Abstract
Background
The success of clinical trials in Epidermolysis Bullosa (EB) is dependent upon the availability of a valid and reliable scoring tool that can accurately assess and monitor disease severity. The Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) and Instrument for Scoring Clinical Outcomes of Research for Epidermolysis Bullosa (iscorEB) were independently developed and validated against the Birmingham Epidermolysis Bullosa Severity Score but have never been directly compared.
Objective
To compare the reliability, convergent validity, and discriminant validity of the EBDASI and iscorEB scoring tools.
Methods
An observational cohort study was conducted in 15 patients with EB. Each patient was evaluated using the EBDASI and iscorEB-clinician scoring tools by 6 dermatologists with expertise in EB. Quality of life was assessed using the iscorEB-patient and Quality of Life in EB measures.
Results
The intraclass correlation coefficients for interrater reliability were 0.942 for the EBDASI and 0.852 for the iscorEB-clinician. The intraclass correlation coefficients for intrarater reliability was 0.99 for both scores. The two tools demonstrated strong convergent validity with each other.
Conclusion
Both scoring tools demonstrate excellent reliability. The EBDASI appears to better discriminate between EB types and disease severities.
Key words: blistering skin disease, dermatology, epidermolysis bullosa, Epidermolysis Bullosa Disease Activity and Scarring Index, Instrument for Scoring Clinical Outcomes of Research for Epidermolysis Bullosa, outcome measure
Abbreviations used: BEBS, Birmingham Epidermolysis Bullosa Severity Score; BMD, bone mineral densitometry; DDEB, dominant dystrophic epidermolysis bullosa; EB, epidermolysis bullosa; EBDASI, Epidermolysis Bullosa Disease Activity and Scarring Index; EBS, epidermolysis bullosa simplex; ICC, intraclass correlation coefficient; iscorEB, Instrument for Scoring Clinical Outcomes of Research for Epidermolysis Bullosa; JEB, junctional epidermolysis bullosa; QoL, quality of life; QOLEB, Quality of Life in Epidermolysis Bullosa score; RDEB, recessive dystrophic epidermolysis bullosa
Capsule Summary.
-
•
The Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) and Instrument for Scoring Clinical Outcomes of Research for EB are the 2 leading outcome measure tools for the assessment and monitoring of disease severity but have never been directly compared.
-
•
The EBDASI demonstrated a superior ability to discriminate between EB types and disease severities, particularly in patients with mild disease.
Introduction
Epidermolysis Bullosa (EB) encompasses multiple heterogenous genodermatoses, grouped by a shared fragility of epithelial lined tissues and surfaces, particularly the skin. Consequently, EB is characterized by recurrent blistering and erosions in response to minimal trauma.1 Although rare, with an incidence of 19.6 cases per million live births, morbidity and mortality in patients with EB is high.2,3 The ability of clinical trials to demonstrate treatment effectiveness is reliant upon the availability of a reliable and valid outcome measure to quantify disease severity and therapeutic response, a notion becoming increasingly important as the therapeutic potential for EB broadens.4
Over the past 2 decades, 4 EB-specific instruments have been designed to monitor disease severity.5, 6, 7, 8 In 2003, The Japanese Study Group for Rare Intractable Skin Diseases devised the first disease-specific severity scores for EB.8 However, these indices provide a broad categorization of disease, which hampers their ability to detect more minor fluctuations in severity. The Birmingham Epidermolysis Bullosa Severity Score (BEBS), developed and validated in 2009, was the first universal scoring system for all EB types.7 However, most of the scoring items in the scale are largely the consequence of scarring: a chronic process inelastic to therapy.
Validated in 2013, the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) is the only EB scoring measure that separates ongoing disease activity from accumulative damage.6 This premise was based on a number of other dermatological scoring systems that distinguish between activity and damage.9, 10, 11 The capacity of the EBDASI to gauge severity and clinical response strongly support its application in longitudinal studies and clinical trials.12
Initially developed in 2014, the Instrument for Scoring Clinical Outcomes of Research for Epidermolysis Bullosa (iscorEB) was partially validated in 2018 as being able to reliably distinguish between EB types and severities.5
The EBDASI and iscorEB are currently considered the most comprehensive and reliable EB-specific scoring measures available, and have both been used in clinical trials for disease monitoring.13,14 However, as both tools were independently validated against the BEBS with differing methodologies, it is currently impossible to determine which tool would be best to use as an outcome measure for clinical trials in EB.
Methods
Patient selection
Based on feasibility, we initially proposed a sample size in order to discriminate excellent from moderate intraclass correlation coefficients (ICC) with 95% confidence intervals. Patients with EB attending regular reviews at the St George Hospital Campus and Premier Dermatology, Sydney, were recruited for the study. Eligibility criteria specified a diagnosis of EB confirmed by consistent clinical findings, immunofluorescence mapping, and/or electron microscopic and genotyping of EB. Patients with all subtypes of EB were eligible. Written consent was obtained from all adult participants and from the parents/guardians of minors. Assent was also obtained from all participants aged 6-17 years.
Scoring procedure
The patient-dependent components of the study were carried out on a single day in August (winter) at Premier Dermatology Research & Development, Sydney. Six dermatologists with prior experience in the assessment and management of EB patients were involved in the scoring on the day. Prior to the assessment of patients, all dermatologists received training in the format of the scoring sessions and the use of the outcome measures.
Three scoring sessions were held throughout the day, each involving 5 patients. As each patient arrived, they completed 2 quality of life (QoL) assessment tools; the iscorEB-p and Quality of Life in EB score (QOLEB), before being taken to a private room to remove their clothes and dressings. The 6 dermatologists rotated independently among the rooms, so only a single doctor was scoring a particular patient at any time. Each doctor completed both the EBDASI and iscorEB-c for each patient, also recording the time taken for completion of each tool. The order of completion of these outcome measures among patients was randomized using a computer-generated random pattern. A clinical photographer took objective photographs of the patients for later comparison with the results (Fig 1). To determine intraobserver reliability, at the conclusion of each scoring session there was an opportunity for patients to be rescored. Each doctor rescored 2 patients among the three sessions, who were predetermined at random using the aforementioned computer random sequence generation. Doctors were blinded to who they would rescore until they had finished scoring all patients from that session. Depending on which patients were rescored, the time interval between the initial and subsequent scoring ranged from 20 to 90 minutes.
Fig 1.
EB photographic examples. A, DDEB. B, Junctional epidermolysis bullosa. C, RDEB. DDEB, Dominant dystrophic epidermolysis bullosa; EB, epidermolysis bullosa; RDEB, recessive dystrophic epidermolysis bullosa.
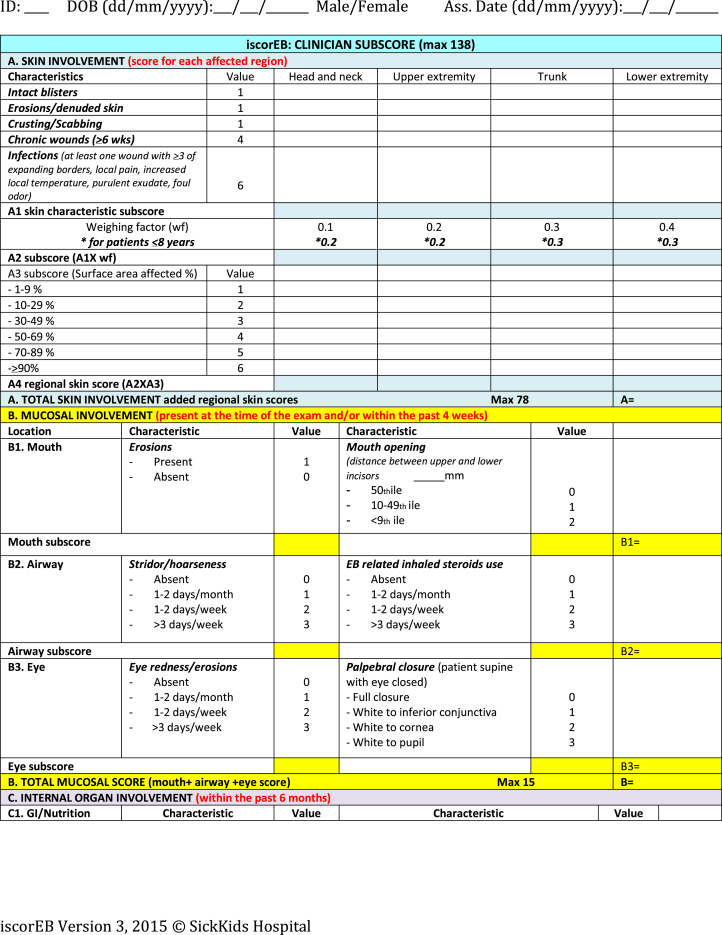
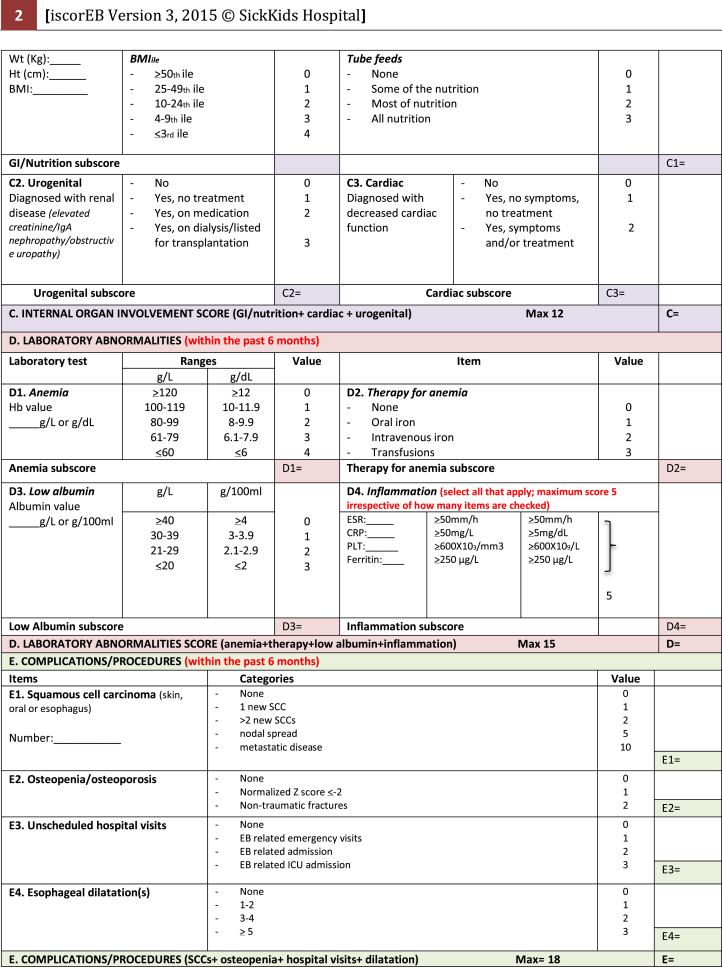
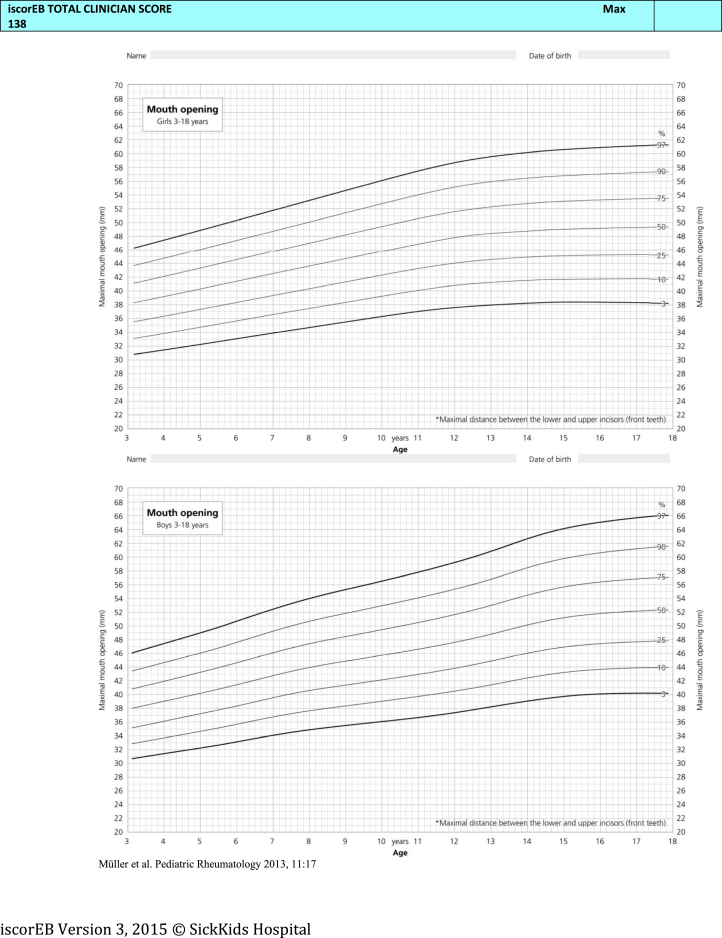
Necessary components of the patient's medical history, as well as blood tests, body mass index (BMI), and bone mineral densitometry (BMD) results were collected by the coinvestigators prior to the study day. This information was summarized on a single A4 sheet and provided in the room of each patient. Percentile charts for BMI and mouth opening were also available in each room. This information was necessary for the iscorEB-c (Fig 2) and the assessors were required to use these details to designate a numerical value in the relevant section of the score.
Fig 2.
Instrument for Scoring Clinical Outcomes of Research for Epidermolysis Bullosa.6
Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI)
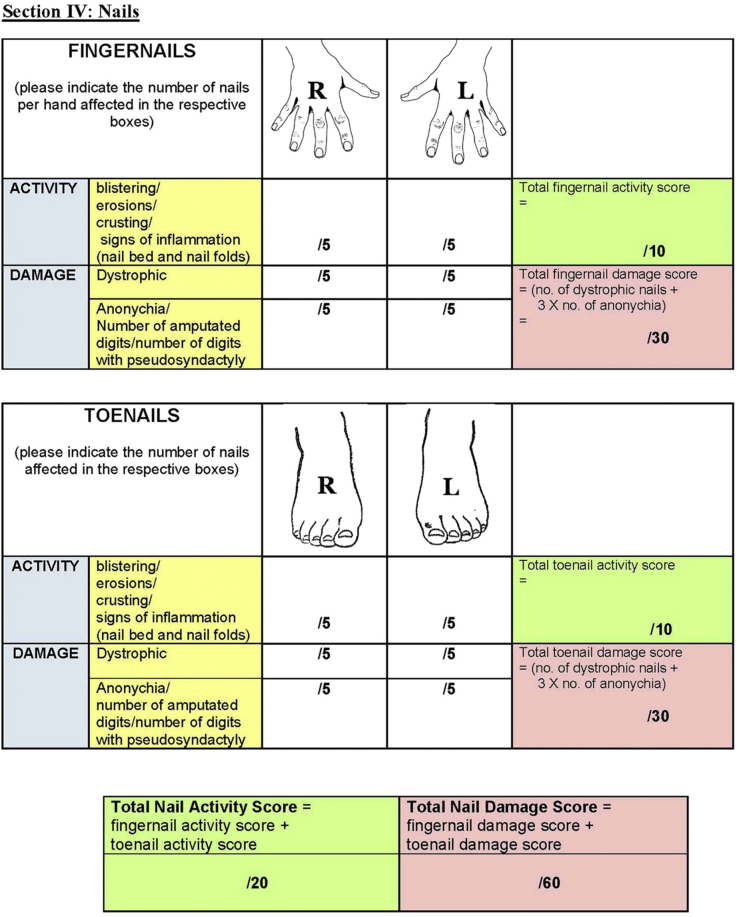
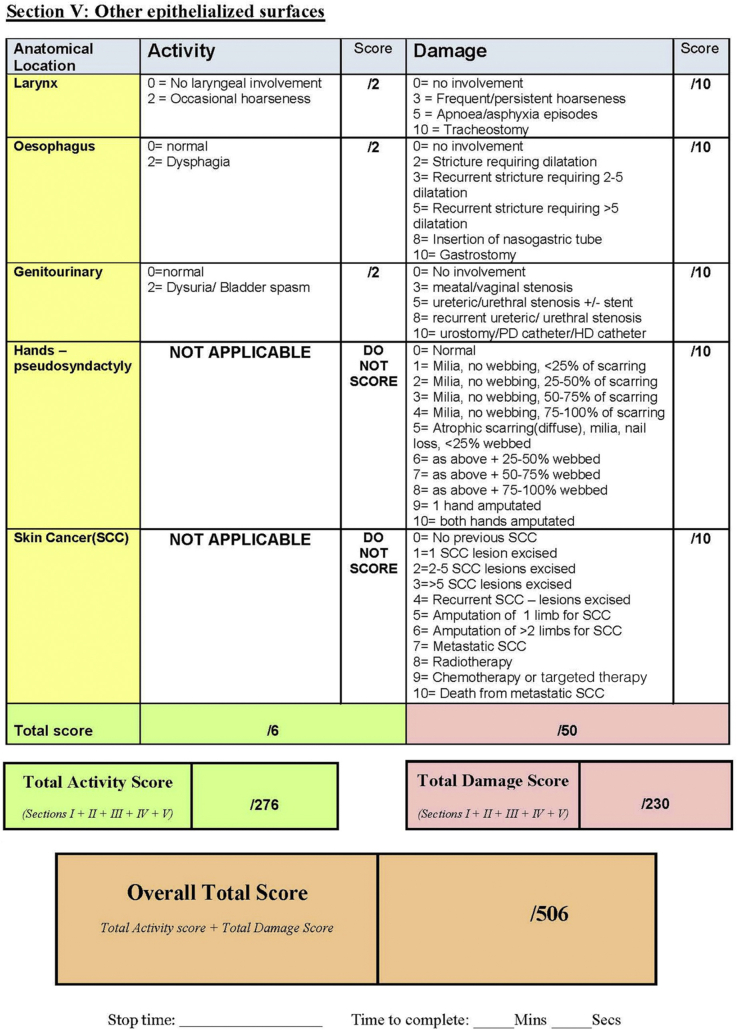
The EBDASI scores disease activity and damage separately across each of its 5 sections: skin, scalp, mucosa, nails, and other epithelialized surfaces. As the most comprehensive section, the “skin” is scored at 12 anatomical sites (Fig 3). All components of the EBDASI can be completed from a detailed examination of the patient, and a basic history taken from the patient (and/or carer) at the time of scoring. Total activity is given a score out of 276, with damage scored out of 230, adding to an overall score out of 506.6
Fig 3.
The Epidermolysis Bullosa Disease Activity and Scarring Index7 quantifies disease severity by scoring activity and damage across 5 domains.
Instrument for Scoring the Clinical Outcomes of Research for Epidermolysis Bullosa (iscorEB)
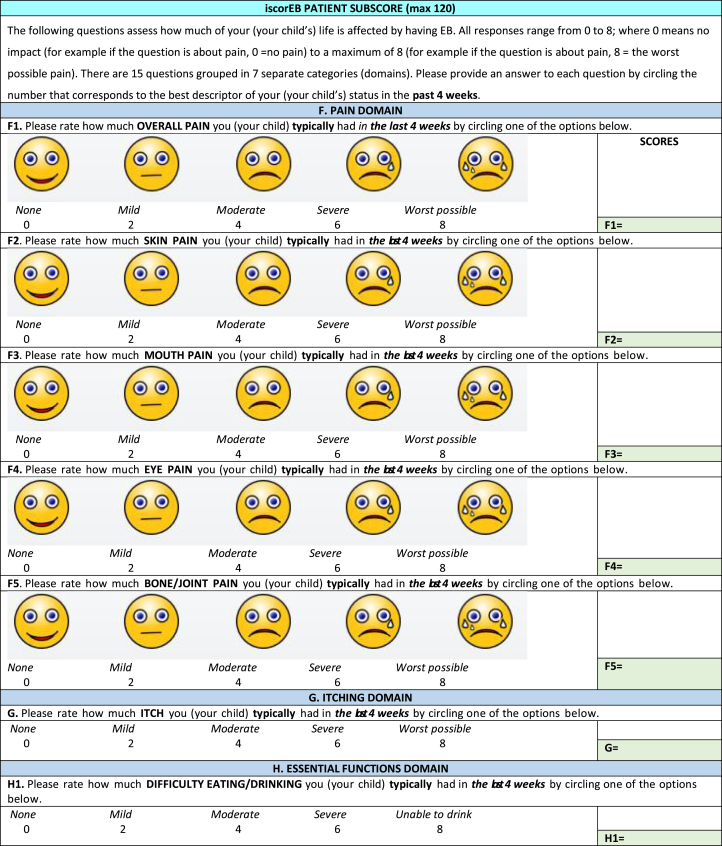
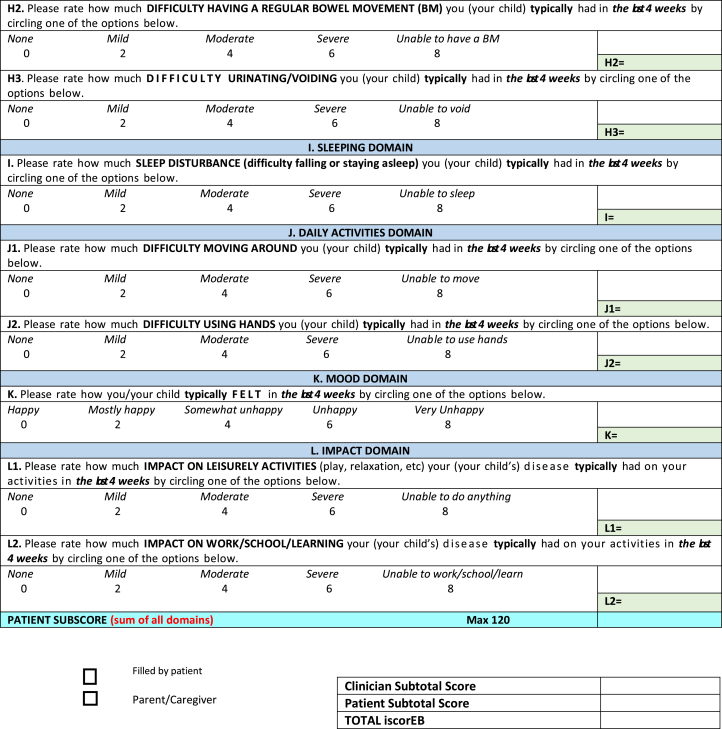
The iscorEB comprises 2 separate sections: the iscorEB-p and iscorEB-c. The iscorEB-p is a QoL measure completed by the patient. The score comprises 15 questions across 7 domains: pain, itch, essential functions, sleep, daily activities, mood, and impact (Fig 2). The patient allocates a numerical value (0-8) to each question that corresponds with the best descriptor of their experience over the last 4 weeks.5 These allocated scores add to a maximum of 120.
The iscorEB-c is the clinician-completed component of the overall iscorEB measure. The score spans 5 sections: skin, mucosal, internal organ involvement, laboratory abnormalities, and complications/procedures, which sum to a total out of 114 (Fig 2). Aside from the first 2 sections involving a thorough examination of the skin and mucosa, the remaining 3 sections require access to a number of blood test results as well as recent BMI and BMD measurements.
Quality of Life in EB (QOLEB) score
The QOLEB is a QoL questionnaire containing 17 questions, with a maximum score of 51. Created and validated by Frew et al in English in 2009, the QOLEB has since been translated and validated in a number of languages.15, 16, 17, 18 All patients in this study completed the English version of this score. Each question requires the patient to identify (from 0 to 3) the degree to which their EB affects a particular emotional or functional aspect of daily life.15,17
Statistical analysis
Statistical analysis was performed using IBM SPSS version 25 (Chicago, IL, USA). Normally distributed data is presented as mean ± one standard deviation. A P-value ≤.05 was considered statistically significant.
Reliability
Intraobserver and interobserver reliabilities were assessed using a one-way, random effects ANOVA model to calculate the ICC. An ICC >0.9 was considered an indicator of excellent reliability.19 Cronbach's alpha was calculated to assess the relevance of each clinical domain to the instrument. The Shapiro-Wilk test was applied to assess the normality of the distributions. Bland-Altman plots were constructed to visualize the intrarater variability in scores for each outcome measure.
Convergent validity
Convergent validity, a subtype of construct validity, was assessed using a linear mixed model. Spearman rho correlation coefficients were determined between the domains of the iscorEB-c, iscorEB-p, QOLEB, and EBDASI.
Discriminant validity
Discriminant validity was assessed to determine whether the EBDASI, iscorEB-c, iscorEB-p, and QOLEB could discriminate between different subtypes of EB. This was assessed using Kruskal-Wallis tests, and the Dunn test for pairwise comparisons if discriminatory significance between EB subtypes was determined.
Feasibility
The feasibility of the EBDASI and iscorEB-c was assessed by requesting assessors document the time taken to complete each scoring measure. An online stopwatch was accessible from the desktop computer in each scoring room for this purpose. The mean scoring time was calculated for each instrument across all assessors and for Dr D. F. Murrell alone (the regular dermatologist of the patient cohort). Simple linear regression analysis was used to determine the relationship between the disease severity and the scoring time.
Results and discussion
Fifteen patients, representing multiple EB subtypes, were recruited for this study. The characteristics of the study cohort are captured in Table I. Mean scores for each patient and instrument are reported in Table II.
Table I.
Demographic and clinical characteristics of the study cohort∗
| Age | |
| Range (mean ± SD) | 16-73 (43.7 ± 14.6) |
| Sex | |
| Female | 4 (27) |
| Race/ethnicity | |
| Caucasian | 11 (73) |
| Asian | 1 (7) |
| Middle-eastern | 3 (20) |
| Epidermolysis Bullosa type, subtype | |
| Simplex, localized | 4 (27) |
| Junctional, generalized intermediate | 2 (13) |
| Dominant dystrophic | 6 (40) |
| Recessive dystrophic, generalized intermediate | 2 (13) |
| Recessive dystrophic, generalized severe | 1 (7) |
| Disease severity; corresponding EBDASI score range12 | |
| Mild; 0-42 | 6 (40) |
| Moderate; 43-106 | 6 (40) |
| Severe; 107-506 | 3 (20) |
| Disease severity; iscorEB validation criteria5 | |
| Mild; localized involvement, 0 non-skin complications | 6 (40) |
| Moderate; widespread involvement, <3 non-skin complications | 4 (27) |
| Severe; generalized involvement, ≥3 non-skin complications | 5 (33) |
EBDASI, Epidermolysis Bullosa Disease Activity and Scarring Index; iscorEB, Instrument for scoring the clinical outcomes of research for Epidermolysis Bullosa; SD, standard deviation.
All values are n (%) unless otherwise specified.
Table II.
Demographics and mean scores by patient
| No. | Age | Sex | EB subtype | Mean score |
|||
|---|---|---|---|---|---|---|---|
| EBDASI | iscorEB-c | iscorEB-p | QOLEB | ||||
| 1 | 16 | F | EBS-L | 8 | 1.6 | 18 | 19 |
| 2 | 73 | M | DDEB | 65 | 15.0 | 44 | 24 |
| 3 | 54 | M | DDEB | 80 | 6.8 | 18 | 8 |
| 4 | 32 | F | RDEB-GS | 222 | 49.1 | 38 | 35 |
| 5 | 25 | M | DDEB | 66 | 4.0 | 16 | 19 |
| 6 | 43 | M | DDEB | 21 | 4.7 | 38 | 14 |
| 7 | 50 | F | RDEB-GI | 92 | 12.4 | 48 | 19 |
| 8 | 44 | F | DDEB | 20 | 2.8 | 2 | 4 |
| 9 | 45 | M | JEB-GI | 86 | 15.8 | 6 | 5 |
| 10 | 58 | M | EBS-L | 14 | 0.4 | 30 | 26 |
| 11 | 25 | M | JEB-GI | 123 | 26.0 | 32 | 22 |
| 12 | 44 | M | DDEB | 50 | 9.1 | 28 | 17 |
| 13 | 41 | M | EBS-L | 6 | 0.8 | 14 | 8 |
| 14 | 56 | M | RDEB-GI | 145 | 14.9 | 28 | 8 |
| 15 | 49 | M | EBS-L | 3 | 0.5 | 16 | 21 |
DDEB, Dominant dystrophic epidermolysis bullosa; EB, epidermolysis bullosa; EBDASI, Epidermolysis Bullosa Disease Activity and Scarring Index; EBS-L, epidermolysis bullosa simplex—localized; iscorEB, Instrument for scoring the clinical outcomes of research for Epidermolysis Bullosa; JEB-GI, junctional epidermolysis bullosa—generalized intermediate; QOLEB, Quality of Life in Epidermolysis Bullosa.; RDEB-GI, recessive dystrophic epidermolysis bullosa—generalized intermediate; RDEB-GS, recessive dystrophic epidermolysis bullosa—generalized severe.
Reliability
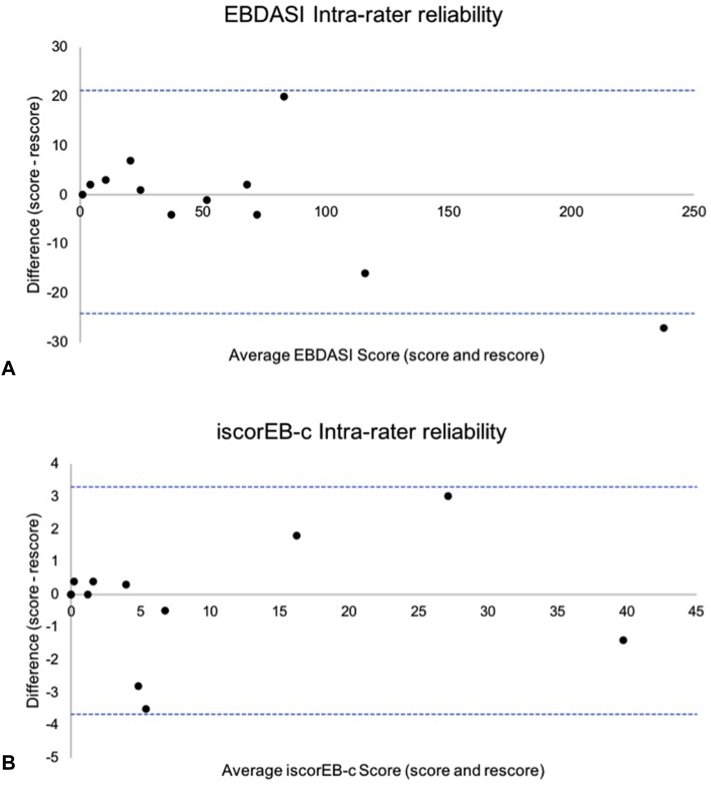
Both the EBDASI and iscorEB-c demonstrated excellent intraobserver reliability across all domains (Table III). The intrarater reliability of the total EBDASI and iscorEB-c scores were further represented on a Bland-Altman plot (Fig 4).
Table III.
Summary, by instrument, of assessments, reliability, and internal consistency
| Instrument or sub-score (reference range) | Mean ± SD | Range | Interobserver reliability (ICC) | Intraobserver reliability (ICC) | Cronbach's alpha | Shapiro-Wilk test of normality∗ |
|---|---|---|---|---|---|---|
| EBDASI (0-506) | 66.6 ± 61.4 | 1-256 | 0.942 | 0.99 (0.98-1) | 0.995 | 0.880 |
| Total activity (0-276) | 17.2 ± 17.6 | 0-89 | 0.867 | 0.96 (0.88-0.99) | 0.974 | 0.841 |
| A1 Skin (0-120) | 11.3 ± 11.1 | 0-49 | 0.903 | 0.96 (0.87-0.99) | 0.984 | 0.875 |
| A2 Scalp (0-10) | 1.2 ± 2.0 | 0-9 | 0.924 | 0.98 (0.94-0.99) | 0.972 | 0.880 |
| A3 Mucosa (0-120) | 2.4 ± 6.7 | 0-45 | 0.767 | 0.93 (0.77-0.98) | 0.781 | 0.648 |
| A4 Nails (0-20) | 1.5 ± 3.4 | 0-15 | 0.173 | 0.99 (0.98-1) | 0.383 | 0.359 |
| A5 Other (0-6) | 0.8 ± 1.1 | 0-4 | 0.575 | 0.74 (0.13-0.92) | 0.895 | 0.504 |
| Total damage (0-230) | 49.4 ± 45.6 | 1-182 | 0.935 | 0.99 (0.99-1) | 0.994 | 0.649 |
| iscorEB-clinician (0-114) | 11.0 ± 13.4 | 0-71.5 | 0.852 | 0.99 (0.98-1) | 0.981 | 0.872 |
| A Skin (0-60) | 5.8 ± 7.7 | 0-42.5 | 0.829 | 0.99 (0.96-1) | 0.939 | 0.930 |
| B Mucosal (0-15) | 1.2 ± 1.4 | 0-6 | 0.617 | 0.93 (0.77-0.98) | 0.909 | 0.741 |
| C Internal organ (0-12) | 1.4 ± 2.9 | 0-11 | 0.795 | 0.99 (0.97-1) | 0.993 | 0.789 |
| D Laboratory abnormalities (0-15) | 2.0 ± 3.7 | 0-13 | 0.860 | 1 (1-1) | 0.982 | 0.566 |
| E Complications/procedures (0-18) | 0.5 ± 0.8 | 0-5 | 0.473 | 0.87 (0.55-0.96) | 0.725 | 0.593 |
| QOLEB (0-51) | 16.6 ± 8.7 | 4-35 | 0.828 | |||
| iscorEB-patient (0-120) | 25.1 ± 13.6 | 2-48 | 0.931 |
EBDASI, Epidermolysis Bullosa Disease Activity and Scarring Index; ICC, intraclass correlation coefficient; iscorEB, Instrument for scoring the clinical outcomes of research for Epidermolysis Bullosa; QOLEB, Quality of Life in Epidermolysis Bullosa; SD, standard deviation.
P-values for Shapiro-Wilk test are not provided as no variables were normally distributed.
Fig 4.
Bland-Altman plots of disagreement between score and rescore results. Dashed lines correspond to the limits of agreement at 95% confidence intervals. A, The Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). B, Instrument for scoring the clinical outcomes of research for Epidermolysis Bullosa-clinician (iscorEB-c). The EBDASI is demonstrated in this figure to have the least variability of scores as compared with the iscorEB-c, as proven by the distribution of the scores close to the x-axis. This implies that the EBDASI has the better intrarater variability as compared with the iscorEB-c.
The “skin” component of the iscorEB-c had a lower interobserver reliability (0.829) than the equivalent “skin” section of the EBDASI (0.903). This may be explained by the designation of 4 points to the presence of “chronic wounds” and 6 points to “infections”, compared to a single point for each: intact blisters, erosions/denuded skin, and crusting/scabbing.5 The subjectivity of determining whether a wound is infected from observation alone reduces the reliability of the score between observers, particularly with such a significant value dependent on the decision.20 Although criteria for chronicity and presence of infection were defined, a similar method employed to identify infection in diabetic foot ulcers determined that the presence of ≥2 of pain, erythema, induration, heat, or edema demonstrated a sensitivity of only 52%.21
Convergent validity
Table IV reports the convergent validity between components of the EBDASI, iscorEB, and QOLEB. The high correlation between the total EBDASI and iscorEB-c scores (Spearman ρ = 0.89 (P <.001)), is a promising result for both scores, as it indicates a good level of agreement on the severity of disease for each patient.22 The correlation was strongest between the iscorEB-c and the EBDASI activity score (Spearman ρ = 0.91 (P <.001)), which was expected as the iscorEB-c does not incorporate many “damage” aspects of the disease.5
Table IV.
Convergent validity between components of the EBDASI, iscorEB, and QOLEB
| Instrument or sub-scores for comparison | Spearman's rho (P-value) | 95% confidence interval |
|---|---|---|
| EBDASI total vs iscorEB total | 0.74 (.002) | 0.37–0.91 |
| EBDASI total vs iscorEB-c | 0.89 (<.001) | 0.68–0.96 |
| EBDASI activity vs iscorEB-c | 0.91 (<.001) | 0.75–0.97 |
| EBDASI damage vs iscorEB-c | 0.90 (<.001) | 0.71–0.97 |
| iscorEB-c vs QOLEB | 0.10 (.711∗) | −0.43–0.59 |
| iscorEB-c vs iscorEB-p | 0.43 (.106∗) | −0.11–0.77 |
| EBDASI total vs QOLEB | 0.09 (.760∗) | −0.45–0.57 |
| EBDASI activity vs QOLEB | 0.10 (.711∗) | −0.43–0.59 |
| EBDASI damage vs QOLEB | 0.06 (.829∗) | −0.47–0.56 |
| EBDASI total vs iscorEB-p | 0.42 (.115∗) | −0.10–0.77 |
| EBDASI activity vs iscorEB-p | 0.46 (.088∗) | −0.07–0.78 |
| EBDASI damage vs iscorEB-p | 0.38 (.165∗) | −0.16–0.75 |
| iscorEB-p vs QOLEB | 0.64 (.01) |
EBDASI, Epidermolysis Bullosa Disease Activity and Scarring Index; iscorEB, Instrument for scoring the clinical outcomes of research for Epidermolysis Bullosa; QOLEB, Quality of Life in Epidermolysis Bullosa.
Not statistically significant.
Discriminant validity
There was a highly statistically significant difference in total score between the different EB types for the EBDASI (Kruskal-Wallis test Χ2(3) = 12.542, P = .006) and iscorEB-c (Kruskal-Wallis test Χ2(3) = 9.05, P = .029). A Dunn test for pairwise comparisons revealed the total EBDASI score could distinguish between epidermolysis bullosa simplex (EBS) and dominant dystrophic epidermolysis bullosa (DDEB) (P = .04), EBS and junctional epidermolysis bullosa (JEB) (P = .007), EBS and recessive dystrophic epidermolysis bullosa (RDEB) (P = .0005), and DDEB and RDEB (P = .02) (Fig 5, A). Comparatively, the iscorEB-c score could distinguish between the EBS and JEB (P = .01), EBS and RDEB (P = .004), and DDEB and RDEB (P = .04) (Fig 5, B).
Fig 5.
Box plots illustrating score distribution by epidermolysis bullosa type. A, Mean total EBDASI score distribution by epidermolysis bullosa type. B, Mean total iscorEB-c score distribution by epidermolysis bullosa type. EBDASI, Epidermolysis Bullosa Disease Activity and Scarring Index; iscorEB-c, Instrument for scoring the clinical outcomes of research for Epidermolysis Bullosa-clinician.
The ability of these scoring measures to discriminate between EB types has not been tested in previous studies that focused on discriminant validity between categorizations of “mild,” “moderate” and “severe” disease—determined by percentage skin involvement and number of non-skin manifestations or systemic complications.5,6 The additional ability of the EBDASI to discriminate EBS from DDEB is an advantage over the iscorEB-c. This is likely owed to its comprehensive examination of damage, which is often the most distinguishing feature in patients with milder forms of EB and potentially minimal active blistering.12 In contrast, by scoring only the active components of skin manifestations based on percentage involvement, the iscorEB-c creates a floor effect that may limit the clinical responsiveness of the tool for milder patients.4 This is supported by the data collected on validation of the tool, whereby the calculated minimally important difference was 5.5 points.5 With a mean iscorEB-c of 11.0 in this study, it is unlikely that a clinical improvement in disease would correspond to a score reduction of this magnitude for most patients. With a mean total EBDASI score of 66.6 in this study, the 9-point reduction in score determined by Jain et al as the minimum clinically significant improvement, is indicative of the greater responsiveness of the EBDASI.12
Scatterplots comparing total scores against the mean rank order were constructed for both the EBDASI (Fig 6, A) and iscorEB-c (Fig 6, B). Comparing the EBDASI and iscorEB-c on this basis further indicates that the EBDASI has a superior ability to distinguish between patients across the severity spectrum. Good discriminant ability by mean rank order was similarly determined on validation of the EBDASI compared with the BEBS.6 The iscorEB-c has never been tested against the mean rank order. However, the confluence of milder iscorEB-c results (Fig 6, B) further supports the floor effect observed in this tool. The heterogeneity of clinical manifestations among EB subtypes makes it difficult to design a comprehensive score that quantifies all potential clinical features. As such, the EBDASI is 4 pages long and certain items are often scored 0 in certain subtypes that are unlikely to exhibit these clinical features, such as skin cancer and poikiloderma. Nevertheless, this avoids floor and ceiling effects observed in the iscorEB.
Fig 6.
Scatterplots illustrating the total score against the mean rank order. Colored dots indicate actual scores, black crosses represent mean scores. A, Mean rank order for EBDASI. B, Mean rank order for iscorEB-c. To determine the mean rank order, the mean total score for each patient was calculated for both instruments and ranked in ascending order. EBDASI, Epidermolysis Bullosa Disease Activity and Scarring Index; iscorEB-c, Instrument for scoring the clinical outcomes of research for Epidermolysis Bullosa-clinician.
Feasibility
The mean scoring times of both the EBDASI (7.6 minutes) and iscorEB-c (4.7 minutes) were acceptable. However, the mean time taken for iscorEB-c data collection was an additional 30 minutes per patient. The EBDASI times were similar to the 7.9-minute mean and 20-minute maximum observed by Loh et al6 and Jain et al,12 respectively. Scoring time was not measured on validation of the iscorEB, so cannot be compared.5 The longer EBDASI scoring time replicates findings of previous studies comparing outcome measures for different dermatological conditions, each of which reported a longer scoring time for tools quantifying lesion number and size.23,24 The mean scoring times for Dr D. F. Murrell, the regular dermatologist of the recruited cohort, were 5.1 and 3.6 minutes for the EBDASI and iscorEB-c, respectively, suggesting reduced scoring time with patient familiarity. There was very strong evidence of a positive linear correlation between the time taken to score and the score severity (Fig 7).
Fig 7.
Scatterplots of the score severity and scoring time. Each color represents the scores obtained by one assessor. A, EBDASI total score severity and scoring time (R2 = 0.4449, P <.001). Pink dots represent scores by assessor 1. EBDASI, Epidermolysis Bullosa Disease Activity and Scarring Index. B, iscorEB-c score severity and scoring time (R2 = 0.1397, P <.001). Each color represents the scores obtained by one assessor. Pink dots represent scores by assessor 1. iscorEB-c, Instrument for scoring the clinical outcomes of research for Epidermolysis Bullosa-clinician. NOTE: This diagram does not take into account the time taken to source the medical data required for score completion.
Further observations
There are few damage components captured by the iscorEB-c, and notably, none on the skin. These damage features such as mitten deformity, nail dystrophy, and skin scarring were omitted as they were less likely to improve with treatment.25 This appears disadvantageous, as the damage caused by EB produces significant burden of disease and should be captured in outcome measures not only for the documentation of total disease severity but to be monitored as treatments are developed that may reduce or prevent accumulative damage.6,26
The “within the past 6 months” condition for sections D (laboratory abnormalities) and E (complications/procedures) of the iscorEB-c presents a number of impracticalities. Firstly, government-subsidized BMD testing is only available on a 12-month basis.27 Therefore, iscorEB-c scoring would incur additional costs to either the patient, doctor, or pharmaceutical company for the second annual test, with minimal probability of clinically significant change.28 Further, the frequency at which patients require supportive interventions may cause false fluctuations in the score. This is particularly relevant to the “therapy for anemia” and “esophageal dilatation” components, as regular treatment at intervals longer than 6 months would only be captured in the score for part of this period, despite no real change to disease severity in the remaining time.
As is almost unavoidable in studies of rare diseases, we recognize that a significant limitation of this study is the size and characteristics of the recruited cohort. This is a common trend in EB research, and the proportion of our cohort with mild disease (40%) is consistent with previous scoring measure comparison studies.5,6 Moreover, conducting this study in winter meant that a number of EBS patients had milder disease than would otherwise be present, as the cooler weather had reduced the sweat and friction contributing toward blistering.29
In conclusion, the independent validation studies for both the EBDASI and iscorEB demonstrated promising characteristics of each tool. Both demonstrate excellent reliability and have strong convergent validity with each other. However, the EBDASI has confirmed responsiveness and a better ability to discriminate among EB types and disease severities across the spectrum, indicating it may be a more appropriate choice for use in clinical trials.
Conflicts of interest
Dr Dedee F. Murrell developed the EBDASI. Dr Dedee F. Murrell and Authors Daniel and Su were involved in the validation of the EBDASI. Dr Kern and authors Rogers, Gibson, Martin, Robertson, Feng, and Oliver G. C. Murrell have no conflicts of interest to declare.
Footnotes
Funding sources: Supported by the University of New South Wales Medicine Honors Research Program (supervised by Professor Dedee F. Murrell), the Australasian Blistering Diseases Foundation, DEBRA AUS/NZ, and Premier Specialists.
Presented as a poster at EB2020 London; the first world congress on Epidermolysis Bullosa, January 22, 2020.
IRB approval status: Reviewed and approved by the Bellberry Human Research Ethics Committee.
References
- 1.Fine J. Inherited epidermolysis bullosa. Orphanet J Rare Dis. 2010;5(12):1–17. doi: 10.1186/1750-1172-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fine J. Epidemiology of inherited epidermolysis bullosa based on incidence and prevalence estimates from the National Epidermolysis Bullosa Registry. JAMA Dermatol. 2016;152(11):1231–1238. doi: 10.1001/jamadermatol.2016.2473. [DOI] [PubMed] [Google Scholar]
- 3.Jeon K., On H., Kim S. Quality of life and economic burden in recessive dystrophic epidermolysis bullosa. Ann Dermatol. 2016;28(1):6–14. doi: 10.5021/ad.2016.28.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coster W. Making the best match: selecting outcome measures for clinical trials and outcome studies. Am J Occup Ther. 2013;67(2):162–170. doi: 10.5014/ajot.2013.006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruckner A., Fairclough D., Feinstein J. Reliability and validity of the instrument for scoring clinical outcomes of research for epidermolysis bullosa (iscorEB) Br J Dermatol. 2018;178(5):1128–1134. doi: 10.1111/bjd.16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loh C., Kim J., Su J. Development, reliability, and validity of a novel Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) J Am Acad Dermatol. 2014;70(1):89–97. doi: 10.1016/j.jaad.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Moss C., Wong A., Davies P. The Birmingham Epidermolysis Bullosa Severity Score: development and validation. Br J Dermatol. 2009;160(5):1057–1065. doi: 10.1111/j.1365-2133.2009.09041.x. [DOI] [PubMed] [Google Scholar]
- 8.Tamai K., Hashimoto I., Hanada K. Japanese guidelines for diagnosis and treatment of junctional and dystrophic epidermolysis bullosa. Arch Dermatol Res. 2003;295(1):S24–S28. doi: 10.1007/s00403-002-0379-y. [DOI] [PubMed] [Google Scholar]
- 9.Hanna S., Kim M., De La Mata N. Responsiveness, minimally clinically important difference, and cut-offs for the Pemphigus Disease Area Index and Autoimmune Bullous Intensity Score for pemphigus. J Investig Dermatol. 2017;137(10):S196. [Google Scholar]
- 10.Klein R., Moghadam-Kia S., LoMonico J. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol. 2011;147(2):203–208. doi: 10.1001/archdermatol.2010.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murrell D., Daniel B., Joly P. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66(3):479–485. doi: 10.1016/j.jaad.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain S., Harris A., Su J. The Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI): grading disease severity and assessing responsiveness to clinical change in epidermolysis bullosa. J Eur Acad Dermatol Venereol. 2017;31(4):692–698. doi: 10.1111/jdv.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phase III efficacy and safety study of Oleogel-S10 in epidermolysis bullosa (EASE). ClinicalTrials.gov identifier: NCT03068780. https://clinicaltrials.gov/ct2/show/NCT03068780 Accessed September 4, 2019. Available at:
- 14.Using topical sirolimus 2% for patients with epidermolysis bullous simplex (EBS) study. ClinicalTrials.gov identifier NCT03016715. https://clinicaltrials.gov/ct2/show/NCT03016715 Accessed September 4, 2019. Available at:
- 15.Frew J.W., Martin L.K., Nijsten T., Murrell D.F. Quality of life evaluation in epidermolysis bullosa (EB) through the development of the QOLEB questionnaire: an EB-specific quality of life instrument. Br J Dermatol. 2009;161(6):1323–1330. doi: 10.1111/j.1365-2133.2009.09347.x. [DOI] [PubMed] [Google Scholar]
- 16.Cestari T., Prati C., Menegon D. Translation, cross-cultural adaptation and validation of the Quality of Life Evaluation in Epidermolysis Bullosa instrument in Brazilian Portuguese. Int J Dermatol. 2015;55(2):94–99. doi: 10.1111/ijd.12819. [DOI] [PubMed] [Google Scholar]
- 17.Frew J., Cepeda-Valdes R., Fortuna G. Cross cultural adaptation of the ‘Quality of Life in Epidermolysis Bullosa’ (QOLEB) questionnaire: validation and results of a Hispanic translation. Australas J Dermatol. 2013;54(2):39–40. [Google Scholar]
- 18.Yuen W.Y., Frew J.W., Veerman K., van den Heuvel E.R., Murrell D.F., Jonkman M.F. Health-related quality of life in epidermolysis bullosa: validation of the Dutch QOLEB questionnaire and assessment in the Dutch population. Acta Derm Venereol. 2014;94(4):442–447. doi: 10.2340/00015555-1758. [DOI] [PubMed] [Google Scholar]
- 19.Mokkink L., Terwee C., Patrick D. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19(4):539–549. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goreshi R., Okawa J., Rose M. Evaluation of reliability, validity, and responsiveness of the CDASI and the CAT-BM. J Investig Dermatol. 2012;132(4):1117–1124. doi: 10.1038/jid.2011.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbach M., Murrell D., Bystryn J. Reliability and convergent validity of two outcome instruments for pemphigus. J Invest Dermatol. 2009;129(10):2404–2410. doi: 10.1038/jid.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukaka M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy M., Gill S., Wu W. Does this patient have an infection of a chronic wound? JAMA. 2012;307(6):605–611. doi: 10.1001/jama.2012.98. [DOI] [PubMed] [Google Scholar]
- 24.Gardner S., Hillis S., Frantz R. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol Res Nurs. 2009;11(2):119–128. doi: 10.1177/1099800408326169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwieger-Briel A., Chakkittakandiyil A., Lara-Corrales I. Instrument for scoring clinical outcome of research for epidermolysis bullosa: a consensus-generated clinical research tool. Pediatr Dermatol. 2014;32(1):41–52. doi: 10.1111/pde.12317. [DOI] [PubMed] [Google Scholar]
- 26.Uitto J., Bruckner-Tuderman L., Christiano A. Progress toward treatment and cure of epidermolysis bullosa: summary of the DEBRA International Research Symposium EB2015. J Invest Dermatol. 2016;136(2):352–358. doi: 10.1016/j.jid.2015.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bone densitometry. Australian Department of Health. https://www1.health.gov.au/internet/main/publishing.nsf/Content/diagnosticimaging-bd.htm Accessed September 2, 2019. Available at:
- 28.Gourlay M., Fine J., Preissner J. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233. doi: 10.1056/NEJMoa1107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.S Robertson, D Murrell. Epidermolysis bullosa simplex. The Australasian College of Dermatologists. www.dermcoll.edu.au Accessed September 5, 2019.