Abstract
It is hypothesized that liver impairment caused by coronavirus disease 2019 (COVID-19) infection might play a central role in severe clinical presentations. Liver injury is closely associated with severe disease and, even with antiviral drugs, have a poor prognosis in COVID-19 patients. In addition to the common hepatobiliary disorders caused by COVID-19, patients with pre-existing liver diseases demand special considerations during the current pandemic. Thus, it is vital that upon clinical presentation, patients with concurrent pre-existing liver disease associated with metabolic dysfunction and COVID-19 be managed properly to prevent liver failure. Careful monitoring and early detection of liver damage through biomarkers after hospitalization for COVID-19 is underscored in all cases, particularly in those with pre-existing metabolic liver injury. The purpose of this study was to determine most recent evidence regarding causality, potential risk factors, and challenges, therapeutic options, and management of COVID-19 infection in vulnerable patients with pre-existing liver injury. This review aims to highlight the current frontier of COVID-19 infection and liver injury and the direction of liver injury in these patients.
Keywords: Liver injury, COVID-19, SARS-CoV-2, Inflammation, Management
Core Tip: Coronavirus disease 2019 (COVID-19) is associated with respiratory symptoms, digestive complications, and liver injury. Severe inflammatory response, anoxia, drug-induced liver injury, direct cytotoxicity, as well as reactivation of pre-existing liver disease might be the etiologic mechanisms behind liver injury in COVID-19 patients. In this review, we study the clinical manifestations and liver-related events seen in COVID-19 patients, including the pathophysiology, etiology, biomarkers, diagnosis, treatment, and management strategies for liver injury. We aim to increase the awareness of healthcare workers about liver injury and to provide information for hepatic management in COVID-19 patients. Physicians should (1) pay special attention to the management of concurrent liver disorders; (2) boost hepatic function by strengthening supportive therapy; and (3) minimize the risk of drug-induced liver injury.
INTRODUCTION
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has quickly spread across the world[1,2]. The early symptoms of COVID-19 mainly include fever, cough, myalgia, and fatigue. The advanced stages of COVID-19, occurring in up to 15% of patients, are characterized by dyspnea that may gradually end in acute respiratory distress syndrome (ARDS) or multiple organ failure (MOF)[3]. ARDS is a well-known major complication in patients with COVID-19. Besides the respiratory symptoms, SARS-CoV-2 infection may lead to other conditions including liver injury, which is manifested by hepatobiliary symptoms and enzyme elevation. There are many possible underlying causes of liver injury in COVID-19 patients, such as a severe inflammatory response, anoxia, drug-induced liver injury, direct cytotoxicity, as well as the reactivation of pre-existing metabolic liver disease[4]. Liver injury can range from elevation of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and bilirubin levels to hepatic dysfunction in severe cases of COVID-19[5]. If liver damage persists, the disease is expected to advance over the patient’s entire lifetime. Under septic conditions, the liver is one of the most important organs as it plays an important role in regulating a broad range of physiological processes such as metabolic, homeostatic, and host-defense activities. It has been proposed that metabolic liver dysfunction in severe sepsis is associated with the aggravation of MOF[6]. Given these data, patients with metabolic liver injury, especially those with autoimmune liver disorders or post-transplant immunosuppression, are at increased risk of infection because of their altered immune system. For example, patients with liver cirrhosis are at an increased risk of acute decompensation when affected by bacterial, fungal, or viral infections[7]. Liver symptoms are not atypical among patients with COVID-19 and may appear without any respiratory symptoms. Hepatic symptoms have been reported to be associated with worse clinical outcomes and an increased risk of mortality[8]. However, in patients with metabolic liver diseases, special consideration should be given to changes in the primary condition, and the monitoring and assessment of liver function should be meticulously undertaken during treatment[9]. It is worth studying whether the COVID-19-related liver dysfunction is caused by the viral infection or by other comorbid medical conditions, including hepatotoxic antiviral drugs, the coexistence of systemic inflammatory response, acute hypoxemic respiratory failure, and/or MOF[10]. In this review, we address the liver-related adverse events seen in the current COVID-19 pandemic and associated antiviral drugs by detailing the pathophysiology, etiology, biomarkers, and diagnosis. This study aimed to raise awareness of liver injury in COVID-19 and provide information concerning hepatic management of afflicted patients. PubMed, Scopus, Science Direct, and Google Scholar were searched for studies using the following keywords: “COVID-19”, “SARS-CoV-2”, “2019-nCoV”, “liver diseases”, and “hepatobiliary system”.
COVID-19 LIVER INJURY EPIDEMIOLOGY
Prevalence of liver injury in patients with COVID-19
In a systematic review conducted in September 2020, the cumulative prevalence of acute liver injury was estimated at 23.7 (16.1-33.1) per 100 patients with COVID-19[11]. In another systematic review and meta-analysis, the frequency of liver injury in patients with COVID-19 was reported as 19% (range: 1%-53%). The prevalence of hypoalbuminemia (26.3-30.9 g/L), which is more common among patients with severe disease, was 6%. Also, the pooled prevalence of elevated liver enzymes for ALT, AST, and total bilirubin was estimated to be 18% (13%-25%), 21% (14%-29%), and 6% (3%-11%), respectively[12]. Liver injury is more prevalent among patients with severe COVID-19 than nonsevere cases[13]; its incidence in COVID-19 patients with a fatal outcome is estimated to be between 58% and 78%[14].
Age and sex
It has been observed that the rise in ALT level during COVID-19 is significantly higher in men as well as younger patients with liver injury according to univariate analysis, though that was not by multivariate analysis[15]. A study in children with COVID-19 and liver injury showed that in addition to a milder course, fewer radiological and laboratory changes were observed in comparison with adults[16]. Also, the probability of a surge in ALT seems to be less likely in children with liver injury than in adults[13,17,18]. A systematic review and meta-analysis showed that children under 18 years of age with COVID-19 had a lower rate of liver injury than adults (10%[4-22] vs 18%[8-35]), although the difference was not statistically significant (P = 0.32)[12]. It seems that males have a higher risk of experiencing acute liver injury due to COVID-19 than females. Direct bilirubin, indirect bilirubin, ALT, alkaline phosphatase (ALP), and gamma-glutamyltransferase (GGT) levels are reportedly higher in male patients with COVID-19[19,20]. Hence, that population may be more susceptible to liver damage, and male patients should be closely monitored for this condition.
Risk factors and predictive factors
Hypoalbuminemia has been identified as a predictor of mortality in COVID-19 patients with liver injury[21]. Decreased hepatic albumin synthesis, leakage of albumin from capillaries, increased catabolism due to fever, and nutritional problems are the probable causes of hypoalbuminemia in patients with severe COVID-19 and liver injury[22].
In terms of ALT and AST, numerous studies have shown that increased levels of these liver enzymes are potential predictors of COVID-19 severity and mortality[12,15,22-24]. In contrast, the results of a case series published as a letter to the editor revealed that increases in the serum concentrations of these two enzymes did not a predict mortality[25].
Studies in liver transplant recipients with COVID-19 concluded that liver transplantation is not a risk factor for more severe disease during hospitalization. In two studies in Italy, it was observed that the number of liver transplanted patients with COVID-19 was very low, three of 200 patients in the first study, and eight of 640 in the second study. None of these patients required mechanical ventilation during hospitalization[26,27].
The severity of COVID-19 was found to be a predictor of liver injury during hospitalization [odds ratio (OR) 2.20, 95% confidence interval (CI): 1.6-3.02; P < 0.001] in both adults and children[6]. Furthermore, younger age and elevated interleukin (IL)-6 or ferritin level have recently been defined as the strongest predictors of liver injury[8]. The risk of liver injury is reportedly higher in COVID-19 patients who develop gastrointestinal (GI) symptoms during their illness, including diarrhea, nausea and vomiting, anorexia, or abdominal pain (OR: 2.71, 95%CI: 1.52-4.83; P = 0.020)[8].
THE PATHOPHYSIOLOGY OF LIVER INJURY IN COVID-19 PATIENTS
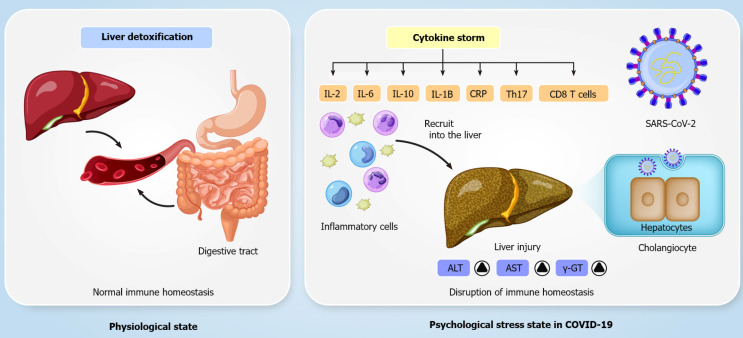
COVID-19 may have both direct and indirect impacts on the hepatobiliary system. The liver biopsy is a procedure that can unravel the pathophysiologic process, disease progression, and prognosis. Liver biopsies are usually done to confirm the presence of viral ribonucleic acid (RNA) in liver tissues, elucidating a direct liver injury mechanism. That SARS-CoV-2 causes direct hepatocyte injury is supported by evidence of cell apoptosis along with ballooned hepatocytes, acidophilic bodies, and lobular inflammation in liver biopsy specimens. The findings are assumed to be a result of direct viral injury[28]. The presence of angiotensin-converting enzyme 2 (ACE2) receptors as a key cell entry receptor for SARS-CoV-2 in cholangiocytes supports a retrograde mode of liver injury following the viral invasion of the bile tree cells[29]. An autopsy examination by Xu et al[16] revealed that SARS-CoV-2 directly contaminated hepatocytes and caused moderate microvascular steatosis and mild hepatic lobular and portal activity[30]. The autopsy results of another COVID-19 fatality revealed that SARS-CoV-2 led to hepatomegaly with dark red, ballooning degeneration along with lobular necrosis and neutrophil infiltration, infiltration of lymphocytes and monocytes in the portal system, sinusoidal dilatation and congestion with microthrombosis[31]. A recent report lends some support to the hypothesis of a potential correlation between expression of the SARS-CoV spike (S) protein and inflammatory responses and hepatitis. At the onset of SARS-CoV infection, host factors elicit an immune response that blocks virus replication, promotes virus removal, and evokes a persistent adaptive immune response against the virus[32]. However, it is important to note that aberrant hepatic biochemistries were also reported in SARS and Middle East respiratory syndrome (MERS) patients, implicating that potential liver damage is closely correlated with coronavirus infections[33,34]. It is not yet clear whether the liver injury is caused directly by SARS-CoV-2. It is well recognized that SARS-CoV-2 is closely associated with SARS-CoV. The two viruses recognize the same ACE2 receptor and do not solely target the lung[35]. Under physiological states, the liver detoxifies the blood coming from the digestive tract, maintaining immune homeostasis across the gut-liver axis. However, immune homeostasis is disrupted under psychological stress states in patients with severe COVID-19 (Figure 1). The induction of immune responses and severe systemic inflammatory responses in SARS-CoV-2 infection, which are known as the cytokine storm syndrome, can drive damage to many organs, including the gut and liver. Studies have shown that Th17 and CD8 T cells, IL-2, IL-6, IL-7, IL-10, tumor necrosis factor-α, granulocyte-colony stimulating factor, interferon-inducible protein-10, monocyte chemotactic protein 1, and macrophage inflammatory protein 1 alpha are involved in the immune response and inflammation in severe cases of COVID-19[3,36,37]. In addition, aberrant hepatic biochemistry results have been found mostly in patients with severe COVID-19. Stress-induced hepatic damage might be related to hypoxia-reoxygenation, activation of Kupffer cells and oxidative stress, intestinal endotoxemia, sympathetic hyperactivity, and adrenocortical system hyperactivity in patients with COVID-19[31].
Figure 1.
Immune homeostasis is disrupted by psychological stress in patients with severe coronavirus disease 2019. ALT: Alanine aminotransferase; AST: Serum aspartate aminotransferase; COVID-19: Coronavirus disease 2019; CRP: C-reactive protein; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Sepsis is typical in cases of severe COVID-19, particularly when patients have gut microbiota imbalance and pre-existing liver cirrhosis[38]. Sepsis is an uncontrolled immune response to an infection, and it causes psychological stress and life-threatening multiple organ failure[39]. Sepsis-related hepatic damage is associated with ischemia and shock, cholestasis, altered bile metabolism, drug toxicity, and overwhelming inflammation[40]. It has been shown that sepsis in COVID-19 patients might be one of the causes of liver injury and leads to a poor prognosis. Moreover, severe tissue hypoxia and hypovolemic shock due to severe dehydration result in ischemic/hypoxic liver injury in COVID-19 patients. Recent findings suggest that ischemic/hypoxic liver injury caused by SARS-CoV-2 infection is related to metabolic acidosis aggravation, calcium overloading, and alterations in the mitochondrial permeability transition pore protein[41]. Additionally, Zhao et al[42] showed that the expression of SARS-CoV-2 can be detected in patchy areas of the liver duct, implying that hepatic ductal organoids are susceptible to SARS-CoV-2 infection. Furthermore, SARS-CoV-2 infection can also disrupt the barrier and bile acid transporting functions of cholangiocytes, implying that SARS-CoV-2 may directly induce cholangiocyte damage leading to the accumulation of toxic bile acid[42]. In view of the points above, viral infection in COVID-19 patients might directly give rise to liver injury. It has been suggested that if AST, ALT, and total bilirubin levels are very high in COVID-19 patients, the hospital stay might be prolonged[3,43]. Besides, the reported findings suggest that among patients with aberrant liver function, moderate and severe cases of COVID-19 are more likely to be associated with the occurrence of liver injury (58.8% and 66.7%, respectively)[44].
ACE2 AND THE LIVER
ACE2 is a metalloproteinase regulator of the renin-angiotensin system, which hydrolyzes angiotensin (Ang) II to Ang-(1-7)[45]. The main physiological function of ACE2 is contributing to the regulation of vasoconstriction and blood pressure[46,47]. Type 2 transmembrane protease serine (TMPRSS2), belonging to the serine protease family, can cleave and activate the coronavirus S protein for membrane fusion[47-49]. It has been demonstrated that ACE2 and TMPRSS2 are key molecules both for the entry of SARS-CoV and SARS-CoV-2 into host cells and for viral spread in the infected host[50-52]. SARS-CoV-2 cell entry is initiated by specific binding of the S protein to the cellular receptor and S protein priming following proteolytic cleavage by host cell proteases. Moreover, each S protein of SARS-CoV-2 contains two subunits, a globular S1 domain at the N-terminal end, and a membrane-proximal S2 domain that mediates attachment and membrane fusion. Similar to SARS-CoV, SARS-CoV-2 utilizes the receptor-binding motif within the S1 domain to bind to the ACE2 receptor, which facilitates the effects of TMPRSS2 on the cleavage of protein S at two distinct sites, termed S1/S2; viral entry then occurs following cell membrane priming and fusion[51,53,54]. The S1 subunit is responsible for host cell receptor binding, while the S2 subunit mediates viral and cell membrane fusion[55]. A recent study by Zhou et al[52] demonstrated that SARS-CoV-2 infection depends on the binding of the ACE2 receptor and the S protein. If the S protein is considered as a key, the ACE2 receptor acts like a lock that is unlocked by its key. A group of researchers also found that the S protein of SARS-CoV-2 displays 10 to 20 fold more affinity to the ACE2 receptor than SARS-CoV, which may explain why SARS-CoV-2 is so contagious[56,57]. Recent studies have determined that the ACE2 receptor and TMPRSS2 are highly expressed not only in lung tissues but also in other organs including the heart, kidney, liver, colon, esophagus, brain, gallbladder, and testis, implying that SARS-CoV-2 might also affect other organs[58,59]. High ACE2 receptor expression was identified in cholangiocytes, with a level 20 fold higher than that in hepatocytes[60]. In addition, researchers have reported that both ACE2 receptors and TMPRSS2 are broadly expressed in the liver[61]. In a recent study, single-cell RNA sequencing analysis and immunohistochemistry confirmed that the ACE2 receptor is predominantly expressed in bile duct epithelial cells[62]. Recently, Seow et al[63] reported that ACE2 receptors and TMPRSS2 are precisely co-expressed in TROP2+ liver progenitors in human hepatic tissue.
THE ROLE OF INFLAMMATION BY COVID-19 IN HEPATIC DYSFUNCTION
The origin of liver damage following COVID-19 infection potentially involves systemic inflammation, viral replication, or drug-related liver injury. Clinical studies indicated a direct association between systemic inflammation with involvement of ferritin, IL-6, and C-reactive protein (CRP), liver injury, and hepatotoxicity[64,65]. The production of IL-6, a pleiotropic cytokine, may stem from immune cells[66], endothelial cells, fibroblasts, and hepatocytes in response to acute hepatic injury and infection[67]. In line with an important effect of systemic inflammation and specifically IL-6 on liver damage, researchers reported a direct association between acute-phase proteins and IL-6 in COVID-19 patients with increased AST levels. Effenberger et al[67] found that the systemic inflammatory response (SIRS) to SARS-CoV-2 infection in COVID-19 patients was responsible for hepatic injury. In agreement with that, the reported findings suggest more pronounced SIRS in COVID-19 cases with a more acute cytokine release syndrome (CRS), that is, need for intensive care measures. Interestingly, IL-6 receptor inhibition by tocilizumab, a recombinant humanized monoclonal antibody, reversed liver injury during CRS[68]. In addition, clinical evidence suggests that inhibition of the inflammatory response in SARS-CoV-2-related CRS ameliorates the course of infection[69]. Data from the MERS-CoV outbreak showed that IL-6 and pro-inflammatory cytokines like IL-2 played important roles in infection and liver injury[70]. In patients with COVID-19, increased IL-6 concentration and other cytokine signatures have been identified[71], correlating with loss of lung function, lung injury, and poor outcomes[72]. As such, it seems that SARS-CoV-2 strongly drives a lethal systemic cytokine response in some patients (as in CRS), although factors that regulate this response are not well understood. This cytokine response is involved in disease severity and patient outcome[73]. Immune pathway hyperactivation and inflammation associated with a cytokine storm in SARS-CoV-2 infection can damage many other organs, such as the gut and liver, through Th17 and CD8 T cells and the activation of inflammatory responses[3,36,74].
THE DIAGNOSIS OF LIVER INJURY IN COVID-19 PATIENTS
Various biomarkers are directly associated with liver injury and indirectly with COVID-19 severity and prognosis. Trends in biomarker research over the course of the disease may help physicians recognize the conditions associated with COVID-19 more quickly and accurately. Liver enzymes, including ALT and AST, are useful biomarkers of hepatic injury in COVID-19 patients[75]. Liver diseases primarily lead to mild clinical symptoms but must be diagnosed early. Hepatic dysfunction could be evaluated by biomarkers that are correlated with liver function (e.g., albumin) and hepatocyte integrity (e.g., ALT and AST), while some are correlated with conditions related to the biliary tract (e.g., ALP and GGT)[75].
The serum levels of several clinical biomarkers such as liver enzymes (e.g., ALT and AST) are elevated in COVID-19 patients. Moreover, recent findings have shown that lactate dehydrogenase (LDH) concentration increases in COVID-19 patients and is significantly higher in patients with increased ALT relative to patients with normal ALT[76,77]. This phenomenon may occur because of cell apoptosis induced by SARS-CoV-2 infection or because of the use of antiviral drugs. It is suggested that the elevated level of LDH reflects the status of liver function and other organs in response to anti-influenza activity of antiviral compounds[78]. Uchide et al[79] also reported that the apoptosis caused by influenza infection was correlated with increasing LDH levels. Moreover, LDH is known as a marker of lung and hepatobiliary diseases. Thus, a marked elevation in LDH values in patients with increased ALT could be indicate the involvement of the lungs and liver. As a critical component of the immune system, CRP is produced in the liver in response to a variety of inflammatory cytokines[80]. Recent studies have shown that an increase of CRP level is detected in almost 80% of patients with COVID-19[81,82]. In addition, a study comparing patients with elevated ALT normal ALT activities found no significant between-group difference in CRP values. Inconsistent with that finding, Li et al[77] found that elevated CRP levels were associated with hepatic dysfunction and increased ALT levels in COVID-19 patients. These discrepancies may be the result of differences in sample sizes and CRP detection kits. In addition, elevated serum ferritin and CRP are particularly useful in prediction of COVID-19 progression or exacerbated secondary bacterial infection, cytokine storm with multiorgan failure, and poor outcome[83].
LIVER-RELATED MANIFESTATIONS IN PATIENTS WITH COVID-19
COVID-19 usually presents as viral pneumonia with primary symptoms of fever, cough, and shortness of breath. However, some patients may develop extrapulmonary signs and symptoms in their clinical picture. Of those, GI and hepatic manifestations are most noticeable[84,85]. Some studies reported that GI symptoms were seen in 26%-53% of COVID-19 patients at any time during their illness. Diarrhea, nausea, vomiting, abdominal pain, anorexia, anosmia, and dysgeusia are among the symptoms reported in decreasing order of prevalence[86-88]. According to the largest, most recent, and most comprehensive systematic reviews, GI symptoms are present in 10%-15% of coronavirus cases, and include diarrhea, 7.7%-9%; nausea/vomiting, 6%-7.8%; loss of appetite, 21%; and abdominal pain, 2.7%-3%[12,89]. Interestingly, few patients with coronavirus infection present with GI symptoms as their only clinical picture or ahead of pulmonary manifestations. On average, 3%-16% of COVID-19 cases may present with digestive symptoms per se in the absence of respiratory symptoms[43,90,91].
Hepatic enzyme derangements are considered as the most common extrapulmonary findings in the context of COVID-19[92]. The incidence of an abnormal liver function test in this context varies from 1% to 76% in the published literature[93-96]. However, three large, recent meta-analyses found that the incidence and prevalence of abnormal liver function tests among COVID-19 patients were 23.1% and 15%-19%, respectively[12,89,97]. Despite the presence of ACE2 receptors on cholangiocytes, the liver injury in such patients was primarily hepatocellular rather than cholestatic, as indicated by elevations in ALT, AST, and LDH levels[60]. A retrospective study evaluating more than 1800 COVID-19 patients at admission and during the hospital course reported enzyme abnormality prevalences of AST 66.9%, ALT 41.6%, and ALP 13.5% at admission with peaks of AST 83.4%, ALT 61.6%, and ALP 80% during hospitalization[84]. Enzyme elevation is usually mild, i.e. 1-2 times the upper limit of normal (ULN); nevertheless, moderate (2-5 times the ULN) and severe (more than 5 times the ULN) elevations of AST and ALT have sometimes been reported. The GGT concentration may also be increased at admission and even escalate during hospitalization. However, that enzyme is not unique to the liver and is distributed in many other tissues including the kidneys, pancreas, spleen, heart, brain, and seminal vesicles. Despite mild elevations in the total bilirubin level, jaundice is rarely seen in COVID-19 patients[86,96,98]. The severity of COVID-19 appears to be associated with increased AST, ALT, and ALP concentrations, as well as a fall in albumin level[84,96,99,100].
To date, no case of acute liver failure (ALF) has been proven to be directly caused by COVID-19. There is only one suspected case of ALF in the context of coronavirus infection[101]. Approximately 3% of all cases of COVID-19 and 3.9% of those with severe COVID-19 have pre-existing chronic liver diseases[97,100]. Few studies concluded that the underlying liver disease, including nonalcoholic fatty liver disease, were associated with more pronounced COVID-19 severity or mortality compared with those with no pre-existing hepatic disorders. Furthermore, a recent meta-analysis failed to find a direct association. Therefore, whether the presence of the underlying liver disease may change the outcome of patients with COVID-19 remains to be shown[97,102-104].
Presumably, liver transplant patients are more vulnerable to coronavirus infection because of their potent immunosuppressive drug regimens. An international registry study (SECURE-Cirrhosis) evaluated 151 Liver transplant recipients with confirmed SARS-CoV-2 infection. The rate of intensive care unit admission and need for invasive ventilation was more frequent in liver transplant patients. However, unlike a few other studies, the study failed to demonstrate that organ transplantation significantly increased the risk of death in the included cases[105-109].
DRUG-INDUCED LIVER INJURY AND COVID-19
The global outbreak of COVID-19 has led to the aggregation of pivotal data on the clinical and epidemiological characteristics of the disease. A number of studies on the topic have revealed that COVID-19 patients can present with different stages of liver disease[110,111]. Cai et al[96] recognized that about half of the patients with COVID-19 showed liver functional test derangements at admission, almost 90% having a mild pattern of liver enzyme elevation, and about 25% had transaminase levels of more than three times the ULN during hospitalization[110,111]. The most common abnormality in liver function tests has been hypoalbuminemia. Although changes in the levels of liver-related blood factors may be caused by direct viral damage, many studies have reported that the use of various drugs during hospitalization caused the abnormalities. Drugs that may be responsible include antivirals such as arbidol, favipiravir, remdesivir, and others; steroids; and antipyretics like acetaminophen or nonsteroid anti-inflammatory drugs[41,112]. Favipiravir is an antiviral drugs that was approved in Japan as a standard treatment for patients with influenza. Favipiravir, also known as T-705, is a pro-drug that is converted to its active metabolite, favipiravir ibofuranosyl-5′-triphosphate, by intracellular phosphorylation. It is a purine nucleotide that is recognized by and inhibits RNA-dependent RNA polymerase. Favipiravir is effective against Ebola, other RNA viruses including West Nile virus, yellow fever virus, foot and mouth disease virus, and Lassa virus[113]. Hyperuricemia and diarrhea have been identified as side effects and were observed in 20% of the treated patients. Psychiatric symptoms, neutropenia, and elevation in transaminases were seen in 2% of the treated patients. Overall, Favipiravir has an excellent reported safety profile worldwide[114]. Another study reported that lopinavir-ritonavir, with or without ribavirin, interferon beta, and/or corticosteroids, was independently associated with increased levels of AST and ALT in patients with COVID-19[115]. Lopinavir is a protease inhibitor used to treat human immunodeficiency virus infection in combination with a low dose of ritonavir, another protease inhibitor, which enhances its biological half-life[116]. Consumption of high-dose ritonavir, 1200 mg once daily, may cause serious side effects including severe hepatotoxicity. It is thus administered at lower doses (200-400 mg) to increase the effectiveness of other medications[117]. Recent studies have reported elevated levels GGT and total bilirubin in hospitalized patients treated with lopinavir/ritonavir, so it should be avoided or used with great caution in patients with a history of metabolic liver disease[117].
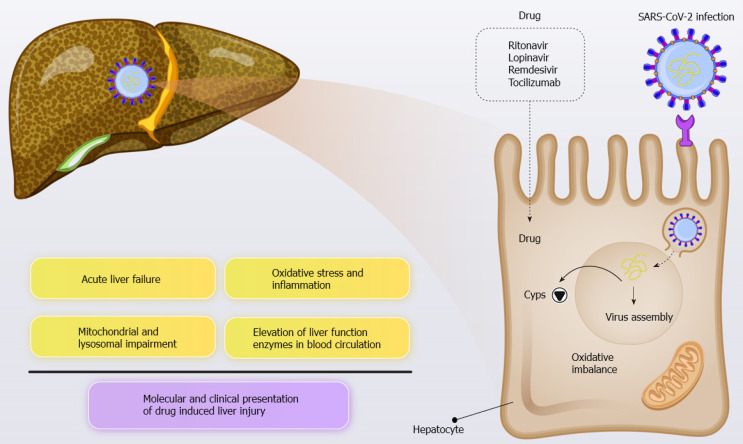
Several studies have highlighted the key role of antiviral drugs targeting COVID-19 in liver dysfunction (Figure 2). For example, Jiang et al[118] found that lopinavir/ ritonavir use in COVID-19 patients was associated with liver injury and abnormal liver function, particularly in patients with noncritical disease. Cai et al[96] reported a marked increase in transaminase levels during hospitalization. Approximately 11% of patients had ALT levels greater than three times the ULN, and about 12% had GGT levels of up to three times the ULN. Lopinavir and ritonavir represent the most important risk factor for liver disease because they leads to a four-fold elevation in the likelihood of hepatic failure[110,111].
Figure 2.
The key role of antiviral drugs targeting coronavirus disease 2019 in liver dysfunction. COVID-19: Coronavirus disease 2019; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Cytochromes P450 (CYPs) are a superfamily of monooxygenase enzymes that mediate clinically relevant drug interactions in various pathological conditions[119]. There is strong support for the hypothesis that the metabolic activity of CYPs will undoubtedly be altered (mostly downregulated) throughout the course of SARS-CoV-2 infection in a similar pattern, leading to a clearance-related pharmacokinetic interaction with the antiviral drugs that are administered. Additionally, liver involvement in SARS-CoV-2 infection may further complicate the clinical course of the disease. In May 2020, the investigational antiviral agent, remdesivir, which was once offered against Ebola, was authorized for emergency use by the United States Food and Drug Administration (FDA) for the treatment of COVID-19. Remdesivir is extensively metabolized by CYPs, particularly CYP3A4. Furthermore, other potential therapeutic candidates for treating COVID-19 including chloroquine[120] and colchicine[121] are also metabolized by enzymes within the liver. A sound understanding of the mechanism of such crosstalk is essential as it may affect the therapeutic/toxic reaction of patients to the agents[122]. Caution should be exercised when anti-cytokine treatment is administered. In this respect, partial or full restoration of normal metabolic conditions can be achieved as a consequence of the immunomodulatory response.
It is well known that use of remdesivir considerably reduces time to recovery of hospitalized COVID-19 patients[123]. Among COVID-19 patients treated with remdesivir, reversible grade 1–2 ALT/AST elevations were seen without pathologic abnormalities in either liver or kidney function. Marked increase in AST and ALT levels were reported in 6% of patients, and life-threatening situations associated with increased AST/ALT levels were present in 2%, which led to a recommendation to reduce or discontinue long-term remedesiver therapy[124]. That is in line with other data that mild hepatocellular injury in the form of increased AST/ALT levels did not progress to ALF in patients without pre-existing chronic liver injury. Therefore, remedesiver can be used with close monitoring of liver toxicity especially in patients with pre-existing liver disease[125].
A recent placebo-controlled study evaluated the protective effect of intravenous remdesivir in hospitalized adult patients with severe COVID-19. It reported premature cessation of treatment in more remdesivir recipients than placebo recipients because of adverse events such as elevation of aminotransferase or bilirubin level. In spite of the association of SARS-CoV-2 infection with hepatic dysfunction, the study found an increased risk of liver damage with remdesivir compared with other therapeutic agents. However, because of the current FDA and European medicines agency approvals to prescribe remdesivir for patients with COVID-19, physicians should be aware of this plausible association and must initiate adequate hepatic monitoring[126].
Considering the fact that there is yet to be a completely effective antiviral therapy for COVID-19, antiviral agents including oseltamivir, arbidol, lopinavir, and ritonavir are used in about half of the serious COVID-19 cases[112]. These antiviral drugs may cause abnormal liver function. Moreover, ribavirin-induced hemolysis might give rise to tissue hypoxia, which may also cause increased serum liver enzyme levels. The pre-existence of chronic liver disease (e.g., hepatitis B or hepatitis C) with or without elevated transaminase levels could pose additional risk of drug-induced liver damage[127]. Accordingly, in patients with pre-existing liver disease, antipyretic drugs, traditional herbal medicines, antiviral medicines or antibiotics should be used cautiously; physicians must take into consideration the risk of liver damage deterioration. For instance, doxycycline is a broad-spectrum tetracycline-class antibiotic that chelates zinc, which is required by the matrix metalloproteinases involved in COVID-19 infection, and inhibits SARS-CoV-2 RNA polymerase activity and viral entry. Doxycycline is a viral serine protease inhibitor that has broad spectrum of antiviral activity including SARS-CoV-2. It exerts anti-inflammatory effects by downregulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Likewise, reduction of secretion of inflammatory cytokines such as tumor necrosis factor-α, IL-1b, and IL-6, and induction of mast cells apoptosis is observed[128]. Hepatotoxicity is seen with doxycycline use, but doxycycline is potentially less hepatotoxic than tetracycline. This medicine is generally safe and effective but has been linked to occasional obviating bile duct injuries[129]. In addition, other studies linking doxycycline with hepatotoxicity reported abrupt hepatic failure and hepatocellular necrosis in patients using potentially hepatotoxic agents, after initiating doxycycline therapy 129].
An increasing number of studies have described azithromycin-induced hepatotoxicity. Azithromycin is a macrolide antibiotic that used for the treatment of Gram-positive and Gram-negative bacterial infections. Azithromycin has synergistic antiviral activity associated with enhanced production of interferon (IFN)-β and IFN-λ and related cytosolic genes that contribute to virus recognition[130]. Azithromycin in vitro and in vivo antiviral activity, especially against respiratory syncytial virus and influenza viruses[130,131], suggests promising antiviral effect against early stage of COVID-19[132].
Like any medication, Azithromycin can have adverse effects on the hepatobiliary system including symptoms and signs of cholestatic hepatitis such as fever, fatigue, jaundice, pruritus, and eosinophilia. However very few reported cases with complications of vanishing bile duct syndrome and hepatic failure requiring liver transplantation have been associated with azithromycin use[133].
Tocilizumab is approved for use in the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis. Although it is not a specific antiviral agent, some researchers have recently suggested tocilizumab for the treatment of severe CRS in COVID-19 patients[68,134]. Tocilizumab is a recombinant humanized monoclonal antibody that blocks the IL-6 signal transduction pathway[68]. The most common set of side effects of tocilizumab are headache, hypertension, and rare occurrences of hepatotoxicity ranging from mild elevation of liver enzymes to serious drug-induced hepatotoxicity[135]. Currently, data on tocilizumab-induced hepatotoxicity in COVID-19 patients have not been established. The evidence shows that tocilizumab might be effective in severe COVID-19, even in patients with elevation of serum transaminase levels of up to 5-fold the ULN and GGT levels of up to ten-fold the ULN. When possible, a baseline liver biopsy is recommended prior to tocilizumab treatment in patients with liver dysfunction to diminish the potentially detrimental effect of other antiviral medicines on liver function during SARS-CoV-2 infection. Some studies reported that the systemic inflammatory reaction to the drugs used in the treatment of SARS-CoV-2 infection and pneumonia might induce liver injury in COVID-19 patients[136]. Larger studies are required to confirm the safety of tocilizumab therapy and to validate its use in chronic liver disease patients[111].
Hepatic dysfunction in COVID-19: Management and challenges
The COVID-19 pandemic is a worldwide threat to human health and global stability. Extensive study has found that in addition to the respiratory system, the digestive tract and liver are involved in SARS-CoV-2 infection. Immune-mediated viral damage, drug-induced hepatotoxicity, secondary liver injury induced by an overwhelming systemic inflammatory reaction, and the deterioration of pre-existing liver injury are leading causes of liver damage in COVID-19. Physicians need to pay particular attention to the management of pre-existing liver injury, the monitoring of liver function, the provision of robust supportive treatment, and minimization of the risk of drug-induced hepatotoxicity in COVID-19 patients.
For all COVID-19 patients, biochemical markers of liver injury, including ALT/AST, bilirubin, albumin, and prothrombin time, should be evaluated to detect liver damage. If plasma LDH and AST values are increased but the ALT value is within the normal range, skeletal muscle or cardiac injury rather than liver damage should be considered. Given that liver failure is also a major risk factor among elderly patients with COVID-19, caregivers need to pay particular attention to the management of hepatic comorbidities (Table 1). In the management of COVID-19 patients with hepatitis B, stopping antiviral treatment should be avoided to prevent the reactivation of hepatitis B, and antihepatitis B virus drugs should be monitored when patients are given glucocorticoid treatment.
Table 1.
Medications used in the treatment of coronavirus disease 2019 and liver-related complications
| Agent | Pharmaceutical property | Mechanism of action | Molecular effects on the liver | Molecular effects on lung | Approved indication(s) | Ref. |
| Tocilizumab | IL-6 receptor-blocking agent | Reduced the expression of TNF-α and IL-10, downregulated inflammasome activation, and inhibited monocyte phagocytic activity, thereby suppressing the cytokine storm | Mild-to-moderate elevation in transaminases and drug-induced liver injury | Reduced the inflammation of lung tissue | Clinical trial stage | [110,141,142] |
| Remdesivir | Antiviral drug | RNA-dependent RNA polymerase inhibitor (Adenosine analog) | Transaminases↑ Bilirubin↑ | Virus lung titers↓, lung hemorrhage↓, and improved pulmonary function | Approved to treat COVID-19 | [126,143] |
| Oseltamivir | Antiviral drug | Neuraminidase inhibitor; inhibits the release of progeny virus from infected host cells | Transaminases↑ Bilirubin↑(Rare) | Interleukin IL-1β↓, TNF-α↓, IL-4↓, IFN-γ↓ | Approved for Influenza A and B | [144,145] |
| Umifenovir (Arbidol) | Antiviral drug | Spike protein/ACE2 membrane fusion inhibitor; inhibits viral entry into target cells and stimulates the immune response | Transaminase↑ | Viral attachment to ACE2↓ | Clinical trial stage | [146] |
| Lopinavir-Ritonavir | Antiviral drug | Antiretroviral protease inhibitor; disruption of viral entry | Golgi fragmentation↑, organelle stress response↑, hepatic injury↑ | p53 DNA damage response activation↑ | Approved for treatment of HIV | [147-149] |
| Bicyclol | Antiviral compound candidate | Directly interacts with viral membranes and minimizes the host’s inflammatory response | Transaminases↓, hepatic triglyceride↓ | Attenuates LPS- acute lung injury | Approved for treatment of liver injury | [150,151] |
| Ribavirin (plus IFN-α) | Antiviral drug | Inhibits viral RNA-dependent RNA polymerase (Guanine analog) | Transaminase↓, liver histological damage↓, liver inflammation↓ | Improved survival in patients with severe MERS-CoV infection | Approved for some viral diseases including hepatitis C, has limited value in treatment of COVID-19 | [152,153] |
| Favipiravir | Antiviral drug | RNA-dependent RNA polymerase inhibitor | Abnormal liver function test (transaminase↑) | Viral load in the lungs↓, pulmonary tissue inflammation ↓ | Approved in some countries to treat influenza, Ebola, and noroviruses | [154,155] |
| Chloroquine and hydroxychloroquine | Antimalarial medication | Inhibits viral fusion and entry into the cell | IL-6↓, TNF-α↓, NF-kB expression↓, liver tissue apoptosis↓ liver inflammation↓ | Lung inflammation↓, oxidative stress↓, pulmonary tissue fibrosis↓ | FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 | [156-158] |
| Probiotics | Live microorganisms | Preventive effects on bacterial and viral infection | Hepatic inflammation↓, transaminases↓, TNF-α↓, ameliorating liver injury and improve liver function | Suppress lung inflammation, preventing effects on acute respiratory tract infections | Approved as dietary supplements | [159-161] |
| N-Acetyl-Cysteine | Antioxidant agent | Precursor of reduced glutathione and mucolytic agent | Liver lactate↓, improve liver function | Inhibition of ACE activity, inhibit virus replication, pulmonary inflammation↓ | Approved for use in humans | [162,163] |
| Glycyrrhizic acid | Natural triterpenoid saponin isolated from Glycyrrhiza spp | Anti-inflammatory, antioxidative, antiviral compound | Oxidative stress↓, apoptosis↓ in the liver tissue | Inhibited the replication and penetration into cells of the SARS-associated coronavirus, IL-33↓ | Approved as a food additive | [164,165] |
| Vitamin E | Fat-soluble vitamin (alpha-tocopherol) | Antioxidant and immunomodulatory agent | ROS↓, GSH↑, NF-κB activation ↓, inflammation↓, fibrosis↓, transaminase↓ | Oxidative stress↓, NO and COX activity↓, T cell proliferation↑ | Approved as dietary supplements | [9,166,167] |
| Vitamin A | Fat-soluble vitamin (all-trans-retinol) | Antioxidant and immunomodulatory agent | Transaminase↓, IL-10↑, NF-κb activation ↓, TNF-α↓ | IL1-β↓, Oxidative stress↓, improved pulmonary function | Approved as dietary supplements | [165,166] |
| Vitamin D | Fat-soluble secosteroids (cholecalciferol) | Reduced viral entry and replication, attenuated inflammation | Modulated liver inflammation and fibrogenesis | TNF-α↓, IFNγ↓, GSH↑, improved pulmonary function | Approved as dietary supplements | [166,168,169] |
| Vitamin C | Water-soluble vitamin (Ascorbic acid) | Cofactor for a number of enzymatic reactions; antioxidant | Transaminase↓, SOD↑, GSH↑, oxidative stress↓, TNF-α↓ | TNFα↓, IL-1β ↓, IL-8↓, NF-κB activation↓, inflammation↓ | Approved as dietary supplements | [166,170] |
| Zinc | Dietary mineral | Anti-inflammatory and antioxidant micronutrient | Decreased pro-inflammatory cytokine production, oxidative stress↓, hepatic tissue apoptosis↓, | Attenuated lung tissue inflammation, TNF-α↓, IL-6↓, IL-1β↓, interferon-γ↑, IL-2↑ | Approved as dietary supplements | [171,172] |
| Mg | Dietary mineral | Anti-inflammatory and antioxidant micronutrient | Liver fibrosis↓, IL-6↓, TNF-α↓, inhibited the NF-κB pathway, transaminase↓ | IL-6↓, IL-1β↓, NF-κB activation↓, COX-2 activity↓, prostaglandin E2↓, lung tissue oxidative stress↓ | Approved as dietary supplements | [173,174] |
| Copper | Dietary mineral | Antiviral activity through damage in virus envelope and surface spikes and destruction of the viral genomes | Ceruloplasmin↑, inflammation↓, IL-6↓, oxidative stress↑ | Viral replication↓, virus particles released from infected cells↓, viral entry↓ | Approved as dietary supplements | [175,176] |
| Dexamethasone | Corticosteroid medication | Reduced aggressive inflammatory response | VLDL↑, HDL↑, IL-1β↓, IL-2↓, TNF-α↓, interferon-γ↓, prostaglandins production↓, liver inflammation↓, prevented the liver fibrosis | Pulmonary inflammation↓, NF-κB activity↓, oxidative markers↓, lung tissue fibrosis↓, mortality in patients with severe COVID-19 disease↓ | WHO-approved treatment for COVID-19 | [177,178] |
ACE: Angiotensin-converting enzyme; COVID-19: Coronavirus disease 2019; COX-2: Cyclooxygenase-2; DNA: Deoxyribonucleic acid; FDA: Food and Drug Administration; GSH: Glutathione; HDL: High density lipoprotein. HIV: Human immunodeficiency virus; IFN-γ: Interferon-gamma; IL-6: Interleukin 6; MERS-CoV: Middle East respiratory syndrome -coronavirus; NF-ΚbB: Nuclear factor kappa-light-chain-enhancer of activated B cells NF-kappa B; RNA: Ribonucleic acid; ROS: Reactive oxygen species; SOD: Superoxide dismutase; TNF-α: Tumor necrosis factor-α; VLDL: Very low-density lipoprotein.
Thus, in the case of severe liver injury in COVID-19 patients, a meticulous evaluation to recognize any underlying disease is needed, and the degree of abnormality in liver function should be recorded to manage the onset of liver failure. The initial screening consists of a careful history of hepatic comorbidities, hepatotoxin exposure (e.g., alcohol, drugs, herbs, and chemicals), hypoxia, and circulation quality. Circulatory and respiratory mechanical support should be considered for COVID-19 patients with hypoxic hepatitis[83].
Administration of L-ornithine-L-aspartate as a promising agent in the management of liver failure could be effective in reducing ammonia levels in hepatic encephalopathy. However, those remedies are only a complementary treatment and should not be overemphasized. Hence, both prebiotics and probiotics could provide various health benefits by regulating the microecological balance and bacterial activity in the intestine[130]. Administration of silymarin 420 mg/d and ursodeoxycholic acid 500 mg/d protect hepatic function from injury, especially if SARS-CoV-2 infection is correlated with increasing serum transaminase levels and serum total bilirubin[133].
A recent study by Ibrahim et al[137] showed that N-acetyl-cysteine (NAC), a drug that reduces the risk of chronic obstructive pulmonary disease, could be used in COVID-19 patients who lack G6PD deficiency. NAC elicited an improvement in clinical manifestations together with an obvious drop in CRP and ferritin levels in COVID-19 patients; rebound increases were seen upon discontinuation of NAC. Resuming intravenous administration of NAC for two additional intervals led to repeated reductions in CRP and ferritin levels as markers of inflammation. NAC administration accelerated the discontinuation of extracorporeal membrane oxygenation (ECMO) and eventual hospital discharge of the patient. In addition, NAC blocked hemolysis and liver enzyme elevation and facilitated removal from the ventilator and venovenous ECMO, leading to full recovery of COVID-19 patients with G6PD deficiency. The mechanism of action of NAC may consist of viral infection blockade and averting the ensuing uncontrolled cytokine storm. Further studies of this agent are needed.
As with other viral infections, liver transplant recipients are at an increased risk of infection with SARS-CoV-2 through hospital exposure during the outbreak[138]. Recipients of liver transplantation may have a strong potential to develop a severe immunosuppressive response, postoperative infection, multiple organ failure, and mortality. There exists a contradiction such that excessive immunosuppression therapy results in acute infections, whereas exposure to insufficient immunosuppression leads to graft loss through the rejection reaction. A study by Qin et al[139] showed that recipients of liver transplantation had a prolonged period of being a virus carrier. Even if the initial SARS-CoV-2 testing is negative during the treatment, there is a chance of a subsequent positive result because of a rise in the viral load accompanying the increased dosage of immunosuppressive drugs. Strict screening guidance and extended follow-up are recommended for recipients of liver transplantation with SARS-CoV-2 infection. Research has shown that corticosteroid treatment of COVID-19 patients with advanced liver disease should be used with caution. In severe respiratory cases of COVID-19, low-dose methylprednisolone may be useful, particularly with increasing lung infiltrates and deteriorating clinical outcome even with invasive mechanical ventilation. Hence, its use is still controversial and in this situation, physicians should pay more attention to hepatic damage in COVID-19 patients[140].
CONCLUSION
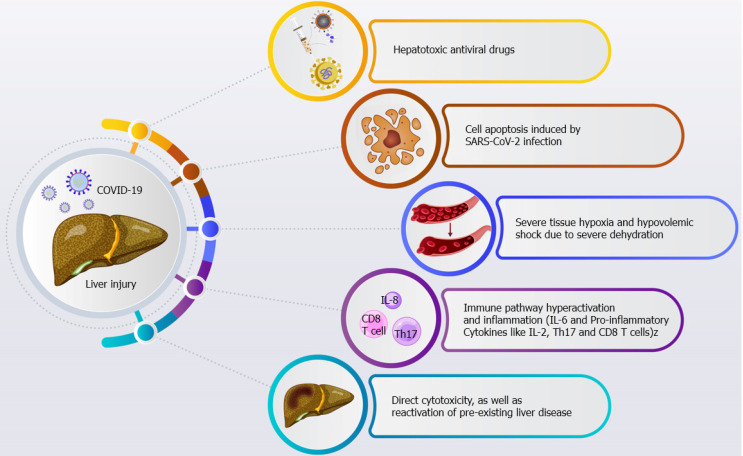
COVID-19 is associated with respiratory symptoms, digestive complications, and liver injury. Severe inflammatory response, anoxia, drug-induced liver injury, direct cytotoxicity, and reactivation of pre-existing liver disease might be the etiologic mechanisms behind liver injury in COVID-19 patients (Figure 3). In this review, we summarized the clinical manifestations and liver-related events seen in COVID-19 patients, including the pathophysiology, etiology, biomarkers, diagnosis, treatment, and management strategies for liver injury. We aimed to increase the awareness of healthcare workers about liver injury and to provide information for hepatic management in COVID-19 patients[141-178]. Physicians should (1) pay special attention to the management of concurrent metabolic liver disorders, (2) boost hepatic function by strengthening supportive therapy, and (3) minimize the risk of drug-induced liver injury. SARS-CoV-2 infection features strong transmission ability and a prolonged incubation time, eliciting an extraordinary threat to human life through various clinical manifestations. SARS-CoV-2 infection can result in liver damage that is associated with the progression of the disease and influences the diagnosis, prognosis, and treatment. Along these lines, liver transplant recipients who acquire COVID-19 have a poor prognosis and a higher risk of mortality. COVID-19-related liver injury can result from moderate microvascular steatosis, severe dehydration, viral infection, hepatotoxic antiviral drugs, the SIRS, acute hypoxemic respiratory failure, and multiple organ failure. SARS-CoV-2 invades the liver directly, causing hepatocyte damage and viral microvascular steatosis. Importantly, ACE2 receptors are highly expressed in liver tissue and play an important role in the hepatobiliary system. Additionally, physicians should pay special attention to protecting the hepatobiliary system over the course of COVID-19 treatment. Some countries have begun SARS-CoV-2 vaccination, which will combat the spread of the disease and may prevent the associated liver damage. Patients should be persuaded to continue their treatments to manage their pre-existing liver diseases. Close monitoring of liver function in COVID-19 patients and the use of diverse therapeutic interventions can blunt liver injury in patients with pre-existing liver diseases and thereby decrease mortality. Additional research is urgently needed to more precisely clarify host-pathogen crosstalk, host immune mechanisms, and mechanisms of viral attachment to host immune cells. Clearly, further investigation of the mechanisms, manifestations, and prognosis of liver injury in patients with COVID-19 is needed to facilitate the development of targeted treatments to improve the patient condition.
Figure 3.
Severe inflammatory response, anoxia, drug-induced liver injury, direct cytotoxicity, and reactivation of pre-existing liver disease might be the etiologic mechanisms behind liver injury in coronavirus disease 2019 patients. Cyps; Cytochrome P450; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Manuscript source: Invited manuscript
Peer-review started: January 18, 2021
First decision: May 2, 2021
Article in press: June 25, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ielasi L, Muthu S S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Gholam Reza Sivandzadeh, Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz 7193635899, Iran.
Hassan Askari, Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz 7193635899, Iran.
Ali Reza Safarpour, Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz 7193635899, Iran. safarpourar@gmail.com.
Fardad Ejtehadi, Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz 7193635899, Iran.
Ehsan Raeis-Abdollahi, Department of Medical Sciences, Qom Medical Branch, Islamic Azad University, Qom 1417613151, Iran.
Armaghan Vaez Lari, Department of Physiology, School of Medicine, Ahvaz Jundishapur University of Medical Science, Ahvaz 6135715794, Iran.
Mohammad Foad Abazari, Research Center for Clinical Virology, Tehran University of Medical Sciences, Tehran 1417653761, Iran.
Firoozeh Tarkesh, Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz 7193635899, Iran.
Kamran Bagheri Lankarani, Health Policy Research Center, Shiraz University of Medical Sciences, Shiraz 71348-45794, Iran.
References
- 1.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askari H, Sanadgol N, Azarnezhad A, Tajbakhsh A, Rafiei H, Safarpour AR, Gheibihayat SM, Raeis-Abdollahi E, Savardashtaki A, Ghanbariasad A, Omidifar N. Kidney diseases and COVID-19 infection: causes and effect, supportive therapeutics and nutritional perspectives. Heliyon. 2021;7:e06008. doi: 10.1016/j.heliyon.2021.e06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 5.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nesseler N, Launey Y, Aninat C, White J, Corlu A, Pieper K, Mallédant Y, Seguin P. Liver Dysfunction Is Associated with Long-Term Mortality in Septic Shock. Am J Respir Crit Care Med. 2016;193:335–337. doi: 10.1164/rccm.201508-1660LE. [DOI] [PubMed] [Google Scholar]
- 7.Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc. 2020;83:521–523. doi: 10.1097/JCMA.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286–2293. doi: 10.3748/wjg.v26.i19.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar-M P, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, Mandavdhare HS, Dutta U, Sharma V. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14:711–722. doi: 10.1007/s12072-020-10071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiehao C, Jin X, Daojiong L, Zhi Y, Lei X, Zhenghai Q, Yuehua Z, Hua Z, Ran J, Pengcheng L, Xiangshi W, Yanling G, Aimei X, He T, Hailing C, Chuning W, Jingjing L, Jianshe W, Mei Z. A Case Series of Children With 2019 Novel Coronavirus Infection: Clinical and Epidemiological Features. Clin Infect Dis. 2020;71:1547–1551. doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GWK Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu L, Fei J, Xu S, Xiang H-X, Xiang Y, Tan Z-X, Li M-D, Liu F-F, Li Y, Han M-F. Acute liver injury and its association with death risk of patients with COVID-19: a hospital-based prospective case-cohort study. Available from: medRxiv 2020.
- 21.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 22.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW the Northwell COVID-19 Research Consortium. Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, Shi CW, Lian X, Chu JG, Chen L, Ren DW, Li GX, Chen XQ, Shen HJ, Chen XM. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113:474–481. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vespa E, Pugliese N, Piovani D, Capogreco A, Danese S, Aghemo A Humanitas Covid-19 Task Force. Liver tests abnormalities in COVID-19: trick or treat? J Hepatol. 2020;73:1275–1276. doi: 10.1016/j.jhep.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Antiga L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 27.Donato MF, Invernizzi F, Lampertico P, Rossi G. Health Status of Patients Who Underwent Liver Transplantation During the Coronavirus Outbreak at a Large Center in Milan, Italy. Clin Gastroenterol Hepatol. 2020;18:2131–2133.e1. doi: 10.1016/j.cgh.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galanopoulos M, Gkeros F, Doukatas A, Karianakis G, Pontas C, Tsoukalas N, Viazis N, Liatsos C, Mantzaris GJ. COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract. World J Gastroenterol. 2020;26:4579–4588. doi: 10.3748/wjg.v26.i31.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 36.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 37.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joung JY, Cho JH, Kim YH, Choi SH, Son CG. A literature review for the mechanisms of stress-induced liver injury. Brain Behav. 2019;9:e01235. doi: 10.1002/brb3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14:417–427. doi: 10.1038/s41581-018-0005-7. [DOI] [PubMed] [Google Scholar]
- 40.Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 41.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Li S, Xu M, Yu P, Zheng S, Duan Z, Liu J, Chen Y, Li J. Risk factors related to hepatic injury in patients with corona virus disease 2019. Available from: medRxiv 2020.
- 45.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 46.Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD, Timens W, Turner AJ, Navis G, van Goor H. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212:1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J Virol. 2019;93 doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 50.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, Yu J, Shan S, Zhou B, Song S, Tang X, Lan J, Yuan J, Wang H, Zhao J, Zhang S, Wang Y, Shi X, Liu L, Wang X, Zhang Z, Zhang L. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 55.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, Du C, Song Y, Wu C, Hu X, Sun Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. doi: 10.1038/s41392-020-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen R, Wang K, Yu J, Howard D, French L, Chen Z, Wen C, Xu Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front Neurol. 2020;11:573095. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. Available from: biorxiv 2020.
- 61.Guo A-X, Cui J-J, OuYang Q-Y, He L, Guo C-X, Yin J-Y. The clinical characteristics and mortal causes analysis of COVID-19 death patients. Available from: medRxiv 2020.
- 62.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seow JJW, Pai R, Mishra A, Shepherdson E, Lim TKH, Goh BK, Chan JK, Chow PK, Ginhoux F, DasGupta R. scRNA-seq reveals ACE2 and TMPRSS2 expression in TROP2+ Liver Progenitor Cells: Implications in COVID-19 associated Liver Dysfunction. Available from: bioRxiv 2020. [DOI] [PMC free article] [PubMed]
- 64.Banks RE, Forbes MA, Patel PM, Storr M, Hallam S, Clarke D, Novick D, Ingham E, Bowmer C, Southgate J, Trejdosiewicz LK, Illingworth J, Perren TJ, Selby PJ. Subcutaneous administration of recombinant glycosylated interleukin 6 in patients with cancer: pharmacokinetics, pharmacodynamics and immunomodulatory effects. Cytokine. 2000;12:388–396. doi: 10.1006/cyto.1999.0556. [DOI] [PubMed] [Google Scholar]
- 65.Weber J, Gunn H, Yang J, Parkinson D, Topalian S, Schwartzentruber D, Ettinghausen S, Levitt D, Rosenberg SA. A phase I trial of intravenous interleukin-6 in patients with advanced cancer. J Immunother Emphasis Tumor Immunol. 1994;15:292–302. doi: 10.1097/00002371-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Effenberger M, Grander C, Grabherr F, Griesmacher A, Ploner T, Hartig F, Bellmann-Weiler R, Joannidis M, Zoller H, Weiss G, Adolph TE, Tilg H. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis. 2021;53:158–165. doi: 10.1016/j.dld.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. 2020;20:277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Tan Y, Ling Y, Lu G, Liu F, Yi Z, Jia X, Wu M, Shi B, Xu S, Chen J, Wang W, Chen B, Jiang L, Yu S, Lu J, Wang J, Xu M, Yuan Z, Zhang Q, Zhang X, Zhao G, Wang S, Chen S, Lu H. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, Sun W, Li J, Chen L, Wang Y, Zhang L, Yu L. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. Available from: MedRxiv 2020.
- 75.Gholizadeh P, Safari R, Marofi P, Zeinalzadeh E, Pagliano P, Ganbarov K, Esposito S, Khodadadi E, Yousefi M, Samadi Kafil H. Alteration of Liver Biomarkers in Patients with SARS-CoV-2 (COVID-19) J Inflamm Res. 2020;13:285–292. doi: 10.2147/JIR.S257078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang , Lin M, Wei L, Xie L, Zhu G, Dela Cruz CS, Sharma L. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li L, Li S, Xu M, Zheng S, Duan Z, Chen Y, Li J. The level of plasma C-reactive protein is closely related to the liver injury in patients with COVID-19. Available from: medRxiv 2020.
- 78.Watanabe W, Sudo K, Asawa S, Konno K, Yokota T, Shigeta S. Use of lactate dehydrogenase to evaluate the anti-viral activity against influenza A virus. J Virol Methods. 1995;51:185–191. doi: 10.1016/0166-0934(94)00103-n. [DOI] [PubMed] [Google Scholar]
- 79.Uchide N, Ohyama K, Bessho T, Toyoda H. Lactate dehydrogenase leakage as a marker for apoptotic cell degradation induced by influenza virus infection in human fetal membrane cells. Intervirology. 2009;52:164–173. doi: 10.1159/000224644. [DOI] [PubMed] [Google Scholar]
- 80.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 81.Wu W, Shi D, Fang D, Guo F, Guo J, Huang F, Chen Y, Lv L, Li L. A new perspective on C-reactive protein in H7N9 infections. Int J Infect Dis. 2016;44:31–36. doi: 10.1016/j.ijid.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 82.Vasileva D, Badawi A. C-reactive protein as a biomarker of severe H1N1 influenza. Inflamm Res. 2019;68:39–46. doi: 10.1007/s00011-018-1188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, Ballard C. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13:217–231. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elmunzer BJ, Spitzer RL, Foster LD, Merchant AA, Howard EF, Patel VA, West MK, Qayed E, Nustas R, Zakaria A, Piper MS, Taylor JR, Jaza L, Forbes N, Chau M, Lara LF, Papachristou GI, Volk ML, Hilson LG, Zhou S, Kushnir VM, Lenyo AM, McLeod CG, Amin S, Kuftinec GN, Yadav D, Fox C, Kolb JM, Pawa S, Pawa R, Canakis A, Huang C, Jamil LH, Aneese AM, Glamour BK, Smith ZL, Hanley KA, Wood J, Patel HK, Shah JN, Agarunov E, Sethi A, Fogel EL, McNulty G, Haseeb A, Trieu JA, Dixon RE, Yang JY, Mendelsohn RB, Calo D, Aroniadis OC, LaComb JF, Scheiman JM, Sauer BG, Dang DT, Piraka CR, Shah ED, Pohl H, Tierney WM, Mitchell S, Condon A, Lenhart A, Dua KS, Kanagala VS, Kamal A, Singh VK, Pinto-Sanchez MI, Hutchinson JM, Kwon RS, Korsnes SJ, Singh H, Solati Z, Willingham FF, Yachimski PS, Conwell DL, Mosier E, Azab M, Patel A, Buxbaum J, Wani S, Chak A, Hosmer AE, Keswani RN, DiMaio CJ, Bronze MS, Muthusamy R, Canto MI, Gjeorgjievski VM, Imam Z, Odish F, Edhi AI, Orosey M, Tiwari A, Patwardhan S, Brown NG, Patel AA, Ordiah CO, Sloan IP, Cruz L, Koza CL, Okafor U, Hollander T, Furey N, Reykhart O, Zbib NH, Damianos JA, Esteban J, Hajidiacos N, Saul M, Mays M, Anderson G, Wood K, Mathews L, Diakova G, Caisse M, Wakefield L, Nitchie H, Waljee AK, Tang W, Zhang Y, Zhu J, Deshpande AR, Rockey DC, Alford TB, Durkalski V North American Alliance for the Study of Digestive Manifestations of COVID-19. Digestive Manifestations in Patients Hospitalized With Coronavirus Disease 2019. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology. 2020;158:2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M. Self-reported Olfactory and Taste Disorders in Patients With Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin Infect Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB AGA Institute. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020;159:320–334.e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo S, Zhang X, Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18:1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Papadopoulos N, Vasileiadi S, Deutsch M. COVID-19 and liver injury: where do we stand? Ann Gastroenterol. 2020;33:459–464. doi: 10.20524/aog.2020.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 94.Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, Tang C, Sang L, Liu J, Ni Z, Hu Y, Liu L, Shan H, Lei C, Peng Y, Wei L, Liu Y, Peng P, Wang J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Liu X, Cheng L, Ye F, Zheng J, Zhang N, Li Y, He J, Li S, Zhong N Medical Treatment Expert Group for COVID-19. Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest. 2020;158:97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.El Ouali S, Romero-Marrero C, Regueiro M. Hepatic manifestations of COVID-19. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc061. [DOI] [PubMed] [Google Scholar]
- 99.Parohan M, Yaghoubi S, Seraji A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;50:924–935. doi: 10.1111/hepr.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316–1320. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- 101.Gurala D, Al Moussawi H, Philipose J, Abergel JR. Acute Liver Failure in a COVID-19 Patient Without any Preexisting Liver Disease. Cureus. 2020;12:e10045. doi: 10.7759/cureus.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/MEG.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen VL, Hawa F, Berinstein JA, Reddy CA, Kassab I, Platt KD, Hsu CY, Steiner CA, Louissaint J, Gunaratnam NT, Sharma P. Hepatic Steatosis Is Associated with Increased Disease Severity and Liver Injury in Coronavirus Disease-19. Dig Dis Sci. 2020 doi: 10.1007/s10620-020-06618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768–771.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garland V, Kumar AB, Borum ML. Gastrointestinal and Hepatic Manifestations of COVID-19: Evolving Recognition and Need for Increased Understanding in Vulnerable Populations. J Natl Med Assoc . 2021;113:142–146. doi: 10.1016/j.jnma.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, González E, Polanco N, Folgueira MD, Lalueza A, Lumbreras C, Aguado JM. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nacif LS, Zanini LY, Waisberg DR, Pinheiro RS, Galvão F, Andraus W, D'Albuquerque LC. COVID-19 in solid organ transplantation patients: A systematic review. Clinics (Sao Paulo) 2020;75:e1983. doi: 10.6061/clinics/2020/e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Serviddio G, Villani R, Stallone G, Scioscia G, Foschino-Barbaro MP, Lacedonia D. Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol. 2020;13:1756284820959183. doi: 10.1177/1756284820959183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6:45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733–742. doi: 10.1136/gutjnl-2020-321726. [DOI] [PubMed] [Google Scholar]
- 116.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sulkowski MS, Mehta SH, Chaisson RE, Thomas DL, Moore RD. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS. 2004;18:2277–2284. doi: 10.1097/00002030-200411190-00008. [DOI] [PubMed] [Google Scholar]
- 118.Jiang S, Wang R, Li L, Hong D, Ru R, Rao Y, Miao J, Chen N, Wu X, Ye Z, Hu Y, Xie M, Zuo M, Lu X, Qiu Y, Liang T. Liver Injury in Critically Ill and Non-critically Ill COVID-19 Patients: A Multicenter, Retrospective, Observational Study. Front Med (Lausanne) 2020;7:347. doi: 10.3389/fmed.2020.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Storelli F, Samer C, Reny JL, Desmeules J, Daali Y. Complex Drug-Drug-Gene-Disease Interactions Involving Cytochromes P450: Systematic Review of Published Case Reports and Clinical Perspectives. Clin Pharmacokinet. 2018;57:1267–1293. doi: 10.1007/s40262-018-0650-9. [DOI] [PubMed] [Google Scholar]
- 120.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 121.Deftereos SG, Siasos G, Giannopoulos G, Vrachatis DA, Angelidis C, Giotaki SG, Gargalianos P, Giamarellou H, Gogos C, Daikos G, Lazanas M, Lagiou P, Saroglou G, Sipsas N, Tsiodras S, Chatzigeorgiou D, Moussas N, Kotanidou A, Koulouris N, Oikonomou E, Kaoukis A, Kossyvakis C, Raisakis K, Fountoulaki K, Comis M, Tsiachris D, Sarri E, Theodorakis A, Martinez-Dolz L, Sanz-Sánchez J, Reimers B, Stefanini GG, Cleman M, Filippou D, Olympios CD, Pyrgakis VN, Goudevenos J, Hahalis G, Kolettis TM, Iliodromitis E, Tousoulis D, Stefanadis C. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): Rationale and study design. Hellenic J Cardiol. 2020;61:42–45. doi: 10.1016/j.hjc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El-Ghiaty MA, Shoieb SM, El-Kadi AOS. Cytochrome P450-mediated drug interactions in COVID-19 patients: Current findings and possible mechanisms. Med Hypotheses. 2020;144:110033. doi: 10.1016/j.mehy.2020.110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, De Rosa R, Corcione A, Durante-Mangoni E. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14:881–883. doi: 10.1007/s12072-020-10077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Montastruc F, Thuriot S, Durrieu G. Hepatic Disorders With the Use of Remdesivir for Coronavirus 2019. Clin Gastroenterol Hepatol. 2020;18:2835–2836. doi: 10.1016/j.cgh.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753–4762. doi: 10.3748/wjg.v26.i32.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sodhi M, Etminan M. Therapeutic Potential for Tetracyclines in the Treatment of COVID-19. Pharmacotherapy. 2020;40:487–488. doi: 10.1002/phar.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heaton PC, Fenwick SR, Brewer DE. Association between tetracycline or doxycycline and hepatotoxicity: a population based case-control study. J Clin Pharm Ther. 2007;32:483–487. doi: 10.1111/j.1365-2710.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 130.Menzel M, Akbarshahi H, Tufvesson E, Persson C, Bjermer L, Uller L. Azithromycin augments rhinovirus-induced IFNβ via cytosolic MDA5 in experimental models of asthma exacerbation. Oncotarget. 2017;8:31601–31611. doi: 10.18632/oncotarget.16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, Lyons K, Schweiger TL, Zheng J, Schechtman KB, Castro M, Bacharier LB. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 Levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135:1171–1178.e1. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bleyzac N, Goutelle S, Bourguignon L, Tod M. Azithromycin for COVID-19: More Than Just an Antimicrobial? Clin Drug Investig. 2020;40:683–686. doi: 10.1007/s40261-020-00933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hanafy AS, Abd-Elsalam S. Challenges in COVID-19 drug treatment in patients with advanced liver diseases: A hepatology perspective. World J Gastroenterol. 2020;26:7272–7286. doi: 10.3748/wjg.v26.i46.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Di Giambenedetto S, Ciccullo A, Borghetti A, Gambassi G, Landi F, Visconti E, Zileri Dal Verme L, Bernabei R, Tamburrini E, Cauda R, Gasbarrini A. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J Med Virol. 2020;92:1787–1788. doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muhović D, Bojović J, Bulatović A, Vukčević B, Ratković M, Lazović R, Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40:1901–1905. doi: 10.1111/liv.14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang J, Zhou L, Yang Y, Peng W, Wang W, Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ibrahim H, Perl A, Smith D, Lewis T, Kon Z, Goldenberg R, Yarta K, Staniloae C, Williams M. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol. 2020;219:108544. doi: 10.1016/j.clim.2020.108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cao J, Cai X, Chen M. Liver Injury in COVID-19: Caution and Management. Liver Cancer. 2020;9:625–626. doi: 10.1159/000508696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qin J, Wang H, Qin X, Zhang P, Zhu L, Cai J, Yuan Y, Li H. Perioperative Presentation of COVID-19 Disease in a Liver Transplant Recipient. Hepatology. 2020;72:1491–1493. doi: 10.1002/hep.31257. [DOI] [PubMed] [Google Scholar]
- 140.Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang XH. for the Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM) A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cortegiani A, Ippolito M, Greco M, Granone V, Protti A, Gregoretti C, Giarratano A, Einav S, Cecconi M. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2021;27:52–66. doi: 10.1016/j.pulmoe.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chaudhry D, Singh PK. Tocilizumab and COVID-19. Indian J Crit Care Med. 2020;24:741–743. doi: 10.5005/jp-journals-10071-23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fang S, Qi L, Zhou N, Li C. Case report on alimentary tract hemorrhage and liver injury after therapy with oseltamivir: A case report. Medicine (Baltimore) 2018;97:e12497. doi: 10.1097/MD.0000000000012497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ye Y, Wang H, Liu J, Zhao F, Xu P. Polygalasaponin F treats mice with pneumonia induced by influenza virus. Inflammopharmacology. 2020;28:299–310. doi: 10.1007/s10787-019-00633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, Peiris M, Yen HL. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marima R, Hull R, Dlamini Z, Penny C. The dual protease inhibitor lopinavir/ritonavir (LPV/r) exerts genotoxic stress on lung cells. Biomed Pharmacother. 2020;132:110829. doi: 10.1016/j.biopha.2020.110829. [DOI] [PubMed] [Google Scholar]
- 149.Khalatbari A, Mishra P, Han H, He Y, MacVeigh-Aloni M, Ji C. Ritonavir and Lopinavir Suppress RCE1 and CAAX Rab Proteins Sensitizing the Liver to Organelle Stress and Injury. Hepatol Commun. 2020;4:932–944. doi: 10.1002/hep4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hensel A, Bauer R, Heinrich M, Spiegler V, Kayser O, Hempel G, Kraft K. Challenges at the Time of COVID-19: Opportunities and Innovations in Antivirals from Nature. Planta Med. 2020;86:659–664. doi: 10.1055/a-1177-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luo Y, Zhang B, Xu DQ, Liu Y, Dong MQ, Zhao PT, Li ZC. Protective effect of bicyclol on lipopolysaccharide-induced acute lung injury in mice. Pulm Pharmacol Ther. 2011;24:240–246. doi: 10.1016/j.pupt.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 152.Hofmann WP, Herrmann E, Sarrazin C, Zeuzem S. Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int. 2008;28:1332–1343. doi: 10.1111/j.1478-3231.2008.01896.x. [DOI] [PubMed] [Google Scholar]
- 153.Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, Almakhlafi GA, Albarrak MM, Memish ZA, Albarrak AM. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scavone C, Brusco S, Bertini M, Sportiello L, Rafaniello C, Zoccoli A, Berrino L, Racagni G, Rossi F, Capuano A. Current pharmacological treatments for COVID-19: What's next? Br J Pharmacol. 2020;177:4813–4824. doi: 10.1111/bph.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kaptein SJF, Jacobs S, Langendries L, Seldeslachts L, Ter Horst S, Liesenborghs L, Hens B, Vergote V, Heylen E, Barthelemy K, Maas E, De Keyzer C, Bervoets L, Rymenants J, Van Buyten T, Zhang X, Abdelnabi R, Pang J, Williams R, Thibaut HJ, Dallmeier K, Boudewijns R, Wouters J, Augustijns P, Verougstraete N, Cawthorne C, Breuer J, Solas C, Weynand B, Annaert P, Spriet I, Vande Velde G, Neyts J, Rocha-Pereira J, Delang L. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci USA. 2020;117:26955–26965. doi: 10.1073/pnas.2014441117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lam S, Lombardi A, Ouanounou A. COVID-19: A review of the proposed pharmacological treatments. Eur J Pharmacol. 2020;886:173451. doi: 10.1016/j.ejphar.2020.173451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dai C, Xiao X, Li D, Tun S, Wang Y, Velkov T, Tang S. Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death Dis. 2018;9:1164. doi: 10.1038/s41419-018-1136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shen H, Wu N, Wang Y, Zhao H, Zhang L, Li T, Zhao M. Chloroquine attenuates paraquat-induced lung injury in mice by altering inflammation, oxidative stress and fibrosis. Int Immunopharmacol. 2017;46:16–22. doi: 10.1016/j.intimp.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 159.Eguchi K, Fujitani N, Nakagawa H, Miyazaki T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci Rep. 2019;9:4812. doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol. 2018;48:39–49. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.De Flora S, Balansky R, La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020;34:13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Onk D, Özçelik F, Onk OA, Günay M, Akarsu Ayazoğlu T, Ünver E. Assessment of Renal and Hepatic Tissue-Protective Effects of N-Acetylcysteine via Ammonia Metabolism: A Prospective Randomized Study. Med Sci Monit. 2018;24:1540–1546. doi: 10.12659/MSM.908172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bailly C, Vergoten G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol Ther. 2020;214:107618. doi: 10.1016/j.pharmthera.2020.107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.From the American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE) Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR) European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS) and World Stroke Organization (WSO) Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, Shazam Hussain M, Jansen O, Jayaraman MV, Khalessi AA, Kluck BW, Lavine S, Meyers PM, Ramee S, Rüfenacht DA, Schirmer CM, Vorwerk D. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J Stroke. 2018;13:612–632. doi: 10.1016/j.jvir.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 166.Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, Holford P, Thornton CA, Whitaker IS. Could Vitamins Help in the Fight Against COVID-19? Nutrients. 2020;12 doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nagashimada M, Ota T. Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life. 2019;71:516–522. doi: 10.1002/iub.1991. [DOI] [PubMed] [Google Scholar]
- 168.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12 doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Udomsinprasert W, Jittikoon J. Vitamin D and liver fibrosis: Molecular mechanisms and clinical studies. Biomed Pharmacother. 2019;109:1351–1360. doi: 10.1016/j.biopha.2018.10.140. [DOI] [PubMed] [Google Scholar]
- 170.Su M, Chen H, Wei C, Chen N, Wu W. Potential protection of vitamin C against liver-lesioned mice. Int Immunopharmacol. 2014;22:492–497. doi: 10.1016/j.intimp.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 171.Pal A, Squitti R, Picozza M, Pawar A, Rongioletti M, Dutta AK, Sahoo S, Goswami K, Sharma P, Prasad R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol Trace Elem Res. 2020 doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract. 2012;27:8–20. doi: 10.1177/0884533611433534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tang CF, Ding H, Jiao RQ, Wu XX, Kong LD. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol. 2020;886:173546. doi: 10.1016/j.ejphar.2020.173546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu M, Yang H, Mao Y. Magnesium and liver disease. Ann Transl Med. 2019;7:578. doi: 10.21037/atm.2019.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Andreou A, Trantza S, Filippou D, Sipsas N, Tsiodras S. COVID-19: The Potential Role of Copper and N-acetylcysteine (NAC) in a Combination of Candidate Antiviral Treatments Against SARS-CoV-2. In Vivo. 2020;34:1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Raha S, Mallick R, Basak S, Duttaroy AK. Is copper beneficial for COVID-19 patients? Med Hypotheses. 2020;142:109814. doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eken H, Ozturk H, Buyukbayram H. Dose-related effects of dexamethasone on liver damage due to bile duct ligation in rats. World J Gastroenterol. 2006;12:5379–5383. doi: 10.3748/wjg.v12.i33.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mokra D, Mikolka P, Kosutova P, Mokry J. Corticosteroids in Acute Lung Injury: The Dilemma Continues. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20194765. [DOI] [PMC free article] [PubMed] [Google Scholar]