Abstract
Aim
To assess the diagnostic utility of an oral rinse active matrix metalloproteinase‐8 (aMMP‐8) point‐of‐care test (POCT) for differentiating periodontal health, gingivitis, as well as different stages and grades of periodontitis.
Materials & Methods
The aMMP‐8 index test was undertaken in 408 consecutive adults, followed by a full‐mouth periodontal examination. The reference standard was the 2017 World Workshop classification of periodontal diseases. Sensitivity, specificity, and the area under the receiver operating characteristic curve (AUROC) were assessed.
Results
68.6% of the participants were diagnosed with periodontitis, including Stages I (15.9%), II (15.9%), III (29.7%) and IV (7.1%). A positive aMMP‐8 POCT was associated with periodontitis after adjusting for age, gender, tobacco smoking and systemic diseases, while it was unable to differentiate among the stages/grades of periodontitis and between gingivitis/periodontal health. This test showed a sensitivity of 33.2% and a specificity of 93.0% for detecting periodontitis (threshold level >10 ng/ml). The levels of aMMP‐8 adjusted by the number of teeth present (aMMP‐8/NTP) performed better for periodontitis (sensitivity: 67.1%; specificity: 68.8%). Notably, aMMP‐8/NTP were strongly predictive for Stage IV periodontitis (threshold level =0.4312 ng/ml) (sensitivity: 89.7%; specificity: 73.6%; and AUROC: 0.856). The test performance greatly improved in combination with age and smoking, with a sensitivity of 82.5%, a specificity of 84.4%, and an AUROC of 0.883.
Conclusion
This aMMP‐8 POCT is able to detect periodontitis with better specificity than sensitivity across the spectrum of its severity. This test may be useful for periodontal screening in conjunction with subject characteristics and/or other sensitive screening tools. Further validation studies are needed.
Keywords: diagnosis, gingivitis, matrix metalloproteinase‐8, periodontal health, periodontitis, point‐of‐care test, screening, sensitivity and specificity
Clinical Relevance.
Scientific rationale for the study: The traditional periodontal diagnostic approach relies on clinical parameters that are difficult to measure, lack precision to detect incipient periodontitis and can only reflect the previous tissue destruction. Point‐of‐care biomarker tests based on oral fluids have the potential to improve screening and diagnosis, in particular whenever reliable clinical examinations are not available.
Principal findings: The aMMP‐8 point‐of‐care test has better specificity than sensitivity to detect periodontitis across its spectrum of severity. The levels of aMMP‐8 adjusted by the number of teeth present yielded moderate‐to‐high accuracy in identifying advanced cases. Moreover, a combined model including the test results with age and smoking performed better than the test alone.
Practical implications: The aMMP‐8 test may be helpful in conjunction with risk factors/indicators of periodontitis or other screening tools.
1. INTRODUCTION
Periodontitis is a dysbiotic biofilm‐initiated, dysregulated host response‐mediated inflammatory disease of the tooth‐supporting apparatus. The diagnosis of periodontitis is conventionally based on the clinical and radiographic examinations, intended to assess the clinical attachment level, the degree of gingival inflammation and alveolar bone loss. These parameters, however, have two sets of limitations: (i) they are difficult to measure and lack adequate precision to enable detection of incipient periodontitis and (ii) they are only able to reflect the previous periodontal destruction, but insufficient to indicate current disease activity and the risk for future progression. Therefore, it is essential to employ supplementary screening and diagnostic methods to identify the potential risk prior to the onset of the noticeable irreversible damage to improve the early detection of periodontitis (Tonetti et al., 2017).
In this respect, salivary biomarkers have been considered simple, non‐invasive and promising diagnostic aids (Giannobile et al., 2009). Biomarkers may allow monitoring of the biochemical processes associated with disease and offer greater insight into the individual case. Among a wide range of potential biomarkers, matrix metalloproteinases have received considerable attention for their crucial role in tissue homeostasis and association with periodontitis‐associated collagen degradation (Birkedal‐Hansen, 1993; Birkedal‐Hansen et al., 1993; Sorsa et al., 2016). These enzymes are tightly controlled and require activation before being able to optimally act on their substrate (DeCarlo et al., 1997, 1998; Van wart & Birkedal‐Hansen, 1990).
Matrix metalloproteinase‐8 (MMP‐8) is emerging as one of the most documented and compelling candidates for the discrimination of periodontal health and disease (Arias‐Bujanda et al., 2020; Kc et al., 2020; de Morais et al., 2018; Zhang et al., 2018). It is the main type of host‐derived collagenases capable of cleaving type I collagen of the periodontal supporting tissues and playing a role in both physiological activity and pathologic tissue destruction (Sapna et al., 2014; Sorsa et al., 2018). MMP‐8 is present in an active and latent form. Its active form has been shown to have (i) a stronger association with the periodontal status and (ii) a better diagnostic accuracy for periodontal disease than its total forms (Leppilahti et al., 2011, 2014).
In recent years, an oral rinse point‐of‐care immunoassay mainly detecting active matrix metalloproteinase‐8 (aMMP‐8) has been developed and assessed in several countries (U.S. Patent No. 10,488,415, 2019). It is noteworthy that the reported performance for detecting periodontal disease is inconsistent across studies. This may be partly explained by several important limitations, including small sample size, the lack of universal periodontal case definitions and selection bias in case‐control designs (Heikkinen et al., 2016; Izadi Borujeni et al., 2015; Leppilahti et al., 2018; Lorenz et al., 2017; Nwhator et al., 2014; Räisänen et al., 2019; Schmidt et al., 2018). Thus, more evidence is needed to evaluate the diagnostic ability of the aMMP‐8 test properly.
The 2017 World workshop proposed a new periodontitis classification, which comprises a staging system to identify disease severity and complexity of management and a grading system to assess the risk for future disease progression (Tonetti et al., 2018). Integral to the new classification system is the possibility to use biomarkers in the diagnosis and case definition. Recently, a study by Sorsa et al., (2020) observed an association between aMMP‐8 concentrations and different stages and grades of periodontitis.
Therefore, this study aimed to (i) test and assess the diagnostic utility of an aMMP‐8 point‐of‐care oral rinse test system for differentiating periodontal health, gingivitis and different stages of periodontitis based on the new classification; and (ii) investigate the correlation between the aMMP‐8 test and periodontitis grade. The study hypothesis was that results of an aMMP‐8 point‐of‐care test (POCT) might improve diagnostic accuracy in a variety of situations where a full clinical assessment may be problematic, and could help to predict individuals at risk for periodontal disease, serving as the first step assessment guide and an adjunct to conventional periodontal diagnostic approaches.
2. METHODS AND MATERIALS
2.1. Study design and population
This cross‐sectional diagnostic accuracy study was conducted in the Prince Philip Dental Hospital, Hong Kong, between July 2019 and August 2020. Details of the design and population have been reported (Deng et al., 2021). In brief, 408 consecutive subjects were recruited from a convenience sample seeking dental care at the hospital. All subjects aged 18 or above were invited to participate. The exclusion criteria were as follows: (i) edentulous adults, (ii) pregnant females, (iii) subjects who received antibiotics within the previous 3 months and (iv) subjects who received professional periodontal treatment (other than supragingival cleaning) within the previous 12 months. The study protocol was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB Approval Number: UW19‐188) and registered on both ClinicalTrials.gov (NCT03928080) and HKU Clinical Trials Registry (HKUCTR‐2631). All procedures were conducted in accordance with the current revision of the Declaration of Helsinki. All participants provided written consent prior to the start of the study. This study followed the Standards for Reporting Diagnostic Accuracy (STARD) guidelines (Cohen et al., 2016).
2.2. Sample size estimation
The sample size was estimated based on the reported specificity of 81% from a previous study using the aMMP‐8 POCT for detecting periodontitis (Lorenz et al., 2017) and observations from a pilot study. In our pilot study of 20 participants, the specificity of the aMMP‐8 POCT for detecting periodontitis was 89%. Therefore, the expected specificity was set as 89%. 128 non‐periodontitis (periodontal health or gingivitis) participants were needed to achieve 80% power with a significance level of 5%. A total of 400 subjects were recruited considering prevalence of periodontitis in Hong Kong (Department of Health, Hong Kong SAR Government, 2011) and allowing 20% of missing or incomplete data.
2.3. Oral rinse aMMP‐8 point‐of‐care index test
A commercial aMMP‐8 POCT system (PerioSafe® PRO, Dentognostics GmbH) and its digital analysis device (ORALyzer®, Dentognostics GmbH) were used prior to the clinical examination. The test is a lateral flow immunoassay that identifies the middle part of activated MMP‐8 fragments with a size between 20 and 35 kDa based on Sorsa’s et al., 2019 invention (U.S. Patent No. 10,488,415, 2019).
The test system comprised the sample pad, conjugate release pad, membrane, test line, control line and absorbent pad (see Appendix S1). The monoclonal MMP‐8‐specific antibody 8706 worked as a tracer antibody conjugated to latex particles in the conjugate pad, while the MMP‐8‐specific antibody 8708 was a catching antibody immobilized in the test line on the membrane (Hanemaaijer et al., 1997; Sorsa et al., 1999). When the oral rinse sample containing aMMP‐8 migrated through the conjugate pad, it bound to the latex particle–tracer antibody complexes and flowed into the test line to react with the catching antibody. The different intensity of the reaction in the test line correlated with the sample concentration that could be quantitatively assessed by the digital reader.
Subjects were required to avoid eating, drinking, brushing and using mouthwash at least 30 min prior to the test. The aMMP‐8 test was administrated to the participants by a trained and calibrated dental surgery assistant according to the manufacturer's instruction by the following steps: (i) a 30‐s pre‐rinse with tap water; (ii) a 60‐s wait after the pre‐rinse; (iii) a 30‐s rinse with a proprietary test buffer; (iv) pouring the oral rinse into a small collection cup that accompanied the test kit; (v) drawing up 3 ml of the rinse into a syringe; and (vi) placing a filter into the syringe and pushing the syringe plunger to add 3–4 droplets of the filtered oral rinse to the test system. Within 5–6 min, the quantitative assessment of aMMP‐8 level is automatically shown on the digital reader; the reported detection level is 10 ng/ml. According to the manufacturer's recommendation, the aMMP‐8 level was considered in the healthy range (concentration ≤ 10 ng/ml); as active periodontal degeneration (10 ng/ml < concentration ≤ 20 ng/ml); or inflammatory tissue destruction (concentration > 20 ng/ml), respectively. Therefore, the diagnostic performance of aMMP‐8 test results was tested using cut‐off values of >10 and >20 ng/ml, respectively.
In this study, aMMP‐8 levels below the detection level were considered to be 10 ng/ml for the statistical analysis. Quantitative results of the aMMP‐8 test were also related to the number of teeth present by dividing the total concentration by the number of teeth present (aMMP‐8/NTP). To ensure consistency, all study personnel were specifically trained and certified by the test manufacturer; all standard procedures were rigorously applied.
2.4. Periodontal examination and case definition—reference standard
Full‐mouth periodontal examinations, as performed and interpreted by a single calibrated examiner (KD), were used as the gold standard for diagnosing periodontal health, gingivitis and different stages and grades of periodontitis as previously reported (Deng et al., 2021). In brief, the clinical examination included probing pocket depth (PPD), full‐mouth bleeding score (FMBS) and clinical attachment level (CAL) measurements at six sites per tooth with a periodontal probe (UNC‐15, Hu‐Friedy), furcation involvement (FI), tooth mobility and numbers of teeth lost attributed to periodontitis. Details of training and reproducibility of the examiner have been reported (Deng et al., 2021). Self‐reported demographic characteristics, smoking status and medical history with particular emphasis on diabetes and its control were also collected.
The diagnoses of different periodontal case definitions, including periodontal health, gingivitis and different stages of periodontitis, were based on the 2017 classification of periodontal diseases (Chapple et al., 2018; Papapanou et al., 2018; Tonetti et al., 2018; Tonetti & Sanz, 2019; Trombelli et al., 2018). The algorithm proposed by Tonetti and Sanz (2019) was used to reach diagnosis of each case by a single periodontist (KD) who was blind to the aMMP‐8 test results. In this study, the term periodontal disease was used to define subjects with plaque‐induced gingivitis or periodontitis. Periodontitis grade was defined using primarily radiographic bone loss divided by age on orthopantomographic digital images and assessing periodontal destruction with reference to deposits as primary criteria. Periodontitis grade was modified based on self‐reported smoking and diabetes control (Tonetti & Sanz, 2019). Subjects with unclear status were discussed among the investigators to reach a consensus. The extent of periodontal disease was defined using the percentage of bleeding on probing for gingivitis cases (Chapple et al., 2018) and the 30% cut‐off of teeth affected at the worse stage to define localized and generalized periodontitis (Sanz et al., 2020).
2.5. Data analysis
Continuous variables are presented as mean values and standard deviations (SD) and categorical variables as frequency distributions. Non‐parametric Kruskal–Wallis test was applied to test differences in continuous variables among patient groups, and chi‐square test and Fisher's exact test were used to compare differences in categorical variables. A Spearman's rank correlation coefficient (Rho) was used for the analysis of the correlation between aMMP‐8 levels and periodontal clinical parameters. To assess the utility of the dichotomized aMMP‐8 POCT to discriminate different periodontal case definitions, a chi‐square test was used to calculate sensitivity, specificity, predictive value positive and predictive value negative based on a cut‐off value of 10 and 20 ng/ml. Sensitivity and specificity values were defined to be low (<60%), moderate (60%–79%) or high (80%) (Nelson et al., 2001). Logistic regression analysis was applied to explore the association of the aMMP‐8 test results with periodontitis and periodontal clinical parameters. Preliminary analyses assessed the unadjusted association in univariate analyses. Next, a multivariable model was constructed to adjust for other confounders (age, gender, etc.). Moreover, the diagnostic accuracy of the aMMP‐8 test results for predicting periodontitis was further evaluated with logistic regression. Model 1 was a crude analysis of a positive aMMP‐8 test. Model 2 was a crude analysis of aMMP‐8/NTP. Model 3 was the selection of the best significant subset of variables using risk factors/indicators and models 1 and 2. For each model, receiver operating characteristic (ROC) curves were constructed, and the area under the ROC curve (AUROC), sensitivity and specificity were estimated using different aMMP‐8/NTP threshold or the predicted probability levels. The corresponding cut‐off value was determined by optimizing sensitivity and specificity from ROC curves. The diagnostic accuracy results derived from the AUROC values were interpreted as low level (0.50–0.70), moderate level (0.71–0.90) and high level (>0.90) (Swets, 1988). p‐values <0.05 were considered to be statistically significant. Statistical analyses were performed using SPSS software, version 26.0 (IBM Corp.).
3. RESULTS
A total of 408 participants were enrolled from a potentially eligible population of 501 patients (Figure 1). Overall, 68.6% of participants were diagnosed with periodontitis and 36.8% with stage III/IV periodontitis, and 31.4% were non‐periodontitis cases. Table 1 displays the demographic and clinical characteristics of the study population. Of all participants, 7.9% were current smokers and 4.9% had diabetes. Subjects with periodontitis were more frequently heavy smokers and had poorly controlled diabetes compared with non‐periodontitis cases. Significantly smaller mean numbers of teeth were present in patients with stage III/IV periodontitis, especially in those with stage IV periodontitis (17.5 ± 7.8) compared with non‐periodontitis or stage I/II periodontitis patients. Other clinical parameters were significantly elevated with increasing severity of periodontal disease.
FIGURE 1.
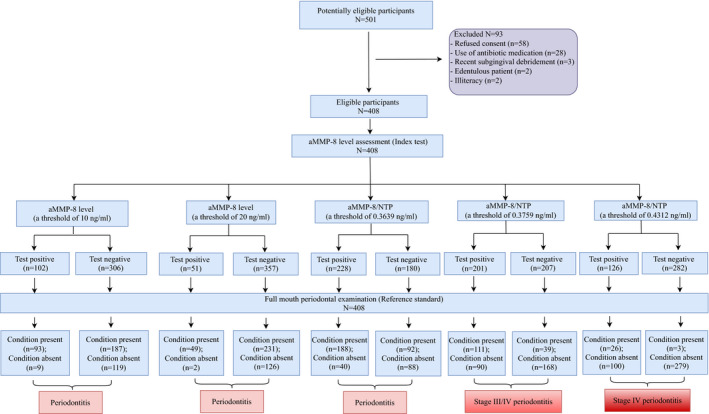
Standards for Reporting Diagnostic Accuracy (STARD) flow diagram of the study for various periodontal case definitions. Different thresholds of aMMP‐8 level or aMMP‐8/NTP showed different diagnostic performance for periodontitis
TABLE 1.
Demographic and clinical characteristics of the study population by periodontal case definitions (n = 408)
| Characteristics | Total (n = 408) | H (n = 66) | G (n = 62) | I (n = 65) | II (n = 65) | III (n − 121) | IV (n = 29) | P (n = 280) | p‐value |
|---|---|---|---|---|---|---|---|---|---|
| Demographic variables | NP/P | ||||||||
| Diabetes | 20 (4.9%) | 0 | 0 | 0 | 5 (7.7%) | 10 (8.3%) | 5 (17.2%) | 20 (7.1%) | 0.002 |
| Poorly controlled diabetesa | 13 (3.2%) | 0 | 0 | 0 | 2 (3.1%) | 6 (5.0%) | 5 (17.2%) | 12 (4.3%) | 0.022 |
| Non‐smokers | 365 (89.5%) | 66 (100.0%) | 61 (98.4%) | 64 (98.5%) | 58 (89.2%) | 98 (81.0%) | 18 (62.1%) | 238 (85.0%) | <0.001 |
| Current smokers | 32 (7.8%) | 0 | 1 (1.6%) | 1 (1.5%) | 5 (7.7%) | 18 (14.9%) | 7 (24.1%) | 31 (11.1%) | <0.001 |
| Heavy smokersb | 19 (4.7%) | 0 | 0 | 1 (1.5%) | 2 (3.1%) | 12 (9.9%) | 4 (13.8%) | 19 (6.8%) | 0.003 |
| Former smokers* | 11 (2.7%) | 0 | 0 | 0 | 2 (3.1%) | 5 (4.1%) | 4 (13.8%) | 11 (3.9%) | 0.020 |
| Clinical parameters | H–IV | ||||||||
| Number of teeth | 26.1 ± 4.3 | 26.7 ± 3.4 | 27.6 ± 1.4 | 28.0 ± 1.5 | 27.6 ± 1.9 | 25.3 ± 3.8 | 17.5 ± 7.8 | 25.6 ± 4.8 | <0.001 |
| Bleeding on probing (%) | 21.4 ± 15.1 | 5.0 ± 2.4 | 24.0 ± 12.5 | 22.5 ± 11.3 | 22.6 ± 10.7 | 23.8 ± 15.1 | 37.9 ± 21.6 | 24.7 ± 14.9 | <0.001 |
| Probing pocket depth (mm) | 2.48 ± 0.65 | 2.01 ± 0.17 | 2.16 ± 0.24 | 2.29 ± 0.21 | 2.39 ± 0.27 | 2.70 ± 0.50 | 3.90 ± 1.18 | 2.66 ± 0.69 | <0.001 |
| Clinical attachment loss (mm) | 1.87 ± 1.70 | 0.76 ± 0.73 | 0.34 ± 0.31 | 0.60 ± 0.42 | 1.82 ± 0.50 | 3.04 ± 0.76 | 5.78 ± 1.89 | 2.47 ± 1.70 | <0.001 |
| Furcation involvement ≥II (%) | 5.48 ± 15.04 | 0 | 0 | 0 | 0 | 11.75 ± 17.70 | 29.78 ± 31.33 | 7.99 ± 17.61 | <0.001 |
| Mobility ≥II (%) | 4.77 ± 14.86 | 0 | 0 | 0 | 0 | 5.32 ± 7.16 | 49.17 ± 31.00 | 7.39 ± 18.09 | <0.001 |
| Periodontitis grades | I–IV | ||||||||
| Grade A periodontitis | n/a | n/a | n/a | 8 (12.3%) | 7 (10.8%) | 0 | 0 | 15 (5.4%) | <0.001 |
| Grade B periodontitis | n/a | n/a | n/a | 52 (80.0%) | 55 (84.6%) | 47 (38.8%) | 0 | 154 (55.0%) | <0.001 |
| Grade C periodontitis | n/a | n/a | n/a | 5 (7.7%) | 3 (4.6%) | 74 (61.2%) | 29 (100%) | 111 (39.6%) | <0.001 |
| Periodontal disease extent | G–IV | ||||||||
| Localized | n/a | n/a | 48 (77.4%) | 5 (7.7%) | 14 (21.5%) | 66 (54.5%) | 0 | 85 (30.4%) | <0.001 |
| Generalized | n/a | n/a | 14 (22.6%) | 60 (92.3%) | 51 (78.5%) | 55 (45.5%) | 29 (100%) | 195 (69.6%) | <0.001 |
Additional demographic information is available in Appendix S1. Data are presented as either mean ± SD or n (%).
Chi‐square tests or Fisher's exact tests (for categorical data) and Kruskal–Wallis tests (for continuous data) were used to assess differences among groups.
H, periodontal health; G, gingivitis; I, stage I periodontitis; II, stage II periodontitis; III, stage III periodontitis; IV, stage IV periodontitis; P, periodontitis; and NP, non‐periodontitis.
n/a, not applicable: grade definitions were only applied to periodontitis subjects; extent assessment was only applied to gingivitis and periodontitis.
Poorly controlled diabetes = glycated haemoglobin ≥ 7.0%.
Heavy smokers (≥10 cigarettes/day) and *former smokers (smoking cessation ≥3 years).
3.1. Associations of a positive aMMP‐8 POCT with periodontal case definitions and periodontitis grade
The discriminative utility of the dichotomized aMMP‐8 POCT to identify different periodontal case definitions is presented in Table 2. The test positivity rates were significantly higher in any periodontitis (stages I‐IV) patients compared with non‐periodontitis cases. However, they were similar among different stages of periodontitis (p > 0.05). No significant difference was noted between gingivitis and periodontal health. At the threshold level of 10 ng/ml, the overall performance was slightly better for periodontitis than other disease categories, with a sensitivity of 33.2% and a specificity of 93.0%. At the threshold level of 20 ng/ml, sensitivity decreased and specificity increased for all case definitions. No significant difference in test positivity rates among periodontitis grades (A‐C) was observed from the chi‐square test.
TABLE 2.
The utility of the dichotomized aMMP‐8 POCT to discriminate various periodontal case definitions
| Diagnostic accuracy measures | Periodontal case definitions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Periodontal disease (n = 342) | Gingivitis (n = 62) | Periodontitis (n = 280) | The stages of periodontitis | p‐value P (I‐IV)/NP | p‐value G/H | p‐value I‐IV | ||||
| I (n = 65) | II (n = 65) | III (n = 121) | IV (n = 29) | |||||||
| threshold level = 10 ng/ml | ||||||||||
| Test positive (n/%) | 99 (28.9%) | 6 (9.7%) | 93 (33.2%) | 23 (35.4%) | 22 (33.8%) | 34 (28.1%) | 14 (48.3%) | <0.001 | 0.314 | 0.209 |
| Test negative (n/%) | 243 (71.1%) | 56 (90.3%) | 187 (66.8%) | 42 (64.6%) | 43 (66.2%) | 87 (71.9%) | 15 (51.7%) | <0.001 | 0.314 | 0.209 |
| Performance | ||||||||||
| Sensitivity | 28.9% | 9.7% | 33.2% | 35.4% | 33.8% | 28.1% | 48.3% | |||
| Specificity | 95.5% | 95.5% | 93.0% | 93.0% | 93.0% | 93.0% | 93.0% | |||
| Predictive value positive | 97.1% | 66.7% | 91.2% | 71.9% | 71.0% | 79.1% | 60.9% | |||
| Predictive value negative | 20.6% | 52.9% | 38.9% | 73.9% | 73.5% | 57.8% | 88.8% | |||
| Threshold level = 20 ng/ml | ||||||||||
| Test positive (n/%) | 51 (14.9%) | 2 (3.2%) | 49 (17.5%) | 10 (15.4%) | 8 (12.3%) | 21 (17.4%) | 10 (34.5%) | <0.001 | 0.233 | 0.065 |
| Test negative (n/%) | 291 (85.1%) | 60 (96.8%) | 231 (82.5%) | 55 (84.6%) | 57 (87.7%) | 100 (82.6%) | 19 (65.5%) | <0.001 | 0.233 | 0.065 |
| Performance | ||||||||||
| Sensitivity | 14.9% | 3.2% | 17.5% | 15.4% | 12.3% | 17.4% | 34.5% | |||
| Specificity | 100% | 100% | 98.4% | 98.4% | 98.4% | 98.4% | 98.4% | |||
| Predictive value positive | 100% | 100% | 96.1% | 83.3% | 80.0% | 91.3% | 83.3% | |||
| Predictive value negative | 18.5% | 52.4% | 35.3% | 69.6% | 68.9% | 55.8% | 86.9% | |||
Periodontal disease, gingivitis + periodontitis.
Chi‐square tests were used to assess differences between groups.
Performance refers to distinguish periodontal disease or gingivitis from periodontal health and distinguish periodontitis (stages I to IV) from non‐periodontitis.
H, periodontal health; G, gingivitis; I, stage I periodontitis; II, stage II periodontitis; III, stage III periodontitis; IV, stage IV periodontitis; P, periodontitis; and NP, non‐periodontitis.
To further explore associations of the aMMP‐8 POCT with periodontitis, logistic regression analysis was performed as displayed in Table 3. There were statistically significant associations between the positive aMMP‐8 POCT results and periodontitis, stage I/II periodontitis and stage IV periodontitis. When adjusting for age, gender, smoking and systemic disease, the correlation remained significant with periodontitis and stage I/II periodontitis, whereas the association was not significant with stage IV periodontitis. Among these variables, age and smoking were significant independent predictors for periodontitis, particularly for stage III/IV periodontitis.
TABLE 3.
Logistic regression analysis for the association of the aMMP‐8 test results with periodontitis case definition and periodontal clinical parameters
| (a) Periodontitis | ||||||
|---|---|---|---|---|---|---|
| Variables | Periodontitis versus periodontal health | Periodontitis versus gingivitis | Periodontitis versus periodontal health +gingivitis | |||
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Male | 1.68 (0.96–2.92) | 1.16 (0.67–2.02) | 1.40 (0.92–2.14) | |||
| Age | 1.09 (1.06 −1.12)*** | 1.09 (1.06 −1.11)*** | 1.19 (1.12–1.25)*** | 1.20 (1.13–1.28)*** | 1.12 (1.09 −1.14)*** | 1.11 (1.08–1.14)*** |
| Current smokers | / | / | 7.59 (1.02–56.73)* | 15.81 (2.13–117.15)** | 10.21 (1.31–79.72)* | |
| Systemic disease | 6.48 (1.97–21.30)** | / | / | 12.85 (3.96–41.73) *** | ||
| A positive test | 10.44 (3.19–34.15)*** | 11.13 (3.25–38.08)*** | 4.64 (1.93–11.17)** | 6.07 (2.26–16.33)*** | 6.58 (3.20–13.53)*** | 7.40 (3.33–16.44)*** |
| aMMP−8/NTP | 10.30 (1.69–62.86)* | 37.94 (2.68–537.07) | 14.28 (3.19–63.93)** | |||
| (b) Different stages of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Variables | Stage I/II periodontitis versus periodontal health + gingivitis | Stage III periodontitis versus periodontal health +gingivitis + stage I/II periodontitis | Stage IV periodontitis versus periodontal health + gingivitis + stage I–III periodontitis | |||
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Male | 1.76 (1.07–2.88)* | 1.81 (1.04–3.17)* | 0.75 (0.48–1.16) | 1.71 (0.79–3.67) | ||
| Age | 1.07 (1.04–1.10)*** | 1.07 (1.04–1.10)*** | 1.12 (1.10–1.14)*** | 1.13 (1.10–1.15)*** | 1.05 (1.03–1.08)*** | 1.05 (1.02–1.08)** |
| Current smokers | 6.15 (0.73–51.78) | 6.27 (2.54–15.45)*** | 10.74 (3.78–30.51)*** | 4.51 (1.76–11.56)** | 4.13 (1.30–13.10)* | |
| Systemic disease | 5.44 (1.53–19.26)** | 6.58 (3.58–12.13)*** | 3.38 (1.52–7.53)** | |||
| A positive test | 7.00 (3.25–15.097)*** | 6.73 (3.00–15.08)*** | 1.48 (0.90–2.43) | 3.09 (1.43–6.64)** | ||
| aMMP−8/NTP | 5.42 (1.51–19.43)** | 1.41 (0.98–2.04) | 2.55 (1.79–3.64)*** | 2.26 (1.56–3.27)*** | ||
| (c) Periodontal clinical parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | ≥2 sites with PDD ≥4 mm versus no sites with PDD ≥4 mm and 1 site with PPD = 4 mm | ≥1 site with bleeding pocket (PPD ≥ 4 mm) versus no sites with bleeding pocket (PPD ≥ 4 mm) | ≥1 site with PDD ≥6 mm versus no sites with PDD ≥6 mm | ≥1 site with bleeding pocket (PPD ≥ 6 mm) versus no sites with bleeding pocket (PPD ≥6 mm) | ||||
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Male | 1.83 (1.03–3.27)* | 1.98 (1.07–3.69)* | 1.57 (0.96–2.56) | 1.31 (0.89–1.94) | 1.33 (0.90–1.99) | |||
| Age | 1.07 (1.04–1.10)*** | 1.07 (1.04–1.10)*** | 1.05 (1.03–1.07)*** | 1.05 (1.03–1.07)*** | 1.06 (1.04–1.07)*** | 1.05 (1.04–1.07)*** | 1.05 (1.03–1.06)*** | 1.04 (1.03–1.06)*** |
| Current smokers | / | 8.92 (1.20–66.30) * | 4.87 (2.06–11.55)*** | 4.79 (1.96–11.68)** | 5.06 (2.21–11.56)*** | 5.22 (2.22–12.27)*** | ||
| Systemic disease | 6.77 (1.61–28.44)** | 2.65 (1.16–6.02)* | 3.70 (2.11–6.51)*** | 2.57 (1.52–4.37)*** | ||||
| A positive test | 23.62 (3.23–172.85)** | 19.43 (2.62–144.05)** | 6.87 (2.70–17.48)*** | 6.19 (2.40–15.95)*** | 2.47 (1.56–3.91)*** | 2.33 (1.40–3.88)** | 2.78 (1.75–4.40)*** | 2.71 (1.65–4.46)*** |
| aMMP−8/NTP | 2.46 (0.91–6.63) | 1.66 (0.94–2.94) | 1.75 (1.22–2.50)** | 1.63 (1.18–2.24)** | ||||
After adjustment for gender, age, smoking and systemic condition, p‐value and OR are provided when the variables remain in the final model; OR, odds ratio; 95% CI, confidence interval of 95%.
aMMP‐8/NTP, the total aMMP‐8 concentration divided by the number of teeth present.
PPD, probing pocket depth and bleeding pocket, PPD ≥4 mm or PPD ≥6 mm concurrent with bleeding on probing.
p < 0.05.
p < 0.01.
p <0.001.
3.2. Associations of the quantitative aMMP‐8 levels with periodontal case definitions and periodontitis grade
The quantitative aMMP‐8 levels according to case definitions or periodontitis grade are illustrated in Figure 2. The modified aMMP‐8 concentration was significantly higher in subjects with periodontitis than in non‐periodontitis cases. However, the concentration showed no association with the severity and grades of periodontitis. Notably, levels of aMMP‐8/NTP in subjects with stage IV periodontitis (median: 1.60 ng/ml; IQR: 0.46–2.58 ng/ml) were significantly higher than those with stage III/IV periodontitis (median: 0.43 ng/ml; IQR: 0.37–0.81 ng/ml), stage I/II periodontitis (median: 0.37 ng/ml; IQR: 0.36–0.52 ng/ml) and non‐periodontitis (median: 0.36 ng/ml; IQR: 0.36–0.37 ng/ml) cases (non‐periodontitis <stages I/II <stage III <stage IV periodontitis, p < 0.001). The grade C periodontitis patients had significantly higher levels of aMMP‐8/NTP (median: 0.43 ng/ml; IQR: 0.37–0.95 ng/ml) than grade B (median: 0.38 ng/ml; IQR: 0.35–0.55 ng/ml) and grade A (median: 0.37 ng/ml; IQR: 0.35–0.38 ng/ml) patients (A = B < C, p < 0.001). After adjusting for the confounders, aMMP‐8/NTP was only markedly correlated with stage IV periodontitis (Table 3). Additionally, aMMP‐8/NTP had odds ratio (OR) of 1.72 (CI: 1.18–2.51, p = 0.005) for grade C periodontitis.
FIGURE 2.
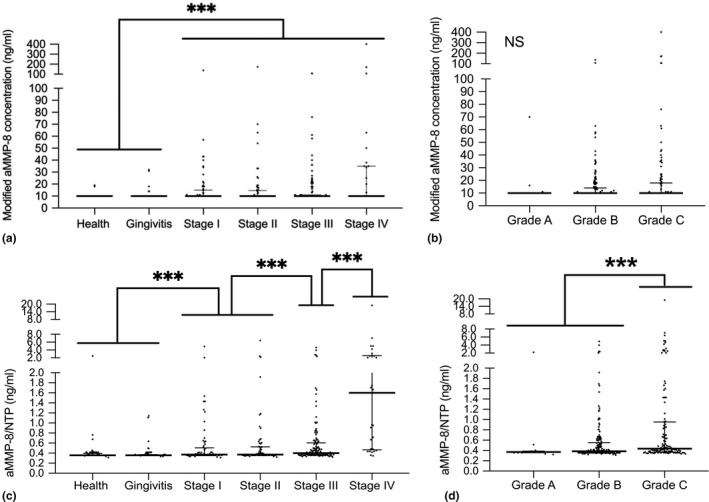
The quantitative aMMP‐8 levels by periodontal case definitions and periodontitis grade. Modified aMMP‐8 levels according to (a) case definitions and (b) periodontitis grade; aMMP‐8/NTP according to (c) case definitions and (d) periodontitis grade. Each dot represents one participant; the horizontal bars in each graph display the medians and interquartile ranges (IQR). Kruskal–Wallis tests were used to assess aMMP‐8 level or aMMP‐8/NTP differences among case definitions and the grading of periodontitis. *** p < 0.001. NS, not significant. Stage I, stage I periodontitis; stage II, stage II periodontitis; stage III, stage III periodontitis; and stage IV, stage IV periodontitis
3.3. Associations of the aMMP‐8 POCT and quantitative aMMP‐8 levels with periodontal clinical parameters
The correlation between the aMMP‐8 POCT and periodontal clinical parameters is presented in Table 3. Subjects with positive POCT results had increased OR (crude and adjusted) for having periodontal pockets (PPD ≥ 4 mm), deep pockets (PPD ≥ 6 mm) and bleeding pockets compared with those with negative results. Scatterplots were created for further analysis of the quantitative aMMP‐8 levels in relation to periodontal parameters (Figure 3). The aMMP‐8 concentrations of subjects with positive POCT results were positively correlated with the number of periodontal pockets, deep pockets, bleeding pockets and bleeding sites (p < 0.05). The strength of correlations between the aMMP‐8 levels from the whole population and those periodontal parameters was somewhat attenuated but still significant.
FIGURE 3.
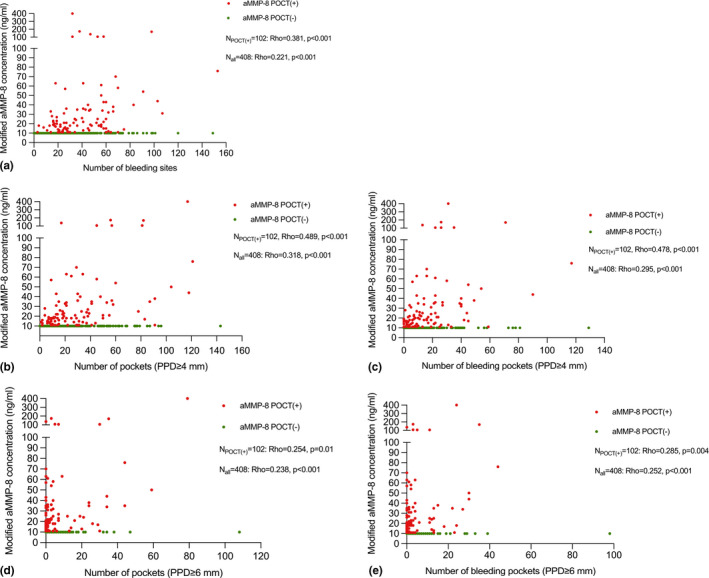
Associations between the quantitative aMMP‐8 levels and periodontal clinical parameters using Spearman's rank correlation coefficient: (a) aMMP‐8 concentrations and the number of bleeding sites: Rho = 0.381, p < 0.001 (subjects with positive POCT, n = 102), Rho = 0.221, p < 0.001 (all subjects, n = 408); (b) aMMP‐8 concentrations and the number of periodontal pockets (PD ≥ 4 mm): Rho = 0.489, p < 0.001 (subjects with positive POCT, n = 102), Rho = 0.318, p < 0.001 (all subjects, n = 408); (c) aMMP‐8 concentrations and the number of bleeding pockets (PD ≥ 4 mm): Rho = 0.478, p < 0.001 (subjects with positive POCT, n = 102), Rho = 0.295, p < 0.001 (all subjects, n = 408); (d) aMMP‐8 concentrations and the number of pockets (PD ≥ 6 mm): Rho = 0.254, p = 0.01 (subjects with positive POCT, n = 102), Rho = 0.238, p < 0.001 (all subjects, n = 408); and (e) aMMP‐8 concentrations and the number of bleeding pockets (PD ≥ 6 mm): Rho = 0.285, p = 0.004 (subjects with positive POCT, n = 102), Rho = 0.252, p < 0.001 (all subjects, n = 408). Red dot, subject with a positive aMMP‐8 POCT and green dot, subject with a negative aMMP‐8 POCT
3.4. Diagnostic utility of aMMP‐8‐based models for periodontitis
The diagnostic performance of models 1–3 for periodontitis is summarized in Table 4, and ROC curves are shown in Figure 4. Overall, the aMMP‐8 POCT (model 1) had a similar performance for differentiating between stage I/II periodontitis and non‐periodontitis compared with aMMP‐8/NTP (model 2); model 2 performed slightly better for the identification of stage III/IV periodontitis than model 1. More specifically, model 2 yielded much better screening ability for stage IV periodontitis than model 1, with an AUROC of 0.856, a sensitivity of 89.7% and a specificity of 73.6%. The model including subject age and smoking status (model 3) performed best for predicting periodontitis (including its different stages) than the other two models: it was 82.5% sensitive and 84.4% specific for detecting periodontitis, with an AUROC of 0.883.
TABLE 4.
The diagnostic utility of the aMMP‐8 test results and demographic factors to discriminate periodontitis
| Variables | Periodontitis (from the whole population) | Stage I/II periodontitis (from non‐periodontitis) | Stage III/IV periodontitis (from the whole population) | Stage IV periodontitis (from the whole population) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Male | ||||||||||||
| Age | X | X | X | X | ||||||||
| Current smokers | X | X | X | |||||||||
| Systemic disease | ||||||||||||
| A positive test | X | X | X | X | X | X | ||||||
| aMMP−8/NTP | X | X | X | X | X | |||||||
| Performance | ||||||||||||
| AUROC (95% CI) | 0.631 (0.576–0.685) | 0.690 (0.639–0.742) | 0.883 (0.845–0.915) | 0.638 (0.570–0.706) | 0.605 (0.536–0.674) | 0.785 (0.729–0.841) | 0.555 (0.497–0.614) | 0.706 (0.652–0.760) | 0.921 (0.895–0.947) | 0.625 (0.513–0.738) | 0.856 (0.775–0.936) | 0.865 (0.807–0.924) |
| Threshold or probability level | 10 ng/ml | 0.3639 ng/ml | 0.523 | 10 ng/ml | 0.4354 ng/ml | 0.48 | 10 ng/ml | 0.3759 ng/ml | 0.212 | 10 ng/ml | 0.4312 ng/ml | 0.067 |
| Sensitivity | 33.2% | 67.1% | 82.5% | 34.6% | 30.0% | 66.2% | 32.0% | 74.0% | 94.7% | 48.3% | 89.7% | 86.2% |
| Specificity | 93.0% | 68.8% | 84.4% | 93.0% | 93.0% | 82.8% | 79.1% | 65.1% | 79.5% | 76.8% | 73.6% | 74.9% |
Model 1 was a crude analysis of a positive aMMP‐8 test; model 2 was a crude analysis of aMMP‐8/NTP; and model 3 was the selection of the best significant subset of variables using the demographic factors and models 1–2.
Abbreviations: AUROC, area under receiver operator characteristic curve; 95% CI, confidence interval of 95%.
aMMP‐8/NTP, the total aMMP‐8 concentration divided by the number of teeth present and non‐periodontitis = periodontal health + gingivitis.
FIGURE 4.
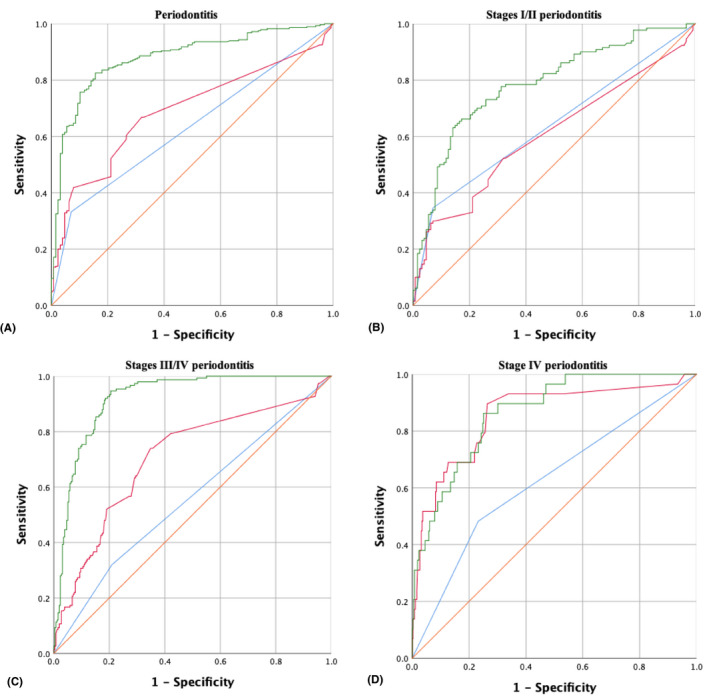
Receiver operating characteristic (ROC) curves: (a) ROC curves of model 1 (blue line), model 2 (red line) and model 3 (green line) for predicting periodontitis from the whole population; (b) ROC curves of model 1 (blue line), model 2 (red line) and model 3 (green line) for predicting stage I/II periodontitis from non‐periodontitis; (c) ROC curves of model 1 (blue line), model 2 (red line) and model 3 (green line) for predicting stage III/IV periodontitis from the whole population; and (d) ROC curves of model 1 (blue line), model 2 (red line) and model 3 (green line) for predicting stage IV periodontitis from the whole population. Please see text for definition of the various models
4. DISCUSSION
This study reports the diagnostic characteristics of an aMMP‐8 POCT to identify subjects with periodontal health and disease using the 2017 classification system. The key findings are that (i) positive test results and elevated aMMP‐8 levels were significantly associated with periodontitis, measures of periodontal pockets and bleeding on probing; (ii) the test has better specificity than sensitivity across the spectrum of disease and, therefore, the diagnostic performance of the test depends greatly upon the diagnostic question being asked; (iii) the diagnostic accuracy of the test was moderate; and (iv) multivariate models including the test and subject characteristics performed better than the test alone.
The present study observed increased odds ratios for the presence of moderate periodontal pocketing and bleeding pockets among subjects with a positive test. Positive associations of the aMMP‐8 levels with the number of periodontal pockets, bleeding pockets and bleeding sites were also observed across the entire population, particularly in subjects with positive POCT results. These data are interesting as they confirm previous studies and support the biological mechanism of the test: (i) microbe–host dysbiosis up‐regulating the expression and activation of MMP‐8 and (ii) the cleavage of collagen fibrils of the periodontium by aMMP‐8 resulting in pocket deepening (Golub et al., 1997; Sorsa et al., 1988; Sorsa et al., 2016). The aMMP‐8 levels correlated better with periodontal pockets than bleeding on probing, consistent with previous findings (Räisänen et al., 2018). This may explain the unsatisfactory diagnostic performance for gingivitis.
A statistically significant correlation between the positive test and periodontitis was found in the present study, and the strong correlation remained significant after adjustment for potential confounders. Despite a significant difference, this aMMP‐8 POCT alone may not be considered a suitable screening tool for periodontitis owing to its low sensitivity (lower than 40%). The aMMP‐8 POCT, however, is highly specific (greater than 90%) and less likely to produce false‐positive results. Thus, a positive test result is useful for “ruling in” disease (Akobeng, 2007).
Although the aMMP‐8 POCT does not seem to be a sensitive tool for the identification of periodontitis, the discriminatory capability greatly improved when the test and the common risk factors/indicators (i.e. age and tobacco smoking) of periodontitis were combined. This test may therefore be usefully incorporated in a multi‐test strategy. Consistent with these findings, recent data showed that the use of an MMP‐9 point‐of‐care test in conjunction with demographic and lifestyle behavioural factors could facilitate the detection of periodontitis (Kim et al., 2020). Building upon and expanding the above understandings, the aMMP‐8 POCT might be used in combination with other sensitive screening methods for detecting periodontitis. The initial screening test is required to reveal as many potential disease cases as possible, while a subsequent aMMP‐8 POCT might be useful to confirm that a positive test detected in the screening process is a true‐positive case.
The sensitivity and specificity values observed in this study differ from those reported in two recent systemic reviews, which indicate that the sensitivity of salivary MMP‐8 ranged from 56% to 93% and the specificity ranged from 48% to 87%, with an overall synthesis of 72.5% sensitivity and 70.5% specificity in the meta‐analysis (Arias‐Bujanda et al., 2020; Kc et al., 2020). The discrepancy may arise from the disparate detection methods (ELISA, IFMA, multiplex cytometry assay, lateral flow immunoassay, etc.) and detection thresholds (ranging from 10 to 36,733 ng/ml) adopted. Different detection methods performed differently in previous studies (Gursoy et al., 2010; Sorsa et al., 2010). One explanation may be that various techniques may detect different molecular forms (pro or active), types (neutrophil or mesenchymal) and sizes of MMP‐8. The detection method highly relies on antibody specificity and high affinity. For example, IFMA and lateral flow immunoassay used the same monoclonal antibody that mainly identifies active forms of neutrophil‐type and fibroblast‐type MMP‐8 (Sorsa et al., 2010), while ELISA detects almost all forms of MMP‐8, including pro‐ and active forms as well as its TIMP complexes (Gul et al., 2020; Lobmann et al., 2002; Sorsa et al., 2016). It must also be noted that no studies assessing the performance of the aMMP‐8 POCT were included in one of the two discussed systematic reviews, and only 2 out 5 studies were included in the other because those studies based on the aMMP‐8 POCT failed to meet the inclusion criteria. A comparison with other original research studies evaluating the validity of the aMMP‐8 POCT, however, is still challenging largely due to variability in study designs, case definition and study populations. A systematic search of the evidence for this report identified 11 relevant studies (see Appendix S1; Heikkinen et al., 2016; Heikkinen et al., 2019; Izadi Borujeni et al., 2015; Johnson et al., 2016; Leppilahti et al., 2018; Lorenz et al., 2017; Nwhator et al., 2014; Räisänen et al., 2019; Schmalz et al., 2019; Schmidt et al., 2018; Sorsa et al., 2020). Five of them were eligible for assessing the risk of bias using the critical review checklist of the revised Quality Assessment of Diagnostic Studies (QUADAS‐2) (Whiting et al., 2011). In the patient selection domain, 4 of 5 papers had a high risk of bias due to the case–control designs, the inappropriate exclusions and sampling methods. In the reference standard domain, 4 of 5 studies did not assess a full‐mouth clinical attachment level, indicating a high risk of bias. Only two studies reported the blinding protocols for index test interpretation and reference standard assessment, and only two studies reported the calibration for clinical measurements. Additionally, most of these studies had a relatively small sample size and no power analysis was performed. In view of the aforementioned aspects, the sensitivity and specificity values in previous studies may deviate from the “true” value, thereby resulting in overestimation or underestimation of diagnostic accuracy to a certain extent.
Notably, most of the previous studies did not consider a scenario with extensive tooth loss, which is particularly important for analysing oral fluids containing gingival crevicular fluid (GCF). GCF includes both physiological transudate and pathological exudate components originating from blood vessels of periodontal tissue and is thus considered potentially valuable for indicating disease activity (Taylor & Preshaw, 2016; Wassall & Preshaw, 2016). Since oral rinse contains the pooled samples of GCF, it is reasonable to propose “aMMP‐8/NTP” to assess periodontal status. In more advanced stages of periodontitis, the inflammatory exudates through periodontal pockets of periodontally compromised teeth might be decreased due to tooth extractions since the aMMP‐8 levels in oral rinse are conceivably influenced by the amount of GCF contributed from each tooth. Interestingly, aMMP‐8/NTP performed similarly in detecting stage I/II periodontitis (basically without tooth loss), slightly better for predicting stage III/IV periodontitis (with some tooth loss) and remarkably better in the identification of stage IV periodontitis (with extensive tooth loss) compared with the positive aMMP‐8 test. Such findings may suggest reasonable consideration for the influence of extensive tooth loss in oral rinse aMMP‐8 test for future studies.
Another important aspect of this study is the association between periodontitis grade and POCT test results when the number of teeth present corrected aMMP‐8 levels. This finding needs to be interpreted with caution, as many grade C subjects were stage IV periodontitis subjects with extensive tooth loss. Previous reports have suggested that aMMP‐8 may be strongly associated with periodontitis grade (Sorsa et al., 2020). Additional well‐designed and conducted studies are required to better understand the diagnostic utility aMMP‐8 test results as an estimate of periodontitis grade. Moreover, previous work showed that this test was associated with gene polymorphisms linked to initial periodontitis in adolescents (Heikkinen et al., 2017). In the present study, the test detected different stages of periodontitis with similar diagnostic values. However, a recent study showed no significant differences in aMMP‐8 levels between stage I periodontitis and periodontal health using aMMP‐8 POCT (Sorsa et al., 2020). More studies are needed in this area.
The present study has a number of strengths including (i) use of the most up‐to‐date classification of periodontal diseases; (ii) adoption of a comprehensive periodontal examination protocol by a single trained and calibrated examiner; (iii) recruitment of an appropriate sample size with sufficient statistical power; (iv) control of the potential confounders; (v) avoidance of a case‐control design; (vi) consideration of blinding in interpreting results of the index test and the reference standard; and (vii) inclusion of a broad spectrum of disease (periodontal health, gingivitis and different stages of periodontitis). Therefore, controlling the risk of bias as mentioned above minimized underestimation or overestimation of test accuracy.
An important limitation of this study is the limit of detection of the POCT (10 ng/ml), which largely restricts the possibility of exploring more optimal thresholds, which may be lower than the detection limit. In this study, 75% of test results were below detection level.
In conclusion, the adopted aMMP‐8 POCT has better specificity than sensitivity across the spectrum of disease. The test combined with subject characteristics helps to detect periodontitis, indicating its potential application in screening of periodontitis in conjunction with other risk factors/indicators and/or additional sensitive tests. More investigations are needed in this area. Moreover, aMMP‐8/NTP may have some additional potentials for indicating periodontitis patients with extensive tooth loss and predicting a rapid disease progression. Future studies should perform validation analyses to verify the present findings and fine‐tune the threshold values of a POCT kit assisting in disease detection for different screening and diagnostic questions and populations.
CONFLICT OF INTERESTS
The authors report no conflict of interest with this study.
Supporting information
Appendix S1
Funding information
The Hong Kong Human Medical Research Fund (HMRF) Grant No: 07182796 to MST supported this study. KD is the recipient of a Hong Kong higher education fellowship
DATA AVAILABILITY STATEMENT
The author elects to not share data.
REFERENCES
- Akobeng, A. K. (2007). Understanding diagnostic tests 1: sensitivity, specificity and predictive values. Acta Paediatrica, 96(3), 338–341. 10.1111/j.1651-2227.2006.00180.x [DOI] [PubMed] [Google Scholar]
- Arias‐Bujanda, N., Regueira‐Iglesias, A., Balsa‐Castro, C., Nibali, L., Donos, N., & Tomas, I. (2020). Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(1), 2–18. 10.1111/jcpe.13202 [DOI] [PubMed] [Google Scholar]
- Birkedal‐Hansen, H. (1993). Role of cytokines and inflammatory mediators in tissue destruction. Journal of Periodontal Research, 28(6 Pt 2), 500–510. 10.1111/j.1600-0765.1993.tb02113.x [DOI] [PubMed] [Google Scholar]
- Birkedal‐Hansen, H., Moore, W. G., Bodden, M. K., Windsor, L. J., Birkedal‐Hansen, B., DeCarlo, A., & Engler, J. A. (1993). Matrix metalloproteinases: a review. Critical Reviews in Oral Biology and Medicine, 4(2), 197–250. 10.1177/10454411930040020401 [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. C., Mealey, B. L., Van Dyke, T. E., Bartold, P. M., Dommisch, H., Eickholz, P., Geisinger, M. L., Genco, R. J., Glogauer, M., Goldstein, M., Griffin, T. J., Holmstrup, P., Johnson, G. K., Kapila, Y., Lang, N. P., Meyle, J., Murakami, S., Plemons, J., Romito, G. A., … Yoshie, H. (2018). Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl 20), S68–S77. 10.1111/jcpe.12940 [DOI] [PubMed] [Google Scholar]
- Cohen, J. F., Korevaar, D. A., Altman, D. G., Bruns, D. E., Gatsonis, C. A., Hooft, L., Irwig, L., Levine, D., Reitsma, J. B., de Vet, H. C. W., & Bossuyt, P. M. M. (2016). STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. British Medical Journal Open, 6(11), e012799. 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morais, E. F., Pinheiro, J. C., Leite, R. B., Santos, P. P. A., Barboza, C. A. G., & Freitas, R. A. (2018). Matrix metalloproteinase‐8 levels in periodontal disease patients: A systematic review. Journal of Periodontal Research, 53(2), 156–163. 10.1111/jre.12495 [DOI] [PubMed] [Google Scholar]
- DeCarlo, A. A., Grenett, H. E., Harber, G. J., Windsor, L. J., Bodden, M. K., Birkedal‐Hansen, B., & Birkedal‐Hansen, H. (1998). Induction of matrix metalloproteinases and a collagen‐degrading phenotype in fibroblasts and epithelial cells by secreted Porphyromonas gingivalis proteinase. Journal of Periodontal Research, 33(7), 408–420. 10.1111/j.1600-0765.1998.tb02337.x [DOI] [PubMed] [Google Scholar]
- DeCarlo, A. A.Jr, Windsor, L. J., Bodden, M. K., Harber, G. J., Birkedal‐Hansen, B., & Birkedal‐Hansen, H. (1997). Activation and novel processing of matrix metalloproteinases by a thiol‐proteinase from the oral anaerobe Porphyromonas gingivalis. Journal of Dental Research, 76(6), 1260–1270. 10.1177/00220345970760060501 [DOI] [PubMed] [Google Scholar]
- Deng, K., Pelekos, G., Jin, L. J., & Tonetti, M. S. (2021). Diagnostic accuracy of self‐reported measures of periodontal disease: a clinical validation study using the 2017 care definitions. Journal of Clinical Periodontology, 10.1111/jcpe.13484 [DOI] [PubMed] [Google Scholar]
- Department of Health, Hong Kong SAR Government (2011). Oral Health Survey 2011. Retrieved from: http://www.toothclub.gov.hk/en/en_pdf/Oral_Health_Survey_2011/Oral_Health_Survey_2011_WCAG_20141112_(EN_Full).pdf (accessed on 24 November 2018). [Google Scholar]
- Giannobile, W. V., Beikler, T., Kinney, J. S., Ramseier, C. A., Morelli, T., & Wong, D. T. (2009). Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontology 2000, 50(1), 52–64. 10.1111/j.1600-0757.2008.00288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub, L. M., Lee, H. M., Greenwald, R. A., Ryan, M. E., Sorsa, T., Salo, T., & Giannobile, W. V. (1997). A matrix metalloproteinase inhibitor reduces bone‐type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflammation Research, 46(8), 310–319. 10.1007/s000110050193 [DOI] [PubMed] [Google Scholar]
- Gul, S. S., Abdulkareem, A. A., Sha, A. M., & Rawlinson, A. (2020). Diagnostic accuracy of oral fluids biomarker profile to determine the current and future status of periodontal and peri‐implant diseases. Diagnostics (Basel, Switzerland), 10(10), 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy, U. K., Könönen, E., Pradhan‐Palikhe, P., Tervahartiala, T., Pussinen, P. J., Suominen‐Taipale, L., & Sorsa, T. (2010). Salivary MMP‐8, TIMP‐1, and ICTP as markers of advanced periodontitis. Journal of Clinical Periodontology, 37(6), 487–493. 10.1111/j.1600-051X.2010.01563.x [DOI] [PubMed] [Google Scholar]
- Hanemaaijer, R., Sorsa, T., Konttinen, Y. T., Ding, Y., Sutinen, M., Visser, H., van Hinsbergh, V. W., Helaakoski, T., Kainulainen, T., Rönkä, H., Tschesche, H., & Salo, T. (1997). Matrix metalloproteinase‐8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor‐alpha and doxycycline. The Journal of Biological Chemistry, 272(50), 31504–31509. 10.1074/jbc.272.50.31504 [DOI] [PubMed] [Google Scholar]
- Heikkinen, A. M., Nwhator, S. O., Rathnayake, N., Mantyla, P., Vatanen, P., & Sorsa, T. (2016). Pilot Study on Oral Health Status as Assessed by an Active Matrix Metalloproteinase‐8 Chairside Mouthrinse Test in Adolescents. Journal of Periodontology, 87(1), 36–40. 10.1902/jop.2015.150377 [DOI] [PubMed] [Google Scholar]
- Heikkinen, A. M., Raisanen, I. T., Tervahartiala, T., & Sorsa, T. (2019). Cross‐sectional analysis of risk factors for subclinical periodontitis; active matrix metalloproteinase‐8 as a potential indicator in initial periodontitis in adolescents. Journal of Periodontology, 90(5), 484–492. 10.1002/JPER.18-0450 [DOI] [PubMed] [Google Scholar]
- Heikkinen, A. M., Raivisto, T., Kettunen, K., Kovanen, L., Haukka, J., Pakbaznejad Esmaeili, E., Elg, J., Gieselmann, D. R., Rathnayake, N., Ruokonen, H., Tervahartiala, T., & Sorsa, T. (2017). Pilot Study on the Genetic Background of an Active Matrix Metalloproteinase‐8 Test in Finnish Adolescents. Journal of Periodontology, 88(5), 464–472. [DOI] [PubMed] [Google Scholar]
- Izadi Borujeni, S., Mayer, M., & Eickholz, P. (2015). Activated matrix metalloproteinase‐8 in saliva as diagnostic test for periodontal disease? A case‐control study. Medical Microbiology and Immunology, 204(6), 665–672. 10.1007/s00430-015-0413-2 [DOI] [PubMed] [Google Scholar]
- Johnson, N., Ebersole, J. L., Kryscio, R. J., Danaher, R. J., Dawson, D.3rd, Al‐Sabbagh, M., & Miller, C. S. (2016). Rapid assessment of salivary MMP‐8 and periodontal disease using lateral flow immunoassay. Oral Diseases, 22(7), 681–687. 10.1111/odi.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kc, S., Wang, X. Z., & Gallagher, J. E. (2020). Diagnostic sensitivity and specificity of host‐derived salivary biomarkers in periodontal disease amongst adults: Systematic review. Journal of Clinical Periodontology, 47(3), 289–308. 10.1111/jcpe.13218 [DOI] [PubMed] [Google Scholar]
- Kim, H.‐D., Lee, C.‐S., Cho, H.‐J., Jeon, S., Choi, Y.‐N., Kim, S. T., Kim, D. H., Jin Lee, H., Vu, H., Jeong, H.‐J., & Kim, B. G. (2020). Diagnostic ability of salivary matrix metalloproteinase‐9 lateral flow test point‐of‐care test for periodontitis. Journal of Clinical Periodontology, 47(11), 1354–1361. 10.1111/jcpe.13360 [DOI] [PubMed] [Google Scholar]
- Leppilahti, J. M., Ahonen, M.‐M., Hernández, M., Munjal, S., Netuschil, L., Uitto, V.‐J., Sorsa, T., & Mäntylä, P. (2011). Oral rinse MMP‐8 point‐of‐care immuno test identifies patients with strong periodontal inflammatory burden. Oral Diseases, 17(1), 115–122. 10.1111/j.1601-0825.2010.01716.x [DOI] [PubMed] [Google Scholar]
- Leppilahti, J., Harjunmaa, U., Järnstedt, J., Mangani, C., Hernández, M., Tervahartiala, T., Lopez, R., Ashorn, U., Ashorn, P., Gieselmann, D.‐R., & Sorsa, T. (2018). Diagnosis of newly delivered mothers for periodontitis with a novel oral‐rinse aMMP‐8 point‐of‐care test in a rural malawian population. Diagnostics (Basel, Switzerland), 8(3), 67. 10.3390/diagnostics8030067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppilahti, J. M., Hernández‐Ríos, P. A., Gamonal, J. A., Tervahartiala, T., Brignardello‐Petersen, R., Mantyla, P., Sorsa, T., & Hernández, M. (2014). Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site‐specific diagnostic value for chronic periodontitis. Journal of Clinical Periodontology, 41(4), 348–356. 10.1111/jcpe.12223 [DOI] [PubMed] [Google Scholar]
- Lobmann, R., Ambrosch, A., Schultz, G., Waldmann, K., Schiweck, S., & Lehnert, H. (2002). Expression of matrix‐metalloproteinases and their inhibitors in the wounds of diabetic and non‐diabetic patients. Diabetologia, 45(7), 1011–1016. [DOI] [PubMed] [Google Scholar]
- Lorenz, K., Keller, T., Noack, B., Freitag, A., Netuschil, L., & Hoffmann, T. (2017). Evaluation of a novel point‐of‐care test for active matrix metalloproteinase‐8: agreement between qualitative and quantitative measurements and relation to periodontal inflammation. Journal of Periodontal Research, 52(2), 277–284. 10.1111/jre.12392 [DOI] [PubMed] [Google Scholar]
- Nelson, D. E., Holtzman, D., Bolen, J., Stanwyck, C. A., & Mack, K. A. (2001). Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS). Sozial‐ Und Präventivmedizin, 46(Suppl 1), S3–S42. [PubMed] [Google Scholar]
- Nwhator, S. O., Ayanbadejo, P. O., Umeizudike, K. A., Opeodu, O. I., Agbelusi, G. A., Olamijulo, J. A., Arowojolu, M. O., Sorsa, T., Babajide, B. S., & Opedun, D. O. (2014). Clinical correlates of a lateral‐flow immunoassay oral risk indicator. Journal of Periodontology, 85(1), 188–194. 10.1902/jop.2013.130116 [DOI] [PubMed] [Google Scholar]
- Papapanou, P. N., Sanz, M., Buduneli, N., Dietrich, T., Feres, M., Fine, D. H., Flemmig, T. F., Garcia, R., Giannobile, W. V., Graziani, F., Greenwell, H., Herrera, D., Kao, R. T., Kebschull, M., Kinane, D. F., Kirkwood, K. L., Kocher, T., Kornman, K. S., Kumar, P. S., … Tonetti, M. S. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl 20), S162–S170. 10.1111/jcpe.12946 [DOI] [PubMed] [Google Scholar]
- Räisänen, I. T., Heikkinen, A. M., Siren, E., Tervahartiala, T., Gieselmann, D. R., van der Schoor, G. J., van der Schoor, P., & Sorsa, T. (2018). Point‐of‐care/Chairside aMMP‐8 analytics of periodontal diseases’ activity and episodic progression. Diagnostics (Basel, Switzerland), 8(4), 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räisänen, I. T., Sorsa, T., van der Schoor, G. J., Tervahartiala, T., van der Schoor, P., Gieselmann, D. R., & Heikkinen, A. M. (2019). Active Matrix Metalloproteinase‐8 Point‐of‐Care (PoC)/Chairside Mouthrinse Test vs. Bleeding on Probing in Diagnosing Subclinical Periodontitis in Adolescents. Diagnostics (Basel, Switzerland), 9(1), 10.3390/diagnostics9010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz, M., Papapanou, P. N., Tonetti, M. S., Greenwell, H., & Kornman, K. (2020). Guest Editorial: Clarifications on the use of the new classification of periodontitis. Journal of Clinical Periodontology, 47(6), 658–659. [DOI] [PubMed] [Google Scholar]
- Sapna, G., Gokul, S., & Bagri‐Manjrekar, K. (2014). Matrix metalloproteinases and periodontal diseases. Oral Diseases, 20(6), 538–550. 10.1111/odi.12159 [DOI] [PubMed] [Google Scholar]
- Schmalz, G., Hubscher, A. E., Angermann, H., Schmidt, J., Schmickler, J., Legler, T. J., & Ziebolz, D. (2019). Associations of chairside salivary aMMP‐8 findings with periodontal parameters, potentially periodontal pathogenic bacteria and selected blood parameters in systemically healthy adults. Diagnostic Microbiology and Infectious Disease, 95(2), 179–184. 10.1016/j.diagmicrobio.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Schmidt, J., Guder, U., Kreuz, M., Löffler, M., Kiess, W., Hirsch, C., Ziebolz, D., & Haak, R. (2018). aMMP‐8 in correlation to caries and periodontal condition in adolescents‐results of the epidemiologic LIFE child study. Clinical Oral Investigations, 22(1), 449–460. 10.1007/s00784-017-2132-0 [DOI] [PubMed] [Google Scholar]
- Sorsa, T., Alassiri, S., Grigoriadis, A., Räisänen, I. T., Pärnänen, P., Nwhator, S. O., Gieselmann, D.‐R., & Sakellari, D. (2020). Active MMP‐8 (aMMP‐8) as a Grading and Staging Biomarker in the Periodontitis Classification. Diagnostics (Basel, Switzerland), 10(2), 61. 10.3390/diagnostics10020061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsa, T., Gieselmann, D.‐R., Korvuo, A., Maier, K., Mäntylä, P., Råman, I., & Tiisala, S. (2019). MMP‐8 activation product, its determination and use. U.S. Patent No. 10,488,415, U.S. Patent and Trademark Office [Google Scholar]
- Sorsa, T., Gursoy, U. K., Nwhator, S., Hernandez, M., Tervahartiala, T., Leppilahti, J., Gursoy, M., Könönen, E., Emingil, G., Pussinen, P. J., & Mäntylä, P. (2016). Analysis of matrix metalloproteinases, especially MMP‐8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontology 2000, 70(1), 142–163. 10.1111/prd.12101 [DOI] [PubMed] [Google Scholar]
- Sorsa, T., Heikkinen, A. M., Leppilahti, J., Tervahartiala, T., Nwhator, S., Rathnayake, N., Mäntylä, P., Gieselmann, D.‐R., & Netuschil, L. (2018). Active matrix metalloproteinase‐8: contributor to periodontitis and a missing link between genetics, dentistry, and medicine. In Pathogenesis of periodontal diseases (pp. 51–57). Cham: Springer International Publishing. [Google Scholar]
- Sorsa, T., Hernandez, M., Leppilahti, J., Munjal, S., Netuschil, L., & Mantyla, P. (2010). Detection of gingival crevicular fluid MMP‐8 levels with different laboratory and chair‐side methods. Oral Diseases, 16(1), 39–45. 10.1111/j.1601-0825.2009.01603.x [DOI] [PubMed] [Google Scholar]
- Sorsa, T., Mäntylä, P., Rönkä, H., Kallio, P., Kallis, G. B., Lundqvist, C., Kinane, D. F., Salo, T., Golub, L. M., Teronen, O., & Tikanoja, S. (1999). Scientific basis of a matrix metalloproteinase‐8 specific chair‐side test for monitoring periodontal and peri‐implant health and disease. Annals of the New York Academy of Sciences, 878(1), 130–140. 10.1111/j.1749-6632.1999.tb07679.x [DOI] [PubMed] [Google Scholar]
- Sorsa, T., Uitto, V. J., Suomalainen, K., Vauhkonen, M., & Lindy, S. (1988). Comparison of interstitial collagenases from human gingiva, sulcular fluid and polymorphonuclear leukocytes. Journal of Periodontal Research, 23(6), 386–393. 10.1111/j.1600-0765.1988.tb01618.x [DOI] [PubMed] [Google Scholar]
- Swets, J. A. (1988). Measuring the accuracy of diagnostic systems. Science, 240(4857), 1285–1293. 10.1126/science.3287615 [DOI] [PubMed] [Google Scholar]
- Taylor, J. J., & Preshaw, P. M. (2016). Gingival crevicular fluid and saliva. Periodontology 2000, 70(1), 7–10. 10.1111/prd.12118 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S., Greenwell, H., & Kornman, K. S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Clinical Periodontology, 45(Suppl 20), S149–S161. 10.1111/jcpe.12945 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S., Jepsen, S., Jin, L., & Otomo‐Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology, 44(5), 456–462. 10.1111/jcpe.12732 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S., & Sanz, M. (2019). Implementation of the new classification of periodontal diseases: Decision‐making algorithms for clinical practice and education. Journal of Clinical Periodontology, 46(4), 398–405. 10.1111/jcpe.13104 [DOI] [PubMed] [Google Scholar]
- Trombelli, L., Farina, R., Silva, C. O., & Tatakis, D. N. (2018). Plaque‐induced gingivitis: Case definition and diagnostic considerations. Journal of Clinical Periodontology, 45(Suppl 20), S44–S67. 10.1111/jcpe.12939 [DOI] [PubMed] [Google Scholar]
- Van Wart, H. E., & Birkedal‐Hansen, H. (1990). The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proceedings of the National Academy of Sciences, 87(14), 5578–5582. 10.1073/pnas.87.14.5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassall, R. R., & Preshaw, P. M. (2016). Clinical and technical considerations in the analysis of gingival crevicular fluid. Periodontology 2000, 70(1), 65–79. 10.1111/prd.12109 [DOI] [PubMed] [Google Scholar]
- Whiting, P. F., Rutjes, A. W., Westwood, M. E., Mallett, S., Deeks, J. J., Reitsma, J. B., & Bossuyt, P. M. (2011). QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine, 155(8), 529–536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- Zhang, L., Li, X., Yan, H., & Huang, L. (2018). Salivary matrix metalloproteinase (MMP)‐8 as a biomarker for periodontitis: A PRISMA‐compliant systematic review and meta‐analysis. Medicine (Baltimore), 97(3), e9642. 10.1097/MD.0000000000009642 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The author elects to not share data.


