Abstract
Background
Evidence of factors associated with psoriasis from large population-based cohort studies is scarce.
Objective
We aimed to explore the risk factors of late-onset psoriasis.
Methods
This study included 487,835 Japanese participants aged 40-107 years, who were followed prospectively from 2012 to 2018 using individually linked databases between annual health checkups and medical claims.
Results
During the study period, 2793 patients (0.57%) newly developed psoriasis; 13.8% had moderate-to-severe psoriasis. In the multivariate analysis, factors associated with psoriasis onset were age (hazard ratio [HR] 1.11 {95% confidence interval [CI]: 1.06-1.16}), male sex (HR: 1.11 [95% CI: 1.02-1.21]), body mass index (HR: 1.09 [95% CI: 1.05-1.14]), smoking (HR: 1.46 [95% CI: 1.31-1.63]), not exercising ≥1 hour per week (HR: 1.13 [95% CI: 1.05-1.22]), and gamma-glutamyl transpeptidase (HR: 1.04 [95% CI: 1.01-1.06]). When we used weight increment of ≥10 kg since the age of 20 years instead of body mass index in the multivariate model, this was also a risk factor (HR: 1.12 [95% CI: 1.04-1.21]).
Limitations
This study targeted people aged >40 years, thereby narrowing the search to the risk factors of late-onset psoriasis.
Conclusion
We showed that increasing age, male sex, body mass index, smoking, low physical activity, weight gain, and gamma-glutamyl transpeptidase are associated with late-onset development of psoriasis and revealed a relationship between liver dysfunction and psoriasis development.
Key words: incidence, onset, predictive factor, psoriasis, risk
Abbreviations used: BMI, body mass index; CI, confidence interval; GGT, gamma-glutamyl transpeptidase; HR, hazard ratio
Capsule Summary.
-
•
Mild liver dysfunction, monitored using gamma-glutamyl transpeptidase, was associated with the involvement of metabolic syndrome in late-onset psoriasis.
-
•
Physicians may wish to consider lifestyle-related risk factors such as weight gain and smoking that may be associated with the development of psoriasis.
Introduction
Psoriasis is a chronic inflammatory skin disease, which has been estimated to affect 0.51%-11.43% of the global population.1 The prevalence of psoriasis in the United States and Europe is 2.3%-3.2%2,3; this prevalence is lower in Asian countries, from 0.23% to 0.47%.4,5 About 13%-30% of patients with psoriasis also have psoriatic arthritis.6,7 Psoriasis causes skin and joint disorders, which lead to decreased quality of life.8 Recent studies show that psoriasis is related to various comorbidities such as diabetes, hypertension, and cardiovascular disease.9, 10, 11
Population-based studies indicate a relationship between psoriasis and metabolic syndrome.12 Owing to an increased risk of comorbidities, the risk of death in patients with psoriasis is also increased and life expectancy is shortened in patients with severe psoriasis.13 Thus, it is considered that early intervention for people with a high risk of developing psoriasis can prevent psoriasis onset and avoid greater severity of psoriasis, leading to improved social productivity and increased life expectancy.
Several studies have identified age, family history of psoriasis, smoking, alcohol consumption, obesity, and physical inactivity as factors associated with psoriasis.14, 15, 16, 17, 18 However, the published studies have limitations of research design and provide data on specific aspects of factors associated with psoriasis from cross-sectional surveys or case-control studies; little has been reported on the risk factors of psoriasis in the general population.
In recent years, several studies have been conducted using databases to research the epidemiology of psoriasis.5,19 The Kokuho database system of the Federation of National Health Insurance Association in Japan includes the Japanese national database of health insurance claims and health checkup data. This database includes the information of Japanese citizens insured through the National Health Insurance and Late Elderly Care Insurance; thus, these data are useful for analyzing the acquired risk factors of psoriasis onset in Japanese population. Using these data, we conducted a population-based cohort study to identify the risk factors associated with the occurrence of psoriasis.
Methods
Data resource
The Shizuoka Kokuho database is derived from a database that provides the linked data of Federation of National Health Insurance Association subscribers, including demographic and registration data, medical claims data, and health checkup data of enrollees in Shizuoka Prefecture. Shizuoka Prefecture has a population of about 3.6 million people and is located nearly in the center of Japan, with representative climatic conditions and population distribution of Japan. The database represented 25% of the population aged below 65 years and 75% of the population aged above 65 years in Shizuoka Prefecture as it included beneficiaries of the Japanese National Health Insurance and Late Elderly Care Insurance.
Japanese medical insurance and health checkups
Japan's medical system is based on an exhaustive insurance system. In Japan, there are 2 types of health insurance for people aged <75 years. One is Employees Health Insurance for the employees of companies; the other is the National Health Insurance for small business owners and their employees. Health insurance for people aged ≥75 years is the Latter-Stage Elderly Medical Care System. The Ministry of Health, Labour, and Welfare in Japan recommends registrants to have a health checkup once a year. The annual health checkups are specifically focused on visceral fat obesity starting at the age of 40 years.
Population and observational period
We used a dataset that comprised 6.5 years of longitudinal data from April 2012 to September 2018. All enrollees were investigated by using individually linked databases between their annual health checkups and medical claims. We excluded patients who received a diagnosis of psoriasis before the start date of health checkups, those who did not undergo a health checkup, and individuals aged <40 years.
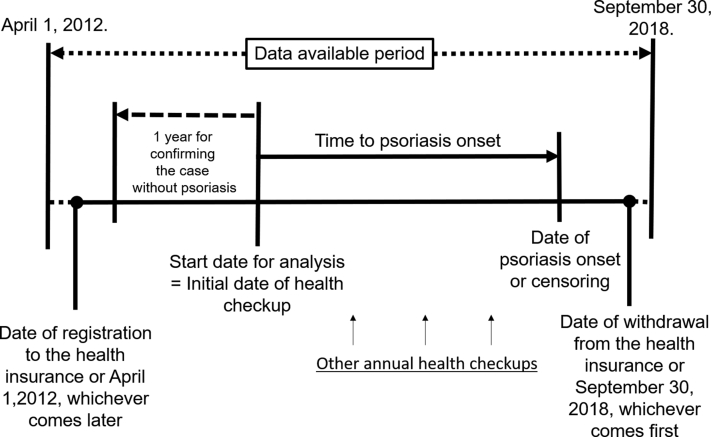
Data pick-up points are shown in Fig 1. Each enrollees' observation period was defined as the time from the date of insurance registration or April 2012, whichever came earlier to the date of insurance withdrawal or September 2018, whichever came later.
Fig 1.
Data pick-up points in the dataset.
Potential factors associated with psoriasis onset
Patients with psoriasis were defined as those who had a medical claim with an International Classification of Diseases, 10th Revision code for psoriasis (L40). Japanese disease codes and the corresponding types of psoriasis with L40.x are shown in Table I.
Table I.
Japanese disease codes and names for the definition of psoriasis
| ICD-10 | Japanese disease code | Disease name |
|---|---|---|
| L400 | 6961004 | Psoriasis vulgaris |
| L400 | 6961013 | Diffuse psoriasis |
| L400 | 6961014 | Nummular psoriasis |
| L400 | 6961015 | Follicular psoriasis |
| L400 | 6961017 | Seborrheic psoriasis |
| L400 | 8832096 | Facial psoriasis vulgaris |
| L400 | 8832675 | Plaque psoriasis |
| L400 | 8834312 | Psoriasis vulgaris of extremities |
| L400 | 8836536 | Psoriasis vulgaris of entire body |
| L400 | 8838120 | Psoriasis vulgaris of head |
| L400 | 8840843 | Psoriasis vulgaris of lower back |
| L401 | 6961007 | Pustular psoriasis |
| L401 | 8846041 | Generalized pustular psoriasis |
| L401 | 8846111 | Acute generalized pustular psoriasis |
| L401 | 8846144 | Pediatric generalized pustular psoriasis |
| L404 | 6961006 | Guttate psoriasis |
| L404 | 6961012 | Psoriasis punctata |
| L405 | 7133001 | Psoriatic arthritis |
| L405 | 8831630 | Psoriatic spondylitis |
| L405 | 8846362 | Psoriatic arthritis/shoulder joint |
| L405 | 8846363 | Psoriatic arthritis/hip joint |
| L405 | 8846364 | Psoriatic arthritis/finger joints |
| L405 | 8846365 | Psoriatic arthritis/knee joint |
| L405 | 8846366 | Psoriatic arthritis/wrist joint |
| L405 | 8846367 | Psoriatic arthritis/sacroiliac joint |
| L405 | 8846368 | Psoriatic arthritis/ankle joint |
| L405 | 8846369 | Psoriatic arthritis/elbow joint |
| L405 | 8846418 | Multiple psoriatic arthritis |
| L408 | 6961002 | Psoriatic erythroderma |
| L408 | 8832799 | Flexural psoriasis |
| L409 | 6961009 | Psoriasis |
| L409 | 8834298 | Psoriasis of extremities |
ICD-10, International Classification of Diseases, 10th Revision.
Psoriasis severity
We categorized the severity of psoriasis as mild or moderate-to-severe, according to the treatment. We defined patients with moderate-to-severe psoriasis as those who received phototherapy or systemic therapy, including oral retinoids (etretinate), apremilast, methotrexate, cyclosporine, azathioprine, granulocyte and monocyte adsorption apheresis, or biologics (adalimumab, infliximab, ustekinumab, secukinumab, ixekizumab, brodalumab, or guselkumab). Patients were classified as having mild psoriasis if they had never received any of these therapies. Several studies have used this approach to approximate the severity of psoriasis.20,21 We referred to treatment for 6 months after a diagnosis of psoriasis was given.
Statistical analysis
The missing covariates used in the multivariate analysis did not occur completely at random among all participants; therefore, we did not impute the missing data. Because the characteristics of the multivariate model with risk factor analysis differed from those of all cases (Table II), the participants included in the multivariate model were analyzed as a full analysis set.
Table II.
Comparison between datasets of all cases and full analysis set∗
| Variable | Full analysis set N = 487,835 | Cases with missing covariates N = 74,134 |
|---|---|---|
| Age (y) | 67.9 ± 10.8 | 72.1 ± 11.2 |
| Sex (male) | 203,927 (41.8) | 30,506 (41.1) |
| BMI | 22.7 ± 3.4 | 22.4 ± 3.4 Missing: 478 |
| Current smoker (Yes) | 57,630 (11.8) | 6843 (9.2) |
| Walking or physical exercise ≥1 h/wk (No) | 240,255 (49.2) | 10,084 (48.2) Missing: 53,203 |
| Increment in weight ≥10 kg since the age of 20 y (Yes) | 142,332 (29.2) | 5340 (24.7) Missing: 52,545 |
| Eats dinner within 2 h before bedtime, ≥3 times/wk | 61,906 (12.7) | 2467 (11.5) Missing: 52,669 |
| Use of hypotensive agents | 204,934 (42.0) | 37,103 (50.0) |
| Use of lipid-lowering agents | 129,618 (26.6) | 21,441 (28.9) |
| Triglyceride (mg/dL) | 115.4 ± 76.4 Missing: 31 | 109.5 ± 67.7 Missing: 291 |
| LDL cholesterol (mg/dL) | 124.7 ± 31.4 | 119.3 ± 30.2 Missing: 352 |
| HDL cholesterol (mg/dL) | 62.5 ± 16.7 | 61.3 ± 16.4 Missing: 342 |
| Use of antidiabetic agents | 43,174 (8.9) | 7212 (9.7) |
| HbA1c (%) | 5.7 ± 0.7 Missing: 2948 | 5.8 ± 0.7 Missing: 7175 |
| AST (IU/L) | 24.2 ± 12.4 Missing: 2 | 24.7 ± 10.8 Missing: 146 |
| ALT (IU/L) | 20.3 ± 14.3 Missing: 5 | 19.3 ± 12.6 Missing: 146 |
| GGT (IU/L) | 32.9 ± 47.1 | 31.0 ± 40.3 Missing: 352 |
| Estimated GFR (mL/min/1.73 m2) | 69.6 ± 15.7 | 66.0 ± 16.5 Missing: 26,671 |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GFR, glomerular filtration rate; GGT, gamma-glutamyl transpeptidase; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The full analysis set did not include missing values for explanatory variables in the multivariate models (see Table VI).
We summarized the data as mean and standard deviation for continuous variables and frequency and percentage for categorical variables. Univariate and multivariate Cox proportional hazards regression were performed to explore the factors associated with psoriasis onset. We calculated the hazard ratio (HR), 95% confidence interval (CI), and P value and conducted the Wald test. The reported risk factors such as age,14 smoking,22 body mass index (BMI),23 weight increment of ≥10 kg since the age of 20 years,23 and potential risk factors such as key etiologic and epidemiologic factors were used in the regression analysis.24 Variables with a relatively large number of missing values (>55,000) in the health checkup data were excluded from the multivariate model. One of 2 variables with high correlation was not used in the multivariate model owing to multicollinearity, based on the criterion of an absolute Spearman correlation coefficient >0.4 (Table III). For internal validation, we also performed multivariate analyses stratified by areas with 42 municipalities in Shizuoka Prefecture. A P value of .05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Table III.
Matrix of Spearman's correlation coefficient∗
| Matrix of Spearman's correlation coefficient | Age | Sex | BMI | Current smoker | Increment in weight ≥10 kg since the age of 20 y | Exercise to sweat lightly for ≥30 min, 2 times/wk, for ≥1 y | Walking or physical exercise ≥1 h/wk | Eats dinner within 2 h before bedtime, ≥3 times/wk | Frequency and volume per day of drink | Systolic blood pressure | Use of hypotensive agents | Triglyceride | LDL cholesterol | HDL cholesterol | Use of lipid-lowering agents | HbA1c | Use of antidiabetic agents | AST | ALT | GGT | Uric acid | Serum creatinine | Estimated GFR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | ||||||||||||||||||||||
| Sex | 0.023 | 1 | |||||||||||||||||||||
| BMI | −0.044 | −0.169 | 1 | ||||||||||||||||||||
| Current smoker | −0.186 | −0.244 | 0.014 | 1 | |||||||||||||||||||
| Increment in weight ≥10 kg since the age of 20 y | 0.100 | 0.130 | −0.581 | −0.045 | 1 | ||||||||||||||||||
| Exercise to sweat lightly for ≥30 min, 2 times/wk, for ≥1 y | −0.110 | 0.068 | 0.001 | 0.056 | −0.037 | 1 | |||||||||||||||||
| Walking or physical exercise ≥1 h/wk | −0.064 | 0.027 | 0.044 | 0.034 | −0.066 | 0.417 | 1 | ||||||||||||||||
| Eats dinner within 2 h before bedtime, ≥3 times/wk | 0.057 | 0.117 | −0.057 | −0.125 | 0.071 | −0.031 | −0.014 | 1 | |||||||||||||||
| Frequency and volume per day of drink | 0.157 | 0.423 | −0.068 | −0.197 | 0.058 | 0.047 | 0.031 | 0.154 | 1 | ||||||||||||||
| Systolic blood pressure (mm Hg) | 0.242 | −0.050 | 0.186 | −0.034 | −0.093 | −0.027 | −0.016 | −0.009 | −0.045 | 1 | |||||||||||||
| Use of hypotensive agents | 0.333 | −0.047 | 0.202 | −0.062 | −0.120 | −0.018 | 0.005 | −0.012 | −0.004 | 0.249 | 1 | ||||||||||||
| Triglyceride (mg/dL) | 0.009 | −0.132 | 0.333 | 0.110 | −0.246 | 0.020 | 0.057 | −0.029 | −0.036 | 0.130 | 0.106 | 1 | |||||||||||
| LDL cholesterol (mg/dL) | −0.126 | 0.122 | 0.066 | −0.038 | −0.044 | 0.012 | 0.011 | 0.041 | 0.100 | 0.018 | −0.186 | 0.185 | 1 | ||||||||||
| HDL cholesterol (mg/dL) | −0.099 | 0.300 | −0.351 | −0.128 | 0.248 | −0.025 | −0.060 | 0.033 | −0.050 | −0.070 | −0.141 | −0.501 | −0.001 | 1 | |||||||||
| Use of lipid-lowering agents | 0.171 | 0.111 | 0.119 | −0.089 | −0.073 | −0.038 | −0.009 | 0.046 | 0.126 | 0.055 | 0.253 | 0.100 | −0.188 | −0.049 | 1 | ||||||||
| HbA1c (%) | 0.135 | −0.031 | 0.203 | −0.030 | −0.154 | −0.035 | −0.003 | 0.021 | 0.090 | 0.086 | 0.141 | 0.165 | 0.037 | −0.162 | 0.198 | 1 | |||||||
| Use of antidiabetic agents | 0.090 | −0.089 | 0.118 | 0.009 | −0.090 | −0.032 | 0.002 | −0.013 | 0.015 | 0.055 | 0.158 | 0.058 | −0.115 | −0.119 | 0.160 | 0.415 | 1 | ||||||
| AST (IU/L) | 0.112 | −0.089 | 0.052 | −0.033 | −0.048 | −0.047 | −0.042 | −0.035 | −0.112 | 0.105 | 0.074 | 0.054 | −0.047 | 0.030 | 0.074 | 0.041 | 0.009 | 1 | |||||
| ALT (IU/L) | −0.158 | −0.219 | 0.287 | 0.047 | −0.239 | −0.023 | −0.009 | −0.052 | −0.131 | 0.065 | 0.036 | 0.206 | 0.024 | −0.121 | 0.085 | 0.143 | 0.073 | 0.664 | 1 | ||||
| GGT (IU/L) | −0.121 | −0.385 | 0.260 | 0.173 | −0.229 | −0.006 | 0.020 | −0.100 | −0.370 | 0.108 | 0.086 | 0.277 | −0.002 | −0.131 | 0.014 | 0.107 | 0.057 | 0.352 | 0.516 | 1 | |||
| Uric acid (mg/dL) | 0.025 | −0.453 | 0.277 | 0.116 | −0.202 | −0.029 | 0.006 | −0.074 | −0.256 | 0.098 | 0.146 | 0.247 | −0.010 | −0.259 | −0.010 | 0.079 | 0.032 | 0.134 | 0.208 | 0.333 | 1 | ||
| Serum creatinine (mg/dL) | 0.152 | −0.629 | 0.173 | 0.086 | −0.112 | −0.072 | −0.016 | −0.047 | −0.198 | 0.053 | 0.159 | 0.160 | −0.081 | −0.272 | 0.004 | 0.029 | 0.076 | 0.077 | 0.091 | 0.209 | 0.534 | 1 | |
| Estimated GFR (mL/min/1.73 m2) | −0.401 | 0.027 | −0.075 | 0.123 | 0.017 | 0.072 | 0.019 | −0.054 | −0.100 | −0.086 | −0.226 | −0.099 | 0.023 | 0.122 | −0.128 | −0.052 | −0.046 | −0.058 | 0.074 | 0.048 | −0.303 | −0.749 | 1 |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GFR, glomerular filtration rate; GGT, gamma-glutamyl transpeptidase; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Categorical variables were treated as ordered-category variables, then Spearman's correlation coefficient was calculated. The category for frequency and volume per day of alcohol included “rarely (cannot) drink,” “sometimes,” “<180 mL/d,” “180 to <360 mL/d,” “360 to <540 mL/d,” and “≥540 mL/d.” Bold type without and with underlining indicates that the absolute coefficient was >0.3 and >0.4, respectively.
Ethics
The data of all enrollees were anonymized in the Federation of National Health Insurance Association to protect participant confidentiality. The study protocol was approved by the ethics committee of Shizuoka General Hospital (SGHIRB#2019009, 2019).
Results
Participant characteristics
Among the 2,188,609 individuals in the Shizuoka Kokuho database, 718,140 underwent health checkups. Among them, 148,964 patients had an observation period of <1 year, 3151 had already developed psoriasis, and 4056 patients aged <40 years were excluded. A total of 561,969 individuals without psoriasis were included in our study. We analyzed the data of 487,835 individuals (203,927 men [41.8%], mean [standard deviation] age 67.9 [10.8] years) in the analysis; this full analysis set did not include individuals with missing values for explanatory variables in the multivariate models.
Psoriasis onset and severity
During the observation period (median 5.50 years), 2793 patients (0.57%) were newly diagnosed with psoriasis. Among these 2793 patients, 86.2% and 13.8% had mild and moderate-to-severe psoriasis, respectively. The baseline characteristics for new-onset psoriasis patients and others are shown in Table IV. We assumed that the severity of psoriasis could be defined according to the treatment within 6 months after the onset of psoriasis. Systemic therapies used at least once within 6 months after onset in patients with moderate-to-severe psoriasis are shown in Table V.
Table IV.
Participant characteristics at baseline health checkup∗
| Variable | Psoriasis onset in cohort period (median 5.5 y) N = 2793 | Controls N = 485,042 |
|---|---|---|
| Age (y) | 69.0 ± 10.0 | 67.9 ± 10.8 |
| Sex (male) | 1331 (47.7) | 202,596 (41.8) |
| BMI | 23.0 ± 3.5 | 22.7 ± 3.4 |
| Current smoker (Yes) | 450 (16.1) | 57,180 (11.8) |
| Walking or physical exercise ≥1 h/wk (No) | 1462 (52.3) | 238,793 (49.2) |
| Increment in weight ≥10 kg since the age of 20 y (Yes) | 958 (34.3) | 141,374 (29.1) |
| Eats dinner within 2 h before bedtime, ≥3 times/wk (Yes) | 369 (13.2) | 61,537 (12.7) |
| Use of hypotensive agents | 1303 (46.7) | 203,631 (42.0) |
| Use of lipid-lowering agents | 784 (28.1) | 128,834 (26.6) |
| Triglyceride (mg/dL) | 120.0 ± 73.3 | 115.3 ± 76.5 |
| LDL cholesterol (mg/dL) | 123.3 ± 31.7 | 124.7 ± 31.4 |
| HDL cholesterol (mg/dL) | 61.2 ± 16.8 | 62.5 ± 16.7 |
| Use of antidiabetic agents | 258 (9.2) | 42,916 (8.8) |
| HbA1c (%) | 5.7 ± 0.6 | 5.7 ± 0.7 |
| AST (IU/L) | 25.0 ± 13.1 | 24.2 ± 12.4 |
| ALT (IU/L) | 20.9 ± 13.3 | 20.3 ± 14.3 |
| GGT (IU/L) | 36.8 ± 52.1 | 32.9 ± 47.0 |
| Estimated GFR (mL/min/1.73 m2) | 68.7 ± 15.7 | 69.6 ± 15.7 |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GFR, glomerular filtration rate; GGT, gamma-glutamyl transpeptidase; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Continuous and categorical variables are summarized using mean ± standard deviation and frequency (percentage), respectively.
Table V.
Systemic therapies used at least once within 6 months after onset in patients with moderate-to-severe psoriasis∗
| Systemic therapy used at least once within 6 mo after onset | Moderate-to-severe psoriasis |
|
|---|---|---|
| N = 385 | ||
| Frequency | % | |
| Phototherapy | 239 | 62.1 |
| Cyclosporine | 55 | 14.3 |
| Oral retinoids (etretinate) | 45 | 11.7 |
| Methotrexate | 28 | 7.3 |
| Apremilast | 6 | 1.6 |
| Adalimumab | 3 | 0.8 |
| Azathioprine | 3 | 0.8 |
| Infliximab | 2 | 0.5 |
| Secukinumab | 2 | 0.5 |
| Ustekinumab | 1 | 0.3 |
| Granulocyte and monocyte adsorption apheresis | 1 | 0.3 |
Systemic therapies for patients with moderate-to-severe psoriasis (385 of 2793 patients, 13.8%) used at least once in the first 6 months after onset. In patients with mild severity (2408 of 2793 patients, 86.2%), treatments other than the above systemic therapies or observation were used.
Identification of factors associated with psoriasis onset
We evaluated the potential risk factors and significant variables using univariate Cox regression analysis (Table VI, univariate model). In the multivariate analysis, we identified the following as risk factors for the onset of psoriasis: age per 10 years: (HR: 1.11 [95% CI: 1.06-1.16]), male sex (versus female sex; HR: 1.11 [95% CI: 1.02-1.21]), BMI per 1 unit (HR: 1.09 [95% CI: 1.05-1.14]), current smoker (versus non-smoker; HR: 1.46 [95% CI: 1.31-1.63]), not walking or exercising ≥1 hour per week (HR: 1.13 [95% CI: 1.05-1.22]), and gamma-glutamyl transpeptidase (GGT) 100 IU/L (HR: 1.04 [1.01-1.06]) (Table VI, multivariate model 1). When weight increment of ≥10 kg since the age of 20 years was used instead of BMI in the multivariate model, although the Spearman's correlation coefficient for these factors was −0.581, it was also a risk factor (HR: 1.12 [95% CI: 1.04-1.21]) (Table VI, multivariate model 2). Further, similar results were obtained in the sensitivity analysis (Table VII).
Table VI.
Results of univariate and multivariate Cox regression analysis for developing psoriasis∗
| Variable (reference) N = 487,835 | Category or unit | Univariate model |
Multivariate model 1 |
Multivariate model 2 |
|||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (y) | 10.8 | 1.08 (1.04-1.12) | <.001 | 1.11 (1.06-1.16) | <.001 | 1.11 (1.06-1.16) | <.001 |
| Sex (women) | Men | 1.21 (1.12-1.30) | <.001 | 1.11 (1.02-1.21) | .012 | 1.11 (1.02-1.20) | .016 |
| BMI | 3.4 | 1.10 (1.06-1.14) | <.001 | 1.09 (1.05-1.14) | <.001 | ||
| Current smoker (No) | Yes | 1.43 (1.29-1.58) | <.001 | 1.46 (1.31-1.63) | <.001 | 1.45 (1.30-1.62) | <.001 |
| Walking or exercise ≥1 h/wk (Yes) | No | 1.14 (1.05-1.22) | .001 | 1.13 (1.05-1.22) | .002 | 1.25 (1.15-1.36) | <.001 |
| Weight increment ≥10 kg since age 20 y (No) | Yes | 1.27 (1.18-1.37) | <.001 | 1.12 (1.04-1.21) | .002 | ||
| Eats dinner within 2 h before bedtime ≥3 times/wk (No) | Yes | 1.04 (0.93-1.16) | .458 | 0.97 (0.87-1.09) | .604 | 0.97 (0.86-1.08) | .548 |
| Use of hypotensive agents (No) | Yes | 1.18 (1.10-1.27) | <.001 | 1.07 (0.98-1.16) | .125 | 1.08 (0.99-1.17) | .088 |
| Use of lipid-lowering agents (No) | Yes | 1.06 (0.97-1.15) | .190 | 1.05 (0.96-1.15) | .275 | 1.05 (0.96-1.15) | .269 |
| Triglyceride (mg/dL) | 76.4 | 1.05 (1.02-1.09) | .002 | ||||
| LDL cholesterol (mg/dL) | 31.4 | 0.96 (0.92-0.99) | .018 | 0.98 (0.95-1.02) | .401 | 0.98 (0.95-1.02) | .429 |
| HDL cholesterol (mg/dL) | 16.7 | 0.94 (0.90-0.97) | <.001 | 1.01 (0.97-1.06) | .577 | 1.01 (0.97-1.05) | .704 |
| Use of antidiabetic agents (No) | Yes | 1.03 (0.90-1.18) | .654 | 0.92 (0.81-1.05) | .217 | 0.93 (0.81-1.06) | .244 |
| HbA1c (%) | 1 | 1.01 (0.96-1.06) | .769 | ||||
| AST (IU/L) | 12.4 | 1.02 (1.01-1.03) | <.001 | ||||
| ALT (IU/L) | 14.3 | 1.03 (1.00-1.06) | .021 | ||||
| GGT (IU/L) | 47.1 | 1.05 (1.03-1.07) | <.001 | 1.04 (1.01-1.06) | .006 | 1.04 (1.01-1.06) | .007 |
| Estimated GFR (mL/min/1.73 m2) | 15.7 | 0.97 (0.95-0.99) | .011 | 0.99 (0.95-1.03) | .637 | 0.99 (0.95-1.03) | .605 |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; GFR, glomerular filtration rate; GGT, gamma-glutamyl transpeptidase; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein.
Bold type in the multivariate model indicates statistical significance. Because the Spearman's correlation coefficient between BMI and weight increment of ≥10 kg since the age of 20 years was −0.581, multivariate model 1 included BMI but not weight increment of ≥10 kg since the age of 20 years; model 2 included weight increment of ≥10 kg since the age of 20 years but not BMI.
Table VII.
Results of the multivariate model stratified by areas with 42 municipalities in Shizuoka Prefecture
| Variable (reference), N = 487,719∗ | Category or unit | Multivariate model 3† |
Multivariate model 4† |
||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 10.8 | 1.11 (1.06-1.16) | <.001 | 1.11 (1.06-1.16) | <.001 |
| Sex (women) | Men | 1.11 (1.02-1.2) | .015 | 1.1 (1.02-1.2) | .019 |
| BMI | 3.4 | 1.09 (1.05-1.14) | <.001 | ||
| Current smoker (No) | Yes | 1.47 (1.32-1.64) | <.001 | 1.46 (1.31-1.63) | <.001 |
| Walking or exercise ≥1 h/wk (Yes) | No | 1.12 (1.04-1.21) | .003 | 1.25 (1.15-1.35) | <.001 |
| Weight increment ≥10 kg since the age of 20 y (No) | Yes | 1.12 (1.04-1.2) | .004 | ||
| Eats dinner within 2 h before bedtime ≥3 times/wk (No) | Yes | 0.96 (0.86-1.08) | .499 | 0.96 (0.86-1.07) | .455 |
| Use of hypotensive agents (No) | Yes | 0.16 (1.06-0.98) | .158 | 1.07 (0.99-1.16) | .108 |
| Use of lipid-lowering agents (No) | Yes | 0.98 (0.94-1.02) | .319 | 0.98 (0.94-1.02) | .353 |
| LDL cholesterol (mg/dL) | 31.4 | 1.01 (0.97-1.05) | .601 | 1.01 (0.97-1.05) | .752 |
| HDL cholesterol (mg/dL) | 16.7 | 0.31 (1.05-0.96) | .313 | 1.05 (0.96-1.15) | .300 |
| Use of antidiabetic agents (No) | Yes | 0.16 (0.91-0.8) | .160 | 0.92 (0.8-1.04) | .184 |
| GGT (IU/L) | 47.1 | 1.04 (1.01-1.06) | .003 | 1.04 (1.01-1.06) | .004 |
| Estimated GFR (mL/min/1.73 m2) | 15.7 | 0.98 (0.94-1.03) | .425 | 0.98 (0.94-1.02) | .396 |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; GFR, glomerular filtration rate; GGT, gamma-glutamyl transpeptidase; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein.
The categorical variable for area was missing in 116 cases.
Multivariate models 3 and 4 included a categorical variable for area in models 1 and 2 (Table VI). The 42 municipalities (number of individuals [% against overall patients]) in Shizuoka Prefecture were, in descending order, Fuji (34,085 cases [7.06%]); Shimizu-ku, Shizuoka (33,708 cases [6.98%]); Aoi-ku, Shizuoka (32,781 cases [6.79%]); Naka-ku, Hamamatsu (32,686 cases [6.77%]); Numazu (30,917 cases [6.41%]); Suruga-ku, Shizuoka (25,395 cases [5.26%]); Fujieda (22,440 cases [4.65%]); Nishi-ku, Hamamatsu (16,704 cases [3.46%]); Higashi-ku, Hamamatsu (16,250 cases [3.37%]); Ito (15,034 cases [3.12%]); Hamakita-ku, Hamamatsu (14,402 cases [2.98%]); Fujinomiya (13,722 cases [2.84%]); Gotenba (13,640 cases [2.83%]); Iwata (13,488 cases [2.79%]); Minami-ku, Hamamatsu (13,465 cases [2.79%]); Kita-ku, Hamamatsu (13,409 cases [2.78%]); Mishima (13,347 cases [2.77%]); Kakegawa (10,680 cases [2.21%]); Shimada (10,166 cases [2.11%]); Izunokuni (9820 cases [2.03%]); Susono (7853 cases [1.63%]); Kosai (7772 cases [1.61]); Izu (7726 cases [1.6%]); Atami (7177 cases [1.49%]); Tenryu-ku, Hamamatsu (6294 cases [1.3%]); Kannami-cho, Tagata-cho (5989 cases [1.24%]); Nagaizumi-cho, Sunto-gun (5367 cases [1.11%]); Shimizu-cho, Sunto-gun (4743 cases [0.98%]); Omaezaki (4371 cases [0.91%]); Kikugawa (4288 cases [0.89%]); Fukuroi (4225 cases [0.88%]); Shimoda (3894 cases [0.81%]); Oyama-cho, Sunto-gun (3581 cases [0.74%]); Yoshida-cho, Haibara (2793 cases [0.58%]); Higashiizu-cho, Kamo-gun (2656 cases [0.55%]); Yaizu (2480 cases [0.51%]); Mori-machi, Syuti-gun (2258 cases [0.47%]); Nishiizu-cho, Kamo-gun (1800 cases [0.37%]); Kawanehon-cho, Haibara-gun (1758 cases [0.36%]); Minamiizu-cho, Kamo-gun (1599 cases [0.33%]); Kawazu-cho, Kamo-gun (1503 cases [0.31%]); and Matsuzaki-cho, Kamo-gun (1349 cases [0.28%]).
Discussion
Our objective was to evaluate the risk factors associated with the onset of psoriasis. We analyzed 487,835 individuals who received health checkups and identified age, sex, smoking, BMI, weight gain since the age of 20 years, low physical activity level, and GGT as factors associated with psoriasis development in the Japanese population.
From the data presented, the prevalence of psoriasis in the population who had undergone health checkups would appear to be 0.44% (3151 out of 718,140). Kubota et al reported that the national prevalence of psoriasis was 0.34% in a Japanese national claims database.19 The higher figure would be expected as our cohort included patients with psoriasis over the age of 40 years. During this study period, 13.8% of newly diagnosed patients with psoriasis progressed to moderate or severe psoriasis. Subgroup analysis of older-onset psoriasis is shown in Table VIII. Liver dysfunction may be higher in young patients with psoriasis. In addition, smoking and weight gain since the age of 20 years were also higher in young patients with psoriasis.
Table VIII.
A comparison of patient backgrounds among psoriasis patients by age
| Variable | Psoriasis onset in cohort period (median 5.5 y) |
P value | |
|---|---|---|---|
| ≥60 y old |
<60 y old |
||
| N = 2414 | N = 379 | ||
| Age (y) | 71.7 ± 7.6 | 51.7 ± 5.9 | NE |
| Sex (male) | 1164 (48.2) | 167 (44.1) | .132 |
| BMI | 22.9 ± 3.3 | 23.6 ± 4.1 | .030 |
| Current smoker (Yes) | 340 (14.1) | 110 (29.0) | <.001 |
| Walking or physical exercise ≥1 h/wk (No) | 1183 (49.0) | 148 (39.1) | <.001 |
| Increment in weight ≥10 kg since the age of 20 y (Yes) | 795 (32.9) | 163 (43.0) | <.001 |
| Eats dinner within 2 h before bedtime, ≥3 times/wk (Yes) | 299 (12.4) | 70 (18.5) | .001 |
| Use of hypotensive agents | 1215 (50.3) | 88 (23.2) | <.001 |
| Use of lipid-lowering agents | 741 (30.7) | 43 (11.3) | <.001 |
| Triglyceride (mg/dL) | 118.9 ± 69.7 | 127.0 ± 92.6 | .913 |
| LDL cholesterol (mg/dL) | 122.9 ± 31.6 | 125.5 ± 3.2.3 | .207 |
| HDL cholesterol (mg/dL) | 60.9 ± 16.6 | 63.4 ± 17.9 | .013 |
| Use of antidiabetic agents | 236 (9.8) | 22 (5.8) | .013 |
| HbA1c (%) | 5.8 ± 0.6 | 5.6 ± 0.7 | <.001 |
| AST (IU/L) | 25.1 ± 13.4 | 24.6 ± 10.7 | .045 |
| ALT (IU/L) | 20.3 ± 12.8 | 24.6 ± 15.9 | <.001 |
| GGT (IU/L) | 36.0 ± 53.2 | 41.7 ± 44.3 | .003 |
| Estimated GFR (mL/min/1.73 m2) | 67.3 ± 15.6 | 77.6 ± 13.5 | <.001 |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GFR, glomerular filtration rate; GGT, gamma-glutamyl transpeptidase; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NE, not evaluate.
In recent years, the concept of “psoriatic march” has been used to explain psoriatic inflammation leading to insulin resistance and atherosclerosis and contributing to cardiovascular comorbidity.25 According to this concept, as a chronic systemic inflammatory disease, the control of psoriasis is essential in terms of its impact on comorbidities.
Several studies have reported the relationship between psoriasis and obesity.26 BMI, weight change since the age of 18 years, waist circumference, and hip circumference are associated with an increased risk of psoriasis.23 Our research also indicated that BMI, weight gain since the age of 20 years, and low levels of physical activity were the factors associated with psoriasis. Thus, weight loss may be useful for the prevention and control of psoriasis. Dietary intervention together with physical exercise significantly reduced the Psoriasis Area and Severity Index.27 Further, it was proven that reduced physical activity is associated with an increased risk of developing psoriasis18,28 Physical activity can have anti-inflammatory effects because increasing the physical activity can decrease some inflammatory molecules such as tumor necrosis factor-α, interferon γ, interleukin 6, and C-reactive protein.29,30 Therefore, physical activity may improve psoriasis symptoms and reduce the risks related to systemic inflammation and psoriasis-linked comorbidities.
Smoking has been found to be an independent risk factor for psoriasis, and the increase in the number of cigarettes is proportional to the risk of psoriasis.17,22 In the present study, multivariate analysis demonstrated that smoking was a risk factor (HR: 1.46 [95% CI: 1.31-1.63], P < .001 in model 1 of Table VI). A meta-analysis of smoking in psoriasis indicated a strong association with current and ex-smokers.31 Smoking cessation is essential to prevent the onset of psoriasis and control psoriatic comorbidities.
A previous study indicated that moderate-to-severe psoriasis is associated with an increased risk of chronic kidney disease.32 However, our study did not identify a relationship between psoriasis onset and renal function (estimated glomerular filtration rate). Impaired renal function was not a risk factor for developing psoriasis but might be a prognostic factor for the severity of psoriasis.
Compared with other well-known comorbidities such as obesity and myocardial infarction, the relationship between psoriasis and liver disease is not well understood. Recently, several studies have revealed an increased frequency of nonalcoholic fatty liver disease in patients with psoriasis.33,34 Patients with psoriasis and psoriatic arthritis have higher scores on enhanced liver fibrosis tests, indicative of liver fibrosis.35 However, there are no prospective data on the relationship between liver disease and the onset of psoriasis. In liver function tests, alanine transaminase and aspartate transaminase are increased when hepatocytes are damaged, but GGT is increased in alcoholic liver injury and fatty liver. Alcohol consumption is reported to be a risk factor for developing psoriasis.16 Furthermore, GGT is a marker of nonalcoholic fatty liver disease and is frequently elevated in patients with nonalcoholic fatty liver disease.36,37 Fatty liver disease occurs with obesity at a high rate, but inflammatory cytokines such as tumor necrosis factor-α are secreted from adipose cells, which causes low levels of persistent general inflammation in the body as well as insulin resistance.38, 39, 40, 41 Such inflammation has recently attracted attention as a risk factor in the development of various diseases, and its involvement in psoriasis has been suggested.25 Therefore, our results may be related to alcohol consumption or fatty liver and the related inflammation. Liver dysfunction is a prominent indicator of the involvement of metabolic syndrome at psoriasis onset. It is possible that alcohol consumption may confound the relationship between psoriasis and liver disease, and further study is warranted.
The onset of psoriasis before the age of 40 years is strongly associated with genetic factors. The onset after this age may be related to acquired factors, such as lifestyle and chronic diseases.42 This study targeted people over the age of 40 years, thereby narrowing the search to primarily acquired risk factors of psoriasis onset.
There are several limitations in the current study. First, it is difficult to determine the exact severity of psoriasis because our claims data did not include the index of severity and extent of psoriasis, such as the Psoriasis Area and Severity Index score. Second, we defined the development of psoriasis based on the International Classification of Diseases, 10th Revision code, but this was not validated using medical chart data. Third, the exact incidence of psoriasis is unknown in Shizuoka Prefecture because we did not have data for all individuals in the prefecture; however, this would not influence the results of risk factor analysis. In this study, we were unable to truly determine the age of onset, and we used the age at diagnosis as a surrogate. Finally, in this study, we ensured that enrollees who were newly diagnosed with psoriasis had not received any psoriatic treatment using the insurance during the 1-year baseline period. Therefore, patients with psoriasis who did not use the insurance for the treatment of psoriasis during this period were considered as new onset. However, owing to the universal insurance system in Japan, such patients are thought to be extremely rare. Despite these limitations, we identified several factors associated with psoriasis onset in this study.
In conclusion, our data confirmed that increasing age, male sex, smoking, BMI, weight gain since the age of 20 years, low physical activity levels, and GGT are associated with the late-onset development of psoriasis in the Japanese population. Further research on the relationship between liver function disorders and the development of psoriasis are needed.
Acknowledgments
Comments and data management were provided by Dr Kiyoshi Tanaka (Kobe Gakuin University), Dr Yasuharu Tabara (Kyoto University), Dr Noriko Kojimahara, Yoshimi Iwasaki, Fumihiro Makita, and Junpei Minami (Shizuoka General Hospital). The manuscript was edited by Edanz Group (https://en-author-services.edanzgroup.com/).
Footnotes
Drs Goto and Nakatani contributed equally to this article.
Funding sources: None.
Conflict of interest: None disclosed.
IRB approval status: The study protocol was approved by the ethics committee of Shizuoka General Hospital (SGHIRB#2019009, 2019).
References
- 1.Michalek I.M., Loring B., John S.M. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212. doi: 10.1111/jdv.13854. [DOI] [PubMed] [Google Scholar]
- 2.Rachakonda T.D., Schupp C.W., Armstrong A.W. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Springate D.A., Parisi R., Kontopantelis E., Reeves D., Griffiths C.E., Ashcroft D.M. Incidence, prevalence and mortality of patients with psoriasis: a U.K. population-based cohort study. Br J Dermatol. 2017;176(3):650–658. doi: 10.1111/bjd.15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding X., Wang T., Shen Y. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol. 2012;22(5):663–667. doi: 10.1684/ejd.2012.1802. [DOI] [PubMed] [Google Scholar]
- 5.Tsai T.-F., Wang T.-S., Hung S.-T. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011;63(1):40–46. doi: 10.1016/j.jdermsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim G., Waxman R., Helliwell P.S. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis Rheum. 2009;61(10):1373–1378. doi: 10.1002/art.24608. [DOI] [PubMed] [Google Scholar]
- 7.Mease P.J., Gladman D.D., Papp K.A. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–735. doi: 10.1016/j.jaad.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Bhosle M.J., Kulkarni A., Feldman S.R., Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4(1):35. doi: 10.1186/1477-7525-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong A.W., Harskamp C.T., Armstrong E.J. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi A.A., Choi H.K., Setty A.R., Curhan G.C. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145(4):379–382. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelfand J.M., Neimann A.L., Shin D.B., Wang X., Margolis D.J., Troxel A.B. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong A.W., Harskamp C.T., Armstrong E.J. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68(4):654–662. doi: 10.1016/j.jaad.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Abuabara K., Azfar R.S., Shin D.B., Neimann A.L., Troxel A.B., Gelfand J.M. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163(3):586–592. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Queiro R., Tejon P., Alonso S., Coto P. Age at disease onset: a key factor for understanding psoriatic disease. Rheumatology (Oxford) 2014;53(7):1178–1185. doi: 10.1093/rheumatology/ket363. [DOI] [PubMed] [Google Scholar]
- 15.Jankovic S., Raznatovic M., Marinkovic J., Jankovic J., Maksimovic N. Risk factors for psoriasis: a case-control study. J Dermatol. 2009;36(6):328–334. doi: 10.1111/j.1346-8138.2009.00648.x. [DOI] [PubMed] [Google Scholar]
- 16.Brenaut E., Horreau C., Pouplard C. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):30–35. doi: 10.1111/jdv.12164. [DOI] [PubMed] [Google Scholar]
- 17.Naldi L., Chatenoud L., Linder D. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125(1):61–67. doi: 10.1111/j.0022-202X.2005.23681.x. [DOI] [PubMed] [Google Scholar]
- 18.Frankel H.C., Han J., Li T., Qureshi A.A. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148(8):918–924. doi: 10.1001/archdermatol.2012.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubota K., Kamijima Y., Sato T. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5(1):e006450. doi: 10.1136/bmjopen-2014-006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta N.N., Azfar R.S., Shin D.B., Neimann A.L., Troxel A.B., Gelfand J.M. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiesa Fuxench Z.C., Shin D.B., Ogdie Beatty A., Gelfand J.M. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152(3):282–290. doi: 10.1001/jamadermatol.2015.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Han J., Choi H.K., Qureshi A.A. Smoking and risk of incident psoriasis among women and men in the United States: a combined analysis. Am J Epidemiol. 2012;175(5):402–413. doi: 10.1093/aje/kwr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setty A.R., Curhan G., Choi H.K. Obesity, waist circumference, weight change, and the risk of psoriasis in women: nurses' Health Study II. Arch Intern Med. 2007;167(15):1670–1675. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 24.Harrell F.E., Jr. Springer; New York: 2015. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. [Google Scholar]
- 25.Boehncke W.H., Boehncke S., Tobin A.M., Kirby B. The 'psoriatic march': a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20(4):303–307. doi: 10.1111/j.1600-0625.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 26.Aune D., Snekvik I., Schlesinger S., Norat T., Riboli E., Vatten L.J. Body mass index, abdominal fatness, weight gain and the risk of psoriasis: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2018;33(12):1163–1178. doi: 10.1007/s10654-018-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naldi L., Conti A., Cazzaniga S. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170(3):634–642. doi: 10.1111/bjd.12735. [DOI] [PubMed] [Google Scholar]
- 28.Torres T., Alexandre J.M., Mendonça D., Vasconcelos C., Silva B.M., Selores M. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15(2):129–135. doi: 10.1007/s40257-014-0061-0. [DOI] [PubMed] [Google Scholar]
- 29.Nicklas B.J., Hsu F.C., Brinkley T.J. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56(11):2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo T., Kobayashi I., Murakami M. Effect of exercise on circulating adipokine levels in obese young women. Endocr J. 2006;53(2):189–195. doi: 10.1507/endocrj.53.189. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong A.W., Harskamp C.T., Dhillon J.S., Armstrong E.J. Psoriasis and smoking: a systematic review and meta-analysis. Br J Dermatol. 2014;170(2):304–314. doi: 10.1111/bjd.12670. [DOI] [PubMed] [Google Scholar]
- 32.Wan J., Wang S., Haynes K., Denburg M.R., Shin D.B., Gelfand J.M. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi: 10.1136/bmj.f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Candia R., Ruiz A., Torres-Robles R., Chavez-Tapia N., Mendez-Sanchez N., Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29(4):656–662. doi: 10.1111/jdv.12847. [DOI] [PubMed] [Google Scholar]
- 34.Gisondi P., Targher G., Zoppini G., Girolomoni G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol. 2009;51(4):758–764. doi: 10.1016/j.jhep.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 35.van der Voort E.A.M., Wakkee M., Veldt-Kok P., Darwish Murad S., Nijsten T. Enhanced liver fibrosis test in patients with psoriasis, psoriatic arthritis and rheumatoid arthritis: a cross-sectional comparison with procollagen-3 N-terminal peptide (P3NP) Br J Dermatol. 2017;176(6):1599–1606. doi: 10.1111/bjd.15220. [DOI] [PubMed] [Google Scholar]
- 36.Banderas D.Z., Escobedo J., Gonzalez E., Liceaga M.G., Ramírez J.C., Castro M.G. γ-Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol. 2012;24(7):805–810. doi: 10.1097/MEG.0b013e328354044a. [DOI] [PubMed] [Google Scholar]
- 37.Dowman J.K., Tomlinson J.W., Newsome P.N. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33(5):525–540. doi: 10.1111/j.1365-2036.2010.04556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220(2):T47–T59. doi: 10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Versini M., Jeandel P.Y., Rosenthal E., Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13(9):981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Brembilla N.C., Boehncke W.H. Dermal adipocytes' claim for fame in psoriasis. Exp Dermatol. 2017;26(5):392–393. doi: 10.1111/exd.13074. [DOI] [PubMed] [Google Scholar]
- 41.Wellen K.E., Hotamisligil G.S. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffiths C.E., Barker J.N. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]