Abstract
Channelrhodopsin‐2 (ChR2) is a light‐gated cation channel and was used to lay the foundations of optogenetics. Its dark state X‐ray structure has been determined in 2017 for the wild‐type, which is the prototype for all other ChR variants. However, the mechanistic understanding of the channel function is still incomplete in terms of structural changes after photon absorption by the retinal chromophore and in the framework of functional models. Hence, detailed information needs to be collected on the dark state as well as on the different photointermediates. For ChR2 detailed knowledge on the chromophore configuration in the different states is still missing and a consensus has not been achieved. Using DNP‐enhanced solid‐state MAS NMR spectroscopy on proteoliposome samples, we unambiguously determined the chromophore configuration in the desensitized state, and we show that this state occurs towards the end of the photocycle.
Keywords: channelrhodopsin, dynamic nuclear polarization, membrane proteins, photocycle, solid-state NMR spectroscopy
Channelrhodopsin‐2, a light driven cation channel with a retinal chromophore, undergoes a cyclic photoreaction with several photointermediates. Here, we analyze the chromophore configuration of the intermediates, which can be cryo‐trapped, in detail by DNP‐enhanced solid‐state NMR spectroscopy and reveal that the desensitized state is in a 13‐cis,15‐syn configuration. Our results also shed further light on the photocycle of the protein.
Introduction
Microbial rhodopsins are heptahelical membrane proteins with a retinal chromophore covalently bound to a conserved lysine in helix G. A large variety of light‐driven functions is carried out by this protein family comprising proton and ion pumps, channels as well as sensors.[1] In the dark‐adapted state the chromophore is either purely in the all‐trans,15‐anti configuration or a mixture of configurations is observed. Some microbial rhodopsins show light‐adaption which refers to light‐induced changes in the protein that remain even after the light has been switched off for some time. Usually, the function of the protein is conveyed by the photoreaction that starts from the all‐trans,15‐anti chromophore configuration. Illumination leads to retinal isomerization around the C13=C14 double bond resulting in a 13‐cis,15‐anti configuration in the first photointermediate. The system relaxes then via several photointermediates to the initial dark state with the all‐trans,15‐anti chromophore. The details of this photocycle vary depending on the protein and its function. Knowing which (photo)‐intermediates are adopted during the photocycle is a prerequisite for understanding the protein function as this varies between the different types of microbial rhodopsins.
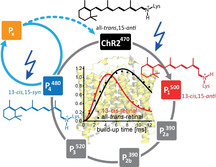
Here, we focus on Channelrhodopsin‐2 from Chlamydomonas reinhardtii (ChR2).[2] ChR2 is a cation channel and has found wide spread application in optogenetics.[3] A crystal structure of the dark state of ChR2 has been determined but not of the photointermediates so far.[4] These were investigated though with different spectroscopic techniques providing information about the changes of the protein and the chromophore compared to the initial dark state. In the dark‐adapted state (ChR2470), the protein has an absorption maximum at 470 nm and the retinal Schiff base chromophore is in the all‐trans,15‐anti configuration.[5] Upon illumination, initial photoisomerization of the C13=C14 bond leads to the first photointermediate (P1 500). Schiff base de‐protonation of P1 500 results in at least two deprotonated states (P2a 390 and P2b 390) and is accompanied by channel opening.[6] The channel remains open during Schiff base re‐protonation (P3 520) but closes before the initial dark state is reached again. During continuous illumination, desensitization of the protein is observed and full photocurrents are only recovered after prolonged time in the dark.[2] This is unique to channelrhodopsins in the microbial retinal family and usually unwanted in optogenetic applications. Understanding the channelrhodopsin photocycle in general and desensitization in particular is therefore of general interest to the biophysical community.
Desensitization is associated with the non‐conducting P4 480 photointermediate which itself is photo active.[5, 7] No consensus has been achieved with respect to the position of P4 480 in the photocycle and its chromophore configuration. Based on FT‐IR spectroscopy it was concluded that P4 480 contains an all‐trans,15‐anti chromophore whereas a Resonance Raman study indicated that the chromophore configuration of P4 480 is 13‐cis,15‐syn.[8] In this work,[8b] it was also postulated that P4 480 is generated directly from the dark state via a photo reaction. This is in contrast to a previously published photocycle model in which P4 480 is only formed during the open state decay.[7] A comparison of the different models and P4 480 configurations is given in Figure S1.
To resolve these contradicting views, it is necessary to characterize the chromophore in the desensitized state of ChR2 in detail. In principle such information can be obtained from crystallographic data. However, to distinguish different chromophore configurations the resolution has to be extremely high and conditions have to be found under which the photointermediate can be studied. From the dark ChR2 X‐ray structure, the configuration of the chromophore could not be determined. Retinal extraction experiments and Resonance Raman spectroscopy had hinted towards a mixture of all‐trans,15‐anti and 13‐cis,15‐syn chromophore.[9] However, the first technique is invasive and the second often suffers from problems with band assignments. Using solid‐state NMR spectroscopy of ChR2 in lipid bilayers circumvents both problems. Making use of signal‐enhancement by dynamic nuclear polarization, we and others could unambiguously show that in the dark the chromophore is purely in the all‐trans, 15‐anti configuration.[5, 10] With this technique it is also possible to study the chromophore in photointermediate states as long as they can be cryo‐trapped as demonstrated by us before.[5] As low temperatures are required for cryo‐trapping and a high detection sensitivity is desired, the experiments are ideally suited for sensitivity‐enhanced MAS‐NMR spectroscopy based on dynamic nuclear polarization (DNP).[11] In Figure 1 a our experimental setup is shown where DNP enhanced solid‐state NMR has been combined with in situ sample illumination in the optical range.
Figure 1.
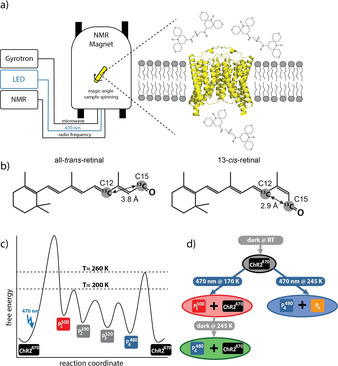
a) Cartoon of the experimental setup used in this work. Microwave, optical and radiofrequency irradiation from a gyrotron, an LED and the NMR console, respectively, can be applied simultaneously to the sample spinning with 8 kHz at the magic angle at cryogenic conditions. The sample consists of a proteoliposome pellet containing channelrhodopsin‐2, PDB code 6EID,[4] which is surrounded by the polarizing agent AMUPol. b) Illustration of the isotope labelling Scheme and comparison of the C12 and C15 distances in all‐trans‐retinal and 13‐cis‐retinal. Distances are taken from the X‐ray structures of crystalline all‐trans‐retinal and 13‐cis‐retinal,[12] respectively. [12,15‐13C2]‐all‐trans‐retinal was used to prepare the ChR2 sample. c) Schematic free energy landscape of the channelrhodopsin‐2 photocycle. The first photointermediate P1 500 is formed after photoexcitation of ChR2470 and is stable at temperatures below ≈200 K. The following P2 390 and P3 520 states cannot be trapped as they decay already below 200 K and P4 480 is reached. P4 480 is stable below ≈260 K and returns to ChR2470 above this temperature. d) Illumination Scheme for the differently trapped states. Keeping the sample in the dark results in the pure ground state (ChR2470). Depending on the temperature, different states are populated by 470 nm illumination. At 170 K a mixture between P1 500 and ChR2470 is generated and at 245 K, a mixture between P4 480 and Px is obtained. Heating the sample which was illuminated at 170 K to 245 K in the dark causes thermal relaxation and leads to a mixture of P4 480 and ChR2470. The color of the circles corresponds to the color of the respective NMR‐spectrum in Figure 2.
Cryo‐trapping of a photointermediate is possible when the energy barrier for its generation is lower (or can be overcome by photoexcitation) than the energy needed for its decay. We have visualized this in Figure 1 c for ChR2 with a free energy diagram based on our previous work.[5] The photointermediate that can be trapped upon illumination at temperatures between 100 and 190 K was assigned to P1 500 and always occurs in a mixture with ChR2470. This assignment was confirmed by optical spectroscopy under cryogenic condition. Another photointermediate can be generated by rising the temperature above 200 K. The same photointermediate, in a mixture with the dark state, is obtained by freeze quenching a sample that has been continuously illuminated at room temperature. This intermediate was assigned to P4 480 as it is the state that will be enriched during continuous illumination due to its long life time. The same NMR signals are obtained when illuminating the sample at 245 K. Interestingly, in this case the ground state population is completely depleted but additional signals occur. This new photo intermediate is a photo product of P4 480. As it is not known to which of the several postulated P4 480 photo intermediates the cryo‐trapped intermediate corresponds, it was termed Px.
The 13C chemical shifts of the retinal Schiff base chromophore are very sensitive to the configuration of the chromophore: The C12 chemical shift is a readout for the configuration about the C13=C14 bond whereas the C14 chemical shift is directly related to the C15=N bond configuration.[13] C12 and C14 are significantly shielded in the 13‐cis and 15‐syn configurations, respectively. In order to resolve any ambiguity about the chromophore configuration in the photointermediates, we analyzed their chemical shifts, and we determined the distance between C12 and C15, which is significantly shorter in the 13‐cis compared with the all‐trans configuration (Figure 1 b). In addition, we performed a thermal relaxation experiment to elucidate the position of P4 480 within the photocycle. All experimental details are given in the SI.
Results and Discussion
For detailed analysis of the retinal Schiff base chromophore in ChR2, [12,15‐13C2]‐all‐trans‐retinal‐ChR2 proteoliposomes were prepared. The proteoliposomes were doped with the radical AMUPol to enable DNP‐enhanced NMR experiments.[14] The obtained signal enhancement was 50 (Figure S2). The associated dramatic improvement turned out to be crucial for the NMR experiments on the cryo‐trapped sample in order to deconvolute the signals of the different photointermediates. Furthermore, the long‐distance double quantum filtered experiments described below would not have been possible without the enhancement provided by the DNP experiment. 13C‐cross polarization spectra were recorded using three different illumination schemes: Without exposure to light, 470 nm illumination at 170 K and 470 nm illumination at 245 K (Figure 1 d). The spectra are shown in Figure 2 a and clear differences are observed around 165 ppm, 136 ppm, 132 ppm and 124 ppm. However, the spectra are dominated by the natural abundance contribution from the protein, the lipids and spinning side bands of the glycerol signal. Double quantum filtering should be able to suppress these signals. The large distance between 13C12 and 13C15 and the large chemical shift anisotropy (CSA) of these atoms make these experiments challenging. The standard Post‐C7 experiment used in our previous study for a one bond distance did not work for this sample.[5, 15] Therefore, we resorted to the CSA compensated SR26 sequence.[16] This experiment, to our knowledge, has so far been applied only to small molecules at ambient temperature. Here we show that DNP‐enhanced solid‐state NMR enables the application of this sequence to a pair of quite distant atoms in the chromophore incorporated in a membrane protein. In addition, we could also quantify these long distances. Although some natural abundance signal intensity remains, the 13C12 and 13C15 signals can be much better identified in the spectra from the SR26 experiment compared to the CP spectra (Figure 2 b–d), especially in the region around 123 ppm which shows a background signal in the CP spectra which overlaps with the 13C12 signal in the spectra of the illuminated samples. The assignment of the 13C12 and 13C15 signals in the spectra is described in the SI and given in Table 1 together with the assignment of 13C14 which was obtained in our earlier work and recalled here to aid the analysis of the chromophore configuration.[5]
Figure 2.
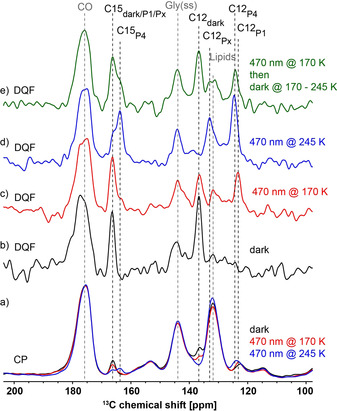
a) Cross polarization (CP) and b–e) SR26 double quantum filtered (DQF) spectra of [12,15‐13C2]‐all‐trans‐retinal‐ChR2 recorded after application of different illumination schemes: dark (black), 470 nm illumination at 170 K (red), 470 nm illumination at 245 K (blue), 470 nm illumination at 170 K followed by a temperature increase in the dark to 245 K (green), see Figure 1 b. Natural abundance background signals are labelled in grey (CO: carbonyl of protein and lipids, Gly(ss): Spinning side band of the glycerol signal and Lipids: olefinic lipid atoms) and [12,15‐13C2]‐retinal signals in black.
Table 1.
Selected 13C‐retinal chemical shifts of the retinal chromophore in ChR2 and its photointermediates. A detailed description of the assignment is given in the SI.
|
State |
13C12 [ppm] |
13C14 [ppm][a] |
13C15 [ppm] |
|---|---|---|---|
|
ChR2470 |
136.8 |
126.0 |
166.5 |
|
P1 500 |
123.2 |
124.2 |
not resolved |
|
P4 480 |
124.5 |
119.3 |
163.8 |
|
Px |
132.8 |
122.7 |
166.1 |
[a] 13C14 chemical shifts were taken from our previous publication.[5]
The 13C12 chemical shift clearly changes (−13.6 ppm) when trapping the first photointermediate, P1 500, as expected for the isomerization around the C13=C14 bond. The effect on the 13C14 chemical shift in this intermediate is only minor showing that the C15=N bond remains in an anti‐configuration. Interestingly, the desensitized state, P4 480, shows a very similar chemical shift for 13C12 compared with P1 500 indicating a C13=C14 cis‐configuration in P4 480. In addition, the 13C14 chemical shift shows a large shielding, which is expected for a C15=N syn‐bond. Px, the photointermediate of P4 480, also shows an increased shielding of 13C12 and 13C14 but the effect is smaller compared to P4 480 and interpretation is more ambiguous.
As additional support for the C13‐cis configuration in P4 480 and to further elucidate the chromophore configuration in Px, we wanted to take advantage of the sensitivity of the 13C12‐13C15 distance to the C13=C14 configuration. This distance is shorter in the C13=C14‐cis confirmation (2.9 Å) than in the C13=C14‐trans configuration (3.8 Å) and can be used to distinguish them (Figure 1 b).[12] To prove the feasibility of this approach we measured the 13C12‐13C15 distance in free retinal using SR26 build‐up experiments. We determined the distance from fitting the experimental data to SR26 build‐up curves generated using the Simpson software (Figure 3 a).[17] The curves differ significantly from each other and the fitted distances correspond well to the expected distances in both molecules. We repeated the experiment on ChR2470 (Figure S3). A sufficient signal‐to‐noise ratio was difficult to obtain in this experiment and is an even greater challenge in the experiment with the illuminated samples as the signal intensity is split between different states. Therefore, based on the free retinal build‐up curves we picked only four different build‐up time points (3, 5, 7 and 9 ms) by which the all‐trans and 13‐cis curves can be clearly distinguished. In this way we could record the SR26 spectra with a sufficient signal‐to‐noise ratio to deconvolute the spectra and obtain the signal intensities (for the deconvolution see Figure S4 and supplementary section 1 for the details of the data analysis).
Figure 3.
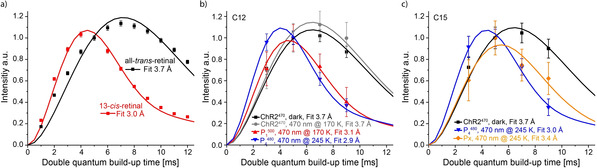
a) Double quantum build‐up curves for [12,15‐13C2]‐all‐trans retinal and [12,15‐13C2]‐13‐cis‐retinal in frozen solution. b) Double quantum build‐up curve of the 13C12 and c) of the 13C15 signals of [12,15‐13C2]‐retinal‐ChR2 recorded following the different illumination protocols shown in Figure 1 d. The best fits of the build‐up curves are shown together with the corresponding C12‐C15 distances. The error ranges of the fits are shown in Figure S5.
Figure 3 b shows the SR26 build‐up curves of the 13C12 signal. The ChR2470 signal in the dark sample as well as the ChR2470 signal that remains in the 470 nm illumination at 170 K sample showed similar build‐up curves which were fitted to similar distances corresponding to the all‐trans configuration as expected. In contrast the curve of 13C12‐P1 500 in the 170 K illuminated sample looked very different and fitted to 3.1 Å which is in good agreement with the expected 13‐cis configuration. The 13C12‐P4 480 curve in the sample illuminated at 245 K looks similar to the 13C12‐P1 500 curve and unambiguously confirms the 13‐cis configuration of the chromophore in this state. The Px 13C12 signal overlaps with the natural abundance lipid signal and the build‐up curve of this signal cannot be easily interpreted. For analysis of the Px state, we therefore resorted to analysis of the 13C15 signal. Figure 3 c shows the results for 13C15‐ChR2470 in the dark sample and 13C15‐P4 480 and 13C15‐Px in the sample illuminated at 245 K. In agreement with the results on 13C12, ChR2470 and P4 480 curves are fitted to 3.7 Å and 3.0 Å confirming the all‐trans and 13‐cis configuration, respectively. Interestingly, the 13C15‐Px build‐up curve fits to a distance of 3.4 Å which is neither in agreement with a planer all‐trans nor a planar 13‐cis configuration. Thus, the retinal is in a twisted configuration which also explains the intermediate position of the chemical shift of the 13C12 signal.
The observed 13‐cis,15‐syn configuration of P4 480 shows that the desensitized state does not correspond to the all‐trans,15‐anti O‐state in the BR photocycle. Thus, to revert to the dark state, the chromophore configuration has to change which could explain the long lifetime of the desensitized state. Interestingly, the observed configuration is the same as the population that arises during thermal equilibration of Bacteriorhodopsin in the dark.[13c] The twisted chromophore structure of the Px state has not been observed so far in any microbial rhodopsin. We speculate that the photoreaction of P4 480 leads to a C13 cis‐trans isomerization similar to the initial photoreaction. This would result in a 13‐anti,15‐syn configuration in Px. The shape of the chromophore thus resembles somewhat the K‐like P1 500 state. The energy stored in the P1 500 state is transferred to the protein resulting in Schiff base deprotonation. For Px no such reaction chain follows and the steric hindrances caused by this configuration can only be released by distorting the chromophore. Further experimental evidence is needed to support this idea.
In addition to time resolved experiments, cryo‐trapping photointermediates using different illumination and temperature schemes can provide insights into the sequence of photointermediates in a photo cycle. We therefore compared our observations with the different models postulated (Figure S1). We observe that the only photo product obtained below 200 K is P1 500. As the signal‐to‐noise ratio of the spectra in Figure 2 b–d) is limited due to the long distance of the atoms used for double quantum filtering, we replotted the double quantum filtered spectra on [14,15‐13C2]‐retinal‐ChR2 from our previous work to unambiguously show that the P4 480 photointermediate is not present in samples illuminated below 200 K (Figure S6).[5] This is in contrast to the idea that P4 480 is also a direct product of the photo reaction. To generate P4 480 additional thermal energy is needed and we could trap the state when illuminating at 245 K. We have shown that P4 480 can also be reached by thermal relaxation of P1 500 (without further illumination). This again is in contrast to the proposal that P4 480 is generated directly from the dark state. Our observations agree with a photocycle model where P4 480 is generated at a later state in the photocycle, for example, during the open state decay as suggested before.[7] We did not discus these observation in detail in our previous work,[5] as the results were in agreement with the photocycle models at that time and a model with the early P4 480 state had not been postulated. To reproduce and confirm our results we performed a thermal relaxation experiment on the [12,15‐13C2]‐retinal‐ChR2 sample. The proteoliposomes were illuminated at 170 K to form the P1 500 state and subsequently the sample temperature was increased to 245 K in the dark (Figure 1 d, Figure 2 e). The 13C12‐P1 500 signal completely disappeared and instead the 13C12‐P4 480 signal at 124.5 ppm is seen confirming that P4 480 follows P1 500 in the photocycle. Figure 4 shows a photocycle model that agrees with our experimental data. The ChR470 population in the spectrum from the thermal relaxation experiment (Figure 2 e) is increased compared to the P1 500 cryo‐trapping condition (470 nm @ 170 K, Figure 2 c), showing that the open state decays not exclusively towards P4 480 but also a direct shortcut to ChR2470 may exist (dashed grey arrow in Figure 4). It can also be expected that Px can undergo a thermal conversion to the dark state (dashed blue arrow in Figure 4).
Figure 4.
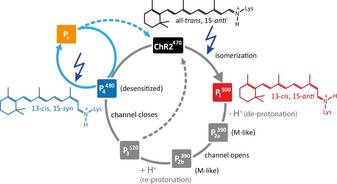
Revised photocycle of ChR2. ChR2470 has an all‐trans,15‐anti chromophore and illumination results in the 13‐cis,15‐anti P1 500 state. The deprotonated P2a 390, P2b 390 and open channel P3 520 states follow but no trapping protocol exists for these states. A 13‐cis,15‐syn chromophore is observed in the desensitized P4 480 state which is populated after channel closure. P4 480 itself is photoactive and we term its photo product Px. Px contains a twisted chromophore structure.
Conclusion
In summary, for the first time, double quantum filtering of atoms at distances up to 3.7 Å has been shown in a reconstituted membrane protein. Based on this method, we have resolved the controversy of the chromophore configuration of the desensitized state of ChR2 (P4 480) using solid‐state NMR spectroscopy as a readout which circumvents the assignment problems which pose a challenge to the interpretation of vibrational spectroscopy data. The P4 480 chromophore is in the 13‐cis,15‐syn configuration which fits well to its long lifetime. In addition, we could show that P4 480 is not directly formed after light excitation but occurs at a later state in the photocycle, probably during channel closure. P4 480 itself is photoactive and we could trap and analyze its photoproduct Px suggesting that it has a non‐planar chromophore structure. Here, we have shown that DNP‐enhanced solid‐state NMR spectroscopy in combination with cryo‐trapping of photointermediates reveals details of the chromophore configuration in ChR2, which are difficult to obtain by other methods. In addition, we could show that reaction pathways can be deduced from the thermal relaxation pathways of cryo‐trapped samples. Therefore, we would like to advocate the use of solid‐state NMR spectroscopy in studying photoactive proteins.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We like to thank Heike Biehl for help with the purification of ChR2. The work was funded by Deutsche Forschungs‐gemeinschaft/Sonderforschungsbereich 807 Transport and Communications across Membranes. The dynamic nuclear polarization experiments were enabled through DFG Equipment Grant GL 307/4‐1 and the Cluster of Excellence Frankfurt: Macromolecular Complexes Frankfurt. Work at the Center for Biomolecular Magnetic Resonance is supported by the State of Hesse. Open access funding enabled and organized by Projekt DEAL.
J. Becker-Baldus, A. Leeder, L. J. Brown, R. C. D. Brown, C. Bamann, C. Glaubitz, Angew. Chem. Int. Ed. 2021, 60, 16442.
Contributor Information
Dr. Johanna Becker‐Baldus, Email: j.baldus@em.uni-frankfurt.de.
Prof. Clemens Glaubitz, Email: glaubitz@em.uni-frankfurt.de.
References
- 1.Ernst O. P., Lodowski D. T., Elstner M., Hegemann P., Brown L. S., Kandori H., Chem. Rev. 2014, 114, 126–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E., Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi J., Rubart M., Zhu W., Front. Bioeng. Biotechnol. 2019, 7, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkov O., Kovalev K., Polovinkin V., Borshchevskiy V., Bamann C., Astashkin R., Marin E., Popov A., Balandin T., Willbold D., Büldt G., Bamberg E., Gordeliy V., Science 2017, 358, eaan8862. [DOI] [PubMed] [Google Scholar]
- 5.Becker-Baldus J., Bamann C., Saxena K., Gustmann H., Brown L. J., Brown R. C., Reiter C., Bamberg E., Wachtveitl J., Schwalbe H., Glaubitz C., Proc. Natl. Acad. Sci. USA 2015, 112, 9896–9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bamann C., Gueta R., Kleinlogel S., Nagel G., Bamberg E., Biochemistry 2010, 49, 267–278. [DOI] [PubMed] [Google Scholar]
- 7.Saita M., Pranga-Sellnau F., Resler T., Schlesinger R., Heberle J., Lorenz-Fonfria V. A., J. Am. Chem. Soc. 2018, 140, 9899–9903. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a.Lórenz-Fonfría V. A., Schultz B. J., Resler T., Schlesinger R., Bamann C., Bamberg E., Heberle J., J. Am. Chem. Soc. 2015, 137, 1850–1861; [DOI] [PubMed] [Google Scholar]
- 8b.Kuhne J., Vierock J., Tennigkeit S. A., Dreier M. A., Wietek J., Petersen D., Gavriljuk K., El-Mashtoly S. F., Hegemann P., Gerwert K., Proc. Natl. Acad. Sci. USA 2019, 116, 9380–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nack M., Radu I., Bamann C., Bamberg E., Heberle J., FEBS Lett. 2009, 583, 3676–3680. [DOI] [PubMed] [Google Scholar]
- 10.Bruun S., Stoeppler D., Keidel A., Kuhlmann U., Luck M., Diehl A., Geiger M. A., Woodmansee D., Trauner D., Hegemann P., Oschkinat H., Hildebrandt P., Stehfest K., Biochemistry 2015, 54, 5389–5400. [DOI] [PubMed] [Google Scholar]
- 11.Becker-Baldus J., Glaubitz C., eMagRes 2018, 7, 79–92. [Google Scholar]
- 12.
- 12a.Hamanaka T., Mitsui T., Ashida T., Kakudo M., Acta Crystallogr. Sect. B 1972, 28, 214–222; [Google Scholar]
- 12b.Simmons C. J., Liu R. S. H., Denny M., Seff K., Acta Crystallogr. Sect. B 1981, 37, 2197–2205. [Google Scholar]
- 13.
- 13a.Englert G., Helv. Chim. Acta 1975, 58, 2367–2390; [DOI] [PubMed] [Google Scholar]
- 13b.Harbison G. S., Mulder P. P. J., Pardoen H., Lugtenburg J., Herzfeld J., Griffin R. G., J. Am. Chem. Soc. 1985, 107, 4809–4816; [Google Scholar]
- 13c.Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G., Proc. Natl. Acad. Sci. USA 1984, 81, 1706–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauvée C., Rosay M., Casano G., Aussenac F., Weber R. T., Ouari O., Tordo P., Angew. Chem. Int. Ed. 2013, 52, 10858–10861; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 11058–11061. [Google Scholar]
- 15.Hohwy M., Jakobsen H. J., Edén M., Levitt M. H., Nielsen N. C., J. Chem. Phys. 1998, 108, 2686. [Google Scholar]
- 16.Kristiansen P. E., Carravetta M., Lai W. C., Levitt M. H., Chem. Phys. Lett. 2004, 390, 1–7. [Google Scholar]
- 17.Bak M., Rasmussen J. T., Nielsen N. C., J. Magn. Reson. 2000, 147, 296–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


