Abstract
Introduction
Fungal skin diseases are highly prevalent worldwide, but few existing studies focus on the burden of dermatomycoses.
Methods
An analysis of fungal skin disease trends in 2017 in 195 countries worldwide was conducted using the Global Burden of Disease Study database, including prevalence rates, age and sex patterns, and fungal burden, using disability-adjusted life years (DALYs). Age-standardized DALYs were also compared to the sociodemographic index values of all the countries in 2017.
Results
The age-specific fungal skin disease DALYs in 2017 showed a right-skewed distribution, with a peak between 1 and 5 years of age. The world region with the greatest burden of fungal skin disease was sub-Saharan Africa (DALY rate 89.3 per 100,000 males, 78.42 for females), and the individual country with the greatest DALY rate was Mali (122). The Global Burden of Disease super region with the lowest fungal skin disease burden had high incomes (DALY rate 33.12 per 100,000 males, 30.16 for females), which includes southern Latin America, western Europe, high-income North America, Australasia, and high-income southern Pacific.
Conclusion
Skin mycoses place a substantial burden on patients worldwide. This burden is the greatest in resource-poor countries, tropical regions, and children between 1 and 5 years of age. DALYs can potentially serve as a purposeful measure for directing health policy resources to improve the global impact of fungal skin disease.
Key words: age-standardized prevalence rates, dermatomycoses, disability-adjusted life years (DALYs), fungal, fungal skin disease, Global Burden of Disease Study (GBD) database, global medicine, gross domestic product per capita, health care disparities, health equity, mycoses, socioeconomic status
Abbreviations used: DALY, disability-adjusted life year; GBD, global burden of disease; SDI, sociodemographic index
Capsule Summary.
-
•
Understanding the epidemiology and impact of fungal cutaneous diseases is critical to developing concerted and sustained global efforts toward reducing this burden.
-
•
Fungal skin infections cause a significant disease burden worldwide. Disability-adjusted life years may serve as a purposeful measure for directing resources and interventions to help reduce this burden.
Introduction
Fungal skin diseases are extremely common, with nearly a billion people worldwide estimated to suffer from dermatomycoses or fungal infections of the skin, hair, and nails.1 The prevalence and incidence of dermatomycoses vary with socioeconomic and geographic differences.2 For example, the frequency of some fungal skin diseases is greater in lower-socioeconomic regions because of crowded living conditions, close proximity to animals, and poor hygiene.2,3 Additionally, dermatophytes thrive in warm, humid climates, and dermatomycoses are more common in tropical countries.2
Dermatophytes, specifically Trichophyton and Microsporum species, are the primary fungal pathogens responsible for skin infections. Some fungal species that cause superficial mycoses are distributed worldwide, such as T rubrum, T mentagrophytes var. interdigitale, and M canis. Other species are restricted by geographic region, such as T soudanense (Africa), T schoenleinii (Eurasia and Africa), T violaceum (Asia, Africa, and Europe), and T concentricum (Pacific Islands, India, and far East).4 T rubrum is the most common dermatophyte reported in developed countries and urban areas within developing countries.5,6 M canis is common in central and southern Europe, T rubrum and T mentagrophytes are prevalent in Asia, and dominant dermatophyte species vary in Latin America, Africa, and the middle East.5,7, 8, 9 In addition to the geographic location and climate, immunocompetence of the host, pathogenicity of the infectious agent, and availability of medical treatment are all factors that can influence the incidence of fungal infections in a population.6
Disease burden can be measured using the metric of disability-adjusted life years (DALYs), which is the sum of the years of potential life lost due to a disease and the years lost due to disability caused by the disease.10 Furthermore, the sociodemographic index (SDI) is a metric utilized to measure development in different geographic regions by capturing the aspects of income, education, and fertility.11 The utilization of metrics, such as DALYs and SDI, may assist dermatologists and researchers in developing policies and interventions to reduce global health disparities resulting from fungal skin diseases.
Fungal skin diseases were the most prevalent skin disease globally in 2017 (10.09%) and contributed a considerable amount (0.17%) to the total DALYs caused by skin disorders (1.76%).12 In 2016, fungal skin diseases were ranked fourth highest in the incidence of disease (2.1 billion cases) when compared to 328 different diseases and injuries globally.13 Additionally, there is a considerable economic burden associated with dermatomycoses. Dermatophyte infections contributed to over half of all fungal disease outpatient visits in the United States and led to estimated costs totaling up to $802 million in 2017.14 The immense burden of fungal skin diseases must be considered in health policy decisions to improve population health and quality of life.
Information about the burden of dermatomycoses can inform and optimize interventions required to minimize morbidity and the economic impact of those affected. This observational study compares the relationship between the burden of fungal skin disease and socioeconomic status of 195 countries worldwide in 2017.
Methods
Our data were obtained from the publicly available Global Burden of Disease (GBD) datasets from 2017. The GBD database contains data about premature death and disability for over 350 diseases in 195 countries and allows the comparison of the impact of diseases, injuries, and risk factors across age groups, sexes, countries, and regions from 1990 to the present day.11 The GBD project is led by the Institute for Health Metrics and Evaluation at the University of Washington and collaborates with over 145 countries and 3600 researchers worldwide.11 An in-depth protocol is available from the Institute for Health Metrics and Evaluation on how data are obtained, incorporated, calculated, and published in the GBD study.15
We performed a cross-sectional observational study to analyze the global trends of fungal skin diseases in 2017 in 195 countries worldwide using the GBD database. We provided age patterns by sex and the total number of DALYs caused by fungal skin diseases and age-specific DALY rates of fungal skin diseases on a global level and by GBD super regions in 2017 (Figs 1 and 2). Seven super regions were created by GBD based on not only the geographic location but also the country's gross domestic product. Age-standardized DALYs caused by fungal skin disease per 100,000 were also compared to the absolute SDI values of all 195 GBD countries globally in 2017 (Fig 3).
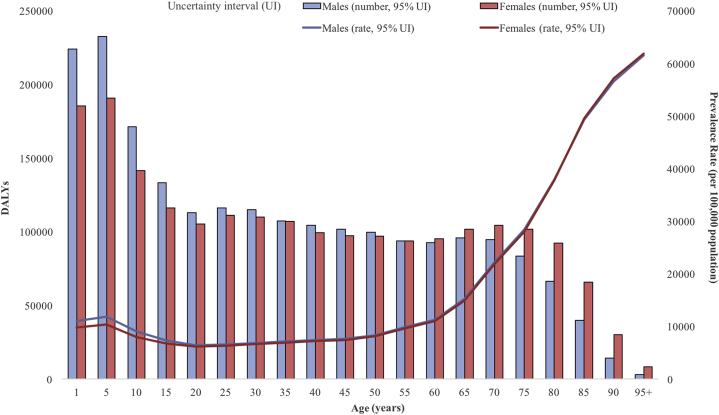
Fig 1.
Global 2017 fungal skin disease age-standardized DALYs per 100,000 people in males and females. DALY, Disability-adjusted life year.
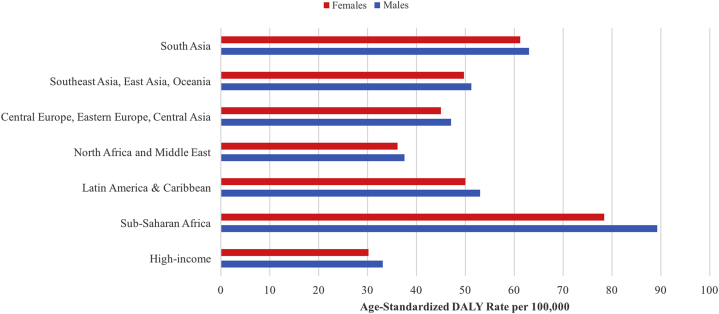
Fig 2.
Age-standardized DALY rate of fungal skin diseases based on sex and geographic GBD super regions in 2017. DALY, Disability-adjusted life year; GBD, global burden of disease.
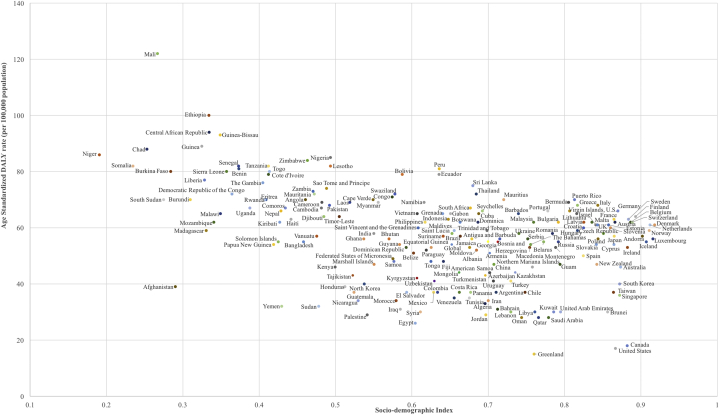
Fig 3.
Age-standardized DALY rates of fungal skin diseases by SDI for 195 countries and territories in 2017. DALY, Disability-adjusted life year; SDI, sociodemographic index.
Results
The GBD 2017 global prevalence of fungal skin disease in both the sexes was approximately 750 million (95% CI 681,568,410.28-808,149,747.09).16 The global age-standardized DALY per 100,000 people with fungal skin disease was 54.86 (95% CI 21.82-114.22) for both the sexes, 56.48 (22.37-117.36) for men, and 53.17 (21.17-110.51) for women.
The age-specific DALYs showed a right-skewed distribution, with fungal skin diseases peaked between 1 and 5 years of age (Fig 1). The increased DALY rate in the age range of 1-5 years attracts attention to the current global health interventions that may not be meeting the needs of children who experience fungal skin disease. Males showed higher DALYs caused by fungal skin diseases at younger ages, but females showed higher DALYs as the population aged. The global age-standardized DALY rates of fungal skin diseases differed between the 7 GBD-defined super regions, but males consistently showed higher DALY burden rates compared to females in all the regions (Fig 2). Sub-Saharan Africa showed the highest DALY rate in both males and females compared to all the super regions (DALY rate 89.3 and 78.42 per 100,000, respectively). The high-income super region, comprising of southern Latin America, western Europe, high-income North America, Australasia, and high-income southern Pacific, exhibited the lowest DALY rate caused by fungal skin diseases (DALY rate 33.12 per 100,000 for males and 30.16 for females).
The top 10 countries with the highest age-standardized DALY rate caused by fungal skin diseases per 100,000 people were Mali (122), Ethiopia (100), central African Republic (94), Guinea-Bissau (93), Guinea (89), Chad (88), Niger (86), Nigeria (85), Zimbabwe (84), and Senegal (82) (Fig 3). Of these countries with the highest fungal burden, Niger, Chad, Mali, and Guinea are among the 10 lowest-SDI countries.17
Discussion
Our results showed that the burden of fungal skin disease was the highest in younger patients, but the prevalence rate was the highest in the elderly population. Fungal skin diseases, such as tinea capitis, are common infections seen in young children worldwide.2 Poor personal hygiene, use of local barbers, and close contact with playmates at school and younger siblings at home lead to an increased risk of disease transmission in this age group.18,19 Superficial fungal infections have also been reported to be more common in males than in females, especially at younger ages, as seen in our study.20 This could be due to the males having more frequent contact with playmates, and they may be less concerned about hygiene and personal grooming compared to females.2,20 Additionally, fungal infections are highly prevalent in the elderly population, varying from 10.4% to 64% in a hospital setting.21,22 In particular, onychomycosis and tinea pedis are more common in middle-aged to elderly men.23,24 Despite this, we showed that elderly patients have the lowest DALYs of fungal disease, indicating that this population does not experience as much burden from their fungal skin infections as younger people.
Fungal infections can significantly decrease the quality of life in patients. In children, the social disapproval and psychological trauma of tinea infections can impact their performance in class.25 Cutaneous fungal infections have also been reported to negatively affect psychosocial well-being in patients by lowering self-esteem and causing embarrassment and social withdrawal.26,27 Additionally, discomfort and inflammation may be associated with fungal infections, resulting in a disability and an inability to perform daily activities.28 If cutaneous fungal diseases are left untreated, secondary infections may occur and cause significant morbidity in patients, such as cellulitis and ulcers.29, 30, 31
Fungal infections have been reported to be the most commonly diagnosed skin disease in Africa.2 This is in agreement with our results, which showed that sub-Saharan Africa and south Asia have the highest global burden of fungal skin disease. Dermatophytes flourish in the keratinized tissue of individuals living in these warm, tropical areas. The resulting cutaneous fungal infections are further exacerbated by wearing clothing for extended periods of time.2 In fact, clothing could present a significant health risk as a vector for dermatophyte transmission. Muthiani et al isolated pathogenic fungi from garments in a popular second-hand clothing market in Kenya.32 Popular disinfecting techniques were applied to the clothing, but they were unable to prevent fungal isolates from growing, likely due to the innate resistance of spores to soaps and detergents. Used clothing markets, which may provide a cheaper clothing option for poorer individuals, have been gaining popularity in several African countries and could be contributing to the rise of endemic cutaneous infections in these regions.32
As previously discussed, dermatophyte species vary by geographic region. However, the prevalence of dermatophytes within different regions are subject to change because of increased migration, tourism, and changes in socioeconomic conditions.1,4,33 For example, T soudanese, T violaceum, and M audouinii are dermatophytes endemic to Africa and Asia but have increased in frequency in North America and Europe in recent years.4 The observation of these epidemiological migrations is critical to the development of interventions to address the high burden of fungal skin diseases.
A cluster of several low-SDI countries was found to have a high burden of fungal skin diseases in our study. The socioeconomic conditions in Africa and certain regions of Asia tended to be poorer compared to those in Europe and the Americas, creating optimal conditions for dermatophytes to spread readily from crowded living conditions, with consistent close human-to-human contact, close proximity to animal vectors, suboptimal hygiene, lack of awareness of dermatophytes, and poor environmental health practices.2,34,35 Additionally, developing countries may have poor health facilities and limited access to health care, resulting in the misdiagnosis or delayed diagnosis of infections.36
There are several important limitations to keep in mind when assessing the global burden of fungal skin diseases. Studies measuring the prevalence and incidence of fungal skin diseases may differ in focus based on the age group, gender category, and target population, making it difficult to compare the data because of different study methodologies. Further limitations include the descriptions of case definitions (eg, self-reported or a physician's or dermatologist's diagnosis) or the definition of prevalence (eg, point prevalence, period prevalence, or lifetime prevalence). The available studies for comparisons among the different GBD regions may be limited by geographic coverage, where certain populations have a relative over- or under-representation of total studies in comparison to their total population. Furthermore, reporting about the global incidence of fungal diseases may be impaired due to the lack of obligatory reporting of fungal diseases, poor diagnostic test performance, and poor clinician suspicion in certain regions.1
The widespread prevalence and therapeutic costs of fungal skin diseases are major public health concerns. Increased education and awareness of dermatophytes are promising steps in reducing the burden and prevalence of fungal skin diseases. These data serve to bring about awareness of cutaneous fungal infections and their substantial impact on the global population.
Footnotes
Funding sources: The GBD was partially funded by the Bill & Melinda Gates Foundation; the funders had no role in the study design, data analysis, data interpretation, or writing of the report.
Conflicts of interest: This research has been conducted as part of the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), co-ordinated by the Institute for Health Metrics and Evaluation. The lead author is a collaborator with the GBD. This article was not developed with consultation or support from the GBD research team. None of the other authors have any conflicts to declare.
IRB approval status: Not applicable.
References
- 1.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. 2017;3(4):57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havlickova B., Czaika V.A., Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51:2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 3.Seth D., Cheldize K., Brown D., Freeman E.F. Global burden of skin disease: inequities and innovations. Curr Dermatol Rep. 2017;6(3):204–210. doi: 10.1007/s13671-017-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol. 2010;28(2):197–201. doi: 10.1016/j.clindermatol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Borman A.M., Campbell C.K., Fraser M., Johnson E.M. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45(2):131–141. doi: 10.1080/13693780601070107. [DOI] [PubMed] [Google Scholar]
- 6.Foster K.W., Ghannoum M.A., Elewski B.E. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50(5):748–752. doi: 10.1016/s0190-9622(03)02117-0. [DOI] [PubMed] [Google Scholar]
- 7.Tao-Xiang N., Zhi-Cheng L., Sao-Mao W., Wen-Zhu L. Analysis of dermatomycoses in Lanzhou district of Northwestern China. Mycopathologia. 2005;160(4):281–284. doi: 10.1007/s11046-005-1156-1. [DOI] [PubMed] [Google Scholar]
- 8.Tan H.H. Superficial fungal infections seen at the National Skin Centre, Singapore. Nihon Ishinkin Gakkai Zasshi. 2005;46(2):77–80. doi: 10.3314/jjmm.46.77. [DOI] [PubMed] [Google Scholar]
- 9.Ellabib M.S., Khalifa Z., Kavanagh K. Dermatophytes and other fungi associated with skin mycoses in Tripoli, Libya. Mycoses. 2002;45(3-4):101–104. doi: 10.1046/j.1439-0507.2002.00731.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Health statistics and information systems. Glob Health Estimates. 2014;13(08):2014. [Google Scholar]
- 11.Institute for Health Metrics and Evaluation Frequently asked questions. http://www.healthdata.org/gbd/faq Accessed March 17, 2020. Available at:
- 12.Mehrmal S., Uppal P., Giesey R.L., Delost G.R. Identifying the prevalence and disability-adjusted life years of the most common dermatoses worldwide. J Am Acad Dermatol. 2020;82(1):258–259. doi: 10.1016/j.jaad.2019.09.066. [DOI] [PubMed] [Google Scholar]
- 13.Vos T., Abajobir A.A., Abate K.H. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedict K., Jackson B.R., Chiller T., Beer K.D. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis. 2019;68(11):1791–1797. doi: 10.1093/cid/ciy776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Protocol for the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) Institute for Health Metrics and Evaluation; 2018. [Google Scholar]
- 16.Institute for Health Metrics and Evaluation (IHME) 2018. Data from: Findings from the Global Burden of Disease Study 2017. [Google Scholar]
- 17.Global Burden of Disease Collaborative Network . Institute for Health Metrics and Evaluation (IHME); Seattle, United States: 2018. Global Burden of Disease Study 2017 (GBD 2017) Socio-Demographic Index (SDI) 1950–2017. [Google Scholar]
- 18.Elewski B.E. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42(1):1–20. doi: 10.1016/s0190-9622(00)90001-x. quiz 21-24. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y.H., Su H.Y., Hsieh Y.J. Survey of infectious skin diseases and skin infestations among primary school students of Taitung County, eastern Taiwan. J Formos Med Assoc. 2000;99(2):128–134. [PubMed] [Google Scholar]
- 20.Oke O.O., Onayemi O., Olasode O.A., Omisore A.G., Oninla O.A. The prevalence and pattern of superficial fungal infections among school children in Ile-Ife, South-Western Nigeria. Dermatol Res Pract. 2014;2014:842917. doi: 10.1155/2014/842917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilgili S.G., Karadag A.S., Ozkol H.U., Calka O., Akdeniz N. The prevalence of skin diseases among the geriatric patients in Eastern Turkey. J Pak Med Assoc. 2012;62(6):535–539. [PubMed] [Google Scholar]
- 22.Watanabe S., Harada T., Hiruma M. Epidemiological survey of foot diseases in Japan: results of 30,000 foot checks by dermatologists. J Dermatol. 2010;37(5):397–406. doi: 10.1111/j.1346-8138.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A.K., Jain H.C., Lynde C.W., Macdonald P., Cooper E.A., Summerbell R.C. Prevalence and epidemiology of onychomycosis in patients visiting physicians' offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43(2):244–248. doi: 10.1067/mjd.2000.104794. [DOI] [PubMed] [Google Scholar]
- 24.Scher R.K. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35(3):S2–S5. doi: 10.1016/s0190-9622(96)90061-4. [DOI] [PubMed] [Google Scholar]
- 25.Chepchirchir A., Bii C., Ndinya-Achola J.O. Dermatophyte infections in primary school children in Kibera slums of Nairobi. East Afr Med J. 2009;86(2):59–68. doi: 10.4314/eamj.v86i2.46934. [DOI] [PubMed] [Google Scholar]
- 26.Chacon A., Franca K., Fernandez A., Nouri K. Psychosocial impact of onychomycosis: a review. Int J Dermatol. 2013;52(11):1300–1307. doi: 10.1111/ijd.12122. [DOI] [PubMed] [Google Scholar]
- 27.Chan H.H., Wong E.T., Yeung C.K. Psychosocial perception of adults with onychomycosis: a blinded, controlled comparison of 1,017 adult Hong Kong residents with or without onychomycosis. Biopsychosoc Med. 2014;8(1):1–9. doi: 10.1186/1751-0759-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elewski B.E. Onychomycosis. Treatment, quality of life, and economic issues. Am J Clin Dermatol. 2000;1(1):19–26. doi: 10.2165/00128071-200001010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Hahnel E., Lichterfeld A., Blume-Peytavi U., Kottner J. The epidemiology of skin conditions in the aged: a systematic review. J Tissue Viability. 2017;26(1):20–28. doi: 10.1016/j.jtv.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Gunaydin S.D., Arikan-Akdagli S., Akova M. Fungal infections of the skin and soft tissue. Curr Opin Infect Dis. 2020;33(2):130–136. doi: 10.1097/QCO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 31.Aly R. Microbial infections of skin and nails. In: Baron S., editor. Medical Microbiology. 4th ed. 1996. [Google Scholar]
- 32.Muthiani Y., Matiru V., Bii C. 2017. Potential Skin Pathogens on Second Hand Clothes and the Effectiveness of Disinfection Methods. [Google Scholar]
- 33.Hay R.J. Tinea Capitis: current Status. Mycopathologia. 2017;182(1-2):87–93. doi: 10.1007/s11046-016-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chikoi R., Nyawale H.A., Mghanga F.P. Magnitude and associated risk factors of superficial skin fungal infection among primary school children in Southern Tanzania. Cureus. 2018;10(7):e2993. doi: 10.7759/cureus.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalu E.I., Wagbatsoma V., Ogbaini-Emovon E., Nwadike V.U., Ojide C.K. Age and sex prevalence of infectious dermatoses among primary school children in a rural South-Eastern Nigerian community. Pan Afr Med J. 2015;20(1):182. doi: 10.11604/pamj.2015.20.182.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nweze E.I., Eke I.E. Dermatophytes and dermatophytosis in the eastern and southern parts of Africa. Med Mycol. 2018;56(1):13–28. doi: 10.1093/mmy/myx025. [DOI] [PubMed] [Google Scholar]