Abstract
Objective
To compare the humoral response after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with multiple sclerosis (MS) receiving different disease-modifying treatments (DMTs).
Methods
Patients with MS with coronavirus disease 2019 (COVID-19) and available anti–SARS-CoV-2 serology were included. The primary endpoint was the anti–SARS-CoV-2 immunoglobulin G (IgG) index. The multivariate analysis was adjusted for COVID-19 severity, SARS-CoV-2 PCR result, and the time between COVID-19 onset and the serology.
Results
We included 61 patients with available IgG index. The IgG index was lower in patients with fingolimod or anti-CD20 monoclonal antibodies compared with patients without treatment (p < 0.01), patients with interferon β-1a or glatiramer (p < 0.01), and patients with another DMT (p = 0.01). The IgG index was correlated with the time between COVID-19 onset and serology (r = −0.296 [−0.510; −0.0477], p = 0.02).
Conclusions
Humoral response after COVID-19 was lower in patients with MS with fingolimod or anti-CD20 mAb. These patients could therefore be at risk of recurrent infection and could benefit from anti–SARS-CoV-2 vaccination. The humoral response after vaccination and the delay before vaccination need to be evaluated.
Classification of Evidence
This study provides Class IV evidence that patients treated with fingolimod or anti-CD20 monoclonal antibodies for MS have a lower humoral response after COVID-19 compared with patients without DMTs or with another DMTs.
Since 2019 and the pandemic outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the related disease (coronavirus disease 2019 [COVID-19]), physicians have been particularly concerned about patients with multiple sclerosis (MS) on disease-modifying treatments (DMTs), especially those with anti-CD20 monoclonal antibodies, such as ocrelizumab or rituximab.1,2 Indeed, patients with rituximab, ocrelizumab, or fingolimod had a higher risk of infection and a reduced humoral response to vaccines.3,4
The objective of our study was to analyze the humoral response after SARS-CoV-2 infection according to different DMTs.
Methods
Patients
Patients from MS centers in Alsace (France) were included prospectively in the French registry of COVID-19 in patients with MS or neuromyelitis optica spectrum disorder (NCT04355611, approval from the ethics committee of Sorbonne University #CER-2020–19).
COVID-19 diagnostic criteria were positive SARS-CoV-2 PCR on nasopharyngeal swab, thoracic CT abnormalities suggesting of COVID-19 (ground-glass opacities), rapidly evolving anosmia or ageusia without rhinitis or nasal obstruction, or COVID-19 typical symptoms (association of cough, fever, and asthenia) in an epidemic zone of COVID-19.
Data
Collected data were age, sex, Expanded Disability Status Scale (EDSS) score, DMT, comorbidity, date of COVID-19 onset, results of SARS-CoV-2 PCR, COVID severity, and date and results of SARS-CoV-2 serology.
DMTs were grouped as: “no treatment,” “no risk” (interferon β-1a and glatiramer acetate), “low risk” (teriflunomide, dimethyl fumarate, natalizumab, methotrexate, and mycophenolate), and “moderate to high risk” (fingolimod, rituximab, and ocrelizumab).
Comorbidities associated with severe COVID-19 were cardiovascular disease, pulmonary disease, diabetes, obesity (body mass index > 30 kg/m2), and smoking.
COVID-19 severity was scored on a 7-point ordinal scale:5
1–2 = not hospitalized
1, normal activities
2, reduction of daily activities
3–7 = hospitalized
3, hospitalized but without supplemental oxygen
4, required supplemental oxygen
5, noninvasive ventilation or high-flow oxygen
6, invasive mechanical ventilation or extracorporeal membrane oxygenation
7, death
SARS-CoV-2 Antibody Detection
Serum samples were tested using the Abbott or Roche chemiluminescent immunoassay detecting immunoglobulin G (IgG) against the SARS-CoV-2 spike protein. Tests were classified as positive or negative with a threshold according to the manufacturer's instructions in the range of 0.72–1.54 U/mL.
Statistical Analysis
The primary endpoint was the IgG index, and the secondary endpoint was the serologic status (negative or positive).
We excluded from the analysis patients with a SARS-CoV-2 serology within 6 weeks after the onset of COVID-19 because immunization against the virus was potentially not finished.
For the multivariate analysis, we selected variables with p < 0.20 in the univariate analysis among the following variables: age, sex, EDSS score, DMT, comorbidity, results of SARS-CoV-2 PCR, COVID severity, and the time between COVID-19 onset and serology. If a quantitative variable was not normally distributed, we changed it to a categorical variable according to the median. The candidate covariates were included in a linear regression model for the anti–SARS-CoV-2 IgG index endpoint and a logistic regression model for the serologic status endpoint (R software).
Data Availability
Anonymized data will be shared on request from any qualified investigator.
Results
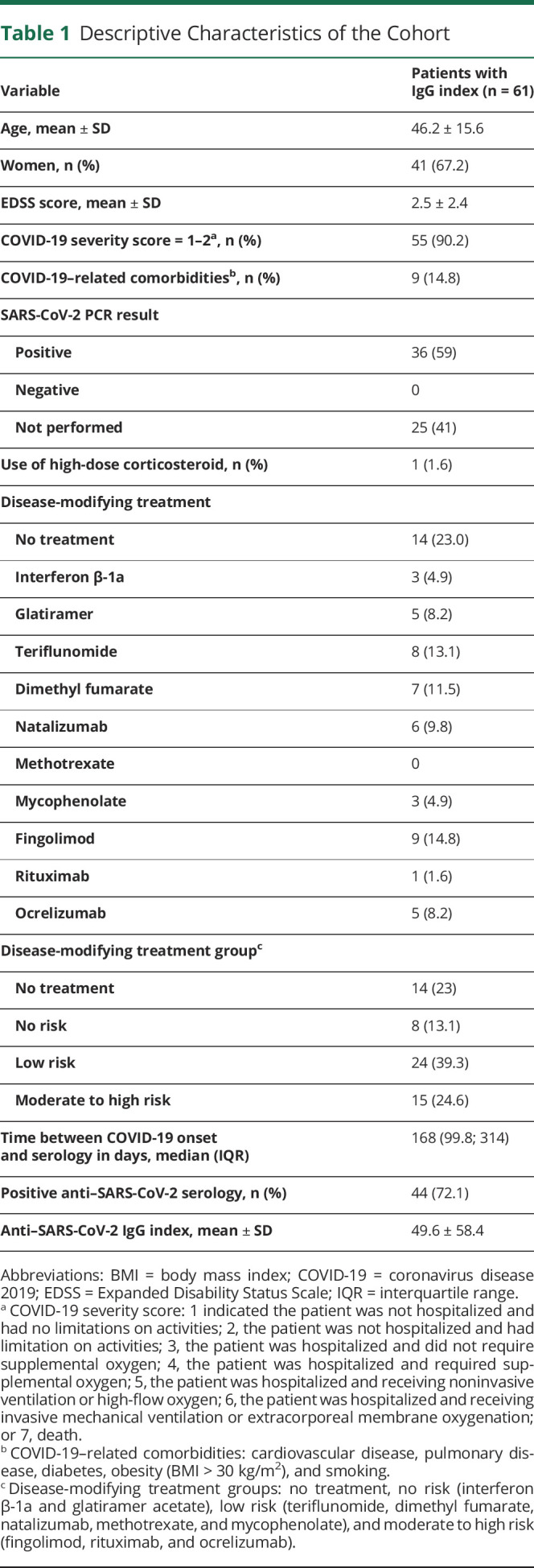
In our registry in the period of the study, 94 patients were infected by SARS-CoV-2, a serology with IgG index >6 weeks from the COVID-19 onset was available for 61 patients, and a serology without IgG index (only qualitative result: positive or negative) >6 weeks from the COVID-19 onset was available for 64 patients. Two patients from the registry died of COVID-19. No patients were vaccinated because no vaccines were available in France during the period of the study. DMTs were introduced in the 3 months before COVID-19 onset for only 3 patients and in the 6 months for 6 patients. Descriptive results are summarized in Table 1.
Table 1.
Descriptive Characteristics of the Cohort
Anti–SARS-CoV-2 Antibodies
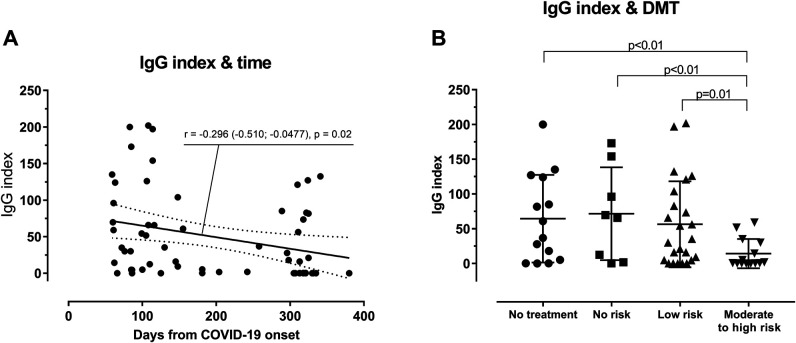
After univariate analysis (eTable 1, links.lww.com/NXI/A537), we selected the following relevant variables: DMT group (no treatment, no risk, low risk, and moderate to high risk), time between COVID-19 onset and the serology (≤168 and >168 days), COVID severity (grade 1–2 and grade ≥3), and the result of SARS-CoV-2 PCR (negative, positive, or not performed). The longer the time between COVID-19 onset and the serology, the lower the IgG index (r = −0.296 [−0.510; −0.0477], p = 0.02; Figure, A). Only 1 patient had a high dose of corticosteroid within the month before the onset of COVID-19 (grade of severity = 1). This variable was therefore not included in the model.
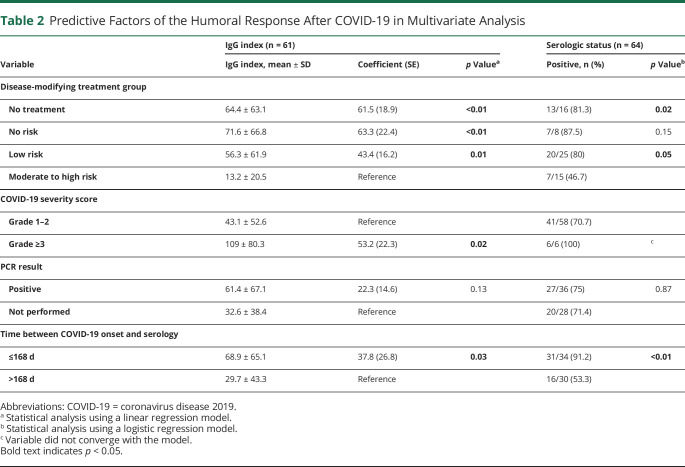
Figure. Anti–SARS-CoV-2 IgG Index According to Time and DMTs.
(A) Anti–SARS-CoV-2 IgG index according to the time from the onset of COVID-19. Statistical analysis was performed using a Pearson correlation coefficient r. (B) Anti–SARS-CoV-2 IgG index according to the DMT group (no treatment; no risk: interferon β-1a and glatiramer acetate; low risk: teriflunomide, dimethyl fumarate, natalizumab, methotrexate, and mycophenolate; and moderate to high risk: fingolimod, rituximab, and ocrelizumab). The analysis was adjusted for COVID-19 severity score, SARS-CoV-2 PCR result, and the time between the onset of COVID-19 and the serology using a linear regression model. COVID-19 = coronavirus disease 2019; DMT = disease-modifying treatment; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
In the multivariate analysis, the IgG index was significantly lower in the moderate to high risk DMT group compared with the other groups (Table 2 and Figure, B). Similarly, patients who had a severe COVID-19 (grade ≥3) had a higher IgG index than patients who did not (p = 0.02, Table 2). This analysis also confirmed that the IgG index decreased with time (p = 0.03, Table 2).
Table 2.
Predictive Factors of the Humoral Response After COVID-19 in Multivariate Analysis
For the serologic status in multivariate analysis (Table 2), serology was more often negative in the moderate to high risk DMT group (8/15 patients; 4/6 patients with anti-CD20 mAb; and 4/9 patients with fingolimod) than in the group without treatment (3/16 patients, p = 0.04) and the low-risk group (5/25 patients, p = 0.05), but no differences were found with the no-risk DMT group (1/8 patients, p = 0.15). Serology was more often negative after 168 days (p < 0.01). No differences were found for the result of PCR.
Patients With Repeated Serologies
At least 2 serologies were available in 15 patients. The mean time from COVID-19 onset to serology 1 was 95.3 ± 57.7 days and to serology 2 was 271.3 ± 84.3 days. The IgG index remained low for 2 of 4 patients in the moderate to high risk DMT group, whereas it increased before decreasing over time in the other groups (eFigure, links.lww.com/NXI/A537).
Discussion
We reported here a reduced humoral response after COVID-19 in patients treated with anti-CD20 mAb and fingolimod compared with patients without DMT or with other DMTs.
These results are consistent with previous case reports or case series, which suggest an absence or a poor humoral response after COVID-19 in patients with anti-CD20 mAb.6-9 Indeed, depleting B cells from pre–B cells to mature B cells, ocrelizumab affects humoral responses to infection and, subsequently, antibody production.3 A case series on DMTs in SARS-CoV-2 antibody-positive patients with MS showed that among the 14 patients, 6 patients were treated with fingolimod, suggesting a humoral response.10 However, in our study, 4 of 9 patients with fingolimod had a negative serology, suggesting a reduced humoral response, as previously demonstrated with immune responses against influenza vaccine.4
Thus, our results suggest that patients with fingolimod or anti-CD20 mAb could be at risk of reinfection with SARS-CoV-2. However, a study showed that patients with asymptomatic or mild COVID-19 had memory T-cell responses without detectable circulating antibodies leading to potential prevention of recurrent infection.11 In view of the high proportion of patients with a score of 1–2 on the COVID-19 severity scale in our cohort (≈90%), these patients could be protected by cellular mechanism than humoral mechanism.
Our study has some limitations. First, the time between the onset of COVID-19 and the anti–SARS-CoV-2 serology was not predetermined and was dependent on the availability of the serology. Second, only the humoral immunity was analyzed and not the cellular immunity, which plays an important role in the defense against viruses and the protection against COVID-19 through vaccination.12 Third, repetitive anti–SARS-CoV-2 serologies were not systematically performed and were available for only 13 patients, thus limiting the interpretation of the humoral response over time.
In conclusion, the humoral response after COVID-19 was lower in patients with MS with fingolimod or anti-CD20 mAb. Consequently, these patients could be at risk of recurrent infection and could benefit from anti–SARS-CoV-2 vaccination. The humoral response after vaccination and the delay before vaccination need to be evaluated.
Acknowledgment
The authors thank Thomas Senger for his assistance in collecting data.
Glossary
- COVID-19
coronavirus disease 2019
- DMT
disease-modifying treatment
- EDSS
Expanded Disability Status Scale
- IgG
immunoglobulin G
- IQR
interquartile range
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Appendix. Authors
Contributor Information
Laurent Kremer, Email: laurentdaniel.kremer@chru-strasbourg.fr.
Thibaut Fabacher, Email: thibaut.fabacher@chru-strasbourg.fr.
Livia Lanotte, Email: livia.lanotte@chru-strasbourg.fr.
Marie-Celine Fleury, Email: marie-celine.fleury@chru-strasbourg.fr.
Nicolas Collongues, Email: nicolas.collongues@chru-strasbourg.fr.
Jerome de Seze, Email: jerome.deseze@chru-strasbourg.fr.
Study Funding
The authors report no targeted funding.
Disclosure
The authors declare no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;70(9):1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):e1999-e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999-e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84(9):872-879. [DOI] [PubMed] [Google Scholar]
- 5.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-Ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382(19):1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maillart E, Papeix C, Lubetzki C, Roux T, Pourcher V, Louapre C. Beyond COVID-19: DO MS/NMO-SD patients treated with anti-CD20 therapies develop SARS-CoV2 antibodies?. Mult Scler Relat Disord. 2020;46:102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornton JR, Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in Two MS patients treated with ocrelizumab. Mult Scler Relat Disord. 2020;44:102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte WL. Attenuation of antibody response to SARS-CoV-2 in a patient on ocrelizumab with hypogammaglobulinemia. Mult Scler Relat Disord. 2020;44:102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallach AI, Picone MA. The presence of SARS-CoV2 antibodies in MS patients. Mult Scler Relat Disord. 2021;50:102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallucci G, Zito A, Baldanti F, et al. Safety of disease-modifying treatments in SARS-CoV-2 antibody-positive multiple sclerosis patients. Mult Scler Relat Disord. 2021;49:102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158.e14-168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594-599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on request from any qualified investigator.