Abstract
Objective
To describe clinical, radiologic, and pathologic features of Baló concentric sclerosis (BCS) and assess overlap between BCS and other CNS inflammatory demyelinating diseases.
Methods
Retrospective review of BCS cases from US and Australian tertiary care centers.
Results
We identified 40 BCS cases with 38 available MRIs. Solitary MRI lesions were present in 26% (10/38). We saw >1 active concurrent BCS lesion in 45% (17/38). A third (13/38) had multiple sclerosis–suggestive lesions on the index MRI, of which 10 fulfilled Barkhof criteria. In patients with serial MRI performed within 1 month of the index MRI, lesions expanded radially with sequentially increased numbers of T2 hyperintense rings 52% (14/27). Initially nonenhancing or centrally enhancing lesions subsequently developed single or multiple enhancing rings (41%; 9/22) and incomplete enhancing rings (14%; 3/22). Discordance between rings as they appear on apparent diffusion coefficient, diffusion-weighted imaging, and gadolinium-enhanced imaging was observed in 67% (22/33). Aquaporin-4 immunoglobulin G (n = 26) and myelin oligodendrocyte glycoprotein immunoglobulin G (n = 21) were negative in all patients with serum available. Clinical response to steroid treatment was seen in 46% (13/28). A monophasic clinical course was present in 56% (18/32) at last follow-up (median 27.5 months; range 3–100 months). The initial attack was fatal in 10% (4/40). Median time from symptom onset to death was 23 days (range 19–49 days). All 17 patients with pathology available demonstrated typical findings of multiple sclerosis. Patients with active demyelinating lesions all demonstrated oligodendrocytopathy (pattern III).
Conclusions
BCS may be a distinct subtype of multiple sclerosis characterized by pattern III immunopathology.
Baló concentric sclerosis (BCS) is a CNS inflammatory demyelinating disease characterized by alternating rings of demyelination and relatively preserved myelin.1 Historically, BCS was thought to have a uniformly poor prognosis. However, earlier cases were based on autopsy studies and milder or self-limiting cases may have been missed or misdiagnosed.2 The availability of MRI has led to a better appreciation of the variable natural history of patients presenting with radiologically evident Baló lesions.3 BCS has been described in association with multiple sclerosis (MS) and seropositive neuromyelitis optica spectrum disorder (NMOSD), indicating a common mechanism of injury or response to injury in these disorders.4 It has also been associated with viral infections, including human herpesvirus 65 and hepatitis C.6 The frequency of oligoclonal bands in patients with Baló lesions is lower than that of typical MS, suggesting that Baló lesions represent a distinct etiology to MS.7
Case reports of patients with BCS have described acute plaque pathology consistent with MS type III immunopathologic pattern, characterized by cerebral white matter distal oligodendrocyte loss within demyelinated areas.8 The pathophysiology underlying the concentricity of Baló lesions is debated.1 One hypothesis is that of a hypoxic response to demyelination causing ischemic preconditioning at the leading edge of demyelination, which protects a ring of tissue, leading to alternating myelinated and demyelinated areas.9 Whether BCS is an MS variant, a disease in its own right, or simply a pattern of injury common to a number of demyelinating diseases remains uncertain.
Our objective was to describe the clinical, radiologic, and pathologic features of patients with BCS and to assess the overlap between BCS and other CNS demyelinating syndromes.
Methods
We used our institution's medical record diagnostic-linkage system to identify all patients with BCS treated at Mayo Clinic from January 1996 through December 2017 (n = 24). Additional cases were identified from an original cohort of 760 patients belonging to the Multiple Sclerosis Lesion Project (MSLP)–US cohort (n = 12)10 and a cohort based in Sydney, Australia (n = 4). The MSLP study is an international collaborative effort to study the clinical, radiologic, and pathologic correlates of the MS lesion. Patients were included from the MSLP who had (1) tissue diagnosis of inflammatory demyelination confirmed by a specialist in neuropathology of demyelinating disease (Y.G., C.F.L.) to be consistent with MS, with the presence of confluent plaques in active stage of myelin destruction, relative sparing of axons, and glial scarring; (2) no clinical, radiologic, serologic, or pathologic evidence of neoplasm, infection, vascular, or nondemyelinating inflammatory etiology; and (3) no structural or immunocytochemical evidence for an inflammatory demyelinating disease induced by known virus infections, such as subacute sclerosing panencephalitis or progressive multifocal leukoencephalopathy. We conducted a retrospective review of the clinical, radiologic, and pathology records of patients in whom a diagnosis of BCS was considered, MRI demonstrated demyelinating lesions with concentricity, or lesional brain pathology demonstrated alternating bands of demyelination and relatively preserved myelin.
Clinical and radiologic data were reviewed by 2 neurologists (E.A.J., W.O.T.) to confirm the diagnosis of BCS by consensus agreement based on interpretation of clinical and radiologic features. The index attack was defined as the constellation of neurologic symptoms leading to diagnosis. Encephalopathy was defined as behavioral change or alteration in consciousness. Improvement in attack-related, targeted neurologic deficits was graded as follows: no improvement if there was no gain in neurologic function; mild improvement if there was improvement in neurologic status without affecting function; moderate improvement if there was definite improvement in function; and marked improvement if there was major functional improvement.11 Disability was measured with the Expanded Disability Status Scale (EDSS).12
“Baló concentric sclerosis” lesions were defined radiologically as 2 or more concentric rings of alternating hyperintensity and hypointensity on T2-weighted MRI sequences.13 “Baló-like” lesions were defined as alternating bands of signal intensity (≥2 alternations) on non-T2 weighted MRI sequences or lesions with atypical radiologic features (layered alternating bands, or mosaic patterns) with biopsy evidence of alternating bands of demyelination and relatively preserved myelin. Barkhof criteria for MS were applied to each available MRI.14
Neuroimaging was independently evaluated by an experienced neuroradiologist (P.P.M.) blinded to the clinical diagnosis. Radiographic features of interest were index lesion location, size, mass effect, edema, T2-weighted lesion load, diffusion-weighted imaging (DWI) characteristics, and gadolinium enhancement pattern. When multiple active lesions were present, the largest lesion was characterized for analysis. Pathologic tissue from brain biopsy or autopsy was examined by neuropathologists with expertise in CNS demyelinating disorders (C.F.L. and Y.G.). Pathologic material was collected in the Department of Neuropathology at the Mayo Clinic, Rochester, MN; the Neuropathologic Institute at the University of Göttingen, Germany; and the Institute of Brain Research at the University of Vienna, Austria. Active MS lesions were grouped into 1 of 4 distinct immunopathologic patterns, as described previously.15
Neuropathologic Techniques and Immunocytochemistry
All tissue blocks were classified with regard to lesional activity. Paraffin-embedded 5-μm sections were stained with hematoxylin & eosin, Luxol fast blue myelin stain, periodic acid–Schiff reaction, and Bielschowsky silver impregnation axonal stain.15 Immunohistochemistry was performed without modification on paraffin sections using an avidin-biotin or an alkaline phosphatase/anti–alkaline phosphatase technique as described in detail previously.16 The primary antibodies were omitted in negative controls. In situ hybridization was performed using digoxigenin-labeled riboprobes specific for proteolipoprotein. The source and specificity of the probes, the labeling techniques, and the methods of in situ hybridization have been described in detail previously.17 To visualize degenerating cells in tissue sections, DNA fragmentation within cell nuclei was determined with the method of in situ tailing.18 The sections were then processed for immunohistochemistry with antibodies against myelin oligodendrocyte glycoprotein (MOG), glial fibrillary acidic protein, T cells, and macrophages as described above. Apoptotic oligodendrocytes were defined by nuclear condensation and fragmentation in cells stained by either MOG or cyclic nucleotide phosphodiesterase antibodies. Stored serum was examined for aquaporin-4 (AQP4) immunoglobulin G (IgG) and MOG-IgG with fluorescence-activated cell-sorting assays.19 Other methods used at the time of presentation for AQP4-IgG included cell-based assay (1 patient), ELISA (2 patients), and tissue immunofluorescence (2 patients).20
Standard Protocol Approvals, Registrations, and Patient Consents
The institutional review boards of the Mayo Clinic, Rochester, MN (IRB 16-010508) and Royal Prince Alfred Hospital, Sydney, Australia (HREC/17/RPAH/145) approved this study. All patients consented to the use of their medical records for research purposes at the time of their clinical evaluation.
Statistical Methods
All statistical analyses were performed with the patient as the unit of analysis. Even though some patients had biopsy followed by autopsy, they contributed only 1 data point to any analysis, thus maintaining statistical independence among observations. Chi-square tests and Fisher exact tests were used to assess associations. p Values were interpreted as goodness of fit measures indicating how consistent observed data were with the null hypothesis, recognizing that hypothesis testing depends on an idealized set of assumptions such as random sampling from a population.
Data Availability
Anonymized data within this article will be shared by request from any qualified investigator.
Results
Clinical Characteristics
Of the 40 patients identified, 29 patients had T2-weighted imaging findings consistent with BCS lesion, 7 patients had Baló-like lesions, 2 patients had atypical lesions with biopsy evidence of alternating bands of demyelination, and 2 patients did not have MRI available but had BCS confirmed on autopsy.
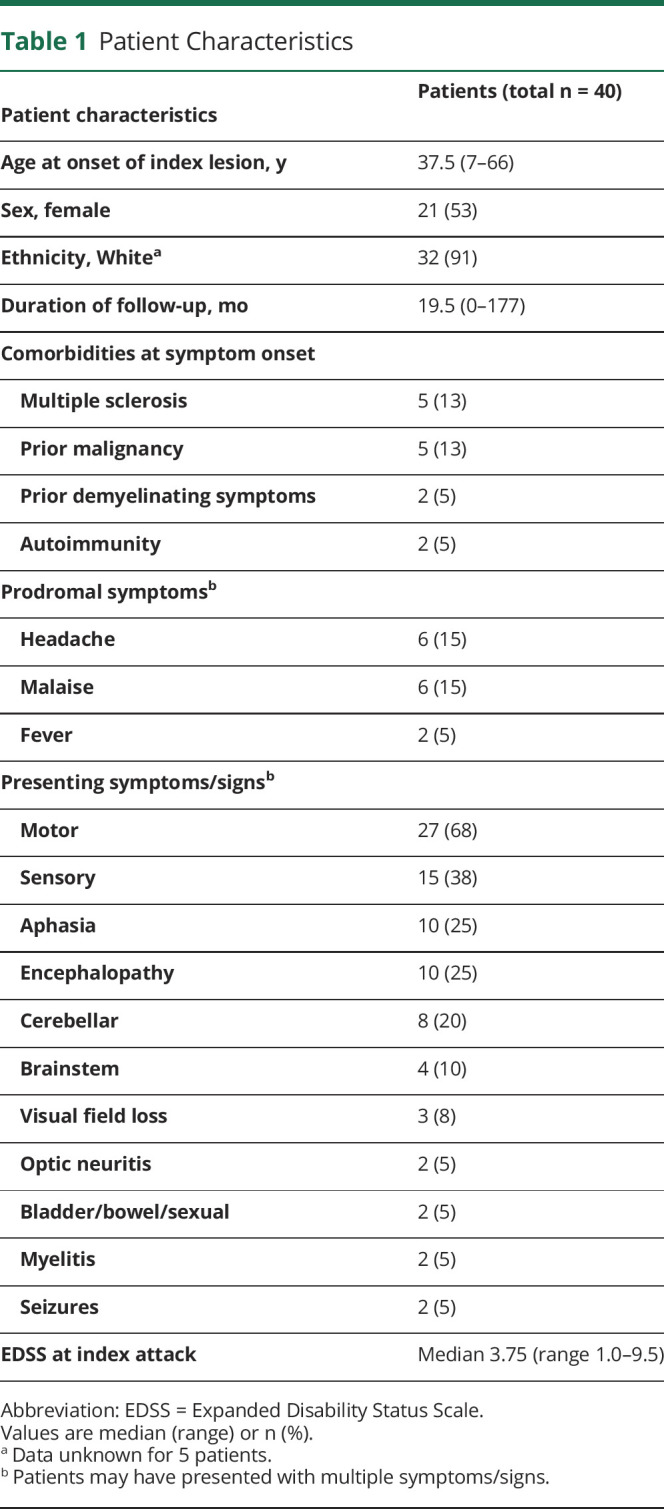
The clinical characteristics of the cohort are shown in table 1. At presentation, 7 patients had either an established diagnosis of demyelinating disease (MS, n = 5) or a history suggestive of prior demyelinating events (2 patients). One patient was taking dimethyl fumarate. No other patients were on disease-modifying therapy at presentation. Approximately one-third of patients (13/40) reported prodromal symptoms of fever, malaise, or headache. The most common neurologic features were motor (27/40 [68%]) or sensory deficits (15/40 [38%]) and dysphasia (10/40 [25%]). Ten patients (25%) presented with behavioral change or impaired consciousness consistent with encephalopathy. The median EDSS at presentation was 3.75 (range 1.0–9.5). Initial misdiagnosis was not uncommon: 3 patients were initially thought to have a brain tumor, 1 patient a cerebral abscess, and 1 patient received IV thrombolysis for treatment of suspected stroke.
Table 1.
Patient Characteristics
Neuroimaging Results
Brain MRI was available to review for 38 patients (table 2). Two patients identified by autopsy did not have MRI for review. Ten patients (26%) presented with solitary brain lesions. Additional T2-weighted high-signal lesions were encountered in 74% (28/38), and almost half of the patients (17/38) had more than 1 concurrent gadolinium-enhancing Baló lesion (figure 1). A third of patients (13/38) had lesions suggestive of MS on the index MRI, of whom 10 met Barkhof criteria. Of the patients who underwent biopsy, 5 had an MRI prior to biopsy that fulfilled Barkhof criteria.
Table 2.
Radiographic Characteristics of Lesions Demonstrating Bands of Concentric Sclerosis
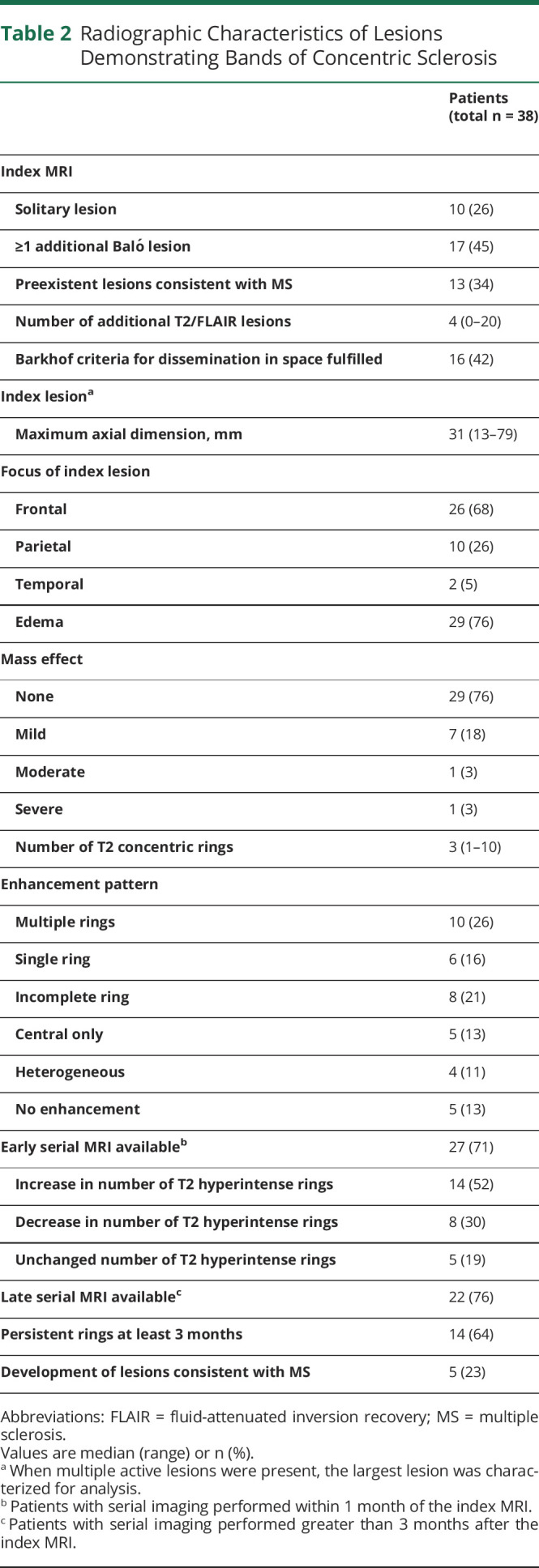
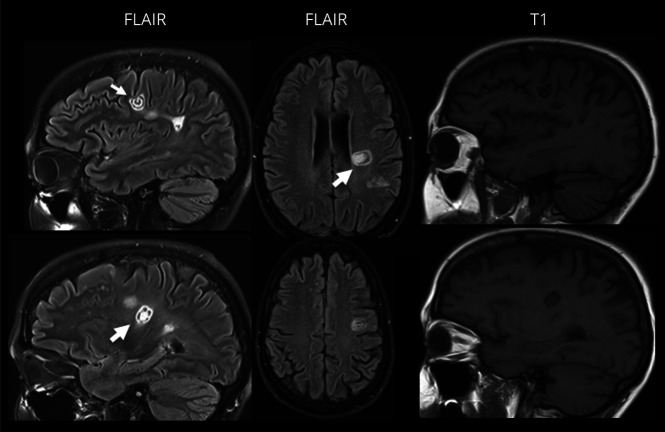
Figure 1. Concurrent Baló and Baló-like Lesions.
Fluid-attenuated inversion recovery (FLAIR) and T1-weighted MRI demonstrating concurrent Baló (small arrow) and Baló-like (large arrow) lesions in a single patient.
Index lesions were exclusively located in the cerebral hemispheres (frontal lobe, n = 26; parietal lobe, n = 10; temporal lobe, n = 2). The median lesion size in maximum axial dimension on the index T2-weighted images was 31 mm (range 13–79). Edema was present in 76% (29/38), and 24% (9/38) had mass effect, which was predominantly mild. Index lesions had a median of 3 concentric rings (range 2–10). Concentric rings were better visualized on T2-weighted imaging compared to T2/fluid-attenuated inversion recovery (FLAIR) and were better visualized on apparent diffusion coefficient (ADC) imaging compared to DWI. When evaluating the index MRI, concentric rings were better appreciated on ADC when compared to DWI in 25/32 patients with ADC imaging available and were better appreciated on DWI in 9/32 patients when compared to ADC. In 1 patient, the concentric rings were equally apparent on ADC and DWI. Similarly, concentric rings were seen in 31 patients on T2 imaging on the index MRI, compared to 16 patients on FLAIR imaging. In patients in whom both T2 and FLAIR were available, concentric rings were better visualized on T2 in 19/29 patients, they were equally apparent in T2 and FLAIR in 9/29 patients, and were more evident on FLAIR when compared to T2 imaging in 1 patient. Single or multiple gadolinium-enhancing rings were observed in 42% (single, n = 6; multiple, n = 10), and open enhancing rings were observed in 21% (n = 8) of index lesions. Homogenous enhancement within a lesion was not observed.
Early MRI performed within 1 month of the index scan was available for 27 patients. The index lesions expanded radially with a sequential increase in the number of T2 rings in 52% (14/27). Discordance between the visualized rings as they appear on diffusion-weighted and gadolinium-enhanced imaging was observed in 70% (19/27) of index lesions with DWI. The lesions often demonstrated an initial phase of DWI restriction followed by a T2 hypointense ring and then later evolution of gadolinium enhancement (figure 2). Multiple enhancing rings were present either on the index MRI (n = 10) or developed on subsequent scans (n = 9). Enhancement was most prominent in the outer ring in 74% (14/19). MRI performed at least 3 months after the index MRI was available for 22 patients. T2 rings remained visible in 64% (14/22). In no patient did the T2/FLAIR abnormality disappear completely.
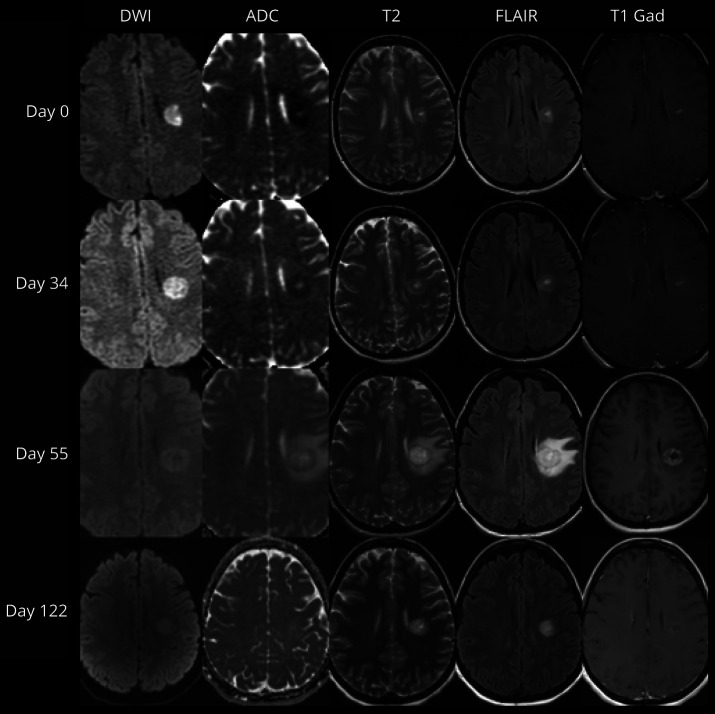
Figure 2. Temporal Evolution of a Baló Concentric Sclerosis.
Diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC), T2-weighted (T2), fluid-attenuated inversion recovery (FLAIR), and postgadolinium T1-weighted (T1 Gad) MRI demonstrating evolution of a single lesion with initial peripheral diffusion restriction associated with central enhancement progressing to develop multiple concentric rings, associated with peripheral enhancement, followed by resolution of enhancement and ADC changes with T2-weighted abnormalities being persistently visible at 122 days from the initial MRI.
Cerebrospinal Fluid
CSF analysis was completed in 33 patients (median 20 days after symptom onset; range 1–544 days). Where recorded, the white cell count was elevated in 25% (7/28) (range 6–27 cells/μL; normal 0–5); protein was elevated in 67% (16/24) (range 40–134 mg/dL; normal 0–35); 2 or more unique CSF oligoclonal bands were detected in 67% (20/30).
Antibody Evaluation
Patient serum was evaluated for AQP4-IgG or MOG-IgG either at the time of presentation or subsequently on stored serum in 26 and 21 patients respectively and were all negative.
Pathology
Pathologic specimens from 17 patients (biopsy, n = 15; autopsy, n = 4) were evaluated at Mayo Clinic. Two patients underwent serial biopsy and autopsy. All pathologic specimens demonstrated alternating bands of demyelination and relatively preserved myelin. Immunopathologic phenotyping was performed in 13 patients, and all demonstrated an oligodendrocytopathy pattern (pattern III).15
Treatment Outcome
The treating physician's diagnosis at the time of index attack was BCS (n = 21), tumefactive demyelination (TD) (n = 10), MS (n = 8), or acute disseminated encephalomyelitis (ADEM) (n = 1). The initial treatment strategy was corticosteroids in 70% (28/40) and concurrent plasmapheresis and corticosteroids in 5% (2/40). A moderate or marked response to corticosteroids alone was seen in 46% (13/28); however, 11% (3/28) had a mild response and 43% (12/28) had no response. One patient who received plasmapheresis in addition to corticosteroids had a moderate response, and the second had no response.
In patients who had an incomplete response to the initial treatment for the index attack, a range of therapy options was utilized. A moderate or marked response to treatment was seen in 79% (15/19) of patients. These were treated with additional corticosteroids (6/7); plasmapheresis (5/5); corticosteroids and plasmapheresis (2/2); corticosteroids, IV immunoglobulin (IVIg), and plasmapheresis (1/1); or IV cyclophosphamide (1/1). Mild or no response was seen in 21% (4/19) of patients. These were treated with IVIg (2/2), corticosteroids (1/7), or corticosteroids and cyclophosphamide (1/1).
Six patients received prolonged therapy for the index attack, and 4 patients had a moderate or marked response to corticosteroids (2/5), plasmapheresis (1/1), and plasmapheresis and cyclophosphamide (1/1) at 6 months. Seven patients did not receive any treatment. One patient had transient symptoms improving within days, and 5 others either markedly improved or completely recovered in the absence of treatment over 1–6 months. One patient who presented in 1964 did not receive immunotherapy treatment and died 22 days after symptom onset. The initial treatment for 3 patients was unknown.
Four patients (10%) (BCS, n = 2; TD, n = 2) died during the index attack. The median time from first manifesting symptoms to death was 23 days (range 19–49). One patient did not receive treatment and died of brainstem compression leading to respiratory arrest. One patient was treated with corticosteroids and plasmapheresis, followed by additional corticosteroids and cyclophosphamide, and ultimately died of pulmonary embolus. One patient had a rapid progression to coma over days and died despite receiving corticosteroids. The cause of death in the fourth patient was unknown.
Long-term Outcome
Four patients died during the index attack, and 4 patients did not have follow-up data available. Clinical follow-up data of more than 3 months were available for 32 patients (median follow-up 26.5 months; range 3–177). Seventeen patients (53%) were treated with MS disease-modifying medications (interferon, n = 10; natalizumab, n = 5; glatiramer, n = 2) after the index attack, and 2 patients began treatment after further relapses (dimethyl fumarate, n = 1; glatiramer, n = 1). There was no significant difference in the rates of treatment with disease-modifying medication between patients who presented with multiple additional T2 hyperintense lesions on the index MRI (15/24 [63%]) and patients who presented with isolated BCS lesions (2/8 [25%]) (p = 0.11).
At a medium follow-up of 27.5 months (range 3–100), 56% (18/32) of patients had a monophasic disease with no further relapse. Of the patients who relapsed, 4 had presented with isolated BCS lesions and 10 had multiple T2 hyperintense lesions on the index MRI (p = 0.70). The median time to relapse was 5 months (range 1–18). Two patients presented with another BCS lesion when they relapsed (2 and 9 months after the index attack). Of the patients with a clinical diagnosis of BCS, 50% (9/18) relapsed during follow-up, compared to 43% (3/7), 33% (2/6), and 0% (0/1) of patients initially diagnosed with RRMS, TD, and ADEM, respectively (p = 0.94). Physician diagnosis at last follow-up was MS (n = 20), BCS (n = 13), TD (n = 6), and ADEM (n = 1). Ten patients (48%) initially diagnosed with BCS had been reclassified by the treating physician as MS, and 2 patients initially diagnosed with TD were reclassified as BCS. The median EDSS at last follow-up was 2.5 (range 0–10). Of 9 patients who were followed for more than 5 years after symptom onset, 4/9 (44%) had subsequent clinical attacks. One patient developed secondary progressive MS and died 14.5 years after the initial event at age 58 years. The cause of death was not recorded.
Discussion
Although BCS has previously been described as a monophasic fulminant disease, this study describes 40 patients, 10% of whom died from their initial attack. The majority of the cohort did not have a diagnosis of CNS demyelinating disease at presentation. Whereas the majority of patients were ultimately diagnosed with MS, there are clinical, radiologic, and pathologic features in this cohort that point to this, representing a specific subset of MS. The median age of this cohort was 38, which is slightly older than the typical age at onset of MS. Only 67% of patients had positive oligoclonal bands, in contrast with 85%–95% of patients with typical MS. This is consistent with findings of Jarius and colleagues.7 Half of the patients had monophasic disease. Only one-third had imaging features consistent with MS, and most compellingly, all patients with active demyelinating disease had pattern III immunopathology, indicating a common underlying mechanism of injury.
We observed BCS lesions occurring de novo, in patients with preexisting MRI changes suggestive of demyelinating disease, and in the context of an established diagnosis of MS. Almost half of the patients diagnosed with BCS experienced subsequent clinical or radiologic relapses consistent with MS. While considered a variant of MS, BCS lesions may not be exclusive to MS and could represent a pattern of injury common among demyelinating lesions. Concentric lesions have also been observed in cases of NMOSD21 and loss of AQP4 expression as can be seen in neuromyelitis optica has also been described in BCS lesions.22 Nonetheless, we did not detect AQP4-IgG or MOG-IgG in any patient with available serum.
Serial MRI in the acute phase of the disease reveals how BCS lesions evolve over time. We observed lesions expanding radially with a sequential increase in the number of T2 hyperintense rings. The visualized rings did not precisely match, and discordance between the rings as they appear on ADC, DWI, and gadolinium-enhanced imaging was frequently observed (67%). The lesions often demonstrated an initial phase of DWI restriction followed by a T2 hypointense ring and then later evolution of gadolinium enhancement. Early and dynamic ischemia in the layers of BCS lesions has been reported previously23,24 and observed in MRI studies of biopsy-proven, Baló-like demyelinating brain lesions.25 Diffusion restriction prior to gadolinium enhancement suggests that the initial insult in patients with BCS is a primary brain insult, with secondary breakdown of the blood–brain barrier and ingress of autoinflammatory cells. In a quantitative study, Koelblinger et al26 demonstrated that diffusion restriction in Baló lesions was more prominent in the outer layers of active lesions, consistent with an initial central lesion, growing outward. A hypoxia-like event may precede the demyelinating process in BCS, and tissue preconditioning may give rise to the laminated appearance of BCS lesions.9 Expression of hypoxia-induced tissue preconditioning–related proteins has been observed at the rim of periplaque regions and may provide relative resistance to tissue injury in expanding lesions.9,24
Four fundamentally different patterns of demyelination have been described in MS lesions suggesting different targets of injury and mechanisms of demyelination between different subgroups of MS and possibly at different stages of disease development.15 Pattern III lesions are characterized by a distal oligodendrocyte dystrophy. They are composed mainly of T lymphocytes with macrophages and activated microglia and are distinguished from pattern I and II lesions by the absence of immunoglobulin and complement. In a pathologic cohort of MS lesions, a Baló-like concentric pattern was seen at the edge of immunopathologic pattern III lesions only, in the absence of typical Baló-like MRI features.15 In a separate autopsy series of acute MS or fulminant exacerbations of chronic MS with Baló-like lesions seen only on pathology, all cases with concentric demyelination demonstrated immunopathologic pattern III.9 The current series is the first one to our knowledge to demonstrate that radiologic Baló and Baló-like features correlate strongly with an underlying pattern III immunopathology.
BCS was historically considered to have a uniformly poor prognosis. MRI has shown that the natural history of the disease varies and favorable outcomes with treatment are not uncommon.27 Recently, in a cohort of patients with Baló-like lesions, marked clinical improvement or full recovery from the initial attack was reported in 83%.27 In our cohort of patients with BCS lesions, we observed a spectrum of possible outcomes, from relatively benign disease, in which symptoms resolved without any immunotherapy intervention, to fulminant disease, resulting in patient death. Of the 4 cases that resulted in death, 1 occurred in an era before immunotherapy was standard clinical care for MS or demyelinating disease.
The majority of patients received corticosteroids initially, but almost half required additional therapy. Plasmapheresis is effective in patients with severe demyelinating disease who fail to respond to corticosteroids.11 However, plasmapheresis is thought to be effective in patients with antibody- and complement-mediated tissue destruction and less effective in other patterns of injury. Patients with MS with pattern II pathology are more likely to respond favorably to plasmapheresis than patients with patterns I or III.28 Patients with ring-enhancing lesions, which tend to be more common in pattern II, have been observed to have a more favorable response to plasmapheresis.29 In this cohort, a range of therapy options were utilized and often in combination, making it difficult to comment on specific outcomes. However, 14 patients received plasmapheresis and 11 reported a moderate or marked improvement upon treatment. Two of the 3 patients with biopsy pattern III who received plasmapheresis reported a moderate response.
The number of patients in previous studies examining outcomes in patients with BCS lesions has been small. In 1 study, 4 of 7 patients followed for a mean of 8 years remained relapse-free.30 In other studies, all 5 patients2 and 4 of 6 patients27 with a mean follow-up of 2.5 and 1.8 years, respectively, remained relapse-free. In our cohort, 18 of 32 patients had a monophasic disease with no further relapse at last follow-up (median 27.5 months; range 3–100 months). Three patients had greater than 5 years of follow-up without further relapses. Patients who presented with multiple T2 lesions on the index MRI were no more likely to start disease-modifying medication than patients presenting with an isolated BCS lesion, and the rates of relapse on treatment were the same.
Limitations of this study include the lack of agreed diagnostic criteria for BCS. Baló-like lesions are often defined radiologically as lesions with multiple concentric rings or a pattern of alternating bands of signal intensity (≥2 alternations) on any sequence, and pathologically as alternating rings of demyelination and relatively preserved myelin. We included patients who met the radiologic definition or had biopsy evidence to supporting a diagnosis of BCS. As a retrospective study, data were not always available, and follow-up was limited in some cases. The quality of MRI varied considerably among cases as MRI technology improved over the years, and earlier scans did not include diffusion-weighted imaging as part of routine MRI evaluations. Clinical and pathologic cases were identified from a tertiary referral-based population. Smaller lesions or lesions that respond to first-line treatments may not initiate a referral. Therefore, the nature of the cases identified here may not be representative of the spectrum of disease.
BCS is a rare disorder previously thought to be a fulminant demyelinating disorder with poor outcomes or patient death. However, BCS lesions have a spectrum of possible presentations, which include benign disease. BCS lesions may occur in patients with coexisting MS or may herald the onset of a demyelinating disease that is subsequently clinically and radiologically similar to typical MS. When immunopathologic phenotyping was performed, all lesions demonstrated an oligodendrocytopathy pattern (pattern III). Mechanisms leading to the characteristic concentric layering are not completely understood, but tissue ischemia and tissue preconditioning may give rise to the laminated appearance of BCS lesions.
Glossary
- ADC
apparent diffusion coefficient
- ADEM
acute disseminated encephalomyelitis
- AQP4
aquaporin-4
- BCS
Baló concentric sclerosis
- DWI
diffusion-weighted imaging
- EDSS
Expanded Disability Status Scale
- FLAIR
fluid-attenuated inversion recovery
- IgG
immunoglobulin G
- IVIg
IV immunoglobulin
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- MSLP
Multiple Sclerosis Lesion Project
- NMOSD
neuromyelitis optica spectrum disorder
- TD
tumefactive demyelination
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
The authors report no targeted funding.
Disclosure
E.A. Joliffe and Y. Guo have no disclosures relevant to this manuscript. T.A. Hardy attended the Mayo Clinic as a recipient of the Ian Ballard Travel Award funded by MS Research Australia. P.P. Morris and E.P. Flanagan have no disclosures relevant to this manuscript. C.F. Lucchinetti receives funding from Novartis (CFTY720DUS37T) and the NIH (R01NS49577-7). W.O. Tobin has received research funding from the NIH (1R01NS113803-01A1), Mallinckrodt Inc. and the Mayo Clinic Center for MS and Autoimmune Neurology. Go to Neurology.org/N for full disclosures.
References
- 1.Hardy TA, Reddel SW, Barnett MH, Palace J, Lucchinetti CF, Weinshenker BG. Atypical inflammatory demyelinating syndromes of the CNS. Lancet Neurol. 2016;15(9):967-981. [DOI] [PubMed] [Google Scholar]
- 2.Karaarslan E, Altintas A, Senol U, et al. Baló's concentric sclerosis: clinical and radiologic features of five cases. Am J Neuroradiol. 2001;22(7):1362-1367. [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy TA, Miller DH. Baló's concentric sclerosis. Lancet Neurol. 2014;13(7):740-746. [DOI] [PubMed] [Google Scholar]
- 4.Graber JJ, Kister I, Geyer H, Khaund M, Herbert J. Neuromyelitis optica and concentric rings of Baló in the brainstem. Arch Neurol. 2009;66(2):274-275. [DOI] [PubMed] [Google Scholar]
- 5.Pohl D, Rostasy K, Krone B, Hanefeld F. Balo's concentric sclerosis associated with primary human herpesvirus 6 infection. J Neurol Neurosurg Psychiatry. 2005;76(12):1723-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoftberger R, Garzuly F, Dienes HP, et al. Fulminant central nervous system demyelination associated with interferon-alpha therapy and hepatitis C virus infection. Mult Scler. 2007;13(9):1100-1106. [DOI] [PubMed] [Google Scholar]
- 7.Jarius S, Wurthwein C, Behrens JR, et al. Balo's concentric sclerosis is immunologically distinct from multiple sclerosis: results from retrospective analysis of almost 150 lumbar punctures. J Neuroinflamm. 2018;15(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy TA, Tobin WO, Lucchinetti CF. Exploring the overlap between multiple sclerosis, tumefactive demyelination and Baló's concentric sclerosis. Mult Scler. 2016;22(8):986-992. [DOI] [PubMed] [Google Scholar]
- 9.Stadelmann C, Ludwin S, Tabira T, et al. Tissue preconditioning may explain concentric lesions in Balo's type of multiple sclerosis. Brain. 2005;128(pt 5):979-987. [DOI] [PubMed] [Google Scholar]
- 10.Pittock SJ, McClelland RL, Achenbach SJ, et al. Clinical course, pathological correlations, and outcome of biopsy proved inflammatory demyelinating disease. J Neurol Neurosurg Psychiatry. 2005;76(12):1693-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinshenker Brian G, O'Brien Peter C, Petterson Tanya M, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46(6):878-886. [DOI] [PubMed] [Google Scholar]
- 12.Kurtzke JF. Rating neurologic impairment in multiple sclerosis. Neurology. 1983;33:1444. [DOI] [PubMed] [Google Scholar]
- 13.Seewann A, Enzinger C, Filippi M, et al. MRI characteristics of atypical idiopathic inflammatory demyelinating lesions of the brain: a review of reported findings. J Neurol. 2008;255(1):1-10. [DOI] [PubMed] [Google Scholar]
- 14.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120(Pt 11):2059-2069. [DOI] [PubMed] [Google Scholar]
- 15.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47(6):707-717. [DOI] [PubMed] [Google Scholar]
- 16.Vass K, Lassmann H, Wekerle H, Wisniewski HM. The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol. 1986;70(2):149-160. [DOI] [PubMed] [Google Scholar]
- 17.Breitschopf H, Suchanek G, Gould RM, Colman DR, Lassmann H. In situ hybridization with digoxigenin-labeled probes: sensitive and reliable detection method applied to myelinating rat brain. Acta Neuropathol. 1992;84(6):581-587. [DOI] [PubMed] [Google Scholar]
- 18.Gold R, Schmied M, Giegerich G, et al. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest. 1994;71(2):219-225. [PubMed] [Google Scholar]
- 19.Jitprapaikulsan J, Chen JJ, Flanagan EP, et al. Aquaporin-4 and myelin oligodendrocyte glycoprotein autoantibody status predict outcome of recurrent optic neuritis. Ophthalmology. 2018;125(10):1628-1637. [DOI] [PubMed] [Google Scholar]
- 20.Waters PJ, McKeon A, Leite MI, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78(9):665-671. discussion 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda H, Mori M, Katayama K, Kikkawa Y, Kuwabara S. Anti-Aquaporin-4 antibody-seronegative NMO spectrum disorder with Balo's concentric lesions. Intern Med. 2013;52:1517-1521. [DOI] [PubMed] [Google Scholar]
- 22.Matsuoka T, Suzuki SO, Iwaki T, Tabira T, Ordinario AT, Kira J. Aquaporin-4 astrocytopathy in Balo's disease. Acta Neuropathol. 2010;120(7):651-660. [DOI] [PubMed] [Google Scholar]
- 23.Ripellino P, Khonsari R, Stecco A, Filippi M, Perchinunno M, Cantello R. Clues on Balo's concentric sclerosis evolution from serial analysis of ADC values. Int J Neurosci. 2016;126(1):88-95. [DOI] [PubMed] [Google Scholar]
- 24.Takai Y, Misu T, Nishiyama S, et al. Hypoxia-like tissue injury and glial response contribute to Balo concentric lesion development. Neurology. 2016;87(19):2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abou Zeid N, Pirko I, Erickson B, et al. Diffusion-weighted imaging characteristics of biopsy-proven demyelinating brain lesions. Neurology. 2012;78(21):1655-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koelblinger C, Fruehwald-Pallamar J, Kubin K, et al. Atypical idiopathic inflammatory demyelinating lesions (IIDL): conventional and diffusion-weighted MR imaging (DWI) findings in 42 cases. Eur J Radiol. 2013;82(11):1996-2004. [DOI] [PubMed] [Google Scholar]
- 27.Wallner-Blazek M, Rovira A, Fillipp M, et al. Atypical idiopathic inflammatory demyelinating lesions: prognostic implications and relation to multiple sclerosis. J Neurol. 2013;260(8):2016-2022. [DOI] [PubMed] [Google Scholar]
- 28.Keegan M, König F, McClelland R, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366(9485):579-582. [DOI] [PubMed] [Google Scholar]
- 29.Magaña SM, Keegan B, Weinshenker BG, et al. Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol. 2011;68(7):870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Zhang KN, Wu XM, et al. Baló's disease showing benign clinical course and co-existence with multiple sclerosis-like lesions in Chinese. Mult Scler J. 2008;14(3):418-424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data within this article will be shared by request from any qualified investigator.