Abstract
Beta-carotene is an important natural pigment that is very beneficial to human health. It is widely found in vegetables and fruits. The three main functions are antioxidant effects, cell gap junction-related functions and immune-related functions. Because of its diverse functions, beta-carotene is believed to prevent and treat many chronic diseases. Gastric cancer is one of the most important diseases it can treat. Gastric cancer is a type of cancer with a high incidence. Its etiology varies, and the pathogenesis is complex. Gastric cancer seriously affects human health. The role of beta-carotene, a natural nutrient, in gastric cancer has been explored by many researchers, including molecular mechanisms and epidemiological studies. Molecular studies have mainly focused on oxidative stress, cell cycle, signal transduction pathways and immune-related mechanisms of beta-carotene in gastric cancer. Many epidemiological surveys and cohort studies of patients with gastric cancer have been conducted, and the results of these epidemiological studies vary due to the use of different research methods and analysis of different regions. This paper will summarize the results of these studies, mainly in terms of molecular mechanisms and epidemiological research results, which will provide a systematic basis for future studies of the treatment and prognosis of gastric cancer. This paper will help researchers identify new research directions.
Keywords: Beta-carotene, Gastric cancer, Nutrient, Tumor, Stomach, Epidemiology
Core Tip: Beta-carotene is believed to prevent and treat many chronic diseases. Gastric cancer is one of the most important diseases it can treat. A few studies in the literature database describe the pathophysiology, therapy and clinical trials for gastric cancer and beta-carotene. However, this review summarizes the latest studies on the specific effect of beta-carotene on gastric cancer. It includes molecular mechanisms and clinical trials.
INTRODUCTION
Overview of beta-carotene
Carotenoids are a group of important natural yellow, orange-red or red pigments commonly found in animals, higher plants, fungi and algae[1]. The consumption of carotenoid-rich foods of animal and plant origin has been associated with numerous health benefits. However, carotenoids cannot be synthesized in the human body, and they must be consumed through the diet[2]. Approximately 750 carotenoids, except E (trans) and Z (cis) isomers, have been isolated from natural sources[3]. Among these various carotenoids, beta-carotene is a major category. As the most nutritionally active compound, beta-carotene is particularly attractive for the food industry[4]. In addition, many of the properties and functions of beta-carotene have been certified to be beneficial to human health.
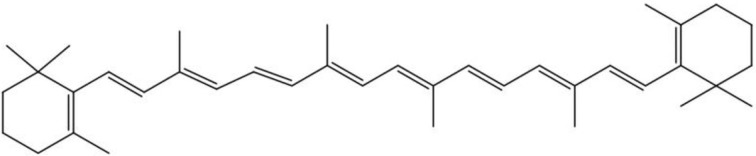
Beta-carotene, a carotene that does not contain an O atom[1], is an orange-red and fat-soluble natural pigment that is mainly found in plants. It is enzymatically cleaved in the intestinal mucosa by beta-carotene 15,15’-monooxygenase at the central double bond, forming two molecules of vitamin A (retinol)[5]. Beta-carotene is also called provitamin A. As the main precursor of vitamin A, beta-carotene is widely distributed in orange and yellow fruits and green leafy vegetables, including carrots, pumpkins and mangoes[6]. Beta-carotene is a type of (C40H56) carotene in which both ends of the molecule are cycled into rings. The most prominent structural feature is that the center is a long system of double bonds and single bonds. The molecule has 11 conjugated double bonds, two of which are located on the ring[1]. The chemical structure of beta-carotene is shown in Figure 1. The structure of conjugated double bonds with a center of symmetry makes beta-carotene highly hydrophobic[7]. This conjugated double-bond system constitutes the light-absorbing chromophore that gives carotenoids color and their special properties and functions[1,8].
Figure 1.
The chemical structure of beta-carotene.
Beta-carotene can be dispersed into a lipid phase. Oil-in-water emulsions serve as a delivery system that produces a physically stable beta-carotene dispersion[9]. It is easily oxidized when exposed to air, light and high temperature. Beta-carotene is a common and stable natural pigment that exists in nature, with low water solubility, a high melting point and low oral bioavailability.
THE EXTRACTION AND SYNTHESIS OF BETA-CAROTENE
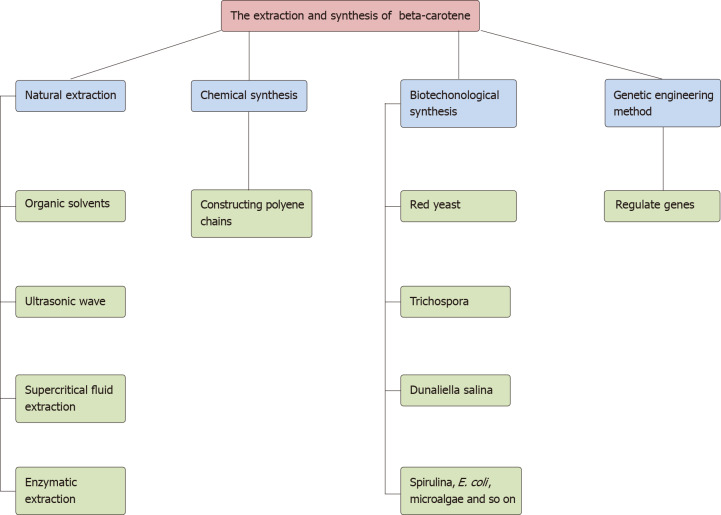
Four main sources of beta-carotene have been identified: extraction from natural resources, chemical synthesis, biosynthesis and genetic engineering methods. In the natural extraction of beta-carotene from natural plants or algae, physical extraction methods mainly include organic solvent extraction[10], ultrasonic wave extraction[11] and supercritical fluid extraction (SFE)[12]; another method is enzymatic extraction. The other three synthesis methods will be described in detail in the following sections. Sometimes, researchers try to combine two or more methods to obtain higher beta-carotene production. In addition, some special and innovative methods to synthesize beta-carotene have been developed, such as air-assisted, low-density solvent-based liquid-liquid microextraction, solidified floating organic droplets and centrifugal partition extraction[13,14]. The extraction and synthesis process of beta-carotene is shown in Figure 2.
Figure 2.
The extraction and synthesis of beta-carotene. E. coli: Escherichia coli.
The natural extraction of beta-carotene
Extraction with organic solvents: Solvent extraction usually requires multiple extraction steps to achieve the desired level of carotenoid recovery, and thus this method takes considerable time. Then, the pretreatment of materials is necessary, which can increase extraction efficiency. Pretreatment includes mechanical methods such as grinding, ball milling, ultrasonication and high-pressure homogenization and nonmechanical methods such as enzymatic or chemical hydrolysis and osmotic shock[15,16]. The most commonly used extractants are acetone and anhydrous ether. The type of extraction solvent and its volume, pH of the sample solution, extraction time and type of salt and its concentration are all main factors that influence the extraction efficiency[17].
Extraction by ultrasonic wave: Ultrasound-assisted extraction has been widely used to extract nutritional material. Compared with traditional solvent extraction, this method can achieve a higher extraction rate by decreasing the processing time, reducing the cost of extraction, preventing thermal damage and enhancing food quality[18]. Ultrasound-assisted extraction is performed with a probe ultrasonic processor. It extracts the active components into the solvent through ultrasonic vibration and cavitation[19]. Several studies have indicated that many factors, including the particle size, solvent, solid/solvent ratio, temperature, extraction time, liquid height and duty cycle of ultrasound exposure exert significant effects on the extraction yield of beta-carotene[17,18]. We should set different conditions according to different materials to modify the extraction.
SFE: SFE increases the efficiency of carotenoid extraction compared with conventional solvent extraction. With a high diffusion coefficient and low viscosity, supercritical “CO2” quickly penetrates the pores of the extracellular matrix, thus reducing the time required for extraction[12,20]. In addition, SFE is considered a green process because of its potential for carbon dioxide recovery and the elimination of harmful organic solvents[20]. Many factors affect the extraction rate and efficiency, including the extraction pressure, extraction time, flow rate and temperature[21]. The extraction temperature acts as a threshold value beyond which the extraction yields will decrease. The threshold temperature of thermal-sensitive beta-carotene in SFE is 70 ℃; once the temperature exceeds 70 ℃, beta-carotene will decrease and isomerize[20].
Enzymatic extraction: Some studies have reported a method to extract more beta-carotene by enzymatic extraction. This method is mainly used to increase the beta-carotene content in juice drinks. Pectolytic and cellulolytic enzymes significantly increase juice recovery. The combination of pectolytic and cellulolytic enzymes as a pretreatment also increases the beta-carotene content of carrot juice by 1.6 times[16]. In a previous study, carotenoid extraction yields increased to greater than 96% using noncommercial enzymes in a sequential and selective two-stage extraction method. Therefore enzymatic extraction is potentially useful to extract carotenoids in industrial practices[22]. Marigold flowers are the most important source of carotenoids in the food industry, and some researchers have tried a new method, simultaneous enzymatic treatment and solvent extraction, to extract carotenoids. The recovery yield reached 97%[23]. Moreover, after juice extraction, byproducts such as cashews, apples and bagasse are still sources of carotenoids. The use of pectinolytic and cellulolytic enzyme complexes to dispose of waste obtains more carotenoids, with an overall increase of 79% compared with the group treated without the enzyme complex[24]. Therefore, the enzymatic extraction method is easy and efficient but requires the right amount of enzyme.
Chemical synthesis method
Chemical synthesis is a method of synthesizing beta-carotene from organic chemical materials through chemical reactions. This method was developed in the 1950s and has been a major source of carotenoid pigments for many years[25]. We can obtain beta-carotene by constructing polyene chains, which involve Wittig reactions or Grignard compounds.
The Wittig reaction forms C=C bonds in organic synthesis and is widely used to synthesize medicine and complex organic compounds. The Wittig reaction of carotenoids involves the combination of two phosphonium salt molecules containing 15 carbon atoms and a dialdehyde molecule containing 10 carbon atoms. Then, the products will be isomerized to form symmetrical compounds with 40 carbon atoms, such as beta-carotene. The synthesis process is designed as C15 + C10 + C15[26].
With the help of the Grignard compound, a diketone molecule with two methanol molecules can produce a compound containing 40 carbon atoms[27].
Biotechnological synthesis method
Biotechnology is an important method to obtain carotene. This method produces carotene through microbial fermentation, which is better than chemical synthesis in terms of quality, technology, resources and cost. A few studies have focused on red yeast and filamentous fungi (Blakeslea trispora) as materials to synthesize beta-carotene.
Red yeast is a saprophyte with strong resistance. The advantages of using red yeast to produce beta-carotene are its low cost, short cycle and convenient fermentation control. The insertion of functional genes such as crtYB and crtE into the oleaginous red yeast, Rhodotorula glutinis, genome could further improve the beta-carotene production[28]. According to one study, the oleaginous red yeast Sporidiobolus pararoseus strain KM281507 is a good potential source of natural carotenoid production, while the percent of beta-carotene production relative to total carotenoids increased[29].
Trichospora is a fungus that grows rapidly. It is commonly used as a raw material to synthesize beta-carotene in biotechnological processes. Researchers have also extracted beta-carotene using the conventional reference extraction methods mentioned above. In addition, the extraction efficiency is affected by many factors, such as the extraction time, shaking speed, size of grinding beads and fixed angle of shelves. Therefore, researchers have developed a new method, high-throughput extraction, to increase the extraction efficiency and yield[30]. In the process of generating beta-carotene from Blakeslea trispora, the yield was determined by culture conditions such as the concentration of carbon source and particularly the pH. The highest production of beta-carotene was obtained when the pH was 7, and the production was also affected by linoleic acid-kerosene and linoleic acid-antioxidant interactions as well as the negative quadratic effects of these variables[31]. The biosynthesis of beta-carotene from Blakeslea trispora was significantly promoted by ultrasonic treatment. After ultrasonic simulation, the beta-carotene yield increased by 40.7% in the study by Wang et al[32].
Dunaliella salina (D. salina) is a monocytic eukaryote that lives in NaCl water. D. salinais has high carotenoid contents of up to 10%-14% dry weight, and thus it is an ideal raw material for the extraction of beta-carotene[33,34]. As the product of photosynthesis in D. salina, beta-carotene is affected by the salinity, temperature, light irradiation conditions and phosphorus sources in the growing environment. A biocompatible method for the extraction of beta-carotene from D. salina was developed, and high extraction yields were achieved using centrifugal partition extraction. The solvent choice, flow rate, rotational speed and extraction mode are significant factors influencing the extraction process[34]. Several researchers developed a blue-red LED wavelength-shifting system (B-R system) combined with an adaptive laboratory evolution blue light-adapted D. salina (ALE-D. salina). Beta-carotene production increased by approximately 19.7% with this system[35].
In addition, some studies have used other microorganisms such as Spirulina[36], Escherichia coli[37] and microalgae[38]. Many new methods have been developed to obtain more beta-carotene from these raw materials. The application of biotechnological methods requires the assistance of physical extraction as well.
Genetic engineering method
With the rapid development of transgenic technology, the production of carotenoids by genetically engineered bacteria has become a focus of research. The production of beta-carotene is increased by regulating the expression of lycopene cyclization enzyme (CrtY) and blocking the pentose phosphate pathway[39,40]. Moreover, the yield of beta-carotene has also been improved by transforming beta-carotene biosynthesis genes (crtI, crtE, crtYB and tHMG1) into the Rhodotorula glutinis (an oleaginous red yeast) genome[28]. Another study combined classical forward and modern reverse genetic techniques to more precisely regulate carotenoid synthesis. The repressor crgA and mutations in genes involved in the newly identified regulatory mechanism resulted in the accumulation of high levels of beta-carotene[41].
Nanoparticles and nanodispersions of beta-carotene
Nanodispersions have particle sizes in the nanometer range and are more physically stable than traditional dispersions containing micron-sized particles; thus, nanoparticles and nanodispersions have received increasing attention. Beta-carotene nanoparticles exhibit bioactivity beyond their high hydrophobicity limit in aqueous media[7]. Protein-stable beta-carotene nanodispersions have been prepared with solvent displacement techniques. Solvent displacement remains as an attractive technique to prepare nanodispersions due to its simplicity and low-energy input[42]. The beta-carotene particle size and distribution are determined by the type of protein, molecular weight and structural flexibility. The nanoencapsulation of carotenoids can be used in the food industry to broaden the application of these pigments. Yellow passion fruit albedo flour is a possible substitute in the production of carotenoid nanodispersions in various food matrices[43].
FUNCTIONS OF BETA-CAROTENE
The functions of beta-carotene are roughly divided into the following aspects: Antioxidant function, facilitating gap-junction intercellular communication and anti-inflammatory effect and immune-related function. Antioxidant is short for antioxidant free radicals, which is a chemical substance (atom, molecule or ion) that contains one or more unpaired electrons in its outer orbital and usually exhibits significant reactivity[44]. Beta-carotene shows its antioxidant activity by neutralizing reactive oxygen species. Gap junctions are plasma membrane domains containing an array of intercellular channels that are formed by the docking of two connexons, and they are responsible for the direct intercellular transfer of ions and small molecules[45]. In some studies, it has been found that beta-carotene has a regulatory effect on the expression of connexin. Beta-carotene is an antioxidant that can also activate the immune system and promote immune responses by activating spleen cells and macrophages[46]. Moreover, beta-carotene is the most abundant dietary precursor of vitamin A, an essential nutrient sustaining normal embryonic development in mammals[47].
The antioxidant effect of beta-carotene
Beta-carotene is a natural compound with significant antioxidant activity[8]. The antioxidant activity of beta-carotene is related to the neutralization of reactive oxygen species, including free radicals, inhibiting their propagation and participation in peroxidative processes. When beta-carotene is present as nanoparticles, its biological activity in aqueous media is substantially improved[7]. The main antioxidant effects include reducing oxidative stress and oxidative damage to DNA.
The use of molecular oxygen by aerobic organisms results in the formation of many oxygen-containing active substances, collectively known as reactive oxygen species (ROS). ROS play an important role in the physiology and pathophysiology of aerobic processes[48]. Carotenoids are effective antioxidants that reduce oxidative stress[49]. As shown in a previous study, beta-carotene exerts its antioxidant effect by scavenging ROS, inhibiting the expression of NADPH oxidase subunits and increasing the expression/activity of antioxidant enzymes[50]. A large number of lipid peroxidation and free radical reactions occurs in organisms that damage nucleic acids, proteins, the cell membrane and cells, resulting in a decrease in cell function, aging of the body and the occurrence of disease. The presence of beta-carotene reduces lipid peroxidation as a radical-scavenging antioxidant[51]. Some studies indicated weak antioxidant activity of beta-carotene, but it showed pro-oxidant activity at higher concentrations. In a study on keratinocytes that produced ROS and glutathione after exposure to beta-carotene and ultraviolet–visible spectroscopy/near infrared, researchers administered beta-carotene to cells using nanocrystals without additional solvents. Low concentrations of beta-carotene protect against oxidative stress. However, the higher the concentrations of beta-carotene, the less effective the molecule[52]. Another study suggested that beta-carotene functions as a potential antioxidant in mouse oocytes. Part of the mechanism is that beta-carotene reduces ROS formation and cell apoptosis and restores actin expression, cortical granule-free domain formation, the homogeneous distribution of mitochondria and nuclear maturation[53].
On the other hand, the antioxidant effect of beta-carotene is related to oxidative damage to DNA. Carotenoids are capable of exerting two overlapping but distinct effects: antioxidant protection by scavenging DNA-damaging free radicals and modulation of DNA repair mechanisms. An inverse association of carotenoid (particularly beta-carotene) consumption with lipid and oxidative stress biomarkers and DNA damage has been observed[54]. The intake of antioxidants such as beta-carotene from the diet is proposed to exert beneficial health effects, but the potential effect of taking extra antioxidants as supplements is controversial. For example, beta-carotene can reduce and enhance the DNA damage caused by genotoxic agents such as catechol. The extent of catechol-induced DNA damage determines whether beta carotene is beneficial or harmful when used as a dietary supplement[55].
Beta-carotene influences gap junctional intercellular communication
Gap junctions are membrane channels found in all cells of the human body that are essential to cellular physiology. They allow the cytoplasm of adjacent cells to communicate and regulate metabolic responses by exchanging small molecules[56]. Gap junctional intercellular communication (GJIC), a form of cell-to-cell communication, also plays an important physiological role[57]. Beta-carotene exerts protective effects on the H2O2-induced inhibition of GJIC in WB-F344 rat liver epithelial cells. In this study, researchers found that beta-carotene restored connexin 43 (Cx43) mRNA expression and prevented the phosphorylation of the Cx43 protein[58]. In a study of beta-carotene and adrenocorticotropic hormone-secreting pituitary adenoma cells, beta-carotene negatively modulated the malignant phenotype of AtT-20 cells. The mechanism underlying this process involves intercellular communication and the expression of Cx43, Skp2 and p27 (kip1)[59]. Another study using a human lung cancer cell line (A549) indicated that oxidized beta-carotene (obtained by heating beta-carotene at 60 °C in open air for 1 h) inhibited GJIC. A high dose of beta-carotene also inhibited GJIC, which may be attributed to the effect of oxidized beta-carotene[60]. In summary, many studies have shown that beta-carotene affects cancer cells by modulating Cx43 expression.
Immune-related function of beta-carotene
Beta-carotene is an important antioxidant that quenches singlet oxygen, inhibits lipid peroxidation and activates the immune system. Many studies have been performed to explore the immune-related function of beta-carotene. In a study on the effective component of Cucurbita moschata Duch, beta-carotene promoted immune responses by activating splenocytes and macrophages. Beta-carotene may exert an immune-enhancing effect through the activation of nuclear factor kappa-B (NF-κB) pathways and activation of immune cells that produce Th1 cytokines[46]. M2 macrophages and activated fibroblasts may modulate the behavior of cancer cells in the tumor microenvironment. Beta-carotene exerts potential therapeutic effects on colorectal cancer by inhibiting M2 macrophage polarization and fibroblast activation[61]. In animal experiments, supplementation of weaned mice with beta-carotene enhanced mucosal immunoglobulin A production in the jejunum or ileum, and the effects were mainly due to the retinoic acid (RA)-mediated immune response[62]. Beta-carotene functionally increases Mmp-9 mRNA levels in murine RAW264.7 (a murine macrophage cell line) macrophages in a concentration- and time-dependent manner, thus enhancing macrophage phagocytosis[63]. Other studies on the relationship between beta-carotene and immunity showed that low-dose supplementation with beta-carotene does not enhance cell-mediated immunity in healthy free-living elderly humans[64]. However, this study was conducted many years ago and has low reference value. A randomized controlled trial showed that dietary supplementation with beta-carotene has no effect on the antioxidant status and immune responses in allergic adults[65].
GASTRIC CANCER
Gastric cancer is a malignant tumor derived from the gastric mucosal epithelium and the second leading cause of cancer-related mortality and the fourth most common cancer globally[66,67]. Gastric cancer is a common disease that threatens human health. The incidence of gastric cancer varies in different regions and is related to various risk factors[66]. Some environmental and genetic factors are involved in the development of gastric cancer, and 52 risk factors for gastric cancer have been identified[68]. Helicobacter pylori (H. pylori), a bacterial carcinogen, infection is the strongest risk factor for the development of gastric cancer[69]. Common risk factors include older age, male sex, tobacco smoking, radiation and family history. Race also influences the incidence of gastric cancer[70]. A first-degree relative diagnosed with gastric cancer is an important risk factor for gastric cancer, and some unclear pathogenic mechanisms underlie this familial aggregation[71]. Atrophic gastritis and intestinal metaplasia are significant risk factors for gastric cancer as well[72].
Gastric cancer is divided into early and advanced gastric cancer according to the depth of tumor invasion. In the pathological classification, the Lauren type is the most widely used type in clinical practice and trials now. According to the histologic features, gastric cancers can be divided into three types: Intestinal, diffuse and mixed[73]. Paris classification was proposed by a workshop in Paris in 2002, and it was based on the Japanese classification. The Paris classification is related to the definition of the subtypes used in endoscopy and the evaluation of the depth of invasion into the submucosa. Type 1-4 is used to describe advanced cancers of the digestive tract mucosa. If the endoscopic appearance is superficial lesions, it is called type 0. Type 0 is divided into three categories: protruding (0-I), nonprotruding and nonexcavated (0-II) and excavated (0-II). Type 0-II lesions are then subdivided into slightly elevated (IIa), flat (IIb) or depressed (IIc)[74]. Gastric cancer results from the accumulation of genetic changes in oncogenes and tumor suppressor genes, which leads to the imbalance of multiple signaling pathways and disrupts the balance between the cell cycle, cell proliferation and death[75]. Dysregulation of developmental pathways such as Wnt/β-catenin signaling, Hedgehog signaling, the Hippo pathway, Notch signaling, NF-kB and epidermal growth factor receptor may be related to gastric cancer[76]. However, some detailed mechanisms have not yet been extensively studied.
Gastric cancer is a common malignant tumor of the digestive system, and surgery is the only way to cure it. The surgery of advanced gastric cancer should include D2 lymphadenectomy. Patients with advanced gastric cancer are mainly treated by surgery in combination with intraperitoneal chemotherapy and hyperthermic perfusion therapy so that we can improve the effect of surgical treatment[77]. The endoscopic treatments of endoscopic mucosal resection and endoscopic submucosal dissection have been widely used for the treatment of early gastric cancers with only little risk of lymph node metastasis[78]. Neoadjuvant chemotherapy, radiotherapy and molecular targeted therapy have become effective methods to improve the prognosis of patients with gastric cancer[79]. In addition, the targeted therapies of gastric cancer include trastuzumab (HER2-positive patient’s first line), ramucirumab (anti-angiogenic second line) and nivolumab or pembrolizumab (anti-programmed cell death protein 1 third line)[80].
Gastritis refers to inflammation of the gastric mucosa, which currently affects more than half of people worldwide[81]. It is divided into acute gastritis and chronic gastritis in accordance to the condition and time of the onset. Gastritis is caused by many factors, including infection, stress, injury, certain medications and immune system diseases. The etiologies of different types of gastritis are different. Acute gastritis is caused by infections. Metaplastic atrophic gastritis with an autoimmune origin and H. pylori-induced inflammation are the two important forms of chronic gastritis[82]. Chronic gastritis is the reaction of process of gastric mucosa to various injuries, including epithelial injury, mucosal inflammation and epithelial regeneration. The common cause is infection, particularly H. pylori infection. H. pylori infection is a major risk factor for gastroduodenal ulcers, gastric cancer and other types of gastric and extragastric diseases[83]. H. pylori can survive for decades in harsh gastric conditions, disrupt the gastric mucosa and change the pattern of hormone release. It uses a variety of virulence factors and targets different cellular proteins to regulate the inflammatory response of the host, launching multiple “hits” on the gastric mucosa, leading to chronic gastritis and peptic ulcer[84].
THE MECHANISM BY WHICH OF BETA-CAROTENE MODULATES GASTRIC CANCER
As an important dietary nutrient, beta-carotene exerts large effects on many diseases. Next, we will discuss every specific mechanism of beta-carotene against gastric cancer, including the functions of beta-carotene itself and different aspects of the mechanism of gastric cancer. The review will elaborate on two aspects: cell experiments and human studies.
In vitro experiments
Several years ago, some researchers conducted in vitro experiments using beta-carotene and gastric cancer cells, which indicated that beta-carotene fights gastric cancer cells mainly through apoptosis, cell signal transduction and oxidative stress.
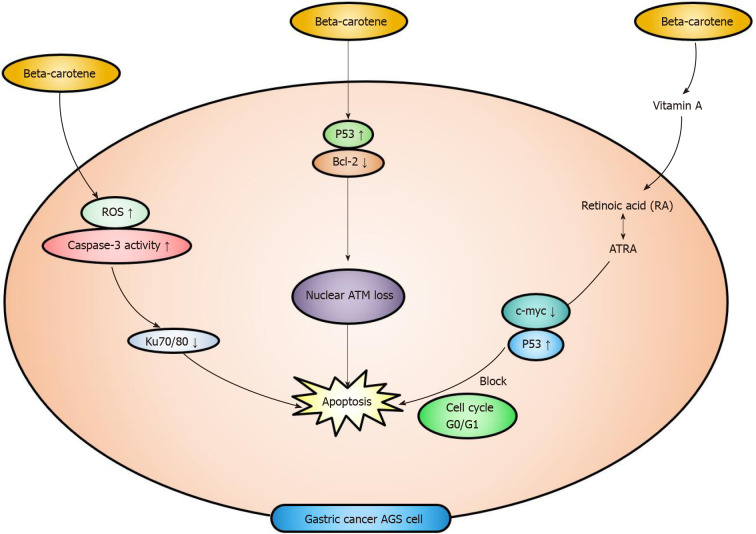
Beta-carotene and the cell cycle: Ku proteins are DNA binding regulatory subunits of DNA-dependent protein kinases composed of Ku70 (70 kDa) and Ku80 (80 kDa). The reduction in Ku70/80 levels is related to the apoptosis of human gastric adenocarcinoma AGS cells. One of the studies proved that beta-carotene decreased the levels of Ku70/80 through an increase in ROS levels and caspase-3 activity in AGS gastric cancer cells. Therefore, ROS-mediated Ku protein loss may be a potential mechanism of beta-carotene-induced apoptosis of AGS gastric cancer cells[85]. As a sensor for DNA-damaging agents, ataxia-telangiectasia-mutated activates many effectors in different signaling pathways, such as DNA repair and apoptosis. Beta-carotene induces the apoptosis of AGS cells by increasing the level of the apoptotic protein p53 and decreasing levels of the antiapoptotic protein Bcl-2 and nuclear ataxia-telangiectasia-mutated. Nuclear ataxia-telangiectasia-mutated loss may be another potential mechanism of beta carotene induced apoptosis in gastric cancer cells[86].
Beta-carotene is converted into vitamin A in animals, which is also called provitamin A. Vitamin A (retinol) will be transformed into RA in cells. All-trans RA (ATRA) is one of the isomers of RA and is also involved in the apoptosis of gastric cancer cells. Some in vitro studies showed that ATRA blocked the cell cycle and enhanced apoptosis in gastric cancer cell lines[87]. Studies of the human gastric cancer cell lines MKN-45 and MKN-28 have explored the ability of RA or folic acid to induce gastric cancer cell apoptosis and DNA fragmentation[88]. RA induces growth inhibition and apoptosis and regulates the cell cycle in gastric cancer cell lines. ATRA inhibits the growth of gastric cancer cell lines by inducing G0/G1 arrest, which is associated with the downregulation of c-myc and hyperphosphorylated Rb levels and upregulation of p21WAF1/CIP1 and p53 expression[89,90]. This part is about different mechanisms of apoptosis in AGS cells of gastric cancer. Figure 3 is the schematic diagram of the apoptotic mechanism.
Figure 3.
Beta-carotene can induce the apoptosis of gastric cancer AGS cells by different pathways. Beta-carotene can decrease the levels of Ku70/80 mediated with increased reactive oxygen species levels and caspase-3 activity in gastric cancer AGS cells, thus making the AGS cells apoptose. By increasing the level of p53 and decreasing the level of Bcl-2, the nuclear ataxia-telangiectasia-mutated decreases and cell apoptosis occurs. Beta-carotene can also turn into all-trans retinoic acid in gastric cancer AGS cells, then c-myc decreases and p53 increases. This process will block the cell cycle G0/G1, and apoptosis occurs. ROS: Reactive oxygen species; ATM: Ataxia-telangiectasia-mutated; RA: Retinoic acid; ATRA: All-trans retinoic acid.
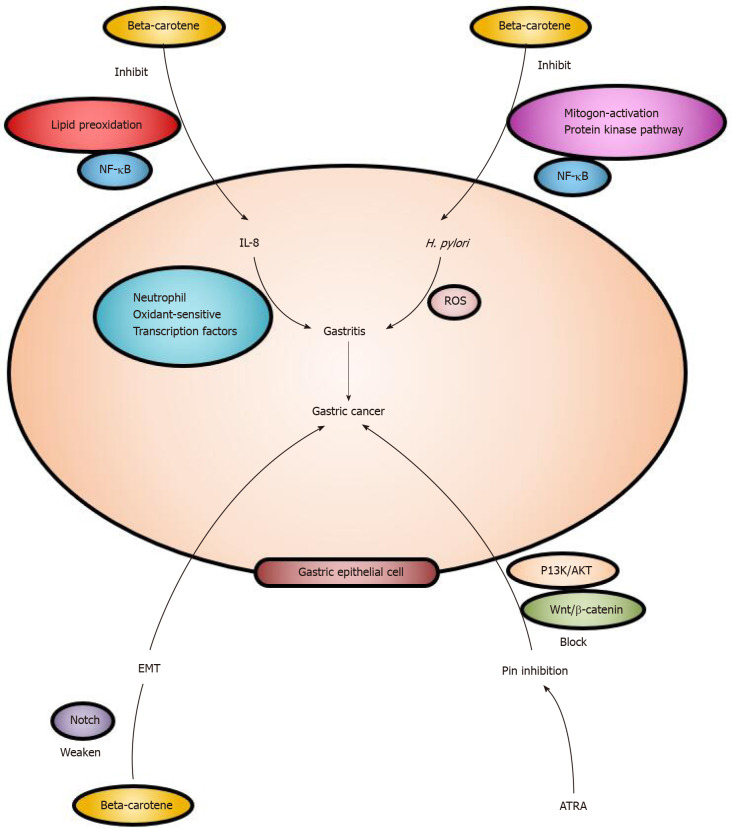
The effect of beta-carotene on oxidative stress: In cell experiments, beta-carotene acted on the oxidative stress mechanism of gastric cancer. ROS protect infected tissues from pathogens by inducing the innate immune response. Interleukin-8 mediates inflammatory responses by recruiting neutrophils expressing oxidant-sensitive transcription factors to gastric epithelial cells. Beta-carotene improves the treatment of oxidative stress-mediated gastric inflammation. Beta-carotene reduces interleukin-8 production by inhibiting lipid peroxidation and oxidant-mediated activation of NF-κB. It also exerts inhibitory effects on intracellular ROS levels in AGS cells[91]. Under acidic conditions, stomach polyunsaturated fatty acids, O2 and dietary iron are mixed to create conditions that promote further lipid peroxidation. Carotenoids inhibit the propagation phase of lipid peroxidation by directly scavenging lipid peroxyl radicals[92].
Beta-carotene shows anticancer activity by reducing NADPH oxidase-mediated ROS production and decreasing NF-κB activation[93]. H. pylori infection can lead to superficial gastritis, atrophic gastritis and finally gastric cancer. Many carotene-rich medical plants inhibit H. pylori-induced inflammatory activity, and the mechanisms are related to the suppression of NF-κB and mitogen-activated protein kinase pathway activation and inhibition of oxidative stress[94]. In H. pylori-infected tissues, infiltrated inflammatory cells produce ROS that cause gastric inflammation. Beta-carotene inhibits ROS-mediated inflammatory signals and reduces the expression of inflammatory mediators, including interleukin-8, inducible nitric oxide synthase and cyclooxygenase-2, in infected tissues[95].
Beta-carotene and signaling pathways: A central common signaling mechanism in cancer is proline-directed phosphorylation, which is regulated by the unique proline isomerase Pin1. Pin1 inhibition results in anticancer activity by blocking multiple cancer-driving pathways, such as the PI3K/AKT and Wnt/β-catenin signaling pathways. ATRA is an inhibitor of Pin1, and inhibition results in inactivation of the PI3K/AKT and Wnt signaling pathways and suppression of tumor growth in gastric cancer[96]. H. pylori induces the hyperproliferation of gastric epithelial cells. Beta-carotene inhibits this process by suppressing β-catenin signaling and oncogene expression. Beta-carotene-rich foods may prevent the development of gastric disorders related to H. pylori infection[97]. The Notch signaling pathway is important for gastric epithelial homeostasis and maintains a balance between cell proliferation and apoptosis. Therefore, the Notch signaling pathway may play an important role in the development of various malignant tumors[98]. It regulates several cellular processes, including cell proliferation, migration, invasion, apoptosis and angiogenesis as well as the epithelial-mesenchymal transition[99]. The epithelial-mesenchymal transition is involved in the initiation of tumorigenesis. In the study mentioned above, researchers found that tobacco smoke-induced gastric epithelial-mesenchymal transition was associated with the Notch pathway, but beta-carotene effectively attenuated this process in the mouse stomach[100]. Beta-carotene can prevent the occurrence of gastric cancer through these mechanisms, which are summarized in Figure 4.
Figure 4.
Beta-carotene can prevent the process of gastric cancerization. It influences the oxidative stress in gastric cells. Beta-carotene inhibits the process of inflammation and cancerization and nuclear factor kappa-B plays an important role. Beta-carotene prevents the gastric cancer through signaling pathways, and it can weaken the Notch pathway. All-trans retinoic acid causes Pin inhibition by blocking PI3K/AKT and Wnt/β-catenin so that the gastric cancer will not appear. NF-κB: Nuclear factor kappa-B; IL: Interleukin; EMT: Epithelial-mesenchymal transition; ROS: Reactive oxygen species; Helicobacter pylori: H. pylori; ATRA: All-trans retinoic acid.
Human epidemiological studies
In the past few years, researchers in different countries have conducted epidemiological studies on the relationship between carotene supplementation and gastric cancer, but some differences in the results of these studies have been noted. In this section, we summarize the results of these studies.
In 2018, South Korean researchers published a case-control study about dietary carotenoid intake and the risk of gastric cancer. Their findings confirmed that higher dietary lycopene intake might be inversely associated with the risk of gastric cancer. In the subgroups stratified by gender, H. pylori-positive subjects and participants who had ever smoked, the association was still significant[101]. Some researchers studied dietary factors associated with the most frequent cancer sites from the European Prospective Investigation into Cancer and Nutrition. One of the findings showed that gastric cancer risk was inversely related to high plasma vitamin C levels, some carotenoids, retinol and α-tocopherol, high intake of cereal fiber and strong adhesion to a Mediterranean diet[102].
In a follow-up study of the Linxian general population nutrition intervention trial, researchers wanted to determine the effects of “factor D,” a combination of 50 µg of selenium, 30 mg of vitamin E and 15 mg of beta-carotene. The cumulative gastric cancer-related mortality of participants receiving factor D treatment decreased from 4.28% to 3.84%, which was lower than participants who did not receive factor D treatment. The beneficial effects of selenium, vitamin E and beta-carotene on mortality were still evident 10 years after the supplement was discontinued, particularly among young adults[103].
Some researchers performed a large nested case-control study on the effects of carotenoids, retinols and tocopherols on gastric cancer in Japanese patients with H. pylori infection. The plasma level of beta-carotene was inversely associated with the risk of gastric cancer[104]. In a prospective cohort study of Swedish adults, intake of vitamin A, retinol and the provitamin A carotenoids alpha-carotene and beta-carotene was inversely associated with the risk of gastric cancer. Participants who consumed the largest amounts of these nutrients had an approximately 40% to 60% lower risk of gastric cancer than participants in the lowest quartile of intake[105].
A group of researchers investigated the relationship between prediagnostic levels of serum micronutrients and gastric cancer risk in Shanghai, China. Their findings showed that high serum levels of alpha-carotene, beta-carotene and lycopene were inversely associated with the risk of developing gastric cancer. Therefore, dietary carotenes, lycopene and vitamin C are potential chemoprevention agents for gastric cancer in humans[106].
In addition to some studies examining the protective effect of beta-carotene on gastric cancer, other studies have found that beta-carotene has no protective effect on gastric cancer and does not reduce the risk of gastric cancer. A meta-analysis in 2014 tried to determine an association between dietary antioxidant and vitamin intake/blood level and the risk of gastric cancer. Dietary intake of vitamins C and E, beta-carotene and alpha-carotene was inversely associated with the risk of stomach cancer, but blood levels of these antioxidant vitamins did not display this association[107]. Another systematic review and meta-analysis found that beta-carotene supplementation does not exert any beneficial effect on cancer prevention.
In contrast, in smokers and asbestos workers, a daily dose of 20 mg to 30 mg increased the risk of lung cancer and stomach cancer[108]. In Linxian, China, a region with epidemic rates of esophageal and gastric cardia cancer, researchers performed a prospective study of serum retinol, beta-carotene, beta-cryptoxanthin and lutein/zeaxanthin levels and esophageal and gastric cancers. In this population, low retinol and high lutein/zeaxanthin concentrations increased the risk of cardia cancer and non-cardia cancer, respectively. No strong association was observed between other analytes and cancer at different sites[109]. In 2016, a meta-analysis of the association of carotenoids with the risk of gastric cancer was conducted. Data from the case-control study suggested that beta-carotene and alpha-carotene were inversely associated with the risk of gastric cancer, while results from the cohort study were inconsistent[110]. A summary of these studies is shown in Table 1.
Table 1.
A summary about beta-carotene and its effects on gastric cancer in human studies
|
Ref.
|
Region
|
Study type
|
Result
|
| Kim et al[101] | South Korea | Case-control study | Higher dietary lycopene intake might be inversely associated with the risk of gastric cancer, especially in Helicobacter pylori-positive subjects and participants who had ever smoked |
| Gonzalez and Riboli[102] | European countries | Prospective investigation | Gastric cancer risk was inversely related to high plasma vitamin C levels, some carotenoids, retinol and α-tocopherol, high intake of cereal fiber and strong adhesion to a Mediterranean diet |
| Qiao et al[103] | Linxian, China | Follow-up study | The cumulative gastric cancer-related mortality of participants receiving “factor D” treatment, a combination of 50 µg of selenium, 30 mg of vitamin E and 15 mg of beta-carotene, decreased from 4.28% to 3.84%, which was lower than participants who did not receive factor D treatment |
| Persson et al[104] | Japan | Nested case-control study | The plasma level of beta-carotene was inversely associated with the risk of gastric cancer |
| Larsson et al[105] | Sweden | Prospective cohort study | Intake of vitamin A, retinol and the provitamin A carotenoids alpha-carotene and beta-carotene was inversely associated with the risk of gastric cancer, approximately 40% to 60% lower risk of gastric cancer than participants in the lowest quartile of intake of the nutrients |
| Yuan et al[106] | Shanghai, China | Cohort study | High serum levels of alpha-carotene, beta-carotene and lycopene were inversely associated with the risk of developing gastric cancer |
| Harvie[107] | - | Meta-analysis | Dietary intake of vitamins C and E, beta-carotene and alpha-carotene was inversely associated with the risk of stomach cancer, but blood levels of these antioxidant vitamins did not display this association |
| Druesne-Pecollo et al[108] | - | Systematic review and meta-analysis | Beta-carotene supplementation does not exert any beneficial effect on cancer prevention. In smokers and asbestos workers, a daily dose of 20 mg to 30 mg increased the risk of lung cancer and stomach cancer |
| Abnet et al[109] | Linxian, China | Prospective study | Low retinol and high lutein/zeaxanthin concentrations increased the risk of cardia cancer and non-cardia cancer, respectively |
| Zhou et al[110] | - | Meta-analysis | Data from the case-control study suggested that beta-carotene and alpha-carotene were inversely associated with the risk of gastric cancer, while results from the cohort study were inconsistent |
CONCLUSION
In conclusion, many epidemiological studies have been conducted on the relationship between beta-carotene and gastric cancer, and differences may exist between the results of each study. However, our mainstream view is that intake of beta-carotene is beneficial to gastric cancer, including reducing the risk of gastric cancer and improving the prognosis. However, the results of epidemiological surveys vary due to factors such as the region and research methods. As an important nutrient, beta-carotene exerts a protective effect on gastric cancer, and the mechanism is still worth exploring.
Footnotes
Conflict-of-interest statement: We declare no conflicts of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: January 12, 2021
First decision: May 13, 2021
Article in press: June 22, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cho JH, Moradi L S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Qian-Hui Chen, Department of Intensive Care Unit, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Bao-Kang Wu, Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, China.
Dan Pan, Department of Geriatrics, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Li-Xuan Sang, Department of Geriatrics, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Bing Chang, Department of Gastroenterology, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China. cb000216@163.com.
References
- 1.Rodriguez-Amaya DB. Structures and Analysis of Carotenoid Molecules. Subcell Biochem. 2016;79:71–108. doi: 10.1007/978-3-319-39126-7_3. [DOI] [PubMed] [Google Scholar]
- 2.Schweiggert RM, Carle R. Carotenoid deposition in plant and animal foods and its impact on bioavailability. Crit Rev Food Sci Nutr. 2017;57:1807–1830. doi: 10.1080/10408398.2015.1012756. [DOI] [PubMed] [Google Scholar]
- 3.Maoka T. Recent progress in structural studies of carotenoids in animals and plants. Arch Biochem Biophys. 2009;483:191–195. doi: 10.1016/j.abb.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Garcia AL, Mohan R, Koebnick C, Bub A, Heuer T, Strassner C, Groeneveld MJ, Katz N, Elmadfa I, Leitzmann C, Hoffmann I. Plasma beta-carotene is not a suitable biomarker of fruit and vegetable intake in german subjects with a long-term high consumption of fruits and vegetables. Ann Nutr Metab. 2010;56:23–30. doi: 10.1159/000262295. [DOI] [PubMed] [Google Scholar]
- 5.Wyss A, Wirtz GM, Woggon WD, Brugger R, Wyss M, Friedlein A, Riss G, Bachmann H, Hunziker W. Expression pattern and localization of beta,beta-carotene 15,15'-dioxygenase in different tissues. Biochem J. 2001;354:521–529. doi: 10.1042/0264-6021:3540521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durante M, Lenucci MS, D'Amico L, Piro G, Mita G. Effect of drying and co-matrix addition on the yield and quality of supercritical CO₂ extracted pumpkin (Cucurbita moschata Duch.) oil. Food Chem. 2014;148:314–320. doi: 10.1016/j.foodchem.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 7.Rocha F, Yumi Sugahara L, Leimann FV, de Oliveira SM, da Silva Brum E, Calhelha RC, Barreiro MF, Ferreira ICFR, Porto Ineu R, Gonçalves OH. Nanodispersions of beta-carotene: effects on antioxidant enzymes and cytotoxic properties. Food Funct. 2018;9:3698–3706. doi: 10.1039/c8fo00804c. [DOI] [PubMed] [Google Scholar]
- 8.Paiva SA, Russell RM. Beta-carotene and other carotenoids as antioxidants. J Am Coll Nutr. 1999;18:426–433. doi: 10.1080/07315724.1999.10718880. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Hou Z, Yang J, Gao Y. Effects of antioxidants on the stability of β-Carotene in O/W emulsions stabilized by Gum Arabic. J Food Sci Technol. 2015;52:3300–3311. doi: 10.1007/s13197-014-1380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mojaat M, Foucault A, Pruvost J, Legrand J. Optimal selection of organic solvents for biocompatible extraction of beta-carotene from Dunaliella salina. J Biotechnol. 2008;133:433–441. doi: 10.1016/j.jbiotec.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Altemimi A, Lightfoot DA, Kinsel M, Watson DG. Employing response surface methodology for the optimization of ultrasound assisted extraction of lutein and β-carotene from spinach. Molecules. 2015;20:6611–6625. doi: 10.3390/molecules20046611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel AS, Kar A, Dash S, Dash SK. Supercritical fluid extraction of β-carotene from ripe bitter melon pericarp. Sci Rep. 2019;9:19266. doi: 10.1038/s41598-019-55481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sricharoen P, Limchoowong N, Techawongstien S, Chanthai S. A novel extraction method for β-carotene and other carotenoids in fruit juices using air-assisted, low-density solvent-based liquid-liquid microextraction and solidified floating organic droplets. Food Chem. 2016;203:386–393. doi: 10.1016/j.foodchem.2016.02.093. [DOI] [PubMed] [Google Scholar]
- 14.Marchal L, Mojaat-Guemir M, Foucault A, Pruvost J. Centrifugal partition extraction of β-carotene from Dunaliella salina for efficient and biocompatible recovery of metabolites. Bioresour Technol. 2013;134:396–400. doi: 10.1016/j.biortech.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Svelander CA, Lopez-Sanchez P, Pudney PD, Schumm S, Alminger MA. High pressure homogenization increases the in vitro bioaccessibility of α- and β-carotene in carrot emulsions but not of lycopene in tomato emulsions. J Food Sci. 2011;76:H215–H225. doi: 10.1111/j.1750-3841.2011.02418.x. [DOI] [PubMed] [Google Scholar]
- 16.Yu LJ, Rupasinghe HP. Improvement of cloud stability, yield and β-carotene content of carrot juice by process modification. Food Sci Technol Int. 2013;19:399–406. doi: 10.1177/1082013212455342. [DOI] [PubMed] [Google Scholar]
- 17.Dey S, Rathod VK. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason Sonochem. 2013;20:271–276. doi: 10.1016/j.ultsonch.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Liu D, Chen J, Ye X, Yu D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrason Sonochem. 2011;18:243–249. doi: 10.1016/j.ultsonch.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Rashed MM, Tong Q, Abdelhai MH, Gasmalla MA, Ndayishimiye JB, Chen L, Ren F. Effect of ultrasonic treatment on total phenolic extraction from Lavandula pubescens and its application in palm olein oil industry. Ultrason Sonochem. 2016;29:39–47. doi: 10.1016/j.ultsonch.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Baysal T, Ersus S, Starmans DA. Supercritical CO(2) extraction of beta-carotene and lycopene from tomato paste waste. J Agric Food Chem. 2000;48:5507–5511. doi: 10.1021/jf000311t. [DOI] [PubMed] [Google Scholar]
- 21.Zaghdoudi K, Framboisier X, Frochot C, Vanderesse R, Barth D, Kalthoum-Cherif J, Blanchard F, Guiavarc'h Y. Response surface methodology applied to Supercritical Fluid Extraction (SFE) of carotenoids from Persimmon (Diospyros kaki L.) Food Chem. 2016;208:209–219. doi: 10.1016/j.foodchem.2016.03.104. [DOI] [PubMed] [Google Scholar]
- 22.Salgado-Roman M, Botello-Alvarez E, Rico-Martínez R, Jiménez-Islas H, Cárdenas-Manríquez M, Navarrete-Bolaños JL. Enzymatic treatment to improve extraction of capsaicinoids and carotenoids from chili (Capsicum annuum) fruits. J Agric Food Chem. 2008;56:10012–10018. doi: 10.1021/jf801823m. [DOI] [PubMed] [Google Scholar]
- 23.Barzana E, Rubio D, Santamaria RI, Garcia-Correa O, Garcia F, Ridaura Sanz VE, López-Munguía A. Enzyme-mediated solvent extraction of carotenoids from marigold flower (Tagetes erecta) J Agric Food Chem. 2002;50:4491–4496. doi: 10.1021/jf025550q. [DOI] [PubMed] [Google Scholar]
- 24.Macedo M, Robrigues RD, Pinto GA, de Brito ES. Influence of pectinolyttic and cellulotyc enzyme complexes on cashew bagasse maceration in order to obtain carotenoids. J Food Sci Technol. 2015;52:3689–3693. doi: 10.1007/s13197-014-1411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon HT, Bauernfeind JC. Carotenoids as food colorants. Crit Rev Food Sci Nutr. 1982;18:59–97. doi: 10.1080/10408398209527357. [DOI] [PubMed] [Google Scholar]
- 26.Farfán P, Gómez S, Restrepo A. Dissection of the Mechanism of the Wittig Reaction. J Org Chem. 2019;84:14644–14658. doi: 10.1021/acs.joc.9b02224. [DOI] [PubMed] [Google Scholar]
- 27.Álvarez R, Vaz B, Gronemeyer H, de Lera ÁR. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem Rev. 2014;114:1–125. doi: 10.1021/cr400126u. [DOI] [PubMed] [Google Scholar]
- 28.Pi HW, Anandharaj M, Kao YY, Lin YJ, Chang JJ, Li WH. Engineering the oleaginous red yeast Rhodotorula glutinis for simultaneous β-carotene and cellulase production. Sci Rep. 2018;8:10850. doi: 10.1038/s41598-018-29194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaiyaso T, Manowattana A. Enhancement of carotenoids and lipids production by oleaginous red yeast Sporidiobolus pararoseus KM281507. Prep Biochem Biotechnol. 2018;48:13–23. doi: 10.1080/10826068.2017.1381620. [DOI] [PubMed] [Google Scholar]
- 30.Dong HW, Li HQ, Xu DG, Wu XY, Wu XS, Zhong JJ. High-throughput extraction of β-carotene from Blakeslea trispora based on a newly developed setup. Biotechnol Appl Biochem. 2014;61:446–452. doi: 10.1002/bab.1178. [DOI] [PubMed] [Google Scholar]
- 31.Mantzouridou F, Roukasa T, Kotzekidoua P, Liakopoulou M. Optimization of beta-carotene production from synthetic medium by Blakeslea trispora: a mathematical modeling. Appl Biochem Biotechnol. 2002;101:153–175. doi: 10.1385/abab:101:2:153. [DOI] [PubMed] [Google Scholar]
- 32.Wang HB, Xu RG, Yu LJ, Luo J, Zhang LW, Huang XY, Zou WA, Zhao Q, Lu MB. Improved beta-carotene and lycopene production by Blakeslea trispora with ultrasonic treatment in submerged fermentation. Z Naturforsch C J Biosci. 2014;69:237–244. doi: 10.5560/znc.2013-0122. [DOI] [PubMed] [Google Scholar]
- 33.Francavilla M, Trotta P, Luque R. Phytosterols from Dunaliella tertiolecta and Dunaliella salina: a potentially novel industrial application. Bioresour Technol. 2010;101:4144–4150. doi: 10.1016/j.biortech.2009.12.139. [DOI] [PubMed] [Google Scholar]
- 34.Monte J, Ribeiro C, Parreira C, Costa L, Brive L, Casal S, Brazinha C, Crespo JG. Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour Technol. 2020;297:122509. doi: 10.1016/j.biortech.2019.122509. [DOI] [PubMed] [Google Scholar]
- 35.Han SI, Kim S, Lee C, Choi YE. Blue-Red LED wavelength shifting strategy for enhancing beta-carotene production from halotolerant microalga, Dunaliella salina. J Microbiol. 2019;57:101–106. doi: 10.1007/s12275-019-8420-4. [DOI] [PubMed] [Google Scholar]
- 36.Gutiérrez-Salmeán G, Fabila-Castillo L, Chamorro-Cevallos G. Nutritional and toxicological aspects of Spirulina (Arthrospira) Nutr Hosp. 2015;32:34–40. doi: 10.3305/nh.2015.32.1.9001. [DOI] [PubMed] [Google Scholar]
- 37.Yoon SH, Lee SH, Das A, Ryu HK, Jang HJ, Kim JY, Oh DK, Keasling JD, Kim SW. Combinatorial expression of bacterial whole mevalonate pathway for the production of beta-carotene in E. coli. J Biotechnol. 2009;140:218–226. doi: 10.1016/j.jbiotec.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 38.León R, Martín M, Vigara J, Vilchez C, Vega JM. Microalgae mediated photoproduction of beta-carotene in aqueous-organic two phase systems. Biomol Eng. 2003;20:177–182. doi: 10.1016/s1389-0344(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 39.Qiang S, Su AP, Li Y, Chen Z, Hu CY, Meng YH. Elevated β-Carotene Synthesis by the Engineered Rhodobacter sphaeroides with Enhanced CrtY Expression. J Agric Food Chem. 2019;67:9560–9568. doi: 10.1021/acs.jafc.9b02597. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Yan P, Li Y, Liu X, Wang Z, Chen T, Zhao X. Enhancing β-Carotene Production in Escherichia coli by Perturbing Central Carbon Metabolism and Improving the NADPH Supply. Front Bioeng Biotechnol. 2020;8:585. doi: 10.3389/fbioe.2020.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Navarro E, Cánovas-Márquez JT, Almagro L, Chen H, Chen YQ, Zhang H, Torres-Martínez S, Chen W, Garre V. A new regulatory mechanism controlling carotenogenesis in the fungus Mucor circinelloides as a target to generate β-carotene over-producing strains by genetic engineering. Microb Cell Fact. 2016;15:99. doi: 10.1186/s12934-016-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu BS, Ichikawa S, Kanafusa S, Nakajima M. Preparation and characterization of beta-carotene nanodispersions prepared by solvent displacement technique. J Agric Food Chem. 2007;55:6754–6760. doi: 10.1021/jf063609d. [DOI] [PubMed] [Google Scholar]
- 43.Bezerra PQM, Matos MFR, Ramos IG, Magalhães-Guedes KT, Druzian JI, Costa JAV, Nunes IL. Innovative functional nanodispersion: Combination of carotenoid from Spirulina and yellow passion fruit albedo. Food Chem. 2019;285:397–405. doi: 10.1016/j.foodchem.2019.01.181. [DOI] [PubMed] [Google Scholar]
- 44.Di Meo S, Venditti P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid Med Cell Longev. 2020;2020:9829176. doi: 10.1155/2020/9829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 46.Kim HY, Nam SY, Yang SY, Kim HM, Jeong HJ. Cucurbita moschata Duch. and its active component, β-carotene effectively promote the immune responses through the activation of splenocytes and macrophages. Immunopharmacol Immunotoxicol. 2016;38:319–326. doi: 10.1080/08923973.2016.1202960. [DOI] [PubMed] [Google Scholar]
- 47.Spiegler E, Kim YK, Wassef L, Shete V, Quadro L. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim Biophys Acta. 2012;1821:88–98. doi: 10.1016/j.bbalip.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li R, Jia Z, Trush MA. Defining ROS in Biology and Medicine. React Oxyg Species (Apex) 2016;1:9–21. doi: 10.20455/ros.2016.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gammone MA, Riccioni G, D'Orazio N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar Drugs. 2015;13:6226–6246. doi: 10.3390/md13106226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Oliveira BF, Costa DC, Nogueira-Machado JA, Chaves MM. β-Carotene, α-tocopherol and ascorbic acid: differential profile of antioxidant, inflammatory status and regulation of gene expression in human mononuclear cells of diabetic donors. Diabetes Metab Res Rev. 2013;29:636–645. doi: 10.1002/dmrr.2439. [DOI] [PubMed] [Google Scholar]
- 51.Tsuchihashi H, Kigoshi M, Iwatsuki M, Niki E. Action of beta-carotene as an antioxidant against lipid peroxidation. Arch Biochem Biophys. 1995;323:137–147. doi: 10.1006/abbi.1995.0019. [DOI] [PubMed] [Google Scholar]
- 52.Lohan SB, Vitt K, Scholz P, Keck CM, Meinke MC. ROS production and glutathione response in keratinocytes after application of β-carotene and VIS/NIR irradiation. Chem Biol Interact. 2018;280:1–7. doi: 10.1016/j.cbi.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Yu S, Zhao Y, Feng Y, Zhang H, Li L, Shen W, Zhao M, Min L. β-carotene improves oocyte development and maturation under oxidative stress in vitro. In Vitro Cell Dev Biol Anim. 2019;55:548–558. doi: 10.1007/s11626-019-00373-0. [DOI] [PubMed] [Google Scholar]
- 54.Cocate PG, Natali AJ, Alfenas RC, de Oliveira A, dos Santos EC, Hermsdorff HH. Carotenoid consumption is related to lower lipid oxidation and DNA damage in middle-aged men. Br J Nutr. 2015;114:257–264. doi: 10.1017/S0007114515001622. [DOI] [PubMed] [Google Scholar]
- 55.Åsgård R, Hellman B. Effect of β-carotene on catechol-induced genotoxicity in vitro: evidence of both enhanced and reduced DNA damage. Free Radic Res. 2013;47:692–698. doi: 10.3109/10715762.2013.815346. [DOI] [PubMed] [Google Scholar]
- 56.Hervé JC, Derangeon M. Gap-junction-mediated cell-to-cell communication. Cell Tissue Res. 2013;352:21–31. doi: 10.1007/s00441-012-1485-6. [DOI] [PubMed] [Google Scholar]
- 57.Rossello RA, H D. Cell communication and tissue engineering. Commun Integr Biol. 2010;3:53–56. doi: 10.4161/cib.3.1.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JS, Lee WM, Rhee HC, Kim S. Red paprika (Capsicum annuum L.) and its main carotenoids, capsanthin and β-carotene, prevent hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Chem Biol Interact. 2016;254:146–155. doi: 10.1016/j.cbi.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Haddad NF, Teodoro AJ, Leite de Oliveira F, Soares N, de Mattos RM, Hecht F, Dezonne RS, Vairo L, Goldenberg RC, Gomes FC, de Carvalho DP, Gadelha MR, Nasciutti LE, Miranda-Alves L. Lycopene and beta-carotene induce growth inhibition and proapoptotic effects on ACTH-secreting pituitary adenoma cells. PLoS One. 2013;8:e62773. doi: 10.1371/journal.pone.0062773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeh SL, Hu ML. Oxidized beta-carotene inhibits gap junction intercellular communication in the human lung adenocarcinoma cell line A549. Food Chem Toxicol. 2003;41:1677–1684. doi: 10.1016/s0278-6915(03)00192-3. [DOI] [PubMed] [Google Scholar]
- 61.Lee NY, Kim Y, Kim YS, Shin JH, Rubin LP. β-Carotene exerts anti-colon cancer effects by regulating M2 macrophages and activated fibroblasts. J Nutr Biochem. 2020;82:108402. doi: 10.1016/j.jnutbio.2020.108402. [DOI] [PubMed] [Google Scholar]
- 62.Nishida K, Sugimoto M, Ikeda S, Kume S. Effects of supplemental β-carotene on mucosal IgA induction in the jejunum and ileum of mice after weaning. Br J Nutr. 2014;111:247–253. doi: 10.1017/S0007114513002195. [DOI] [PubMed] [Google Scholar]
- 63.Lo HM, Wang SW, Chen CL, Wu PH, Wu WB. Effects of all-trans retinoic acid, retinol, and β-carotene on murine macrophage activity. Food Funct. 2014;5:140–148. doi: 10.1039/c3fo60309a. [DOI] [PubMed] [Google Scholar]
- 64.Corridan BM, O'Donoghue M, Hughes DA, Morrissey PA. Low-dose supplementation with lycopene or beta-carotene does not enhance cell-mediated immunity in healthy free-living elderly humans. Eur J Clin Nutr. 2001;55:627–635. doi: 10.1038/sj.ejcn.1601187. [DOI] [PubMed] [Google Scholar]
- 65.Dunstan JA, Breckler L, Hale J, Lehmann H, Franklin P, Lyons G, Ching SY, Mori TA, Barden A, Prescott SL. Supplementation with vitamins C, E, beta-carotene and selenium has no effect on anti-oxidant status and immune responses in allergic adults: a randomized controlled trial. Clin Exp Allergy. 2007;37:180–187. doi: 10.1111/j.1365-2222.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- 66.Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621–628. doi: 10.11622/smedj.2014174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–4490. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z. Risk Factors for Gastric Cancer: A Systematic Review Asian Pac J Cancer Prev 2018; 19: 591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hardbower DM, Peek RM Jr, Wilson KT. At the Bench: Helicobacter pylori, dysregulated host responses, DNA damage, and gastric cancer. J Leukoc Biol. 2014;96:201–212. doi: 10.1189/jlb.4BT0214-099R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi YJ, Kim N. Gastric cancer and family history. Korean J Intern Med. 2016;31:1042–1053. doi: 10.3904/kjim.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver. 2015;9:5–17. doi: 10.5009/gnl14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 74.Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 75.Berger H, Marques MS, Zietlow R, Meyer TF, Machado JC, Figueiredo C. Gastric cancer pathogenesis. Helicobacter. 2016;21 Suppl 1:34–38. doi: 10.1111/hel.12338. [DOI] [PubMed] [Google Scholar]
- 76.Molaei F, Forghanifard MM, Fahim Y, Abbaszadegan MR. Molecular Signaling in Tumorigenesis of Gastric Cancer Iran Biomed J 2018; 22: 217-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med Sci Monit. 2019;25:3537–3541. doi: 10.12659/MSM.916475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3–15. doi: 10.1111/den.12518. [DOI] [PubMed] [Google Scholar]
- 79.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. doi: 10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- 80.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 81.Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015;50:657–667. doi: 10.3109/00365521.2015.1019918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mihály E, Micsik T, Juhász M, Herszényi L, Tulassay Z. [Gastritis and gastropathy] Orv Hetil. 2014;155:43–61. doi: 10.1556/OH.2014.29807. [DOI] [PubMed] [Google Scholar]
- 83.Fischbach W, Malfertheiner P. Helicobacter Pylori Infection. Dtsch Arztebl Int. 2018;115:429–436. doi: 10.3238/arztebl.2018.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Park Y, Choi J, Lim JW, Kim H. β-Carotene-induced apoptosis is mediated with loss of Ku proteins in gastric cancer AGS cells. Genes Nutr. 2015;10:467. doi: 10.1007/s12263-015-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jang SH, Lim JW, Kim H. Mechanism of beta-carotene-induced apoptosis of gastric cancer cells: involvement of ataxia-telangiectasia-mutated. Ann N Y Acad Sci. 2009;1171:156–162. doi: 10.1111/j.1749-6632.2009.04711.x. [DOI] [PubMed] [Google Scholar]
- 87.Bouriez D, Giraud J, Gronnier C, Varon C. Efficiency of All-Trans Retinoic Acid on Gastric Cancer: A Narrative Literature Review. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19113388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang JY, Xiao SD. Effect of trans-retinoic acid and folic acid on apoptosis in human gastric cancer cell lines MKN-45 and MKN-28. J Gastroenterol. 1998;33:656–661. doi: 10.1007/s005350050152. [DOI] [PubMed] [Google Scholar]
- 89.Chen ZM, Wu Q, Chen YQ, Su WJ. [Regulation of cell cycle by retinoic acid in gastric cancer cells] Shi Yan Sheng Wu Xue Bao. 1999;32:135–140. [PubMed] [Google Scholar]
- 90.Wu Q, Chen Z, Su W. Growth inhibition of gastric cancer cells by all-trans retinoic acid through arresting cell cycle progression. Chin Med J (Engl) 2001;114:958–961. [PubMed] [Google Scholar]
- 91.Kim Y, Seo JH, Kim H. β-Carotene and lutein inhibit hydrogen peroxide-induced activation of NF-κB and IL-8 expression in gastric epithelial AGS cells. J Nutr Sci Vitaminol (Tokyo) 2011;57:216–223. doi: 10.3177/jnsv.57.216. [DOI] [PubMed] [Google Scholar]
- 92.Sy C, Caris-Veyrat C, Dufour C, Boutaleb M, Borel P, Dangles O. Inhibition of iron-induced lipid peroxidation by newly identified bacterial carotenoids in model gastric conditions: comparison with common carotenoids. Food Funct. 2013;4:698–712. doi: 10.1039/c3fo30334a. [DOI] [PubMed] [Google Scholar]
- 93.Wang YC. Medicinal plant activity on Helicobacter pylori related diseases. World J Gastroenterol. 2014;20:10368–10382. doi: 10.3748/wjg.v20.i30.10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park Y, Lee H, Lim JW, Kim H. Inhibitory Effect of β-Carotene on Helicobacter pylori-Induced TRAF Expression and Hyper-Proliferation in Gastric Epithelial Cells. Antioxidants (Basel) 2019;8 doi: 10.3390/antiox8120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jang SH, Lim JW, Kim H. Beta-carotene inhibits Helicobacter pylori-induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in human gastric epithelial AGS cells. J Physiol Pharmacol. 2009;60 Suppl 7:131–137. [PubMed] [Google Scholar]
- 96.Zhang Z, Yu W, Zheng M, Liao X, Wang J, Yang D, Lu W, Wang L, Zhang S, Liu H, Zhou XZ, Lu KP. Pin1 inhibition potently suppresses gastric cancer growth and blocks PI3K/AKT and Wnt/β-catenin oncogenic pathways. Mol Carcinog. 2019;58:1450–1464. doi: 10.1002/mc.23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim D, Lim JW, Kim H. β-carotene Inhibits Expression of c-Myc and Cyclin E in Helicobacter pylori-infected Gastric Epithelial Cells. J Cancer Prev. 2019;24:192–196. doi: 10.15430/JCP.2019.24.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;11:745–751. doi: 10.2174/138945010791170860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 100.Lu L, Chen J, Li M, Tang L, Wu R, Jin L, Liang Z. βcarotene reverses tobacco smokeinduced gastric EMT via Notch pathway in vivo. Oncol Rep. 2018;39:1867–1873. doi: 10.3892/or.2018.6246. [DOI] [PubMed] [Google Scholar]
- 101.Kim JH, Lee J, Choi IJ, Kim YI, Kwon O, Kim H, Kim J. Dietary Carotenoids Intake and the Risk of Gastric Cancer: A Case-Control Study in Korea. Nutrients. 2018;10 doi: 10.3390/nu10081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gonzalez CA, Riboli E. Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer. 2010;46:2555–2562. doi: 10.1016/j.ejca.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 103.Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, Johnson LL, Gail MH, Dong ZW, Yu B, Mark SD, Taylor PR. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–518. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Persson C, Sasazuki S, Inoue M, Kurahashi N, Iwasaki M, Miura T, Ye W, Tsugane S JPHC Study Group. Plasma levels of carotenoids, retinol and tocopherol and the risk of gastric cancer in Japan: a nested case-control study. Carcinogenesis. 2008;29:1042–1048. doi: 10.1093/carcin/bgn072. [DOI] [PubMed] [Google Scholar]
- 105.Larsson SC, Bergkvist L, Näslund I, Rutegård J, Wolk A. Vitamin A, retinol, and carotenoids and the risk of gastric cancer: a prospective cohort study. Am J Clin Nutr. 2007;85:497–503. doi: 10.1093/ajcn/85.2.497. [DOI] [PubMed] [Google Scholar]
- 106.Yuan JM, Ross RK, Gao YT, Qu YH, Chu XD, Yu MC. Prediagnostic levels of serum micronutrients in relation to risk of gastric cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2004;13:1772–1780. [PubMed] [Google Scholar]
- 107.Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. Am Soc Clin Oncol Educ Book. 2014:e478–e486. doi: 10.14694/EdBook_AM.2014.34.e478. [DOI] [PubMed] [Google Scholar]
- 108.Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, Hercberg S. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. 2010;127:172–184. doi: 10.1002/ijc.25008. [DOI] [PubMed] [Google Scholar]
- 109.Abnet CC, Qiao YL, Dawsey SM, Buckman DW, Yang CS, Blot WJ, Dong ZW, Taylor PR, Mark SD. Prospective study of serum retinol, beta-carotene, beta-cryptoxanthin, and lutein/zeaxanthin and esophageal and gastric cancers in China. Cancer Causes Control. 2003;14:645–655. doi: 10.1023/a:1025619608851. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Y, Wang T, Meng Q, Zhai S. Association of carotenoids with risk of gastric cancer: A meta-analysis. Clin Nutr. 2016;35:109–116. doi: 10.1016/j.clnu.2015.02.003. [DOI] [PubMed] [Google Scholar]