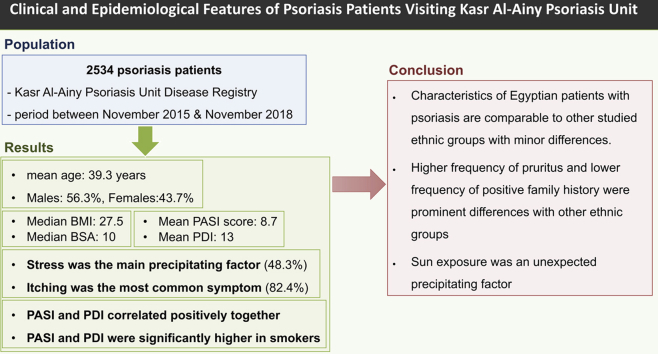
Abstract
Background
Identification of epidemiologic and phenotypic variations of psoriasis among different ethnic groups can further our understanding of this perplexing disease, aiming at better management of patients worldwide.
Objective
To provide a descriptive analysis of psoriasis patients registered at Kasr Al-Ainy Psoriasis Unit Disease Registry.
Methods
This retrospective single-center registry study included patient records between November 2015 and November 2018 (2534 patients). Sociodemographic and phenotypic data were analyzed.
Results
The mean age of the registered patients was 39.3 years and 56.3% were men. Stress was the main precipitating factor (48.3%), whereas the most common symptom reported was itching (82.4%). The median body mass index was 27.5, and the median percentage of body surface area involved was 10.0. The mean Psoriasis Area Severity Index score was 8.7, and the mean Psoriasis Disability Index score was 13.0. Both parameters correlated positively, and both showed significantly higher means in smokers.
Limitations
Despite that the study was performed at a highly specialized tertiary care center with a high flow of patients, this was still a single-center registry.
Conclusions
This work shows that the characteristics of Egyptian patients with psoriasis are comparable to those of other studied ethnic groups, with minor differences.
Key words: ethnic groups, pruritus, psoriasis, registry, retrospective studies
Abbreviations used: HCV, hepatitis C virus; PASI, Psoriasis Area Severity Index; PDI, Psoriasis Disability Index
Graphical abstract
Capsule Summary.
-
•
Psoriasis varies in epidemiology, morphology, distribution, and severity among ethnic groups. This study describes psoriasis characteristics in 2534 patients who received a diagnosis at a tertiary care center.
-
•
Higher frequency of pruritus and lower frequency of positive family history were prominent differences compared with other ethnic groups. Sun exposure was an unexpected precipitating factor.
Introduction
Psoriasis is a chronic, immunomediated, polygenic, inflammatory dermatosis affecting 1% to 3% of the general population.1,2 It is a lifelong disease with a negative effect on patients' quality of life.2 Although the exact causes of psoriasis are not fully understood, several risk factors are recognized, including family history and environmental risk factors, such as smoking, stress, and obesity.3
Not only can psoriasis be highly variable in morphology, distribution, and severity4 but also there is a considerable difference in the incidence of disease because of environmental, genetic, and geographic factors. Various national and international patient registries have been established for proper documentation and follow-up of psoriasis patients. These registries are located in Europe, Malaysia, the North America, United States, the American continent, Australia, and Israel.5 Data and information regarding the epidemiologic and clinical characteristics of psoriasis in Egypt are severely lacking. This work aimed to provide a descriptive analysis of more than 2500 Egyptian psoriasis patients.
Patients and methods
Study setting and design
This single-center, retrospective, observational, registry-based study was conducted at Kasr Al-Ainy's Psoriasis Unit. The unit is specialized and affiliated with the Department of Dermatology, Kasr Al-Ainy hospitals, Faculty of Medicine, Cairo University, which was established in November 2015 and is one of the earliest and largest specialized psoriasis clinics in Egypt. Since then, the unit has served and registered more than 3200 psoriasis patients, whether outpatients or inpatients. The Kasr Al-Ainy Psoriasis Unit Disease Registry database provides a wealth of information regarding various aspects of psoriasis, including demography, epidemiology, disease characteristics, management, and therapeutic response of the Egyptian patients.
Patient population
Data were collected from the records of 2534 psoriasis patients between November 2015 and November 2018 who had been registered in the Kasr Al-Ainy Psoriasis Unit Disease Registry database.
Data collection
The registry variables used in this study are detailed in Table I.
Table I.
Patients' data variables recorded in the Kasr Al-Ainy Psoriasis Unit Disease Registry database
| Patient sociodemographic data | Anthropometric measurements | Psoriasis disease assessment |
|---|---|---|
| Patient ID | Weight | Disease onset, course, and duration, mo |
| Age | Height | Body sites currently affected |
| Sex | Body mass index | Site first affected |
| Occupation | Previous treatments and psoriasis response to them | |
| Marital status | Family history of psoriasis | |
| Residence | Symptoms (itching, dryness, skin tenderness) | |
| Telephone number | Precipitating factors | |
| Smoking | % of body surface area involved | |
| Pregnancy | Baseline Psoriasis Area Severity Index score at first presentation to KAPU | |
| The contraceptive method used if any | Psoriasis Disability Index score for adult patients |
ID, Identification; KAPU, Kasr Al-Ainy Psoriasis Unit.
Ethical considerations
The Dermatology Research Ethical Committee reviewed and approved the study protocol. The need for consent was waived according to institutional regulations for observational studies. The study followed the principles of the declaration of Helsinki.
Statistical analysis
Data entry and analysis were carried out with SPSS (version 24.0, SPSS Inc IBM, Chicago, IL). All variables were checked for normality. Descriptive statistics were summarized as mean ± standard deviation or median (range) as appropriate. χ2 Test was used to assess group differences for categoric variables and the Student t test was used to assess differences between continuous variables. A comparison of nonparametric data was performed with the Mann-Whitney U test. Missing data when less than 5% were considered to be missing completely at random, and this was tested statically because it was not different from the recorded data. All statistical testing was 2 tailed, with a significance level of ≤ .05.
Results
From November 2015 to November 2018, a total of 2534 psoriasis patients were included in the study. The mean age of the study group was 39.3 ± 17.9 years (range 1-94 years), with men representing 56.3% of patients. Positive family history was reported in 23.1% and 26.9% were smokers.
The main precipitating factors reported by the patients were stress (48.3%), followed by cold (39.7%) and sunlight (15.4%). Other less common precipitating factors included infections (4.6%), drugs (2.6%), trauma, and food (0.2% each). More than one-quarter of the study group reported no precipitating factors (26.4%).
The mean disease duration was 106.6 ± 60.0 months. The most common phenotype of psoriasis in the current study was plaque psoriasis (84.1%), followed by guttate psoriasis (10.3%), whereas other less common types included chronic palmoplantar pustulosis and pustular psoriasis (1.8%). Associated symptoms, affected sites, and psoriasis phenotypes of studied patients are listed in Table II. Anthropometric measurements and disease severity among the studied psoriasis patients are listed in Table III.
Table II.
Clinical characteristics of the studied patients with psoriasis (n = 2534)
| Clinical characteristics | Results |
|---|---|
| Age at disease onset (mean ± SD), y | 30.5 ± 17.1 |
| Disease duration, mo | |
| Mean ± SD | 106.6 ± 60.0 |
| Range | 0.25-720 |
| Disease onset | |
| Gradual | 2154 (85.0) |
| Sudden | 380 (15.0) |
| Disease course | |
| Progressive | 1153 (45.5) |
| Regressive | 56 (2.2) |
| Remissions and exacerbations | 1325 (52.3) |
| Symptoms∗ | |
| Itching | 2088 (82.4) |
| Dryness | 926 (36.5) |
| Skin pain and tenderness | 491 (19.4) |
| None | 263 (10.4) |
| Affected sites∗ | |
| Lower limbs | 1921 (75.8) |
| Upper limbs | 1774 (70.0) |
| Scalp | 1560 (61.6) |
| Trunk | 1476 (58.2) |
| Back | 1399 (55.2) |
| Knees | 1362 (53.7) |
| Elbows | 1346 (53.1) |
| Face | 549 (21.7) |
| Nails | 524 (20.7) |
| Palms | 489 (19.3) |
| Flexures | 482 (19.0) |
| Genitalia | 444 (17.5) |
| Neck | 430 (17.0) |
| Soles | 429 (16.9) |
| Phenotype of psoriasis∗ | |
| Plaque psoriasis | 2132 (84.1) |
| Guttate | 261 (10.3) |
| Erythrodermic psoriasis | 95 (3.7) |
| Chronic palmoplantar pustulosis | 46 (1.8) |
| Generalized pustular | 45 (1.8) |
Data are presented as No. (%) unless otherwise indicated.
SD, Standard deviation.
Patients may present by multiple symptoms, sites, and phenotypes simultaneously.
Table III.
Anthropometric measurements and disease severity among the psoriasis patients (n = 2534)
| Variables | Mean ± SD | Median | Minimum | Maximum |
|---|---|---|---|---|
| BMI | 28.0 ± 7.3 | 27.5 | 10.6 | 58.0 |
| The extent of skin lesions (% BSA) | 21.3 ± 23.5 | 10.0 | 1.0 | 100.0 |
| PASI (baseline) | 8.7 ± 0.09 | 5.4 | 0.1 | 60.4 |
| PDI | 13.0 ± 10.0 | 11.0 | 0.0 | 45.0 |
BMI, Body mass index; BSA, body surface area; PASI, Psoriasis Area Severity Index; PDI, Psoriasis Disability Index; SD, standard deviation.
Relevant demographic and clinical characteristics and anthropometric differences between men and women are presented in Tables IV and V.
Table IV.
Comparison between male and female psoriasis patients according to demographic and clinical characteristics (n = 2534)
| Variables | Sex |
P value | |
|---|---|---|---|
| Women (n = 1107) | Men (n = 1427) | ||
| Age at disease onset (mean ± SD), y∗ | 28.1 ± 17.1 | 32.7 ± 16.9 | <.001 |
| Positive family history of psoriasis | 191 (17.3) | 252 (17.7) | .79 |
| Smoking | 38 (3.4) | 648 (45.4) | <.001 |
| Symptoms | |||
| Itching | 933 (84.3) | 1155 (8.9) | .03 |
| Dryness | 415 (37.5) | 511 (35.8) | .38 |
| Skin pain and tenderness | 243 (22.0) | 248 (17.4) | .004 |
| None | 99 (8.9) | 164 (11.5) | .04 |
| Phenotype of psoriasis† | |||
| Plaque | 895 (80.8) | 1237 (86.7) | <.001 |
| Guttate | 126 (11.4) | 135 (9.5) | .11 |
| Erythrodermic | 30 (2.7) | 65 (4.6) | .02 |
| Chronic palmoplantar pustulosis | 15 (1.4) | 31 (2.2) | .13 |
| Generalized pustular | 26 (2.3) | 19 (1.3) | .05 |
| Precipitating factors∗ | |||
| Stress | 466 (42.1) | 555 (38.9) | .10 |
| Cold | 455 (41.1) | 551 (38.6) | .20 |
| None | 268 (24.2) | 402 (28.2) | .03 |
| Sun | 157 (14.2) | 243 (17.0) | .05 |
| Infection | 52 (4.7) | 64 (4.5) | .81 |
| Drugs | 32 (2.9) | 33 (2.3) | .36 |
| Trauma | 1 (0.1) | 4 (0.3) | .29 |
| Food | 2 (0.2) | 2 (0.1) | >.99 |
Data are presented as No. (%) unless otherwise indicated. P ≤ .05 was considered statistically significant. The analysis was conducted by χ2 test.
SD, Standard deviation.
Analysis conducted by independent t test.
Patients may present by multiple phenotypes simultaneously.
Table V.
Comparison between male and female psoriasis patients according to anthropometric and clinical characteristics (n = 2534)
| Variables | Women |
Men |
P value | ||||
|---|---|---|---|---|---|---|---|
| Median | Minimum | Maximum | Median | Minimum | Maximum | ||
| Age at presentation, y | 36 | 1 | 76 | 43 | 2 | 94 | <.001 |
| Duration, mo | 60 | 0.25 | 708 | 72 | 0.25 | 720 | .005 |
| BMI | 29.7 | 10.6 | 58 | 26 | 11.11 | 51.78 | <.001 |
| Extent of skin lesions (% BSA) | 10 | 0 | 97 | 15 | 0 | 100 | <.001 |
| PASI (baseline) | 4.4 | 0.1 | 57.4 | 6.5 | 0.1 | 60.4 | <.001 |
| PDI | 10 | 0 | 45 | 11 | 0 | 45 | .005 |
P ≤ .05 was considered statistically significant. The analysis was conducted with a Mann-Whitney U test.
BMI, Body mass index; BSA, body surface area; PASI, Psoriasis Area Severity Index; PDI, Psoriasis Disability Index.
The Psoriasis Area Severity Index (PASI) score ranged from 0.1 to 60.4, with a mean of 8.7 ± 0.09, whereas the mean Psoriasis Disability Index (PDI) score was 13.0 ± 10.0 (range 0 to 45). There was a statistically significant positive correlation between PDI and PASI scores (r = 0.421; P < .001). PDI score was also positively correlated with extent of disease (r = 0.397; P < .001). PASI score was significantly higher in patients on whom psoriasis exerted a major influence in their daily lives (P < .001). The baseline median PASI and PDI scores among smokers (7.6 and 14, respectively) were significantly higher (P < .001) than for nonsmokers (4.8 and 10, respectively). Patients complaining of pruritus had a higher median PASI score than those without pruritus, and the difference was statistically significant (P < .001).
Baseline PASI correlated positively with patients' age, duration of illness, extent of illness, and PDI score (Supplemental Table I). Additional analyses exploring the relation to smoking, skin tenderness, and hepatitis C virus (HCV) infection are displayed in Supplemental Tables II and III and discussed later.
Discussion
In the northern African and Middle East areas, a few studies11,12 have reported sociodemographic criteria of psoriasis, but they have not yet been reported in Egypt, where an estimated 500,000 persons are affected by psoriasis.13 To our knowledge, this is the largest Egyptian single-center registry analysis to date describing the characteristics of more than 2500 Egyptian psoriasis patients. This study is in line with the worldwide increasing interest in defining regional specificities of psoriasis, particularly in low- and middle-income countries, where epidemiologic studies are lacking, to better identify and address health needs in each country.13
The percentage of patients with psoriasis among all dermatology patients attending our outpatient skin clinic from November 2015 until November 2018 was 1.3%, which was higher than that reported from clinics in several West African countries (0.05%-0.9%), almost half that reported in a Nepali dermatology clinic (2.9%), and much lower than that reported in a Turkish clinic (5.5%).6, 7, 8 The sociodemographic characteristics of our patients were comparable to those of patients from Asian countries such as Nepal and Malaysia. Male predominance (56%) was similar to that reported from Nepal, Maghreb, and Malaysia (53.7%, 55.7%, and 56.6%, respectively).2,7,11 In contrast, a female predominance was reported by some Western counties such as Denmark (53.2%) and in Minnesota.8, 9, 10
In accordance with previous reports,11 the mean age at onset was 30.5 years, with 69.8% of patients classified as having type 1 psoriasis, 14.2% of whom were younger than 16 years. This was minimally different than onset at aged 26.4, 29.1, 33.6 to 33.4, and 35.1 years in Nepal, Spain, Italy, and Malaysia, respectively.2,7,14,15 Regardless of disease onset, we observed an increase in PASI score with both the age of the patient and the duration of disease (P < .001). In concordance with a UK population-based study and Chinese nationwide and Italian surveys,15, 16, 17 the mean age of onset of psoriasis in our female patients was significantly lower than that in male patients.
Family history of psoriasis, which is considered one of the risk factors for the development of the disease,3 was present in 17.5% of our patients. This is lower than the percentage in studies carried out in Italy, Spain, Maghreb, China, and Malaysia, which reported family history for 45.9%, 40.7%, 28.6%, 23.1%, and 23.1% of patients, respectively.2,14, 15, 16,18 This may be due to cultural and social factors in which patients may deny affliction in their family. Such a denial may provide patients some reassurance regarding avoidance of social stigmatization and exclusion,19,20 a fear that is even more accentuated by the local culture of the “perfect mate” to the extent that social network groups have been created by psoriasis patients to socialize for marriage purposes. Further investigation of the rate of family history is needed, ideally by family physicians for more accurate data.
A meta-analysis acknowledged the association between smoking and psoriasis prevalence as well as severity.21 Earlier meta-analyses additionally reported more resistance to treatment among smokers compared with nonsmokers.22,23 More than one-quarter of our patients (26.9%) were smokers, and those were mostly men (94%) whose baseline median PASI score was significantly higher than that of nonsmokers. Smoking was significantly more common among patients with plaque, nail, palmoplantar, and erythrodermic psoriasis compared with other types of psoriasis.
Psychological stress is associated with psoriasis exacerbation and may even trigger the onset of disease. It is estimated that 31% to 88% of patients report stress as a trigger for their psoriasis.24,25 In our patients, 40.3% reported that stress was a precipitating factor. This percentage is comparable to that in data from China (34.5%) and Malaysia (48.3%) but is much lower than that reported in Maghreb (79.4%).2,16,18
Psoriasis affected the activities of daily living of 83.8% of our patients. Higher baseline PASI score was associated with a significantly higher major effect on activities of daily living. This was further demonstrated by the positive correlation between PASI and PDI scores (P < .001) and between PDI and extent of disease (P < .001). Patients with erythrodermic psoriasis showed significantly higher PDI values compared with other subtypes.
Although sun exposure may have a positive clinical effect on psoriasis, presumably involving immunoregulatory mechanisms,26 15.8% of our patients reported sun exposure without sunburn as an exacerbating factor of their disease. Although often overlooked, worsening of psoriasis after sun exposure had been documented to occur in 5% to 20% of patients.27, 28, 29, 30, 31, 32 Several mechanisms had been proposed for this phenomenon, including Koebnerization, an associated photosensitive disorder, or a direct ultraviolet exacerbation of psoriasis.32 Rutter and colleagues32 reported an association of photosensitive psoriasis with low-dose broadband ultraviolet A, the HLA-Cw∗0602 allele, and ultraviolet-induced histologic evidence of early psoriasis. Photosensitive psoriasis may not be related to only fair-skinned patients, and dermatologists should be aware of this risk factor when dealing with ethnic skin and advise susceptible patients accordingly.
The most common symptom we recorded was itching, which was reported by 82.4% of our patients. Itching has been shown to be a very common symptom among psoriasis patients, and its incidence ranges from 60% to 90%, depending on the population studied.33 Egyptian psoriasis patients in this study showed one of the highest incidences of psoriasis-associated pruritus. Patients complaining of pruritus had a higher median PASI score than those without pruritus (P < .001), which was comparable to the findings of Bahali and colleagues34 among Turkish patients with psoriasis. In the current work, itching was most commonly found in patients with plaque psoriasis and least with chronic palmoplantar pustulosis and palmoplantar psoriasis.
Skin pain and tenderness, a commonly neglected and underreported symptom, was reported by 19.4% of our patients irrespective of any joint pain. Tenderness was more commonly reported by female patients (P = .004) as well as those with erythrodermic, pustular, or palmoplantar psoriasis (P = .001, .001, and .009, respectively). Moreover, patients with dry skin or itching complained of skin tenderness (P < .001), a more significant finding. Ljosaa and colleagues35 reported skin pain in up to 42% of their psoriasis patients, and it was related to quality of life.
Similar to previous reports from different regions of the world,2,14,16,18 the most common type of psoriasis in our patients was the chronic plaque type, recorded in 84.1% of patients. The less common forms of psoriasis—namely, erythrodermic and pustular psoriasis—were observed in 3.7% and 1.8% of patients, respectively. On comparison of the latter figures with observations from other countries, both erythrodermic and pustular psoriasis were still uncommon compared with other forms of psoriasis; however, differences in incidence were noted among different ethnicities. In Maghreb, erythrodermic and pustular psoriasis was observed in 13.6% and 5.7% of psoriasis patients, respectively,18 more than double that of our findings. On the other hand, in China, erythrodermic psoriasis was reported in only 0.6% of patients.16 Access to health care and genetic and epigenetic mechanisms may be responsible for such diversities and may warrant further study.
The most commonly affected sites with psoriasis in the current work were the lower limbs (75.8%) and upper limbs (70%), whereas the least affected were the neck (17%) and soles (16.9%). Scalp involvement was evident in 61.6% of patients, whereas genitalia were affected in 17.5% of patients. Both latter sites may significantly affect patients' quality of life, which seems to be related not only to the body surface area affected but also the site.36 The relatively high prevalence of scalp involvement among Egyptian patients with psoriasis, and possibly Middle Eastern patients—men and women—who share head coverings for cultural or religious reasons, requires further research to determine the effect of head coverings on scalp psoriasis. It also should alert physicians to inquire thoroughly about scalp involvement to avoid missing a diagnosis that has deleterious effects on quality of life.
The mean body mass index was calculated at 28.0 ± 7.3, denoting a tendency for overweight that was comparable to the mean body mass index for the Egyptian population (28.2) reported in 201737 Even though most studies have demonstrated that patients with psoriasis have significantly higher odds of having obesity,38,39 some have shown a lack of evidence to support this notion.38,40,41 We did not detect any correlation between body mass index and PASI or PDI score. Nonetheless, obesity is assumed to decrease treatment response, complicate drug therapy, increase psoriasis severity,38 and even present a causal relationship between high body mass index and psoriasis.42
The association of cutaneous psoriasis with other diseases has been the focus of several types of research during the past few years. Apart from classic psoriatic arthritis, inflammatory bowel disease, and psychological disorders, evidence suggests that cardiovascular disease, obesity, diabetes, hypertension, dyslipidemia, metabolic syndrome, nonalcoholic fatty liver disease, and cancer have a higher prevalence in psoriasis patients compared with the general population.43,44 We detected 1 or more of these comorbidities in 38.3% of our patients, with hypertension and diabetes being the most common (11.9% and 10.9% of all patients, respectively). In total, 5.5% of our patients had either active HCV or had received recent treatment for it. The prevalence of HCV among patients with psoriasis has been previously reported to be significantly higher than among nonpsoriatic controls (1.03% vs 0.56%),45 and this was attributed to hepatitis C–induced upregulation of key psoriasis cytokines; namely, cathelicidin, TLR9, and interferon gamma.46
The mean PASI score in our registry records was low (8.7 ± 0.09), probably because most patients were receiving treatment for psoriasis at registry enrollment. A similar explanation was suggested by Kimbal and colleagues47 to justify their findings of body surface area in the PSOLAR registry. On the other hand, a relatively high mean PDI score and mean percentage body surface area in our registry (13.0% ± 10.0% and 21.0% ± 24.0%, respectively), contrasting with the relatively low PASI score, reflects the importance of using several parameters for proper evaluation of disease severity. We observed significantly lower values for PASI score, PDI score, and percentage body surface area affected among female patients compared with male patients. This may be related to female patients' younger age and shorter disease duration at first visit, with consequently an earlier therapeutic intervention in comparison with that for male patients.
To summarize, in response to the global interest for the sociodemographic specificities of psoriasis, this registry-based study is the largest descriptive analysis of Egyptian patients with psoriasis, to our knowledge. It shows that the characteristics of Egyptian patients with psoriasis are comparable to those of other studied ethnic groups, apart from minor differences. Paradoxically, sun exposure without sunburn is an exacerbating factor in 15.8% of Egyptian psoriasis patients. The positive family history of psoriasis among Egyptian patients is one of the lowest worldwide. Pruritus was the commonest symptom associated with psoriasis in more than 80% of our cases, is associated with higher PASI score, and should be further investigated as predictive of psoriasis severity. Further descriptive studies using the Kasr Al-Ainy Psoriasis Unit Disease Registry may help assess patterns of management and outcomes of this cohort of patients.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
Reviewed and approved by the Dermatology Research Ethical Committee.
Appendix
Supplemental Table I.
Baseline Psoriasis Area Severity Index score in relation to various parameters
| PASI (baseline) | r | P value |
|---|---|---|
| Age, y | 0.143 | <.001 |
| Duration, mo | 0.095 | <.001 |
| BMI | −0.024 | .26 |
| Extent (% BSA) | 0.79 | <.001 |
| PDI | 0.421 | <.001 |
BMI, Body mass index; BSA, body surface area; PASI, Psoriasis Area Severity Index; PDI, Psoriasis Disability Index.
Supplemental Table II.
Smoking in relation to psoriasis phenotypes and symptoms
| Psoriasis phenotype and symptoms | Smoking |
P value | |
|---|---|---|---|
| No | Yes | ||
| Itching | 1529 (82.7) | 559 (81.5) | .46 |
| Dryness | 681 (36.9) | 245 (35.7) | .60 |
| Tenderness | 374 (20.2) | 117 (17.1) | .07 |
| None | 184 (10.0) | 79 (11.5) | .25 |
| Plaque psoriasis | 1527 (82.6) | 605 (88.2) | .001 |
| Scalp psoriasis | 760 (41.1) | 311 (45.3) | .06 |
| Nail psoriasis | 238 (12.9) | 154 (22.4) | <.001 |
| Flexural | 216 (11.7) | 91 (13.3) | .28 |
| Guttate | 199 (10.8) | 62 (9.0) | .203 |
| Palmoplantar psoriasis | 159 (8.6) | 78 (11.4) | .03 |
| Erythrodermic psoriasis | 58 (3.1) | 37 (5.4) | .008 |
| Chronic palmoplantar pustulosis | 31 (1.7) | 15 (2.2) | .39 |
| Pustular | 38 (2.1) | 7 (1.0) | .08 |
Supplemental Table III.
Skin tenderness in relation to itching, dryness, and psoriasis phenotypes
| Psoriasis variables | Tenderness |
P value | |
|---|---|---|---|
| No (n = 2043) | Yes (n = 491) | ||
| Itching | 1652 (80.9) | 436 (88.8) | <.001 |
| Dryness | 663 (32.5) | 263 (53.6) | <.001 |
| None | 263 (12.9) | 0 | <.001 |
| Plaque psoriasis | 1744 (85.4) | 388 (79.0) | .001 |
| Scalp psoriasis | 870 (42.6) | 201 (40.9) | .51 |
| Nail psoriasis | 304 (14.9) | 88 (17.9) | .09 |
| Flexural | 237 (11.6) | 70 (14.3) | .11 |
| Guttate | 215 (10.5) | 46 (9.4) | .45 |
| Palmoplantar psoriasis | 176 (8.6) | 61 (12.4) | .009 |
| Erythrodermic psoriasis | 64 (3.1) | 31 (6.3) | .001 |
| Chronic palmoplantar pustulosis | 34 (1.7) | 12 (2.4) | .25 |
| Pustular | 26 (1.3) | 19 (3.9) | <.001 |
References
- 1.Michalek I.M., Loring B., John S.M. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212. doi: 10.1111/jdv.13854. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Affandi A., Khan I., Ngah Saaya N. Epidemiology and clinical features of adult patients with psoriasis in Malaysia: 10-year review from the Malaysian Psoriasis Registry (2007–2016) Dermatol Res Pract. 2018;2018:4371471. doi: 10.1155/2018/4371471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huerta C., Rivero E., Rodríguez L.A. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143(12):1559–1565. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 4.Langley R.G., Krueger G.G., Griffiths C.E. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18–ii23. doi: 10.1136/ard.2004.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eissing L., Rustenbach S.J., Krensel M. Psoriasis registries worldwide: systematic overview on registry publications. J Eur Acad Dermatol Venereol. 2016;30(7):1100–1106. doi: 10.1111/jdv.13634. [DOI] [PubMed] [Google Scholar]
- 6.Chandran V., Raychaudhuri S.P. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun. 2010;34(3):J314–J321. doi: 10.1016/j.jaut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Mikrani J.A., Shrestha A. Clinical and epidemiological features of psoriasis in patients visiting Lumbini Medical College. J Lumbini Med Coll. 2014;2(1):1–3. [Google Scholar]
- 8.Bilgili M.E., Yildiz H., Sarici G. Prevalence of skin diseases in a dermatology outpatient clinic in Turkey. A cross-sectional, retrospective study. J Dermatol Case Rep. 2013;7(4):108–112. doi: 10.3315/jdcr.2013.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egeberg A., Skov L., Gislason G.H., Thyssen J.P., Mallbris L. Incidence and prevalence of psoriasis in Denmark. Acta Derm Venereol. 2017;97(6-7):808–812. doi: 10.2340/00015555-2672. [DOI] [PubMed] [Google Scholar]
- 10.Bell L.M., Sedlack R., Beard C.M., Perry H.O., Michet C.J., Kurland L.T. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127(8):1184–1187. [PubMed] [Google Scholar]
- 11.Henseler T., Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13(3):450–456. doi: 10.1016/s0190-9622(85)70188-0. [DOI] [PubMed] [Google Scholar]
- 12.Shalom G., Zisman D., Babaev M. Psoriasis in Israel: demographic, epidemiology, and healthcare services utilization. Int J Dermatol. 2018;57(9):1068–1074. doi: 10.1111/ijd.14130. [DOI] [PubMed] [Google Scholar]
- 13.IPC, ILDS and IFPA. Global Psoriasis Atlas. Prevalence Data: Egypt. Available at: https://globalpsoriasisatlas.org/statistics/prevalence. Accessed November 29, 2019.
- 14.Ferrándiz C., Pujol R.M., García-Patos V., Bordas X., Smandía J.A. Psoriasis of early and late onset: a clinical and epidemiologic study from Spain. J Am Acad Dermatol. 2002;46(6):867–873. doi: 10.1067/mjd.2002.120470. [DOI] [PubMed] [Google Scholar]
- 15.Altobelli E., Petrocelli R., Marziliano C. Family history of psoriasis and age at disease onset in Italian patients with psoriasis. Br J Dermatol. 2007;156(6):1400–1401. doi: 10.1111/j.1365-2133.2007.07906.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen K., Wang G., Jin H. Clinic characteristics of psoriasis in China: a nationwide survey in over 12000 patients. Oncotarget. 2017;8(28):46381–46389. doi: 10.18632/oncotarget.18453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Springate D.A., Parisi R., Kontopantelis E., Reeves D., Griffiths C.E., Ashcroft D.M. Incidence, prevalence and mortality of patients with psoriasis: a UK population-based cohort study. Br J Dermatol. 2017;176(3):650–658. doi: 10.1111/bjd.15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ammar-Khodja A., Benkaidali I., Bouadjar B. EPIMAG: international cross-sectional epidemiological psoriasis study in the Maghreb. Dermatology. 2015;231(2):134–144. doi: 10.1159/000382123. [DOI] [PubMed] [Google Scholar]
- 19.Case D.O., Andrews J.E., Johnson J.D., Allard S.L. Avoiding versus seeking: the relationship of information seeking to avoidance, blunting, coping, dissonance, and related concepts. J Med Libr Assoc. 2005;93(3):353–362. [PMC free article] [PubMed] [Google Scholar]
- 20.Claassen L., Henneman L., Janssens A.C. Using family history information to promote healthy lifestyles and prevent diseases; a discussion of the evidence. BMC Public Health. 2010;10(1):248. doi: 10.1186/1471-2458-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richer V., Roubille C., Fleming P. Psoriasis and smoking: a systematic literature review and meta-analysis with qualitative analysis of effect of smoking on psoriasis severity. J Cutan Med Surg. 2016;20(3):221–227. doi: 10.1177/1203475415616073. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong A.W., Harskamp C.T., Dhillon J.S., Armstrong E.J. Psoriasis and smoking: a systematic review and meta-analysis. Br J Dermatol. 2014;170(2):304–314. doi: 10.1111/bjd.12670. [DOI] [PubMed] [Google Scholar]
- 23.Fowles J., Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12(4):424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rousset L., Halioua B. Stress and psoriasis. Int J Dermatol. 2018;57(10):1165–1172. doi: 10.1111/ijd.14032. [DOI] [PubMed] [Google Scholar]
- 25.Roque Ferreira B., Pio-Abreu J.L., Reis J.P., Figueiredo A. Analysis of the prevalence of mental disorders in psoriasis: the relevance of psychiatric assessment in dermatology. Psychiatr Danub. 2017;29(4):401–406. doi: 10.24869/psyd.2017.401. [DOI] [PubMed] [Google Scholar]
- 26.Søyland E., Heier I., Rodríguez-Gallego C. Sun exposure induces rapid immunological changes in skin and peripheral blood in patients with psoriasis. Br J Dermatol. 2011;164(2):344–355. doi: 10.1111/j.1365-2133.2010.10149.x. [DOI] [PubMed] [Google Scholar]
- 27.Lane C.G., Crawford G.M. Psoriasis: a statistical study of two hundred and thirty-one cases. Arch Derm Syph. 1937;35(6):1051–1061. [Google Scholar]
- 28.Lomholt G. GEC Gad; Copenhagen: 1963. Prevalence, spontaneous course, and genetics. A census study on the prevalence of skin disease on the Faroe Islands. In: Psoriasis. Volume 5 of Fródskaparrit: Suppl; pp. 31–33. [Google Scholar]
- 29.Ros A.M., Eklund G. Photosensitive psoriasis: an epidemiologic study. J Am Acad Dermatol. 1987;17(5):752–758. doi: 10.1016/s0190-9622(87)70258-8. [DOI] [PubMed] [Google Scholar]
- 30.Ros A.M., Wennersten G. Photosensitive psoriasis–clinical findings and phototest results. Photodermatology. 1986;3(6):317–326. [PubMed] [Google Scholar]
- 31.Farber E.M., Bright R.D., Nall M.L. Psoriasis: a questionnaire survey of 2,144 patients. Arch Dermatol. 1968;98(3):248–259. doi: 10.1001/archderm.98.3.248. [DOI] [PubMed] [Google Scholar]
- 32.Rutter K.J., Watson R.E., Cotterell L.F., Brenn T., Griffiths C.E., Rhodes L.E. Severely photosensitive psoriasis: a phenotypically defined patient subset. J Invest Dermatol. 2009;129(12):2861–2867. doi: 10.1038/jid.2009.156. [DOI] [PubMed] [Google Scholar]
- 33.Szepietowski J.C., Reich A. Pruritus in psoriasis: an update. Eur J Pain. 2016;20(1):41–46. doi: 10.1002/ejp.768. [DOI] [PubMed] [Google Scholar]
- 34.Bahali A.G., Onsun N., Su O. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92(4):470–473. doi: 10.1590/abd1806-4841.20175402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ljosaa T.M., Mork C., Stubhaug A., Moum T., Wahl A.K. Skin pain and skin discomfort is associated with quality of life in patients with psoriasis. J Eur Acad Dermatol Venereol. 2012;26(1):29–35. doi: 10.1111/j.1468-3083.2011.04000.x. [DOI] [PubMed] [Google Scholar]
- 36.Dopytalska K., Sobolewski P., Błaszczak A., Szymańska E., Walecka I. Psoriasis in special localizations. Reumatologia. 2018;56(6):392–398. doi: 10.5114/reum.2018.80718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization Egypt national STEPwise survey for noncommunicable diseases risk factors. https://www.who.int/ncds/surveillance/steps/egypt/en/
- 38.Jensen P., Skov L. Psoriasis and obesity. Dermatology. 2016;232(6):633–639. doi: 10.1159/000455840. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong A.W., Harskamp C.T., Armstrong E.J. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2(12):e54. doi: 10.1038/nutd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herron M.D., Hinckley M., Hoffman M.S. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141(12):1527–1534. doi: 10.1001/archderm.141.12.1527. [DOI] [PubMed] [Google Scholar]
- 41.Mallbris L., Granath F., Hamsten A., Ståhle M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J Am Acad Dermatol. 2006;54(4):614–621. doi: 10.1016/j.jaad.2005.11.1079. [DOI] [PubMed] [Google Scholar]
- 42.Budu-Aggrey A., Brumpton B., Tyrrell J. Evidence of a causal relationship between body mass index and psoriasis: a Mendelian randomization study. PLoS Med. 2019;16(1):e1002739. doi: 10.1371/journal.pmed.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni C., Chiu M.W. Psoriasis and comorbidities: links and risks. Clin Cosmet Investig Dermatol. 2014;7:119–132. doi: 10.2147/CCID.S44843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliveira M.D., Rocha B.D., Duarte G.V. Psoriasis: classical and emerging comorbidities. Anais Bras Dermatol. 2015;90(1):9–20. doi: 10.1590/abd1806-4841.20153038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen A.D., Weitzman D., Birkenfeld S., Dreiher J. Psoriasis associated with hepatitis C but not with hepatitis B. Dermatology. 2010;220(3):218–222. doi: 10.1159/000286131. [DOI] [PubMed] [Google Scholar]
- 46.Chun K., Afshar M., Audish D. Hepatitis C may enhance key amplifiers of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(4):672–678. doi: 10.1111/jdv.13578. [DOI] [PubMed] [Google Scholar]
- 47.Kimball A.B., Leonardi C., Stahle M., PSOLAR Steering Committee Demography, baseline disease characteristics and treatment history of patients with psoriasis enrolled in a multicentre, prospective, disease-based registry (PSOLAR) Br J Dermatol. 2014;171(1):137–147. doi: 10.1111/bjd.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]