Abstract
Objective
To evaluate the clinical consequences of extended interval dosing (EID) of ocrelizumab in relapsing-remitting multiple sclerosis (RRMS) during the coronavirus disease 2019 (COVID-19) pandemic.
Methods
In our retrospective, multicenter cohort study, we compared patients with RRMS on EID (defined as ≥4-week delay of dose interval) with a control group on standard interval dosing (SID) at the same period (January to December 2020).
Results
Three hundred eighteen patients with RRMS were longitudinally evaluated in 5 German centers. One hundred sixteen patients received ocrelizumab on EID (median delay [interquartile range 8.68 [5.09–13.07] weeks). Three months after the last ocrelizumab infusion, 182 (90.1%) patients following SID and 105 (90.5%) EID patients remained relapse free (p = 0.903). Three-month confirmed progression of disability was observed in 18 SID patients (8.9%) and 11 EID patients (9.5%, p = 0.433). MRI progression was documented in 9 SID patients (4.5%) and 8 EID patients (6.9%) at 3-month follow-up (p = 0.232). Multivariate logistic regression showed no association between treatment regimen and no evidence of disease activity status at follow-up (OR: 1.266 [95% CI: 0.695–2.305]; p = 0.441). Clinical stability was accompanied by persistent peripheral CD19+ B-cell depletion in both groups (SID vs EID: 82.6% vs 83.3%, p = 0.463). Disease activity in our cohort was not associated with CD19+ B-cell repopulation.
Conclusion
Our data support EID of ocrelizumab as potential risk mitigation strategy in times of the COVID-19 pandemic.
Classification of Evidence
This study provides Class IV evidence that for patients with RRMS, an EID of at least 4 weeks does not diminish effectiveness of ocrelizumab.
Immunotherapy for relapsing-remitting multiple sclerosis (RRMS) is critical for maintaining disease stability, but potentially increases the risk of infection. This is of particular importance in light of the ongoing coronavirus disease 2019 (COVID-19) pandemic. In general, pulsed depletion of CD20-expressing B cells by ocrelizumab or rituximab can increase the risk of respiratory infections for several months.1-3 Regarding COVID-19 disease, it has recently been discussed that B cell–depleting therapies may not only be accompanied with higher rates of infection, but could also influence the severity and mortality,4,5 albeit well-controlled data are still lacking.
Drug-free intervals are long between 2 courses of ocrelizumab as its treatment effect is determined by long-lasting (selective) immune suppression eventually appraisable by peripheral B-cell reconstitution.6 This provides the opportunity to individually delay therapy during the pandemic.7,8 In addition, extended interval dosing (EID) might also be favorable in terms of severe acute respiratory syndrome coronavirus 2 vaccine response, which is probably reduced under therapeutic approaches with B-cell depletion.9-11 During the first peak of the COVID-19 outbreak between January 2020 and September 2020 in Germany, several treatment courses of ocrelizumab were delayed due to safety concerns. Although some smaller studies suggest longer treatment-free intervals of B cell–depleting therapies in RRMS without lack of efficacy,8,12,13 real-world data on EID in a larger cohort of ocrelizumab-treated patients with RRMS are still lacking.
We here report clinical outcomes of delayed ocrelizumab infusions during the COVID-19 pandemic in 116 patients on EID compared with 202 patients on standard interval dosing (SID).
Methods
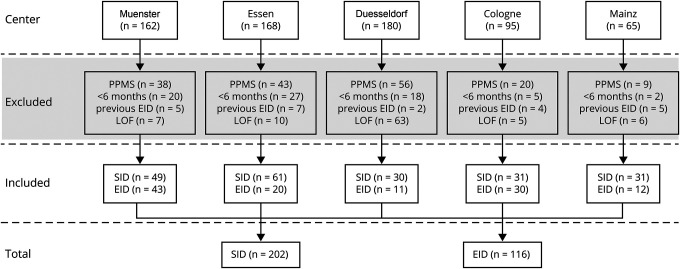
We performed an ad hoc analysis of our observational, multicentric cohort of adult patients with RRMS undergoing ocrelizumab treatment. Ocrelizumab therapy was performed at the German University Hospitals Muenster, Mainz, Essen, Duesseldorf, and Cologne in accordance with national and international guidelines. We included patients with RRMS who received at least both initial treatment cycles of ocrelizumab (2 × 300 mg with a 2-week interval) before experiencing SID or EID during the observation period. In other words, the observation period in which either the SID or EID took place always related to maintenance cycle (600 mg). The SID was defined as regular maintenance interval of ocrelizumab infusion after 6 months, whereas the EID group included patients with an ocrelizumab infusion delay of at least 4 weeks (6 months + ≥4 weeks delay). Patients were excluded if (1) they were treated with ocrelizumab for primary progressive MS, (2) if only the 2 induction cycles with 300 mg were administered (treatment duration with ocrelizumab <6 months), (3) experienced EID before the observation period (before January 2020), (4) or if no follow-up data were available (Figure 1).
Figure 1. Flowchart of Case Ascertainment.
This flowchart depicts how the 318 ocrelizumab-treated patients with relapsing-remitting multiple sclerosis (RRMS) were identified. The source population was all patients with multiple sclerosis (MS) treated with ocrelizumab in 5 German centers during the period between January 2020 and September 2020. We excluded patients with MS who were treated with ocrelizumab due to a primary progressive disease course (PPMS), if only the 2 induction cycles with 300 mg were administered (treatment duration with ocrelizumab <6 months (Mo) during the observational period). SID and EID do not refer to the first 2 half doses with 300 mg, respectively, if patients experienced an extended interval dosing (EID) before Infusion C (the infusion administered between January 2020 and September 2020 that led to the division of the 2 groups–standard interval dosing [SID] and EID), or if the patient for any other reason did not satisfy the inclusion criteria (e.g., loss of follow-up [LOF]).
Looking at the period between January 2020 and September 2020, patients receiving ocrelizumab EID were compared with patients receiving ocrelizumab on SID (Figure 2). The 2 infusions defining SID vs EID (Infusions B and C, Figure 2) were defined as follows: Infusion B was the last ocrelizumab infusion (second 300 mg cycle or 600 mg maintenance infusion) before January 2020, and Infusion C (always 600 mg standard maintenance dose) was the subsequent infusion, administered between January 2020 and September 2020. A relapse was defined as a neurologic deficit related with an acute inflammatory demyelinating event that lasts at least 24 hours in the absence of infection or fever.
Figure 2. Flowchart of the Study Procedure.
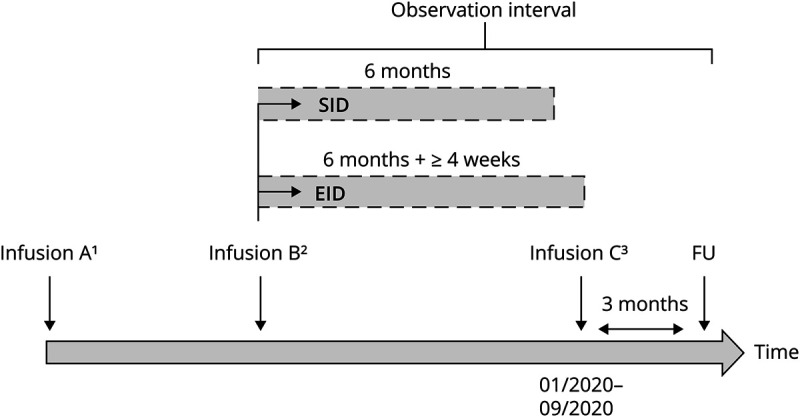
1Infusion A was defined as the second last ocrelizumab infusion (300 mg or 600 mg cycles) before January 2020 (before the coronavirus disease 2019 pandemic started in Germany). 2Infusion B was defined as the last ocrelizumab infusion (second 300 mg infusion or 600 mg dose) before January 2020 and as the beginning of the observation interval. 3The infusion that followed on, further referred to as Infusion C, was the infusion administered between January 2020 and September 2020 (always 600 mg maintenance cycle). EID = extended interval dosing; FU = follow-up; SID = standard interval dosing.
Confirmed progression of disability (CPD) was determined by standardized neurologic examinations 3 months following Infusion C, further referred to as follow-up (3 months ± 10 days after the last ocrelizumab infusion). Clinical and MRI outcomes were collected at the end of the observation interval (Figure 2). MRI progression was defined as new or enlarged T2-weighted or T1-weighted gadolinium-enhancing lesions. Expanded Disability Status Scale (EDSS) progression was considered clinically relevant if 2 independent clinical assessments 3 months apart (at Infusion C and follow-up) indicated an increase of the EDSS as follows: +1.5 points (baseline = 0.0), +1.0 point (baseline = 1.0–4.0), and +0.5 points (baseline ≥ 4.5). Treatment success was further classified with the concept of no evidence of disease activity (NEDA-3). While at Infusion B, NEDA-3 status was related to the time period of 6 months before this infusion B (in other words between Infusion A and Infusion B), NEDA-3 status during the observation period (between Infusion B and follow-up) was calculated based on the time period between Infusion B to follow-up (including Infusion C, Figure 2).14 Peripheral blood CD19+ B-cell depletion was defined as < 10 cells/μL.
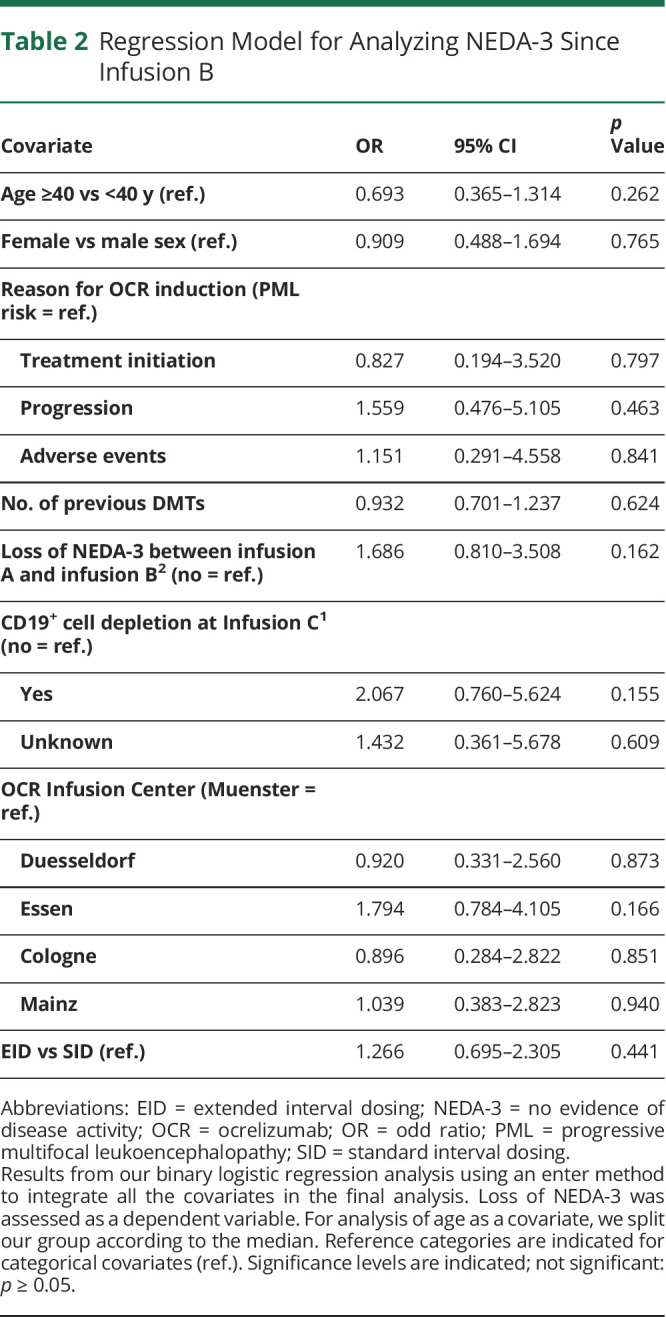
The Mann-Whitney U test (ordinal) or χ2 test (categorical) was used for comparison of demographic and clinical features where appropriate. Binary logistic regression was performed, using loss of NEDA-3 status as the dependent variable and sex, age (above vs below median), reason for ocrelizumab initiation (treatment-naive patients, disease progression, adverse events, or risk of progressive multifocal leukoencephalopathy number of previous disease-modifying therapies, loss of NEDA-3 before the observational period, and CD19+ B-cell depletion at Infusion C as covariates in an enter method. Statistical analysis was conducted using SPSS Statistics 26 (IBM, NY).
Standard Protocol Approvals, Registrations, and Patient Consents
Ethical approval was obtained from local authorities (2016-002937-31; 2019-712-f-S; 2017044238), and patients gave informed consent.
Data Availability
Data will be shared with qualified investigators on request; please contact meuth@uni-duesseldorf.de.
Results
Three hundred eighteen patients with RRMS treated with ocrelizumab between January 2020 and September 2020 were included in our study (Figure 1). One hundred sixteen patients received ocrelizumab on EID (median delay [interquartile range, IQR] 8.68 [5.09–13.07] weeks), and 202 patients received ocrelizumab on SID (median delay [IQR] −0.07 [−1.07 to 1.07] weeks). Baseline parameters were evenly balanced between groups (Table 1). Moreover, no significant differences between the SID and EID group in terms of disease activity before the observation period (before Infusion B) were evident (number of patients with relapses [SID vs EID]: 14 [6.9%] vs 9 [7.8%], p = 0.783; with CPD: 10 [5.0%] vs 6 [5.2%], p = 0.466; with MRI progression: 24 [11.9%] vs 11 [9.5%], p = 0.943; with loss of NEDA-3 Infusion B: 39 patients [19.3%] vs 17 patients [14.6%], p = 0.860).
Table 1.
Baseline Characteristics of the Ocrelizumab Cohort and Subgroups (Total N = 318)
Regarding the interval between Infusion B and follow-up, no significant differences in clinical and radiologic measurements of disease progression between SID and EID were visible. In total, 29 patients (9.1%) showed 3-month CPD at follow-up, with 18 patients on SID (8.9%) and 11 (9.5%) on EID (p = 0.433). Moreover, 20 patients (9.9%) on SID experienced a relapse since Infusion B vs 11 patients (9.5%) on EID (p = 0.903). MRI progression was evident in 9 patients (4.5%) on SID vs 8 patients (6.9%) on EID (p = 0.232). Of note, 39 patients (19.3%) on SID experienced loss of NEDA-3 at follow-up, compared with 25 patients (21.6%) on EID (p = 0.312). The adjusted OR for loss of NEDA-3 since Infusion B was 1.266 (95% CI: 0.695–2.305; p = 0.441), with no selection of further covariates (Table 2). Of note, NEDA-3 status at follow-up (p = 0.262) as well as the 3-month CPD rate (p = 0.814), the relapse rate (p = 0.086), and MRI activity (p = 0.754) since Infusion B were not related to the duration of EID.
Table 2.
Regression Model for Analyzing NEDA-3 Since Infusion B
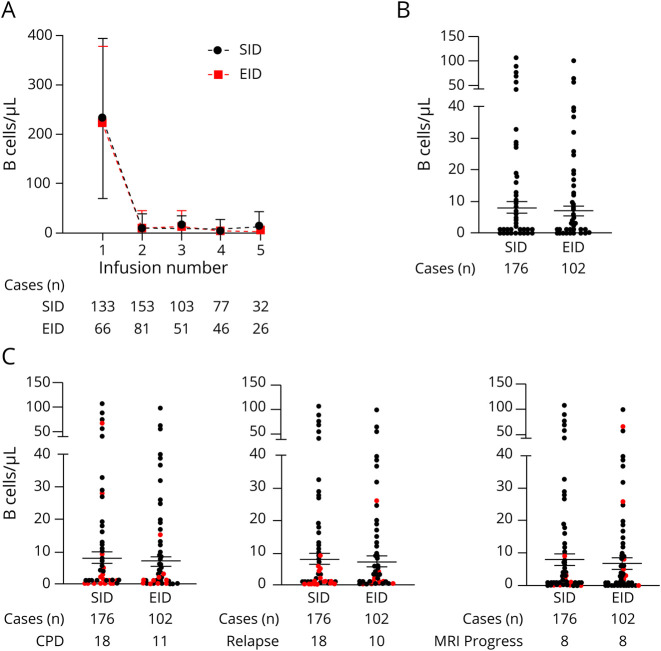
Next, we analyzed the available longitudinal B-cell levels of our cohort (also data that were available before the COVID-19 pandemic, Figure 3A) to illustrate the dynamic of B-cell depletion and repopulation. The absolute B-cell counts decreased after the first ocrelizumab infusion and remained low over the entire treatment period. No differences in longitudinal B-cell counts between the SID and the EID cohort were visible. At Infusion C, absolute peripheral CD19+ B-cell counts were available in 278 of 318 patients (87.4%). Of note, CD19+ B-cell depletion was widely persistent (Figure 3B), with a percentage of patients depleted at Infusion C of 82.6% (150/176) on SID vs 83.3% (85/102) on EID (p = 0.463). Moreover, CD 19+ B-cell depletion at Infusion C was not related to the duration of EID (p = 0.337).
Figure 3. B-Cell Levels Before and During Ocrelizumab Treatment in Patients With Relapsing-Remitting Multiple Sclerosis.
(A) CD19+ B-cell levels at sampling immediately before ocrelizumab infusions 1 to 5 in the standard interval dosing (SID) group compared with the extended interval dosing (EID) group. Infusion number 1 represents both baseline ocrelizumab infusions (2 × 300 mg with a 2-week interval). The numbers of cases used to estimate the means and SDs are shown below the figure. B-cell counts are presented as mean absolute counts of CD19+ cells/μL of blood. (B) The CD19+ B-cell levels immediately before ocrelizumab Infusion C (the infusion administered between January 2020 and September 2020 that led to the division of the 2 groups—SID and EID). B-cell counts are presented as absolute numbers of CD19+ cells/μL of peripheral blood. The number of patients with absolute CD19+ B-cell counts available at Infusion C is shown below the figure. (C) CD19+ B-cell counts immediately before ocrelizumab Infusion C are depicted. CD19+ B-cell numbers of patients who experienced either a 3-month confirmed progression of disability (CPD, left), a relapse (center), or MRI progression (either new or enlarged T2-weighted lesions or T1-weighted gadolinium enhancement, right) between Infusion B and follow-up are highlighted in red. The number of patients with absolute CD19+ B-cell counts available at Infusion C as well as those with disease activity is shown below the figure.
With regard to Infusion C, we did not observe a significant difference in re-emerging disease activity between the patients with persistent B-cell depletion (n = 235) and those with evidence of B-cell repopulation (n = 43, relapse: p = 0.616, MRI progression: p = 0.828, CPD: p = 0.671, graphical illustration of individual B-cell counts at Infusion C and disease activity is shown in Figure 3C).
Of interest, 4 patients (1.3%) of our multicentric cohort had COVID-19 disease during the observation period. Two of them were in the EID cohort (1.7%), and the other 2 received ocrelizumab on SID (0.9%). Two patients were female, and they were aged 46, 33, 23, and 40 years at the time of COVID-19 disease. Apart from RRMS and the associated ocrelizumab treatment, none of these patients had other existing chronic conditions or an otherwise compromised immune system. All of them experienced a mild to moderate disease course and had classical symptoms of fever, dry cough, and tiredness. Two patients reported loss of taste and smell and headache, and 1 had diarrhea during the infection. Only 1 patient (from the SID group) required hospitalization; however, not for COVID-19 symptoms, but rather due to acute but short-lasting clinical deterioration of RRMS. All patients recovered from COVID-19 without sequelae.
Discussion
Considering the potential infection risks in times of COVID-19 and the future vaccine response, it is crucial to evaluate whether dosing intervals of immune cell–depleting therapies can be extended.10,15 Furthermore, as general infection risks may increase with treatment duration and age while benefits may decrease,16 long-term B cell–depleting treatment strategy studies are needed.
Here, we show real-world data of patients who received ocrelizumab on EID (median delay [IQR] 8.68 [5.09–13.07] weeks) compared with patients treated at regular intervals. The rate of patients reaching NEDA-3 did not differ significantly between both groups, suggesting that EID of at least 4 weeks did not diminish effectiveness of ocrelizumab, at least after short-term evaluation. Although a substantial proportion of our cohort had an aggressive disease course and had been on highly active immunotherapies before ocrelizumab initiation, our EID results are consistent with the high NEDA-3 rates observed in phase III clinical trials.14
Our findings support previous results from smaller studies in patients with RRMS receiving ocrelizumab12,13 or rituximab,8 indicating long-term disease stability after few treatment cycles. Albeit most of our patients showed persistent B-cell depletion on EID, recurrence of CD19+ B cells may occur in the absence of disease activity.8 Although our data did not reveal an association between absolute peripheral CD19+ B-cell number and re-emerging disease activity, low levels have been discussed to serve as surrogate marker to justify delaying B cell–depleting infusions,17 in particular in other disease entities.18,19
Considering that the incidence of upper respiratory tract infection was increased in ocrelizumab phase III clinical trials in RRMS,3 there are some concerns about the infection risk and severity of COVID-19 in patients with MS treated with ocrelizumab. In our cohort, 4 patients had COVID-19 disease regardless of the dosing interval. Besides the severe B-cell impairment (documented B-cell counts in 3 patients at COVID-19 infection were 0, 0, and 4 cells/μL, respectively) and partly higher disability (EDSS in the affected patients was 2.0, 2.5, 4.0, and 8.0, respectively), representing an additional risk factor for COVID-19 severity,20,21 clinical presentation was mild to moderate in all patients. Of note, it is currently still unclear whether the CD19+ B-cell level in the peripheral blood correlates with the severity of a COVID-19 disease.22,23 Although our study was not designed to identify the effect of EID on the clinical outcomes of a COVID-19 disease, the fact that B-cell depletion is maintained in our EID cohort suggests that this strategy might not mitigate the risk of severe COVID-19 disease. However, we cannot formally rule out differential effects in tissues like spleen, lymph nodes, bone marrow, or the CNS. Thus, an EID might lead to an earlier B-cell repopulation simultaneously in the bone marrow and spleen before the B cells reappear in the peripheral blood, resulting in an earlier immunity.24,25
Our findings of a favorable outcome in the absence of severe complications reflect the preliminary results of an Italian study in patients with MS, in which only 5% of 232 cases of COVID-19 disease were defined as severe or critical,21 and those of several case series on COVID-19–related pneumonia in patients with MS under ocrelizumab treatment.26-28 Contrastingly, other authors reported a more severe, even fatal, COVID‐19 disease course in RRMS cases treated with ocrelizumab.4,5,29 As such, the data published are conflicting, possibly explained by selection bias and confounding factors (e.g., age, EDSS) not sufficiently controlled for in the mostly retrospective cohort studies available so far.
In addition, given the effect of ocrelizumab in compromising the immune system, an impact on immunization responses cannot be ruled out, introducing new challenges in the rapid pandemic outbreak of COVID-19. Although the B-cell response to a variety of different vaccines is markedly inhibited by CD20 depletion,11,30,31 an EID might probably increase the likelihood of repopulation of naive B cells and thus the response to the current COVID-19 vaccines.10,13 However, in our study, we did not observe a difference in CD19+ B-cell repopulation rates between the SID and the EID group, probably due to a relatively short EID interval (median delay 8.68 weeks). Thus, the immunogenicity of SARS-CoV2 vaccines in patients with RRMS during treatment with ocrelizumab and whether the immune response mounted by antigenic stimulation of these vaccines is enhanced in EID need to be investigated in future studies.
As a limitation, we would like to stress the short observation period and possible selection bias of our study, including individual physician and/or patient decisions to potentially delay ocrelizumab infusion irrespective of disease activity in the light of the infection risk during the COVID-19 pandemic. These preclude a general recommendation for EID in patients treated with ocrelizumab. In particular, it might be important to determine whether the extension of a single infusion interval has a significant impact on disease progression over a longer period of time. For this purpose, follow-up of the cohort over a period of more than 12 months might be useful. Moreover, future prospective, noninferiority studies should investigate the long-term approach of continuous EID in terms of clinical outcomes and safety concerns. In addition, other outcome parameters such as Multiple Sclerosis Functional Composite score or sub scores as well as neurofilament light chain levels should be considered to evaluate disease progression.3,32
Nevertheless, in light of the current COVID-19 pandemic, clinicians can benefit from our results obtained from a well-characterized, large, multicenter cohort, when evaluating risk-based treatment strategies on an individual level. Given the successful transfer of real-world retrospective data33,34 into the considerations of a prospective clinical trial of natalizumab in RRMS (NCT03689972), our findings may help when designing future studies for long-term therapy with B cell–depleting agents.
Acknowledgment
The authors thank all patients who were part of this study. The current work was conducted outside of third-party funding.
Glossary
- COVID-19
coronavirus disease 2019
- CPD
confirmed progression of disability
- EDSS
Expanded Disability Status Scale
- EID
extended interval dosing
- IQR
interquartile range
- NEDA-3
no evidence of disease activity
- RRMS
relapsing-remitting multiple sclerosis
- SID
standard interval dosing
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Contributor Information
Leoni Rolfes, Email: leoni.rolfes@ukmuenster.de.
Marc Pawlitzki, Email: marc.pawlitzki@ukmuenster.de.
Steffen Pfeuffer, Email: steffen.pfeuffer@ukmuenster.de.
Christopher Nelke, Email: christopher.nelke@ukmuenster.de.
Anke Lux, Email: anke.lux@med.ovgu.de.
Refik Pul, Email: refik.pul@uk-essen.de.
Christoph Kleinschnitz, Email: christoph.kleinschnitz@uk-essen.de.
Konstanze Kleinschnitz, Email: konstanzekleinschnitz@hotmail.com.
Rebeca Rogall, Email: rebecca.rogall@gmx.de.
Katrin Pape, Email: katrin.pape@unimedizin-mainz.de.
Stefan Bittner, Email: bittner@uni-mainz.de.
Frauke Zipp, Email: zipp@uni-mainz.de.
Clemens Warnke, Email: clemens.warnke@uk-koeln.de.
Yasemin Goereci, Email: yasemin.goereci@uk-koeln.de.
Michael Schroeter, Email: michael.schroeter@uk-koeln.de.
Jens Ingwersen, Email: jens.ingwersen@uni-duesseldorf.de.
Orhan Aktas, Email: orhan.aktas@med.uni-duesseldorf.de.
Luisa Klotz, Email: luisa.klotz@ukmuenster.de.
Tobias Ruck, Email: tobias.ruck@med.uni-duesseldorf.de.
Heinz Wiendl, Email: heinz.wiendl@ukmuenster.de.
Study Funding
No targeted funding reported.
Disclosure
L. Rolfes received travel reimbursements from Merck Serono and Sanofi-Aventis. M. Pawlitzki received speaker honoraria and travel/accommodation/meeting expenses and research funding from Novartis. His research is founded by the German Multiple Sclerosis Society North Rhine-Westphalia (DMSG) and the program “Innovative Medizinische Forschung” (IMF) of the Medical Faculty of the University of Muenster. S. Pfeuffer received travel grants from Sanofi-Aventis and Merck Serono, lecturing honoraria from Sanofi-Aventis, Mylan Healthcare, and Biogen Idec, and research support from Diamed, Merck Serono, and the German Multiple Sclerosis Society North Rhine-Westphalia. C. Nelke and A. Lux report no disclosures relevant to the manuscript. R. Pul received honoraria for lecturing and consulting from Alexion, Bayer HealthCare, Biogen Idec, Bristol-Myers Squibb, MedDay, Merck Serono, Mylan, Novartis, Roche, and Sanofi-Aventis. He received research fund from HERZ Burgdorf, Merck Serono, and Novartis. C. Kleinschnitz received honoraria for lecturing and consulting as well as financial research support from Ablynx, Almirall, Amgen, Bayer Vital, Bristol-Myers Squibb, Biotronik, Boehringer Ingelheim, Biogen Idec, CSL Behring, Daiichi-Sankyo, Desitin, Eisai, Ever Pharma, Sanofi-Aventis, Merck Serono, Mylan, MedDay, Novartis, Pfizer, Roche, Siemens, Stago, and Teva. K. Kleinschnitz and K. Pape report no disclosures relevant to the manuscript. S. Bittner has received funding for travel expenses for attending meetings from Novartis and Merck Serono and honoraria from Biogen Idec, Merck Serono, Novartis, Roche, Sanofi-Aventis, and Teva. His research is funded by Deutsche Forschungsgemeinschaft (DFG) and Hertie Foundation. F. Zipp received research grants and/or consultation funds from the DFG, BMBF, PMSA, Genzyme, Merck Serono, Roche, Novartis, Sanofi-Aventis, Celgene, ONO, and Octapharma. S. Bittner received research grants and/or consultation funds from the DFG, BMBF, PMSA, Genzyme, Merck Serono, Roche, Novartis, Sanofi-Aventis, Celgene, ONO, and Octapharma. C. Warnke has received institutional compensation for research, serving on scientific advisory boards, or lecturing from Novartis, Sanofi-Aventis, Alexion, Janssen, Biogen, and Roche. Y. Göreci and J. Ingwersen report no disclosures relevant to the manuscript. O. Aktas received honoraria for lecturing and travel expenses for attending meetings from Alexion, Almirall, Bayer HealthCare, Biogen, Celgene, Genzyme, Merck Serono, Novartis, Roche, Sanofi-Aventis, Teva, and VielaBio. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), and by Biogen and Novartis. L. Klotz received compensation for serving on scientific advisory boards for Genzyme, Janssen, Novartis, and Roche. She received speaker honoraria and travel support from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, and Teva. She receives research support from the German Ministry for Education and Research, the German Research Foundation, the IZKF Münster, IMF Münster, Biogen Idec, Novartis, and Merck Serono. T. Ruck reports grants from German Ministry of Education, Science, Research and Technology; grants and personal fees from Sanofi-Aventis and Alexion; personal fees from Biogen Idec, Roche, and Teva; and personal fees and nonfinancial support from Merck Serono, outside the submitted work. Heinz Wiendl received grants from German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, the European Union, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and RE Children's Foundation, Biogen Idec, GlaxoSmithKline GmbH, Roche, and Sanofi-Aventis, consulting fees from AbbVie, Actelion, Biogen Idec, IGES, Johnson & Johnson, Novartis, Roche, and Sanofi-Aventis, support for travel to meetings for other purposes from AbbVie, Actelion, Biogen, IGES, Johnson & Johnson, Novartis, Roche, and Sanofi-Aventis, fees for participation in review activities such as data monitoring boards from PSI CRO Deutschland GmbH, Swiss Multiple Sclerosis Society, payment for lectures from Alexion, Biogen Idec, Cognomed, Roche, Hertie Foundation, Merck Serono, Novartis, Roche, Genzyme, Teva, and WebMD Global, and honorarium for expert testimony from Alexion, Biogen Idec, Merck Serono, Novartis, and Sanofi-Aventis, outside the submitted work. He has filed patents No SEP-103.323-1/08, EP2769223, WO2013057092 (A1), and 15001186.4–1402. S.G. Meuth received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer HealthCare, Biogen Idec, Celgene, Diamed, Sanofi-Aventis, MedDay, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Bundesinstitut für Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology, and by Alexion, Almirall, Amicus Therapeutics Germany, Biogen Idec, Diamed, Fresenius Medical Care, Sanofi-Aventis, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. Go to Neurology.org/NN for full disclosures.
References
- 1.Ancau M, Berthele A, Hemmer B. CD20 monoclonal antibodies for the treatment of multiple sclerosis: up-to-date. Expert Opin Biol Ther. 2019;19(8):829-843. [DOI] [PubMed] [Google Scholar]
- 2.Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. [DOI] [PubMed] [Google Scholar]
- 4.Müller T. Antikörper gegen CD 20: ein Risiko in der Pandemie? InFo Neurologie. 2020;22(10):71-72. [Google Scholar]
- 5.Zabalza A, Cardenas-Robledo S, Tagliani P, et al. COVID-19 in MS patients: susceptibility, severity risk factors and serological response. Eur J Neurol. 2020. Dec 19. doi: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- 6.Lunemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16(1):56-62. [DOI] [PubMed] [Google Scholar]
- 7.Pawlitzki M, Zettl UK, Ruck T, Rolfes L, Hartung HP, Meuth SG. Merits and culprits of immunotherapies for neurological diseases in times of COVID-19. EBioMedicine. 2020;56:102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maarouf A, Rico A, Boutiere C, et al. Extending rituximab dosing intervals in patients with MS during the COVID-19 pandemic and beyond? Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker D, Roberts CAK, Pryce G, et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol. 2020;202(2):149-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999-e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barun B, Gabelic T, Adamec I, et al. Influence of delaying ocrelizumab dosing in multiple sclerosis due to COVID-19 pandemics on clinical and laboratory effectiveness. Mult Scler Relat Disord. 2020;48:102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker D, Pryce G, James LK, Marta M, Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult Scler Relat Disord. 2020;44:102279. [DOI] [PubMed] [Google Scholar]
- 14.Havrdova E, Arnold DL, Bar-Or A, et al. No evidence of disease activity (NEDA) analysis by epochs in patients with relapsing multiple sclerosis treated with ocrelizumab vs interferon beta-1a. Mult Scler J Exp Transl Clin. 2018;4(1):2055217318760642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korsukewitz C, Reddel SW, Bar-Or A, Wiendl H. Neurological immunotherapy in the era of COVID-19 - looking for consensus in the literature. Nat Rev Neurol. 2020;16(9):493-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweitzer F, Laurent S, Fink GR, et al. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Curr Opin Neurol. 2019;32(3):305-312. [DOI] [PubMed] [Google Scholar]
- 17.Ellrichmann G, Bolz J, Peschke M, et al. Peripheral CD19(+) B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J Neurol. 2019;266(1):57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Huh SY, Lee SJ, Joung A, Kim HJ. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70(9):1110-1117. [DOI] [PubMed] [Google Scholar]
- 19.Pellkofer HL, Krumbholz M, Berthele A, et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology. 2011;76(15):1310-1315. [DOI] [PubMed] [Google Scholar]
- 20.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sormani MP; Italian Study Group on C-iims. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes R, Whitley L, Fitovski K, et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord. 2020;49:102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bittner S, Ruck T, Wiendl H, Grauer OM, Meuth SG. Targeting B cells in relapsing-remitting multiple sclerosis: from pathophysiology to optimal clinical management. Ther Adv Neurol Disord. 2017;10(1):51-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hausler D, Hausser-Kinzel S, Feldmann L, et al. Functional characterization of reappearing B cells after anti-CD20 treatment of CNS autoimmune disease. Proc Natl Acad Sci U S A. 2018;115(39):9773-9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iannetta M, Cesta N, Stingone C, et al. Mild clinical manifestations of SARS-CoV-2 related pneumonia in two patients with multiple sclerosis under treatment with ocrelizumab. Mult Scler Relat Disord. 2020;45:102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghajarzadeh M, Mirmosayyeb O, Barzegar M, et al. Favorable outcome after COVID-19 infection in a multiple sclerosis patient initiated on ocrelizumab during the pandemic. Mult Scler Relat Disord. 2020;43:102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero-Escribano P, Matias-Guiu J, Gomez-Iglesias P, Porta-Etessam J, Pytel V, Matias-Guiu JA. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord. 2020;42:102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazi I, Kelton JG, Larche M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122(11):1946-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim W, Kim SH, Huh SY, et al. Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. Eur J Neurol. 2013;20(6):975-980. [DOI] [PubMed] [Google Scholar]
- 32.Cross A, Bennett J, von Büdingen HC, et al. Ocrelizumab treatment reduced levels of neurofilament light chain and numbers of B cells in the cerebrospinal fluid of patients with relapsing multiple sclerosis in the OBOE study (S56.008). Neurology. 2019(15 suppl):92.31068157 [Google Scholar]
- 33.Ryerson LZ, Foley J, Chang I, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology. 2019;93(15):e1452-e1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(8):885-889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared with qualified investigators on request; please contact meuth@uni-duesseldorf.de.