Abstract
Background
There is a need for improvement in informed medical consent to address the lack of standardization and to increase patient engagement.
Objective
To investigate the use of a video to aid informed consent for Mohs micrographic surgery and evaluate patient understanding, satisfaction, anxiety, and time savings relative to verbal consent.
Methods
A 2-armed randomized controlled trial involving 102 patients compared video-assisted consent with a control group who underwent consent in the standard verbal manner. All participants underwent questionnaire-based testing of knowledge, satisfaction, and anxiety, and the time of each consultation was measured.
Results
Patients who watched the video performed significantly better in the knowledge questionnaire compared with the control group (P = .02), were more satisfied with their understanding of the risks of Mohs micrographic surgery (P = .013), and spent less time with their physician (P = .008). Additionally, 78.4% of video group patients reported that they preferred seeing the video before speaking with their physician.
Limitations
The study design may not replicate day-to-day clinical practice.
Conclusion
Video-assisted consent for Mohs micrographic surgery improves patient knowledge, leads to a better understanding of the risks, and saves physicians time without compromising patient satisfaction and anxiety levels in this study setting.
Capsule Summary.
-
•
There are many disadvantages to the traditional consent process, including a lack of standardization and poor information retention.
-
•
Videos aid the consent process for Mohs micrographic surgery, saving physician time and improving patient knowledge of the procedure, without compromising the patient experience.
Introduction
Informed consent is an ethical and legal necessity in the practice of medicine and involves an exchange of information between a patient and physician. Typically, this includes, but is not limited to, an explanation of the method of the procedure or treatment, the risks and benefits, and alternative treatment options.1
Traditionally, the consent process involves an oral exchange of information between a medical practitioner and his or her patient, often accompanied by a signature on a paper document to validate the exchange of understanding and formalize the patient's agreement to the proposed treatment. However, consent is often poorly done, with as few as 9% of the processes being valid.2 This may place individuals and institutions at risk in any subsequent litigation.3 Furthermore, the consent process is idiosyncratic and largely unstandardized between different medical practitioners and indeed within and between institutions.4,5
There are many reasons for deficiencies in the consent process, including truncation caused by high patient volumes, excessive and time-consuming explanations of complex multistep procedures, or both. Physicians and patients may be dissatisfied with current consent methods.6 There is a clear requirement for improvement to address the lack of standardization of information delivery and to increase patient engagement, understanding, and satisfaction.
Audiovisual media offer a standardized medium for delivery of information because every patient receives the same information in an unbiased manner. Furthermore, the process of preparing and editing the video provides the opportunity to reflect on the most informative and least biased language. Videos have been successfully used for consent for a range of medical procedures,7, 8, 9, 10, 11, 12, 13, 14, 15, 16 in clinical trials,17,18 and in dermatology for treatment options for basal cell carcinoma19 and skin biopsies,20 and have been used in Mohs micrographic surgery.21,22 In most cases in which videos were used in the consent process, there were improvements in patient satisfaction,9,11,13, 14, 15,20 patient knowledge, or both.7,8,10,13, 14, 15, 16,19, 20, 21,23 Several studies have demonstrated that videos take the same amount of time as standard processes19 or even save time,9,12,21 and reduce patient anxiety.11
Mohs micrographic surgery is a complex multistage procedure, with waiting times in between stages, depending on the extent of the cancer mass before surgical repair of the defect. The procedure is often unfamiliar to patients and may be difficult to explain adequately during a routine consultation.
We therefore conducted a randomized controlled trial comparing standard consent procedures to evaluate whether video-assisted consent improved the consent process. The primary outcome measure was patient comprehension, assessed with a postconsent knowledge questionnaire. Secondary outcomes included patient satisfaction, anxiety before and after the consent process, patient preference, and the time per physician consultation.
We hypothesized that video-assisted consent compared with verbal consent would lead to improvements in patient knowledge of Mohs micrographic surgery while enhancing patient satisfaction, decreasing anxiety levels, and saving physician time.
Materials and methods
Trial design
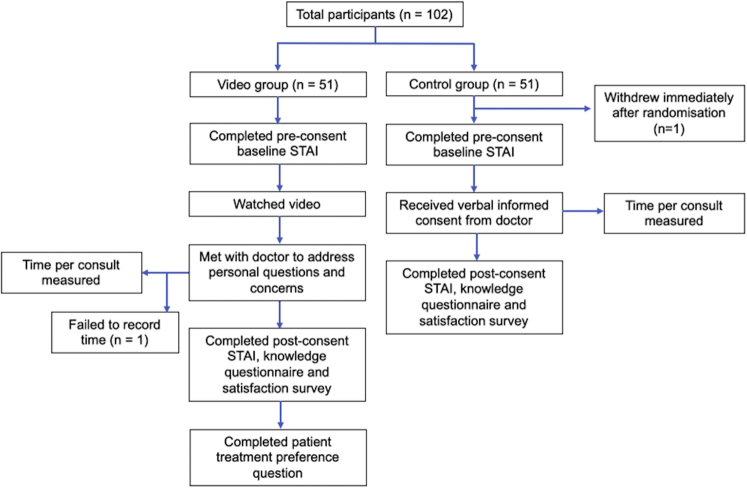
This study was a 2-armed randomized controlled trial with a 1:1 allocation ratio (Fig 1).
Fig 1.
Participant flow. STAI, Spielberger State-Trait Anxiety Inventory.
Participants and sample size
Patients who were aged 18 years or older, English speaking, and referred for Mohs micrographic surgery were recruited from 2 large outpatient dermatology facilities in Sydney, Australia, from March to November 2019. Patients were excluded if they had a visual or hearing impairment, were from a non-English-speaking background, or did not have the capacity to consent for the procedure. The sample size was precalculated at 102 to achieve a power of greater than 0.8, and participants were randomized in a 1:1 ratio into 2 study arms (n = 51 in each arm).
Randomization
The allocation sequence was generated by a computer using a permuted block randomization scheme and the allocation was stored in sequentially numbered sealed envelopes. Both hospitals involved in the study shared the same sequence and participants were assigned to their respective envelope chronologically according to the time of their arrival at the clinics.
Interventions
Participants were randomized to either the control or the video-assisted group. In the video group, participants first watched the video on an iPad, with adherence ensured through direct observation by the study personnel. The video animation of 5 minutes and 50 seconds' duration outlined the standard consent for Mohs micrographic surgery, including the reasons for the procedure, how the procedure is performed, benefits, risks, and alternative treatment options. The video was designed to supplement rather than replace the patient's consultation with his or her physician.
Video group patients then met with their physicians, who were instructed not to repeat the information already provided in the video, but rather to address the participant's personal questions and concerns about the procedure.
Control group patients received informed consent according to the usual practice of the physicians, and they were provided with a standardized checklist of items to cover in the consent process.
Outcomes
All participants were asked to complete a series of questionnaires. Before the consent, basic demographic information was obtained. Participants then completed the preconsent 6-item short form of the Spielberger State-Trait Anxiety Inventory, in which a greater numeric score equated to greater anxiety. This was administered to gain a baseline measure of each participant's anxiety level before viewing of the video and the consent process.
Participants then carried out their respective consent procedures according to the group they were randomized to. Immediately after completion of the consent process, participants once again completed the 6-item Spielberger State-Trait Anxiety Inventory.
All participants were then instructed to complete a knowledge questionnaire consisting of 10 multiple-choice questions that evaluated their understanding of Mohs micrographic surgery. One point was given for each correct answer, with a total maximum possible score of 10. The knowledge questionnaire was pretested with 2 independent dermatologists before the study for appropriate coverage of knowledge.
Last, participants in both groups completed a satisfaction survey measured on a Likert scale.
Additionally, video group patients were asked whether they preferred to only watch a video, only talk with a physician, or watch a video and then talk with a physician, and to provide any reasons for their preferences.
The time per consultation was measured by study personnel using a stopwatch. For the video group, the recorded time did not include the time taken to watch the video because this did not involve the physician. The start of the consultation was taken as the point when the patient and physician first met, and completion of the consent was taken as the point when the participant signed the physical consent form and had no further questions about the procedure.
Statistical methods
Statistical analysis was performed and checked with IBM SPSS Statistics software (version 26.0, IBM Corp, Armonk, NY). Continuous variables were compared with independent 2-sample t tests and nominal variables were compared with χ2. An analysis of covariance was used to compare between baseline (preconsent) and final (postconsent) Spielberger State-Trait Anxiety Inventory scores, and an analysis of variance was used to compare level of education versus knowledge questionnaire scores.
Results
Baseline characteristics
A total of 102 patients were enrolled in the study, with 51 from each study group (Fig 1). A total of 36 participants were recruited from Royal North Shore Hospital (19 in the video group and 17 in the control group), and 65 participants were recruited from The Skin Hospital, Darlinghurst (32 in the video group and 33 in the control group). One participant withdrew from the study immediately after randomization because of time constraints and did not complete any surveys, including demographic information. Because of such early withdrawal, no responses were given and therefore none were included in the analysis of results. Overall, 91% of participants completed the survey in its entirety, and the numbers of participants who answered each question are shown in the relevant tables (Table I, Table II to Table III, Table IV). There were no statistically significant differences in baseline characteristics between the 2 study groups (Table I).
Table I.
Patient characteristics by study group
| Characteristic | Control group (n = 50) | Video group (n = 51) |
|---|---|---|
| Mean age ± SD, y∗ | 65.1 ± 13.0 | 63.1 ± 10.9 |
| Sex, No. (%)† | ||
| Men | 32 (64) | 24 (47.1) |
| Level of schooling completed, No. (%)† | ||
| Year 10 or below | 8 (16) | 13 (25.5) |
| Diploma or year 12 | 20 (40) | 18 (35.3) |
| Bachelor's or master's | 17 (34) | 17 (33.3) |
| Professional or doctorate/PhD | 5 (10) | 3 (5.9) |
| Skin cancer type, No. (%)† | ||
| BCC | 37 (74) | 44 (86.3) |
| SCC | 3 (6) | 4 (7.8) |
| Other or unsure | 10 (20) | 3 (5.9) |
| Previous skin cancers, No. (%)† | ||
| 0 | 13 (26) | 15 (29.4) |
| 1–5 | 20 (40) | 22 (43.1) |
| 5–10 | 9 (18) | 7 (13.7) |
| ≥10 | 8 (16) | 7 (13.7) |
| Site recruited, No. (%)† | ||
| Royal North Shore Hospital | 17 (34) | 19 (37.3) |
| The Skin Hospital, Darlinghurst | 33 (66) | 32 (62.7) |
BCC, Basal cell carcinoma; PhD, doctor of philosophy; SCC, squamous cell carcinoma; SD, standard deviation.
No comparisons had a P < .05.
Independent-samples t test.
χ2 Test.
Table II.
The knowledge questionnaire with percentages answered correctly for each study group
| Question | Possible answers | Control group, % correct (n) | Video group, % correct (n) | P value |
|---|---|---|---|---|
| 1. The anesthetic for my Mohs micrographic surgery will be: |
|
82 (50) | 90.2 (51) | .23† |
| 2. After Mohs micrographic surgery, most patients go home: |
|
84 (50) | 96.1 (51) | .04† |
| 3. Most patients require this much time off work: |
|
10 (50) | 76.5 (51) | <.001† |
| 4. If I have stitches, they will: |
|
60 (50) | 90.2 (51) | <.001† |
| 5. The Mohs micrographic surgery can last for: |
|
62 (50) | 82.4 (51) | .02† |
| 6. Most patients receive their results: |
|
69.4 (49) | 88.2 (51) | .02† |
| 7. After the procedure, most patients will have: |
|
63.3 (49) | 88.2 (51) | .003† |
| 8. The benefit of Mohs micrographic surgery is that: |
|
69.4 (49) | 86.3 (51) | .04† |
| 9. After the procedure, most patients will: |
|
79.6 (49) | 80.4 (51) | .92† |
| 10. Patients at increased risk of complications from Mohs micrographic surgery are: |
|
53.1 (49) | 78.4 (51) | .007† |
| Total score out of 10, mean ± SD | 6.33 ± 2.61 | 8.57 ± 1.85 | .02‡ | |
SD, Standard deviation.
Correct answer.
χ2 Test.
Independent-samples t test.
Table III.
Six-item STAI scores by study group
| Control group (n = 50) | Video group (n = 50) | P value | |
|---|---|---|---|
| Baseline, mean ± SD | 34.20 ± 13.09 | 34.40 ± 11.12 | .22 |
| Final, mean ± SD | 30.73 ± 10.57 | 33.07 ± 13.43 | |
| Baseline, final, mean ± SD | 3.47 ± 8.52 | 1.34 ± 10.60 |
SD, Standard deviation; STAI, Spielberger State-Trait Anxiety Inventory.
Table IV.
The satisfaction survey with scores by study group
| Statement | Mean Likert scale score (1–5) |
P value | |
|---|---|---|---|
| Control group, mean ± SD (n) | Video group, mean ± SD (n) | ||
| 1. The information presented to me about my procedure was easy to understand. | 4.43 ± 0.71 (49) | 4.59 ± 0.61 (51) | .23 |
| 2. I will go into my procedure with confidence. | 4.18 ± 0.95 (49) | 4.35 ± 0.627 (51) | .29 |
| 3. Other treatment options available for my condition were explained to me adequately. | 4.04 ± 0.94 (49) | 4.24 ± 0.68 (51) | .24 |
| 4. I understand the risks of having this procedure. | 4.12 ± 0.97 (49) | 4.51 ± 0.51 (51) | .013 |
| 5. I understand the benefits of having this procedure. | 4.41 ± 0.89 (49) | 4.63 ± 0.49 (49) | .13 |
| 6. My questions about the procedure have been addressed adequately. | 4.53 ± 0.54 (49) | 4.58 ± 0.54 (50) | .65 |
| 7. I was satisfied with the way I provided consent for Mohs micrographic surgery. | 4.43 ± 0.79 (49) | 4.62 ± 0.53 (50) | .16 |
| Total out of 35 | 30.14 ± 4.43 (49) | 31.52 ± 3.15 (50) | .08 |
Knowledge outcomes
The video group performed significantly better (P = .02) in the knowledge questionnaire than the control group, with a mean improvement in total score of 2.24 points (95% confidence interval 1.35-3.14) (Table II). Furthermore, a greater percentage of video group patients than control patients answered each question correctly. This was statistically significant (P < .05) for 8 of the 10 questions. There were no statistically significant correlations between educational level of patients and their knowledge scores (P = .14).
Anxiety levels
Overall, both groups experienced a decrease in anxiety levels after the consent process, and the difference between the groups was not statistically significant (Table III).
Participant satisfaction
Overall satisfaction was improved by the video, although not to a statistically significant extent (P = .08) (Table IV). In particular, video group patients rated higher satisfaction with their understanding of the risks of Mohs micrographic surgery (P = .013).
Participant preference
The majority of patients (78.4%) preferred to see a video before speaking with their physician, whereas 5.9% preferred to watch the video only and 9.8% preferred to talk with their physician only. The most commonly cited reason for preferring both video and physician was that the video gave an easy-to-understand overview of the procedure, which then allowed patients to discuss their specific questions and concerns with their physician.
Time spent with physician
On average, video group patients spent a mean of 14.34 minutes (standard deviation 3.62 minutes) with their physician, whereas control group patients spent a mean of 16.41 minutes (standard deviation 4.05 minutes). This difference was statistically significant (P = .02), with a mean saving of 2.07 minutes (95% confidence interval 0.55-3.59 minutes) by the video group.
Discussion
Our results show that the addition of our standardized video to aid informed consent for Mohs micrographic surgery increased patient knowledge of the procedure, as demonstrated by higher scores on the knowledge questionnaire. This result is consistent with that of other studies from areas of medicine outside of dermatology.7,8,10,13, 14, 15 In contrast, the study in Mohs micrographic surgery by Delcambre et al22 showed no significant difference in patient comprehension between patients who watched a short video before informed consent (intervention group) and those who underwent only informed consent (control group). However, the video used in their experiment was only 1 minute and 40 seconds long, as opposed to our video, which was 5 minutes and 50 seconds. Furthermore, a longer and more comprehensive video (6 minutes and 38 seconds' duration) was freely available for all participants to view online before randomization, with approximately 10% of both the control and intervention groups accessing it before the experiment.22
There was no correlation between educational level of the patients and their performance in the knowledge questionnaire, suggesting that video-assisted consent has the potential to improve comprehension in patients from all educational backgrounds.
For 3 of the 10 questions in the knowledge questionnaire, 60% or less of control group patients answered correctly. Because the questionnaire was administered immediately after the conclusion of the consent process, it is reasonable to infer that the information required to correctly answer these questions may not have been discussed with patients adequately, or perhaps that audio-only consent by the physician resulted in less information retention. Knowledge scores were significantly improved with the video because more than 75% of video patients answered each question correctly.
Both groups displayed diminished anxiety after the consent process compared with the baseline obtained before consent, with levels lower in the physician-only group, which was not significantly different.
The majority of video group patients (78.4%) said they would prefer to watch a video explaining the procedure to them, followed by talking with their physician. In a similar study, 100% of patients who watched a video detailing wound care instructions for Mohs micrographic surgery said they would recommend the video to a friend having Mohs micrographic surgery.21 Sonne et al17 also revealed that patients preferred video format over an equivalent paper format for the delivery of consent information, with 96.7% reporting that the videos improved their understanding of the procedures.
Although the video group was more satisfied with the consent process than the control group, this did not reach a level of statistical significance. Other studies have also shown that the addition of a video to the consent process improves patient satisfaction.9,11,13, 14, 15,20 However, video group patients were significantly more satisfied with their understanding of the risks of Mohs micrographic surgery, which is a crucial element of informed consent.24 This finding is supported by another study in which subjects who watched an informative video before undergoing traditional consent for Mohs micrographic surgery believed themselves to be better informed of the risks and benefits of the surgery.22
Our results confirm those of other studies that demonstrated the time-saving benefit of video-assisted consent.9,12,21
Despite promising results, our study had some limitations. The study design may not replicate day-to-day practice, in which a video may be shown after a physician consultation rather than before.
It might be argued that the video-assisted group simply received more information than the standard group, and further studies might need to compare the video-assisted group with a group that has received other forms of information before meeting with the physician, such as written information. Last, a larger sample size in future studies may produce results of a greater power, which may allow for detecting statistically significant differences in patient satisfaction and anxiety.
Conclusions
Our study demonstrates the positive and promising effects of video-assisted consent in Mohs micrographic surgery; most notably, improvements in patient knowledge, understanding of procedural risks, and time savings. Video-assisted consent offers standardized information delivery without compromising the patient experience, and may have the potential to reduce medicolegal risk by providing a more robust consent process. Further exploration of this modality should be considered for physicians and institutions undertaking Mohs micrographic surgery.
Acknowledgments
We would like to acknowledge all patients who agreed to participate in this research, as well as Jessica Bale, MD, Philippa Dickison, MD, Lisa Abbott, MD, Geoffrey Lee, MD, and Erin Mewton for their assistance in patient management.
Footnotes
Funding sources: Supported by an Avant Doctor in Training scholarship awarded to Dr Saunderson.
Conflicts of interest: Drs Rhodes and Saunderson are cofounders of Pracway Pty Ltd, an online consent management platform. Since study completion, Ms Miao has been employed by Pracway Pty Ltd. Drs Venning, Mallitt, Isserman, Moreno, Lee, Ryman, and Fischer have no conflicts of interest to declare.
Drs Saunderson and Rhodes were not involved in the execution of the study.
This clinical trial was approved by Nepean Blue Mountains LHD HREC and Northern Sydney LHD.
References
- 1.O'Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4–7. doi: 10.1136/jme.29.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braddock C.H., III, Edwards K.A., Hasenberg N.M., Laidley T.L., Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA. 1999;282(24):2313–2320. doi: 10.1001/jama.282.24.2313. [DOI] [PubMed] [Google Scholar]
- 3.Gogos A.J., Clark R.B., Bismark M.M., Gruen R.L., Studdert D.M. When informed consent goes poorly: a descriptive study of medical negligence claims and patient complaints. Med J Aust. 2011;195(6):340–344. doi: 10.5694/mja11.10379. [DOI] [PubMed] [Google Scholar]
- 4.Fortun P., West J., Chalkley L., Shonde A., Hawkey C. Recall of informed consent information by healthy volunteers in clinical trials. QJM. 2008;101(8):625–629. doi: 10.1093/qjmed/hcn067. [DOI] [PubMed] [Google Scholar]
- 5.Brehaut J.C., Carroll K., Elwyn G. Informed consent documents do not encourage good-quality decision making. J Clin Epidemiol. 2012;65(7):708–724. doi: 10.1016/j.jclinepi.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 6.McGaughey I. Informed consent and knee arthroscopies: an evaluation of patient understanding and satisfaction. Knee. 2004;11(3):237–242. doi: 10.1016/S0968-0160(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 7.Rossi M.J., Guttmann D., MacLennan M.J., Lubowitz J.H. Video informed consent improves knee arthroscopy patient comprehension. Arthroscopy. 2005;21(6):739–743. doi: 10.1016/j.arthro.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Agre P., Kurtz R.C., Krauss B.J. A randomized trial using videotape to present consent information for colonoscopy. Gastrointest Endosc. 1994;40(3):271–276. doi: 10.1016/s0016-5107(94)70054-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Ruan X., Tang H., Yang W., Xian Z., Lu M. Video-assisted informed consent for cataract surgery: a randomized controlled trial. J Ophthalmol. 2017;2017:1–6. doi: 10.1155/2017/9593631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tipotsch-Maca S.M., Varsits R.M., Ginzel C., Vecsei-Marlovits P.V. Effect of a multimedia-assisted informed consent procedure on the information gain, satisfaction, and anxiety of cataract surgery patients. J Cataract Refract Surg. 2016;42(1):110–116. doi: 10.1016/j.jcrs.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Jamshidi N., Abbaszadeh A., Kalyani M.N., Sharif F. Effectiveness of video information on coronary angiography patients' outcomes. Collegian. 2013;20(3):153–159. doi: 10.1016/j.colegn.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Goldberger J.J., Kruse J., Kadish A.H., Passman R., Bergner D.W. Effect of informed consent format on patient anxiety, knowledge, and satisfaction. Am Heart J. 2011;162(4):780–785.e781. doi: 10.1016/j.ahj.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Mednick Z., Irrcher I., Hopman W.M., Sharma S. Assessing a narrated white board animation as part of the consent process for intravenous fluorescein angiography: a randomized educational study. Can J Ophthalmol. 2016;51(6):471–475. doi: 10.1016/j.jcjo.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Bowers N., Eisenberg E., Montbriand J., Jaskolka J., Roche-Nagle G. Using a multimedia presentation to improve patient understanding and satisfaction with informed consent for minimally invasive vascular procedures. Surgeon. 2017;15(1):7–11. doi: 10.1016/j.surge.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Sahai A., Kucheria R., Challacombe B., Dasgupta P. Video consent: a pilot study of informed consent in laparoscopic urology and its impact on patient satisfaction. JSLS. 2006;10(1):21–25. [PMC free article] [PubMed] [Google Scholar]
- 16.Tompsett E., Afifi R., Tawfeek S. Can video aids increase the validity of patient consent? J Obstet Gynaecol. 2012;32(7):680–682. doi: 10.3109/01443615.2012.698329. [DOI] [PubMed] [Google Scholar]
- 17.Sonne S.C., Andrews J.O., Gentilin S.M. Development and pilot testing of a video-assisted informed consent process. Contemp Clin Trials. 2013;36(1):25–31. doi: 10.1016/j.cct.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraft S.A., Constantine M., Magnus D. A randomized study of multimedia informational aids for research on medical practices: implications for informed consent. Clin Trials. 2017;14(1):94–102. doi: 10.1177/1740774516669352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love E.M., Manalo I.F., Chen S.C., Chen K.-H., Stoff B.K. A video-based educational pilot for basal cell carcinoma (BCC) treatment: a randomized controlled trial. J Am Acad Dermatol. 2016;74(3):477–483.e477. doi: 10.1016/j.jaad.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong A.W., Alikhan A., Cheng L.S., Schupp C., Kurlinkus C., Eisen D.B. Portable video media for presenting informed consent and wound care instructions for skin biopsies: a randomized controlled trial. Br J Dermatol. 2010;163(5):1014–1019. doi: 10.1111/j.1365-2133.2010.10067.x. [DOI] [PubMed] [Google Scholar]
- 21.Migden M., Chavez-Frazier A., Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27(1):89–93. doi: 10.1016/j.sder.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Delcambre M., Haynes D., Hajar T. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2019;46(5):591–598. doi: 10.1097/DSS.0000000000002213. [DOI] [PubMed] [Google Scholar]
- 23.Orringer J.S., Fendrick A.M., Trask P.C. The effects of a professionally produced videotape on education and anxiety/distress levels for patients with newly diagnosed melanoma: a randomized, prospective clinical trial. J Am Acad Dermatol. 2005;53(2):224–229. doi: 10.1016/j.jaad.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 24.Nijhawan L.P., Janodia M.D., Muddukrishna B. Informed consent: issues and challenges. J Adv Pharm Technol Res. 2013;4(3):134–140. doi: 10.4103/2231-4040.116779. [DOI] [PMC free article] [PubMed] [Google Scholar]