Abstract
Objective
Magnesium has been implicated in regulating blood pressure and vascular endothelial cell function, but its role in the pathophysiology of intracranial aneurysm is not known. Here we performed a Mendelian randomization analysis to investigate the association between serum magnesium concentration and risk of intracranial aneurysm.
Methods
Five single-nucleotide polymorphisms strongly associated with serum magnesium concentrations in a genome-wide association study in 23,829 individuals of European ancestry were used as genetic instruments. Genetic association estimates for intracranial aneurysm were obtained from a genome-wide association study in 79,429 individuals (7,495 cases and 71,934 controls). The inverse variance weighted method was used in the primary analyses to obtain the causal estimates.
Results
Higher genetically predicted serum magnesium concentrations were associated with lower risk of intracranial aneurysm. The odds ratios per 0.1 mmol/L increment in genetically predicted serum magnesium concentrations were 0.66 (95% confidence interval [CI] 0.49–0.91) for intracranial aneurysm (unruptured and ruptured combined), 0.57 (95% CI 0.30–1.06) for unruptured intracranial aneurysm, and 0.67 (95% CI 0.48–0.92) for aneurysmal subarachnoid hemorrhage.
Conclusion
This study provides evidence to support that increased serum magnesium concentrations reduce the risk of intracranial aneurysm and associated hemorrhage.
Intracranial aneurysm rupture and resultant subarachnoid hemorrhage is associated with a high rate of morbidity and mortality.1,2 Serum magnesium concentrations have been implicated in regulating blood pressure and function of the vascular endothelium.3-5 However, whether increased serum magnesium concentrations affect the risk of intracranial aneurysm and related subarachnoid hemorrhage is not known.
To investigate this, we leveraged randomly allocated genetic variants related to serum magnesium concentrations as instrumental variables in a 2-sample mendelian randomization (MR) study assessing whether higher genetically predicted serum magnesium concentrations are associated with a reduced risk of intracranial aneurysm. A multivariable MR analysis was further performed to investigate whether any potential association may be mediated through effects on blood pressure.
Methods
Genetic Instruments and Data Sources
As instrumental variables for the primary analyses, we used single nucleotide polymorphisms (SNPs) associated with serum magnesium concentrations at genome-wide significance (p < 5 × 10−8) in a genome-wide association study in 23,829 individuals of European ancestry.6 Six independent SNPs in different genetic loci and on different chromosomes were identified and proposed as instrumental variables for serum magnesium concentrations. In complementary analyses, we added 2 uncorrelated SNPs in or near known magnesium transport genes and associated with serum magnesium concentrations after applying a Bonferroni correction for the number of genetic variants examined in each region.6 Summary statistics data for intracranial aneurysm in individuals of European ancestry were obtained from a genome-wide association study of 23 cohorts comprising 79,429 individuals (7,495 cases [69% with ruptured intracranial aneurysm, 28% with unruptured intracranial aneurysm, and 3.8% with unknown rupture status] and 71,934 controls).7 Genetic association estimates for systolic blood pressure were taken from a genome-wide association study of 757,601 individuals of European ancestry.8
Standard Protocol Approvals, Registrations, and Patient Consents
Only summary-level data were analyzed in this study, for which appropriate ethical approval and participant consent had previously been acquired in the original genome-wide association studies. The analyses were approved by the Swedish Ethical Review Authority (2019–03986).
Statistical Analysis
In our main analyses, we applied the multiplicative random-effects inverse-variance weighted method to estimate the total association of serum magnesium concentrations with intracranial aneurysm.9 To assess the potential presence of directional pleiotropy, MR-Egger regression analysis was performed.9 Finally, to evaluate whether the results may be driven by potential outliers, we used the MR pleiotropy residual sum and outlier method.9 To evaluate whether blood pressure may mediate any association, we implemented a multivariable MR model with adjustment for genetically predicted systolic blood pressure. The weighted median method was used as a complementary method.9 We scaled all odds ratios (ORs) per 0.1 mmol/L increase in serum magnesium concentrations, which corresponds to an approximate 1 SD. Analyses were conducted using the mrrobust package10 for Stata (StataCorp) and the MendelianRandomization package11 for R.
Data Availability
All data generated or analyzed during this study are included in the table.
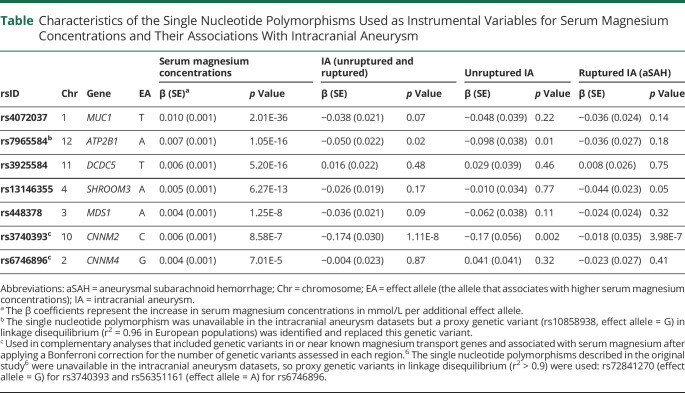
Table.
Characteristics of the Single Nucleotide Polymorphisms Used as Instrumental Variables for Serum Magnesium Concentrations and Their Associations With Intracranial Aneurysm
Results
Of the 6 SNPs associated with serum magnesium at the genome-wide significance threshold, 1 SNP was unavailable in the intracranial aneurysm datasets and no proxy SNP was available at a linkage disequilibrium (LD) R2 > 0.6. The other 5 SNPs (including 1 proxy SNP in strong LD with the original SNP) were used as instrumental variables for serum magnesium in the primary analyses. For the 2 SNPs in or near magnesium transport genes, we used proxy SNPs in strong or complete LD with the original SNPs (table). Information on the SNPs used as instrumental variables and their associations with intracranial aneurysm is shown in the table.
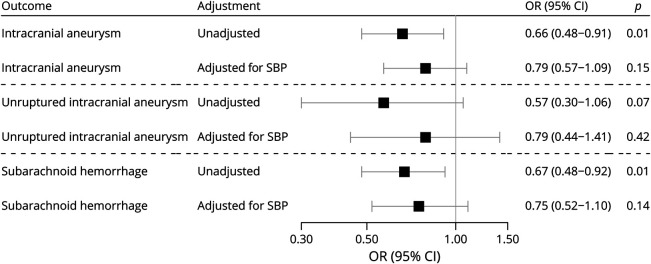
Genetically predicted serum magnesium concentrations were inversely associated with risk of intracranial aneurysm. In the analyses based on the 5 genome-wide significant SNPs, the ORs per 0.1 mmol/L increase in genetically predicted magnesium concentrations were 0.66 (95% confidence interval [CI] 0.49–0.91) for intracranial aneurysm, 0.57 (95% CI 0.30–1.06) for unruptured intracranial aneurysm, and 0.67 (95% CI 0.48–0.92) for aneurysmal subarachnoid hemorrhage. The sensitivity analysis based on the weighted median method provided similar results (corresponding ORs 0.64 [95% CI 0.45–0.91], 0.62 [95% CI 0.32–1.20], and 0.66 [95% CI 0.44–0.98]). There was no evidence of directional pleiotropy (all p values for the MR-Egger intercept >0.60), and no outlier was found in the MR pleiotropy residual sum and outlier method. Adjustment for genetically predicted systolic blood pressure through a multivariable MR model attenuated the associations of genetically predicted serum magnesium with all 3 considered outcomes, consistent with a partial mediating effect (figure).
Figure. Association Between Genetically Predicted Serum Magnesium Concentrations and Intracranial Aneurysm.
Estimates are scaled per genetically predicted 0.1 mmol/L (about 1 SD) increase in serum magnesium concentrations. Adjustment for genetically predicted systolic blood pressure (SBP) was made using a multivariable mendelian randomization model. CI = confidence interval; OR = odds ratio.
The complementary analyses incorporating 2 SNPs in or near magnesium transport genes yielded similar results. In these analyses based on 7 SNPs, the ORs per 0.1 mmol/L increase in genetically predicted magnesium concentrations were 0.55 (95% CI 0.32–1.00) for intracranial aneurysm, 0.53 (95% CI 0.25–1.10) for unruptured intracranial aneurysm, and 0.55 (95% CI 0.32–0.97) for aneurysmal subarachnoid hemorrhage.
Discussion
This MR study provides evidence to support that higher serum magnesium concentrations reduce the risk of intracranial aneurysm and aneurysmal subarachnoid hemorrhage, with systolic blood pressure mediating part of this effect. Whereas magnesium supplementation has been demonstrated to increase serum magnesium concentrations and reduce blood pressure in randomized clinical trials,3 to our knowledge this is the first MR study to identify a potential causal association between serum magnesium concentrations and risk of intracranial aneurysm and aneurysmal subarachnoid hemorrhage. The associations of genetically predicted serum magnesium with risk of intracranial aneurysm and aneurysmal subarachnoid hemorrhage did not fully attenuate after adjustment for genetically predicted systolic blood pressure, suggesting that magnesium may also affect the risk of these outcomes via other mechanisms. In addition to a blood pressure–lowering effect, increased magnesium concentrations may reduce the risk of intracranial aneurysm rupture by improving endothelial function4,5 and reducing oxidative stress.12
A strength of this study is the MR design, which is less prone to confounding compared with conventional observational studies. Other major strengths are the relatively large number of cases of intracranial aneurysm and the robustness of the findings in different MR sensitivity analyses that are more robust to the inclusion of pleiotropic variants. Population stratification bias was minimized because all analyses were restricted to populations of European ancestry and adjustment was made for principal components for ancestry in the original genome-wide association studies from which summary data were used. In terms of limitations, we cannot rule out that our genetic proxies for serum magnesium affect intracranial aneurysm risk through alternative pathways that may violate the requisite assumptions of MR. Another limitation is that we did not have access to appropriate data for a bidirectional MR analysis to investigate whether biological mechanisms predisposing to intracranial aneurysm influence circulating magnesium levels. Finally, the genetic variants used to proxy the effect of modifying serum magnesium concentration reflect small, lifelong effects in serum magnesium concentrations, while in contrast a clinical intervention would typically exert a greater change in serum magnesium concentrations later in life. Caution should be taken when extrapolating findings from MR to infer the effect of a clinical intervention, and clinical trials are warranted to guide optimal practice.
This MR study provides evidence to support that higher serum magnesium concentrations reduce the risk of intracranial aneurysm and aneurysmal subarachnoid hemorrhage. These findings add to the growing body of evidence highlighting a beneficial role of higher magnesium for preventing cerebrovascular and cardiovascular diseases.13-15
Acknowledgment
The authors thank the International Stroke Genetics Consortium (ISGC) Intracranial Aneurysm working group. Summary statistics data for intracranial aneurysm were accessed through the ISGC Cerebrovascular Disease Knowledge Portal.
Glossary
- CI
confidence interval
- LD
linkage disequilibrium
- MR
mendelian randomization
- OR
odds ratio
- SNP
single nucleotide polymorphism
Appendix. Authors
Footnotes
Editorial, page 157
Study Funding
This study was supported by the Swedish Research Council for Health, Working Life and Welfare (Forte, 2018-00123). Dipender Gill is supported by the British Heart Foundation Research Centre of Excellence (RE/18/4/34215) at Imperial College London and a National Institute for Health Research Clinical Lectureship (CL-2020-16-001) at St. George's, University of London.
Disclosure
S.C. Larsson reports no disclosures. D. Gill is employed part time by Novo Nordisk. The study is not industry-sponsored. Go to Neurology.org/N for full disclosures.
References
- 1.Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389(2):655-666. [DOI] [PubMed] [Google Scholar]
- 2.Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med. 2017;377(3):257-266. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Li Y, Del Gobbo LC, et al. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension. 2016;68(2):324-333. [DOI] [PubMed] [Google Scholar]
- 4.Cunha AR, D'El-Rei J, Medeiros F, et al. Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J Hypertens. 2017;35(1):89-97. [DOI] [PubMed] [Google Scholar]
- 5.Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000;102(19):2353-2358. [DOI] [PubMed] [Google Scholar]
- 6.Meyer TE, Verwoert GC, Hwang SJ, et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet. 2010;6(8):e1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakker MK, van der Spek RAA, van Rheenen W, et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat Genet. 2020;52(12):1303-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evangelou E, Warren HR, Mosen-Ansorena D, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50(10):1412-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess S, Davey Smith G, Davies NM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019;4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiller W, Davies NM, Palmer TM. Software application profile: mrrobust: a tool for performing two-sample summary Mendelian randomization analyses. Int J Epidemiol. 2019;48:684-690. [Google Scholar]
- 11.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morais JB, Severo JS, Santos LR, et al. Role of magnesium in oxidative stress in individuals with obesity. Biol Trace Elem Res. 2017;176(1):20-26. [DOI] [PubMed] [Google Scholar]
- 13.Larsson SC, Traylor M, Burgess S, et al. Serum magnesium and calcium levels in relation to ischemic stroke: Mendelian randomization study. Neurology. 2019;92(9):e944-e950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson SC, Burgess S, Michaëlsson K. Serum magnesium levels and risk of coronary artery disease: mendelian randomisation study. BMC Med. 2018;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson SC, Drca N, Michaelsson K. Serum magnesium and calcium levels and risk of atrial fibrillation. Circ Genom Precis Med. 2019;12(1):e002349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the table.
Table.
Characteristics of the Single Nucleotide Polymorphisms Used as Instrumental Variables for Serum Magnesium Concentrations and Their Associations With Intracranial Aneurysm