Abstract
Background
Despite efforts toward the earlier detection and prevention of skin cancer, the prevalence of skin cancers continues to increase. Identifying trends in skin cancer burdens among populations can lead to impactful and sustainable interventions.
Methods
We assessed the global trends in skin cancer from 1990 to 2017 in 195 countries worldwide through the Global Burden of Disease Study (GBD) 2017 database.
Results
The rate of change in skin cancers between 1990 to 2017 varied among countries. Squamous cell carcinomas increased by 310% during this time, the highest among any neoplasm tracked by the GBD. Men experienced greater age-specific prevalence rates of keratinocyte carcinoma across all ages (P < .05). Women had a greater prevalence of melanoma until approximately age 50 years, after which the trend reversed until age 85 years. Men experienced greater age-specific death rates across all ages. The disability-adjusted life years (DALYs) of melanoma and keratinocyte carcinoma increased exponentially with age (P < .05).
Conclusion
The incidence, prevalence, and DALYs of skin cancers are increasing disproportionately among different demographic groups. As a worldwide epidemiological assessment, the GBD 2017 provides frequently updated measures of the skin cancer burden, which may help to direct resources and allocate funding to close the gap in global skin cancer disparities.
Key words: basal cell carcinoma, disability-adjusted life years (DALYs), Global Burden of Disease Study (GBD) database, melanoma, sociodemographic index (SDI), squamous cell carcinoma
Abbreviations used: BCC, basal cell carcinomas; DALYs, disability-adjusted life years; KC, keratinocyte carcinoma; SCC, squamous cell carcinomas; SDI, sociodemographic index; UVR, ultraviolet radiation
Capsule Summary.
-
•
The prevalence of skin cancer continues to increase and is a large contributor to skin-related disability. This article demonstrates these trends on a global scale.
-
•
Recognizing global trends in skin cancer epidemiology and socioeconomic status may help to maximize public health interventions to reduce this global health disparity.
Introduction
The overall incidence and prevalence of melanoma and keratinocyte carcinoma (KC), which comprise basal cell carcinomas (BCC) and squamous cell carcinomas (SCC), have increased in recent decades. One in every 3 diagnosed cancers is a skin cancer, and 132,000 new cases of melanoma occur each year.1 Among the different types of skin cancers, KC is the most common, with BCC accounting for 75% of cases in Caucasians.2 The incidence of melanoma has increased by 4%-6% annually in fair-skinned populations in North America, Northern Europe, Australia, and New Zealand.3 Despite accounting for only 2% of all skin cancer cases, invasive melanoma is responsible for 80% of skin cancer deaths.4
These findings have led to numerous campaigns and efforts emphasizing the prevention and early detection of skin cancer. Primary care physicians and dermatologists are encouraged to counsel patients about the risk of using tanning beds, minimizing ultraviolet radiation (UVR) exposure during peak daylight hours, seeking shaded areas, wearing sun-protective clothing, and emphasizing sunscreen use. Despite the implementation of these public health strategies, many countries find themselves in the midst of a possible skin cancer epidemic.5
One measurement of disease morbidity is disability-adjusted life years (DALYs), defined as the years of life lost due to premature mortality plus the years lost due to disability for people living with a health condition or its consequences.6 The sum of DALYs across a population is the burden of disease.6 The differences in prognosis among populations with skin cancer may be reflected in the burden of disease, based on the inclusion of years of life lost in the calculation of DALYs. The sociodemographic index (SDI) was developed in 2016 to track key measures of socioeconomic development, predict health outcomes, monitor inequalities, and monitor the impact of interventions on health outcomes.7,8 The SDI combines the income per capita, years of schooling, and total fertility rate to identify where countries sit on a spectrum of 0 to 1 in terms of development.7 Metrics such as DALYs and the SDI may help dermatologists and key policy and decision makers to focus resources on interventions to maximize the public health impact.
In this study, we highlight multiple global trends in skin cancer from 1990 to 2017 in 195 countries worldwide through the Global Burden of Disease Study (GBD) database. We include age and sex patterns, present the melanoma and KC burdens through DALYs, and provide comparisons to the SDI. A detailed cross-sectional analysis of the global burden of melanoma using 2015 GBD study data has been published.9 To our knowledge, our study comparisons have not yet been made using the most recent 2017 GBD study results. Currently published GBD literature has not yet addressed longitudinal trends in melanoma or KC in association with SDI. This study aims to contribute to the growing body of research addressing global trends in and the global prevalence of skin cancer.
Methods
Our data were derived from publicly available GBD datasets in 2017. The GBD datasets provide data to compare the magnitude of diseases, injuries, and risk factors across age groups, sexes, countries, regions, and time periods from 1990 to the present day for more than 350 diseases in 195 countries.7 An available in-depth protocol describes how the data are obtained, incorporated, calculated, and published in the GBD study from the Institute for Health Metrics and Evaluation.10
Melanoma and KC were included in our analysis based on the high incidence and available data in the GBD study. Other non-keratinocytic cancers such as Kaposi sarcoma were excluded from the GBD study, and were therefore excluded from our analysis. In addition to melanoma and KC, we provide a list of all neoplasms tracked by the GBD and their respective global incidence rates (Table I). The global percent changes in the age-standardized prevalence rate per 100,000 population members from 1990 to 2017 are given for melanoma and KC (Fig 1, A and B). The age patterns (in 5-year intervals) in 2017 were organized by sex for both melanoma and KC, and both the total prevalence and age-specific prevalence rate are provided (Fig 2, A and B). A similar figure describing the total deaths and age-specific death rate for melanoma is also provided (Fig 2, C).
Table I.
Global incidence ranks of neoplasms in 2017
| Cause∗ | Number of new cases (2017) | Percent change (1990-2017) | 2017 Incidence rank |
|---|---|---|---|
| Other benign and in situ neoplasms | 9,714,953 | 42.8% | 1 |
| Skin cancer: basal cell carcinoma | 5,884,759 | 77.4% | 2 |
| Lung cancer | 2,163,132 | 100.4% | 3 |
| Breast cancer | 1,960,682 | 123.1% | 4 |
| Colorectal cancer | 1,833,451 | 121.9% | 5 |
| Skin cancer: squamous cell carcinoma | 1,778,829 | 309.7% | 6 |
| Prostate cancer | 1,334,315 | 179.1% | 7 |
| Stomach cancer | 1,220,662 | 41.2% | 8 |
| Benign and in situ intestinal neoplasms | 1,010,854 | 60.9% | 9 |
| Other malignant neoplasms | 715,546 | 132.0% | 10 |
| Cervical cancer | 601,186 | 44.5% | 11 |
| Lymphoma | 487,964 | 135.9% | 12 |
| Bladder cancer | 473,800 | 90.1% | 13 |
| Esophageal cancer | 472,525 | 52.3% | 14 |
| Pancreatic cancer | 447,665 | 129.1% | 15 |
| Uterine cancer | 406,793 | 121.6% | 16 |
| Brain cancer | 405,218 | 108.4% | 17 |
| Liver cancer (due to hepatitis B) | 403,964 | 83.8% | 18 |
| Kidney cancer | 393,043 | 89.6% | 19 |
| Lip and oral cavity cancer | 389,760 | 109.6% | 20 |
| Melanoma | 308,684 | 161.3% | 21 |
| Benign and in situ cervical and uterine neoplasms | 299,385 | 14.6% | 22 |
| Ovarian cancer | 286,127 | 88.1% | 23 |
Benign and in situ neoplasms: International Statistical Classification of Diseases and Related Health Problems, Tenth Revision ([ICD-10] codes D00-D49).
Fig 1.
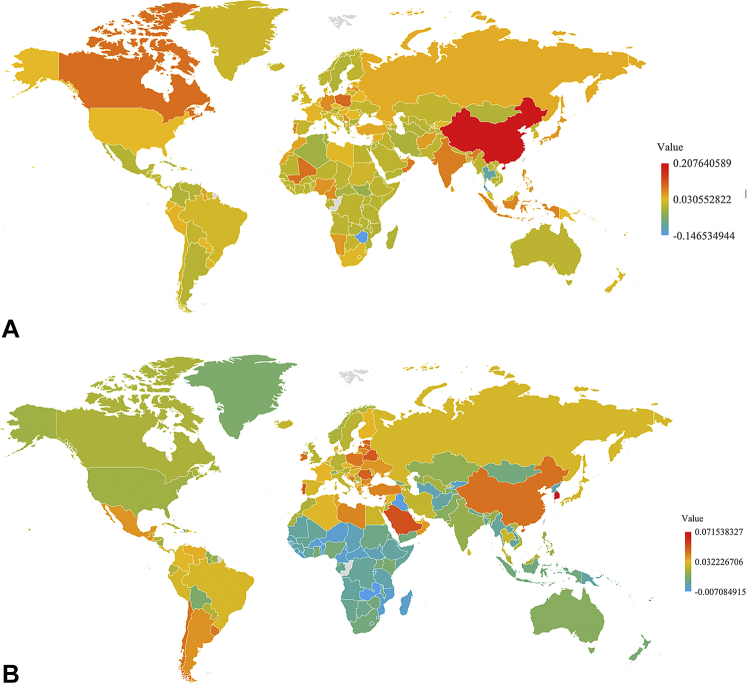
A, Percent changes in the age-standardized prevalence rate of keratinocyte carcinoma per 100,000 population from 1990 to 2017. The top 10 countries with the largest increases were: China, Trinidad and Tobago, Poland, Canada, Mali, Oman, Lebanon, India, Indonesia, and Portugal. The top 10 countries with the largest decrease were: Zimbabwe, Thailand, Burundi, South Sudan, Algeria, Jordan, Tunisia, Central African Republic, Iran, and Brunei. B, Percent changes in the age-standardized prevalence rate of melanoma per 100,000 population from 1990 to 2017. The top 10 countries with the largest increases were: South Korea, Lebanon, Cyprus, Saudi Arabia, Portugal, Belarus, Romania, Estonia, Singapore, and Latvia. The top 10 countries with the largest decreases were: Burundi, Zambia, Iraq, Burkina Faso, Liberia, Guinea-Bissau, Niger, Mozambique, Cameroon, and Kyrgyzstan.
Fig 2.
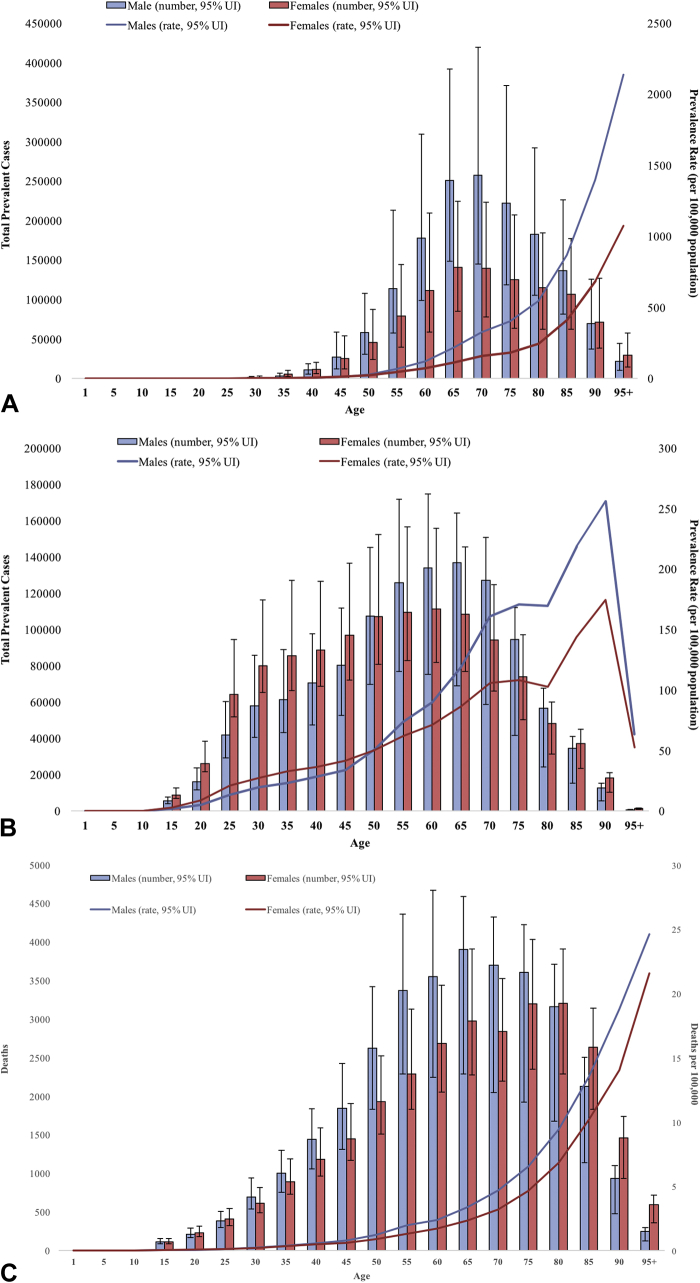
A, Age patterns by sex of the total number of prevalent cases in 2017 and age-specific prevalence rates of keratinocyte carcinoma at the global level. B, Age patterns by sex of the total number of prevalent cases in 2017 and age-specific prevalence rate of melanoma at the global level. C, Age patterns by sex of the total number of deaths in 2017 and age-specific death rate of melanoma at the global level.
The global DALYs per 100,000 population in 2017 by age range are provided for melanoma and KC (Fig 3). Comparisons of the global DALYs of melanoma and KC per 100,000 population members between 1990 and 2017 between all 7 GBD super regions, the global average (Fig 4, A and B), and the geographic regions of the world are also presented (Fig 5). Lastly, we compared the age-standardized DALYs for melanoma and KC per 100,000 population members in 2017 with the SDIs for all 195 countries and territories in the GBD study (Fig 6, A and B). Statistical analyses were performed using a 2-tailed linear regression and SPSS Statistics, version 25.0 (IBM Corp.). The significance threshold was set to P < .05.
Fig 3.
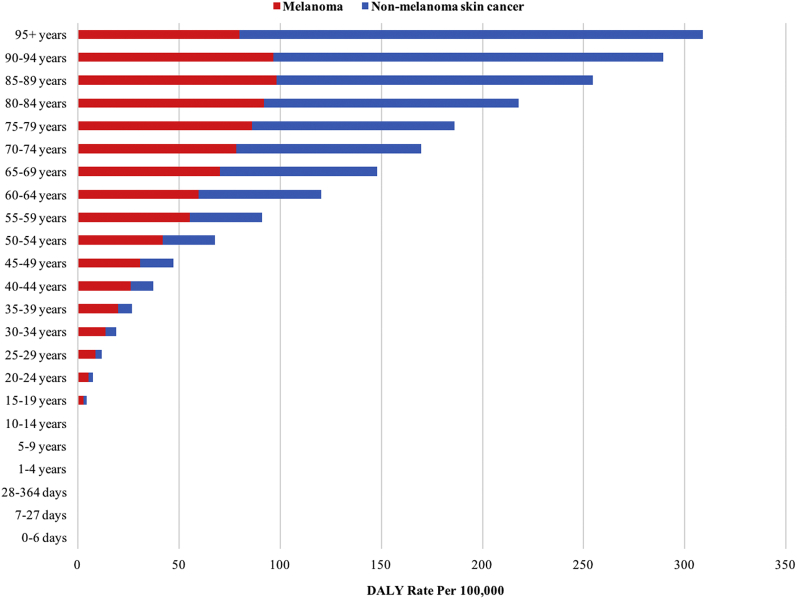
Melanoma and keratinocyte carcinoma disability-adjusted life years per 100,000 population at the global level by age in 2017.
Fig 4.
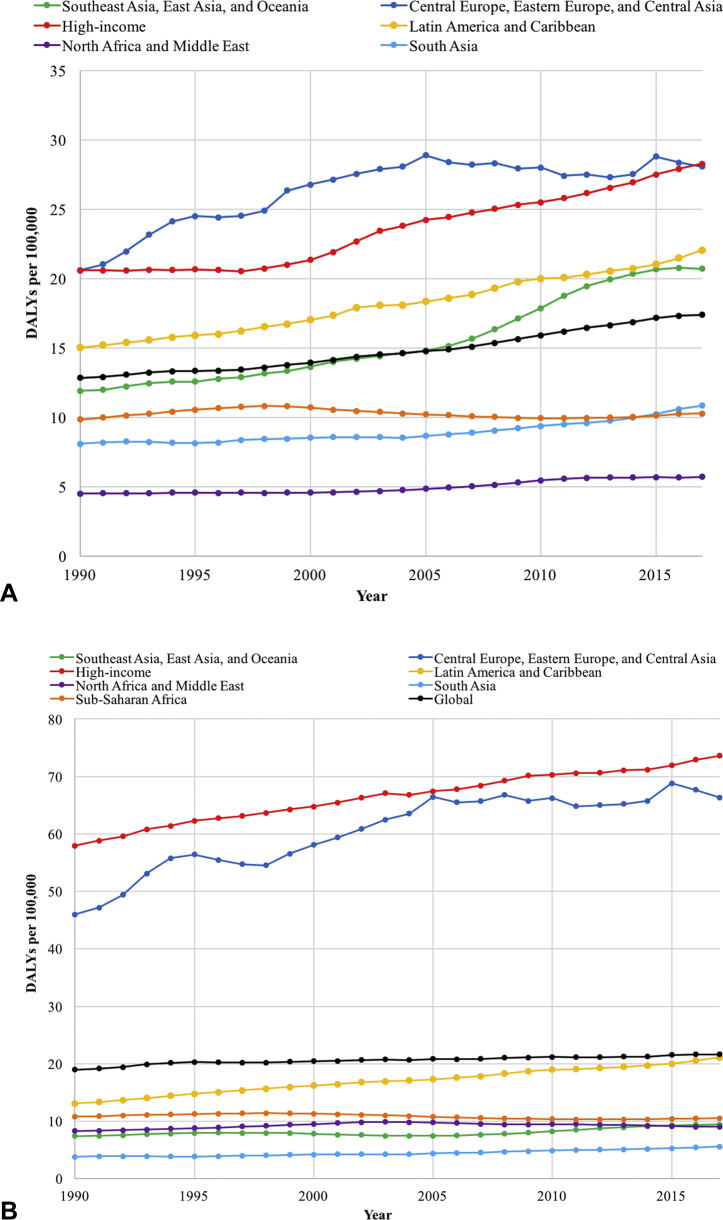
A, Trends in DALYs per 100,000 cases of keratinocyte carcinoma in 7 GBD super regions along with the global value from 1990 to 2017. B, Trends in the DALYs per 100,000 cases of melanoma in 7 GBD super regions along with the global value from 1990 to 2017. DALYs, Disability-adjusted life years; GBD, Global Burden of Disease.
Fig 5.
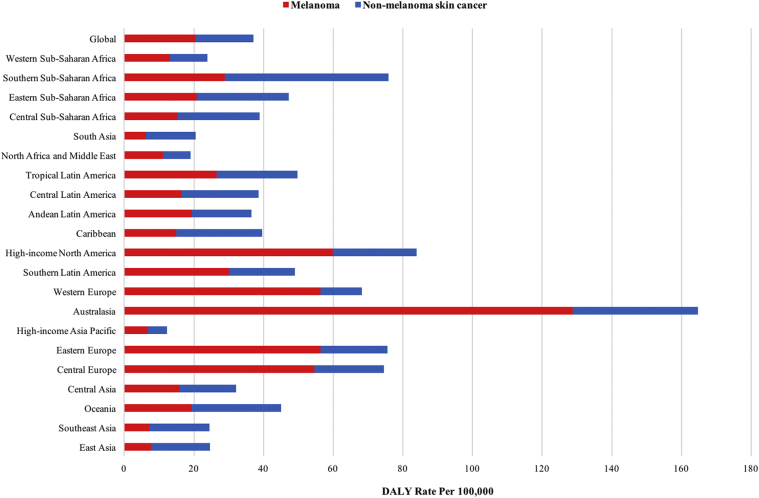
Melanoma and keratinocyte carcinoma disability-adjusted life years per 100,000 population by Global Burden of Disease world regions in 2017.
Fig 6.
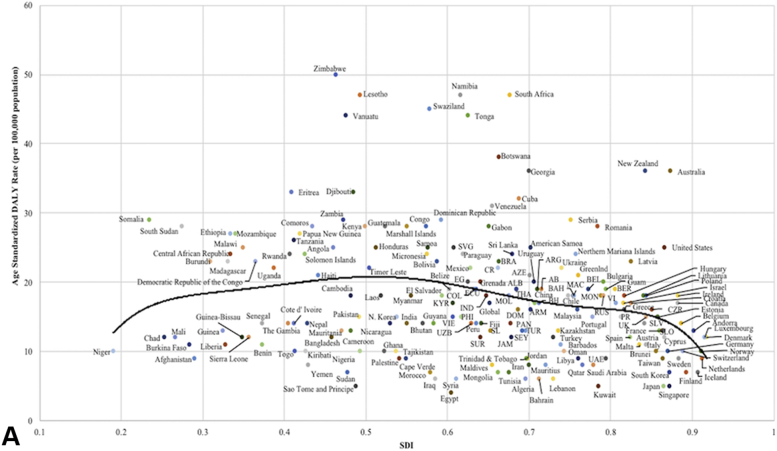
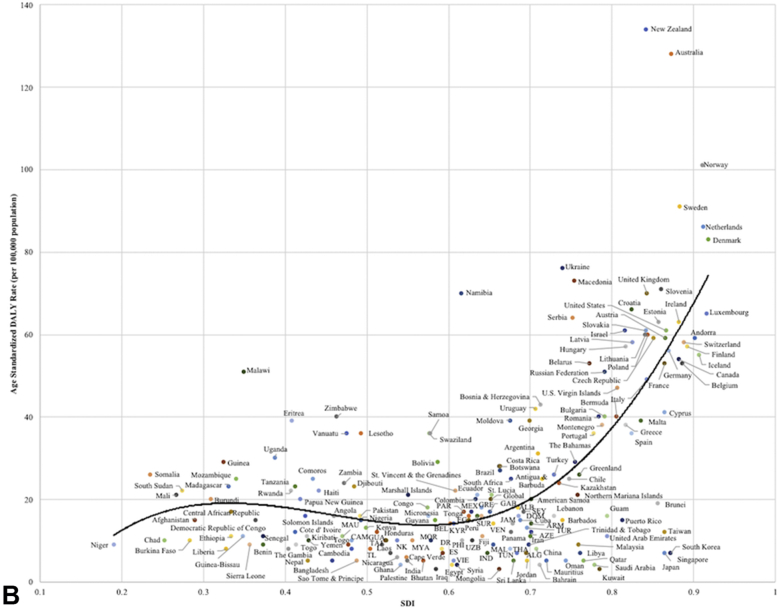
A, Age-standardized disability-adjusted life years from keratinocyte carcinoma by sociodemographic index for all 195 countries and territories in 2017. Expected values are shown as the black line. ALB, Albania; AB, Antigua and Barbuda; ARG, Argentina; ARM, Armenia; AZE, Azerbaijan; BAH, Bahamas; BEL, Belarus; BER, Bermuda; BH, Bosnia and Herzegovina; BRA, Brazil; COL, Colombia; CR, Costa Rica; CZR, Czech Republic; DOM, Dominica; ECU, Ecuador; EG, Equatorial Guinea; IND, Indonesia; JAM, Jamaica; KYR, Kyrgyzstan; MAC, Macedonia; MOL, Moldova; MON, Montenegro; PAN, Panama; PHI, Philippines; PR, Puerto Rico; RUS, Russian Federation; SEY, Seychelles; SLV, Slovakia; SLO, Slovenia; SL, St. Lucia; SVG, St. Vincent and the Grenadines; SUR, Suriname; THA, Thailand; TUR, Turkmenistan; UAE, United Arab Emirates; UK, United Kingdom; VI, U.S. Virgin Islands; UZB, Uzbekistan; VIE, Vietnam. B, Age-standardized disability-adjusted life years from melanoma by sociodemographic index for all 195 countries and territories in 2017. Expected values are shown as the black line. ALB, Albania; ARM, Armenia; AZE, Azerbaijan; BEL, Belize; CAM, Cameroon; DOM, Dominica; DR, Dominican Republic; ES, El Salvador; EG, Equatorial Guinea; GAB, Gabon; GRE, Grenada; GUA, Guatemala; IND, Indonesia; JAM, Jamaica; KYR, Kyrgyzstan; MAL, Maldives; MAU, Mauritania; MEX, Mexico; MOR, Morocco; MYA, Myanmar; NK, North Korea; PAR, Paraguay; PHI, Philippines; SEY, Seychelles; SUR, Suriname; TAJ, Tajikistan; THA, Thailand; TL, Timor Leste; TUN, Tunisia; TUR, Turkmenistan; UZB, Uzbekistan; VEN, Venezuela; VIE, Vietnam.
Results
Countries across the globe were found to exhibit varying levels of change in the age-standardized prevalence rates of melanoma and KC between 1990 and 2017 (Fig 1, A and B). BCC, SCC, and melanoma were the first, fifth, and 20th leading causes of invasive neoplasms (excluding “other benign and in situ neoplasms”), respectively, in 2017 (Table I). The percent changes from 1990 to 2017 were 310% for SCC, 161% for melanoma, and 77% for BCC.
In 2017, the global prevalence of KC was greatest between the ages of 65 and 75 years, with a large male predominance between ages 50 and 85 years (P < .05) (Fig 2, A). Men experienced greater age-specific prevalence rates of KC across all ages. Women had greater prevalence of melanoma until approximately 50 years of age, after which the trend reversed until 85 years of age (P < .05) (Fig 2, B). Melanoma-related deaths peaked between the ages of 50 and 85 years, and men experienced greater age-specific death rates across all ages (P < .05) (Fig 2, C). An exponential increase in the DALYs from both melanoma and KC was observed with increasing age (P < .05) (Fig 3).
Across the GBD super regions, Central Europe, Eastern Europe, Central Asia, Latin America, the Caribbean, and high-income countries had consistently higher DALYs for KC than the global average between 1990 and 2017 (P < .05) (Fig 4, A). The DALY rates in Southeast Asia, East Asia, and Oceania increased since 1990, eventually surpassing the global average in 2005. When the melanoma DALY rates were compared between 1990 and 2017, Central Europe, Eastern Europe, Central Asia, and high-income countries consistently had more than twice and up to nearly 4 times the global average DALY rate (P < .05) (Fig 4, B). The highest KC DALY rates were seen in Southern Sub-Saharan Africa, Australasia, and Eastern Sub-Saharan Africa, and the highest melanoma DALY rates were seen in Australasia, followed by high-income North America and Europe (P < .05) (Fig 5).
When comparing the age-standardized DALY rates from KC with the SDI, the largest deviations from the expected values were seen in the African countries of Zimbabwe, Lesotho, Swaziland, Namibia, Tonga, Botswana and South Africa and in Vanuatu, Georgia, Australia, and New Zealand (P < .05) (Fig 6, A). The expected age-standardized DALY rates from melanoma were compared to SDI. An exponential trend line was revealed, where higher SDI countries were estimated to have far larger age-standardized DALY rates (P < .05) (Fig 6, B). Australia and New Zealand were found to have much higher-than-predicted DALY rates for both melanoma and KC.
Discussion
The global prevalence and associated DALYs of melanoma and KC have increased to the present day. The global prevalence of melanoma was 0.03% in 2017, compared to 0.02% in 1990.11 The global prevalence of KC increased from 0.01% in 1990 to 0.03% in 2017.11 The percentage of total DALYs due to melanoma and KC increased from 0.04% in 1990 to 0.07% in 2017 and from 0.03% in 1990 to 0.05% in 2017 respectively.11 The 310% increase in SCC between 1990 and 2017 was the highest of any of the malignancies tracked by the GBD study.
The large global growth in the prevalence and morbidity of melanoma and KC demonstrates an important public health opportunity for increased prevention. KC is typically excluded from cancer registries, making the true prevalence difficult to estimate and likely underestimated.12 While UVR is the most important risk factor in the development of KC, other risk factors include immunosuppression and occupational exposure to tar and mineral oils.12 Immunosuppression may contribute to the large SCC growth, as evidenced by the increased incidence of SCC in organ transplant recipients and individuals with HIV/AIDS or hematological malignancies.13 Melanoma growth ranked third, at 161%, between 1990 and 2017. Melanoma growth may be related to overdiagnosis from increased biopsies, the reclassification of non-malignant diagnoses as melanoma, and the increased sensitivity of diagnostics techniques.5,14 By 2050, the proportion of the world population older than 60 years will nearly double to 22%.15 This rapid increase in longevity combined with the high age-specific rate of melanoma in the elderly may also contribute to the large global increase in melanoma rates.5
A large database in the United Kingdom similarly showed that men experienced greater rates of BCC at a mean age of 70.5 years.16 High incidence rates of KC in the older population may be due to the accumulation of intermittent sun exposure beginning in adolescence and recent strategies to increase screening and diagnosis in the elderly.17 Adult women experience higher prevalence rates of melanoma until approximately 50 years of age because they are more likely to participate in indoor tanning, which is associated with a subsequent diagnosis of melanoma.18 Despite higher prevalence rates, female melanoma patients may experience better outcomes, as estrogen likely stimulates an immune response by blocking the inhibitory signals that prevent tumor recognition.19
In addition to differences in the innate sensitivity of melanin to UVR, part of the wide geographic variance in skin cancer rates may be attributed to different levels of background UVR exposure due to ozone depletion, urbanization, and altitude and latitude variations.20 The ambient UVR strength is greater at higher altitudes due to a thinner atmosphere through which light can traverse, and a 2% increase in the melanoma risk is observed with every 10-meter elevation gain.21 UVR is also highest near the equator, as sunlight hits the earth more directly.20 Urbanization and a higher socioeconomic status are associated with up to a 50% increase in the risk of melanoma, which is likely explained by increased exposure to occupational chemicals and UVR, easier access to indoor tanning, and increased holiday travel.22,23
The large attributable risk of UVR with respect to skin cancer demonstrates an opportunity for improving sun-safe behaviors, and population-based primary prevention strategies have demonstrated some efficacy. Between 2003 and 2004, whole-body screening exams were performed on more than 360,000 adults in Schleswig-Holstein, Germany. By 2009, the intervention led to a 47% and 49% decline in melanoma mortality in men and women, respectively.24 Nationwide, biennial screening for adults older than 35 years was implemented in Germany in 2008, but unfortunately failed to lead to a decline in melanoma-related mortality.25 Melanoma mortality rates in Schleswig-Holstein have returned to the overall level in Germany (2.4 per 100,000), possibly due to less stringent screening guidelines compared to the pilot study.25 Regions of Southern Africa, including Lesotho, Swaziland, Namibia, Botswana, and South Africa, all of which had large deviations from the expected values when comparing DALYs rates from KC to SDI (Fig 6, A), lack robust skin cancer prevention campaigns. The limited resources for primary prevention is of concern in Southern Africa, especially with current behavioral changes that increase UVR exposure, such as spending time outdoors when outside temperatures are cooler than indoor dwellings.26
As a high SDI continent, Australasia often leads discussions in the literature on skin cancer prevalence. Risk factors and prevention strategies in areas with the highest rates of skin cancer may be of interest to other populations. Due to a colonial period in Australasian history, the majority of the population trace their ancestry to English, Scottish, or Irish descent.27 These populations are known to lack much of the innate intrinsic photoprotection provided by cutaneous melanin.28 One study estimated that 63% of all melanomas and virtually all KC are attributed to the 3 to 5 times higher UVR levels in Australia.29 Regular use of sunscreen with a sun protection factor of 15 or higher prevented approximately 9.3% of SCC and 14% of new melanoma cases in this population.30 As part of a more comprehensive sun protection strategy, sunscreen appears key to the prevention of skin cancer in high-risk populations.
Although sunscreen is part of the public health campaigns in many high-income countries, it may be cost-prohibitive. For many populations on the low end of the socioeconomic spectrum, sunscreen is less of a priority than other necessities of daily living. Sunscreen is not on the World Health Organization list of essential medications. The lack of financial means, occupational exposure from outdoor work, and the entry of sunlight into homes with low density materials may all contribute to high levels of skin cancer morbidity.31 The amount of UVR exposure may be largely out of an individual's control if they are employed in an outdoor profession. Sun-protective clothing and advisement to seek shade is necessary but not always possible. In addition to promoting education on minimizing exposure to UVR during peak daylight hours, a cost-effective solution involves making homemade sunscreen. An 85-gram mixture of 75% almond oil, 16% zinc oxide, and 9% beeswax provides a sun protection factor rating of approximately 15, and costs 11 times less than a similar-strength commercial alternative.31
There are limitations to consider in the context of the global burden of skin cancer. Studies measuring KC are limited because of their exclusion from large cancer registries, which makes data comparison difficult. Questionnaires that do not include the staging of melanoma may not adequately assess the true burden of disease. The GBD did not assign different weights based on the depth of melanoma, although the years lost due to disability is likely higher with an increased Breslow thickness.32 GBD disability estimates for skin disease may only reflect symptoms such as itching and appearance changes and may exclude complications such as secondary infection or mental health effects that exist far beyond the recommended follow-up.32,33 Available studies for comparison among the different GBD regions may be limited by geographical coverage, where certain populations are relatively over or underrepresented among of total studies in comparison to their total populations. Despite limitations inherent to the GBD and the global reporting of skin cancer, large-scale epidemiological data continue to help dermatologists and key decision makers to shape public policy and lower the global burden of skin cancer.
In 2050, 80% of older people will be living in low- and middle-income countries.15 In addition, access to dermatologists is rare in developing and rural settings.33 As elderly populations and corresponding rates of skin cancer grow, we must keep the accessibility and financial burden of skin cancer prevention in mind. As estimates point toward future generations with larger elderly populations, we believe an emphasis on skin cancer prevention will lead to more sustainable interventions with greater impacts. Practice-based changes such as the implementation of a chronic disease plan may also reduce the anxiety experienced by patients, prevent unneeded health care visits, and reduce health care costs.34 The increased community engagement, outreach, and development of new preventive strategies are promising future steps to overcome the challenge of reducing the global burden of skin cancer.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: This research has been conducted as part of the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), coordinated by the Institute for Health Metrics and Evaluation. The GBD was partially funded by the Bill & Melinda Gates Foundation.
Disclaimer: The funders had no role in the study design, data analysis, data interpretation, or writing of the report. All authors are collaborators with the Global Burden of Disease. This article was not developed with the consultation or support of the Global Burden of Disease research team.
IRB approval status: Not applicable.
References
- 1.Skin cancers. World Health Organization. https://www.who.int/uv/faq/skincancer/en/index1.html Available at:
- 2.Samarasinghe V., Madan V. Nonmelanoma skin cancer. J Cutan Aesthet Surg. 2012;5:3–10. doi: 10.4103/0974-2077.94323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward W.H., Farma J.M., editors. Cutaneous Melanoma: Etiology and Therapy [Internet] Codon Publications; Brisbane (AU): 2017. https://www.ncbi.nlm.nih.gov/books/NBK481860/doi:10.15586/codon.cutaneousmelanoma.2017 Available at: [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force, Grossman D.C., Curry S.J. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1134–1142. doi: 10.1001/jama.2018.1623. [DOI] [PubMed] [Google Scholar]
- 5.Apalla Z., Lallas A., Sotiriou E., Lazaridou E., Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7:1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metrics: Disability-Adjusted Life Year (DALY). World Health Organization. https://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/ Available at:
- 7.Frequently asked questions. IHME. http://www.healthdata.org/gbd/faq Available from:
- 8.Leach-Kemon K. A new way of measuring development helps assess health system performance. IHME. 2017. http://www.healthdata.org/acting-data/new-way-measuring-development-helps-assess-health-system-performance Available at:
- 9.Karimkhani C., Green A.C., Nijsten T. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177:134–140. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Protocol for the global burden of diseases, injuries, and risk factors study (GBD) [Internet]. Institute for Health Metrics and Evaluation. 2018. http://www.healthdata.org/sites/default/files/files/Projects/GBD/March2020_GBD%20Protocol_v4.pdf Accessed March 17, 2020. Available at:
- 11.Network GBoDC Global Burden of Disease Study 2017 Results [Internet]. Evaluation IfHMa, ed. Seattle, United States: Global Burden of Disease Collaborative Network. 2018. http://www.healthdata.org/policy-report/findings-global-burden-disease-study-2017 Accessed March 17, 2020. Available at:
- 12.Leiter U., Eigentler T., Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol. 2014;810:120–140. doi: 10.1007/978-1-4939-0437-2_7. [DOI] [PubMed] [Google Scholar]
- 13.Nagarajan P., Asgari M.M., Green A.C. Keratinocyte carcinomas: current concepts and future research priorities. Clin Cancer Res. 2019;25:2379–2391. doi: 10.1158/1078-0432.CCR-18-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welch H.G., Woloshin S., Schwartz L.M. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481. doi: 10.1136/bmj.38516.649537.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization Ageing and health. 2018. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health Accessed April 24, 2020. Available at:
- 16.Bath-Hextall F., Leonardi-Bee J., Smith C., Meal A., Hubbard R. Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. Int J Cancer. 2007;121:2105–2108. doi: 10.1002/ijc.22952. [DOI] [PubMed] [Google Scholar]
- 17.Garcovich S., Colloca G., Sollena P. Skin cancer epidemics in the elderly as an emerging issue in geriatric oncology. Aging Dis. 2017;8:643–661. doi: 10.14336/AD.2017.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colantonio S., Bracken M.B., Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847–857.e1. doi: 10.1016/j.jaad.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 19.Smalley K.S. Why do women with melanoma do better than men? Elife. 2018;7:e3351. doi: 10.7554/eLife.33511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaro-Ortiz A., Yan B., D'Orazio J.A. Ultraviolet radiation, aging and the skin: prevention of damage by topical cAMP manipulation. Molecules. 2014;19:6202–6219. doi: 10.3390/molecules19056202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haluza D., Simic S., Moshammer H. Temporal and spatial melanoma trends in Austria: an ecological study. Int J Environ Res Public Health. 2014;11:734–748. doi: 10.3390/ijerph110100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharp L., Donnelly D., Hegarty A. Risk of several cancers is higher in urban areas after adjusting for socioeconomic status. Results from a two-country population-based study of 18 common cancers. J Urban Health. 2014;91:510–525. doi: 10.1007/s11524-013-9846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouse C.H., Hillyer G.C., Basch C.E., Neugut A.I. Geography, facilities, and promotional strategies used to encourage indoor tanning in New York City. J Community Health. 2011;36:635–639. doi: 10.1007/s10900-010-9354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katalinic A., Waldmann A., Weinstock M.A. Does skin cancer screening save lives?: an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395–5402. doi: 10.1002/cncr.27566. [DOI] [PubMed] [Google Scholar]
- 25.Katalinic A., Eisemann N., Waldmann A. Skin cancer screening in Germany. Documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629–634. doi: 10.3238/arztebl.2015.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright C.Y., du Preez D.J., Millar D.A., Norval M. The epidemiology of skin cancer and public health strategies for its prevention in Southern Africa. Int J Environ Res Public Health. 2020;17:1017. doi: 10.3390/ijerph17031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Australia community profile. 2016. https://profile.id.com.au/australia/ancestry Accessed April 20, 2020. Available at:
- 28.Brenner M., Hearing V.J. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen C.M., Wilson L.F., Green A.C. Cancers in Australia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use. Aust N Z J Public Health. 2015;39:471–476. doi: 10.1111/1753-6405.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gies P., Roy C., Javorniczky J., Henderson S., Lemus-Deschamps L., Driscoll C. Global Solar UV Index: Australian measurements, forecasts and comparison with the UK. Photochem Photobiol. 2004;79:32–39. [PubMed] [Google Scholar]
- 31.Miyar M.E., Diven D.G., Cosulich M.T. Serving the underserved: creating a low-cost sunscreen with natural ingredients for humanitarian medical trips to the developing world. Br J Dermatol. 2014;171:415–417. doi: 10.1111/bjd.12993. [DOI] [PubMed] [Google Scholar]
- 32.Holterhues C., Hollestein L.M., Nijsten T., Koomen E.R., Nusselder W., de Vries E. Burden of disease due to cutaneous melanoma has increased in the Netherlands since 1991. Br J Dermatol. 2013;169:389–397. doi: 10.1111/bjd.12346. [DOI] [PubMed] [Google Scholar]
- 33.Seth D., Cheldize K., Brown D., Freeman E.F. Global Burden of Skin Disease: inequities and innovations. Curr Dermatol Rep. 2017;6:204–210. doi: 10.1007/s13671-017-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollestein L.M., de Vries E., Aarts M.J., Schroten C., Nijsten T.E. Burden of disease caused by keratinocyte cancer has increased in The Netherlands since 1989. J Am Acad Dermatol. 2014;71:896–903. doi: 10.1016/j.jaad.2014.07.003. [DOI] [PubMed] [Google Scholar]