Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been associated with several neurologic manifestations including the development of cerebral lesions resembling CNS vasculitis in elderly patients with severe coronavirus disease 2019 (COVID-19).1-4 Here, we report additional evidence for COVID-19–related CNS vasculitis, confirmed by biopsy, in a young healthy patient with otherwise mild COVID-19 infection.
Case Report
A 26-year-old woman experienced 4 days of anosmia, dysgeusia, malaise, and fatigue 6 days after an airplane flight in mid-March 2020. Approximately 2–3 weeks later, she noticed difficulty keeping “flip-flops” on her left foot slowly progressing to dragging of her left foot in July 2020. Neurologic examination revealed left pyramidal tract dysfunction with left-sided weakness (4+/5 Medical Research Council Scale of left elbow flexion, hip flexion, knee flexion, and foot dorsiflexion) and hyperreflexia (3+ in biceps, brachioradialis, triceps, patellar, and Achilles reflexes with unsustained foot clonus and pathologic reflexes). The rest of her examination was normal, and she denied headache or any change in mental status or cognition.
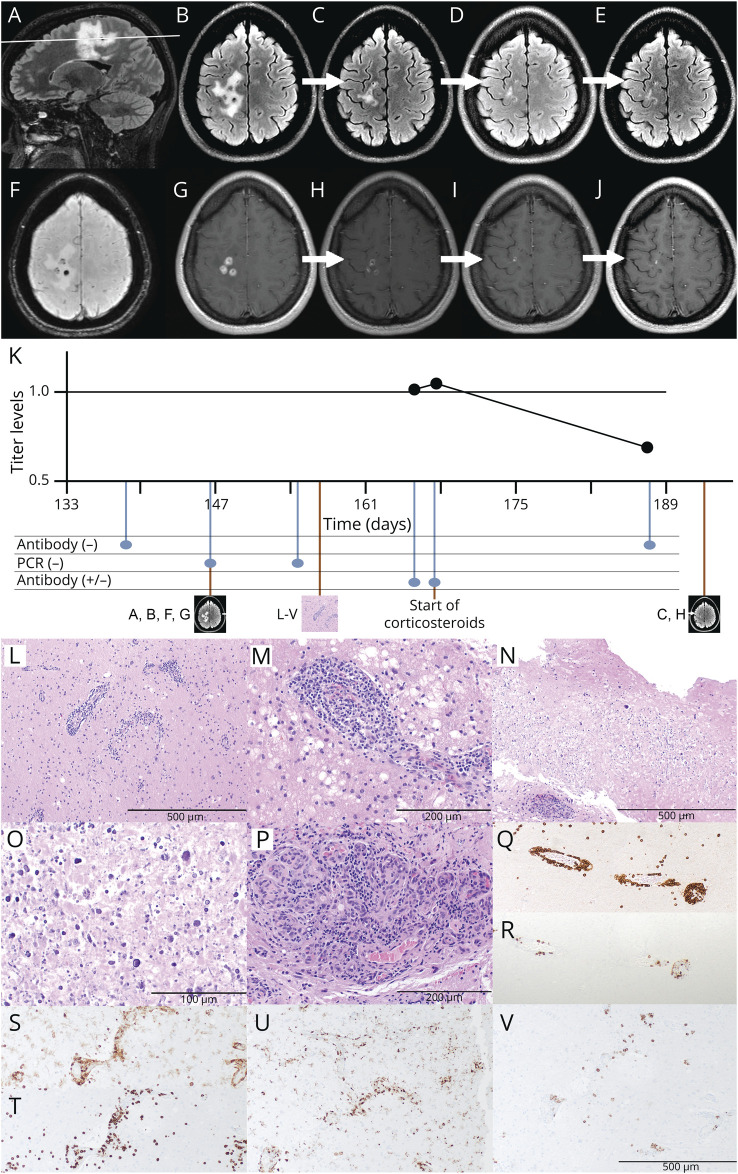
Her initial brain MRI demonstrated multiple, irregular, peripherally enhancing lesions clustered within the right frontoparietal white matter. These lesions also had peripheral diffusion restriction and surrounding T2/fluid-attenuated inversion recovery hyperintensity suggestive of edema and internal susceptibility likely reflective of blood products (Figure, A, B, F, G). Cervical spine MRI was normal. CSF analysis showed a cell count of 1/mm3, protein level of 29 mg/dL, and glucose of 59 mg/dL. SARS-CoV-2 PCR testing was not available during the episode of presumed COVID-19 symptoms in March 2020 and was nonreactive in August 2020 during her initial hospitalization. SARS-CoV-2 antinucleocapsid antibody testing was significant for 2 positive test results (Roche assay) in September 2020, although she had negative results in simultaneously obtained additional serologic assays (Diazyme, Abbott; Figure, K). No SARS-CoV-2 PCR was performed in the CSF. Evaluation in a COVID-19 infectious disease clinic resulted in confirmation of a prior COVID-19 infection by clinical criteria. Otherwise, extensive laboratory workup of the patient's serum and CSF for other infectious, neoplastic, or autoimmune causes was negative.
Figure. Serial MRIs, Time Course, and Histopathology.
(A, B, F, G) Initial brain MRI (5 months after presumed COVID-19 infection). The white line in A depicts the level of the axial sections in B–D. Sagittal (A) and axial (B) T2/FLAIR, susceptibility-weighted (F), and gadolinium-enhanced T1-weighted (G) sequences show multiple peripherally irregularly enhancing ovoid lesions within the right frontoparietal white matter with surrounding T2/FLAIR hyperintensity suggestive of vasogenic edema. (C–E, H–J) Repeat MRI at 1 (C, H), 3 (D, I), and 6 (E, J) months after the initiation of steroid treatment. Axial T2/FLAIR-weighted (C–E) and contrast-enhanced T1-weighted (H–J) sequences show a decreased size of T2/FLAIR hyperintensities and decreased contrast enhancement. (K) Time course and results of antinucleocapsid SARS-CoV-2 antibody testing. Time is shown as days since COVID-19 infection. The upper graph depicts the time course of available Roche titer levels (1.03, 1.05, and 0.7; the assay's cutoff of 1.0 is marked as a solid black line). Time point of initial and repeat MRI, biopsy, and initiation of corticosteroids is shown below the timeline. (L–V) Stereotactic biopsy of the right frontal lesion. (L–P) H&E-stained sections show edematous and gliotic white matter with extensive lymphoplasmacytic perivascular inflammation, as well as infiltration of vessel walls by lymphocytes, consistent with lymphocytic vasculitis (L: ×100; M: ×200). Multiple foci of eosinophilic coagulative necrosis with extensive dystrophic calcification were also noted (N: ×100; O: ×400). Focal endothelial swelling and hypertrophy, as well as vascular proliferation were present (P: ×200). (Q–V) Immunohistochemistry showed the perivascular and intravascular lymphocytes to be predominantly CD3-positive T cells (Q: CD3 IHC, ×100) with a small subset of CD20-positive B cells (R: CD20 IHC, ×100). Numerous perivascular CD4-positive T lymphocytes (S: CD4 IHC, ×100), many perivascular and parenchymal CD8-positive T lymphocytes (T: CD8 IHC, ×100), abundant perivascular macrophages and parenchymal activated microglia (U: CD68 IHC, ×100), and scattered perivascular plasma cells (V: CD138 IHC, ×100) were observed. Special stains for bacterial and fungal organisms including Gram, AFB, GMS, and HSV1/2 stains were negative (not shown) as well as tissue cultures. No viral inclusions were seen. AFB = acid-fast bacteria; COVID-19 = coronavirus disease 2019; FLAIR = fluid-attenuated inversion recovery; GMS = Grocott's methenamine silver; H&E = hematoxylin and eosin; HSV = herpes simplex virus; IHC = immunohistochemistry.
Stereotactic biopsy of one of the right frontoparietal lesions demonstrated findings consistent with lymphocytic vasculitis, including infiltration of vessel walls by lymphocytes (predominantly CD3+ [mixture of CD4+ and CD8+] T cells with a sparse admixture of CD20+ B cells) with endothelial hypertrophy. In addition, edematous and gliotic white matter changes with extensive lymphoplasmacytic perivascular inflammation were noted, along with parenchymal necrosis and abundant dystrophic calcification. No viral inclusions were seen (Figure, L–V).
The patient was treated with IV methylprednisolone 1,000 mg daily for 3 days, followed by an oral prednisone taper over 6 months starting at 60 mg daily. After 2 months, mycophenolate mofetil was added and uptitrated over 3 weeks to 1,000 mg twice daily with a 4-month overlap with the ongoing prednisone taper. The patient showed continued clinical improvement of her left-sided hemiparesis with minimal residual left lower extremity weakness on most recent examination. Follow-up brain MRIs at 1, 3, and 6 months after initiation of corticosteroids showed continuing decrease in the size and enhancement of right frontoparietal white matter lesions, with no new lesions (Figure, C–E, H–J).
Discussion
We report a case of biopsy-confirmed CNS vasculitis that shortly followed a COVID-19 infection. To our knowledge, this is the fifth reported case of COVID-19–related CNS vasculitis and the first to be confirmed by biopsy or to occur in a young patient with otherwise mild COVID-19 infection. As the patient's slowly progressive neurologic deficit began 2–3 weeks after her COVID-19 infection, this temporal correlation suggests a causal relationship, although there remains diagnostic uncertainty as PCR testing was not performed during her acute infection.
Notably, all 4 previous cases demonstrating CNS vasculitis were diagnosed based solely on imaging findings with no biopsy confirmation. All 4 were older (aged 64–69 years), experienced a more severe COVID-19 infection requiring intensive care unit level care, and developed earlier severe manifestation of neurologic symptoms compared with our case.1-4
A possible underlying mechanism is an endotheliitis caused by direct SARS-CoV-2 infection of endothelial cells through binding to the angiotensin-converting enzyme 2 receptor, which has been shown in renal, gastrointestinal, pulmonal, and cerebral vasculature.5,6 Alternatively, a proinflammatory state may have unmasked a primary CNS vasculitis. Interesting differences between this case and typical primary CNS vasculitis include the lack of headache or alteration in mental status/cognition, although a variety of phenotypes can be seen in this condition. Regardless of the mechanism, our case demonstrates that young patients with mild COVID-19 infection are still at risk for neurologic complications. Further studies of long-term outcomes in similar cases are needed to optimize the choice and duration of immunosuppression.
Acknowledgment
The authors thank the UC San Diego Neuropathology Division Fund for making possible the immunohistologic stains included in this article.
Appendix. Authors
Contributor Information
Garrett M. Timmons, Email: garrett.timmons@gmail.com.
Torge Rempe, Email: trempe@health.ucsd.edu.
Elizabeth A. Bevins, Email: eareed@health.ucsd.edu.
Vanessa Goodwill, Email: vgoodwill@health.ucsd.edu.
Annalise Miner, Email: aeminer@health.ucsd.edu.
Arthur Kavanaugh, Email: akavanaugh@health.ucsd.edu.
Michele Ritter, Email: mlritter@health.ucsd.edu.
Study Funding
The authors report no targeted funding.
Disclosure
The authors declare that they have no competing interests. Unrelated to the work, the authors declare the following: G.M. Timmons reports no disclosures relevant to the manuscript. T. Rempe receives grant funding from the National MS Society. E.A. Bevins, V. Goodwill, and A. Miner report no disclosures relevant to the manuscript. A. Kavanaugh conducted research sponsored by AbbVie, Amgen, Eli Lilly, Novartis, and Pfizer. M. Ritter reports no disclosures relevant to the manuscript. J.S. Graves over the past year has grant/contract research support from the National MS Society, Biogen, and Octave Bioscience. She serves on a steering committee for a trial supported by Novartis; she has received honoraria for a nonpromotional, educational activity for Sanofi-Genzyme; and she has received speaker fees from Alexion and BMS and served on an advisory board for Genentech. Go to Neurology.org/NN for full disclosures.
References
- 1.Dixon L, Coughlan C, Karunaratne K, et al. Immunosuppression for intracranial vasculitis associated with SARS-CoV-2: therapeutic implications for COVID-19 cerebrovascular pathology. J Neurol Neurosurg Psychiatry. 2021;92:103-104. [DOI] [PubMed] [Google Scholar]
- 2.Hanafi R, Roger PA, Perin B, et al. COVID-19 neurologic complication with CNS vasculitis-like pattern. AJNR Am J Neuroradiol. 2020;41(8):1384-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira RMC, Santos DH, Olivetti BC, et al. Bilateral trochlear nerve palsy due to cerebral vasculitis related to COVID-19 infection. Arq Neuropsiquiatr. 2020;78(6):385-386. [DOI] [PubMed] [Google Scholar]
- 4.Vaschetto R, Cena T, Sainaghi PP, et al. Cerebral nervous system vasculitis in a Covid-19 patient with pneumonia. J Clin Neurosci. 2020;79:71-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vargas G, Medeiros Geraldo LH, Gedeão Salomão N, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and glial cells: insights and perspectives. Brain Behav Immun Health. 2020;7:100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92(7):699-702. [DOI] [PMC free article] [PubMed] [Google Scholar]