Abstract
Background
Little is known about the effectiveness and drug survival associated with apremilast under real-world conditions.
Objective
To investigate the influence of patient and disease characteristics on drug survival associated with apremilast and to elucidate clinical effectiveness with regard to the psoriasis area and severity index (PASI) reduction.
Methods
This was an observational, retrospective, multicenter analysis from the Austrian Psoriasis Registry.
Results
Data from 367 patients were eligible for analysis. The 12-month drug survival rate associated with apremilast (ie, the proportion of patients on the drug) was 57.3% and decreased significantly in patients younger than 40 years (relative hazard ratio = 1.49, P = .007918). Sex; concomitant arthritis; previous biologic therapy; obesity; and palmoplantar, scalp, nail, and intertriginous involvement did not significantly affect drug survival. At 12 months, the response rates in patients receiving apremilast per protocol with a PASI of 50, 75, 90, and 100 were 80.0%, 56.4%, 38.2%, and 22.7%, respectively.
Limitations
Inclusion of a substantial number of patients with no record of absolute PASI at study entry and lack of PASI reduction follow-up data of 103 patients (28.1%) after starting apremilast treatment.
Conclusion
Apremilast is a robust antipsoriatic drug for which the drug survival is not strongly influenced by most patient- or disease-related factors except age. Drug survival is significantly shorter in patients younger than 40 years.
Key words: apremilast, drug survival, psoriasis
Abbreviations used: HR, hazard ratio; LOCF, last observation carried forward; PASI, psoriasis area and severity index; PP, per protocol; PsoRA, Psoriasis Registry Austria; SD, standard deviation
Capsule Summary.
-
•
Little is known about the effectiveness and factors influencing the drug survival of apremilast.
-
•
Apremilast drug survival is not strongly influenced by most patient or disease-related factors. However, drug survival is significantly shorter in patients younger than 40 years of age.
Introduction
Since its introduction in Europe in 2015, the antipsoriatic drug apremilast has become a valuable treatment option for both moderate-to-severe plaque psoriasis and psoriatic arthritis.1, 2, 3, 4 It is especially useful for patients in whom the use of biologic drugs is to be avoided (eg, those with cancer, latent tuberculosis infection, or infective hepatitis)5, 6, 7 or in those with psoriasis-related diseases such as palmoplantar pustulosis.8 However, little is known about the drug survival associated with apremilast (ie, the proportion of patients on apremilast treatment at certain time points), effectiveness, and safety in real-world patients.9, 10, 11, 12, 13, 14 Biologic treatments for psoriasis tend to perform more poorly in real-world settings than in clinical trials. Therefore, it is important to evaluate the long-term effectiveness and drug survival of small molecules such as apremilast.15, 16, 17, 18
We use the term “drug survival” as it best reflects real-life outcomes by encompassing many reasons for treatment discontinuation that are both related and unrelated to the drug performance, including safety reasons19,20 (ie, adverse events), pregnancy, complete remission or lack of improvement, denial of reimbursement, availability of alternative treatment options, increasing expectations of physicians and patients, or unconsidered patient needs.21, 22, 23
Most biologics have similar overall drug survival rates (per drug within a certain range), but the 12-month survival rates of apremilast range widely by study, from 2.6% to 55.4%.24,25 Decreased biologic drug survival is associated with female sex, previous biologic exposure, and obesity.26 For most biologics, metabolic conditions (ie, hypertension, diabetes, and metabolic syndrome and its associated comorbidities) increase the risk of treatment discontinuation, although this was not the case for apremilast in a previous study.27 However, 1 study has shown that the risk of apremilast discontinuation does increase in obese patients receiving it [hazard ratio (HR): 1.2].25 The risk of apremilast discontinuation also appears to increase in patients with palmoplantar pustulosis suffering from depression8 but not in patients with concomitant psoriatic arthritis.28 Note, however, that most studies of apremilast drug survival (except 1 study from Spain with 377 patients)25 enrolled relatively few patients (ie, 35, 94, and 138 patients) and were therefore insufficiently powered to fully determine what parameters influence drug survival.8,28,29
Therefore, we aimed to evaluate the influence of patient and disease characteristics on apremilast drug survival and the effectiveness of apremilast in reducing the extent and severity of psoriasis in a large psoriasis registry.
Methods
Analytical design
This study was an observational retrospective multicenter analysis of clinical data extracted from the Austrian Psoriasis Registry (PsoRA) on November 30, 2019. The design of this nationwide Austrian database has been described previously.30, 31, 32, 33, 34 Detailed information about PsoRA is available at www.psoriasisregistry.at. The registry defines 1 treatment as the time from a patient's allocation to a specific therapy, followed by at least 1 visit, until the last observation or discontinuation of treatment. For every visit entered in the registry, the continuous prescription of a drug has to be confirmed; otherwise, the reason for treatment discontinuation has to be entered. PsoRA also collects data on the psoriasis area and severity index (PASI), which can be entered at the start of treatment and at every recorded visit. This allows the automatic calculation of the percent PASI change from baseline, ranging from complete remission (PASI 100) to partial remission (PASI 90, PASI 75, PASI 50, and PASI <50) to worsening. For patients with a missing PASI at baseline (at treatment start), the PASI reduction category can be manually entered at each visit thereafter. The registry was approved by the ethics committee of the Medical University of Graz (application number 21-094 ex 09/10). The present analysis was conducted in accordance with the principles of the Declaration of Helsinki.
Data analysis and statistics
All patients >18 years of age who had psoriasis of any clinical type started apremilast before November 2019 and had at least 1 follow-up visit were eligible for this study, irrespective of previous systemic treatment, psoriatic arthritis, or comorbidities. Drug survival was calculated using Kaplan-Meier estimates and log-rank tests. Patients were censored at the last date of follow-up if the end of treatment had not occurred until then. Relative HRs were calculated for patient characteristics [sex, age at therapy start (<40 vs ≥40 years of age), body mass index (BMI, <30 vs ≥30), concomitant psoriatic arthritis, biologic naïvety], and disease characteristics (palmar and/or plantar, scalp, nail, or inverse involvement). For the purposes of this analysis, patients with an unknown history of concomitant arthritis were considered not to have psoriatic arthritis.
The effectiveness of apremilast treatment was evaluated in terms of the absolute change in PASI and reduction in PASI. The change in PASI was calculated and analyzed per protocol (PP) and per last observation carried forward (LOCF) together with worst-case analysis by considering all patients with no follow-up as treatment failures (ie <PASI 50). Patients included in the PP analysis received no concomitant systemic therapy or phototherapy; for those included in the LOCF analysis, we carried forward their PASI score from the last visit at discontinuing apremilast or starting concomitant systemic therapy or phototherapy. The chi-square test was used to test for differences in concomitant psoriatic arthritis prevalence by sex and for differences in treatment discontinuation by age at treatment start (<40 vs ≥40 years of age). Calculations were performed using R 3.6.2 (www.r-project.org) with the statistical analysis package survival 3.1-8.
Results
General patient characteristics
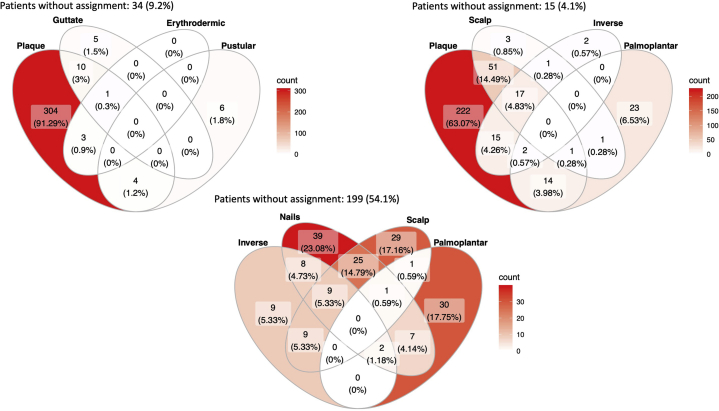
At the time of data extraction, PsoRA contained data on 4348 patients who had undergone a total of 7002 systemic treatments. A total of 367 patients, including 138 (37.6%) women and 229 (62.4%) men, had received apremilast and were eligible for this analysis (Table I), and at least 1 follow-up visit had been recorded for 264 (71.9%) patients. Concomitant psoriatic arthritis was present in 89 (24.3%) patients and of unknown status in 20 patients (5.4%) (Table I). The prevalence of psoriatic arthritis did not differ by sex (P = .21) (Table II). At the start of apremilast treatment, the mean age (standard deviation, SD) was 50.0 years ± 15.0, and a large proportion of patients (28.1%) were <40 years of age (Table I). Other characteristics of the patients at the start of treatment, such as disease duration, weight, BMI, and concomitant psoriatic arthritis, are summarized in Table I. The most common psoriasis type was plaque (322 patients, 87.7%). Nail psoriasis or involvement was present in 91 (24.8%) patients, and scalp psoriasis or involvement was present in 74 (20.2%) (Table III and Fig 1). Previous treatments had been administered to 305 (83.1%) of patients, of which UVB phototherapy (20.3%), fumaric acid (19.6%), methotrexate (20.1%), and biologics (15.5%) were most frequent (Table IV).
Table I.
Patient characteristics
| Number of patients | 367 |
| Women (%) | 138 (37.6) |
| Men (%) | 229 (62.4) |
| Age (years), mean (SD) | 50.0 (±15.0) |
| Age < 40 years (%) | 103 (28.1) |
| Number (%) of patients with psoriatic arthritis∗ | 89 (24.3) |
| BMI, mean (SD) | 28.5 (±6.3) |
| PASI, mean (SD) | 7.0 (±6.4) |
| PASI (non-naïve), mean (SD) | 8.0 (±7.6) |
BMI, Body mass index; PASI, psoriasis area and severity index; SD, standard deviation.
For 20 (5.4%) patients, presence and/or history of psoriatic arthritis was unknown.
Table II.
Prevalence of psoriatic arthritis∗
| Sex | Number (%) of patients |
||
|---|---|---|---|
| All | Without arthritis | With arthritis | |
| Male | 229 | 179 (78.2) | 50 (21.8) |
| Female | 138 | 99 (71.7) | 39 (28.3) |
Prevalence numbers (percentages) of all patients (N = 367) regarding concomitant arthritis and sex. A chi-square test indicated no significant differences between patients with or without psoriatic arthritis with respect to sex (P = .21).
Table III.
Psoriasis types
| Psoriasis type | Plaque | Guttata | Erythrodermic | Pustular | Palmar and/or plantar | Inverse | Nails | Scalp |
|---|---|---|---|---|---|---|---|---|
| Plaque | 322∗ | |||||||
| Guttata | 11 | 16 | ||||||
| Erythrodermic | 4 | 1 | 4 | |||||
| Pustular | 4 | NA | NA | 10 | ||||
| Palmar and/or plantar | 17 | NA | 1 | 10 | 41 | |||
| Inverse | 34 | 3 | 1 | 1 | 2 | 37 | ||
| Nails | 73 | 1 | 0 | 3 | 10 | 19 | 91 | |
| Scalp | 69 | 3 | 0 | 1 | 2 | 18 | 35 | 74 |
NA, Not applicable.
Numbers in bold represent the total numbers of patients with certain types of psoriasis. Some patients had more than one type of psoriasis thus the total number of specific types of psoriasis exceeds the total number of patients (N = 367).
Fig 1.
Distribution of psoriasis types. Distribution numbers (%) of patients regarding psoriasis types and body site involvement (N = 367).
Table IV.
Previous treatments
| Previous systemic treatment | Number (%) of patients with previous systemic treatment or not∗ | Type of treatment | Number (%) of administered treatments† | |
|---|---|---|---|---|
| Yes | 305 (83.1) | Phototherapy | UVB | 87 (20.3) |
| PUVA | 49 (11.4) | |||
| Conventional systemic | Cyclosporine | 6 (1.4) | ||
| Fumaric acid | 84 (19.6) | |||
| Methotrexate | 86 (20.1) | |||
| Retinoids | 30 (7.0) | |||
| Biologics | Adalimumab | 16 (3.7) | ||
| Etanercept | 19 (4.4) | |||
| Golimumab | 1 (0.2) | |||
| Infliximab | 2 (0.5) | |||
| Ixekizumab | 1 (0.2) | |||
| Secukinumab | 10 (2.3) | |||
| Ustekinumab | 18 (4.2) | |||
| Other | 19 (4.4) | |||
| Total number of treatments | 428 (100) | |||
| No | 62 (16.9) | NA | NA | |
NA, Not applicable; PUVA, psoralen plus ultraviolet A; UVB, ultraviolet B.
Percentages of patients with (N = 305, 83.1%) and without (N = 62, 16.9%) therapy before starting apremilast.
Certain patients received more than one previous treatment; thus the total number of specific treatment (N = 428) for psoriasis exceeds the total number of patients who had received previous treatment.
Effectiveness
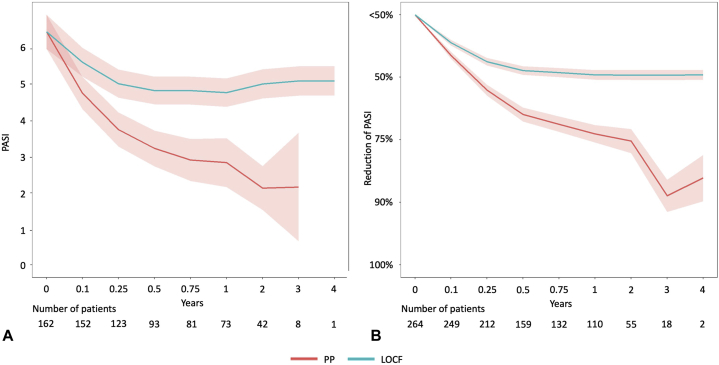
PASI values at the start of treatment were documented for 162 (44.1%) patients. The mean (SD) PASI of those patients at treatment start was 6.48 (±6.37) (Fig 2 and Table V). In the PP analysis, the mean (SD) PASI was 3.76 (±5.58) at 3 months and improved to 2.84 (±6.13) at 12 months. In the LOCF analysis, the mean (SD) PASI was 5.04 (±5.96) at 3 months and did not improve much until 12 months and beyond (until last observation) (Fig 2 and Table V).
Fig 2.
Effectiveness of apremilast. A, Absolute PASI value (± 95% confidence interval) and (B) mean PASI reduction score (± 95% confidence interval) plotted over time for patients analyzed in PP (red line) and LOCF (blue line). LOCF, Last observation carried forward; PASI, psoriasis area and severity index; PP, per protocol.
Table V.
Effectiveness of apremilast
| Timepoint (months) | PASI, mean (SD) |
PASI reduction category, mean (SD) |
||
|---|---|---|---|---|
| PP | LOCF | PP | LOCF | |
| 0 | 6.48 (6.37) | 6.48 (6.37) | NA | NA |
| 3 | 3.76 (5.58) | 5.04 (5.96) | 3.79 (1.33) | 4.25 (1.26) |
| 6 | 3.24 (5.02) | 4.85 (5.94) | 3.40 (1.46) | 4.07 (1.48) |
| 12 | 2.84 (6.13) | 4.79 (6.21) | 3.09 (1.54) | 4.03 (1.54) |
| 24 | 2.14 (4.15) | 5.03 (6.43) | 2.98 (1.50) | 4.03 (1.58) |
| 36 | 2.16 (4.50) | 5.12 (6.46) | 2.11 (1.14) | 4.03 (1.62) |
| 48 | NA | 5.12 (6.48) | 2.39 (0.55) | 4.04 (1.61) |
LOCF, Last observation carried forward/worst-case scenario; NA, not applicable; PASI, psoriasis area and severity index; PP, per protocol; SD, standard deviation. PASI reduction category is defined as follows: 5 (<50%), 4 (50% to <75%), 3 (75% to <90%), 2 (90% to <100%) and 1 (100%).
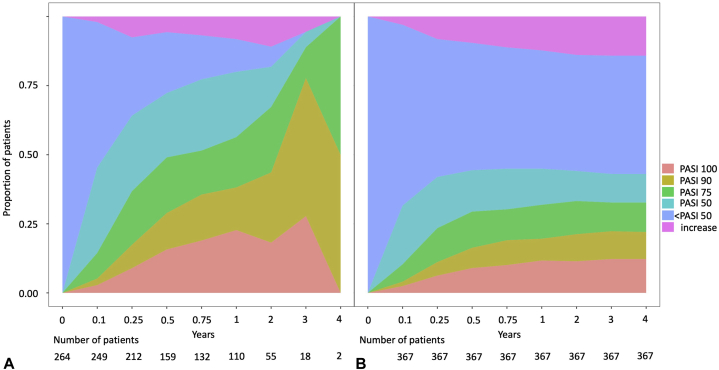
In the PP analysis, the mean (SD) PASI reduction score was 3.79 (±1.33) at 3 months, which improved to 3.09 (±1.54) at 12 months. In the LOCF analysis, the PASI reduction score was 4.25 (±1.26) at 3 months, which improved slightly to 4.03 (±1.54) at 12 months (Fig 2 and Table V). Three months after the start of treatment, 9.0% of patients in the PP analysis had achieved a complete remission of psoriatic plaques and 36.8% had achieved a PASI 75 reduction (Fig 3 and Table VI). After the first treatment year, complete remission was observed in 22.7% of patients and partial remission (PASI 75) was observed in 56.4% of patients in the PP analysis (Fig 3 and Table VI).
Fig 3.
Achievement of skin goals. Relative number of PP (A) and LOCF/worst-case scenario (B) patient treatment cycles in which a certain PASI improvement was achieved, plotted over time. LOCF, Last observation carried forward; PASI, psoriasis area and severity index; PP, per protocol.
Table VI.
Achievement of treatment goals
| Timepoint (months) | Number of patients (PP/LOCF) | Percentage of patients achieving a certain PASI reduction (PP/LOCF) |
|||||
|---|---|---|---|---|---|---|---|
| PASI 100 | >PASI 90 | >PASI 75 | >PASI 50 | <PASI 50 | Increase of PASI | ||
| 3 | 212/367 | 9.0/6.3 | 17.5/11.2 | 36.8/23.5 | 64.2/42.0 | 28.3/49.9 | 7.5/8.2 |
| 6 | 159/367 | 15.7/9.0 | 28.9/16.4 | 49.0/29.5 | 72.3/44.5 | 22.0/46.0 | 5.7/9.5 |
| 12 | 110/367 | 22.7/11.7 | 38.2/19.6 | 56.4/31.9 | 80.0/45.0 | 11.8/42.8 | 8.2/12.3 |
| 24 | 55/367 | 18.2/11.4 | 43.7/21.2 | 67.3/33.2 | 81.8/44.1 | 7.3/42.0 | 10.9/13.9 |
| 36 | 18/367 | 27.8/12.3 | 77.8/22.4 | 88.9/32.8 | 94.5/43.2 | NA/42.8 | 5.6/14.2 |
| 48 | 2/367 | NA/12.3 | 50.0/22.1 | 100/32.7 | NA/43.1 | NA/42.8 | NA/14.2 |
LOCF, Last observation carried forward; NA, not applicable; PASI, psoriasis area and severity index; PP, per protocol.
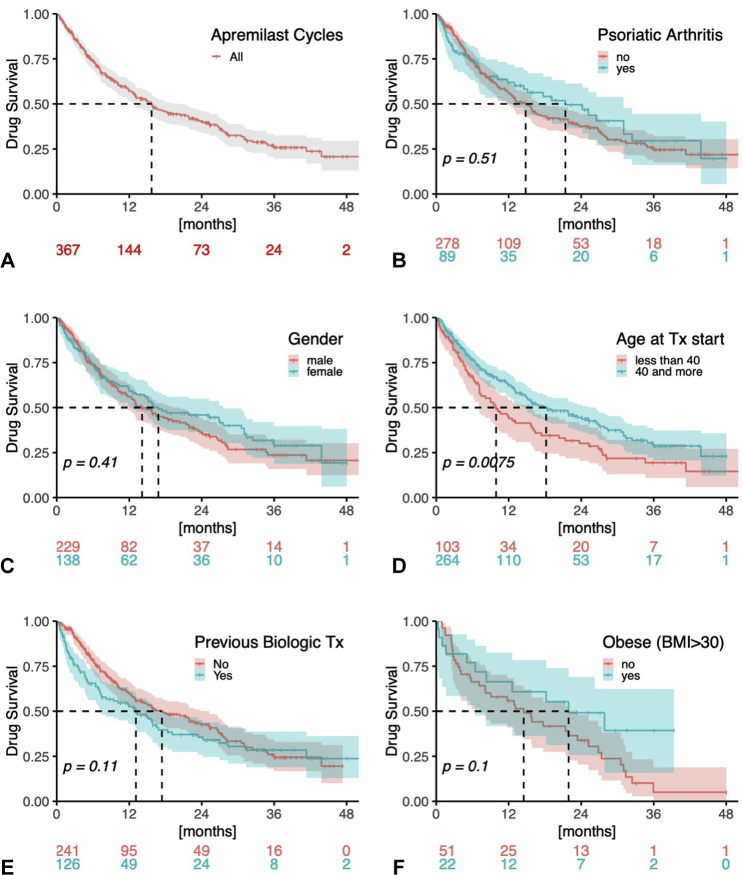
Drug survival
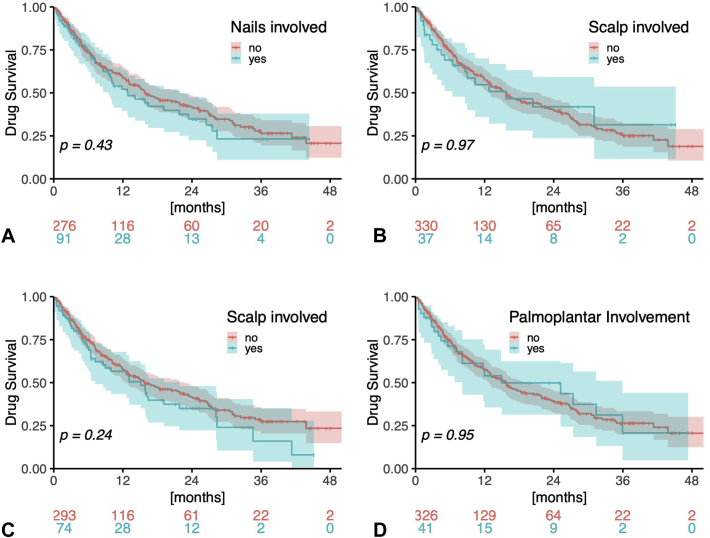
The overall drug survival rate at 12 months was 57.3%, and the median survival was 15.7 months (Fig 4 and Table VIII). Five patients (1.4%) temporarily paused apremilast treatment (for up to several weeks) mainly to observe whether or not psoriasis would reoccur. Most of the patient characteristics (female sex, concomitant psoriatic arthritis, BMI, and biologic naïvety) and disease characteristics (scalp, nail, inverse or palmar, and/or plantar involvement) analyzed were not significantly associated with an increased risk of drug discontinuation (Figs 4 and 5 and Table VIII). However, an age <40 years at treatment start was significantly associated with an increased risk of treatment discontinuation [relative HR (CI): 1.493 (1.111-2.007), P = .007918) (Fig 4 and Table VIII)]. An analysis for confounding factors revealed that a significantly higher proportion of patients <40 years at treatment start suffered from inverse (48.7% vs 7.2%, P = .004) and scalp (33.0% vs 15.2%, P = .000127) involvement (Table IX). In addition, a higher percentage of patients ≥40 years had psoriatic arthritis (29.2% vs 11.7%, P = .001).
Fig 4.
Drug survival of apremilast. Relative drug survival rates (± 95% confidence intervals) of apremilast (N = 367) with regard to different factors possibly influencing survival, using Kaplan-Meier estimates and log-rank tests.
Table VIII.
Risk ratios for apremilast discontinuation
| Risk factor | Relative risk (CI) | P value |
|---|---|---|
| Female sex | 0.885 (0.662-1.182) | .4077 |
| Concomitant psoriatic arthritis | 1.095 (0.777-1.542) | .6046 |
| Age <40 years at start of treatment | 1.493 (1.111-2.007) | .007918∗ |
| BMI ≥30 | 0.576 (0.294-1.128) | .1075 |
| Previous biologic treatment | 1.269 (0.949-1.696) | .1083 |
| Palmar and/or plantar involvement | 0.986 (0.627-1.551) | .9526 |
| Scalp involvement | 1.228 (0.872-1.729) | .2396 |
| Nail involvement | 1.143 (0.821-1.593) | .4288 |
| Inverse involvement | 0.989 (0.616-1.590) | .9662 |
BMI, Body mass index; CI, confidence interval.
Significant P values are in bold.
Fig 5.
Drug survival regarding body site involvement. Relative drug survival rates (± 95% confidence intervals) of apremilast (N = 367) with regard to the involvement of body sites that possibly influence survival, using Kaplan-Meier estimates and log-rank tests.
Table IX.
Patient and disease characteristics regarding age
| Characteristics | Number (%) of patients/mean value (SD) |
P value | |
|---|---|---|---|
| <40 years (N = 103) | ≥40 years (N = 264) | ||
| Patient characteristics | .403 | ||
| Sex | |||
| Male | 68 (66.0) | 161 (61.0) | |
| Female | 35 (34.0) | 103 (39.0) | |
| Arthritis | |||
| No | 91 (88.3) | 187 (70.8) | .001∗ |
| Yes | 12 (11.7) | 77 (29.2) | |
| PASI at therapy start | 6.9 (5.5) | 7.0 (6.7) | .929 |
| BMI | 26.6 (7.5) | 29.3 (5.6) | .095 |
| Biologic naïvety | |||
| No | 69 (67.0%) | 172 (65.2) | .807 |
| Yes | 34 (33.0) | 92 (34.8) | |
| Disease characteristics | |||
| Palmar and/or plantar involvement | |||
| No | 94 (91.3) | 232 (87.9) | .368 |
| Yes | 9 (8.7) | 32 (12.1) | |
| Scalp involvement | |||
| No | 69 (67.0) | 224 (84.8) | .000127∗ |
| Yes | 34 (33.0) | 40 (15.2) | |
| Nail involvement | |||
| No | 77 (74.8) | 199 (75.4) | 1.000 |
| Yes | 26 (25.2) | 65 (24.6) | |
| Inverse involvement | |||
| No | 19 (51.3) | 245 (92.8) | .004∗ |
| Yes | 18 (48.7) | 19 (7.2) | |
BMI, Body mass index; PASI, psoriasis area and severity index; SD, standard deviation.
Significant P values are in bold. N = 367
Reasons for treatment discontinuation
Treatment was stopped early in 195 (53.1%) patients (Table X). In an analysis by the number of stopped treatments, the most common reasons for treatment discontinuation were primary therapeutic failure (ie, no skin improvement at all, 32.3%), side effects (31.3%), and secondary loss of efficacy (ie, relapse after initial skin improvement, 20.5%) (Table X). In an analysis by patient number, gastrointestinal symptoms (8.7%) were the most frequently occurring side effects with regard to the total patient number. Eleven patients (2.9%), including 5 women and 6 men, stopped treatment because of depression (including potential signs of depression such as dysthymia, energy loss, and sleeping changes) (Table XI). Ten of those patients (90.9%) were >40 years of age. One patient in whom depression had been previously diagnosed reported suicidal ideation. Other common side effects leading to treatment discontinuation were headache (2.1%) and infection (1.1%). Seven (1.9%) patients discontinued treatment due to ≥2 side effects (Table XI). An analysis of the reason for treatment discontinuation (ie, primary and secondary treatment failure, side effects, patient request, denial of reimbursement) with regard to patients age (<40 vs ≥40 years at treatment start) revealed no differences (Table XII).
Table X.
Reason for drug discontinuation∗
| Reason for treatment discontinuation | Number (%) of discontinued treatment cycles per stopped/per total treatments |
|---|---|
| Remission | |
| Complete | NA |
| None | 43 (22.1/11.7) |
| Partial | 20 (10.3/5.5) |
| No and partial | 63 (32.3/17.2) |
| Loss of efficacy | 40 (20.5/10.9) |
| Denial of reimbursement | 2 (1.0/0.5) |
| Patient request | 13 (6.6/3.5) |
| Pregnancy | NA |
| Side Effect | 61 (31.3/16.6) |
| Other | 16 (8.2/4.4) |
| All | 195 (100/53.1) |
NA, Not applicable.
Total number of patients and treatments (N = 367).
Table XI.
Reason for treatment discontinuation due to side effects∗
| Type of side effect | Number (%) of discontinued treatments∗ (per total number of stopped treatments†/per total treatments‡) |
|---|---|
| Depression | 11 (5.6/2.9) |
| Gastrointestinal symptoms | 32 (16.3/8.7) |
| Headache | 8 (4.1/2.1) |
| Infection | 4 (2.0/1.1) |
| Liver toxicity | 1 (0.5/0.3) |
| Kidney toxicity | 1 (0.5/0.3) |
| Neurological symptoms | 2 (1.0/0.5) |
| Sleep disorder | 2 (1.0/0.5) |
| Rash | 1 (0.5/0.3) |
| Skin cancer | 1 (0.5/0.3) |
| Other cancer | 1 (0.5/0.3) |
| Other | 5 (2.5/1.3) |
Number of patients (N = 61) who discontinued apremilast due to side effects (N = 69).
Total number of stopped treatments (N = 195).
Total number of patients and treatments (N = 367). Note that treatment was stopped due to 2 side effects in 6 patients and due to 3 side effects in 1 patient.
Table XII.
Reason for treatment discontinuation regarding age
| Reason for treatment discontinuation | Number (%) of discontinued treatment cycles per stopped stopped/per total treatments∗ |
|
|---|---|---|
| <40 years | ≥40 years | |
| Remission | ||
| Complete | NA | NA |
| None | 13 (19.4/12.6) | 30 (23.4/11.3) |
| Partial | 9 (13.4/8.7) | 11 (8.6/4.2) |
| No and partial | 22 (32.8/21.3) | 41 (32.9/15.5) |
| Loss of efficacy | 15 (22.4/14.6) | 25 (19.5/9.5) |
| Denial of reimbursement | 1 (1.5/0.9) | 1 (0.8/0.4) |
| Patient request | 5 (7.5/4.8) | 8 (6.3/3.0) |
| Pregnancy | NA | NA |
| Side Effect | 20 (29.9/19.4) | 41 (32.0/15.5) |
| Other | 4 (6.0/3.9) | 12 (9.4/4.5) |
| All | 67/103 | 128/264 |
NA, Not applicable.
Prevalence numbers (percentages) of all patients (N = 367) regarding the reason for treatment discontinuation in patients < or ≥40 years of age at the start of therapy. The chi-square test indicates no significant differences in patients with or without psoriatic arthritis regarding sex (P = .21).
Most patients who discontinued apremilast treatment were subsequently treated with biologics (61.6%). Those most frequently used were ustekinumab (29.2%), ixekizumab (11.3%), and secukinumab (10.3%) (Table XIII).
Table XIII.
Treatments after apremilast discontinuation
| Treatment discontinuation | Number (%) of patients with systemic treatment or not∗ | Type of treatment | Number (%) of treatments | |
|---|---|---|---|---|
| Yes | 195 (53.1) | Phototherapy | UVB | 1 (0.5) |
| PUVA | 2 (1.0) | |||
| Conventional systemic | Fumaric acid | 4 (2.1) | ||
| Methotrexate | 12 (6.2) | |||
| Retinoids | 3 (1.5) | |||
| Biologics | Adalimumab | 9 (4.6) | ||
| Brodalumab | 6 (3.1) | |||
| Etanercept | 4 (2.1) | |||
| Guselkumab | 7 (3.6) | |||
| Ixekizumab | 22 (11.3) | |||
| Risankizumab | 3 (1.5) | |||
| Secukinumab | 20 (10.3) | |||
| Tildrakizumab | 1 (0.5) | |||
| Ustekinumab | 57 (29.2) | |||
| All biologics | 120 (61.6) | |||
| Other | 1 (0.5) | |||
| No treatment specified | 43 (22.1) | |||
| No | 172 (46.9) | NA | NA | |
NA, Not applicable; PUVA, psoralen plus ultraviolet A; UVB, ultraviolet B.
Percentages of patients starting with another treatment after apremilast discontinuation. Certain patients received more than one biologic treatment after apremilast discontinuation, therefore the total number of biologics (N = 129) exceeds the total number of patients who had received a biologic (N = 120).
Discussion
This analysis of 367 patients is one of the largest registry-based studies of effectiveness and drug survival in patients treated with apremilast. Our analysis of treatment sequences helped us to evaluate the role of apremilast in psoriasis treatment. UVB-phototherapy (20.3%) and PUVA (11.4%), as well as fumaric acid (19.6%) and methotrexate (20.1%) as traditional systemic agents were the most frequently administered treatments before apremilast (Table IV); biologic therapy (61.6%) was the most frequently administered treatment after apremilast discontinuation (Table XIII).
As shown by PP analysis, apremilast was clinically effective when evaluated in terms of PASI reduction. At 3 months after treatment start, PASI 100 had been achieved in 9.0% of patients, PASI 90 in 17.5%, PASI 75 in 36.8%, and PASI 50 in 64.2% (Table VI). At 12 months, the rates had increased to PASI 100 in 22.7%, PASI 90 in 38.2%, PASI 75 in 56.4%, and PASI 50 in 80.0% (Table VI). Similar findings for PASI 75 and PASI 90 responses at 3 and 12 months were recently reported from Spanish and Italian cohorts.25,35 However, in our LOCF/worst-case scenario analysis, the clinical effectiveness of apremilast plateaued at 3 to 6 months after treatment start (Figs 2 and 3), in accordance with recently published guidelines suggesting that drug effectiveness should be evaluated at 16 weeks after the start of treatment.5
Overall, the drug survival rate at 12 months in our study was 57.3%. This is in the upper range of previously published results (Table VII), which vary widely due to presumed differences in the methodical approaches used by the groups reporting them. For instance, lower 12-month survival rates were detected in insurance claims databases from France (30.7%) and the United States (2.6%)24,36 and in the Slovenian psoriasis registry (20.0%).13 However, rates similar to ours were seen in retrospective observational studies from Spain (54.9%)25 and Japan (53.4%),28 although the apremilast-treated cohorts in most of those studies were smaller than ours.
Table VII.
Drug survival with regard to different characteristics
| Characteristics | Drug survival rates [percentage (CI)] for a specific drug∗ |
Median drug survival (CI) | ||
|---|---|---|---|---|
| 3 months | 6 months | 12 months | ||
| Patient characteristics | ||||
| Sex | ||||
| Male | 88.2 (83.1-91.8) | 74.2 (67.6-79.6) | 56.0 (48.4-62.9) | 14.1 (11.5-20-3) |
| Female | 83.0 (75.5-88.3) | 74.1 (65.7-80.8) | 59.1 (49.8-67.3) | 16.8 (12.0-27.5) |
| Arthritis | ||||
| No | 88.3 (83.6-91.7) | 75.0 (69.0-80.1) | 56.4 (49.4-62.8) | 14.8 (11.9-17.4) |
| Yes | 79.2 (69.1-86.4) | 74.0 (63.2-82.1) | 61.9 (49.9-71.8) | 21.4 (11.8-31-1) |
| Age at therapy start | ||||
| ≥40 years | 87.8 (83.2-91.3) | 76.7 (70.9-81.6) | 62.6 (55.8-68.6) | 18.2 (14.5-25.2) |
| <40 years | 81.9 (72.8-88.2) | 67.4 (56.9-75.9) | 44.0 (3.3-54.2) | 9.9 (7.1-15.8) |
| BMI | ||||
| <30 | 78.4 (64.4-87.4) | 66.3 (51.5-77.5) | 55.9 (41.0-68.4) | 14.5 (7.1-23.4) |
| ≥30 | 81.8 (58.5-92.8) | 77.0 (53.2-89.7) | 66.3 (41.8-82.5) | 21.9 (6.5-NA) |
| Biologic naïvety | ||||
| No | 76.2 (67.6-82.8) | 65.5 (56.2-73.3) | 52.5 (42.8-61.4) | 13.1 (7.3-16.8) |
| Yes | 91.4 (86.9-94.3) | 78.6 (72.5-83.5) | 59.6 (52.3-66.2) | 17.4 (12.9-25.2) |
| Disease characteristics | ||||
| Palmar and/or plantar involvement | ||||
| No | 86.4 (82.1-89.7) | 74.3 (68.9-78.8) | 57.5 (51.3-63.1) | 15.7 (12.8-19.1) |
| Yes | 82.5 (66.7-91.3) | 71.2 (53.9-83.0) | 54.0 (35.6-69.2) | 15.0 (8.1-35.9) |
| Scalp involvement | ||||
| No | 86.9 (82.4-90.4) | 75.0 (69.4-79.8) | 57.4 (50.9-63.4) | 15.8 (12.4-22.8) |
| Yes | 83.1 (72.2-90.1) | 70.7 (58.2-80.0) | 56.6 (43.4-67.9) | 15.1 (7.2-21.8) |
| Nail involvement | ||||
| No | 86.5 (81.8-90.1) | 74.0 (68.2-79.0) | 58.8 (52.2-64.8) | 15.9 (13.1-21.9) |
| Yes | 85.2 (75.9-91.1) | 74.7 (63.8-82.8) | 52.1 (39.5-63.2) | 12.9 (9.5-21.8) |
| Inverse involvement | ||||
| No | 87.1 (82.9-90.4) | 74.7 (69.4-79.2) | 57.6 (51.4-63.2) | 15.8 (12.9-21.4) |
| Yes | 78.0 (60.8-88.4) | 69.1 (51.1-81.6) | 54.7 (35.9-70.1) | 15.7 (6.4-NA) |
| Overall survival per drug | 86.2 (82.1-89.4) | 74.1 (69.1-78.5) | 57.3 (51.5-62.6) | 15.7 (12.8-20.3) |
CI, Confidence interval; NA, not applicable.
Percentages (confidence interval) of drug survival at 12 months (N = 367).
Furthermore, our analysis indicates that apremilast is a robust antipsoriatic drug for which drug survival is not strongly influenced by most patient or disease-related factors (Figs 4, 5, and Tables VII, VIII). For instance, previous studies of biologics identified female sex as an independent risk factor for treatment discontinuation; however, this was not the case for apremilast in our study. Moreover, the drug survival of apremilast was not influenced by previous biologic exposure, obesity, concomitant psoriatic arthritis, or clinical psoriasis type in our study (Figs 4, 5, and Tables VII, VIII). However, drug survival was significantly influenced by the age at treatment start. When compared with patients aged ≥40 years, those <40 years at the start of treatment had an increased risk of treatment discontinuation (relative HR: 1.49, P = .007918) (Fig 4 and Table VIII) and had a significantly higher rate of inverse (48.7% vs 7.2%) and scalp (33.0% vs 15.2%) involvement (Table IX). However, a statistical subgroup analysis of a potential interaction between age and psoriasis type would have been underpowered, and therefore, we did not perform this investigation. Although data on the effects of age on biologic and nonbiologic drug survival are limited,26 it is well known that younger patients place more importance on clinical efficacy than do older patients, as this enables the former group to lead normal working lives, feel comfortable being in public, be less burdened in partnerships and have normal sex lives23; therefore, younger patients may be tempted to discontinue apremilast more quickly for a lack of effectiveness. Furthermore, the increased inverse and scalp involvement in younger patients may have additionally contributed to worse drug survival in patients <40 years old (Table IX). Moreover, a significantly higher percentage of patients ≥40 years of age had psoriatic arthritis (29.2% vs 11.7%), which possibly contributed to prolonged drug survival in this group, as increased drug survival was previously observed for patients with psoriatic arthritis and biologic treatment.26 Overall, the age-dependent decrease in drug survival among conventional systemic therapies in younger patients was described in a retrospective database analysis for psoriasis patients receiving acitretin (HR: 0.992 per year) and methotrexate (HR: 0.99 per year) in Israel.37
The main reasons for drug discontinuation in our analysis were primary treatment failure (32.3%), secondary loss of efficacy (20.5%), and side effects (31.3%) (Table X). While the observed rates of primary and secondary treatment failure are in the ranges of previously published results, the rate of drug discontinuation due to side effects is higher (31.3% vs 5.1-26.9%).25,28,38, 39, 40, 41, 42, 43 Gastrointestinal symptoms (8.7%) were the most common side effects, followed by headache (2.1%) and infection (1.1%) (Table XI). Eleven patients (2.9%) stopped apremilast because of signs of depression, beginning depression, or worsening depression, and 1 patient reported suicidal ideation. When we compared the treatment discontinuation rates for apremilast in this analysis with those in previously reported studies, we observed similar rates of discontinuation due to depression and headache44,45 but a lower rate of discontinuation due to gastrointestinal symptoms in our study (8.7% vs 13.0-19.2%).44,45
Limitations
No PASI follow-up data were available for 28.1% of patients after the start of apremilast (Fig 2, B). Our analysis of effectiveness included a substantial number of patients who had no record of absolute PASI at therapy start (Fig 2, A). However, a much higher proportion of patients had documented PASI reduction values throughout our follow-up period (Fig 2, B).
Conclusions
Apremilast is a robust antipsoriatic drug for which the drug survival is not strongly influenced by the psoriasis subtype; female sex; obesity; psoriatic arthritis; previous biologic exposure; or palmoplantar, nail, scalp, and inverse involvement. However, drug survival is decreased in patients <40 years of age. Furthermore, apremilast seems to be an effective treatment option, although it does not target a specific cytokine or receptor. However, factors predicting the therapeutic response remain to be identified.
Acknowledgments
We thank the patients participating in PsoRA and all members and investigators of PsoRA (see https://psora.medunigraz.at/) who provided data for this analysis. Special thanks go to Matthias Wagner, Vienna, for development of the original electronic PsoRA database; Maximilian Errath for technical support; and Andrea Berghold, Department chair, Institute for Medical Informatics, Statistics, and Documentation, for continuous support in further development of the PsoRA database. We also thank Martina Praszl-Posch and Kirsten Sommer for support of data entry in the PsoRA database and Jude Richard, Austin, Texas, for editing of the manuscript. This work has been conducted as part of a dissertation thesis (TG).
Footnotes
Funding sources: Psoriasis Registry Austria (PsoRA) was supported by unrestricted research grants or educational grants from the following pharmaceutical companies: AbbVie (2015-2020), Amgen GmbH (2019-2020), Almirall (2017-2020), Celgene (2016-2018), Eli Lilly (2015-2020), Janssen (2014-2016), Leo Pharma (2014-2020), Novartis (2019), Merck Sharp & Dohme (2014), Sandoz (2019-2020), and Pfizer (2008-2018).
Conflicts of interest: Dr Wolf has received research grants, speaker and/or consulting honoraria, and/or travel refunds from AbbVie, Amgen GmbH, Almirall, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Merck Sharp & Dohme, Sandoz, and Pfizer. Dr Graier has received a travel grant from Novartis. Dr Jonak has received research grants, speaker and/or consulting honoraria, and/or travel refunds from AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO Pharma, Mallinckrodt/Therakos, Novartis, Pfizer, and 4SC. Dr Hoetzenecker has received research grants and speaker and consulting honoraria from AbbVie, Amgen GmbH, Almirall, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, and Bencard. Dr Ratzinger reports personal fees from Eli Lilly, personal fees from AbbVie, personal fees from Novartis, grants and personal fees from Leo, personal fees from Janssen, personal fees from Pfizer; personal fees from Eli Lilly, AbbVie, Novartis, Janssen, and Pfizer; and grants and personal fees from Leo, outside the submitted work. Dr Prillinger has received speaker and consulting honoraria from AbbVie, Eli Lilly, Janssen, and Novartis and travel refunds from AbbVie, Almirall, Celgene, Eli Lilly, Janssen, Leo Pharma, and Novartis. Dr Sator has received research grants, speaker and/or consulting honoraria, and/or travel refunds from AbbVie, Actelion, Amgen, Almirall, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Merck Sharp & Dohme, Sandoz, Maruho, ALK, Galderma, UCB, Gilead, and Pfizer. Dr Skvara received honoria/travel refunds as speaker/consultant from AbbVie, Almirall, Celgene, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB. Dr Mlynek has received research grants, speaker and/or consulting honoraria, and/or travel refunds from AbbVie, Amgen GmbH, Almirall, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, and Pfizer. Dr Vujic has received research grants, speaker and/or consulting honoraria, and/or travel refunds from AbbVie, Amgen GmbH, Almirall, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Merck Sharp & Dohme, Sandoz, and Pfizer. Dr Saxinger has received speaker and consulting honoraria from Almirall, AbbVie, and Novartis. Dr Kölli has received travel refunds from Janssen, Celgene, Almirall, and Pelpharma and consulting honoria from Novartis and Lilly. Dr Schütz-Bergmayr has received speaker and consulting honoria from AbbVie, Celgene, Lilly, Janssen, and Novartis. Dr Weger has received speaker and/or consulting honoraria and/or travel refunds from AbbVie, Amgen GmbHm Almirall, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Merck Sharp & Dohme, Sandoz, and Pfizer.
IRB approval status: The registry was approved by the ethics committee of the Medical University of Graz (application number 21-094 ex 09/10).
References
- 1.Gladman D.D., Kavanaugh A., Gómez-Reino J.J. Therapeutic benefit of apremilast on enthesitis and dactylitis in patients with psoriatic arthritis: a pooled analysis of the PALACE 1-3 studies. RMD Open. 2018;4(1):e000669. doi: 10.1136/rmdopen-2018-000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kavanaugh A., Gladman D.D., Edwards C.J. Long-term experience with apremilast in patients with psoriatic arthritis: 5-year results from a PALACE 1-3 pooled analysis. Arthritis Res Ther. 2019;21(1):118. doi: 10.1186/s13075-019-1901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul C., Cather J., Gooderham M. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 4.Papp K., Reich K., Leonardi C.L. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Ghamrawi R.I., Ghiam N., Wu J.J. Comparison of psoriasis guidelines for use of apremilast in the United States and Europe: a critical appraisal and comprehensive review. J Dermatolog Treat. 2020:1–6. doi: 10.1080/09546634.2020.1770176. [DOI] [PubMed] [Google Scholar]
- 6.Afra T.P., Razmi T.M., Dogra S. Apremilast in psoriasis and beyond: big hopes on a small molecule. Indian Dermatol Online J. 2019;10(1):1–12. doi: 10.4103/idoj.IDOJ_437_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Megna M., Fabbrocini G., Camela E., Cinelli E. Apremilast efficacy and safety in elderly psoriasis patients over a 48-weeks period. J Eur Acad Dermatol Venereol. 2020;34(11):e705–e707. doi: 10.1111/jdv.16443. [DOI] [PubMed] [Google Scholar]
- 8.Kromer C., Wilsmann-Theis D., Gerdes S. Drug survival and reasons for drug discontinuation in palmoplantar pustulosis: a retrospective multicenter study. J Ger Soc Dermatol. 2019;17(5):503–516. doi: 10.1111/ddg.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J.J., Pelletier C., Ung B., Tian M. Real-world treatment patterns and healthcare costs among biologic-naive patients initiating apremilast or biologics for the treatment of psoriasis. J Med Econ. 2019;22(4):365–371. doi: 10.1080/13696998.2019.1571500. [DOI] [PubMed] [Google Scholar]
- 10.Wu J.J., Pelletier C., Ung B., Tian M. Treatment patterns and healthcare costs among biologic-naive patients initiating apremilast or biologics for the treatment of psoriatic arthritis: results from a US claims analysis. Curr Med Res Opin. 2020;36(1):169–176. doi: 10.1080/03007995.2019.1668204. [DOI] [PubMed] [Google Scholar]
- 11.Loft N.D., Egeberg A., Rasmussen M.K. Patient-reported outcomes during treatment in patients with moderate-to-severe psoriasis: a Danish nationwide study. Acta Derm Venereol. 2019;99(13):1224–1230. doi: 10.2340/00015555-3331. [DOI] [PubMed] [Google Scholar]
- 12.Nast A., Jacobs A., Rosumeck S., Werner R.N. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: a systematic review and meta-analysis. J Invest Dermatol. 2015;135(11):2641–2648. doi: 10.1038/jid.2015.206. [DOI] [PubMed] [Google Scholar]
- 13.Lunder T., Zorko M.S., Kolar N.K. Drug survival of biological therapy is showing class effect: updated results from Slovenian National Registry of psoriasis. Int J Dermatol. 2019;58(6):631–641. doi: 10.1111/ijd.14429. [DOI] [PubMed] [Google Scholar]
- 14.Yasmeen N., Sawyer L.M., Malottki K., Levin L.Å., Didriksen Apol E., Jemec G.B. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat. 2020:1–15. doi: 10.1080/09546634.2020.1743811. [DOI] [PubMed] [Google Scholar]
- 15.de la Cueva Dobao P., Notario J., Ferrándiz C. Expert consensus on the persistence of biological treatments in moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2019;33(7):1214–1223. doi: 10.1111/jdv.15600. [DOI] [PubMed] [Google Scholar]
- 16.Sruamsiri R., Iwasaki K., Tang W., Mahlich J. Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real-world data study using a claims database. BMC Dermatol. 2018;18(1):1–11. doi: 10.1186/s12895-018-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason K.J., Barker J., Smith C. Comparison of drug discontinuation, effectiveness, and safety between clinical trial eligible and ineligible patients in BADBIR. JAMA Dermatol. 2018;154(5):581–588. doi: 10.1001/jamadermatol.2018.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masson Regnault M., Castañeda-Sanabria J., Diep Tran M.H.T. Users of biologics in clinical practice: would they be eligible for phase III clinical studies? Cohort Study in the French Psoriasis Registry PSOBIOTEQ. J Eur Acad Dermatol Venereol. 2019;34(2):293–300. doi: 10.1111/jdv.15878. [DOI] [PubMed] [Google Scholar]
- 19.Sbidian E., Mezzarobba M., Weill A., Coste J., Rudant J. Persistence of treatment with biologics for patients with psoriasis: a real-world analysis of 16 545 biologic-naïve patients from the French National Health Insurance database (SNIIRAM) Br J Dermatol. 2019;180(1):86–93. doi: 10.1111/bjd.16809. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Doval I., Dávila-Seijo P. How real are ‘real-life studies’ in psoriasis, and the uncertain meaning of drug persistence. Br J Dermatol. 2019;180(1):15–16. doi: 10.1111/bjd.17104. [DOI] [PubMed] [Google Scholar]
- 21.Strohal R., Prinz J.C., Girolomoni G., Nast A. A patient-centred approach to biological treatment decision making for psoriasis: an expert consensus. J Eur Acad Dermatol Venereol. 2015;29(12):2390–2398. doi: 10.1111/jdv.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strober B.E., van der Walt J.M., Armstrong A.W. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb) 2019;9(1):5–18. doi: 10.1007/s13555-018-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maul J.T., Navarini A.A., Sommer R. Gender and age significantly determine patient needs and treatment goals in psoriasis – a lesson for practice. J Eur Acad Dermatol Venereol. 2019;33(4):700–708. doi: 10.1111/jdv.15324. [DOI] [PubMed] [Google Scholar]
- 24.Wu B., Muser E., Teeple A., Pericone C.D., Feldman S.R. Treatment adherence and persistence of five commonly prescribed medications for moderate to severe psoriasis in a U.S. commercially insured population. J Dermatolog Treat. 2020:1–8. doi: 10.1080/09546634.2019.1687828. [DOI] [PubMed] [Google Scholar]
- 25.del Alcázar E., Suárez-Pérez J.A., Armesto S. Real-world effectiveness and safety of apremilast in psoriasis at 52 weeks: a retrospective, observational, multicenter study by the Spanish Psoriasis Group. J Eur Acad Dermatol Venereol. 2020;34(12):2821–2829. doi: 10.1111/jdv.16439. [DOI] [PubMed] [Google Scholar]
- 26.Mourad A., Straube S., Armijo-Olivo S., Gniadecki R. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2019;181(3):450–458. doi: 10.1111/bjd.17738. [DOI] [PubMed] [Google Scholar]
- 27.Feldman S.R., Zhang J., Martinez D.J. Real-world treatment patterns and healthcare costs of biologics and apremilast among patients with moderate-to-severe plaque psoriasis by metabolic condition status. J Dermatolog Treat. 2019:1–9. doi: 10.1080/09546634.2019.1698699. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto M., Komine M., Kamiya K., Sugai J., Ohtsuki M. Drug survival of apremilast in a real-world setting. J Dermatol. 2019;46(7):615–617. doi: 10.1111/1346-8138.14943. [DOI] [PubMed] [Google Scholar]
- 29.Lunder T., Marko P., Koser Kolar N., Kralj B., Kecelj Leskovec N. Drug survival of biologic therapies for the treatment of psoriasis: results of Slovenian national registry. Biologicals. 2018;54:44–49. doi: 10.1016/j.biologicals.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Inzinger M., Legat F.J., Hofer A. Short- to intermediate-term follow-up in patients treated with the combination of 311-nm ultraviolet B phototherapy and biological agents. Br J Dermatol. 2014;171(4):915–917. doi: 10.1111/bjd.12992. [DOI] [PubMed] [Google Scholar]
- 31.Inzinger M., Wippel-Slupetzky K., Weger W. Survival and effectiveness of tumour necrosis factor-alpha inhibitors in the treatment of plaque psoriasis under daily life conditions: report from the psoriasis registry Austria. Acta Derm Venereol. 2016;96(2):207–212. doi: 10.2340/00015555-2214. [DOI] [PubMed] [Google Scholar]
- 32.Inzinger M., Heschl B., Weger W. Efficacy of psoralen plus ultraviolet A therapy vs. biologics in moderate to severe chronic plaque psoriasis: retrospective data analysis of a patient registry. Br J Dermatol. 2011;165(3):640–645. doi: 10.1111/j.1365-2133.2011.10396.x. [DOI] [PubMed] [Google Scholar]
- 33.Inzinger M., Weger W., Heschl B., Salmhofer W., Quehenberger F., Wolf P. Methotrexate vs. fumaric acid esters in moderate-to-severe chronic plaque psoriasis: data registry report on the efficacy under daily life conditions. J Eur Acad Dermatol Venereol. 2013;27(7):861–866. doi: 10.1111/j.1468-3083.2012.04596.x. [DOI] [PubMed] [Google Scholar]
- 34.Busard C.I., Cohen A.D., Wolf P. Biologics combined with conventional systemic agents or phototherapy for the treatment of psoriasis: real-life data from PSONET registries. J Eur Acad Dermatol Venereol. 2018;32(2):245–253. doi: 10.1111/jdv.14583. [DOI] [PubMed] [Google Scholar]
- 35.Balato A., Campione E., Cirillo T. Long-term efficacy and safety of apremilast in psoriatic arthritis: focus on skin manifestations and special populations. Dermatol Ther. 2020;33(3):e13440. doi: 10.1111/dth.13440. [DOI] [PubMed] [Google Scholar]
- 36.Sbidian E., Billionnet C., Weill A., Maura G., Mezzarobba M. Persistence of apremilast in moderate-to-severe psoriasis: a real-world analysis of 14 147 apremilast- and methotrexate-naive patients in the French National Health Insurance database. Br J Dermatol. 2020;182(3):690–697. doi: 10.1111/bjd.18047. [DOI] [PubMed] [Google Scholar]
- 37.Shalom G., Zisman D., Harman-Boehm I. Factors associated with drug survival of methotrexate and acitretin in patients with psoriasis. Acta Derm Venereol. 2015;95(8):973–977. doi: 10.2340/00015555-2130. [DOI] [PubMed] [Google Scholar]
- 38.Ighani A., Georgakopoulos J.R., Zhou L.L., Walsh S., Shear N., Yeung J. Efficacy and safety of apremilast monotherapy for moderate to severe psoriasis: retrospective study. J Cutan Med Surg. 2018;22(3):290–296. doi: 10.1177/1203475418755982. [DOI] [PubMed] [Google Scholar]
- 39.Papadavid E., Rompoti N., Theodoropoulos K., Kokkalis G., Rigopoulos D. Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32(7):1173–1179. doi: 10.1111/jdv.14832. [DOI] [PubMed] [Google Scholar]
- 40.Ohata C., Ohyama B., Kuwahara F., Katayama E., Nakama T. Real-world data on the efficacy and safety of apremilast in Japanese patients with plaque psoriasis. J Dermatolog Treat. 2019;30(4):383–386. doi: 10.1080/09546634.2018.1525480. [DOI] [PubMed] [Google Scholar]
- 41.Mayba J.N., Gooderham M.J. Real-world experience with apremilast in treating psoriasis. J Cutan Med Surg. 2017;21(2):145–151. doi: 10.1177/1203475416676030. [DOI] [PubMed] [Google Scholar]
- 42.Vujic I., Herman R., Sanlorenzo M. Apremilast in psoriasis – a prospective real-world study. J Eur Acad Dermatol Venereol. 2018;32(2):254–259. doi: 10.1111/jdv.14598. [DOI] [PubMed] [Google Scholar]
- 43.Wong T.H., Sinclair S., Smith B., Fraser C., Morton C.A. Real-world, single-centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42(6):675–676. doi: 10.1111/ced.13150. [DOI] [PubMed] [Google Scholar]
- 44.Ighani A., Georgakopoulos J.R., Shear N.H., Walsh S., Yeung J. Short-term reasons for withdrawal and adverse events associated with apremilast therapy for psoriasis in real-world practice compared with in clinical trials: a multicenter retrospective study. J Am Acad Dermatol. 2018;78(4):801–803. doi: 10.1016/j.jaad.2017.09.067. [DOI] [PubMed] [Google Scholar]
- 45.Lee E.B., Amin M., Egeberg A., Wu J.J. Adverse events associated with apremilast use and withdrawal for psoriasis in a real-world setting. J Eur Acad Dermatol Venereol. 2018;32(10):e393–e394. doi: 10.1111/jdv.15061. [DOI] [PubMed] [Google Scholar]