 9 (32%) with headache and 16 (35%) with a prior febrile illness. In the unknown etiology group, the fever at onset group was younger than both the previous fever and no fever groups. Most patients with prodromal headache were in the previous fever group (88%). The median duration of SE in the entire population was 24 (IQR = 7–128) h, and the median intensive care unit (ICU) stay was 3.5 (IQR = 2–24) days. The previous fever group experienced longer SE duration and ICU stays than the fever at onset group (Table 1).
3.3. Electroencephalogram
An EEG was performed in 42 (91%) patients. Among them, 13 (31%) had seizures during the EEG monitoring, and 20 (48%) had epileptiform discharges (Table 1). EEG was performed within 24 h from SE onset in 25 patients, four of them with seizures recorded. EEG was performed later than 24 h in 17 patients, and in nine of these 17 seizures were recorded. Discharges were generalized in 10 (48%), lateralized in 10 (48%), bilateral independent in two (9%), and multifocal in two (9%). The previous fever group had more frequent epileptiform abnormalities than the fever at SE onset group. Thirteen patients required a burst suppression coma, including 11 in the previous fever group. The older patients more frequently had epileptiform discharges (Table 2).
3.4. Imaging
Eight (17%) patients had a computerized tomography only, and all were nonlesional. Thirty‐seven (80%) had brain MRI, which was abnormal in 20 (54%) patients. The most frequent findings were diffuse or focal T2 and fluid‐attenuated inversion recovery (FLAIR) hyperintense changes (n = 16). Hyperintensities were located in the neocortex (n = 6), mesiotemporal lobe (n = 5), and basal ganglia (n = 4). Two patients had leptomeningeal T2/FLAIR enhancement. MRI abnormalities were less frequent in patients with fever at onset. The MRI was repeated in 11 patients. One patient with initially normal MRI had subsequent abnormal diffusion‐weighted imaging and T2/FLAIR hyperintensities in bilateral thalamic areas. Four patients had unchanged or improved results, with a reduction of FLAIR hyperintensities. Five patients showed progression of the disease, by increased T2/FLAIR hyperintensities (n = 3) and/or development of cortical atrophy (n = 3). Normal MRI was associated with better outcomes (73% vs. 31%, p = .03). Abnormal MRI occurred more frequently in patients older than 6 years (Table 2).
3.5. Cerebrospinal fluid
A lumbar puncture was performed in 41 (89%) patients. Results were abnormal in 14 (34%); 12 (80%) had an elevated protein level (range = 43–229 mg/100 ml, median = 72 [IQR = 53–95] mg/100 ml), and 11 (73%) had an elevated white blood cell count (range = 6–211/mm3, median = 42 [IQR = 24.5–49.7]/mm3). Cerebrospinal fluid (CSF) abnormalities were more common in the previous fever group compared to the fever at onset group (Tables 1 and 3).
TABLE 3.
Results of statistical tests for comparisons between the three NORSE subgroups of unknown etiology after Bonferroni correction (statistically significant p‐values [p < .05])
| FSE | No fever | ||
|---|---|---|---|
| Age | FIRES | .047 | NS |
| No fever | .034 | – | |
| SE duration | FIRES | .015 | NS |
| No fever | NS | – | |
| Duration of ICU stay | FIRES | <.001 | .005 |
| No fever | NS | – | |
| Epileptiform discharges on EEG | FIRES | .030 | NS |
| No fever | NS | – | |
| Abnormal MRI | FIRES | .002 | NS |
| No fever | .026 | – | |
| Abnormal CSF | FIRES | .006 | NS |
| No fever | NS | – | |
| ASM | FIRES | .053 | NS |
| No fever | NS | – | |
| Continuous infusion | FIRES | .026 | NS |
| No fever | NS | – | |
| Ketogenic diet | FIRES | <.001 | NS |
| No fever | NS | – | |
| Immunotherapies | FIRES | <.001 | NS |
| No fever | NS | – | |
| Complications, any | FIRES | <.001 | NS |
| No fever | NS | – | |
| Good outcome | FIRES | <.001 | NS |
| No fever | NS | – |
Abbreviations: ASM, antiseizure medication; CSF, cerebrospinal fluid; EEG, electroencephalogram; FIRES, febrile infection‐related epilepsy syndrome; FSE, febrile SE; ICU, intensive care unit; MRI, magnetic resonance imaging; NS, not significant, SE, status epilepticus.
3.6. Treatment
All patients received at least one BZD as first‐line treatment and at least one non‐BZD ASM as second‐line treatment. Fosphenytoin was the most frequent second‐line option (71%). Fifty‐four percent of patients subsequently received at least one continuous infusion of anesthetics, with midazolam being the most common. More ASMs and continuous infusions were required in the previous fever group (Table 1). Twelve patients (26%) received steroids, intravenous immunoglobulins, and plasma exchange, including 11 patients in the previous fever group. Nine patients started the ketogenic diet, all in the previous fever group. The outcome was not improved by this treatment (one with good vs. eight with poor outcome). Overall, immunotherapies and ketogenic diet were both more frequent in the previous fever group and associated with poor outcome (Tables 1 and 3).
3.7. Outcome
Twenty‐eight (61%) patients returned to baseline at discharge, including 17 of 19 (89%) in the fever at onset group and four of 16 (25%) in the previous fever group. Patients in the fever at onset group had fewer medical complications than the previous fever group, and these were often related to therapies used to treat SE (such as hypotension in the setting of continuous infusion therapy). Three (6%) patients died during admission, all in the previous fever group. Previous fever cases with longer SE durations had worse outcomes (p < .05).
Functional outcome at follow‐up was available in 41 patients. At last follow‐up (range = 5 months–5 years) from SE onset, 22 of 41 patients had normal cognitive performance, including 15 of 19 in the fever at onset group, two of 12 in the previous fever group, and two of four in the no fever group. Follow‐up data on seizure recurrence were available in 39 patients. Twenty‐one were seizure‐free (with or without ASM) in the entire cohort, including 13 of 18 patients in the fever at onset group, three of four patients in the no fever group, and one of 11 in the previous fever group. Among 12 previous fever patients with abnormal MRI, one died, eight had residual epilepsy, and follow‐up was not available in three patients.
In the subanalysis by age, 22 of 29 (76%) of the youngest patients returned to baseline, and 17 (72%) of these were in the fever at onset subgroup. However, three of 11 (27%) patients older than 6 years returned to baseline at discharge. Among the previous fever patients, four of nine (44.4%) from the youngest group and none from the oldest group returned to baseline.
3.8. NORSE of unknown etiology without fever
Five patients had NORSE of unknown etiology without fever. We present individual characteristics in Table 4. Three patients (60%) had abnormal MRI findings, two (40%) had interictal discharges on EEG, and one (20%) had elevated CSF protein levels. The outcome was favorable in four (80%).
TABLE 4.
Clinical characteristics of the new onset refractory SE of unknown etiology without fever cases
| Characteristic | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age, years | 10 | 2 | 10 | 13 | 2 |
| Gender | Male | Female | Male | Male | Female |
| Comorbidities | – | – | – | Hearing loss | – |
| Prodromal symptoms | – | – | – | – | URTI |
| Fever at status epilepticus onset | NA | No | No | No | No |
| Epileptiform EEG | Yes | No | No | Yes | No |
| Seizures | – | – | |||
| Periodic discharges | – | – | |||
| Sporadic epileptiform discharges | Lateralized | Multifocal | |||
| MRI | Abnormal | Abnormal | Abnormal | None | None |
| T2/FLAIR hyperintensity | Left hippocampus | Left hippocampus | Right thalamus | ||
| Abnormal CSF | Yes | None | No | No | None |
| Elevated protein level | Yes, 72 mg/dl | ||||
| Elevated white cell count | No, 4/mm3 | ||||
| ASMs | 2 | 3 | 2 | 1 | 1 |
| Continuous infusion | 0 | 0 | 1 | 1 | 0 |
| Ketogenic diet | 0 | 0 | 0 | 0 | 0 |
| Immunotherapies | 1, steroids | 0 | 0 | 0 | 0 |
| ICU duration, days | 2 | 1 | 1.5 | 4 | 1 |
| SE duration, h | 24 | NA | 15 | 9 | 6 |
| Convulsive time, min | 120 | 1320 | 150 | 177 | 90 |
| Complications | No | No | No | Yes, hypotension | No |
| Good outcome | Yes | No | Yes | Yes | Yes |
Abbreviations: ASM, antiseizure medication; CSF, cerebrospinal fluid; EEG, electroencephalogram; FLAIR, fluid‐attenuated inversion recovery; ICU, intensive care unit; MRI, magnetic resonance imaging; NA, not available; SE, status epilepticus; URTI, upper respiratory tract infection.
4. DISCUSSION
4.1. Summary
NORSE cases represented 16% of the 279 prospectively collected children with an episode of RSE. Children with NORSE of unknown etiology were divided into three patient subgroups: (1) fever at SE onset (FSE), (2) previous fever (FIRES), and (3) no fever. FIRES patients had prolonged SE and worse outcomes, with 25% recovering baseline functions. Refractory FSE patients were younger and had shorter SE episodes, and 89% recovered baseline function. Fever occurring more than 24 h before the onset of SE may identify a subgroup with different clinical characteristics and worse outcomes.
4.2. Differences between previous fever and fever at SE onset groups
NORSE of unknown etiology had different profiles across the three subgroups. NORSE with previous fever (FIRES) differed from NORSE with fever at onset (refractory FSE). FIRES cases were older, had more frequent CSF and MRI abnormalities, and had a more severe clinical course. FIRES patients required more aggressive treatment (higher rates of continuous infusions, ketogenic diet, and immunotherapy). This clinical presentation of NORSE of unknown etiology with previous fever is consistent with prior reports on FIRES.6, 9, 10, 11, 19 In a FIRES cohort of 77 patients, an 11% mortality rate was reported, 93% of patients presented with residual epilepsy, and 66% had subsequent mild to severe mental delay.6 Comparatively, our FIRES cohort revealed a mortality of 18%, 94% with residual epilepsy, and 77% with a functional decline. Poor outcomes in the prior study may have been in part attributed to selection bias, as superrefractory or prolonged cases of SE were specifically included. An inclusion criterion in the prior cohort was the diagnosis of “acute onset catastrophic SE,” as defined by continuation of seizures following the first treatment cycle of burst suppression coma or by multiple seizures per day for more than 1 week despite treatment.6 In our study, this group was defined as NORSE with a previous febrile episode, without any criteria for duration or superrefractoriness of SE, thus shifting selection based on treatment response to clinical criteria early in the presentation.
NORSE with fever at onset had a different clinical profile, suggesting at least overlap or congruence with FSE.16, 20 All patients except one were younger than 5 years with favorable outcomes and most often normal results in complementary studies. Moreover, the comparison of the subgroups by age supported the higher frequency of FSE (62% vs. 9%) and more favorable outcomes in the youngest group, consistent with the current definition of febrile seizures. The fever at onset group represents our largest subgroup (40%) of NORSE cases, in line with prior studies highlighting FSE as the most common cause of the first episode of SE, being at least twice as frequent as acute symptomatic SE.21 FSE is refractory in 10%–20% of patients, whereas acute symptomatic SE is refractory in 20%–40%.21, 22 Therefore, our large proportion of refractory FSE (40%) may be explained by the exclusion of acute symptomatic cases.
The refractory FSE profile appears distinct from the usual description of NORSE, but the proposed definitions include those patients within the NORSE entity. The different evolution of FIRES and FSE may be explained by an alternative underlying etiology. In children with FIRES, elevated levels of proconvulsant cytokines (e.g., interleukin‐6) have been documented in the CSF, suggesting an inflammatory cause, potentially in the setting of a genetic predisposition. An underlying immune cause is also supported by the association with polymorphisms in the interleukin‐1 receptor antagonist (IL1RN) gene.23, 24 The time limit of fever onset in the current definitions of FSE and FIRES remains arbitrary and may not prevent the risk of initial misdiagnosis in clinical practice. Thus, additional criteria should be considered, to better distinguish these entities early in the disease course. Based on our findings, age (>2 years) and electroclinical duration of SE (>48 h) could potentially be considered as complementary factors in future diagnostic approaches, as they were the most distinctive features. Eighty‐two percent of FIRES patients were older than 2 years, and 79% of FSE patients were younger. Similarly, SE duration was longer than 48 h in 86% of FIRES and shorter in 89% of FSE cases (Table 5). Other features such as the seizure semiology (generalized/focal), onset characteristics (abrupt single SE episode vs. a flurry of seizures of increasing frequency), and presence of a fever‐free interval before SE may also warrant further investigation.
TABLE 5.
Key clinical features for the differential diagnosis between FSE, FIRES, and NORSE without fever
| Clinical features | FSE | NORSE of unknown etiology | |
|---|---|---|---|
| FIRES | NORSE without fever | ||
| Time from fever onset to SE onset | <24 h | >24 h | No fever |
| Age | 1 month–6 years | School‐aged | Variable |
| <2 years in 79% | >2 years in 81% | >2 years in 80% | |
| SE duration | Most <48 h (90%) | Most >48 h (86%) | All <48 h |
| Abnormal findings on MRI, CSF, or EEG | None in CSF and MRI | At least one abnormal finding: 90% | Variable |
| EEG: focal/diffuse slowing in 47% | CSF 63% | ||
| MRI 69% | |||
| EEG 80% | |||
| Outcome | Generally good | Generally poor | Variable |
Abbreviations: CSF, cerebrospinal fluid; EEG, electroencephalogram; FIRES, febrile infection‐related epilepsy syndrome; FSE, febrile SE; MRI, magnetic resonance imaging; NORSE, new onset refractory SE; SE, status epilepticus.
In our study, FIRES cases had longer SE duration and worse outcomes than refractory FSE. Etiology is often the main determinant of outcome related to SE, possibly playing a larger role than the SE episode and related treatment,25, 26, 27 but others highlighted the independent prognostic role of SE duration.28, 29, 30, 31 We were unable to address this issue in our subset of patients, as most episodes of NORSE of unknown etiology lasted more than 2 days, whereas all episodes of FSE lasted less than 1 day, and therefore, duration may have confounded outcomes. FIRES cases were more frequently treated with immunotherapies and the ketogenic diet than others, which was not associated with better outcomes. Notably, the application of therapies to patients with more severe disease courses and hence worse outcomes may have led to confounding by indication.
4.3. NORSE of unknown etiology and FIRES
Previous pediatric studies have focused on FIRES,8, 9, 10, 11, 32, 33 whereas NORSE series have included mostly adults.1, 5 Our study confirms that pediatric NORSE cases are mostly represented by FIRES if we differentiate FSE cases. In the largest series of FIRES, almost all patients had a prior febrile illness.6 Whether cases of NORSE with unknown etiology without fever are similar to FIRES is unclear, as the fever could sometimes be missed, or perhaps other unidentified triggers may lead to a similar presentation. Although the small size of our cohort prevented any statistically significant differences, three of these cases (Cases 1, 3, and 4) seemed to differ from FIRES, based on older age, shorter SE duration, fewer MRI and CSF abnormalities, and more favorable outcomes. The fifth case had a clinical course compatible with FSE, but no fever was noted.
NORSE with previous fever (FIRES) frequently presented with abnormal EEG (80%), neuroimaging (69%), or CSF (63%) findings. The combination of these findings, including prior febrile episodes and RSE, raises suspicion for autoimmune or infectious encephalitis.34, 35, 36 One patient presented with clinical symptoms of meningitis, including headache and neck pain. However, the severe, monosymptomatic course including seizures only, and lack of response to first‐line immunotherapy in FIRES, may help separate these entities, making an autoimmune etiology less likely.1, 6, 37 Rather, recent studies suggest a key role of innate immune system dysfunction in FIRES patients.38 The most frequent imaging abnormalities were seen in the FIRES subgroup (69%), including T2/FLAIR hyperintensities in the mesial temporal lobe, neocortical regions, or basal ganglia. In the largest prior FIRES series, 45% of MRIs were abnormal, mainly with bi‐ or unilateral hippocampal, peri‐insular, or basal ganglia T2/FLAIR hyperintensities.6 However, proportions of abnormal imaging findings are variable among FIRES studies, ranging from 0 to 58%,8, 13, 19, 33, 39 even if MRI was systematically performed at the “acute phase” of the disease. When repeated after several weeks, additional T2/FLAIR hyperintensities or diffuse atrophies were often noted.6, 8, 39 Furthermore, a recent study showed reversible bilateral claustral MRI T2 hyperintensity in adults and children with NORSE.40 The proportion of abnormal findings in our FIRES group is higher than in prior studies, and this may have been related to relatively late MRI imaging during the disease.
4.4. Discussion of NORSE definition
The current NORSE definition is largely based on the ability to quickly identify the cause of the SE. It is thus expected that the incidence of NORSE of unknown etiology will decrease with time as our understanding of the underlying causes improves and the availability of faster diagnostic methods increases. Our study focused on patients with unknown etiology after extensive workup. This subgroup might correspond to a single disease and may be independent of the speed of testing or an assessment within 72 h, warranting more detailed evaluation.
Our analyses provide suggestions for improvements in the definition, such as the consideration of fever onset, SE duration, and age. These factors, among others, may help distinguish the previously well‐defined FSE group, which is currently included in the current larger NORSE definition. Our data support that refractory FSE is a separate clinical entity based on clinical presentation and course, and should be excluded from the NORSE diagnosis, or distinguished within NORSE subcategories to acknowledge the different clinical presentation, age spectrum, and clinical course, which may also be reflected in etiological differences. Further representation of the clinical characteristics may help improve acute patient assessments and may reduce the importance of etiologic diagnosis in the current definitions. However, similar to other SE presentations, etiological findings remain crucial for prognosis and more specific treatment selection.
4.5. Challenges
Given the observational nature of this study, the selection of patients is not random but includes consenting patients meeting inclusion criteria. Only cases treated in selected pSERG centers were included, and although we used a standardized data acquisition tool, clinical information may have been collected differently between centers, introducing a potential center‐based selection and information bias.
Diagnosis and treatment were guided by physicians' judgment and standard of care, without standardized diagnostic or treatment protocols. The efficiency of drugs cannot be assessed given the large heterogeneity of treatment on a small group of patients. In addition, the subanalysis of patients with refractory and superrefractory SE was limited by the sample size. EEG and MRI results are not comparable if obtained at different time points, as results are likely to change with time. MRI results were not available in all patients at the time of data collection. Information on MRI timing, protocol, or the equipment utilized was not reported in our dataset. Moreover, complete autoimmune, infectious, and genetic workup was not systematically performed, which could have increased the number of NORSE of unknown etiology cases in this series. Also, outcomes were not assessed by official scales of measurement, and follow‐up time was variable. This highlights the importance of more standardized protocols in future studies. The strengths of this study are the prospective design and multicenter large cohort. Prior studies have not highlighted the detailed relationship of previous fever or fever at SE onset.
There is no diagnostic test for NORSE. Although our data support the cutoff time for fever occurrence of 24 h to separate FIRES and FSE, our cohort was not sufficiently large to test other time windows. Therefore, this time window remains arbitrary. Furthermore, a clearer distinction between FSE and NORSE definitions, or related subcategories, may be helpful.
5. CONCLUSIONS
Approximately 16% of RSE patients present as NORSE. Our study identifies the time of the onset of fever as a relevant factor identifying three subgroups of patients within NORSE of unknown etiology and supports the current age cutoff for FSE. In approximately 40% of cases, a febrile illness precedes SE onset by up to 2 weeks, and these patients meet FIRES criteria. FIRES patients are usually older, present with prolonged SE, have more abnormal MRI and CSF findings, and often have an unfavorable outcome. Our study provides clinical differences between these groups, confirming separate clinical features in refractory FSE, which may help improve diagnostic criteria. An additional distinction between NORSE and refractory FSE definitions may offer diagnostic and prognostic opportunities. Further research is needed to improve our understanding of pediatric NORSE, FIRES, and FSE.
CONFLICT OF INTEREST
C.S. is funded by the International Federation of Clinical Neurophysiology. C.B.A. is funded by the Fundación Alfonso Martín Escudero. I.S.F. is funded by the Epilepsy Research Fund and was funded by the Fundación Alfonso Martín Escudero and the HHV6 Foundation. M.A.‐G. was funded by the Fundación Alfonso Martín Escudero. J.N.B. is funded by National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke 1K23NS116225. He also served as a consultant for Novartis. W.D.G. is an editor for Epilepsia and Epilepsy Research. T.A.G. is funded by NIH grants 2U01‐NS045911, U10‐NS077311, R01‐NS053998, R01‐NS062756, R01‐NS043209, R01‐LM011124, R01‐NS065840, U24 NS107200, and 1U01TR002623. He has received consulting fees from Supernus, Sunovion, Eisai, and UCB. He also serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medicolegal cases. He receives royalties from a patent license. D.T. has received research funding from Children's Miracle Network Hospitals and Duke Forge. He has also received consultation fees from Gerson Lehrman Group, Guidepoint, IQVIA, and bioStrategies Group. M.S.W. serves as a scientific consultant and on the clinical advisory board for Sage Pharmaceuticals. A.A.W. receives research funding from Novartis, Eisai, Pfizer, UCB, Acorda, Lundbeck, GW Pharma, Upsher‐Smith, and Zogenix and receives publication royalties from UpToDate. T.L. serves on the board of the NORSE Institute, on the council (and as past president) of the American Clinical Neurophysiology Society, as committee chair at the American Epilepsy Society (Investigator Workshop Committee), as founder and consortium principal investigator of the pediatric status epilepticus research group, as an associate editor for Wyllie's Treatment of Epilepsy 6th and 7th editions. He is part of pending patent applications to detect, treat, and predict seizures and to diagnose epilepsy. He receives research support from the NIH, Patient‐Centered Outcomes Research Institute, Epilepsy Research Fund, Epilepsy Foundation of America, Epilepsy Therapy Project, and Pediatric Epilepsy Research Foundation, and has received research grants from Lundbeck, Eisai, Upsher‐Smith, Mallinckrodt, Sage, and Pfizer. He has served in the past as a consultant for Zogenix, UCB, Engage, Amzell, Upsher‐Smith, Eisai, and Sunovion. He performs video‐electroencephalographic long‐term and ICU monitoring, electroencephalograms, and other electrophysiological studies at Boston Children's Hospital and affiliated hospitals and bills for these procedures, and he evaluates pediatric neurology patients and bills for clinical care. He has received speaker honorariums from national societies including the American Academy of Neurology, American Epilepsy Society, and American Clinical Neurophysiology Society, and for grand rounds at various academic centers. T.L. is a coinventor of the TriVoxHealth technology. In the future, this technology may be sold commercially. If this were to occur, T.L. and Boston Children's Hospital might receive financial benefits in the form of compensation. As in all research studies, the hospital has taken steps designed to ensure that this potential for financial gain does not endanger research subjects or undercut the validity and integrity of the information learned by this research. His wife, Dr. Karen Stannard, is a pediatric neurologist, and she performs video‐electroencephalographic long‐term and ICU monitoring, electroencephalograms, and other electrophysiological studies and bills for these procedures and she evaluates pediatric neurology patients and bills for clinical care. None of the other authors has any conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Claudine Sculier participated in drafting and revising the manuscript for content, including medical writing, study concept and design, data acquisition, analysis and interpretation of data, statistical analysis, and study supervision or coordination. Cristina Barcia Aguilar participated in drafting and revising the manuscript for content, including medical writing, study concept and design, data acquisition, analysis and interpretation of data, statistical analysis, and study supervision or coordination. Nicolas Gaspard participated in drafting and revising the manuscript for content, including medical writing, study concept and design, data acquisition, analysis and interpretation of data, statistical analysis, and study supervision or coordination. Marina Gaínza‐Lein participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Iván Sánchez Fernández participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Marta Amengual‐Gual participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Anne Anderson participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Ravindra Arya participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Brian T. Burrows participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. J. Nicholas Brenton participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Jessica L. Carpenter participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Kevin E. Chapman participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Justice Clark participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. William D. Gaillard participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Tracy A. Glauser participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Joshua L. Goldstein participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Howard P. Goodkin participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Mark Gorman participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Yi‐Chen Lai participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Tiffani L. McDonough participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Mohamad A. Mikati participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Anuranjita Nayak participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Katrina Peariso participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. James Riviello participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Allison Rusie participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Katherine Sperberg participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Coral M. Stredny participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Robert C. Tasker participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Dmitry Tchapyjnikov participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Alejandra Vasquez participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Mark S. Wainwright participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Angus A. Wilfong participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Korwyn Williams participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination. Tobias Loddenkemper participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, and study supervision or coordination.
ACKNOWLEDGMENTS
This study and consortium were funded by the Epilepsy Research Fund, the Epilepsy Foundation of America (EF‐213583, Targeted Initiative for Health Outcomes), the American Epilepsy Society/Epilepsy Foundation of America Infrastructure Award, and the Pediatric Epilepsy Research Foundation.
Sculier C, Barcia Aguilar C, Gaspard N, Gaínza‐Lein M, Sánchez Fernández I, Amengual‐Gual M, et al; pSERG . Clinical presentation of new onset refractory status epilepticus in children (the pSERG cohort). Epilepsia. 2021;62:1629–1642. 10.1111/epi.16950
Claudine Sculier and Cristina Barcia Aguilar contributed equally.
REFERENCES
- 1.Gaspard N, Foreman BP, Alvarez V, Cabrera Kang C, Probasco JC, Jongeling AC, et al. New‐onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology. 2015;85(18):1604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new‐onset refractory status epilepticus (NORSE), febrile infection‐related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59(4):739–44. [DOI] [PubMed] [Google Scholar]
- 3.Costello DJ, Kilbride RD, Cole AJ. Cryptogenic new onset refractory status epilepticus (NORSE) in adults—infectious or not? J Neurol Sci. 2009;277(1–2):26–31. [DOI] [PubMed] [Google Scholar]
- 4.Khawaja AM, DeWolfe JL, Miller DW, Szaflarski JP. New‐onset refractory status epilepticus (NORSE)—the potential role for immunotherapy. Epilepsy Behav. 2015;47:17–23. [DOI] [PubMed] [Google Scholar]
- 5.Gall CRE, Jumma O, Mohanraj R. Five cases of new onset refractory status epilepticus (NORSE) syndrome: outcomes with early immunotherapy. Seizure. 2013;22(3):217–20. [DOI] [PubMed] [Google Scholar]
- 6.Kramer U, Chi C‐S, Lin K‐L, Specchio N, Sahin M, Olson H, et al. Febrile infection‐related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia. 2011;52(11):1956–65. [DOI] [PubMed] [Google Scholar]
- 7.van Baalen A, Vezzani A, Häusler M, Kluger G. Febrile infection‐related epilepsy syndrome: clinical review and hypotheses of epileptogenesis. Neuropediatrics. 2017;48(1):5–18. [DOI] [PubMed] [Google Scholar]
- 8.Caraballo RH, Reyes G, Avaria MFL, Buompadre MC, Gonzalez M, Fortini S, et al. Febrile infection‐related epilepsy syndrome: a study of 12 patients. Seizure. 2013;22(7):553–9. [DOI] [PubMed] [Google Scholar]
- 9.Howell KB, Katanyuwong K, Mackay MT, Bailey CA, Scheffer IE, Freeman JL, et al. Long‐term follow‐up of febrile infection‐related epilepsy syndrome. Epilepsia. 2012;53(1):101–10. [DOI] [PubMed] [Google Scholar]
- 10.Patil SB, Roy AG, Vinayan KP. Clinical profile and treatment outcome of febrile infection‐related epilepsy syndrome in South Indian children. Ann Indian Acad Neurol. 2016;19(2):188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakuma H, Awaya Y, Shiomi M, Yamanouchi H, Takahashi Y, Saito Y, et al. Acute encephalitis with refractory, repetitive partial seizures (AERRPS): a peculiar form of childhood encephalitis. Acta Neurol Scand. 2010;121(4):251–6. [DOI] [PubMed] [Google Scholar]
- 12.Schubert‐Bast S, Zöllner JP, Ansorge S, Hapfelmeier J, Bonthapally V, Eldar‐Lissai A, et al. Burden and epidemiology of status epilepticus in infants, children, and adolescents: a population‐based study on German health insurance data. Epilepsia. 2019;60(5):911–20. [DOI] [PubMed] [Google Scholar]
- 13.Baxter P, Clarke A, Cross H, Harding B, Hicks E, Livingston J, et al. Idiopathic catastrophic epileptic encephalopathy presenting with acute onset intractable status. Seizure. 2003;12(6):379–87. [DOI] [PubMed] [Google Scholar]
- 14.Guterman EL, Betjemann JP, Aimetti A, Li JW, Wang Z, Yin D, et al. Association between treatment progression, disease refractoriness, and burden of illness among hospitalized patients with status epilepticus. JAMA Neurol. 2021;78(5):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez Fernández I, Abend NS, Agadi S, An S, Arya R, Carpenter JL, et al. Gaps and opportunities in refractory status epilepticus research in children: a multi‐center approach by the Pediatric Status Epilepticus Research Group (pSERG). Seizure. 2014;23(2):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinnar S, Hesdorffer DC, Nordli DR, Pellock JM, O'Dell C, Lewis DV, et al. Phenomenology of prolonged febrile seizures: results of the FEBSTAT study. Neurology. 2008;71(3):170–6. [DOI] [PubMed] [Google Scholar]
- 17.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus—report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56(10):1515–23. [DOI] [PubMed] [Google Scholar]
- 18.Patel N, Ram D, Swiderska N, Mewasingh LD, Newton RW, Offringa M. Febrile seizures. BMJ. 2015;351:h4240. [DOI] [PubMed] [Google Scholar]
- 19.Mikaeloff Y, Jambaqué I, Hertz‐Pannier L, Zamfirescu A, Adamsbaum C, Plouin P, et al. Devastating epileptic encephalopathy in school‐aged children (DESC): a pseudo encephalitis. Epilepsy Res. 2006;69(1):67–79. [DOI] [PubMed] [Google Scholar]
- 20.Nordli DR, Moshé SL, Shinnar S, Hesdorffer DC, Sogawa Y, Pellock JM, et al. Acute EEG findings in children with febrile status epilepticus: results of the FEBSTAT study. Neurology. 2012;79(22):2180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin RFM, Neville BGR, Peckham C, Bedford H, Wade A, Scott RC, et al. Incidence, cause, and short‐term outcome of convulsive status epilepticus in childhood: prospective population‐based study. Lancet. 2006;368(9531):222–9. [DOI] [PubMed] [Google Scholar]
- 22.Lambrechtsen FACP, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia. 2008;49(4):615–25. [DOI] [PubMed] [Google Scholar]
- 23.Saitoh M, Kobayashi K, Ohmori I, Tanaka Y, Tanaka K, Inoue T, et al. Cytokine‐related and sodium channel polymorphism as candidate predisposing factors for childhood encephalopathy FIRES/AERRPS. J Neurol Sci. 2016;368:272–6. [DOI] [PubMed] [Google Scholar]
- 24.Sakuma H, Tanuma N, Kuki I, Takahashi Y, Shiomi M, Hayashi M. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection‐related refractory status epilepticus. J Neurol Neurosurg Psychiatry. 2015;86(7):820–2. [DOI] [PubMed] [Google Scholar]
- 25.Camfield P, Camfield C. Unprovoked status epilepticus: the prognosis for otherwise normal children with focal epilepsy. Pediatrics. 2012;130(3):e501–e506. [DOI] [PubMed] [Google Scholar]
- 26.Sillanpää M, Shinnar S. Status epilepticus in a population‐based cohort with childhood‐onset epilepsy in Finland. Ann Neurol. 2002;52(3):303–10. [DOI] [PubMed] [Google Scholar]
- 27.Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA. Long‐term mortality after a first episode of status epilepticus. Neurology. 2002;58(4):537–41. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson KJ, Koivikko MJ. Status epilepticus in children: aetiology, treatment, and outcome. Dev Med Child Neurol. 1997;39(10):652–8. [DOI] [PubMed] [Google Scholar]
- 29.Maegaki Y, Kurozawa Y, Hanaki K, Ohno K. Risk factors for fatality and neurological sequelae after status epilepticus in children. Neuropediatrics. 2005;36(3):186–92. [DOI] [PubMed] [Google Scholar]
- 30.Maytal J, Shinnar S, Moshé SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989;83(3):323–31. [PubMed] [Google Scholar]
- 31.Gaínza‐Lein M, Sánchez Fernández I, Jackson M, Abend NS, Arya R, Brenton JN, et al. Association of time to treatment with short‐term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol. 2018;75(4):410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer U, Shorer Z, Ben‐Zeev B, Lerman‐Sagie T, Goldberg‐Stern H, Lahat E. Severe refractory status epilepticus owing to presumed encephalitis. J Child Neurol. 2005;20(3):184–7. [DOI] [PubMed] [Google Scholar]
- 33.van Baalen A, Häusler M, Boor R, Rohr A, Sperner J, Kurlemann G, et al. Febrile infection‐related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia. 2010;51(7):1323–8. [DOI] [PubMed] [Google Scholar]
- 34.Nabbout R. FIRES and IHHE: delineation of the syndromes. Epilepsia. 2013;54(Suppl 6):54–6. [DOI] [PubMed] [Google Scholar]
- 35.Illingworth MA, Hanrahan D, Anderson CE, O'Kane K, Anderson J, Casey M, et al. Elevated VGKC‐complex antibodies in a boy with fever‐induced refractory epileptic encephalopathy in school‐age children (FIRES). Dev Med Child Neurol. 2011;53(11):1053–7. [DOI] [PubMed] [Google Scholar]
- 36.Glaser CA, Gilliam S, Honarmand S, Tureen JH, Lowenstein DH, Anderson LJ, et al. Refractory status epilepticus in suspect encephalitis. Neurocrit Care. 2008;9(1):74–82. [DOI] [PubMed] [Google Scholar]
- 37.Clarkson BDS, LaFrance‐Corey RG, Kahoud RJ, Farias‐Moeller R, Payne ET, Howe CL. Functional deficiency in endogenous interleukin‐1 receptor antagonist in patients with febrile infection‐related epilepsy syndrome. Ann Neurol. 2019;85(4):526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meletti S, Giovannini G, d'Orsi G, Toran L, Monti G, Guha R, et al. New‐onset refractory status epilepticus with claustrum damage: definition of the clinical and neuroimaging features. Front Neurol. 2017;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Specchio N, Fusco L, Claps D, Vigevano F. Epileptic encephalopathy in children possibly related to immune‐mediated pathogenesis. Brain Dev. 2010;32(1):51–6. [DOI] [PubMed] [Google Scholar]
- 40.Meletti S, Slonkova J, Mareckova I, Monti G, Specchio N, Hon P, et al. Claustrum damage and refractory status epilepticus following febrile illness. Neurology. 2015;85(14):1224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All statistical analyses and results are available in GitHub at https://cristinabarcia.github.io/pSERG‐NORSE/.


