Abstract
Background
Little is known about the targets in the CNS that mediate ethanol analgesia. This study explores the role of spinal astrocyte aldehyde dehydrogenase-2 (ALDH2), a key ethanol-metabolising enzyme, in the analgesic effects of ethanol in mice.
Methods
Astrocyte and hepatocyte ALHD2-deficient mice were generated and tested in acute and chronic pain models. Cell-type-specific distribution of ALDH2 was analysed by RNA in situ hybridisation in spinal slices from astrocytic ALDH2-deficient mice and their wild-type littermates. Spinal ethanol metabolites and γ-aminobutyric acid (GABA) content were measured using gas chromatography/mass spectrometry and liquid chromatography/mass spectrometry.
Results
ALDH2 mRNA was expressed in both astrocytes and neurones in spinal cord slices. Astrocyte ALDH2-deficient mice had decreased expression of ALDH2 mRNA in astrocytes, but not in neurones. Astrocyte ALDH2 deficiency inhibited ethanol-derived acetate, but not acetaldehyde content in spinal cord tissues. Depletion of spinal astrocyte ALDH2 selectively inhibited ethanol-induced anti-nociceptive effect, but not the effect of ethanol, on motor function. Astrocyte ALDH2 deficiency abolished ethanol-induced GABA elevation. The ethanol metabolite acetate produced anti-nociception and increased GABA synthesis in a manner similar to ethanol. I.T. delivery of either GABAA or GABAB receptor antagonists prevented ethanol and acetate-induced analgesia.
Conclusions
These findings provide evidence that ALDH2 in spinal astrocytes mediates spinal ethanol metabolism and ethanol-induced analgesic effects by promoting GABA synthesis and GABAergic transmission in spinal cord.
Keywords: acetate, ALDH2, analgesia, astrocyte, ethanol, GABA, pain, spinal cord
Editor's key points.
-
•
The targets that mediate ethanol analgesia were explored by examining the role of spinal astrocyte aldehyde dehydrogenase-2 (ALDH2), a key ethanol-metabolising enzyme, in the analgesic effects of ethanol in mice.
-
•
ALDH2 was expressed in mouse astrocytes and neurones of wild-type mice, but was selectively absent from astrocyte-specific ALDH2 knockout mice.
-
•
Depletion of spinal astrocyte ALDH2 inhibited the anti-nociceptive effect of ethanol, but not its locomotor effects, and reduced spinal γ-aminobutyric acid (GABA) synthesis.
-
•
Thus, the analgesic mechanism of ethanol requires ALDH2-mediated metabolism in spinal astrocytes, which promotes GABA synthesis and GABA-mediated analgesia.
Ethanol is one of the oldest and most widely used drugs in the world. Ethanol produces a variety of behavioural changes because of its ability to suppress the CNS. Like opioids and cannabinoids, ethanol has been used for pain relief since ancient times. One quarter of people experiencing chronic pain turn to ethanol to relieve their suffering.1,2 Unfortunately, reaching maximum pain relief always requires binge drinking.3 Prolonged and excessive use of ethanol to stop pain leads to a number of harmful health consequences in humans.4 The most notable effect is increased risk for ethanol dependence.2 Besides, chronic ethanol drinking causes peripheral neuropathy, and ethanol withdrawal often results in hyperalgesia in rodents and in humans.5,6 In addition, chronic ethanol use increases dose requirements for general anaesthetic agents and risk of postoperative complications.7
Unlike opioids and cannabinoids, ethanol does not bind to a single specific protein target in the CNS. The metabolites of ethanol can be more active than ethanol alone. Ethanol metabolism is controlled through various genetic factors in humans.3 The key enzyme that converts acetaldehyde to acetate is acetaldehyde dehydrogenase-2 (ALDH2). A major genetic deficiency in ALDH2 is found in nearly one-third of the East Asian population.8 This naturally occurring point mutation disrupts ALDH2 activity, increases blood acetaldehyde content, and produces various adverse reactions to ethanol.
ALDH2 deficiency in humans and rodents is associated with increased pain sensitivity to various noxious stimuli.9,10 Deficiency of ALDH2 results in serum and tissue acetaldehyde elevation and acetate ablation.11,12 Although elevated acetaldehyde is found to produce peripheral neuronal inflammation and pain hypersensitivity,10 little is known about the role of acetate in pain modulation. Brain acetate has been thought to derive from hepatic ethanol metabolism.13 The in vivo consequence of brain ethanol metabolism remains elusive because the levels of the metabolising enzymes, including ALDH2, are very low.8 There is strong evidence suggesting that acetate is utilised and metabolised exclusively by astrocytes in the brain.14 Unfortunately, the cell-type-specific distribution of brain ALDH2 is unknown because of a lack of specific approaches in vivo.
Ethanol produces motor impairment, sedative, and anxiolytic effects through enhancement of γ-aminobutyric acid (GABA)A receptor function.15 Both GABAA and GABAB receptors play a major role in pain control in rodents and humans.16, 17, 18 Several positive modulators of GABAB receptors are used to treat ethanol preference and dependence in animals and humans.19 The relationship between ethanol and GABA synthesis remains controversial. In vivo nuclear magnetic resonance (NMR) studies have demonstrated conversion of systemic [13C]acetate to [13C]GABA in the brain.14 The metabolic pathways from ethanol to GABA are less clear. Emerging evidence suggests that both ALDH1a1 and ALDH2 can promote GABA synthesis through putrescine degradation and utilisation pathways in neurones and astrocytes.20,21
The precise mechanisms underlying ethanol-induced analgesic effects remain elusive. Although ethanol can produce both analgesia and anaesthesia, there is strong evidence suggesting that ethanol produces anti-nociceptive effects in a mechanism independent of ethanol-induced anaesthetic action and motor impairment in human and animal studies.22,23 A supraspinal mechanism of action likely contributes to the relief of stress and emotional pain by ethanol because of its anxiolytic action.2 Although the spinal cord is an important gateway for transmitting nociceptive signals to the brain, the role of the spinal cord in ethanol-induced behavioural effects has not been established. A recent study has suggested that spinal ALDH2 contributes to neuroprotection and recovery after spinal cord injury.24 Nevertheless, little is known about the roles of spinal ALDH2 in ethanol metabolism and ethanol-induced behavioural changes because of a lack of in vivo approaches that can separate peripheral and central ALDH2-mediated effects.
In this study, we asked if spinal ALDH2 regulates ethanol metabolites and the ethanol-induced analgesic effect in mice. We tested this hypothesis by performing various in vitro and in vivo experiments in cell-type-specific ALDH2-deficient mice. We also explored the possible mechanism underlying astrocytic ALDH2 control of ethanol analgesia.
Methods
This study was performed in accordance with National Institutes of Health guidelines for care and use of laboratory animals. All procedures were approved by both National Institute on Alcohol Abuse and Alcoholism Animal Use and Animal Care Committee and the Institutional Animal Care and Use Committee of Anhui Medical University. Expanded methods can be found in the Supplementary material.
Animals
C57BL/6J wild-type and glial cell (Aldh2Gfap–/–) and hepatocellular-specific (Aldh2Hep–/–) ALDH2-deficient male mice were used in this study. The ALDH2 floxed mice and tissue-specific ALDH2 knockout mice were generated as described12 (Supplementary material).
Spinal cord virus injection
For spinal cord astrocyte-specific Aldh2 gene deficiency, AVV5-GFAPCre (105550-AAV5; Addgene, MA, USA) and AVV5-GFP (37825-AAV5; Addgene, MA, USA) lentivirus were injected bilaterally into the dorsal horn of the spinal cord (L3–4) at a depth of 300 μm in ALDH2 floxed male mice. Four weeks after the virus injection, the mice were tested for tail flick reflex (TFR), complete Freund's adjuvant (CFA)-induced inflammatory pain with electronic von Frey, paw thermal stimulating, locomotion activity, and rotarod performance after i.p. administration with ethanol 1.2 or 2 g kg−1 (Supplementary material).
Detection of ALDH2 protein, mRNA, and enzyme activity
ALDH2 protein, mRNA, and enzyme activity were detected by immunoblot, immunofluorescence, RNA in situ hybridisation (ISH), qRT–PCR, and ALDH2 enzyme activity (detailed information in Supplementary materials). The probes used for ISH were designed and generated by Advanced Cell Diagnostics, Inc. (Hayward, CA, USA).
Behavioural tests
C57BL/6J and spinal cord virus-injected male mice and astrocytic-specific ALDH2-deficient hepatocytic-specific ALDH2-deficient male mice and their wild-type littermates were used in behaviour tests. TFR, electronic von Frey, paw thermal stimulating, hot plate, locomotion activity, and rotarod tests were performed to evaluate ethanol- or acetate-induced anti-nociception. All animals were habituated to the procedure room for at least 1 h before testing (see detailed information in the Supplementary material).
Measurement of ethanol and acetaldehyde
Aldh2Gfap–/– and their wild-type littermate mice (Aldh2Gfap+/+) were randomly assigned different groups, weighed, individually housed, and acclimated for at least 1 h before testing. All mice were anaesthetised with isoflurane and decapitated to harvest whole blood and spinal cord tissue after ethanol 2 g kg−1 or an equal volume of saline via i.p. injection. Procedures for determination of serum and spinal cord ethanol and acetaldehyde by gas chromatography/mass spectrometry (GC/MS) are described in the Supplementary material.
Measurement of spinal cord GABA content
Aldh2Gfap+/+ and Aldh2Gfap–/– littermate mice were randomly assigned different groups and acclimated for at least 1 h before testing. At 30 and 60 min after injection with ethanol 2 g kg−1, acetate 1.5 g kg−1, or an equal volume of saline (i.p.), mice were anaesthetised with isoflurane and decapitated to harvest spinal cord tissue. GABA was measured by liquid chromatography/mass spectrometry/mass spectrometry (detailed information in Supplementary material).
Measurement of serum and spinal cord acetate
Spinal cord and serum were used to measure acetate using a spectrophotometric assay kit according to the manufacturer's instructions (ab204719; Abcam, Inc., Cambridge, MA, USA; details in Supplementary digital content).
Statistical analysis
Data are expressed as mean (standard error of the mean) and analysed with GraphPad Prism version 7.0 for Windows (San Diego, CA, USA). The unpaired two-tailed Student's t-test was used for comparison between two groups, and one-way analysis of variance (anova) or two-way anova followed by Tukey's test was used for multiple comparisons of more than two groups. Repeated-measures anova followed by Tukey's test was performed to analyse values for pain latency and threshold at different time points. The sample size in each group was calculated using G∗Power, version 3.1.7 (Franz Faul, University of Kiel, Kiel, Germany). We set significance at 5% and power at 80%, with P<0.05 considered to be significant. Effect sizes between groups are based on our previous work.12,25
Results
We used 98 C57BL/6J mice for the experiments on i.t. drug delivery; two of 51 ALDH2 floxed mice were removed after intra-spinal injection with adeno-associated virus because of motor impairment. From a total of 65 mice, three mice were disqualified based on a criterion test of rotarod performance. A total of 257 ALDH2-deficient mice and their wild-type littermates were used in the studies of ethanol pharmacokinetics and biochemical and behavioural studies. There was no data exclusion from analysis for in vitro experiments.
Astrocytic expression of ALDH2 in spinal cord
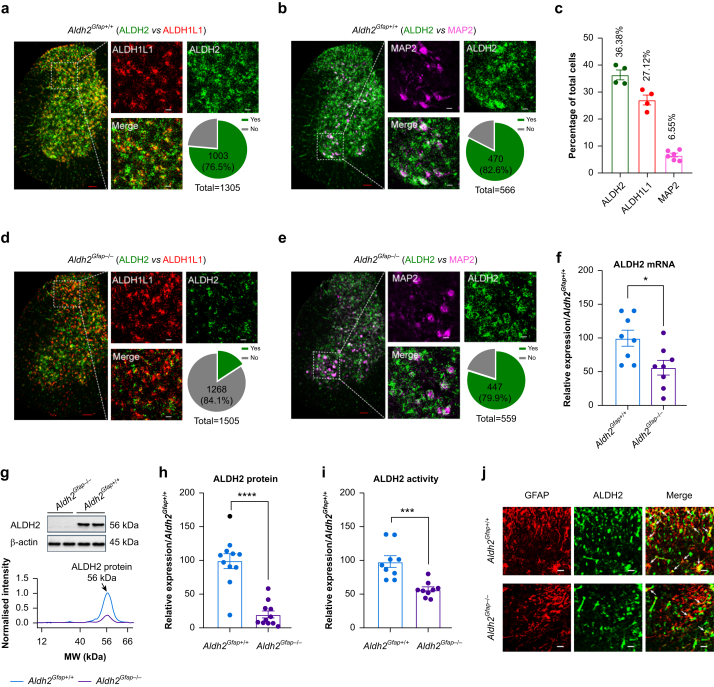
The cell-type-specific distribution of ALDH2 has not been described. We therefore conducted ISH using RNAscope (Advanced Cell Diagnostics, Inc., Hayward, CA, USA) analysis to examine ALDH2 mRNA distribution in mouse spinal cord slices. ALDH1L1 was used to detect spinal astrocyte-selective gene expression,26 and MAP2 was used to detect the signal of a subpopulation of neurones.27 A strong signal of ALHD2 was detected in spinal cells evenly distributed in both white and grey matter (Fig. 1a). ALDH2 mRNA was expressed in 36.4% of total grey matter cells (Fig. 1c). Although ALDH1L1 mRNA was mainly expressed in dorsal horn cells (27.1% of total cells), MAP2 mRNA was primarily detected in the large cells of the anterior grey horn (6.6% of total cells) (Fig. 1b). ALDH2 mRNA was detected in 76.5% of ALDH1L1-positive cells (Figs 1a) and 82.6% of MAP2-positive cells (Fig. 1b). To validate astrocyte ALDH2 expression in vivo, we generated astrocyte ALDH2 conditional knockout mice, Aldh2Gfap–/– mice (target for astrocytes) using the Cre–loxP recombination system. In spinal slices from these transgenic mice, the ALDH2 mRNA signal seemed weak, and there was a substantial reduction in ALDH2 co-localisation with ALDH1L1 compared with slices shown in Fig. 1a (from 76.5% to 15.9%) (Fig. 1d). In contrast, only a slight decrease was observed in the co-localisation of ALDH2- and MAP2-positive cells in the anterior horn of astrocytic ALDH2 knockout mice (Fig. 1e; 82.6% vs 79.9%). This suggests an astrocytic-specific depletion of ALDH2 mRNA in Aldh2Gfap–/– mice. Consistent with this, expression of ALDH2 mRNA, protein, and enzyme activity was significantly reduced in Aldh2Gfap–/– mice (Fig. 1f–i). Similarly, astrocytic ALDH2 deficiency disrupted the co-localisation of ALDH2 with GFAP immunoactivity in spinal slices (Fig. 1j).
Fig 1.
Spinal aldehyde dehydrogenase-2 (ALDH2) expression is reduced in astrocyte ALDH2-deficient mice. (a–b) Microscopy of ALDH2 mRNA (green), ALDH1L1 mRNA (red), and MAP2 mRNA (violet) in spinal cord slices from Aldh2Gfap+/+ mice. The grey area represents the co-localisation of ALDH2 with ALDH1L1 or MAP2 (n=4–6 mice for each group). Red scale bar: 100 μm; white scale bar: 20 μm; Yes: co-localisation; No: no co-localisation. (c) Bars represent the percentage of ALDH2-, ALDH1L1-, and MAP2-positive cells to total cells in grey matter of the spinal cord. (d–e) Microscopy of ALDH2 mRNA (green), ALDH1L1 mRNA (red), and MAP2 mRNA (violet) in spinal cord slices from Aldh2Gfap–/– mice (n=4–6 mice per group). Red scale bar: 100 μm; white scale bar: 20 μm; Yes: co-localisation; No: no co-localisation. (f) Bars represent relative expression of spinal ALDH2 mRNA detected by qRT–PCR, data normalised to the value of Aldh2Gfap+/+ (n=8 mice for each group). (g) Representative bands and peaks of quantitative densitometry of ALDH2 protein. (h) Summary of ALDH2 protein levels in lumbar spinal cord from Aldh2Gfap–/– and Aldh2Gfap+/+ mice (n=11 mice per group). (i) ALDH2 enzymatic activity in lumbar spinal cord from Aldh2Gfap–/– and Aldh2Gfap+/+ mice, normalised to the value of Aldh2Gfap+/+ (n=9 mice per group). (j) Confocal immunofluorescence microscopy of ALDH2 (green) and GFAP (red) in spinal cord slices from Aldh2Gfap–/– and Aldh2Gfap+/+ mice. White scale bar: 20 μm. All data are expressed as mean (standard error of the mean). Analysis was performed using unpaired two-tailed Student's t-test, ∗P<0.05, ∗∗∗P<0.001, and ∗∗∗∗P<0.0001 compared with Aldh2Gfap+/+ mice.
Astrocytic ALDH2 mediates ethanol analgesia
The preceding observations suggest that Aldh2Gfap–/– mice are a valuable approach to study the in vivo consequences of spinal astrocyte ALDH2. We tested if astrocyte ALDH2 deficiency alters ethanol-induced analgesic effects. We tested the effect of systemic ethanol in doses from 1.2 to 2 g kg−1 because such doses produce blood ethanol concentrations (0.07–0.2 g dl−1), as observed in human binge drinking.28 We first measured latency in the TFR test after ethanol administration (Fig. 2a). Systemic administration of ethanol, in a dose-dependent manner (0.5–3 g kg−1, i.p.), produced an analgesic effect in the TFR test in wild-type (Aldh2Gfap+/+) mice (Fig. 2b). The magnitude of ethanol analgesia reached a maximum 15–30 min after systemic ethanol (Fig. 2c). Ethanol-induced analgesia was significantly reduced in astrocyte ALDH2-deficient (Aldh2Gfap–/–) mice (Fig. 2b and c). For instance, astrocyte ALDH2 deficiency decreased percentage of maximum possible effect (MPE) induced by ethanol from 23.9% to 8.2% (P=0.1142; ethanol at 1 g kg−1) and from 47.8% to 27.6% (P=0.0156; ethanol at 2 g kg−1). Although the TFR test represents a spinal reflex, the hot-plate hyperalgesia assay is used generally for supraspinal actions of analgesics.29 In line with a previous study,30 ethanol increased response latency in the hot-plate test. However, astrocyte ALDH2 deficiency did not significantly alter the analgesic effect of ethanol in the hot-plate test (Fig. 2d).
Fig 2.
Astrocyte ALDH2 regulates ethanol-induced anti-nociception in spinal cord. (a) Schematic diagram of tail flick reflex (TFR), hot-plate test, and experimental procedure. (b) The dose–response curves of ethanol (EtOH) anti-nociception in TFR test in Aldh2Gfap–/– and Aldh2Gfap+/+ mice (0.5, 1, and 3 g kg−1: n=13 mice per group; 2 g kg−1: n=12 mice per group). (c) The time courses of ethanol (2 g kg−1, i.p.) anti-nociception in TFR test in Aldh2Gfap–/– and Aldh2Gfap+/+ mice (n=12 mice per group). (d) The time courses of ethanol (1.2 g kg−1, i.p.) induced anti-nociception in hot-plate test in Aldh2Gfap–/– and Aldh2Gfap+/+ mice (Aldh2Gfap+/+: n=18 mice per group; Aldh2Gfap–/–: n=16 mice per group). (e) Representative bands and peaks of quantitative densitometry, and statistic summary of ALDH2 protein in lumbar spinal cord from AldhHep+/+ and Aldh2Hep–/– mice (n=8 mice per group). (f) The time courses of ethanol (2 g kg−1, i.p.) anti-nociception in TFR test in AldhHep+/+ and Aldh2Hep–/– mice (n=11 mice per group). (g) Schematic diagram of complete Freund's adjuvant (CFA)-induced inflammatory pain and experimental procedure. (h–i) Mechanical hypersensitivity after ethanol (1.2 g kg−1, i.p.) administration under the CFA-induced inflammatory model via electronic von Frey in Aldh2Gfap–/– (h: n=10 mice per group), Aldh2Hep–/– (i: n=8 mice per group) and their littermates. (j) Bar graphs showing total distance of locomotion from 0 to 30 min after ethanol (1.2 and 2 g kg−1, i.p.) administration in Aldh2Gfap–/– and Aldh2Gfap+/+ mice (n=11 mice per group). All data are expressed as mean (standard error of the mean). Analysis was performed using unpaired two-tailed Student's t-test (e), or two-way analysis of variance (anova) followed by Tukey's test (b, j), or two-way repeated-measures anova followed by Tukey's test (c, d, f, h, i); ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, and ∗∗∗∗P<0.0001. MPE, maximum possible effect; ns, not significant.
Liver is thought to be the primary organ for alcohol metabolism.13 Hepatocyte ALDH2-deficient (Aldh2Hep–/–) mice have significantly elevated serum acetaldehyde after systemic ethanol.12 To determine whether hepatocyte ALDH2 deficiency affects ethanol analgesia, we first examined expression of ALDH2 protein in spinal cord in Aldh2Hep–/– mice. Spinal ALDH2 protein did not significantly differ between Aldh2Hep–/– mice and their wild-type littermates (Aldh2Hep+/+) (Fig. 2e). Unlike astrocyte ALDH2 deficiency, hepatocyte ALDH2 deficiency did not significantly affect the analgesic effect of ethanol in the TFR test (Fig. 2f).
We next tested whether astrocyte ALDH2 deficiency alters ethanol analgesia in chronic inflammatory pain induced by intra-plantar administration of CFA. Mechanical allodynia was evaluated using electronic von Frey filaments before and after injection of CFA (Fig. 2g). In line with our previous study,25 CFA administration reduced the mechanical pain threshold to tense von Frey stimuli (Fig. 2h). Systemic administration of ethanol significantly reduced mechanical allodynia by enhancing the pain threshold from 0.67 (0.05) to 5.08 (0.22) g. Astrocyte ALDH2 deficiency significantly inhibited ethanol enhancement of pain threshold by ∼42% (P<0.0001; Fig. 2h). Hepatocyte ALDH2 deficiency abolished liver ALDH2 enzymatic activity.12 In contrast to astrocyte ALDH2-deficient mice, hepatocyte ALDH2-deficient mice did not significantly alter ethanol-induced analgesic effect on CFA-induced pain hypersensitivity in mice (Fig. 2i).
Ethanol suppresses motor activity in animals and humans. We next tested if spinal astrocyte ALDH2 deficiency could alter ethanol-induced hypolocomotion. Locomotor activity was reduced after systemic ethanol at both 1.2 and 2 g kg−1 in mice (Fig. 2j). There was no significant difference in locomotion after ethanol between astrocyte ALDH2-deficient mice and their wild-type littermates. Together, these findings suggest that astrocyte ALDH2 deficiency selectively mediates ethanol-induced anti-nociceptive action in acute and chronic pain.
Astrocytic ALDH2 mediates ethanol conversion to acetate in the spinal cord
To examine the impact of spinal ALDH2 on alcohol metabolism, we assessed serum and spinal cord ethanol and acetaldehyde in Aldh2Gfap+/+ and Aldh2Gfap–/– mice using GC/MS. Although serum and spinal ethanol and acetaldehyde rapidly rose after systemic administration of ethanol, there was no difference observed in serum and spinal ethanol metabolites between astrocyte ALDH2-deficient mice and their wild-type littermates (Fig. 3a–d). We next examined serum and spinal acetate levels using a colorimetric kit in the wild-type and astrocyte ALDH2-deficient mice. Both serum and spinal acetate levels were elevated after systemic ethanol in mice (Fig. 3e and f). Astrocyte ALDH2 deficiency did not significantly alter serum acetate derived from ethanol; however, the deficiency in astrocytic ALDH2 completely prevented ethanol-induced elevation of acetate in spinal cord (Fig. 3e and f).
Fig 3.
Astrocytic ALDH2 regulates ethanol metabolism in the spinal cord. (a–d) Graphs showing acetaldehyde (AcH) and ethanol (EtOH) concentrations detected by gas chromatography/mass spectrometry in the serum and spinal cord at 10, 30, and 60 min after ethanol (2 g kg−1, i.p.) administration in Aldh2Gfap–/– and Aldh2Gfap+/+ mice (0 min: n=5 per group; 10 min: n=6 mice per group; 30 min: n=5 mice per group; 60 min: n=6 mice per group). (e–f) Bar graphs showing acetate relative concentration in serum (0 min: n=5 [Aldh2Gfap+/+], n=6 [Aldh2Gfap–/–]; 10 min: n=6 [Aldh2Gfap+/+], n=5 [Aldh2Gfap–/–]; 30 min: n=6 mice per group; 60 min: n=6 mice per group) and spinal cord (0 min: n=9 [Aldh2Gfap+/+], n=8 [Aldh2Gfap–/–]; 10 min: n=8 mice per group; 30 min: n=10 [Aldh2Gfap+/+], n=9 [Aldh2Gfap–/–]; 60 min: n=8 mice per group) at 10, 30, and 60 min after ethanol (2 g kg−1, i.p.) in Aldh2Gfap–/– and Aldh2Gfap+/+ mice. (g) Statistical summary of the potentiation of γ-aminobutyric acid (GABA) by ethanol (2 g kg−1, i.p.) at 30 and 60 min in the spinal cord of Aldh2Gfap+/+ mice (n=7 mice per group). (h) Bar graphs showing GABA concentrations in the spinal cord at 30 and 60 min after ethanol (2 g kg−1, i.p.) in Aldh2Gfap–/– and Aldh2Gfap+/+ mice (baseline: n=7 mice per group; 30 min: n=7 [Aldh2Gfap+/+], n=8 [Aldh2Gfap–/–]; 60 min: n=7 mice per group). (i) Microscopic images showing the GABA immunostaining in spinal dorsal horn at baseline and 30 and 60 min after ethanol (2 g kg−1, i.p.) administration in Aldh2Gfap–/– and Aldh2Gfap+/+ mice. White scale bar: 30 μm. All data are expressed as mean (standard error of the mean). Groups were compared by one-way analysis of variance (anova) followed by Tukey's test (g), or two-way anova followed by Tukey's test (a–f, h), ∗P<0.05 and ∗∗P<0.01 compared with Aldh2Gfap+/+ mice, ##P<0.01 compared with baseline (0 min). ns, not significant.
Ethanol can promote GABA-mediated neurotransmission via presynaptic, postsynaptic, and extra-synaptic mechanisms.31, 32, 33 We next asked whether astrocyte ALDH2 deficiency alters spinal cord GABA content in mice. Systemic ethanol increased spinal cord GABA by 21% after 30 min (P=0.007; Fig. 3g). No such spinal GABA increase by ethanol was observed in astrocyte ALDH2-deficient mice (Fig. 3h). The concentrations of spinal GABA were slightly lower than baseline GABA 30 and 60 min after ethanol in astrocyte ALDH2-deficient mice (Fig. 3h). There was a significant difference in spinal GABA after systemic ethanol between Aldh2Gfap+/+ and Aldh2Gfap–/– mice. Consistent with this, GABA immunoactivity was increased in the dorsal horn of spinal cord slices from wild-type mice, but from astrocyte ALDH2-deficient mice after systemic ethanol (Fig. 3i).
Acetate mediates ethanol analgesia and GABA synthesis
The preceding observations suggest that astrocytic ALDH2 mediates ethanol analgesia and ethanol-derived acetate increases in the brain. To test if acetate can mimic ethanol analgesia, we examined the analgesic effect of systemic acetate in acute and chronic pain models (Fig. 4a). Systemic administration of acetate dose dependently increased the latency of the pain threshold in the TFR test (Fig. 4b), peaking at 30 min and lasting for 90 min (Fig. 4c). Acetate also alleviated mechanical allodynia induced by CFA in mice (Fig. 4d). The time courses of acetate-induced analgesia on both TFR- and CFA-induced pain were nearly identical to ethanol analgesia, suggesting that acetate mediates the ethanol-induced analgesic effect. Like ethanol, systemic acetate enhanced GABA concentrations in lumbar spinal cord from 7.2 (0.3) to 8.7 (0.4) nmol mg−1 (P=0.0237) (Fig. 4e). Consistent with this, GABA immunoactivity was enhanced in the dorsal horn area of the spinal slices from mice 60 min after systemic acetate (Fig. 4f).
Fig 4.
Acetate-induced anti-nociception in spinal cord. (a) Hypothetical diagram of acetate (AcT)-induced anti-nociception in spinal cord by increasing γ-aminobutyric acid (GABA) synthesis. (b) Bar graphs showing the dose–response of acetate (0–2 g kg−1, i.p.) anti-nociception in tail flick reflex (TFR) test in wild-type mice (n=13 mice per group). (c) Time course of anti-nociception of 1.5 g kg−1 acetate i.p. in TFR test (n=13 mice per group). (d) Mechanical hypersensitivity with time after acetate administration and its anti-nociceptive effects in wild-type mice via electronic von Frey test (saline: n=6 mice; acetate: n=8 mice). (e) Bar graphs showing gas chromatography/mass spectrometry spectra of GABA concentrations in the spinal cord at 30 and 60 min after acetate (1.5 g kg−1) or saline in wild-type mice (n=5 mice per group). (f) Microscopic images of GABA immunostaining in the spinal cord at baseline or 30 and 60 min after acetate (1.5 g kg−1) administration. White scale bar: 30 μm. All data are expressed as mean (standard error of the mean). Analysis was performed using one-way analysis of variance (anova) followed by Tukey's test (b, e), or two-way repeated-measures anova followed by Tukey's test (c, d), ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, and ∗∗∗∗P<0.0001 compared with saline or baseline. MPE, maximum possible effect; ns, not significant.
Spinal ALDH2 mediates ethanol analgesia
The dorsal horn of the spinal cord is the first centre for receiving and relaying nociceptive and non-nociceptive inputs. To explore whether localised astrocyte ALDH2 in spinal dorsal horn contributes to ethanol-induced anti-nociception, we injected AAV5-GFAPCre and AAV5-GFP vector virus into both dorsal horns of the spinal cord of L3–4 in Aldh2 floxed mice (Fig. 5a). Both Cre and green fluorescent protein (GFP) immunoactivities were detected in the area of spinal dorsal horn 4 weeks after the viral injections (Fig. 5b). The levels of spinal ALDH2 protein were reduced by 61.9% (P=0.0005) in mice previously injected with AAV5-GFAPCre compared with mice injected with AAV5-GFP (Fig. 5c and d). These findings suggest that mice with spinal dorsal horn injection of AAV-GFAPCre are valuable for exploring the role of spinal astrocytic ALDH2 in ethanol analgesic action. Ethanol increased pain threshold in the TFR test from a baseline of 3.5 (0.6) to 5.5 (0.3) s in AAV-GFP mice (Fig. 5e). Spinal astrocyte ALDH2-deficient mice attenuated the peak of ethanol analgesic magnitude by 22% (P=0.0259). Similarly, spinal astrocyte ALDH2 deficiency significantly inhibited peak magnitudes of ethanol analgesia in both thermal-stimulation-induced hyperalgesia and mechanical-stimulation-induced allodynia in CFA-injected mice (Fig. 5f and g). However, spinal astrocyte ALDH2 deficiency did not significantly alter ethanol-induced hypolocomotion, discoordination, and hypothermia (Fig. 5h–j). These findings suggest that spinal astrocyte ALDH2 selectively mediates ethanol-induced analgesia.
Fig 5.
GFAP-Cre virus abolished ethanol-induced analgesia, but not locomotion in spinal cord. (a) Schematic diagram of adeno-associated virus (AAV) microinjection into the spinal cord (L3–4) and experimental procedure. (b) Microscopic images of Cre (red) and green fluorescent protein (GFP) (green) immunostaining in the spinal cord 4 weeks after AAV5-GFAPCre and AAV5-GFP virus injection. White scale bar: 200 μm. (c–d) Representative bands and statistic summary of aldehyde dehydrogenase-2 (ALDH2) protein in lumbar spinal cord from AAV5-GFAPCre and AAV5-GFP virus-injected mice (n=6 mice per group). (e) Time courses of tail flick reflex (TFR) latency showing ethanol (EtOH; 2 g kg−1, i.p.)-induced anti-nociception in AAV5-GFAPCre and AAV5-GFP virus-injected mice (n=10 mice per group). (f–g) Mechanical and thermal hypersensitivities with time courses of ethanol (1.2 g kg−1, i.p.) anti-nociception in the complete Freund's adjuvant (CFA) model in spinal cord virus-injected mice (n=8 mice per group). (h) Total distance of locomotion from 0 to 30 min after ethanol (1.2 and 2 g kg−1, i.p.) administration in spinal cord virus-injected mice (n=10 mice per group). (i) Bar graphs showing rotarod performance after 30 min of ethanol (1.2 and 2 g kg−1, i.p.) administration in spinal cord virus-injected mice (n=7 mice per group). (j) The core body temperature was measured by a thermometer after 30 min of ethanol (1.2 and 2 g kg−1, i.p.) administration in spinal cord virus-injected mice (n=8 mice per group). All data are expressed as mean (standard error of the mean). Analysis was performed using unpaired two-tailed Student's t-test (d), two-way analysis of variance (anova) followed by Tukey's test (h–j), or two-way repeated-measures anova followed by Tukey's test (e–g), ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, and ∗∗∗∗P<0.0001 compared with AAV5-GFAPCre virus-injected mice or saline. ns, not significant.
Spinal GABA receptors mediate ethanol- and acetate-induced analgesic effects
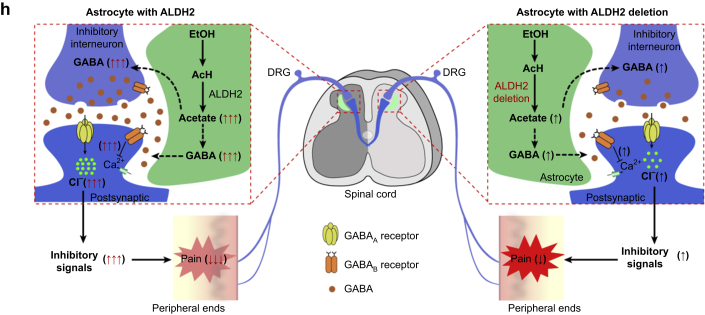
Both GABAA and GABAB receptors are the primary targets for ethanol action in the brain34,35 and are also critical for spinal pain mechanisms.18 To test if spinal GABAA and GABAB receptors are involved in ethanol- or acetate-induced anti-nociception, we delivered a GABAA receptor antagonist, bicuculline (BIC; 200 ng), or a GABAB receptor antagonist, CGP55845 (CGP; 200 ng) via i.t. infusion in mice (Fig. 6a). Either antagonist prevented ethanol-induced analgesia without significantly affecting ethanol-induced discoordination (Fig. 6b–d). Similarly, GABAA and GABAB receptor antagonists inhibited acetate-induced analgesia but not acetate-induced discoordination (Fig. 6e–g). These observations favour a working hypothesis that spinal astrocyte ALDH2 critically regulates the metabolic pathway from ethanol to acetate. Acetate mediates ethanol-induced analgesia through promoting GABA synthesis (Fig. 6h).
Fig 6.
Ethanol- and acetate-induced analgesia via γ-aminobutyric acid (GABA) signalling pathways in spinal cord. (a) Diagram of experimental design to test the effects of i.t. GABAA and GABAB receptor antagonists on ethanol analgesia. (b–c) Time courses of tail flick reflex latency showing abolishment of ethanol (EtOH; 2 g kg−1, i.p.)-induced anti-nociception by intrathecal injection of GABAA receptor antagonist (bicuculline [BIC]; 200 ng) or GABAB receptor antagonist (CGP55845 [CGP]; 200 ng) in C57BL/6J mice (n=8 mice per group). (d) Bar graphs showing that impairment of rotarod performance 30 min after ethanol (2 g kg−1, i.p.) administration was not abolished by an intrathecal injection of GABAA or GABAB receptor antagonist in C57BL/6J mice (n=8 mice per group). (e–g) GABAA or GABAB receptor antagonist only abolished acetate (1.5 g kg−1, i.p.) induced anti-nociception, but not impairment of rotarod performance in C57BL/6J mice (e: n=8 mice per group; f: n=9 mice per group; g: n=8 mice per group). (h) Schematic illustration of proposed aldehyde dehydrogenase-2 (ALDH2)-dependent mechanism underlying ethanol-induced acetate, GABA synthesis, and analgesia. All data are expressed as mean (standard error of the mean). Analysis was performed using two-way analysis of variance (anova) followed by Tukey's test (d and g), or two-way repeated-measures anova followed by Tukey's test (b, c, e, and f). ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, and ∗∗∗P<0.0001 compared with the same time point of ethanol or acetate with GABA receptor antagonist (i.t.) or saline. ns, not significant.
Discussion
About 28% of patients with chronic pain use alcohol to alleviate pain frequently.1 The underlying mechanisms implicated in ethanol-induced analgesia are unknown. The results presented here show that ALDH2 is mainly expressed in astrocytes in the spinal cord. This astrocytic ALDH2 was essential for ethanol metabolism in the spinal cord, which determined the production of acetate and GABA after low to intermediary doses of systemic ethanol. We also provide evidence suggesting that spinal ALDH2 is a potential target that mediates ethanol metabolism and ethanol-induced analgesic effects via acetate–GABA metabolic pathways in the spinal cord.
The role of brain ethanol metabolism has been a controversial topic.36 Brain acetate has until recently been thought to derive from liver ethanol metabolism.37 The in vivo impact of brain ALDH2 has been largely ignored because the level of ALDH2 in the brain is relatively low.8 We observed that ALDH2 mRNA and protein were distributed in both astrocytes and neurones. However, astrocyte ALDH2 is mainly distributed in the spinal dorsal horn area, a centre for receiving and relaying pain sensory signalling. ALDH2 mRNA was found in motor neurones located in the spinal anterior horn, identified by MAP2. There is a possibility that dorsal root ganglion neuronal or astrocytic ALDH2 is involved in ethanol-induced analgesic effect. We injected intra-spinal Cre virus to delete spinal ALDH2 selectively. Deletion of astrocytic ALDH2 protein mainly occurred in the spinal dorsal horn, as indicted by immunostaining and immunoblotting. Using astrocyte ALDH2 conditional knockout mice, we showed that astrocyte ALDH2 controlled acetate production from ethanol metabolism in the spinal cord. We failed to detect any difference in spinal acetaldehyde content in astrocytic ALDH2-deficient mice compared with their wild-type littermates after ethanol administration. One possible explanation is that acetaldehyde can cross between central and peripheral pools after systemic administration of low-dose ethanol (1–2 g kg−1).
Our results show that ALDH2 mediated ethanol-induced analgesic effects in an astrocyte-specific and spinal-cord-specific manner. There was selective disruption of the co-localisation between ALDH2 mRNA and astrocyte-specific markers, but not neuronal markers in spinal slices from astrocyte ALDH2-deficient mice. Selective deletion of astrocyte ALDH2 from either brain or spinal cord inhibited ethanol-induced anti-nociception. Such inhibition of ethanol-induced analgesia appeared to be separable from other ethanol-induced behavioural changes. For example, spinal astrocyte ALDH2 deficiency did not affect ethanol-induced discoordination or hypothermia. Consistent with a spinal ALDH2 mechanism, astrocyte ALDH2 deficiency did not alter pain sensitivity in the hot-plate test, which represents a supraspinal nociceptive pathway.
Ethanol can produce anaesthesia and motor impairment. This raises the question as to whether our assessment of ethanol-induced analgesia is complicated by ethanol-induced anaesthetic effects and motor impairment. Systemic administration of ethanol can produce loss of righting reflex in mice.38 However, this effect was only observed at higher doses of ethanol (>2.5 g kg−1, i.p.). The doses of ethanol given to animals in this study were <2 g kg−1, i.p., which does not induce loss of righting reflex in mice. Such low-to-intermediate doses of ethanol can impair motor function in mice. However, no significant difference was observed in rotarod performance after systemic ethanol between spinal astrocyte ALDH2-deficient and wild-type mice. These findings support the idea that spinal astrocyte ALDH2 selectively contributes to ethanol-induced analgesia.
Amongst ethanol metabolites, acetaldehyde has been described as the most active metabolite in regulating nociception.10,11 Local injection of acetaldehyde increases pain hypersensitivity in rodents,9,10 which is unlikely to contribute to the analgesic effect of ethanol. Astrocyte ALDH2 deficiency did not affect serum or spinal acetaldehyde content. Hepatocyte ALDH2 deficiency did not affect ethanol analgesia even though it led to an elevation of serum acetaldehyde concentrations.12 Rather, we provide evidence suggesting that acetate is the key ethanol metabolite that mediates the analgesic effects of ethanol via promoting GABA synthesis.
There are multiple pathways that may contribute to the conversion of ethanol–acetate to GABA in the brain. Amongst them, the tricarboxylic acid cycle in glia and neurones is likely the primary pathway. There is strong evidence suggesting that acetate is mainly utilised by astrocytes in the brain.37 Several in vivo studies using NMR have revealed a metabolic pathway in the brain that directly mediates conversion of [13C]acetate to [13C]GABA in mice and rats.14,39,40 There is also evidence that astrocytes can directly synthesise GABA in the brain.41,42 Although the concentrations of GABA were found to reach 5–10 mM in cerebellar Bergmann glial cells,43 the source of glial GABA is unknown. Recent studies have shown that astrocytes can produce GABA via a putrescine catabolic pathway.43 One key enzyme in this pathway is ALDH2. Such astrocytic GABA synthesis mediates tonic GABAergic inhibition and motor function in the cerebellum and hippocampus.21,42
We cannot exclude the contribution of neuronal ALDH2 to ethanol-induced analgesic effects. It appears that spinal ALDH2 mRNA is abundantly expressed in the cells of spinal cord grey mater. ALDH2 mRNA co-localised with MAP2 by 82%. Depletion of ALDH2 from astrocytes did not completely inhibit expression of ALDH2 mRNA, protein, and enzymatic activity. Use of Aldh2Gfap–/– mice has a few limitations. One of them is that expression of the Cre gene is brain-region specific in adulthood (http://www.informatics.jax.org/allele/MGI:3522215?romRibbon=open) such that tissue-specific depletion of ALDH2 in Aldh2Gfap–/– mice may not truly represent the actual distribution of astrocyte ALDH2 in normal mice.
In summary, these findings suggest that spinal ALDH2 mediates the production of acetate from ethanol metabolism by an astrocyte-dependent mechanism. Astrocyte ALDH2 regulates ethanol-induced analgesic effects via distinct acetate–GABA synthesis pathways. Spinal astrocyte ALDH2 is an important target not only for the analgesic effect of ethanol, but also for the pathophysiology of pain at the spinal level.
Authors' contributions
Project conception: YZ, LZ
Study design: YZ, LZ, SJ
Performance of experiments: SJ, RC, XH, YL, GL, LZ
Initial data collection/analysis: SJ, RC, XH, YL, LZ
Final data analysis: SJ, YZ, LZ
Writing of paper: SJ, YZ, LZ
Critical revision of paper: LZ, YZ, DML
Funding
US National Institutes of Health (Graduate Partnerships Program); Anhui Medical University; Division of Intramural Clinical and Biological Research, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health; National Natural Science Foundation of China (81801938 to SJ).
Declarations of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors thank Frederic Langevin and Ketan J. Patel (University of Cambridge) for aldehyde dehydrogenase-2 flox mice. The authors thank Harvey-Judith White (National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, USA) for technical assistance with the γ-aminobutyric acid assay.
Handling editor: Hugh C Hemmings Jr
Footnotes
This article is accompanied by an editorial: Alcohol and analgesia: a fine wine getting better with age by Zambelli et al., Br J Anaesth 2021:127:177–181, doi: 10.1016/j.bja.2021.05.003
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.02.035.
Contributor Information
Ye Zhang, Email: zhangye.hassan@aliyun.com.
Li Zhang, Email: lzhang@mail.nih.gov.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Riley J.L., 3rd, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. J Pain. 2009;10:944–952. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egli M., Koob G.F., Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eng M.Y., Luczak S.E., Wall T.L. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Res Health. 2007;30:22–27. [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan P.L., Schutte K.K., Moos R.H. Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction. 2005;100:777–786. doi: 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- 5.Gatch M.B., Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res. 1999;23:328–333. [PubMed] [Google Scholar]
- 6.Jochum T., Boettger M.K., Burkhardt C., Juckel G., Bär K.-J. Increased pain sensitivity in alcohol withdrawal syndrome. Eur J Pain. 2010;14:713–718. doi: 10.1016/j.ejpain.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Spies C.D., Rommelspacher H. Alcohol withdrawal in the surgical patient. Anesth Analg. 1999;88:946–954. doi: 10.1097/00000539-199904000-00050. [DOI] [PubMed] [Google Scholar]
- 8.Edenberg H.J. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L., Li L., Tang H. Alcohol-aggravated episodic pain in humans with SCN11A mutation and ALDH2 polymorphism. Pain. 2020;161:1470–1482. doi: 10.1097/j.pain.0000000000001853. [DOI] [PubMed] [Google Scholar]
- 10.Zambelli V.O., Gross E.R., Chen C.H. Aldehyde dehydrogenase-2 regulates nociception in rodent models of acute inflammatory pain. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C.H., Ferreira J.C., Gross E.R., Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillot A., Ren T., Jourdan T. Targeting liver aldehyde dehydrogenase-2 prevents heavy but not moderate alcohol drinking. Proc Natl Acad Sci U S A. 2019;116:25974–25981. doi: 10.1073/pnas.1908137116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghrayeb A., Gottlieb E., Mor I. Alcohol-derived acetate modulates brain function. Nat Metab. 2019;1:1036–1037. doi: 10.1038/s42255-019-0139-3. [DOI] [PubMed] [Google Scholar]
- 14.Hassel B., Sonnewald U., Fonnum F. Glial–neuronal interactions as studied by cerebral metabolism of [2-13C]acetate and [1-13C]glucose: an ex vivo 13C NMR spectroscopic study. J Neurochem. 1995;64:2773–2782. doi: 10.1046/j.1471-4159.1995.64062773.x. [DOI] [PubMed] [Google Scholar]
- 15.Koob G.F. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Knabl J., Witschi R., Hösl K. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 17.Gwak Y.S., Hulsebosch C.E. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60:799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeilhofer H.U., Neumann E., Munro G. Spinal GABAA receptors for pain control: back to the future? Br J Anaesth. 2019;123:e176–e179. doi: 10.1016/j.bja.2019.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agabio R., Colombo G. GABAB receptor ligands for the treatment of alcohol use disorder: preclinical and clinical evidence. Front Neurosci. 2014;8:140. doi: 10.3389/fnins.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J.I., Ganesan S., Luo S.X. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 2015;350:102–106. doi: 10.1126/science.aac4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo J., Min J.O., Kang D.S. Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum. Proc Natl Acad Sci U S A. 2018;115:E5253. doi: 10.1073/pnas.1721187115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson T., Oram C., Correll C.U., Tsermentseli S., Stubbs B. Analgesic effects of alcohol: a systematic review and meta-analysis of controlled experimental studies in healthy participants. J Pain. 2017;18:499–510. doi: 10.1016/j.jpain.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Neddenriep B., Bagdas D., Contreras K.M. Pharmacological mechanisms of alcohol analgesic-like properties in mouse models of acute and chronic pain. Neuropharmacology. 2019;160:107793. doi: 10.1016/j.neuropharm.2019.107793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin W., Shen K., Liu X. Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/reperfusion injury. Neural Regen Res. 2017;12:1166–1171. doi: 10.4103/1673-5374.211198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong W., Cui T., Cheng K. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha 3 glycine receptors. J Exp Med. 2012;209:1121–1134. doi: 10.1084/jem.20120242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y., Vidensky S., Jin L. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia. 2011;59:200–207. doi: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du Y., Chen C.P., Tseng C.-Y., Eisenberg Y., Firestein B.L. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55:463–472. doi: 10.1002/glia.20472. [DOI] [PubMed] [Google Scholar]
- 28.Durcan M.J., Lister R.G. Time course of ethanol’s effects on locomotor activity, exploration and anxiety in mice. Psychopharmacology. 1988;96:67–72. doi: 10.1007/BF02431535. [DOI] [PubMed] [Google Scholar]
- 29.Grossman M.L., Basbaum A.I., Fields H.L. Afferent and efferent connections of the rat tail flick reflex (a model used to analyze pain control mechanisms) J Comp Neurol. 1982;206:9–16. doi: 10.1002/cne.902060103. [DOI] [PubMed] [Google Scholar]
- 30.Campbell V.C., Taylor R.E., Tizabi Y. Antinociceptive effects of alcohol and nicotine: involvement of the opioid system. Brain Res. 2006;1097:71–77. doi: 10.1016/j.brainres.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 31.Sauguet L., Howard R.J., Malherbe L. Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun. 2013;4:1697. doi: 10.1038/ncomms2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen R.W. Extrasynaptic GABAA receptors in the nucleus accumbens are necessary for alcohol drinking. Proc Natl Acad Sci U S A. 2011;108:4699–4700. doi: 10.1073/pnas.1102818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelm M.K., Criswell H.E., Breese G.R. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev. 2011;65:113–123. doi: 10.1016/j.brainresrev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris R.A., Trudell J.R., Mihic S.J. Ethanol’s molecular targets. Sci Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang J.-H., Chen F., Krstew E. The GABAB receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology. 2006;50:632–639. doi: 10.1016/j.neuropharm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Hipólito L., Sánchez M.J., Polache A., Granero L. Brain metabolism of ethanol and alcoholism: an update. Curr Drug Metab. 2007;8:716–727. doi: 10.2174/138920007782109797. [DOI] [PubMed] [Google Scholar]
- 37.Deelchand D.K., Shestov A.A., Koski D.M., Uğurbil K., Henry P.-G. Acetate transport and utilization in the rat brain. J Neurochem. 2009;109:46–54. doi: 10.1111/j.1471-4159.2009.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slater C.A., Jackson A., Muldoon P.P. Nicotine enhances the hypnotic and hypothermic effects of alcohol in the mouse. Alcohol Clin Exp Res. 2016;40:62–72. doi: 10.1111/acer.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiwari V., Veeraiah P., Subramaniam V., Patel A.B. Differential effects of ethanol on regional glutamatergic and GABAergic neurotransmitter pathways in mouse brain. J Neurochem. 2014;128:628–640. doi: 10.1111/jnc.12508. [DOI] [PubMed] [Google Scholar]
- 40.Wang J., Du H., Ma X. Metabolic products of [2-13C]ethanol in the rat brain after chronic ethanol exposure. J Neurochem. 2013;127:353–364. doi: 10.1111/jnc.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Meur K., Mendizabal-Zubiaga J., Grandes P., Audinat E. GABA release by hippocampal astrocytes. Front Comput Neurosci. 2012;6:59. doi: 10.3389/fncom.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S., Yoon B.E., Berglund K. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- 43.Yoon B.E., Woo J., Chun Y.E. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol. 2014;592:4951–4968. doi: 10.1113/jphysiol.2014.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.