Abstract
BACKGROUND
In recent years, neoadjuvant chemotherapy (NAC) has been increasingly used in patients with resectable colorectal liver metastases. However, the efficacy and safety of NAC in the treatment of resectable colorectal liver metastases (CRLM) are still controversial.
AIM
To assess the efficacy and application value of NAC in patients with resectable CRLM.
METHODS
We searched PubMed, Embase, Web of Science, and the Cochrane Library from inception to December 2020 to collect clinical studies comparing NAC with non-NAC. Data processing and statistical analyses were performed using Stata V.15.0 and Review Manager 5.0 software.
RESULTS
In total, 32 studies involving 11236 patients were included in this analysis. We divided the patients into two groups, the NAC group (that received neoadjuvant chemotherapy) and the non-NAC group (that received no neoadjuvant chemotherapy). The meta-analysis outcome showed a statistically significant difference in the 5-year overall survival and 5-year disease-free survival between the two groups. The hazard ratio (HR) and 95% confidence interval (CI) were HR = 0.49, 95%CI: 0.39-0.61, P = 0.000 and HR = 0.48 95%CI: 0.36-0.63, P = 0.000. The duration of surgery in the NAC group was longer than that of the non-NAC group [standardized mean difference (SMD) = 0.41, 95%CI: 0.01-0.82, P = 0.044)]. The meta-analysis showed that the number of liver metastases in the NAC group was significantly higher than that in the non-NAC group (SMD = 0.73, 95%CI: 0.02-1.43, P = 0.043). The lymph node metastasis in the NAC group was significantly higher than that in the non-NAC group (SMD = 1.24, 95%CI: 1.07-1.43, P = 0.004).
CONCLUSION
We found that NAC could improve the long-term prognosis of patients with resectable CRLM. At the same time, the NAC group did not increase the risk of any adverse event compared to the non-NAC group.
Keywords: Colorectal neoplasm, Neoadjuvant chemotherapy, Systematic review, Randomized controlled trials, Meta-analysis, Colorectal liver metastases
Core Tip: Although hepatectomy is currently recommended as the most reliable treatment for colorectal liver metastasis, there are still a great number of patients who have recurrences and metastases after surgical resection. In recent years, neoadjuvant chemotherapy (NAC) has been increasingly used in patients with resectable colorectal liver metastases (CRLM). However, the efficacy and safety of NAC in the treatment of CRLM are still controversial. Therefore, we conducted a systematic review and meta-analysis to assess the value of NAC in patients with CRLM.
INTRODUCTION
Colorectal cancer (CRC), one of the most common malignant tumors in Japan[1], is also the leading cause of death among cancer patients in Europe and the United States[2]. A report[3] showed that colorectal cancer ranked third among solid cancers in men and second among women, which explained why it is one of the most common malignancies in the world. Every year, many people are diagnosed with colorectal cancer, and the number grows every year. Compared to other organs, colorectal cancer seems more likely to metastasize to the liver. As the largest substantive organ in the human body, the importance of the liver is evident. Therefore, many deaths of patients with colorectal cancer are caused by liver metastasis[2,4,5].
Hepatectomy is currently recommended as the most reliable treatment for colorectal liver metastasis, and hepatic resection can provide significant long-term benefit with 5-year survival rates approaching 50% in many reports[6-9]. However, only 10%-20% of those patients have the opportunity to undergo surgical resection of metastatic colorectal cancer (CRLM) as more than 80% of the patients are not suitable for liver resection because of advanced disease at the time of diagnosis[10-12]. Although hepatectomy remains the only treatment that can ensure prolonged survival[13], there are still a great number of patients who have recurrences and metastases after surgical resection[14]. Many studies have reported that more than half of patients experience a recurrence after hepatectomy[15-17].
In recent years, neoadjuvant chemotherapy (NAC) has been highly effective, and response rates of 50%-80% have been reported[18-20]. Modern systemic chemotherapy has been widely used to increase the cure rate of patients with resectable tumors and to transform some unresectable metastases to enable surgery[21-23]. However, NAC does not show an overall survival benefit for patients with resectable CRLM, and a subset of patients experience disease progression during treatment[10,13,24]. In recent years, some studies have reported that NAC had no significant survival benefit for patients with resectable CRLM[25-27]. At the same time, NAC has also attracted extensive attention for its potential damage to the liver[28,29], and it remains unclear whether the presence of chemotherapy-induced liver injury or impaired liver functional reserve affects the long-term outcomes. Thus, the efficacy and safety of NAC in patients with resectable colorectal cancer liver metastasis remain controversial. Therefore, the purpose of this meta-analysis was to evaluate the application value of NAC in patients with colorectal cancer with liver metastases.
MATERIALS AND METHODS
Search strategy
Up to December 2020, four major databases including PubMed, Embase, Web of Science, and the Cochrane Library were searched. The study was designed and conducted in accordance with the standardized Systematic Reviews and Meta-Analyses (PRISMA) guidelines[30] and PRISMA-P guidelines[31]. We used the following keywords in the retrieval process ”Colorectal Neoplasm”, ”Neoplasms, Colorectal”, “Colorectal Tumor”, “Neoadjuvant Chemotherapies”, ”Neoadjuvant Therapies”, “Neoadjuvant Chemotherapy”, ”Neoadjuvant chemotherapy”, and ” Colorectal liver metastases”, “Colonic liver metastases”, “Rectal liver metastases”. The search for PubMed strategy is provided in the Supplementary Table 1.
Inclusion and exclusion criteria
Two investigators (Zhang Y and Ge L) independently reviewed the title and abstract of the included studies, using the inclusion and exclusion criteria. The Inclusion criteria were: (1) Patients with colorectal cancer with liver metastasis confirmed by computed tomography imaging and pathology; (2) Reports with at least one of the outcome measures below; (3) Studies in which patients with extrahepatic metastases were excluded; and (4) Study designs including clinical, randomized controlled trials (RCTs) or observational studies. The exclusion criteria were: (1) Patients with extrahepatic metastases; (2) Patients with preoperative evaluations indicating non-resectable tumors; and (3) Document types including reviews, meta-analyses, letters, case reports, conference abstracts, or duplicate publications.
Data extraction
Two investigators (Zhang Y and Ge L) extracted the baseline characteristics, major outcome indicators, and secondary outcome indicators from the included and consistent studies. The baseline characteristics included the name of the first author of the included study, country, type of study, and general characteristics of the patients in each group. The major outcome indicators included survival outcomes, including 5-year overall survival (OS) and 5-year disease-free survival (DFS). The secondary outcome indicators included the duration of surgery, blood loss, the length of hospital stay, the number of liver metastases, the size of the largest metastasis, synchronous liver metastases, perioperative complications, bile leakage, surgical site infection, liver failure, blood transfusions, major liver resection, lymph node metastasis, and R0 liver resection. The outcome indicators in this meta-analysis are presented in detail in the results section.
Quality assessment
We used the Cochrane risk of bias tool for RCTs[32] and the Newcastlee Ottawa Scale (NOS)[33] criterion for cohort studies to assess the quality of the included studies. Any discrepancy between the two reviewers was resolved by discussion and mutual agreement. If necessary, disagreements were resolved by discussion and consultation with the third researcher (Ma SX).
Statistical analysis
Stata version 15.0 (Stata Corporation, College Station, TX, United States) and Review Manager 5.0 (Cochrane Collaboration's Information Management System) software were used for the statistical analyses. The odds ratio (OR) and 95% confidence interval (CI) were employed to analyze the dichotomous variables, such as adverse event outcomes and synchronous metastasis. Meanwhile, the standardized mean difference (SMD) with a 95%CI was used to analyze the continuous variables, such as the duration of surgery and blood loss. In addition, the hazard ratio (HR) was used as a summary statistical measure of survival outcome (5-year OS and 5-year DFS). We used Cochran's Q test and I2to evaluate heterogeneity between the studies. An I2of greater than 50% was considered to indicate significant heterogeneity. In this case, a random-effects model and sensitivity analysis or subgroup analysis were needed to analyze the source of the heterogeneity.
Possible publication bias was assessed using funnel plots, Egger's, and Begg's tests. All statistical values were calculated by the 95%CI, and a P value of less than 0.05 was considered statistically significant.
RESULTS
Studies retrieved and characteristics
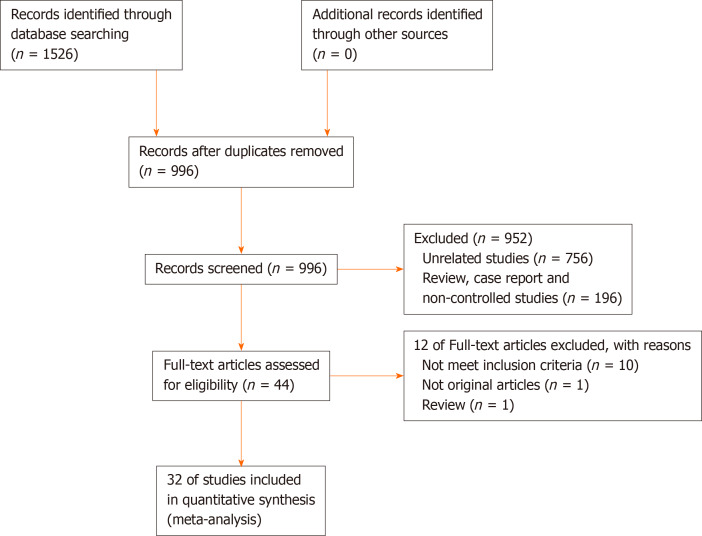
A flow diagram of the study selection is shown in Figure 1, according to PRISMA. Initially, a total of 1526 potentially eligible studies were identified, and then repeated studies, case reports, meeting abstracts, reviews, meta-analyses, and other unrelated studies were excluded. Finally, 32 studies were included in this meta-analysis, which involved 11236 patients (NAC group = 4791; non-NAC group = 6445). The study included 31 retrospective cohort studies[1,3,6-9,34-58] and one RCT[59], in which NAC was compared with non-NAC for patients who underwent surgery for the treatment of CRLM.
Figure 1.
Study identification and selection flow.
The characteristics of the included studies and the summary results of the NOS scores are shown in Table 1. Quality evaluation of all observational studies was conducted using the NOS scale, and the scores ranged from six to nine stars. In general, studies with a score of 6 were considered of high quality. The quality evaluation of the one RCT is presented in Figure 2, which showed that the overall quality of the one RCT was good. The meta-analysis results are shown in Table 2.
Table 1.
Baseline characteristics of the included studies
|
Ref.
|
|
Study design
|
Patients (n)
|
Age (yr) mean ± SD/mean
|
Gender (M/F)
|
Clinical T stage
|
Synchronous/metachronous
|
Tumor location
|
||||
|
Neo chemo
|
No neo chemo
|
Neo chemo
|
No neo chemo
|
Neo chemo
|
No neo chemo
|
Neo chemo
|
No neo chemo
|
|||||
| Allen et al[58], 2003 | 2003 | Retrospective | 52 | 54 | 59 | 63 | 33/19 | 28/26 | T1/T2/T3/T4 | 52/0 | 54/0 | NR |
| Tanaka et al[57], 2003 | 2003 | Prospective | 48 | 23 | 57 (38-69) | 59 (37-79) | 28/20 | 17/6 | NR | 33/15 | 18/5 | a.b |
| Aloia et al[56], 2006 | 2006 | Retrospective | 75 | 17 | 57 ± 12 | 60 ± 11.9 | 46/29 | 8/9 | NR | NR | NR | NR |
| Hewes et al[54], 2007 | 2007 | Retrospective | 80 | 21 | NR | NR | 40/40 | 11/10 | NR | 25/55 | 7/14 | NR |
| Aloysius et al[55], 2007 | 2007 | Prospective | 79 | 25 | 65 (61-72) | 64 (60-70) | 49/30 | 9/16 | NR | NR | NR | NR |
| Mehta et al[53], 2008 | 2008 | Retrospective | 130 | 43 | NR | NR | NR | NR | NR | 51/79 | 17/26 | NR |
| Tamandl et al[49], 2009 | 2009 | Retrospective | 29 | 41 | 75 ± 3 | 75 ± 3 | 16/13 | 28/13 | T1/T2/T3/T4 | NR | NR | NR |
| Boostrom et al[52], 2009 | 2009 | Retrospective | 44 | 55 | 64 | 57.5 | 28/16 | 30/25 | NR | 24/20 | 24/31 | NR |
| Lubezky et al[51], 2009 | 2009 | Prospective | 37 | 19 | 63 | 66 | NR | NR | NR | NR | NR | a.b |
| Scoggins et al[50], 2009 | 2009 | Retrospective | 112 | 74 | 59 | 68.5 | 67/45 | 38/36 | T1/T2/T3/T4 | 19/93 | 9/65 | a.b.c |
| Adam et al[48], 2010 | 2010 | Prospective | 169 | 1302 | NR | NR | 100/69 | 831/471 | NR | NR | NR | a.b.c |
| Scartozzi et al[47], 2011 | 2011 | Prospective | 60 | 44 | NR | NR | 23/37 | 23/31 | T1/T2/T3/T4 | NR | NR | NR |
| Son et al[46], 2011 | 2011 | Retrospective | 20 | 206 | NR | NR | 15/5 | 134/72 | T1/T2/T3/T4 | 12/8 | 126/80 | a.b |
| Cucchetti et al[45], 2011 | 2011 | Prospective | 125 | 117 | 63.9 ±1 0.4 | 64.9 ± 9.8 | 27/20 | 27/20 | T1/T2/T3/T4 | 19/28 | 19/28 | NR |
| Spelt et al[43], 2012 | 2012 | Retrospective | 97 | 136 | 64 (33-90) | 66.5 (30-88) | 61/36 | 81/55 | T1/T2/T3/T4 | 65/97 | 70/66 | NR |
| Pinto et al[44], 2012 | 2012 | Retrospective | 205 | 205 | 58.9 ± 12 | 61.9 ± 12 | 128/77 | 144/61 | T1/T2/T3/T4 | 123/82 | 105/100 | NR |
| Nordlinger et al[59], 2013 | 2013 | RCT | 182 | 182 | 60.7 (9.35) | 62.4 (9.63) | 127/54 | 114/65 | T1/T2/T3/T4 | 61/121 | 67/115 | a.b.c |
| Araujo et al[42], 2013 | 2013 | Retrospective | 175 | 236 | 54.8 (47.5-62.3) | 60.9 (51.1-67.6) | 103/72 | 148/88 | NR | NR | NR | a.b |
| Oh et al[41], 2013 | 2013 | Prospective | 15 | 15 | 54 | 63 | 12/3 | 11/4 | T2/T3/T4 | NR | NR | a.b |
| Scilletta et al[40], 2014 | 2014 | Retrospective | 52 | 129 | 64 ± 13 | 63 ± 9 | 29/23 | 74/55 | NR | NR | NR | a.b.c |
| Zhu et al[39], 2014 | 2014 | Retrospective | 121 | 345 | 58.0 (35-72) | 59.0 (28-84) | 81/40 | 213/142 | T1/T2/T3/T4 | NR | NR | a.b |
| Bonney et al[37], 2015 | 2015 | Retrospective | 693 | 608 | NR | NR | 418/275 | 370/238 | NR | 693 | 608 | NR |
| Schreckenbach et al[36], 2015 | 2015 | Retrospective | 117 | 71 | 61 (35–81) | 69 (34-85) | 86/31 | 74/26 | NR | 87/30 | 26/45 | a. |
| Ayez et al[38], 2015 | 2015 | Retrospective | 65 | 154 | 63 (58-70) | 66 (59-72) | 47/18 | 95/59 | NR | 55/10 | 133/21 | NR |
| Kim et al[6], 2017 | 2017 | Retrospective | 32 | 32 | 59 ± 10 | 59 ± 8 | 23/9 | 22/10 | T2/T3/T4 | NR | NR | a.b. |
| Strowitzki et al[3], 2017 | 2017 | Prospective | 125 | 125 | NR | NR | NR | NR | NR | 69/56 | 69/56 | a.b.c. |
| Inoue et al[7], 2018 | 2018 | Retrospective | 61 | 61 | 66 (33-89) | 63 (41-85) | 31/30 | 32/29 | NR | 30/31 | 30/31 | NR |
| Kumar et al[8], 2018 | 2018 | Prospective | 176 | 271 | 62 (30-82) | 63 (29-86) | 105/71 | 168/103 | NR | NR | NR | NR |
| Makowiec et al[9], 2018 | 2018 | Retrospective | 106 | 228 | 64 (25-80) | 64 (33-87) | 64/42 | 158/70 | NR | 68/38 | 94/134 | a.b |
| Hirokawa et al[1], 2019 | 2019 | Prospective | 20 | 117 | 67 (28-76) | 68 (38-89) | 13/7 | 70/47 | T1/T2/T3/T4 | 6/14 | 36/81 | a.b |
| Ratti et al[35], 2019 | 2019 | Retrospective | 73 | 73 | 62 (37-84) | 60 (35-86) | 39/34 | 41/32 | T1/T2/T3/T4 | 73/0 | 73/0 | a.b |
| Wiseman et al[34], 2019 | 2019 | Retrospective | 1416 | 1416 | 60 ± 7 | 61 ± 12 | 836/580 | 803/613 | NR | NR | NR | NR |
M: Male; F: Female; NAC group: Neoadjuvant chemotherapy group; non-NAC group: No neoadjuvant chemotherapy group; NR: Not reported; a: Colon; b: Rectum; c: Other.
Figure 2.
Quality assessment of 1 randomized controlled trial. A: Risk of bias graph; B: Risk of bias summary.
Table 2.
Meta-analysis results
|
Outcome indicators
|
No. of study
|
Patients (n)
|
HR/OR/SMD (95%CI)
|
P
value
|
Heterogeneity
|
||||
|
NAC
|
non-NAC
|
χ
2
|
I²
|
P
value
|
|||||
| 5-year overall survival | 22 | 2580 | 4218 | 0.49 (0.39-0.61) | P < 0.01 | 14.38 | 0.00% | P = 0.853 | |
| 5-year disease free survival | 13 | 1643 | 2864 | 0.48 (0.36-0.63) | P < 0.01 | 5.68 | 0.00% | P = 0.931 | |
| Duration of surgery | 8 | 1980 | 2396 | 0.41 (0.01-0.82) | P < 0.05 | 172.79 | 95.90% | P = 0.000 | |
| Number of liver metastases | 12 | 1017 | 1096 | 0.73 (0.02-1.43) | P < 0.05 | 549.46 | 98.00% | P = 0.000 | |
| Blood loss during surgery | 6 | 474 | 646 | 0.53 (-0.05-1.10) | P = 0.072 | 90.12 | 94.50% | P = 0.000 | |
| Length of hospital stay (d) | 6 | 605 | 565 | 0.01 (-0.61-1.02) | P = 0.624 | 184.79 | 97.30% | P = 0.000 | |
| Size of largest metastases (cm) | 13 | 1226 | 1539 | 0.03 (-0.31-0.30) | P = 0.980 | 168.39 | 92.90% | P = 0.000 | |
| Synchronous metastases | 19 | 2355 | 2553 | 1.17 (0.90-1.58) | P = 0.221 | 43.91 | 65.80% | P = 0.000 | |
| Major liver resection | 14 | 2780 | 3133 | 1.06 (0.97-1.22) | P = 0.143 | 5.21 | 0.00% | P = 0.970 | |
| Lymph node metastasis | 16 | 1523 | 2128 | 1.24 (1.07-1.43) | P < 0.05 | 29.26 | 49.50% | P = 0.013 | |
| R0 liver resection | 7 | 723 | 976 | 0.85 (0.61-1.18) | P = 0.336 | 6.29 | 4.60% | P = 0.391 | |
| Perioperative complications | 17 | 2886 | 3161 | 1.00 (0.76-1.31) | P = 0.980 | 51.82 | 69.10% | P = 0.000 | |
| Bile leakage | 10 | 2244 | 2364 | 1.10 (0.84-1.43) | P = 0.481 | 4.77 | 0.00% | P = 0.782 | |
| Surgical site infection | 8 | 2065 | 2056 | 0.94 (0.76-1.16) | P = 0.571 | 9.68 | 27.70% | P = 0.208 | |
| Liver failure | 7 | 2025 | 1939 | 1.04 (0.76-1.42) | P = 0.813 | 5.77 | 13.40% | P = 0.329 | |
| Blood transfusion | 5 | 1805 | 1710 | 1.07 (0.90-1.29) | P = 0.438 | 5.95 | 32.80% | P = 0.203 | |
NAC: Neoadjuvant chemotherapy group; non-NAC: No neoadjuvant chemotherapy group; HR: Hazard ratio; OR: Odds ratio; SMD: Standard mean difference.
Survival results
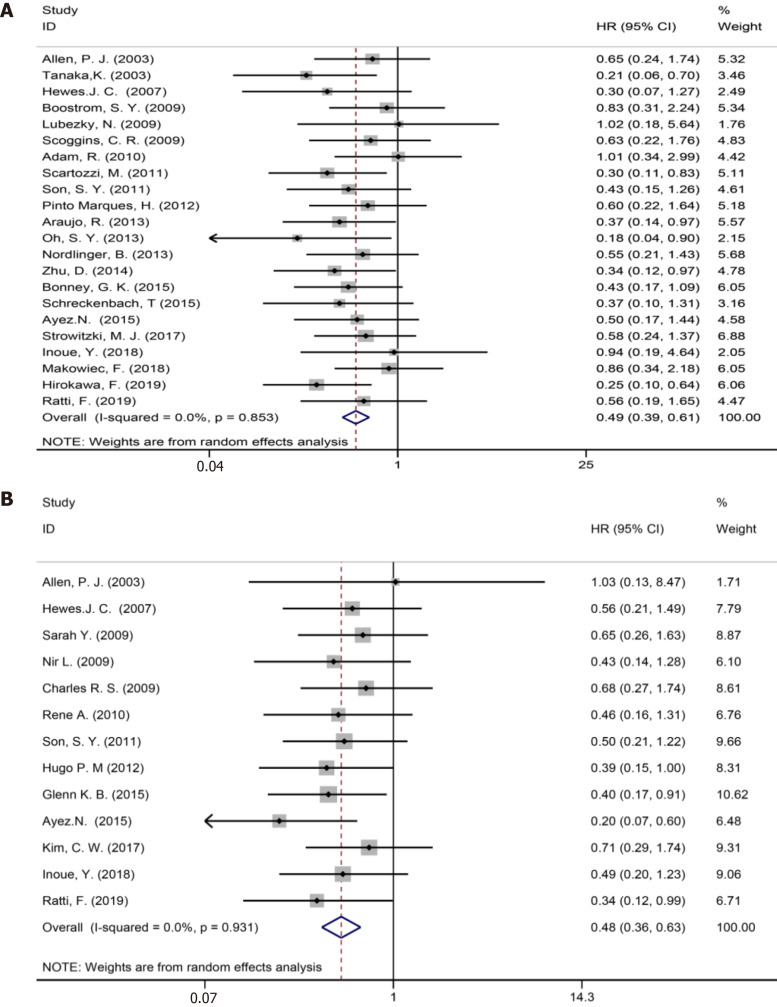
Twenty two studies[1,3,7,9,35-39,41,42,44,47-52,54,57-59] reported 5-year OS (Figure 3A). The results of the meta-analysis showed a significant survival benefit in the NAC group (HR = 0.49, 95%CI: 0.39-0.61, P = 0.000, I2= 0.0%).
Figure 3.
Result of 5-year overall survival and disease-free survival. A: 5-year overall survival for all patients and two groups; B: 5-year disease-free survival for all patients and two groups.
Thirteen included studies[6,7,35,37,38,44,46,48,50-52,54,58] reported 5-year DFS (Figure 3B). The results of the meta-analysis showed a significant survival benefit in the NAC group (HR = 0.48, 95%CI: 0.36-0.63, P = 0.000, I2= 0.0%). Compared to the non-NAC groups, there were significant DFS benefits in the NAC groups.
Perioperative results
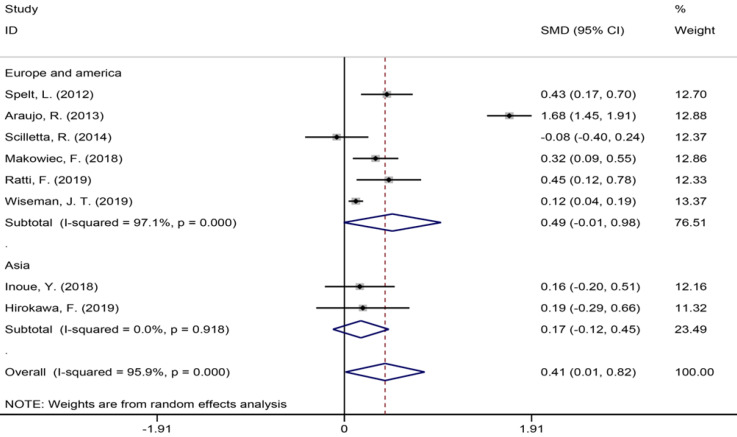
Eight studies[1,7,9,34,35,40,42,43] with 4396 patients assessing the duration of surgery showed an increase in surgery duration (Figure 4) in the NAC group (SMD = 0.41, 95%CI: 0.01-0.82, P = 0.044, I2= 95.9%). The meta-analysis of Europe and America studies (SMD = 0.49, 95%CI: -0.01-0.98, P = 0.054) and Asia studies (SMD = 0.17, 95%CI: -0.12-0.45, P = 0.247). The results showed no heterogeneity in the subgroup of Asia studies (χ2 = 0.01, I2= 0.0%, P = 0.918).
Figure 4.
Duration of operation for all patients and by study region subgroups.
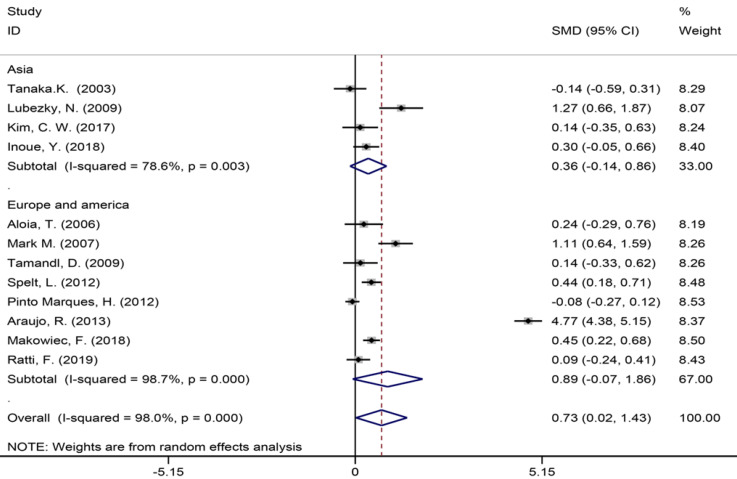
Twelve of the 32 included studies[6,7,9,35,42-44,49,51,55-57] assessing the number of liver metastases (Figure 5) showed a significant statistical difference between the two groups (SMD = 0.73, 95%CI: 0.02-1.43, P = 0.043, I2= 98.0%), indicating that there were more liver metastases in the patients in the NAC group. The meta-analysis of Europe and America studies (SMD = 0.89, 95%CI: -0.07-1.86, P = 0.069) and Asia studies (SMD = 0.36, 95%CI: -0.14-0.86, P = 0.159). High heterogeneity was showed in the subgroup of Asia studies (χ2 = 14.03, I2= 78.6%, P = 0.003).
Figure 5.
Number of liver metastases for all patients and by study region subgroups.
Sixteen of 32 included studies[6,8,35,36,38,39,41-44,46,50-52,58,59] assessing the lymph node metastasis (Figure 6) showed a significant statistical difference between the two groups (SMD = 1.24, 95%CI: 1.07-1.43, P = 0.004, I2= 49.5%), indicating that there were more lymph node metastasis in the patients in the NAC group.
Figure 6.
Lymph node metastasis for all patients and two groups.
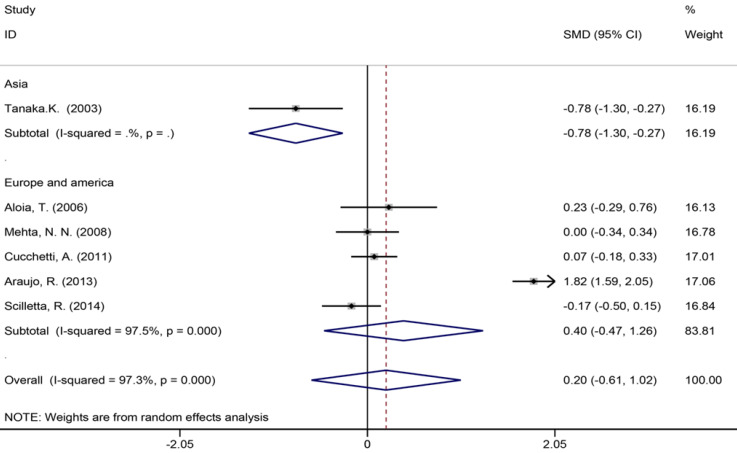
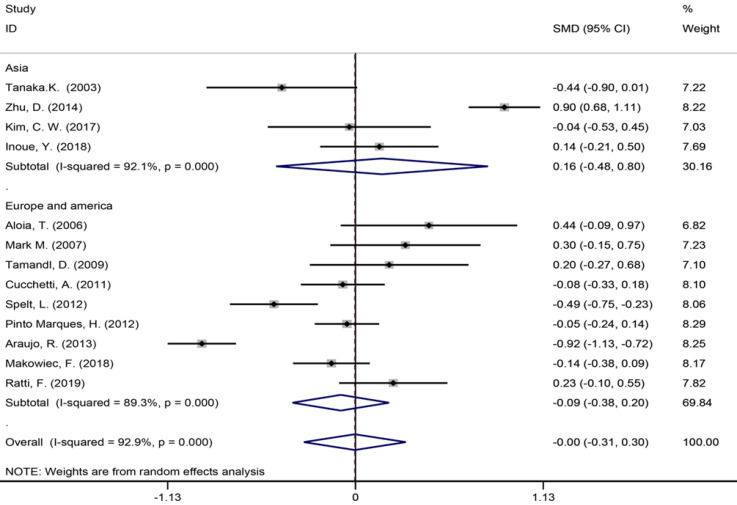
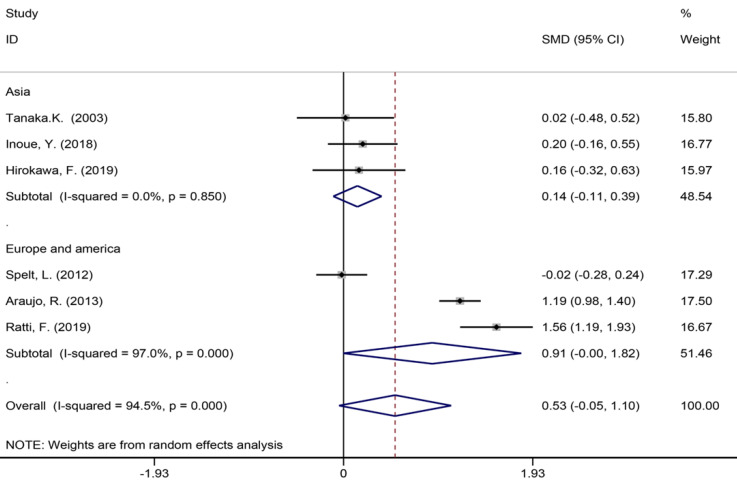
Six studies[40,42,45,53,56,57] reported the length of hospital stay (Figure 7), 13 studies[6,7,9,35,39,42-45,49,55-57] reported the size of the largest metastasis (Figure 8), and six studies[1,7,35,42,43,57] reported blood loss during surgery (Figure 9). The results of the meta–analysis showed no significant statistical difference between these three indicators in the two groups (SMD = 0.20, 95%CI: -0.61-1.02, P = 0.624, I² = 97.3%; SMD = -0.00, 95%CI: -0.31-0.30, P = 0.980, I² = 92.9%; SMD = 0.53, 95%CI: -0.05-1.10 P = 0.072, I² = 94.5%). The results showed that the length of hospital stay in the European and American study subgroup was highly heterogeneous (χ2 = 158.33, I2 = 97.5%, P = 0.000). The size of the largest metastasis in the Asian study subgroup was highly heterogeneous (χ2 = 38, I2= 92.1%, P = 0.000). There was no heterogeneity in blood loss in the Asian study subgroup (χ2 = 0.33, I2= 0.0%, P = 0.850).
Figure 7.
Length of hospital stay and by study region subgroups.
Figure 8.
Size of the largest metastasis for all patients and by study region subgroups.
Figure 9.
Blood loss during surgery for all patients and by study region subgroups.
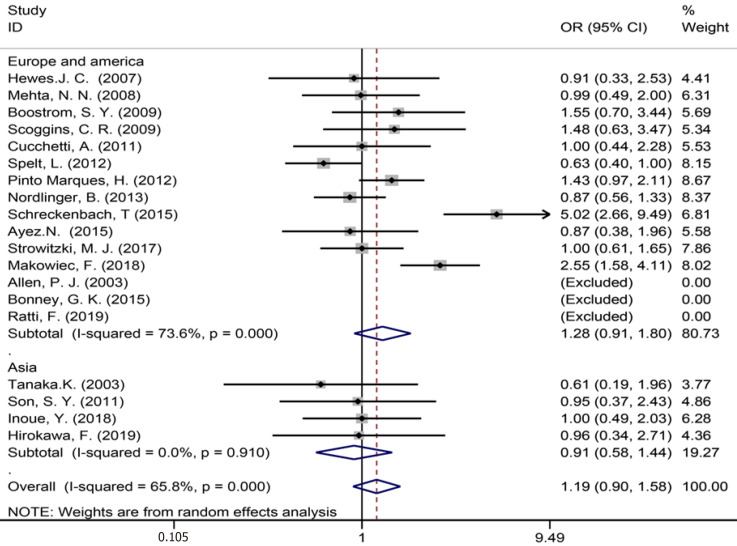
Data were acquired from 19 studies[1,3,7,9,35-38,43-46,50,52-54,57-59] on synchronous metastases .The pooled results (Figure 10) showed that there was no statistical difference between the two groups (OR = 1.19, 95%CI: 0.90–1.58, P = 0.221, I2 = 65.8%). The meta-analysis of Europe and America studies (OR = 1.28, 95%CI: 0.91-1.80, P = 0.153) and Asia studies (OR = 0.91, 95%CI: 0.58-1.44, P = 0.685). The results showed no heterogeneity in the subgroup of Asia studies (χ2 = 0.54, I2= 0.0%, P = 0.910).
Figure 10.
Synchronous metastases for all and by study region subgroups.
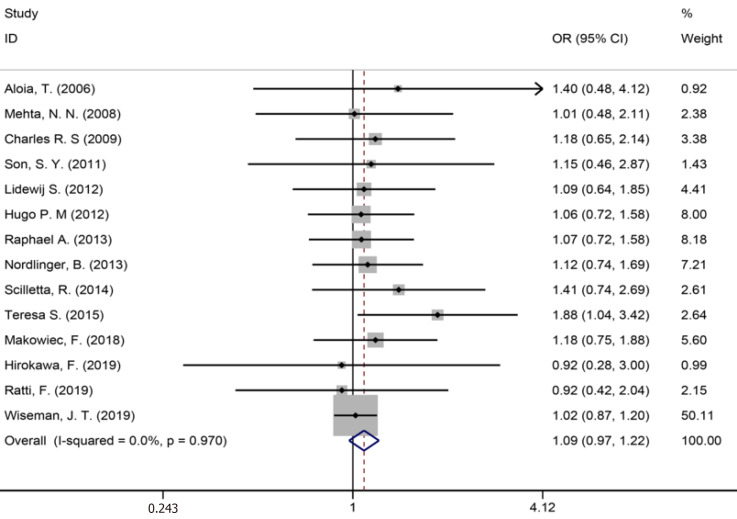
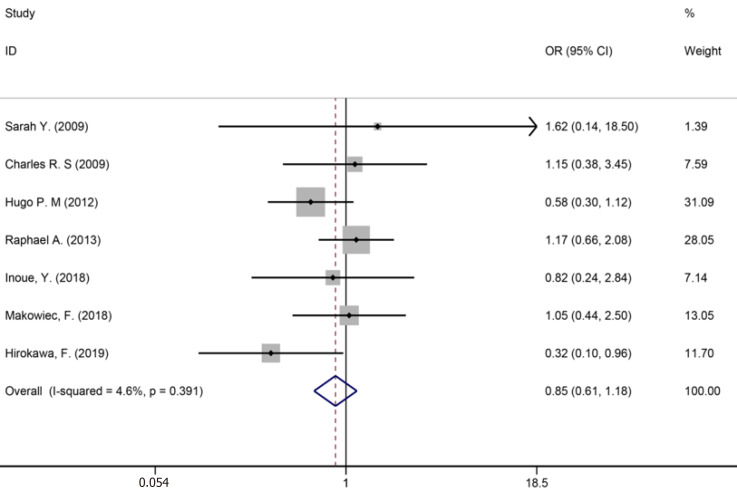
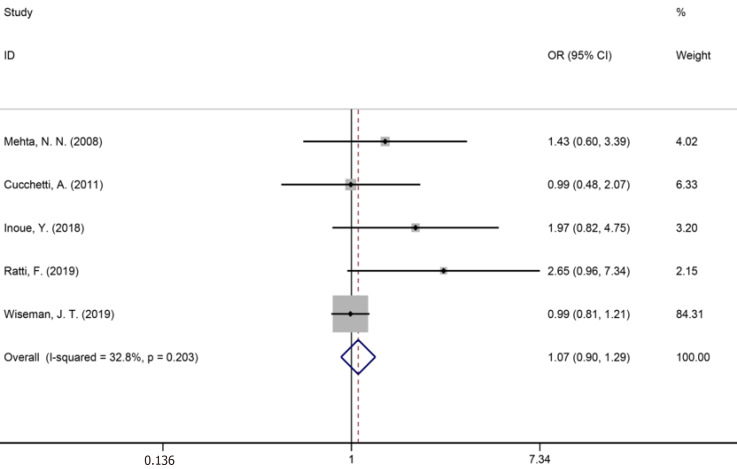
Fourteen studies[1,9,34-36,40,42-44,46,50,53,56,59] reported major liver resection (Figure 11), seven studies[1,7,9,42,44,50,52] reported R0 liver resections (Figure 12), and five studies[7,34,35,45,53] reported blood transfusions (Figure 13). The results of the meta-analysis showed no significant statistical difference between the three indicators in the two groups (OR = 1.09, 95%CI: 0.97-1.22, P = 0.143, I2= 0.0%; OR = 0.85, 95%CI: 0.61-1.18, P = 0.336, I2= 4.6%; OR = 1.07, 95%CI: 0.90-1.29, P = 0.438).
Figure 11.
Major liver resection for all patients and two groups.
Figure 12.
R0 liver resections for all patients and two groups.
Figure 13.
Blood transfusions for all patients and two groups.
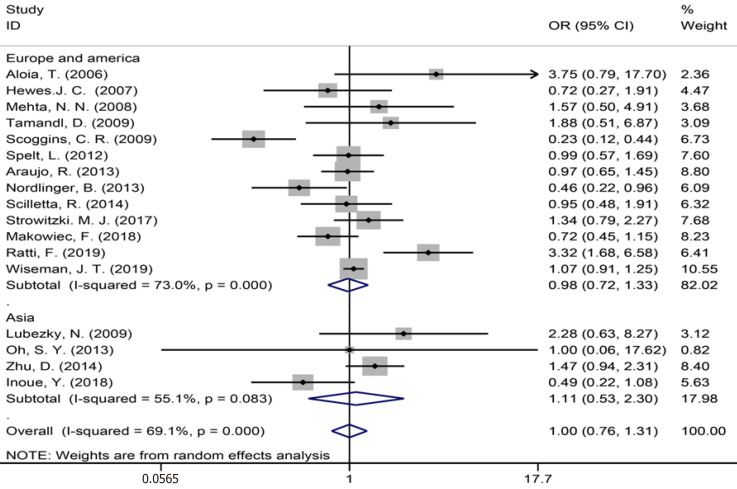
The assembled data from 17 studies[3,7,9,34,35,39-43,49-51,53,54,56,59] assessing perioperative complications showed no statistically significant difference between the groups (OR = 1.00, 95%CI: 0.76-1.31, P = 0.989, I² = 69.1%, Figure 14). The meta-analysis of Europe and America studies (OR = 0.98, 95%CI: 0.72-1.33, P = 0.885) and Asia studies (OR = 1.11, 95%CI: 0.53-2.30, P = 0.783). The results showed high heterogeneity in the subgroup of Europe and America studies (χ2 = 44.37, I2= 73.0%, P = 0.000).
Figure 14.
Perioperative complications for all patients and by study region.
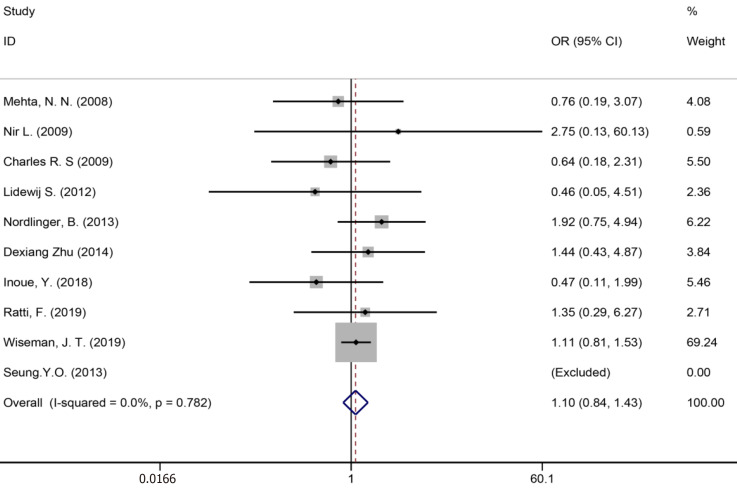
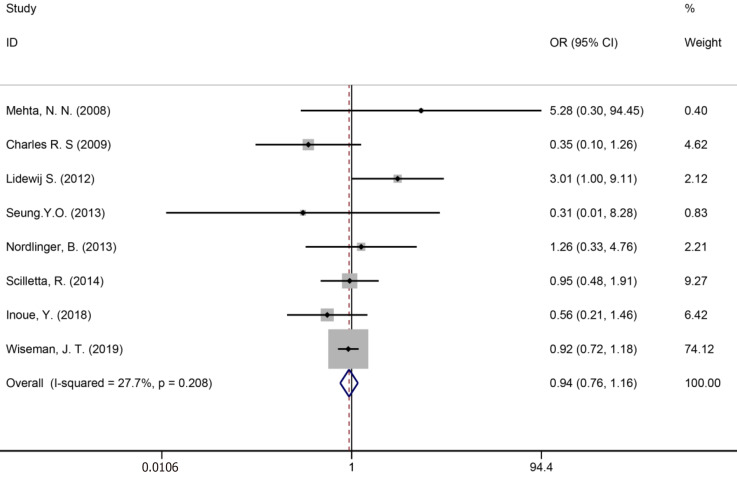
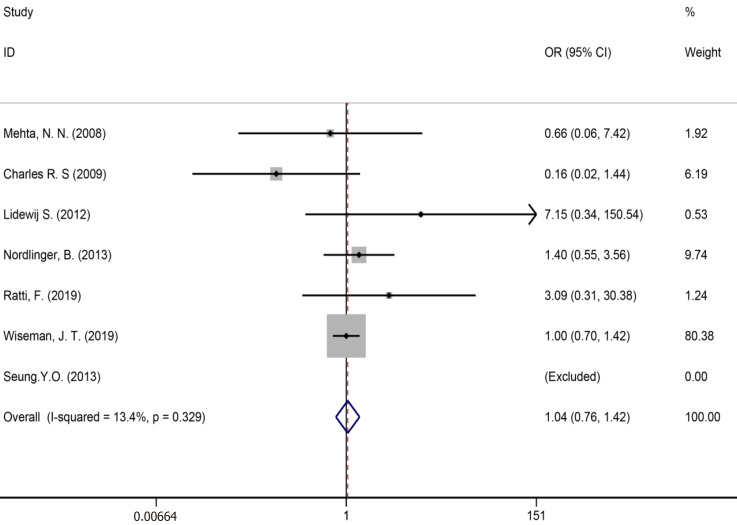
Ten studies[7,34,35,39,41,43,50,51,53,59] reported bile leakage (Figure 15), eight studies[7,34,40,41,43,50,53,59] reported surgical site infections (Figure 16), and seven studies[34,35,41,43,50,53,59] reported liver failure (Figure 17). The results of the meta–analysis showed no significant statistical difference between the three indicators in the two groups (OR = 1.10, 95%CI: 0.84-1.43, P = 0.481, I² = 0.00%; OR = 0.94, 95%CI: 0.76–1.16, P = 0.571, I² = 27.7%; OR = 1.04, 95%CI: 0.76-1.42, P = 0.329, I² = 13.4%).
Figure 15.
Bile leakage for all patients and two groups.
Figure 16.
Surgical site infections for all patients and two groups.
Figure 17.
Liver failure for all patients and two groups.
Publication bias
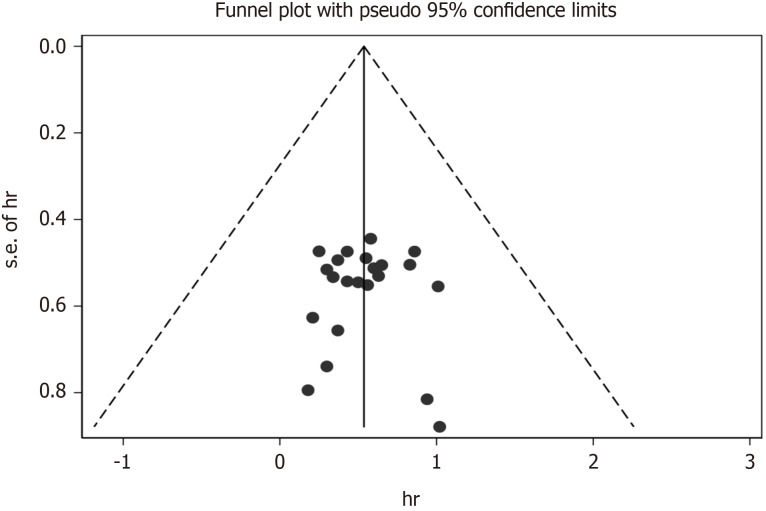
We used Begg’s and Egger’s regression tests to explore the publication bias of the studies in our meta-analysis and a funnel plot based on the NAC was generated to assess publication bias (Figure 18). Publication bias was not observed [Begg’s test (P = 0.888) and Egger’s tests (P= 0.676)].
Figure 18.
Funnel plot for potential publication bias of overall survival.
Sensitivity analysis
Sensitivity analysis of the primary outcomes with high heterogeneity (continuous variables and individual dichotomous variables) was performed to explore their potential source and assess the robustness of the outcomes. After ignoring each included study in turn for each outcome, the results of those indicators were stable. The result of the sensitivity analysis showed in Supplementary Figure 1.
DISCUSSION
A previous meta-analysis comprising 18 studies with a total of 6254 patients concluded that NAC improved the survival of patients with initially resectable CRLM[60]. Our meta-analysis evaluated the safety and efficiency of NAC and found that NAC could provide significant survival benefits for patients with resection of CRLM, consistent with previous studies. This conclusion was also confirmed with recent findings concerning the association between NAC and survival outcomes[10,11,24]. Therefore, we performed this systematic review and meta-analysis to provide an updated viewpoint on this subject.
In this meta-analysis, we analyzed 5-year OS and 5-year DFS. For cancer patients, one of the essential indicators for evaluating a treatment is survival outcomes such as the 5-year OS and the 5-year DFS, which may reflect whether a treatment could benefit those patients. In this study, one study[59] conducted a phase 3 clinical RCT to compare the survival outcomes of patients treated with or without NAC. The results of the study indicated that the 5-year OS was 51.2% (95%CI: 43.6-58.3) in the perioperative chemotherapy group vs 47.8% (95%CI: 40.3-55.0) in the surgery-only group. The results of this phase 3 clinical RCT showed no difference in OS with the addition of perioperative chemotherapy compared to surgery alone for patients with resectable liver metastases from colorectal cancer. However, NAC had an obvious DFS advantage. The perioperative chemotherapy group that subsequently underwent hepatectomy (83%) experienced 9.2% longer PFS (P = 0.025) compared to the group undergoing surgery only.
Many previous studies have compared the survival outcomes of patients treated with or without NAC. However, the findings are not consistent. A study from Japan[1] showed that the overall survival after initial treatment was significantly worse in the NAC group (5.56 years) than that in the non-NAC group. Moreover, a South Korea study[46] reported that the DFS rates in the NAC and non-NAC groups were 23% and 39%, respectively, and the patient survival rates were 42% and 66% (P > 0.05), respectively. One study[19] showed that although NAC can transform a small number of patients with initially inoperable liver metastases into a resectable state, very few patients meet this criterion, and the long-term outcomes of these patients are not significantly different from those of patients who do not receive NAC. However, another South Korean study[6], reported that the DFS rate was significantly higher in the preoperative chemotherapy group than in the primary resection group. The 3-year DFS rates were 34.2% and 16.8%, respectively, and this was also consistent with our findings. Therefore, the discussion and controversy surrounding this conclusion have never stopped, so large sample clinical trials are needed to confirm further it.
High heterogeneity was observed in the continuous variables, such as blood loss and the number of liver metastases, which may be related to study design, ethnic differences, inconsistent measurement methods, and different reporting methods. The included original studies were mostly from Europe and America, which may affect the accuracy and credibility in the measurement results. There were also fewer patients in the NAC group than in the non-NAC group. Therefore, the size of the patient sample was likely to contribute to this result. In addition, a major reason may be the use of neoadjuvant chemotherapy drugs[61]. One study’s multivariate analysis of all study factors potentially contributing to the increased intraoperative transfusion rates determined that preoperative chemotherapy was the only independent prognostic factor[56]. This was most likely related to blood vessel damage caused by preoperative chemotherapy.
Because of the high heterogeneity in the pooled data for continuous variables and individual dichotomous variables, subgroup analysis was conducted according to the different study regions, and we performed a sensitivity analysis to explore their potential source and assess the robustness of the outcomes. After ignoring each included study in turn for each outcome, the results of those indicators were stable.
Our meta-analysis showed that NAC could increase the duration of surgery and that the NAC group had more liver metastases and lymph node metastasis. Moreover, the number of liver lesions invaded by tumor cells and the number of lymph nodes invaded are closely related to the patient prognosis. In this case, the surgical methods involved may be completely different[62,63]. Several previous studies reported that NAC could affect the blood supply to liver tissue and lead to the fibrosis of liver cells. Consequently, this affects the duration of surgery and the amount of blood loss[56,64-67]. In our meta-analysis, the results of the pooled data on blood loss were not statistically significant. Since the data on blood loss were only generated from six studies, this result may be affected by the small limited number of included studies and the insufficient sample size.
In this study, the safety of NAC was also one of the key points of our discussion. In this study, there was no statistical significance in the combined effect size in terms of the incidence of surgical site infection, bile leakage, and liver failure.
Liver failure is a very common and highly fatal complication after NAC[68]. Additionally, NAC has been proven to cause tissue damage to the liver, including vascular lesions of liver parenchyma and steatosis of liver tissue[53,56,63]. Pathologically, these histologic lesions are inextricably linked to the occurrence and prognosis of postoperative complications of NAC[68-70]. However, it should be noted that patients with severe complications such as liver failure often received extensive chemotherapy before surgery, which is also closely associated with confounding factors like type, dose, and duration of chemotherapy drugs[71]. In this study, the combined effect size of liver failure was not statistically significant because of the above confounding factors and the small sample, as the combined effect size of liver failure was only obtained from the research data of seven different studies.
Many studies have shown that a positive margin (< 1 mm) is an indicator of a poor prognosis[72-76]. Although there is a consensus[59] that patients with a negative surgical margin (R0) have a better prognosis, differences remain in the range of the optimal surgical margin of liver lesions during perioperative systemic treatment and its relationship with the survival prognosis of patients. Moreover, Miller et al[77] evaluated the optimal margin of resection, which confirmed the importance of R0 resection for CRLM in the modern era of chemotherapy and suggested that patients with positive margin should receive additional post-resection chemotherapy to improve survival. However, this study did not find an advantage in long-term survival of patients with a larger margin of resection. In addition, other studies showed that among patients undergoing NAC following R0 and R1 resection, no significant difference was found in OS or recurrence-free survival after surgery[21,71].
In this study, we present the pooled analysis of the impact of NAC on long-term oncology outcomes after liver metastases were resected. In 2016, the safety and effectiveness of NAC in the treatment of colorectal cancer was systematically evaluated[60]. Contrasted to previous studies, our research incorporated more original studies and sensitivity analysis, and more indicators were performed.
Some studies[48,78] have shown that additional adjuvant chemotherapy can significantly improve and prolong the survival period of patients with liver metastases after complete resection. The NCCN guidelines[79] recommend that the duration of peri-operative chemotherapy, including neoadjuvant chemotherapy, should not exceed 6 mo. Moreover, European Society for Medical Oncology guidelines[80] explicitly suggest that the perioperative treatment mode should be measured from two dimensions: Surgical technical standards and tumor prognosis. The latest NCCN guidelines for the treatment of colorectal cancer[81] recommend FOLFOX as the preferred preoperative chemotherapy option for patients with resectable CRLM and recommend postoperative adjuvant chemotherapy for patients with CRLM who have not received preoperative NAC treatment but have undergone complete surgical resection. Since the efficacy of NAC in patients with resectable CRLM remains controversial and to control for confounders, the role of NAC in patients with resectable CRLM was only discussed in this study. This is also the limitation of this study.
CONCLUSION
The results of this meta-analysis showed that NAC improved the long-term prognosis of the patients who underwent surgery for the treatment of colorectal liver metastases. At the same time, the NAC group did not increase the risk of any adverse event compared to the non-NAC group. Because this study was a secondary study and the included original research studies were mostly from Europe and America, it was impossible to control the differences among the original studies, which may have affected the reliability of the results. In the future, well-designed prospective RCTs are warranted to define better the treatment effects using NAC.
ARTICLE HIGHLIGHTS
Research background
Surgery is an effective method for the treatment of liver metastases from colorectal cancer, but the risk of recurrence and metastasis is higher after surgery. The use of neoadjuvant chemotherapy (NAC) for the treatment of resectable colorectal cancer liver metastases is still controversial.
Research motivation
Many previous studies have reported the efficacy of adding NAC in the surgical treatment of resectable liver metastases from colorectal cancer. However, their conclusions have been inconsistent. A randomized controlled trial has revealed that NAC can confer a significant survival advantage over disease-free survival (DFS). In order to solve this dispute systematically and comprehensively, it is necessary to conduct a meta-analysis.
Research objective
The purpose of this study is to use a systematic review and meta-analysis to evaluate the application value of NAC in patients with resectable colorectal cancer and liver metastases.
Research method
We searched PubMed, Embase, Web of Science, and the Cochrane Library to collect clinical studies comparing NAC with non-NAC. Data processing and statistical analyses were performed using Stata V.15.0 and Review Manager 5.0 software. The odds ratio (OR) and 95% confidence interval (CI) were employed to analyze the dichotomous variables. Meanwhile, the standardized mean difference (SMD) with a 95%CI was used to analyze the continuous variables. In addition, the hazard ratio (HR) was used as a summary statistical measure of survival outcome [5-year overall survival (OS) and 5-year DFS].
Research results
Thirty-two studies involving 11236 patients were included in this analysis, which included 31 retrospective cohort studies and one randomized controlled trial. Our results showed a statistically significant difference in the 5-year OS (HR = 0.49, 95%CI: 0.39-0.61 P = 0.000), 5-year DFS (HR = 0.48 95%CI: 0.36-0.63 P = 0.000), the duration of surgery (SMD = 0.41, 95%CI: 0.01-0.82, P = 0.044), the number of liver metastases (SMD = 0.73, 95%CI: 0.02-1.43, P = 0.043), and the number of lymph node metastasis (SMD = 1.24, 95%CI: 1.07-1.43, P = 0.004). However, our results showed no statistically significant difference in the combined effect size in terms of the incidence of surgical site infection (OR = 0.94, 95%CI: 0.76-1.16, P = 0.571, I² = 27.7%), bile leakage (OR = 1.10, 95%CI: 0.84-1.43, P = 0.481, I² = 0.00%), and liver failure (OR = 1.04, 95%CI: 0.76-1.42, P = 0.329, I² = 13.4%).
Research conclusions
NAC can significantly improve the long-term survival advantages of colorectal liver metastases patients, including 5-year OS and 5-year DFS. At the same time, it does not increase the incidence of postoperative bile leakage, surgical site infection, liver failure, and other complications.
Research perspectives
This study had several limitations: First, the included original research studies were mostly from Europe and America, which may affect the accuracy and credibility when comparing studies from different regions. Second, the representative sample size was relatively low. Furthermore, most of the studies that we included were observational studies, which may adversely affect the quality of the study results. Moreover, this study was a secondary study, and it was impossible to control the differences among the original studies, which may have affected the reliability of the results. Finally, colorectal liver metastases is a heterogeneous disease, and differences in tumor biology and expressed proteins may cause significant bias.
ACKNOWLEDGEMENTS
The authors thank the DaVinci Surgery System Database (DSSD, www.davincisurgery database.com) and the Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province for their help and support regarding the methodology and meta-analysis process.
Footnotes
Conflict-of-interest statement: No conflict of interest.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Unsolicited manuscript
Peer-review started: February 16, 2021
First decision: April 6, 2021
Article in press: June 16, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rathnaswami A S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Yue Zhang, General Surgery Clinical Medical Center, Gansu Provincial Hospital, Lanzhou 730000, Gansu Province, China.
Long Ge, Evidence Based Social Science Research Center, School of Public Health, Lanzhou University, Lanzhou 730000, Gansu Province, China.
Jun Weng, General Surgery Clinical Medical Center, Gansu Provincial Hospital, Lanzhou 730000, Gansu Province, China.
Wen-Yu Tuo, General Surgery Clinical Medical Center, Gansu Provincial Hospital, Lanzhou 730000, Gansu Province, China.
Bin Liu, General Surgery Clinical Medical Center, Gansu Provincial Hospital, Lanzhou 730000, Gansu Province, China.
Shi-Xun Ma, General Surgery Clinical Medical Center, Gansu Provincial Hospital, Lanzhou 730000, Gansu Province, China.
Ke-Hu Yang, Evidence Based Social Science Research Center, School of Public Health, Lanzhou University, Lanzhou 730000, Gansu Province, China.
Hui Cai, General Surgery Clinical Medical Center, Gansu Provincial Hospital, Lanzhou 730000, Gansu Province, China. Caialonteam@163.com.
Data sharing statement
No additional data are available.
References
- 1.Hirokawa F, Asakuma M, Komeda K, Shimizu T, Inoue Y, Kagota S, Tomioka A, Uchiyama K. Is neoadjuvant chemotherapy appropriate for patients with resectable liver metastases from colorectal cancer? Surg Today . 2019;49:82–89. doi: 10.1007/s00595-018-1716-x. [DOI] [PubMed] [Google Scholar]
- 2.Winter H, Rassam J, Virdee PS, Goldin R, Pitcheshwar P, Weaver K, Primrose J, Berry DP, Wasan HS, Sharma RA. Hepatic Resection Following Selective Internal Radiation Therapy for Colorectal Cancer Metastases in the FOXFIRE Clinical Trial: Clinical Outcomes and Distribution of Microspheres. Cancers (Basel) . 2019;11 doi: 10.3390/cancers11081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strowitzki MJ, Schmidt T, Keppler U, Ritter AS, Mahmoud S, Klose J, Mihaljevic AL, Schneider M, Büchler MW, Ulrich AB. Influence of neoadjuvant chemotherapy on resection of primary colorectal liver metastases: A propensity score analysis. J Surg Oncol . 2017;116:149–158. doi: 10.1002/jso.24631. [DOI] [PubMed] [Google Scholar]
- 4.van Hazel GA, Heinemann V, Sharma NK, Findlay MP, Ricke J, Peeters M, Perez D, Robinson BA, Strickland AH, Ferguson T, Rodríguez J, Kröning H, Wolf I, Ganju V, Walpole E, Boucher E, Tichler T, Shacham-Shmueli E, Powell A, Eliadis P, Isaacs R, Price D, Moeslein F, Taieb J, Bower G, Gebski V, Van Buskirk M, Cade DN, Thurston K, Gibbs P. SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients With Metastatic Colorectal Cancer. J Clin Oncol . 2016;34:1723–1731. doi: 10.1200/JCO.2015.66.1181. [DOI] [PubMed] [Google Scholar]
- 5.Helling TS, Martin M. Cause of death from liver metastases in colorectal cancer. Ann Surg Oncol . 2014;21:501–506. doi: 10.1245/s10434-013-3297-7. [DOI] [PubMed] [Google Scholar]
- 6.Kim CW, Lee JL, Yoon YS, Park IJ, Lim SB, Yu CS, Kim TW, Kim JC. Resection after preoperative chemotherapy versus synchronous liver resection of colorectal cancer liver metastases: A propensity score matching analysis. Medicine (Baltimore) . 2017;96:e6174. doi: 10.1097/MD.0000000000006174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue Y, Fujii K, Tashiro K, Ishii M, Masubuchi S, Yamamoto M, Shimizu T, Asakuma M, Hirokawa F, Hayashi M, Narumi Y, Uchiyama K. Preoperative Chemotherapy May Not Influence the Remnant Liver Regenerations and Outcomes After Hepatectomy for Colorectal Liver Metastasis. World J Surg . 2018;42:3316–3330. doi: 10.1007/s00268-018-4590-1. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Dennison AR, Robertson V, Jones MJ, Neal CP, Garcea G. Clinical risk scores in the current era of neoadjuvant chemotherapy for colorectal liver metastases. ANZ J Surg . 2018;88:E16–E20. doi: 10.1111/ans.13688. [DOI] [PubMed] [Google Scholar]
- 9.Makowiec F, Bronsert P, Klock A, Hopt UT, Neeff HP. Prognostic influence of hepatic margin after resection of colorectal liver metastasis: role of modern preoperative chemotherapy. Int J Colorectal Dis . 2018;33:71–78. doi: 10.1007/s00384-017-2916-3. [DOI] [PubMed] [Google Scholar]
- 10.Fukuoka K, Nara S, Honma Y, Kishi Y, Esaki M, Shimada K. Hepatectomy for Colorectal Cancer Liver Metastases in the Era of Modern Preoperative Chemotherapy: Evaluation of Postoperative Complications. World J Surg . 2017;41:1073–1081. doi: 10.1007/s00268-016-3724-6. [DOI] [PubMed] [Google Scholar]
- 11.Ayez N, van der Stok EP, de Wilt H, Radema SA, van Hillegersberg R, Roumen RM, Vreugdenhil G, Tanis PJ, Punt CJ, Dejong CH, Jansen RL, Verheul HM, de Jong KP, Hospers GA, Klaase JM, Legdeur MC, van Meerten E, Eskens FA, van der Meer N, van der Holt B, Verhoef C, Grünhagen DJ. Neo-adjuvant chemotherapy followed by surgery versus surgery alone in high-risk patients with resectable colorectal liver metastases: the CHARISMA randomized multicenter clinical trial. BMC Cancer . 2015;15:180. doi: 10.1186/s12885-015-1199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yedibela S, Elad L, Wein A, Dimmler A, Merkel S, Hohenberger W, Meyer T. Neoadjuvant chemotherapy does not increase postoperative complication rate after resection of colorectal liver metastases. Eur J Surg Oncol . 2005;31:141–146. doi: 10.1016/j.ejso.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Imai K, Yamashita YI, Miyamoto Y, Nakao Y, Yusa T, Itoyama R, Nakagawa S, Okabe H, Hiyoshi Y, Nitta H, Chikamoto A, Baba H. Implication of primary tumor location for the indication of preoperative chemotherapy in patients with colorectal liver metastases. HPB (Oxford) . 2019;21:405–412. doi: 10.1016/j.hpb.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Barresi V, Fioravanzo A, Pecori S, Tomezzoli A, Reggiani Bonetti L. The histopathologic report of surgically resected colorectal liver metastases: What is clinically relevant? Pathol Res Pract . 2019;215:152547. doi: 10.1016/j.prp.2019.152547. [DOI] [PubMed] [Google Scholar]
- 15.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg . 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 16.D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol . 2011;18:1096–1103. doi: 10.1245/s10434-010-1409-1. [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Allard MA, Benitez CC, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, Baba H, Adam R. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist . 2016;21:887–894. doi: 10.1634/theoncologist.2015-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacchetti S, Itzhaki M, Gruia G, Adam R, Zidani R, Kunstlinger F, Brienza S, Alafaci E, Bertheault-Cvitkovic F, Jasmin C, Reynes M, Bismuth H, Misset JL, Lévi F. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol . 1999;10:663–669. doi: 10.1023/a:1008347829017. [DOI] [PubMed] [Google Scholar]
- 19.Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, Kunstlinger F, Levi F, Bismuth F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol . 2001;8:347–353. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 20.Nikfarjam M, Shereef S, Kimchi ET, Gusani NJ, Jiang Y, Avella DM, Mahraj RP, Staveley-O'Carroll KF. Survival outcomes of patients with colorectal liver metastases following hepatic resection or ablation in the era of effective chemotherapy. Ann Surg Oncol . 2009;16:1860–1867. doi: 10.1245/s10434-008-0225-3. [DOI] [PubMed] [Google Scholar]
- 21.Ayez N, Lalmahomed ZS, Eggermont AM, Ijzermans JN, de Jonge J, van Montfort K, Verhoef C. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol . 2012;19:1618–1627. doi: 10.1245/s10434-011-2114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayez N, Lalmahomed ZS, van der Pool AE, Vergouwe Y, van Montfort K, de Jonge J, Eggermont AM, Ijzermans JN, Verhoef C. Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy? Ann Surg Oncol . 2011;18:2757–2763. doi: 10.1245/s10434-011-1819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulitanò C, Aldrighetti L, Arru M, Vitali G, Ronzoni M, Catena M, Finazzi R, Villa E, Ferla G. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg . 2006;244:833–5; author reply 835. doi: 10.1097/01.sla.0000243600.98464.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichida H, Mise Y, Ito H, Ishizawa T, Inoue Y, Takahashi Y, Shinozaki E, Yamaguchi K, Saiura A. Optimal indication criteria for neoadjuvant chemotherapy in patients with resectable colorectal liver metastases. World J Surg Oncol . 2019;17:100. doi: 10.1186/s12957-019-1641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teoh B, Prabhakaran S, Behrenbruch CC, Hayes I, Hollande F, Heriot AG, Michael M, Knowles B, Thomson BN. Comparison of survival of chemotherapy and surgery vs. surgery alone for resectable colorectal liver metastasis. HPB . 2019;21:S142–S3. [Google Scholar]
- 26.Sui K, Okabayashi T, Iwata J, Morita SM, Matsumoto T, Inada R, Iiyama T, Shimada Y, Kobayashi M. Effect of Neoadjuvant Chemotherapy in Patients with Colorectal Cancer Liver Metastases. Surg Technol Int . 2019;34:208–214. [PubMed] [Google Scholar]
- 27.Solaini L, Gardini A, Passardi A, Mirarchi MT, D'Acapito F, La Barba G, Cucchi M, Casadei Gardini A, Frassineti GL, Cucchetti A, Ercolani G. Preoperative Chemotherapy and Resection Margin Status in Colorectal Liver Metastasis Patients: A Propensity Score-Matched Analysis. Am Surg . 2019;85:488–493. [PubMed] [Google Scholar]
- 28.Desjardin M, Bonhomme B, Le Bail B, Evrard S, Brouste V, Desolneux G, Fonck M, Bécouarn Y, Béchade D. Hepatotoxicities Induced by Neoadjuvant Chemotherapy in Colorectal Cancer Liver Metastases: Distinguishing the True From the False. Clin Med Insights Oncol . 2019;13:1179554918825450. doi: 10.1177/1179554918825450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Sawo P, Rensen SS, Rouflart MMJ, Winstanley A, Vreuls CPH, Verheij J, van Mierlo KMC, Lodewick TM, van Woerden V, van Tiel FH, van Dam RM, Dejong CHC, Olde Damink SWM. Impact of chemotherapy-associated liver injury on tumour regression grade and survival in patients with colorectal liver metastases. HPB (Oxford) . 2018;20:147–154. doi: 10.1016/j.hpb.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 30.reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ . 2016;354:i4086. doi: 10.1136/bmj.i4086. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Chen Y, Yao L, Zhou Q, Wu Q, Estill J, Wang Q, Yang K, Norris SL. Reporting of declarations and conflicts of interest in WHO guidelines can be further improved. J Clin Epidemiol . 2018;98:1–8. doi: 10.1016/j.jclinepi.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ . 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol . 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 34.Wiseman JT, Guzman-Pruneda F, Xourafas D, Chun YS, Ejaz A, Tsung A, Pawlik TM, Cloyd JM. Impact of Neoadjuvant Chemotherapy on the Postoperative Outcomes of Patients Undergoing Liver Resection for Colorectal Liver Metastases: A Population-Based Propensity-Matched Analysis. J Am Coll Surg . 2019;229:69–77.e2. doi: 10.1016/j.jamcollsurg.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Ratti F, Fuks D, Cipriani F, Gayet B, Aldrighetti L. Timing of Perioperative Chemotherapy Does Not Influence Long-Term Outcome of Patients Undergoing Combined Laparoscopic Colorectal and Liver Resection in Selected Upfront Resectable Synchronous Liver Metastases. World J Surg . 2019;43:3110–3119. doi: 10.1007/s00268-019-05142-4. [DOI] [PubMed] [Google Scholar]
- 36.Schreckenbach T, Malkomes P, Bechstein WO, Woeste G, Schnitzbauer AA, Ulrich F. The clinical relevance of the Fong and the Nordlinger scores in the era of effective neoadjuvant chemotherapy for colorectal liver metastasis. Surg Today . 2015;45:1527–1534. doi: 10.1007/s00595-014-1108-9. [DOI] [PubMed] [Google Scholar]
- 37.Bonney GK, Coldham C, Adam R, Kaiser G, Barroso E, Capussotti L, Laurent C, Verhoef C, Nuzzo G, Elias D, Lapointe R, Hubert C, Lopez-Ben S, Krawczyk M, Mirza DF LiverMetSurvey International Registry Working Group. Role of neoadjuvant chemotherapy in resectable synchronous colorectal liver metastasis; An international multi-center data analysis using LiverMetSurvey. J Surg Oncol . 2015;111:716–724. doi: 10.1002/jso.23899. [DOI] [PubMed] [Google Scholar]
- 38.Ayez N, van der Stok EP, Grünhagen DJ, Rothbarth J, van Meerten E, Eggermont AM, Verhoef C. The use of neo-adjuvant chemotherapy in patients with resectable colorectal liver metastases: Clinical risk score as possible discriminator. Eur J Surg Oncol . 2015;41:859–867. doi: 10.1016/j.ejso.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Zhu D, Zhong Y, Wei Y, Ye L, Lin Q, Ren L, Ye Q, Liu T, Xu J, Qin X. Effect of neoadjuvant chemotherapy in patients with resectable colorectal liver metastases. PLoS One . 2014;9:e86543. doi: 10.1371/journal.pone.0086543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scilletta R, Pagano D, Spada M, Mongiovì S, Pesce A, Portale TR, Guardabasso V, Puleo S, Gruttadauria S. Comparative analysis of the incidence of surgical site infections in patients with liver resection for colorectal hepatic metastases after neoadjuvant chemotherapy. J Surg Res . 2014;188:183–189. doi: 10.1016/j.jss.2013.11.1092. [DOI] [PubMed] [Google Scholar]
- 41.Oh SY, Kim DY, Kim YB, Suh KW. Comparison of oncological outcomes between neoadjuvant and adjuvant chemotherapy combined with surgery for resectable synchronous colorectal liver metastases. J Surg Res . 2013;182:257–263. doi: 10.1016/j.jss.2012.10.927. [DOI] [PubMed] [Google Scholar]
- 42.Araujo R, Gonen M, Allen P, Blumgart L, DeMatteo R, Fong Y, Kemeny N, Jarnagin W, D'Angelica M. Comparison between perioperative and postoperative chemotherapy after potentially curative hepatic resection for metastatic colorectal cancer. Ann Surg Oncol . 2013;20:4312–4321. doi: 10.1245/s10434-013-3162-8. [DOI] [PubMed] [Google Scholar]
- 43.Spelt L, Hermansson L, Tingstedt B, Andersson R. Influence of preoperative chemotherapy on the intraoperative and postoperative course of liver resection for colorectal cancer metastases. World J Surg . 2012;36:157–163. doi: 10.1007/s00268-011-1342-x. [DOI] [PubMed] [Google Scholar]
- 44.Pinto Marques H, Barroso E, de Jong MC, Choti MA, Ribeiro V, Nobre AM, Carvalho C, Pawlik TM. Peri-operative chemotherapy for resectable colorectal liver metastasis: does timing of systemic therapy matter? J Surg Oncol . 2012;105:511–519. doi: 10.1002/jso.22133. [DOI] [PubMed] [Google Scholar]
- 45.Cucchetti A, Ercolani G, Cescon M, Di Gioia P, Peri E, Brandi G, Pellegrini S, Pinna AD. Safety of hepatic resection for colorectal metastases in the era of neo-adjuvant chemotherapy. Langenbecks Arch Surg . 2012;397:397–405. doi: 10.1007/s00423-011-0894-4. [DOI] [PubMed] [Google Scholar]
- 46.Son SY, Yi NJ, Hong G, Kim H, Park MS, Choi YR, Suh KS, Kim DW, Jeong SY, Park KJ, Park JG, Lee KU. Is neoadjuvant chemotherapy necessary for patients with initially resectable colorectal liver metastases in the era of effective chemotherapy? Korean J Hepatobiliary Pancreat Surg . 2011;15:206–217. doi: 10.14701/kjhbps.2011.15.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scartozzi M, Siquini W, Galizia E, Stortoni P, Marmorale C, Berardi R, Fianchini A, Cascinu S. The timing of surgery for resectable metachronous liver metastases from colorectal cancer: Better sooner than later? Dig Liver Dis . 2011;43:194–198. doi: 10.1016/j.dld.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Adam R, Bhangui P, Poston G, Mirza D, Nuzzo G, Barroso E, Ijzermans J, Hubert C, Ruers T, Capussotti L, Ouellet JF, Laurent C, Cugat E, Colombo PE, Milicevic M. Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg . 2010;252:774–787. doi: 10.1097/SLA.0b013e3181fcf3e3. [DOI] [PubMed] [Google Scholar]
- 49.Tamandl D, Gruenberger B, Herberger B, Kaczirek K, Gruenberger T. Surgery after neoadjuvant chemotherapy for colorectal liver metastases is safe and feasible in elderly patients. J Surg Oncol . 2009;100:364–371. doi: 10.1002/jso.21259. [DOI] [PubMed] [Google Scholar]
- 50.Scoggins CR, Campbell ML, Landry CS, Slomiany BA, Woodall CE, McMasters KM, Martin RC. Preoperative chemotherapy does not increase morbidity or mortality of hepatic resection for colorectal cancer metastases. Ann Surg Oncol . 2009;16:35–41. doi: 10.1245/s10434-008-0190-x. [DOI] [PubMed] [Google Scholar]
- 51.Lubezky N, Geva R, Shmueli E, Nakache R, Klausner JM, Figer A, Ben-Haim M. Is there a survival benefit to neoadjuvant versus adjuvant chemotherapy, combined with surgery for resectable colorectal liver metastases? World J Surg . 2009;33:1028–1034. doi: 10.1007/s00268-009-9945-1. [DOI] [PubMed] [Google Scholar]
- 52.Boostrom SY, Nagorney DM, Donohue JH, Harmsen S, Thomsen K, Que F, Kendrick M, Reid-Lombardo KM. Impact of neoadjuvant chemotherapy with FOLFOX/FOLFIRI on disease-free and overall survival of patients with colorectal metastases. J Gastrointest Surg . 2009;13:2003–9; discussion 2009. doi: 10.1007/s11605-009-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta NN, Ravikumar R, Coldham CA, Buckels JA, Hubscher SG, Bramhall SR, Wigmore SJ, Mayer AD, Mirza DF. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol . 2008;34:782–786. doi: 10.1016/j.ejso.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Hewes JC, Dighe S, Morris RW, Hutchins RR, Bhattacharya S, Davidson BR. Preoperative chemotherapy and the outcome of liver resection for colorectal metastases. World J Surg . 2007;31:353–64; discussion 365. doi: 10.1007/s00268-006-0103-8. [DOI] [PubMed] [Google Scholar]
- 55.Aloysius MM, Zaitoun AM, Beckingham IJ, Neal KR, Aithal GP, Bessell EM, Lobo DN. The pathological response to neoadjuvant chemotherapy with FOLFOX-4 for colorectal liver metastases: a comparative study. Virchows Arch . 2007;451:943–948. doi: 10.1007/s00428-007-0497-1. [DOI] [PubMed] [Google Scholar]
- 56.Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, Azoulay D, Bismuth H, Castaing D, Adam R. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol . 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka K, Adam R, Shimada H, Azoulay D, Lévi F, Bismuth H. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg . 2003;90:963–969. doi: 10.1002/bjs.4160. [DOI] [PubMed] [Google Scholar]
- 58.Allen PJ, Kemeny N, Jarnagin W, DeMatteo R, Blumgart L, Fong Y. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg . 2003;7:109–117. doi: 10.1016/S1091-255X(02)00121-X. [DOI] [PubMed] [Google Scholar]
- 59.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol . 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 60.Liu W, Zhou JG, Sun Y, Zhang L, Xing BC. The role of neoadjuvant chemotherapy for resectable colorectal liver metastases: a systematic review and meta-analysis. Oncotarget . 2016;7:37277–37287. doi: 10.18632/oncotarget.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welsh FK, Tilney HS, Tekkis PP, John TG, Rees M. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer . 2007;96:1037–1042. doi: 10.1038/sj.bjc.6603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiomi A, Ito M, Maeda K, Kinugasa Y, Ota M, Yamaue H, Shiozawa M, Horie H, Kuriu Y, Saito N. Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1,014 consecutive patients. J Am Coll Surg . 2015;220:186–194. doi: 10.1016/j.jamcollsurg.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 63.Komori H, Beppu T, Baba Y, Horino K, Imsung C, Masuda T, Hayashi H, Okabe H, Ootao R, Watanabe M, Takamori H, Iyama K, Baba H. Histological liver injury and surgical outcome after FOLFOX followed by a hepatectomy for colorectal liver metastases in Japanese patients. Int J Clin Oncol . 2010;15:263–270. doi: 10.1007/s10147-010-0046-9. [DOI] [PubMed] [Google Scholar]
- 64.Esposito A, Mancini R, Ettorre G, Garufi C, Saracca E, Arcieri S, Cosimelli M. A combined approach of neoadjuvant chemotherapy and surgery for colorectal liver metastases. J Exp Clin Cancer Res . 2003;22:197–202. [PubMed] [Google Scholar]
- 65.Müller H, Hilger R. Curative and palliative aspects of regional chemotherapy in combination with surgery. Support Care Cancer . 2003;11:1–10. doi: 10.1007/s00520-002-0355-2. [DOI] [PubMed] [Google Scholar]
- 66.Ong SY. Neoadjuvant chemotherapy in the management of colorectal metastases: A review of the literature. Ann Acad Med Singap . 2003;32:205–211. [PubMed] [Google Scholar]
- 67.Wang P, Chen Z, Huang WX, Liu LM. Current preventive treatment for recurrence after curative hepatectomy for liver metastases of colorectal carcinoma: a literature review of randomized control trials. World J Gastroenterol . 2005;11:3817–3822. doi: 10.3748/wjg.v11.i25.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamashita S, Shindoh J, Mizuno T, Chun YS, Conrad C, Aloia TA, Vauthey JN. Hepatic atrophy following preoperative chemotherapy predicts hepatic insufficiency after resection of colorectal liver metastases. J Hepatol . 2017;67:56–64. doi: 10.1016/j.jhep.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 69.Falcão D, Alexandrino H, Caetano Oliveira R, Martins J, Ferreira L, Martins R, Serôdio M, Martins M, Tralhão JG, Cipriano MA, Castro E Sousa F. Histopathologic patterns as markers of prognosis in patients undergoing hepatectomy for colorectal cancer liver metastases - Pushing growth as an independent risk factor for decreased survival. Eur J Surg Oncol . 2018;44:1212–1219. doi: 10.1016/j.ejso.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 70.Baldin P, Van den Eynde M, Hubert C, Jouret-Mourin A, Komuta M. The role of the pathologist and clinical implications in colorectal liver metastasis. Acta Gastroenterol Belg . 2018;81:419–426. [PubMed] [Google Scholar]
- 71.Narita M, Oussoultzoglou E, Bachellier P, Jaeck D, Uemoto S. Post-hepatectomy liver failure in patients with colorectal liver metastases. Surg Today . 2015;45:1218–1226. doi: 10.1007/s00595-015-1113-7. [DOI] [PubMed] [Google Scholar]
- 72.Elias D, Lasser P, Rougier P, Ducreux M, Bognel C, Roche A. Frequency, technical aspects, results, and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg . 1995;180:213–219. [PubMed] [Google Scholar]
- 73.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg . 2005;241:715–722, discussion 722. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Are C, Gonen M, Zazzali K, Dematteo RP, Jarnagin WR, Fong Y, Blumgart LH, D'Angelica M. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg . 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Figueras J, Burdio F, Ramos E, Torras J, Llado L, Lopez-Ben S, Codina-Barreras A, Mojal S. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol . 2007;18:1190–1195. doi: 10.1093/annonc/mdm106. [DOI] [PubMed] [Google Scholar]
- 76.Liu W, Sun Y, Zhang L, Xing BC. Negative surgical margin improved long-term survival of colorectal cancer liver metastases after hepatic resection: a systematic review and meta-analysis. Int J Colorectal Dis . 2015;30:1365–1373. doi: 10.1007/s00384-015-2323-6. [DOI] [PubMed] [Google Scholar]
- 77.Miller CL, Taylor MS, Qadan M, Deshpande V, Worthington S, Smalley R, Collura C, Ryan DP, Allen JN, Blaszkowsky LS, Clark JW, Murphy JE, Parikh AR, Berger D, Tanabe KK, Lillemoe KD, Ferrone CR. Prognostic Significance of Surgical Margin Size After Neoadjuvant FOLFOX and/or FOLFIRI for Colorectal Liver Metastases. J Gastrointest Surg . 2017;21:1831–1840. doi: 10.1007/s11605-017-3557-0. [DOI] [PubMed] [Google Scholar]
- 78.Schwarz RE, Berlin JD, Lenz HJ, Nordlinger B, Rubbia-Brandt L, Choti MA. Systemic cytotoxic and biological therapies of colorectal liver metastases: expert consensus statement. HPB (Oxford) . 2013;15:106–115. doi: 10.1111/j.1477-2574.2012.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wu CS, Gregory KM, Freedman-Cass D. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw . 2017;15:370–398. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 80.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol . 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 81.Messersmith WA. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J Natl Compr Canc Netw . 2019;17:599–601. doi: 10.6004/jnccn.2019.5014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.