Abstract
Purpose:
To evaluate whether reduction in glioblastoma radiation treatment volume can reduce risk of acute severe lymphopenia (ASL).
Methods and Materials:
A total of 210 patients with supratentorial/nonmetastatic glioblastoma were treated with radiation therapy (RT) plus temozolomide from 2007 to 2016 and had laboratory data on total lymphocyte counts. Before 2015, 164 patients were treated with standard-field RT (SFRT), and limited-field RT (LFRT) was implemented thereafter for 46 patients to reduce treatment volume. Total lymphocyte counts were evaluated at baseline, during RT, and at approximately week 12 from initiating RT. Acute severe lymphopenia was defined as any total lymphocyte count < 500 cells/μL within 3 months (by week 12) of initiating RT. Multivariate analysis for overall survival (OS) was performed with Cox regression and with logistic regression for ASL. Propensity score matching was performed to adjust for variability between cohorts. Acute severe lymphopenia, progression-free survival (PFS), and OS were compared using the Kaplan-Meier method.
Results:
Limited-field RT patients had higher gross tumor volume than SFRT patients yet lower brain dose—volume parameters, including volume receiving 25 Gy (V25 Gy: 41% vs 53%, respectively, P<.01). Total lymphocyte count at week 12 was significantly higher for LFRT than for SFRT (median: 1100 cells/μL vs 900 cells/μL, respectively, P = .02). On multivariate analysis, ASL was an independent predictor of OS, and brain V25 Gy was an independent predictor of ASL. The ASL rate at 3 months was 15.5% for LFRT and 33.8% for SFRT (P = .12). In a propensity-matched comparison of 45 pairs of LFRT and SFRT patients, PFS (median: 5.9 vs 6.2 months, respectively, P = .58) and OS (median: 16.2 vs 13.9 months, respectively, P = .69) were not significantly different.
Conclusions:
Limited-field RT is associated with less lymphopenia after RT plus temozolomide and does not adversely affect PFS or OS. Brain V25 Gy is confirmed as an important dosimetric predictor for ASL.
Summary
Any occurrence of acute grade ≥3 lymphopenia from chemoradiotherapy for glioblastoma patients is associated with worse progression-free survival and overall survival. The resulting lymphopenia is associated with brain volume exposed to moderate dose of radiation. Reduction of radiation treatment volume to the brain seems to reduce lymphopenia and does not negatively impact survival.
Introduction
Glioblastoma (GBM) has a poor prognosis despite multimodality treatment (1, 2). In patients treated with radiation therapy (RT) and concurrent temozolomide (TMZ), prospective data show that significant treatment-induced lymphopenia can result and is associated with tumor progression and worse survival (3). Even in elderly patients receiving a reduced dose of RT, a similar phenomenon has been observed (4). With the emergence of immunotherapy as the fourth modality of cancer treatment (5, 6), there may be an increasing need to understand and modulate iatrogenic immunosuppression.
Before the TMZ era, Hughes et al (7) noticed that 24% of GBM patients treated with RT alone subsequently developed opportunistic infections due to severe lymphopenia with CD4 count < 200 cells/μL. Radiation fields at that time tended to include large margins on T1 enhancing tumor as well as T2 abnormality on magnetic resonance imaging, and plans were delivered with 3-dimensional planning. It was hypothesized that the irradiation of circulating lymphocytes as blood flowed through the brain might represent a possible mechanism. Modeling simulation demonstrated that partial-brain irradiation for 30 fractions could expose the entire blood pool to 0.5 Gy (8), a toxic dose to lymphocytes (9). Radiation therapy dosimetric parameter of brain V25 Gy (brain volume receiving 25 Gy) has been previously demonstrated as an independent predictor of acute severe lymphopenia (ASL) after RT and TMZ (10).
The standard RT of GBM in the United States has traditionally been treating the T1 enhancing mass and T2 abnormality with a generous margin of approximately 2-3 cm to 46 Gy and then boosting the enhancing mass with a 2- to 3-cm margin to 60 Gy (11). However, many institutions have argued for eliminating using the T2 abnormality for designing the planning target volume (PTV) for RT and advocated for using a more-limited margin. These retrospective institutional reports of more limited-field RT have shown comparable patterns of recurrence and have not resulted in worse outcomes (12-15). Since January 2015, we revised our institutional guideline to reduce RT treatment volume for newly diagnosed GBM. This study evaluates the impact of such volume reduction on treatment-induced lymphopenia and survival outcomes after chemoradiotherapy.
Methods and Materials
Patient selection
Adult patients older than 18 years with newly diagnosed World Health Organization grade IV supratentorial/nonmetastatic GBM who received standard fractionated RT of 60 Gy with concurrent TMZ at our institution from January 2007 to December 2016 were retrospectively reviewed. Eligible patients were required to have total lymphocyte count (TLC) measurement before RT and at least 1 TLC value within 3 months after initiating RT. Total lymphocyte count values were recorded using the absolute lymphocyte counts from the clinical complete blood count panel. For comparison at specific time points, TLC data were categorized as at baseline (within 4 weeks before RT), week 2, week 6, and week 12 (±2-week window) after initiating RT. The study was conducted with the approval of the institutional review board.
Radiation therapy
Photon-based RT was delivered with either 3-dimensional conformal RT (3D-CRT) or intensity modulated radiation therapy (IMRT) technique. Patients were typically simulated with computed tomography and magnetic resonance imaging for RT planning. Before January 2015, patients were typically treated using a standard-field RT (SFRT) approach as outlined in the Radiation Therapy Oncology Group guidelines (11). Briefly, the initial gross tumor volume (GTV) was defined as T1 enhancement, surgical cavity, and T2 abnormality; the boost GTV was defined as T1 enhancement and surgical cavity only. The initial and boost GTVs were expanded by approximately 1 to 2 cm to produce the initial and boost clinical target volumes (CTVs), respectively, while respecting anatomic boundaries. Planning target volumes were 0.3 to 0.5-cm expansions from the CTVs. Standard-field RT was delivered using either a sequential boost or concomitant boost technique. For sequential boost, the initial PTV was treated to 46 Gy in 2-Gy fractions, followed by an additional of 14 Gy in 2-Gy fractions to the boost PTV. For concomitant boost, the initial PTV was treated to 54 Gy in 1.8-Gy fractions, whereas the boost PTV was treated to 60 Gy in 2-Gy fractions. In the cases in which the T2 abnormality was the same as T1 enhancement, a single PTV was treated to 60 Gy in 2-Gy fractions. In January 2015 our institution adopted a limited-field RT (LFRT) approach to treat GBM, whereby the GTV was only defined as T1 enhancement and surgical cavity without routine inclusion of T2 abnormality (unless the tumor did not enhance well and the T2 abnormality was thought to reflect gross tumor by the treating physician). The initial and boost CTVs consisted of 1.5-cm and 0.5-cm expansions of the GTV, respectively, again respecting anatomic boundaries. The CTV to PTV expansion was 0.3 to 0.5 cm. The LFRT approach was similar to the European Organization for Research and Treatment of Cancer guidelines (11), except with a reduction of treatment volume after 46 Gy for a limited-margin boost field (14). The SFRT and LFRT volume definitions are summarized in Table E1 (available online at www.redjournal.org). The initial and boost PTVs of LFRT were uniformly treated with a sequential boost technique as above, except their brain V25 Gy parameter was now routinely minimized using IMRT optimization without sacrificing coverage. Brain (which did not include brainstem) dose—volume histogram parameter (V10-60 Gy in 5-Gy increments), GTVs, and PTVs were extracted from archived RT plans using Computational Environment for Radiotherapy Research software as previously described (16).
Chemotherapy
All patients received concurrent TMZ at a dose of 75 mg/m2 given daily during RT. Adjuvant temozolomide was typically initiated 12 weeks from the start of RT and administered at a dose of 150-200 mg/m2 given over 5 days per 28-day cycle. In the SFRT cohort only, 25 patients also received additional agents (or placebo) during RT and TMZ, including bevacizumab/placebo (n = 8), dasatinib/placebo (n = 6), cediranib/placebo (n = 4), carmustine wafers (n = 3), cilengitide (n = 2), and CT-322 (n = 2).
Statistical analysis
Patient characteristics were compared using either the Mann-Whitney U test or χ2 test (or Fisher’s exact testing for smaller cell counts) for continuous or categorical variables, respectively. ASL was defined as any occurrence of TLC < 500 cells/μL within 3 months (by week-12 time point) of starting RT. ASL, progression-free survival (PFS), and overall survival (OS) rates between the 2 treatment strategies were calculated using the Kaplan-Meier method and then compared using log—rank testing. All time to event data were calculated from the start of RT. Univariate and multivariate analyses to identify predictors for OS and ASL were performed using Cox regression and logistic regression modeling, respectively. Variables with P < .10 on univariate analyses were entered in a forward-conditional manner for multivariate analyses. Linear regression was performed to model the relationship between brain V25 Gy and 3-month ASL rate.
Propensity score matching without replacement was performed to minimize unbalanced confounding factors between the SFRT and LFRT cohorts using the nearest-neighbor technique with a caliper distance of 0.20 of the standard deviation of the logit of the propensity score. Matching variables included clinical characteristics known to be predictive of ASL and OS, to minimize the confounding effect. Balance between the propensity-matched cohorts was performed by comparing standardized mean differences between the matching variables, to confirm that no standardized mean difference was >0.25 (17-24). The TLCs and GTVs of the matched cohorts were compared using the Wilcoxon matched-pair signed rank test. Acute severe lymphopenia rates and survival curves were generated using the Kaplan-Meier method and compared using log—rank testing.
All tests were 2-sided, and significance was defined as a P value ≤ .05. Statistical analyses were performed with the Statistical Package for Social Sciences, version 23.0 (IBM SPSS Statistics, Armonk, NY), with the R propensity matching extension package (R, version 2.15.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
Two hundred seventeen adult GBM patients were treated with 60 Gy of RT with concurrent TMZ between 2007 and 2016: 164 before 2015 and 53 thereafter. Seven patients from 2015-2016 were excluded from the analysis because they were treated using SFRT owing to enrollment to clinical protocols in which T2 abnormalities and larger margins were mandated. Thus, a total of 210 patients were eligible for the analysis: 164 patients with SFRT and 46 patients with LFRT.
Age, Karnofsky performance status (KPS), sex, extent of resection (EOR), O6-methylguanine-DNA-methyltransferase (MGMT) methylation, baseline TLCs, and dexamethasone use were well balanced between the 2 groups. Limited-field RT had larger GTVs, lower proportion with unknown MGMT status, and lower proportion treated with 3D-CRT. As expected, the brain dose—volume histogram and initial and boost PTVs were significantly lower with LFRT (Table 1). As seen in Figure 1A, TLCs at baseline, week 2, and week 6 were not significantly different between the 2 cohorts, but TLCs at week 12 were significantly higher for LFRT than for SFRT (median 1100 cells/μL vs 900 cells/μL, respectively, P = .02). As seen in Figure 1B, LFRT also had significantly lower brain V25 Gy than SFRT (41% vs 53%, P < .01). Limited-field RT had an approximately 50% reduction of ASL rate at 3 months compared with SFRT, though not statistically significant (15.5% vs 33.8%, 95% confidence interval [CI] 4.3-25.4% vs 23.7-42.5%, respectively, P = .12; Fig. 2A). Administration of additional agents during RT plus TMZ was not associated with higher ASL (odds ratio [OR] 0.734, 95% CI 0.261-2.065). However, given the imbalance of some SFRT patients receiving additional agents during RT plus TMZ and 3D-CRT, a sensitivity analysis was performed to exclude these patients (n = 51), and a similar difference of ASL rates was observed (Fig. E1; available online at www.redjournal.org).
Table 1.
Patient and treatment characteristics
| Characteristic | All (n = 210) | Standard field (n = 164) | Limited field (n = 46) | P |
|---|---|---|---|---|
| Age (y), median (range) | 57 (21-82) | 57 (21-82) | 57 (21-75) | .890 |
| KPS, median (range) | 80 (50-100) | 80 (50-100) | 80 (50-100) | .241 |
| KPS, n (%) | .219 | |||
| >70 | 125 (60) | 94 (57) | 31 (67) | |
| ≤70 | 85 (40) | 70 (43) | 15 (33) | |
| Sex (%) | .780 | |||
| Male | 127 (60) | 100 (61) | 27 (59) | |
| Female | 83 (40) | 64 (39) | 19 (41) | |
| Race, n (%) | .312 | |||
| White | 196 (93) | 151 (92) | 45 (98) | |
| Other | 14 (7) | 13 (8) | 1 (2) | |
| EOR, n (%) | .584 | |||
| Biopsy | 32 (15) | 26 (16) | 6 (13) | |
| STR | 55 (26) | 45 (27) | 10 (22) | |
| GTR | 123 (59) | 93 (57) | 30 (65) | |
| MGMT status, n (%) | <.001 | |||
| Known | 161 (77) | 115 (70) | 46 (100) | |
| Unknown | 49 (23) | 49 (30) | 0 | |
| MGMT methylated, n (%)* | .645 | |||
| Yes | 62 (38) | 43 (37) | 19 (41) | |
| No | 99 (62) | 72 (63) | 27 (59) | |
| Baseline TLC (cells/μL), median (range) | 1400 (300-4200) | 1400 (400-4200) | 1500 (300-3500) | .874 |
| Baseline Dex use (mg/day), median (range) | 4 (0-24) | 4 (0-16) | 1 (0-24) | .157 |
| Baseline Dex use > 4 mg/day, n (%) | .934 | |||
| Yes | 42 (20) | 33 (20) | 9 (20) | |
| No | 168 (80) | 131 (80) | 37 (80) | |
| Additional agent/placebo during RT, n (%) | .002 | |||
| Yes | 25 (12) | 25 (15) | 0 | |
| No | 185 (88) | 139 (85) | 46 (100) | |
| RT technique, n (%) | .001 | |||
| 3D-CRT | 28 (13) | 28 (17) | 0 | |
| IMRT | 182 (87) | 136 (83) | 46 (100) | |
| GTV (cm3),* median (range) | [n = 184] 33.3 (5.4-250.3) |
[n = 145] 32.0 (5.4-134.3) |
[n = 39] 48.4 (7.6-250.3) |
.014 |
| PTV initial (cm3),* median (range) | [n = 200] 334.0 (50-1225) |
[n = 155] 375 (50-1225) |
[n = 45] 245.7 (112.8-622.4) |
<.001 |
| PTV boost (cm3),* median (range) | [n = 198] 216.3 (50-690.6) |
[n = 155] 230.5 (50-690.6) |
[n = 43] 156.9 (76.1-622.4) |
<.001 |
| Brain DVH (%),* median (range) | [n = 200] | [n = 155] | [n = 45] | |
| V10 Gy | 77 (37-100) | 80 (42-100) | 68 (37-96) | <.001 |
| V15 Gy | 66 (31-100) | 68 (35-100) | 56 (31-90) | <.001 |
| V20 Gy | 57 (24-100) | 60 (27-100) | 48 (24-83) | <.001 |
| V25 Gy | 50 (19-100) | 53 (21-100) | 41 (19-78) | <.001 |
| V30 Gy | 45 (15-100) | 47 (18-100) | 34 (15-74) | <.001 |
| V35 Gy | 39 (13-100) | 42 (15-100) | 30 (13-68) | <.001 |
| V40 Gy | 35 (11-99) | 37 (13-99) | 26 (11-63) | <.001 |
| V45 Gy | 31 (10-98) | 33 (12-98) | 24 (10-58) | <.001 |
| V50 Gy | 27 (8-95) | 29 (10-95) | 21 (8-51) | <.001 |
| V55 Gy | 22 (7-86) | 23 (7-86) | 19 (7-45) | .001 |
| V60 Gy | 14 (3-52) | 14 (3-52) | 13 (5-32) | .183 |
Abbreviations: 3D-CRT = 3-dimensional conformal radiation therapy; Dex = dexamethasone; DVH = dose—volume histogram; EOR = extent of resection; GTR = gross total resection; GTV = gross tumor volume (GTV for standard-field RT cohort represents the boost GTV); IMRT = intensity modulated radiation therapy; KPS = Karnofsky performance status; MGMT = O6-methylguanine-DNA-methyltransferase; PTV = planning target volume; RT = radiation therapy; STR = subtotal resection; TLC = total lymphocyte count; V10-60 Gy = brain volume receiving 10-60 Gy.
Variables with missing values: MGMT methylation (49 patients), GTV (26 patients), PTV initial (10 patients), PTV boost (12 patients), brain DVH (10 patients).
Fig. 1.
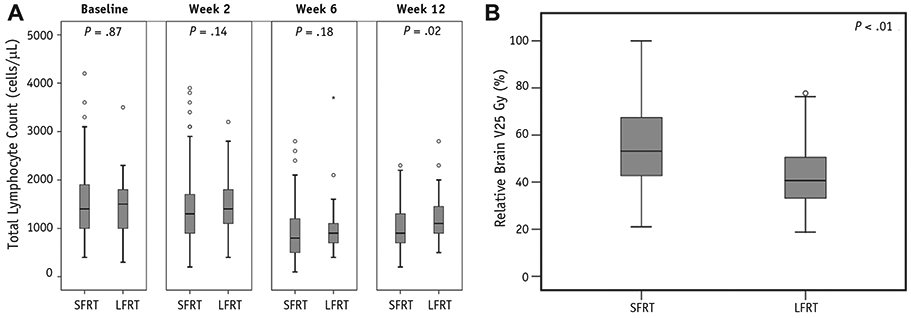
(A) Box and whisker plot illustrating median and quartile distribution of total lymphocyte counts for standard-field radiation therapy (SFRT) and limited-field radiation therapy (LFRT) patients at baseline, week 2, week 6, and week 12. (B) Box and whisker plot illustrating median and quartile distribution of brain V25 Gy (brain volume receiving 25 Gy) for standard-field radiation therapy and limited-field radiation therapy patients.
Fig. 2.
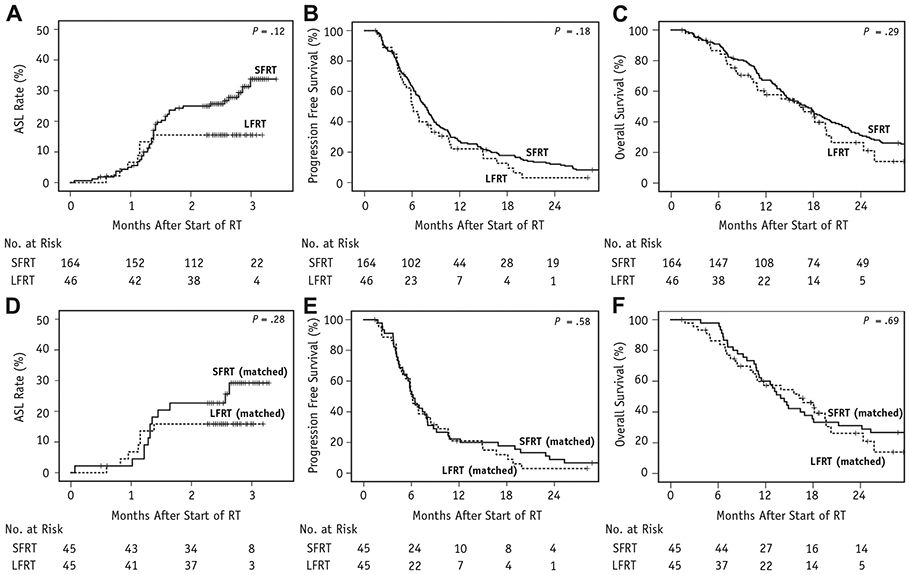
(A) Acute severe lymphopenia (ASL) rate of glioblastoma (GBM) patients treated with standard-field radiation therapy (SFRT) and limited-field radiation therapy (LFRT). (B) Progression-free survival of GBM patients treated with SFRT and LFRT. (C) Overall survival of GBM patients treated with SFRT and LFRT. (D) Acute severe lymphopenia rate of propensity-matched patients treated with SFRT and LFRT. (E) Progression-free survival of propensity-matched patients treated with SFRT and LFRT. (F) Overall survival of propensity-matched patients treated with SFRT and LFRT.
After a median follow-up of 15.4 months for all patients and 24.1 months for surviving patients, median PFS was 6.1 months (95% CI 5.2-7.1 months) for LFRT versus 7.7 months (95% CI 6.8-8.6 months) for SFRT (P = .18; Fig. 2B), and median OS was 16.2 months (95% CI 9.5-23.0 months) for LFRT versus 17.1 months (95% CI 14.4-19.8 months) for SFRT (P = .29; Fig. 2C). As seen in Table 2, Cox regression analyses identified EOR (hazard ratio [HR] 1.619, 95% CI 1.126-2.327), MGMT methylation (HR 0.469, 95% CI 0.322-0.683), and ASL (HR 1.831, 95% CI 1.196-2.803) as factors independently predictive for OS. Notably, TLC < 500 cells/μL (grade 3 or higher lymphopenia) at week 6 or 12 lacked statistically significant association with OS. Patients with ASL had significantly worse PFS and OS than those without ASL: median PFS of 6.4 months (95% CI 5.0-7.9 months) versus 7.7 months (95% CI 6.7-8.7 months, P = .01), respectively (Fig. E2A; available online at www.redjournal.org); median OS of 10.8 months (95% CI 9.7-12.0 months) versus 18.1 months (95% CI 16.1-20.0 months, P < .01), respectively (Fig. E2B; available online at www.redjournal.org). In Table 3, logistic regression analyses identified older age (OR 1.091, 95% CI 1.047-1.137), female sex (OR 2.901, 95% CI 1. 391-6.047), and higher brain V25 Gy (OR 1.048, 95% CI 1.022-1.074) as independent predictors for ASL. Notably, dexamethasone use at baseline lacked statistically significant association with ASL on multivariate analysis (Table 3). A dose—response curve also demonstrated that the risk of ASL increased linearly with higher brain V25 Gy in our cohort (Fig. 3A). As seen in Figure 3B, V25 Gy < 40% was associated with significantly lower ASL rate (12.6%, 95% CI 3.4-21.0%) than V25 Gy > 40% (35.7%, 95% CI 25.4-44.6%, P = .01).
Table 2.
Overall survival univariate and multivariate Cox regression analysis
| Characteristic | Univariate HR (95% CI) | P | Multivariate HR (95% CI) (n = 160) | P |
|---|---|---|---|---|
| Older age | 1.014 (1.001-1.027) | .034 | NS | NS |
| Higher KPS | 0.987 (0.974-1.001) | .062 | NS | NS |
| Female sex | 0.811 (0.595-1.103) | .182 | ||
| Caucasian race | 0.733 (0.406-1.323) | .302 | ||
| EOR | ||||
| GTR | Ref | |||
| STR/biopsy | 1.395 (1.031-1.886) | .031 | 1.619 (1.126-2.327) | .009 |
| Methylated MGMT* | 0.493 (0.339-0.717) | <.001 | 0.469 (0.322-0.683) | <.001 |
| Higher baseline TLC (100 cells/μL) | 0.984 (0.963-1.007) | .166 | ||
| Higher baseline Dex Use (mg/d) | 1.040 (1.003-1.079) | .034 | NS | NS |
| Limited-field RT | 1.246 (0.827-1.877) | .293 | ||
| RT technique (IMRT) | 0.964 (0.634-1.464) | .863 | ||
| TLC < 500 cells/μL at week 6 | 1.454 (0.988-2.139) | .058 | NS | NS |
| TLC < 500 cells/μL at week 12 | 1.488 (0.806-2.748) | .204 | ||
| ASL at any time point | 1.867 (1.326-2.629) | <.001 | 1.831 (1.196-2.803) | .005 |
| GTV* | 1.003 (0.998-1.007) | .222 |
Table 3.
Acute severe lymphopenia univariate and multivariate logistic regression analysis
| Characteristic | Univariate OR (95% CI) | P | Multivariate OR (95% CI) (n = 200) | P |
|---|---|---|---|---|
| Older age | 1.067 (1.030-1.105) | <.001 | 1.091 (1.047-1.137) | <.001 |
| Higher KPS | 0.966 (0.940-0.993) | .015 | NS | NS |
| Female sex | 2.702 (1.422-5.132) | .002 | 2.901 (1.391-6.047) | .004 |
| Caucasian race | 2.055 (0.444-9.499) | .357 | ||
| EOR | Ref | |||
| GTR | 1.590 (0.846-2.988) | .150 | ||
| STR/biopsy | ||||
| Methylated MGMT* | 1.152 (0.533-2.492) | .719 | ||
| Higher baseline TLC (100 cells/μL) | 0.933 (0.884-0.985) | .012 | NS | NS |
| Higher baseline Dex use (mg/d) | 1.078 (1.005-1.155) | .035 | NS | NS |
| Limited-field RT | 0.475 (0.198-1.138) | .095 | NS | NS |
| RT technique (IMRT) | 0.653 (0.275-1.549) | .334 | ||
| Brain DVH* | ||||
| V10 | 1.032 (1.007-1.057) | .012 | NS | NS |
| V15 | 1.029 (1.007-1.051) | .008 | NS | NS |
| V20 | 1.027 (1.007-1.047) | .008 | NS | NS |
| V25 | 1.027 (1.007-1.047) | .008 | 1.048 (1.022-1.074) | <.001 |
| V30 | 1.027 (1.006-1.049) | .012 | NS | NS |
| V35 | 1.026 (1.004-1.049) | .020 | NS | NS |
| V40 | 1.026 (1.002-1.050) | .032 | NS | NS |
| V45 | 1.025 (1.000-1.051) | .049 | NS | NS |
| V50 | 1.026 (0.998-1.054) | .065 | NS | NS |
| V55 | 1.025 (0.994-1.058) | .116 | ||
| V60 | 1.032 (0.986-1.080) | .174 |
Fig. 3.
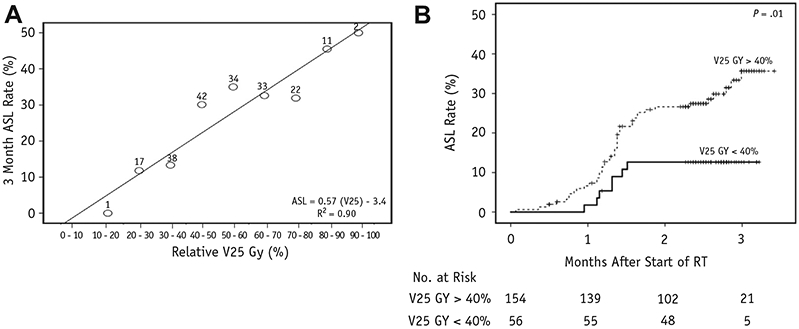
(A) Dose—response plot of brain V25 Gy (brain volume receiving 25 Gy) and 3-month acute severe lymphopenia (ASL) rate. Number above each data point denotes the sample size of that dose range. Linear regression equation estimates the risk of ASL for any given brain V25 Gy. (B) ASL rate stratified by V25 Gy greater than or less than 40%. Abbreviation: RT = radiation therapy.
To reduce unbalanced confounders between LFRT and SFRT, a matched cohort was created using 1:1 propensity score matching with the following variables: age, KPS, sex, EOR, MGMT methylation status, and baseline TLC. Gross tumor volume was not included because its wide variation limited its applicability for matching, but the matched cohort resulted in comparable GTVs between SFRT and LFRT (36.6 cm3 vs 45.6 cm3, respectively, P = .11). Of note, although RT technique was not used as a matching variable owing to its nonsignificant association with ASL or OS, the matched cohort only contained 1 SFRT patient who received 3D-CRT. Baseline characteristics for the matched cohort of 45 pairs were well balanced (Table E2; available online at www.redjournal.org). The TLCs at baseline, week 2, and week 6 were not significantly different between the matched cohorts, but TLCs at week 12 were borderline significant (median 1100 cells/μL for LFRT vs 1000 cells/μL for SFRT, P = .06). Acute severe lymphopenia rate at 3 months for LFRT (15.9%, 95% CI 4.4-26.0%) was again lower but not statistically significantly different than matched SFRT (29.2%, 95% CI 13.3-41.3%, P = .28; Fig. 2D). As seen in Figs. 2E and 2F, matched LFRT did not have significantly different PFS than matched SFRT (median 5.9 vs 6.2 months, 95% CI 5.0-6.9 months vs 5.3-7.1 months, respectively, P = .58) nor OS (median 16.2 vs 13.9 months, 95% CI 9.4-23.0 months vs 11.5-16.3 months, respectively, P = .69).
Discussion
Our study demonstrates that reducing treatment volumes using LFRT for newly diagnosed GBM receiving concurrent TMZ may lead to reduced treatment-induced lymphopenia. This study also confirms that ASL (more so than grade 3 or higher lymphopenia at week 6 or 12) is independently associated with OS and that brain V25 Gy is a significant dosimetric predictor for ASL. Importantly, reduction of treatment volume with LFRT does not seem to impact PFS or OS negatively.
The present study confirms and builds on some of the previous findings on treatment-induced lymphopenia in GBM. Investigators previously analyzed 183 grade 3/4 glioma patients treated with RT (ranging between 54 Gy and 66 Gy) and TMZ from 2007-2012. They showed female sex, older age, lower baseline TLC, and higher brain V25 Gy as the most significant predictors for ASL (10). With a more homogeneous population of only GBM patients and standard RT dose of 60 Gy, we again confirmed brain V25 Gy as the most significant dosimetric predictor of ASL. Unlike the other previously significant variables, baseline TLC did not meet the significance threshold in the multivariate analysis, which may be due to less frequent ASL in the present study. Importantly, this study provides evidence to support the previous hypothesis that reduction of brain V25 Gy may reduce treatment-induced lymphopenia. Grossman et al (3) previously demonstrated that grade 3 lymphopenia at approximately 2 months from the start of RT was independently associated with OS. Although this study confirms a significant association between treatment-induced lymphopenia and OS, it also suggests the occurrence of grade 3 lymphopenia at any time within the 3-month window (rather than its absence or presence at month 2 or 3) may be a more robust indicator.
Our finding that LFRT did not result in worse PFS and OS is reassuring and is consistent with previous retrospective studies. In the pre-TMZ era, Chang et al (12) illustrated that patterns of failure in GBM patients did not differ if the T2 abnormality was not intentionally used to generate CTVs. Subsequently, Paulsson et al (15) reported on survival outcomes in GBM patients (mostly treated with TMZ-based chemoradiotherapy) using a range of CTV expansions (5-20 mm) and noted no significant differences in survival with reduced CTV margins. The 2016 American Society for Radiation Oncology clinical practice guideline for GBM reports considerable variability in CTV margin size among cooperative groups, with the American Brain Tumor Consortium recommending margins of 5 mm for both initial and boost CTVs (11). Our data confirm that CTV margin reduction and elimination of routine inclusion of T2 abnormality not only do not result in worse PFS/OS but also may reduce treatment-induced lymphopenia. Given the dismal prognosis of GBM with standard chemoradiotherapy and increasing trend to incorporate immunotherapy or targeted agents, standardization and adoption of a more limited-field approach to irradiate GBM should be revisited.
Given the strong association between ASL and OS, the lack of improved PFS/OS with reduction of ASL after LFRT is slightly counter-intuitive. The nonsignificant difference of PFS/OS between SFRT and LFRT may be due to limited sample size, which may also explain the lack of statistical significance for ASL rates despite large observed difference. Another possibility may be that ASL is a surrogate rather than a cause for decreased PFS or OS. In the multivariate analysis for OS (Table 2), ASL seemed to replace age and KPS as a prognostic factor for OS, suggesting ASL may be a surrogate for the patient’s overall health status. Interestingly, Campian et al (25) previously performed a feasibility study to administer lymphocyte reinfusion after completion of chemoradiotherapy but failed to reverse lymphopenia as compared with matched historical controls. Clinical trials to test other methods to manage treatment-related lymphopenia in GBM, such as interleukin-7 (NCT02659800) are currently under investigation and will shed further insight on whether treatment of ASL may affect OS.
Currently, the mechanism behind treatment-induced lymphopenia is unclear. Yovino et al (8) previously modeled the effect of RT field for GBM and the direct radiation dose to circulating lymphocytes. This model explains why the brain V25 Gy correlates with ASL and why reduction of treatment volume can reduce ASL in our study. Investigators of other disease sites have noted similar correlations between RT volume and lymphopenia (26). However, lymphopenia can persevere for many months in GBM patients after completion of partial-brain RT without significant radiation exposure to much of the lymphoid organs or bone marrow. This indicates that exposure of circulating lymphocytes to RT is not the sole mechanism of lymphopenia. Preclinical studies have demonstrated that RT can have indirect consequences on circulating lymphocytes. Kapoor et al (27) found that ex vivo irradiation of a small proportion of blood and reinfusion into mice can cause a drastic reduction in systemic lymphocyte count, which can be reversed with autologous stem cell transplantation. Our group is currently conducting additional translational research to further explore the underlying mechanisms.
Limitations of this study stem from the retrospective nature of its design. Without prospective randomization, the SFRT and LFRT cohorts may have unadjusted or unknown confounders that may not be completely accounted for by multivariate analysis or propensity score matching. For example, LFRT is more recent with more uniform treatment design and may also derive benefit from improved general medical care over time. A small percentage of SFRT patients received additional systemic agents during RT plus TMZ or were treated with 3D-CRT instead of IMRT, but these factors did not significantly impact ASL (Table 3 and Fig. E1; available online at www.redjournal.org). Although steroid exposure might play a confounding effect on lymphopenia, our analysis showed that baseline steroid use (either as a continuous variable or a binary variable with 4 mg/d as a cut-off) was not significantly different between the SFRT and LFRT cohorts (Table 1). Furthermore, baseline steroid use did not seem to correlate with ASL when accounting for other clinical factors (Table 3), which is consistent with prior data (3, 10). Limited-field RT has fewer patients and more limited follow-up, so lack of statistical difference for ASL and survival outcomes may be the result of under-powered sample size. Thus, the findings of this study should be considered hypothesis-generating, and more extensive prospective studies should be considered to determine whether treatment reduction of GBM may lead to improvement in ASL and survival.
Conclusion
This study adds to the growing body of literature evaluating the relationship between RT and treatment-induced lymphopenia in GBM patients. In particular, V25 Gy of the brain is an independent predictor for ASL in GBM patients receiving 60 Gy of RT with concurrent TMZ, and reduction of brain volume irradiated may lead to less treatment-induced lymphopenia.
Supplementary Material
Footnotes
Conflict of interest: C.T. reports personal fees from Merck, personal fees from Varian, and personal fees from Novocure outside the submitted work.
Supplementary material for this article can be found at www.redjournal.org.
Presented in part at the 59th Annual Meeting of the American Society for Radiation Oncology, September 24-17, 2017, San Diego, CA.
References
- 1.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA 2015;314:2535–2543. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason W, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 3.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 2011;17:5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendez JS, Govindan A, Leong J, et al. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol 2016;127:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reardon DA, Freeman G, Wu C, et al. Immunotherapy advances for glioblastoma. Neuro Oncol 2014;16:1441–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finocchiaro G, Pellegatta S. Perspectives for immunotherapy in glioblastoma treatment. Curr Opin Oncol 2014;26:608–614. [DOI] [PubMed] [Google Scholar]
- 7.Hughes MA, Parisi M, Grossman S, et al. Primary brain tumors treated with steroids and radiotherapy: Low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys 2005;62:1423–1426. [DOI] [PubMed] [Google Scholar]
- 8.Yovino S, Kleinberg L, Grossman SA, et al. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 2013;31:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res 1990;123:224–227. [PubMed] [Google Scholar]
- 10.Huang J, DeWees TA, Badiyan SN, et al. Clinical and Dosimetric Predictors of Acute Severe Lymphopenia During Radiation Therapy and Concurrent Temozolomide for High-Grade Glioma. Int J Radiat Oncol Biol Phys 2015;92:1000–1007. [DOI] [PubMed] [Google Scholar]
- 11.Cabrera AR, Kirkpatrick JP, Fiveash JB, et al. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol 2016;6:217–225. [DOI] [PubMed] [Google Scholar]
- 12.Chang EL, Akyurek S, Avalos T, et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys 2007;68:144–150. [DOI] [PubMed] [Google Scholar]
- 13.Gebhardt BJ, Dobelbower MC, Ennis WH, et al. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat Oncol 2014;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald MW, Shu HKG, Curran WJ, et al. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int J Radiat Oncol Biol Phys 2011;79:130–136. [DOI] [PubMed] [Google Scholar]
- 15.Paulsson AK, McMullen KP, Peiffer AM, et al. Limited margins using modern radiotherapy techniques does not increase marginal failure rate of glioblastoma. Am J Clin Oncol 2014;37:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deasy JO, Blanco AI, Clark VH. CERR: A computational environment for radiotherapy research. Med Phys 2003;30:979–985. [DOI] [PubMed] [Google Scholar]
- 17.Thoemmes F Propensity score matching in SPSS. Available at: http://arxiv.org/abs/1201.6385.Accessed August 10, 2017.
- 18.Iacus SM, King G, Porro G. cem: Software for coarsened exact matching. J Stat Softw 2009;30:1–27.21666874 [Google Scholar]
- 19.Ho DE, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007;15:199–236. [Google Scholar]
- 20.Ho DE, Imai K, King G, et al. MatchIt : Nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42:1–28. [Google Scholar]
- 21.Hansen BB, Bowers J. Covariate balance in simple, stratified and clustered comparative studies. Stat Sci 2008;23:219–236. [Google Scholar]
- 22.Hansen BB, Klopfer SO. Optimal full matching and related designs via network flows. J Comput Graph Stat 2006;15:609–627. [Google Scholar]
- 23.Hansen BB. Full matching in an observational study of coaching for the SAT. J Am Stat Assoc 2004;99:609–618. [Google Scholar]
- 24.Bertsekas DP, Tseng P. Relaxation methods for minimum cost ordinary and generalized network flow problems. Oper Res 1988;36:93–114. [Google Scholar]
- 25.Campian JL, Ye X, Gladstone DE, et al. Pre-radiation lymphocyte harvesting and post-radiation reinfusion in patients with newly diagnosed high grade gliomas. J Neurooncol 2015;124:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014;89:1084–1091. [DOI] [PubMed] [Google Scholar]
- 27.Kapoor V, Khudanyan A, De La Puente P, et al. Stem cell transfusion restores immune function in radiation-induced lymphopenic C57BL/6 mice. Cancer Res 2015;75:3442–3445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


