Abstract
BACKGROUND
Psoriasis is a chronic autoimmune disease that usually manifests as a red scaly epidermis, induration, and hyperproliferation of basal keratinocytes. About 2% of the world’s population suffers from psoriasis but there are no clear therapeutics yet. Recently, mesenchymal stem cells (MSCs) have been regarded as a therapeutic alternative for autoimmune diseases, as they possess immunosuppressive effects without risks. Human umbilical cord-derived MSCs effectively regulate immune cells and are characterized by low immunogenicity, which has many advantages in treating immune diseases.
CASE SUMMARY
The patient was a 47-year-old male, diagnosed with psoriasis in 1995. He had received various treatments for 25 years, but the psoriatic condition was not significantly improved. He was given three rounds of minimally manipulated umbilical cord-derived MSCs over 2 wk. The erythema gradually disappeared. Three months after the 1st round, all erythema completely disappeared, and the psoriasis did not recur.
CONCLUSION
Minimally manipulated umbilical cord-derived MSC transplantation can potentially treat patients who suffer from psoriasis.
Keywords: Psoriasis, Umbilical cord-derived mesenchymal stem cells, Allogenic, Cell therapy, Minimal manipulation, Case report
Core Tip: In recent decades, interest in biomedical applications of mesenchymal stem cells (MSCs) has increased due to their anti-inflammatory, immunosuppressive, and immunomodulatory abilities. MSCs have been used to study a variety of human diseases without side effects. The human umbilical cord is an excellent source of MSCs. We sought to improve a patient’s psoriatic condition by using minimally manipulated umbilical cord-derived MSCs. In this case study, we report the efficacy of the minimally manipulated umbilical cord-derived MSC therapy to treat a patient with psoriasis.
INTRODUCTION
Psoriasis is an incurable autoimmune disease exhibiting a wide spectrum of clinical symptoms. Psoriasis typically presents with a red scaly epidermis, induration, and hyperproliferation of basal keratinocytes[1]. This serious skin condition most commonly occurs on the head, upper limbs, hands, trunk, and lower limbs, though it can appear on any location. About 2% of the world’s population suffers from psoriasis[1]. Despite the wide variety of treatment options available to treat psoriasis, there are currently no definitive treatment methods[2-4]. Therefore, there is a need for a treatment method that can replace the existing treatment methods.
Previous studies about the effectiveness of mesenchymal stem cells (MSCs) for patients with autoimmune diseases showed positive results because they have anti-inflammation and immunomodulation[5-8]. For this reason, studies using MSCs to treat psoriasis have been conducted[9-12]. As a result, the possibility of treating psoriasis using MSCs has been demonstrated. Previous case reports have shown that psoriasis did not recur after MSC treatment[11,12].
In this study, we present a safer and more effective treatment method than previous studies. It is well known that genetic mutations and aging-related modifications increase as the MSCs are cultured[13-15]. These results show the increasing occurrence of various risks, including tumor formation, and decreasing treatment effect. Therefore, we treated psoriasis using uncultured, minimally manipulated umbilical cord-derived (MM-UC)-MSCs. We evaluated the severity of psoriasis in the patient using the Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI)[16]. As a result of three rounds of MM-UC-MSCs transplantation, the PASI and DLQI score of the patient decreased from 9.9 to 1.7 and from 27 to 3, respectively. We confirmed that there were no side effects and no relapse during the 5-mo follow-up period. Therefore, in this case study, we report the efficacy of MM-UC-MSC therapy for a psoriasis patient.
CASE PRESENTATION
Chief complaints
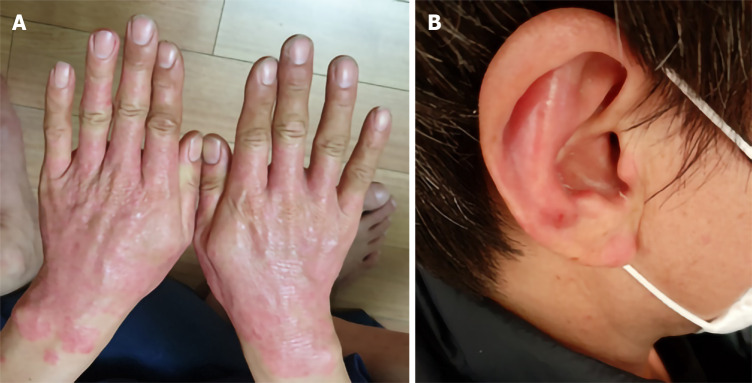
On April 1, 2020, a 47-year-old man, suffering from psoriasis, visited our clinic. The patient had inflammation on the fingers, the backs of the hands, both wrists, and both ears. Erythema was widely spread on both hands, all fingers, and both wrists. Erythema was also found on the inside of the auricle and on the lower part of the earlobe (Figure 1A and B). Also, he complained of itchiness.
Figure 1.
Psoriasis lesion sites before treatment. A: The psoriasis lesion sites on the hands and wrists before transplantation of minimally manipulated umbilical cord-derived mesenchymal stem cells (MM-UC-MSCs); B: The ear before transplantation of MM-UC-MSCs.
History of present illness
He had been diagnosed with psoriasis in 1995. Subsequently, over the next 25 years, he had received herbal treatment, dermatological laser treatment, and drug treatment.
History of past illness
The patient has been no specific disease excepted psoriasis.
Personal and family history
The patient had no diseases personal and family histories.
Physical examination
When the patient visited the hospital, the lesion area was spread over several parts of the body.
Laboratory examinations
The severity of psoriasis was evaluated using the PASI and DLQI at the time of the patient’s visit. The PASI and DLQI scores were 9.9 and 27, respectively.
FINAL DIAGNOSIS
The patient was diagnosed with psoriasis based on the PASI score and previous diagnosis results.
TREATMENT
UC procurement
Disinfected UCs were donated by the Obstetrics and Gynecology Department at Lynn Woman’s Hospital. Informed consent was obtained from the mothers donating the umbilical cord. Seven blood and urine tests were performed on the mothers to confirm the safety of the UCs. These tests included assessment for hepatitis B surface antigen, hepatitis B surface antibody, hepatitis C antigen, hepatitis C antibody, human immunodeficiency virus, syphilis rapid plasma reagin, and human T-cell lymphotropic virus type I and II antibody.
Isolation, quality evaluation, and preparation of MM-UC-MSCs
The donated UCs were isolated, evaluated, and prepared as described in our former work[8,17]. Briefly, the UC tissues were ground and treated with a mixture of collagenase and hyaluronidase. After filtration, the cells were immediately frozen at -197 °C. Each UC produced 2.5-7.0 × 108 cells. MSC expression was confirmed by detection of the markers CD73 (78.68%), CD90 (94.25%), and CD105 (93.72%) using a CyFlow® Cube 6 (Sysmex, Lincolnshire, IL, United States) and FCS Express 5 software (De Novo Software, Glendale, CA, United States). These expression markers were similar to our previous results of CD73 (70%-80%), CD90 (90%-100%), and CD105 (90%-100%). We also confirmed that the extracted cells are safe cells free from contamination by microorganisms or adventitious viruses (data not shown).
For local transplantation, the isolated cells were used at a concentration of 1 × 106 cells/mL in 0.9% physiological saline. For intravenous transplantation, cells were used at a concentration of 3 × 106 cells/mL in 0.9% physiological saline.
Treatment
In the 1st round, the injection solution containing MM-UC-MSCs was transplanted intravenously and by local transplantation on April 1, 2020. For intravenous transplantation, a 10-mL injection solution containing MM-UC-MSCs was mixed with 100 mL of 0.9% sodium chloride injection, USP. Then, the 110 mL mixture was intravenously transplanted for 1-1.5 h. Immediately after the transplant was over, the same process was repeated once more. In the case of local transplantation, 12 Ultra-FineTM II Insulin Syringes containing the injection solution were prepared. Each syringe containing the injection solution was injected in and around the lesion, at 2 cm intervals with 0.25 mL. The injected volume of injection solution for each hand and wrist was 4 mL, for each ear was 1 mL, and for the face was 2 mL. The 2nd and 3rd rounds were local transplantation only and were performed each at 1 wk intervals.
OUTCOME AND FOLLOW-UP
At the 1st hospital visit on April 1, 2020, the severity of psoriasis was diagnosed using PASI and DLQI. The PASI score was 9.9. The extent of psoriasis was determined to be not severe, but the DLQI score was 27, indicating that the skin disease had an enormous influence on daily life. The PASI and DLQI score of the patient decreased from 9.9 to 1.7 and from 27 to 3, respectively, after 122 d from the 1st transplantation (Figure 2).
Figure 2.
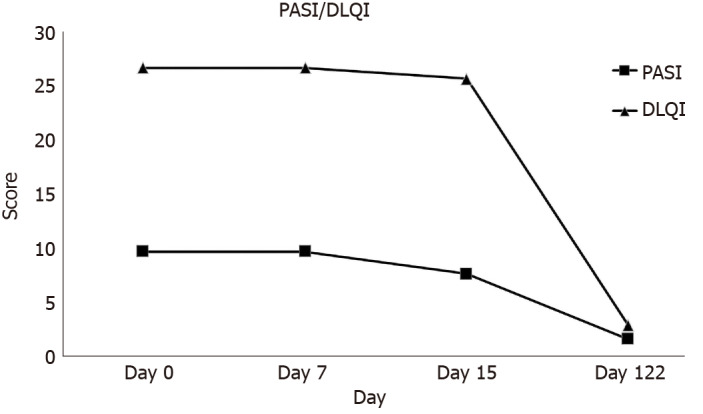
Graph for Psoriasis Area and Severity Index and Dermatology Life Quality Index score change in a patient with psoriasis according to the procedure outcomes. DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index.
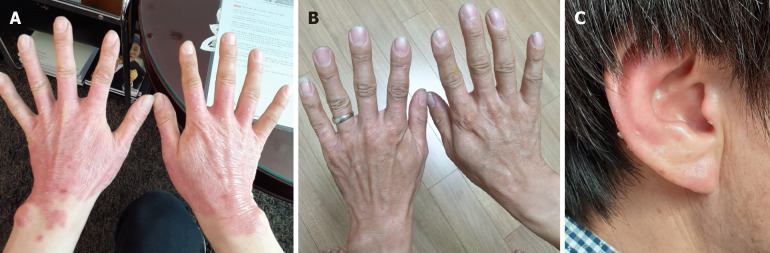
The erythema on both hands was slightly reduced, especially around the wrist area at day 7 after the 1st transplantation (Figure 3A). Itching also decreased, and exfoliation increased. After the 1st transplantation, the erythema gradually disappeared, and all erythema was almost resolved at day 122 after the 1st transplantation. Ultimately, the erythema was not observed on either hand, any fingers, or either wrist (Figure 3B). It was also confirmed that erythema of the ears was significantly reduced (Figure 3C).
Figure 3.
Visible changes at the lesion sites on the patient during and after treatment. A and B: The erythema changes of both hands and wrist observed at day 15 (A) and day 122 (B) after the 1st transplant; C: The erythema of the ear lesion site at day 122 after the 1st transplant.
Report of side effects
The patient reported having experienced no adverse reactions or side effects, including dermatitis, fever, chills, or nausea.
DISCUSSION
Psoriasis is one of the autoimmune diseases that can reduced a patient’s quality of life[2]. Currently, there is no adequate treatment for psoriasis[2-4]. Therefore, there is a need for a new treatment method for psoriasis.
Previous studies have shown that MSCs may be a new alternative to psoriasis treatment[9-12]. However, previous studies have used cultured MSCs. It is well known that genetic mutations and aging-related modifications increase as the MSCs are cultured[13-15]. This results in an increase in the occurrence of various risks, including tumor formation and decreasing the treatment effect.
Here, we describe a case of psoriasis treated using MM-UC-MSCs. The MM-UC-MSCs safer materials than cultured MSCs because MM-UC-MSCs have few genetic mutations by expansion in vitro. Also, the MM-UC-MSCs are expected to be more effective materials than cultured MSCs because of no aging-related modifications[13-15]. The patient was transplanted with the MSCs three times at 1-wk intervals. The patient had not received any other therapies during or after the MM-UC-MSCs treatment. Gradual clearing of the erythema was observed 122 d from the 1st treatment. Follow-up for 5 mo showed the patient’s psoriasis lesions to be mostly cleared. Interestingly, there was no significant change after the 1st and 2nd treatments. This result is hypothesized to be due to the time required for transplanted MM-UC-MSCs to engraft and function.
In general, treatment for psoriasis is aimed at a 75% improvement based on the PASI score[16]. In addition, there is a strong correlation between the PASI score, which represents severity of psoriasis, and the DLQI score, which represents quality of life. Therefore, a reduction in the PASI score of 75% or more can significantly improve the quality of life of the treated patient, which means that the treatment method is highly effective. In this study, the PASI score of the psoriasis patient treated by MM-UC-MSC transplantation decreased from 9.9 to 1.7 at day 122 after the 1st transplantation. During this period, the patient’s DLQI score dropped from 27 to 3, confirming a significant improvement in quality of life as well.
In this patient, treatment of psoriasis by MM-UC-MSC transplantation was safe and effective. However, further studies should be performed in a larger number of patients with psoriasis and for a longer follow-up time to confirm our observations.
CONCLUSION
Based on the PASI and DLQI scores, MM-UC-MSC transplantation had a great effect on treating psoriasis. After 5 mo, psoriasis did not recur and the patient did not report any side effects. Clinical trials with more patients and a longer follow-up time are needed before introducing this method as a safe and efficient psoriasis treatment.
Footnotes
Informed consent statement: The patient involved in this study gave his written informed consent authorizing disclosure of his protected health information.
Conflict-of-interest statement: All authors have no conflicts of interest to declare.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: January 13, 2021
First decision: May 11, 2021
Article in press: July 6, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leung PC, Onsun N S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
Contributor Information
Hyunjun Ahn, bio Beauty&Health Company (bBHC)-Stem Cell Treatment and Research Institute (STRI), Seoul 04420, South Korea; Department of Functional Genomics, University of Science and Technology KRIBB School, Deajeon 34113, South Korea.
Sang Yeon Lee, bio Beauty&Health Company (bBHC)-Stem Cell Treatment and Research Institute (STRI), Seoul 04420, South Korea.
Won-Ju Jung, Stem Cell Treatment, 97.7 B&H Clinic, Seoul 04420, South Korea.
Jia Pi, bio Beauty&Health Company (bBHC)-Stem Cell Treatment and Research Institute (STRI), Seoul 04420, South Korea.
Kye-Ho Lee, bio Beauty&Health Company (bBHC)-Stem Cell Treatment and Research Institute (STRI), Seoul 04420, South Korea. sylee@stc365.com.
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med. 1995;332:581–588. doi: 10.1056/NEJM199503023320907. [DOI] [PubMed] [Google Scholar]
- 3.Winterfield LS, Menter A, Gordon K, Gottlieb A. Psoriasis treatment: current and emerging directed therapies. Ann Rheum Dis. 2005;64 Suppl 2:ii87–90; discussion ii91. doi: 10.1136/ard.2004.032276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radi G, Campanati A, Diotallevi F, Bianchelli T, Offidani A. Novel Therapeutic Approaches and Targets for Treatment of Psoriasis. Curr Pharm Biotechnol. 2021;22:7–31. doi: 10.2174/1389201021666200629150231. [DOI] [PubMed] [Google Scholar]
- 5.Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC, Schrepfer S. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011;20:655–667. doi: 10.3727/096368910X536473. [DOI] [PubMed] [Google Scholar]
- 6.Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 7.Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653–664. doi: 10.1016/j.tips.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn H, Lee SY, Jung WJ, Lee KH. Alopecia treatment using minimally manipulated human umbilical cord-derived mesenchymal stem cells: Three case reports and review of literature. World J Clin Cases. 2021;9:3741–3751. doi: 10.12998/wjcc.v9.i15.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owczarczyk-Saczonek A, Krajewska-Włodarczyk M, Kruszewska A, Placek W, Maksymowicz W, Wojtkiewicz J. Stem Cells as Potential Candidates for Psoriasis Cell-Replacement Therapy. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paganelli A, Tarentini E, Benassi L, Kaleci S, Magnoni C. Mesenchymal stem cells for the treatment of psoriasis: a comprehensive review. Clin Exp Dermatol. 2020;45:824–830. doi: 10.1111/ced.14269. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Niu JW, Ning HM, Pan X, Li XB, Li Y, Wang DH, Hu LD, Sheng HX, Xu M, Zhang L, Zhang B. Treatment of Psoriasis with Mesenchymal Stem Cells. Am J Med. 2016;129:e13–e14. doi: 10.1016/j.amjmed.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang SG, Hsu NC, Wang SM, Wang FN. Successful Treatment of Plaque Psoriasis with Allogeneic Gingival Mesenchymal Stem Cells: A Case Study. Case Rep Dermatol Med. 2020;2020:4617520. doi: 10.1155/2020/4617520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Wang J, Dang J, Zhu W, Chen Y, Zhang X, Xie J, Hu B, Huang F, Sun B, Bellanti JA, Zheng SG. A preclinical study-systemic evaluation of safety on mesenchymal stem cells derived from human gingiva tissue. Stem Cell Res Ther. 2019;10:165. doi: 10.1186/s13287-019-1262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao X, Wang J, Zhou G, Aszodi A, Schönitzer V, Scherthan H, Atkinson MJ, Rosemann M. Extended in vitro culture of primary human mesenchymal stem cells downregulates Brca1-related genes and impairs DNA double-strand break recognition. FEBS Open Bio. 2020;10:1238–1250. doi: 10.1002/2211-5463.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Ding Y, Liu Z, Liang X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front Cell Dev Biol. 2020;8:258. doi: 10.3389/fcell.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28:333–337. doi: 10.1111/jdv.12106. [DOI] [PubMed] [Google Scholar]
- 17.Tong CK, Vellasamy S, Tan BC, Abdullah M, Vidyadaran S, Seow HF, Ramasamy R. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35:221–226. doi: 10.1042/CBI20100326. [DOI] [PubMed] [Google Scholar]