Abstract
Background
Hidradenitis suppurativa (HS) and atopic dermatitis (AD) are both chronic inflammatory skin diseases. An association between these 2 conditions can have important potential implications for elucidating pathogenesis, disease course, and treatment.
Objective
To investigate the association between AD and HS.
Methods
We performed a retrospective cohort study of patients seen at Duke University Medical Center from 2007 to 2017 who had AD compared with a control group without an AD diagnosis. The association of AD and HS was evaluated using a logistic regression model after adjusting for other confounders including age, sex, and race.
Results
Of 28,780 patients with an AD diagnosis, 325 (1.1%) were diagnosed with HS compared with 76 (0.2%) within the 48,383 patients in the non-AD group. An adjusted logistic regression model demonstrated an increased odds ratio of having HS diagnosis in the AD group as compared with the control non-AD group (odds ratio: 5.57, 95% confidence interval: 4.30-7.21, P < .001).
Limitations
This was a retrospective study performed at a single institution with the possibility of surveillance bias being present.
Conclusions
Patients with AD are more likely to be diagnosed with HS than patients without AD. Further research is needed to understand the pathophysiologic mechanism and potential treatment implications.
Key words: atopic dermatitis, barrier defect, clinical research, hidradenitis suppurativa, inflammatory skin diseases, notch signaling
Abbreviations used: AD, atopic dermatitis; AMP, antimicrobial peptide; CI, confidence interval; HS, hidradenitis suppurativa; ICD, International Classification of Diseases; OR, odds ratio; PPV, positive predictive value; TSLP, thymic stromal lymphopoietin
Capsule Summary.
-
•
This article provides early evidence for a clinical association between hidradenitis suppurativa (HS) and atopic dermatitis (AD).
-
•
We recommend judicious use of antiseptic topical washes and solutions in patients with both HS and AD to avoid drying the skin, which could contribute to the barrier defect.
Introduction
Hidradenitis suppurativa (HS) is a chronic relapsing inflammatory condition that presents in the second or third decade of life and affects approximately 0.1%-0.7% of the population.1,2 It is diagnosed clinically based on specific criteria that include the occurrence of chronic, recurrent, deep-seated, painful nodules and draining tunnels in 1 or more predilection skin areas, such as the axilla and groin. Flares of this chronic cutaneous condition are extremely painful, and the acute presentation of these episodes has led to an increased utilization of health care delivery in high-cost settings, such as the emergency department and inpatient hospitalizations.3 Risk factors for HS include smoking, female sex, and African American race.4,5 Furthermore, HS is frequently associated with multiple comorbidities, such as inflammatory bowel disease, metabolic syndrome, obesity, and polycystic ovary syndrome.6,7
Similar to HS, atopic dermatitis (AD) is also a chronic relapsing inflammatory skin disease. This condition affects approximately 20% of the population, with 85% of patients having AD present before 5 years of age.8 AD typically peaks in infancy and in the third decade of life. AD results in pruritic lesions that commonly arise on the elbows, chest, face, neck, and ankles. AD is usually the first clinical manifestation of atopy and is commonly associated with asthma, allergic rhinitis, and food allergies. Similar to HS, risk factors associated with AD include African American race, female sex, and smoking.9, 10, 11 AD is known to result in an increased delivery of health care, and a recent study determined that AD's health care resource utilization was significantly higher when compared with that of non-AD controls, mainly because of health care provider visits and associated costs.12
We are not aware of any published clinical data indicating a link between the 2 diseases. However, based on our clinical experience in our hidradenitis specialty clinic, in addition to the aforementioned factors, we postulated that AD is associated with HS.
Methods
Data sources and study population
Patients who had AD diagnosed at Duke University Medical Center between November 1, 2007, and November 1, 2017, were identified from the Duke Enterprise Data Unified Content Explorer database, a web-based environment that allows Duke-based investigators access to information generated during patient care within the Duke University Health Care System. A random cohort of 50,000 patients seen at Duke University Medical Center during the same time period were also extracted from the Duke Enterprise Data Unified Content Explorer database and used as a control group. Identified AD and non-AD cohorts were evaluated using electronic health records going back to 1997. Age was calculated based on the date of the first encounter.
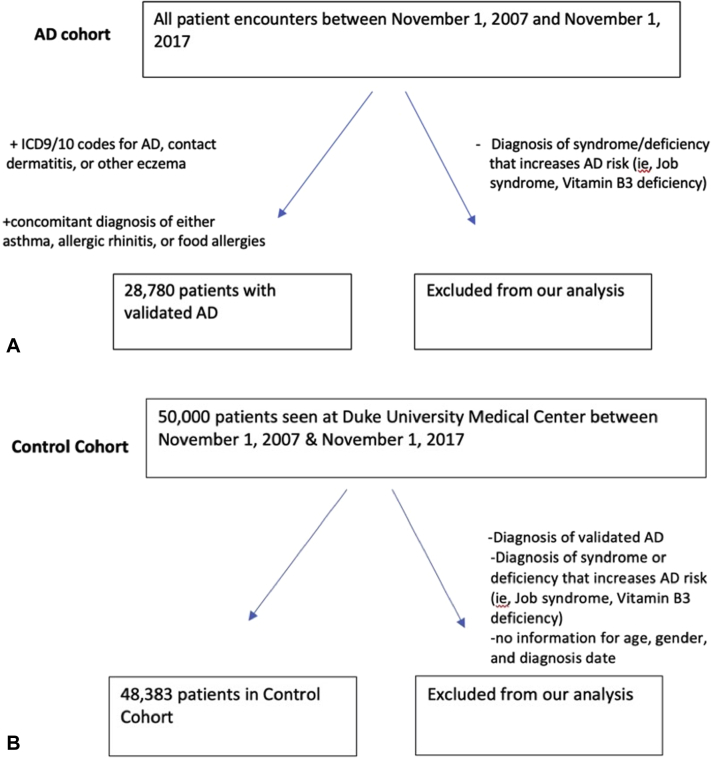
Patients with AD were identified using the International Classification of Diseases Nineth and Tenth Revisions (International Classification of Diseases [ICD 9/10]) codes 691.8 and L20.9 for atopic dermatitis and related conditions and ICD diagnosis codes 692.9 and L25.9 for contact dermatitis and other eczema, unspecified cause. These codes were previously validated by Hsu et al.13 In order to increase the positive predictive value (PPV) for the diagnosis of AD, we only considered a patient to have validated AD if they also had a concomitant diagnosis of either asthma, allergic rhinitis, or food allergies with the caveat that a patient needed a minimum of 2 diagnoses of allergic rhinitis to be considered to have allergic rhinitis.13,14 In validating our own cohort, 50 patients were randomly selected from our AD cohort identified based on ICD codes. Chart reviews were performed in order to determine the AD diagnosis by using the UK criteria. This criterion specifically included history of flexural involvement, history of dry skin, onset under the age of 2, personal history of asthma, history of a pruritic skin condition, and visible flexural dermatitis to determine if a patient had the diagnosis of AD.15 We observed a PPV of 74% for the diagnosis of AD using this method, which is consistent with the range of 68%-80% previously reported in the literature.13,16 Of note, we excluded patients with a syndrome or deficiency that increases the risk for atopic dermatitis, including Jobs syndrome, Wiskott–Aldrich syndrome, ataxia telangiectasia, and Vitamin B3 deficiency.17, 18, 19 After determining the validity of this selection criterion, we identified a cohort of 28,780 patients with a diagnosis of AD (Fig 1, A).
Fig 1.
Atopic dermatitis. Flow diagram of cohort selection of patients with (A) and without (B) validated atopic dermatitis. AD, Atopic dermatitis.
For the non-AD control group, we proceeded to exclude patients with validated AD and any syndromes that increase the risk of AD as mentioned previously and were left with a cohort of 48,383 patients (Fig 1, B). We then identified patients with a diagnosis of HS from the non-AD control cohort and the AD cohort.
We used ICD-9/10 codes 705.83 and L73.2 to identify patients of all ages with a diagnosis of HS from this cohort.20 In validating our own cohort, 50 patients were randomly selected from our HS cohort identified based on ICD codes. We used the following criteria, based on previous studies, to validate the HS diagnosis: confirmation by a dermatologist, pathology results, or accuracy of HS lesion description.7 We observed a PPV of 76% for the diagnosis of HS by using a single ICD-9/10 code. This result is consistent with the previous finding by Strunk et al21 showing that the presence of at least one ICD-9 code for HS provides a 79% PPV in confirming HS diagnosis. The initial diagnosis date for HS was identified as the diagnosis date for boils in patients who initially presented with the ICD-9/10 code for boils and later had HS diagnosed to account for the delay in diagnosis of HS.
Statistical methods
Categorical variables were summarized with frequency counts and percentages for non-missing data. Continuous variables were summarized using the mean, SD, median, 25th and 75th percentiles (Q1 to Q3), and range. A logistic regression model was used to study the association between AD and HS after adjusting for age, sex, and race with results presented using the odds ratio (OR) with 95% confidence interval (CI). A P-value < .05 was considered statistically significant, and all analyses were conducted using SAS version 9.4 (SAS Institute Inc).
Results
In total, we identified 28,780 patients with validated AD and 48,383 non-AD controls (Fig 1, A and B). Demographic characteristics stratified by AD are summarized in Table I. The AD group was composed of a slightly higher percentage of female patients (58.4% vs 54.7%) and Black or African American patients (37.6% vs 22.3%) as compared with the non-AD control group. The median age within the AD group was 23.6 years (Q1-Q3: 0.7-47.8) compared with 38.4 years (Q1-Q3: 20.7-56.9) within the non-AD control group.
Table I.
Demographic characteristics of the atopic dermatitis and non-atopic dermatitis control cohort
| Non-AD controls (N = 48,383) | Atopic dermatitis (N = 28,780) | |
|---|---|---|
| Sex | ||
| Female | 26,453 (54.7%) | 16,819 (58.4%) |
| Race | ||
| Black or African American | 10,797 (22.3%) | 10,811 (37.6%) |
| White or Caucasian | 29,004 (59.9%) | 14,853 (51.6%) |
| Other∗ | 5216 (10.8%) | 2607 (9.1%) |
| Unknown | 3366 (7.0%) | 509 (1.8%) |
| Age at first encounter (years) | ||
| Mean (SD) | 38.6 (23.1) | 26.6 (24.8) |
| Median | 38.4 | 23.6 |
| Q1, Q3 | 20.7, 56.9 | 0.7, 47.8 |
| Range | (0.0-99.7) | (0.0-97.0) |
| Smoking status | ||
| Missing | 20,347 | 8601 |
| Former or current smoker | 10,212 | 6736 |
| Never | 17,824 | 13,443 |
AD, Atopic dermatitis.
Other includes Alaskan native, American Indian, native Hawaiian or other Pacific Islander, or multiracial.
In the AD group, 325 (1.1%) were diagnosed with HS. Among these 325 patients, 171 (52.6%) had HS before AD and 154 (47.4%) had AD before HS. For patients who had both AD and HS, the median age at HS diagnosis was 34 years (Q1-Q3: 19.2-45.0). The median time between the diagnosis of AD and HS was 0.0 years (Q1-Q3: −3.1 to 3.0). In the non-AD control group, 76 (0.2%) patients were diagnosed with HS. For these 76 patients, the median age at HS diagnosis was 29 years (Q1-Q3: 20.2-41.2). The unadjusted odds of having an HS diagnosis for patients who had AD was significantly higher than that for patients in the non-AD control group (OR = 7.26, 95%CI: 5.65- 9.32, P-value < .001).
A multivariable logistic regression model was used to study the effect of AD on the odds of having of HS after adjusting for age, race, and sex and is summarized in Table II. The adjusted model demonstrated an increased OR of having an HS diagnosis in the AD group as compared with the non-AD control group (OR: 5.57, 95% CI: 4.30-7.21, P < .001), indicating that patients with AD were more likely to have HS (Table II). Furthermore, we found sex and race were significantly associated with the incidence of HS with an increased odds of having HS for female compared with male patients (OR: 3.36, 95% CI: 2.59-4.36, P < .001) and for African American race compared with Caucasian (OR: 3.52, 95% CI: 2.80-4.43, P < .001) as expected.
Table II.
Multivariable logistic regression model for assessing the association of hidradenitis suppurativa with atopic dermatitis
| Covariates | Odds ratio (95% confidence interval) | P-value |
|---|---|---|
| Atopic dermatitis (Yes vs no) | 5.57 (4.30-7.21) | <.001 |
| Sex (Female vs male) | 3.36 (2.59-4.36) | <.001 |
| Race (Black vs White)∗ | 3.52 (2.80-4.43) | <.001 |
| Age (with 10-year increase) | 0.99 (0.95-1.03) | .596 |
For race, the odds ratios of “other” vs “White” and “Unknown” vs “White” were not significant and not shown in this table.
Discussion
We determined that patients with a diagnosis of AD have an approximately 5.57-fold increased OR of having HS as compared with those who do not have AD. This relationship is highly significant and suggests a potential unique pathophysiologic link that may be related to similar deficient notch signaling, barrier defect and antimicrobial peptide (AMP) dysregulation seen in both conditions. This association may also reflect a common genetic susceptibility shared between AD and HS.22, 23, 24 In clinical practice, a history of atopy should be considered when deciding on the frequency and use of various topical antiseptic washes typically recommended for patients with HS. Patients should be carefully monitored for clinical signs of eczema in areas affected by HS and treated accordingly. Exacerbation of barrier defect in these areas can also worsen HS by contributing to ongoing inflammatory responses. Future prospective studies are needed to understand whether hidradenitis patients with a concomitant AD diagnosis demonstrate a different disease course and treatment response compared with those of patients with hidradenitis alone. The theoretical similarities in pathophysiology need further confirmation and could result in treatment options with similar therapeutic targets. We therefore suggest several testable hypotheses that can be considered to explain this new observation of an increased association of HS in AD patients.
Recent evidence suggests that in cases of familial HS, there are loss-of-function mutations in the gamma secretase complex, which may lead to decreased proteolytic cleavage of transmembrane notch protein to its active form.25 The role of impaired notch signaling in HS is unclear, but in AD, notch signaling dysregulation can result in epidermal barrier defects and increased inflammation.26, 27, 28 Specifically, notch signaling plays a key role in the regulation of skin differentiation (epidermis and pilosebaceous unit)29 and is important in the processing of filaggrin.30 Mouse models with genetically suppressed notch signaling have shown AD-like skin disease and increased transepidermal water loss.31 Notch signals are also important in the differentiation of regulatory T cells and play a role in the feedback inhibition of innate immunity.24,32 In particular, notch dysfunction has been associated with increases in thymic stromal lymphopoietin (TSLP) in multiple disease processes including AD.26,33,34 Recent studies have suggested that TSLP alone is sufficient to promote the Th2 phenotype, but a combination of TSLP and toll-like receptor 3 is required to promote simultaneous Th17 phenotype, a unique profile seen in severe asthma.35 In HS, there is clear evidence suggesting an increased presence of Th17 cells in lesional skin in addition to an upregulation of the Th2 pathway as well as the Th1 pathway.22,36, 37, 38 We suspect that a combination of TSLP increase secondary to defective notch signaling, as well as other inflammatory mediators, maybe be contributing to the inflammatory cascade witnessed in HS, although this has not yet been examined. Such findings may provide a potential link in the association between AD and HS.
In addition, AMP dysregulation in the setting of altered skin flora acts to promote an inflammatory response in both AD and HS; specifically, both diseases are associated with altered levels of the AMPs such as cathelicidin (LL-37) and dermcidin.39,40 In both AD and HS, a distinct wound response signature similar to what is seen at injury sites, including an upregulation of AMPs, has also been reported.23,41 In circumstances in which AMP levels were altered, the resulting phenotypic changes were shown to lead to an increase in local inflammatory mediators, further propagating tissue inflammation and damage.39,42,43 Therefore, the exact role of AMP dysregulation, similar in both AD and HS, needs to be further elucidated.
Several limitations are present that warrant consideration when interpreting the results of our study. This was a retrospective study performed at a single institution. However, the study did examine a heterogeneous population that is mostly representative of the North Carolina population. Additionally, patients may have moved throughout their lifetime and received health care from multiple medical centers. Therefore, the association between AD and HS may be even stronger, as many of our patients could have had a diagnosis of AD during childhood but did not access health care at Duke University Health Care System until a later age. Another important limitation that warrants consideration is the fact that surveillance bias may be present in our study, as patients who had AD managed by dermatologists may possibly have had a higher chance of being diagnosed with HS as compared with that in the patients in the control group. Unfortunately, we do not have provider information of encounters for each patient over time; therefore, we cannot accurately estimate the percentage of patients being seen by a dermatologist for each of the 2 cohorts (AD vs non-AD control cohort). However, the association between AD and HS was independent of the timing of the AD diagnosis.
In the future, our goal is to repeat this study with a larger US population using Truven Health MarketScan research database. Despite these limitations, this study described an association between AD and HS, as demonstrated by the increased incidence of HS witnessed in patients with AD.
Conflicts of interest
Dr Macleod consulted for Silab when she was an employee at Duke. The MacLeod laboratory has previously received funds from Silab Company; funding from this partnership was not directly used for this study. Silab did not have any influence on the content of this project. Dr Macleod is also consulting for the LEO Foundation. The spouse of Dr Macleod is employed at Precision Biosciences and has stock options. Dr Jaleel is an investigator for UCB and reported consulting for Eli Lilly and Chemocentryx and receiving honoraria. None of the content or the decision to publish has been affected by the authors' involvement with Eli Lilly, Silab, Chemocentryx, Precision Biosciences or the LEO foundation. Drs Kaakati, Tanaka, Liu, Ward, and Green have no conflicts of interest to declare.
Footnotes
Dr Macleod is currently employed by Janssen Pharmaceuticals.
Drs Kaakati and Tanaka contributed equally to this article.
Funding sources: Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002553, which provides support to Duke BERD core (Biostatistics, Epidemiology, and Research Design). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
IRB approval status: Reviewed and exempt by Duke Central IRB.
References
- 1.Garg A., Kirby J.S., Lavian J., Lin G., Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760–764. doi: 10.1001/jamadermatol.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingram J.R., Jenkins-Jones S., Knipe D.W., Morgan C.L.I., Cannings-John R., Piguet V. Population-based clinical practice research datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178(4):917–924. doi: 10.1111/bjd.16101. [DOI] [PubMed] [Google Scholar]
- 3.Khalsa A., Liu G., Kirby J.S. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(4):609–614. doi: 10.1016/j.jaad.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 4.Micheletti R.G. Hidradenitis suppurativa: current views on epidemiology, pathogenesis, and pathophysiology. Semin Cutan Med Surg. 2014;33(3 suppl):S48–S50. doi: 10.12788/j.sder.0091. [DOI] [PubMed] [Google Scholar]
- 5.Reeder V.J., Mahan M.G., Hamzavi I.H. Ethnicity and hidradenitis suppurativa. J Invest Dermatol. 2014;134(11):2842–2843. doi: 10.1038/jid.2014.220. [DOI] [PubMed] [Google Scholar]
- 6.Deckers I.E., Benhadou F., Koldijk M.J. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional study. J Am Acad Dermatol. 2017;76(1):49–53. doi: 10.1016/j.jaad.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Shlyankevich J., Chen A.J., Kim G.E., Kimball A.B. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71(6):1144–1150. doi: 10.1016/j.jaad.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Kay J., Gawkrodger D.J., Mortimer M.J., Jaron A.G. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30(1):35–39. doi: 10.1016/s0190-9622(94)70004-4. [DOI] [PubMed] [Google Scholar]
- 9.Moore M.M., Rifas-Shiman S.L., Rich-Edwards J.W. Perinatal predictors of atopic dermatitis occurring in the first six months of life. Pediatrics. 2004;113(3 Pt 1):468–474. doi: 10.1542/peds.113.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagarajan S., Yan C., Pradhan K.M., Vastardi M.A. The role of gender in the association between obesity and atopic disease in urban children. J Allergy ClinImmunol. 2017;139(2):AB387. [Google Scholar]
- 11.Kantor R., Kim A., Thyssen J.P., Silverberg J.I. Association of atopic dermatitis with smoking: a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75(6):1119–1125.e1. doi: 10.1016/j.jaad.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert L., Gupta S., Amand C., Gadkari A., Mahajan P., Gelfand J.M. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J Am Acad Dermatol. 2018;78(1):54–61.e1. doi: 10.1016/j.jaad.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Hsu D.Y., Dalal P., Sable K.A. Validation of International Classification of Disease ninth Revision codes for atopic dermatitis. Allergy. 2017;72(7):1091–1095. doi: 10.1111/all.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt J., Stadler E., Kuster D., Wustenberg E.G. Medical care and treatment of allergic rhinitis: a population-based cohort study based on routine healthcare utilization data. Allergy. 2016;71(6):850–858. doi: 10.1111/all.12838. [DOI] [PubMed] [Google Scholar]
- 15.De D., Kanwar A.J., Handa S. Comparative efficacy of Hanifin and Rajka's criteria and the UK working party's diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20(7):853–859. doi: 10.1111/j.1468-3083.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- 16.Williams H.C., Burney P.G., Pembroke A.C., Hay R.J. Validation of the UK diagnostic criteria for atopic dermatitis in a population setting. Br J Dermatol. 1996;135(1):12–17. [PubMed] [Google Scholar]
- 17.Buchbinder D., Nugent D.J., Fillipovich A.H. Wiskott-Aldrich syndrome: diagnosis, current management, and emerging treatments. Appl Clin Genet. 2014;7:55–66. doi: 10.2147/TACG.S58444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegyi J., Schwartz R.A., Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43(1):1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 19.Bos J.D. Atopiform dermatitis. Br J Dermatol. 2002;147(3):426–429. doi: 10.1046/j.1365-2133.2002.05010.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim G.E., Shlyankevich J., Kimball A.B. The validity of the diagnostic code for hidradenitis suppurativa in an electronic database. Br J Dermatol. 2014;171(2):338–342. doi: 10.1111/bjd.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strunk A., Midura M., Papagermanos V., Alloo A., Garg A. Validation of a case-finding algorithm for hidradenitis suppurativa using administrative coding from a clinical database. Dermatology. 2017;233(1):53–57. doi: 10.1159/000468148. [DOI] [PubMed] [Google Scholar]
- 22.Thomi R., Cazzaniga S., Seyed Jafari S.M., Schlapbach C., Hunger R.E. Association of hidradenitis suppurativa with T helper 1/T helper 17 phenotypes: a semantic map analysis. JAMA Dermatol. 2018;154(5):592–595. doi: 10.1001/jamadermatol.2018.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coates M., Mariottoni P., Corcoran D.L. The skin transcriptome in hidradenitis suppurativa uncovers an antimicrobial and sweat gland gene signature which has distinct overlap with wounded skin. PLoS One. 2019;14(5):e0216249. doi: 10.1371/journal.pone.0216249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auderset F., Coutaz M., Tacchini-Cottier F. The role of Notch in the differentiation of CD4+ T helper cells. Curr Top Microbiol Immunol. 2012;360:115–134. doi: 10.1007/82_2012_227. [DOI] [PubMed] [Google Scholar]
- 25.Wang B., Yang W., Wen W. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330(6007):1065. doi: 10.1126/science.1196284. [DOI] [PubMed] [Google Scholar]
- 26.Melnik B.C. The potential role of impaired Notch signalling in atopic dermatitis. Acta Derm Venereol. 2015;95(1):5–11. doi: 10.2340/00015555-1898. [DOI] [PubMed] [Google Scholar]
- 27.Troy T.C., Turksen K. The targeted overexpression of a Claudin mutant in the epidermis of transgenic mice elicits striking epidermal and hair follicle abnormalities. Mol Biotechnol. 2007;36(2):166–174. doi: 10.1007/s12033-007-0027-z. [DOI] [PubMed] [Google Scholar]
- 28.Murthy A., Shao Y.W., Narala S.R., Molyneux S.D., Zuniga-Pflucker J.C., Khokha R. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity. 2012;36(1):105–119. doi: 10.1016/j.immuni.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Demehri S., Liu Z., Lee J. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6(5):e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H.Y., Kao C.H., Lin K.M., Kaartinen V., Yang L.T. Notch signaling regulates late-stage epidermal differentiation and maintains postnatal hair cycle homeostasis. PLoS One. 2011;6(1):e15842. doi: 10.1371/journal.pone.0015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franzke C.W., Cobzaru C., Triantafyllopoulou A. Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J Exp Med. 2012;209(6):1105–1119. doi: 10.1084/jem.20112258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maillard I., Fang T., Pear W.S. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 33.Indra A.K. Epidermal TSLP: a trigger factor for pathogenesis of atopic dermatitis. Expert Rev Proteomics. 2013;10(4):309–311. doi: 10.1586/14789450.2013.814881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boguniewicz M., Leung D.Y. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242(1):233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka J., Watanabe N., Kido M. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy. 2009;39(1):89–100. doi: 10.1111/j.1365-2222.2008.03151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran B., Sweeney C.M., Hughes R. Hidradenitis suppurativa is characterized by dysregulation of the Th17:Treg cell axis, which is corrected by anti-TNF therapy. J Invest Dermatol. 2017;137(11):2389–2395. doi: 10.1016/j.jid.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 37.Shanmugam V.K., Jones D., McNish S., Bendall M.L., Crandall K.A. Transcriptome patterns in hidradenitis suppurativa: support for the role of antimicrobial peptides and interferon pathways in disease pathogenesis. Clin Exp Dermatol. 2019;44(8):882–892. doi: 10.1111/ced.13959. [DOI] [PubMed] [Google Scholar]
- 38.Schlapbach C., Hanni T., Yawalkar N., Hunger R.E. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(4):790–798. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Reinholz M., Ruzicka T., Schauber J. Cathelicidin LL-37: an antimicrobial peptide with a role in inflammatory skin disease. Ann Dermatol. 2012;24(2):126–135. doi: 10.5021/ad.2012.24.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann S.C., Saborowski V., Lange S., Kern W.V., Bruckner-Tuderman L., Rieg S. Expression of innate defense antimicrobial peptides in hidradenitis suppurativa. J Am Acad Dermatol. 2012;66(6):966–974. doi: 10.1016/j.jaad.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Ballardini N., Johansson C., Lilja G. Enhanced expression of the antimicrobial peptide LL-37 in lesional skin of adults with atopic eczema. Br J Dermatol. 2009;161(1):40–47. doi: 10.1111/j.1365-2133.2009.09095.x. [DOI] [PubMed] [Google Scholar]
- 42.Harder J., Dressel S., Wittersheim M. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J Invest Dermatol. 2010;130(5):1355–1364. doi: 10.1038/jid.2009.432. [DOI] [PubMed] [Google Scholar]
- 43.Wolk K., Warszawska K., Hoeflich C. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186(2):1228–1239. doi: 10.4049/jimmunol.0903907. [DOI] [PubMed] [Google Scholar]