Summary
The use of high-dose intravenous immune globulin (IVIG) plus anticoagulation is recommended for the treatment of vaccine-induced immune thrombotic thrombocytopenia (VITT), a rare side effect of adenoviral vector vaccines against coronavirus disease 2019 (Covid-19). We describe the response to IVIG therapy in three of the first patients in whom VITT was identified in Canada after the receipt of the ChAdOx1 nCoV-19 vaccine. The patients were between the ages of 63 and 72 years; one was female. At the time of this report, Canada had restricted the use of the ChAdOx1 nCoV-19 vaccine to persons who were 55 years of age or older on the basis of reports that VITT had occurred primarily in younger persons. Two of the patients in our study presented with limb-artery thrombosis; the third had cerebral venous and arterial thrombosis. Variable patterns of serum-induced platelet activation were observed in response to heparin and platelet factor 4 (PF4), indicating the heterogeneity of the manifestations of VITT in serum. After the initiation of IVIG, reduced antibody-induced platelet activation in serum was seen in all three patients. (Funded by the Canadian Institutes of Health Research.)
Recently, vaccination with adenoviral vector vaccines against coronavirus disease 2019 (Covid-19) has been implicated in a rare prothrombotic disorder that has been termed vaccine-induced immune thrombotic thrombocytopenia (VITT).1-5 Most patients in whom VITT has been diagnosed have been between the ages of 20 and 55 years and have presented with unusual thromboses, such as cerebral venous sinus thrombosis and splanchnic-vein thrombosis.1-5 The pathogenesis involves the production of IgG antibodies that recognize platelet factor 4 (PF4) and that strongly activate platelets through their FcγIIa receptors, leading to a decrease in the number of platelets (platelet consumption) and activation of coagulation.1,3 The disorder strongly mimics autoimmune heparin-induced thrombocytopenia (HIT)6-8 on the basis of both clinical and serologic evidence, even though patients with VITT usually have not received heparin.
High-dose intravenous immune globulin (IVIG) competitively inhibits the interaction of VITT antibodies with the platelet FcγIIa receptors, thus reducing platelet activation — a result that may be an important treatment consideration. Since minimal data exist for treating patients with VITT, the recommended use of IVIG is based primarily on an analogy with treatment of autoimmune HIT, in which the administration of IVIG rapidly increases the platelet count and reduces hypercoagulability.9-11 Our report documents the inhibition of serum-induced platelet activation after treatment with IVIG in three patients in whom VITT was diagnosed between March 31 and April 13, 2021. Our study also shows how the serotonin-release assay — the most common laboratory test of platelet activation that is performed to detect HIT in North American reference laboratories — can be adapted to detect VITT antibodies.
Case Reports
Patient 1
Patient 1 was a 72-year-old woman with an unremarkable medical history who reported having an onset of left limb pain and claudication 7 days after vaccination with ChAdOx1 nCoV-19 (Covishield, an AstraZeneca vaccine licensed for production by the Serum Institute of India). Her symptoms progressed, and she was admitted to the hospital 8 days after symptom onset. Imaging showed a suprarenal aortic thrombus, with occlusion of the left superficial and deep femoral arteries, plus partial thromboses of the celiac and right peroneal arteries. At that time, a disorder resembling heparin-induced thrombocytopenia was not suspected, so unfractionated heparin was started; 3 days later, the patient underwent surgical embolectomy. By that time, VITT was suspected, and argatroban was initiated. Since there was no improvement in the platelet count during a 5-day period, high-dose IVIG was administered. The patient’s platelet count increased, and she was discharged home while receiving oral apixaban.
Patient 2
Patient 2 was a 63-year-old man without cardiovascular risk factors or a history of thrombosis who reported having cramping in his left leg beginning 18 days after vaccination. Four days later, acute dyspnea developed. The following day, his left leg became painful and cold. He presented to the emergency department (24 days after vaccination), at which time computed tomographic angiography showed acute arterial thrombosis in the left leg, plus extensive pulmonary embolism. He received tinzaparin (low-molecular-weight heparin) and underwent surgical embolectomy. Lower-limb ultrasonography revealed nonocclusive right popliteal deep-vein thrombosis. Suspicion of VITT prompted a switch from heparin to fondaparinux and the administration of IVIG. Although no new thromboses occurred after IVIG treatment, residual distal lower-limb thrombosis resulted in distal foot ischemic necrosis, and at the time of this report, the patient was awaiting amputation.
Patient 3
Patient 3 was a 69-year-old man with non–insulin-dependent diabetes mellitus, hypertension, obstructive sleep apnea, recently diagnosed prostate cancer (not yet staged), and no history of thrombosis; he had a history of heparin exposure 9 months earlier during transcatheter aortic-valve replacement, which was followed by the administration of aspirin (81 mg) daily. Twelve days after vaccination, he reported having headache and confusion and was admitted to the hospital with progressive left-sided weakness. The diagnosis of VITT was made on hospital day 3 when further left-sided weakness became evident; right middle cerebral-artery stroke with hemorrhagic transformation was identified. Additional thromboses were documented in the right internal carotid artery, right cerebral transverse and sigmoid sinuses, right internal jugular vein, hepatic vein, and distal lower-limb vein; pulmonary embolism was also found. He was treated with fondaparinux and IVIG. No new clinically evident thromboses were subsequently observed, although the patient continued to have hemiplegia.
A recurrence of thrombocytopenia led to the administration of additional IVIG, which was followed by a transient improvement in the platelet count, along with a switch to rivaroxaban owing to a concern about VITT antibody cross-reactivity with fondaparinux; however, this cross-reactivity was ruled out by laboratory testing. Subsequently, the patient underwent therapeutic plasma exchange with the use of solvent detergent plasma as replacement fluid, which was administered in 13 exchanges from day 47 to day 62 after vaccination. The patient had subsequent gradual improvement in his platelet count, which reached a normal level of 158,000 per cubic millimeter on day 62.
Methods
Written informed consent for publication of their data was obtained from all three patients or their substitute decision makers. Serum was obtained both before and after the administration of IVIG to identify any changes in platelet-activating reactivity (ex vivo studies). We used a commercial immunoassay to detect antibodies against PF4–polyanion complexes (LIFECODES PF4 IgG/IgA/IgM enzyme-linked immunosorbent assay [ELISA], Immucor), according to the manufacturer’s instructions. We also performed a serotonin-release assay, in which the patient’s serum was incubated with donor platelets containing radioactive 14C serotonin and various levels of heparin. On this assay, when antibody that is present in the serum binds and activates donor platelets, it releases radiolabeled serotonin from the platelet granules. Thus, a higher percentage of serotonin release indicates greater platelet activation.12 However, we also used an assay that included a modification in which increasing doses of PF4 were added to the serum rather than heparin.13 We performed other assays with high levels of unfractionated heparin (100 U per milliliter), Fc receptor–blocking monoclonal antibody (IV.3) (5 μg per milliliter), and IVIG (Gammagard Liquid, Shire Pharma) (10 mg per milliliter). Weak reactivity was defined as a serotonin release of 20% to 49.9%, and strong reactivity as a release of more than 80%.
Results
Platelet Changes after IVIG
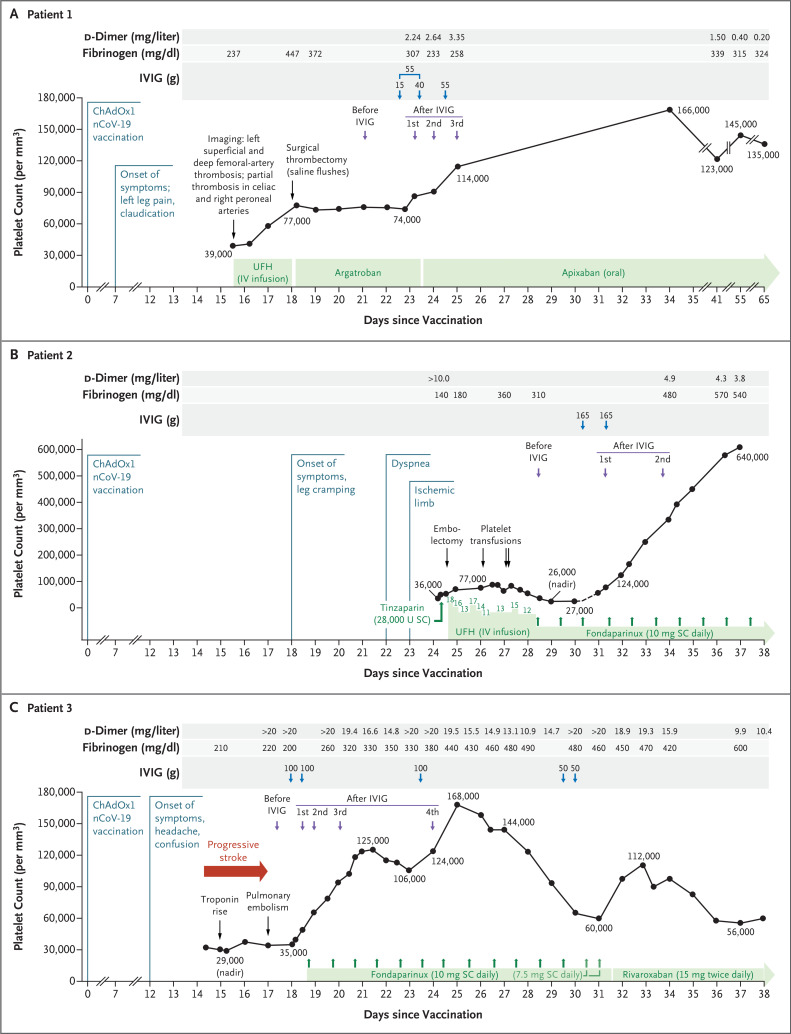
Figure 1 shows serial platelet counts for the three patients in relation to treatment with anticoagulant and IVIG. Data regarding the patients’ height, weight, and dosing considerations for IVIG administration (according to the Ontario dose calculator14) are provided in the Figure 1 legend.
Figure 1. Clinical and Laboratory Data for the Three Study Patients with VITT.
Serial platelet counts and coagulation tests for d-dimer and fibrinogen levels are shown in relation to clinical events in the three patients. The timing of blood samples obtained before and after the administration of intravenous immune globulin (IVIG) correspond to the performance of enzyme-linked immunosorbent assays and platelet-activation assays.
Panel A shows the findings in Patient 1, a 72-year-old woman in whom vaccine-induced immune thrombotic thrombocytopenia (VITT) was complicated by limb-artery thrombosis and partial celiac-artery thrombosis. The calculation of the IVIG dose was based on both weight and height, according to the “dosing weight” designation (1 g per kilogram of body weight) of the Ontario dose calculator.14 Thus, for a female patient weighing 59 kg with a height of 162 cm, the dose would be 55 g, which the patient received. However, the first dose was divided into portions of 15 g and 40 g, since the patient had an adverse reaction (severe chills) after the initial 15-g infusion of IVIG; the remaining 40 g was given the next day without incident.
Panel B shows the findings in Patient 2, a 63-year-old man with VITT that was complicated by limb-artery thrombosis, pulmonary embolism, and deep-vein thrombosis. According to the “dosing weight” on the Ontario dose calculator, for a male patient weighing 158 kg with a height of 198 cm, the dose of IVIG would be 120 g; the patient’s actual dose was 165 g because the ordering physician opted to use a dose closer to the patient’s actual body weight.
Panel C shows the findings in Patient 3, a 69-year-old man with VITT that was complicated by stroke involving the right middle cerebral artery, cerebral venous sinus thrombosis (right cerebral transverse and sigmoid sinuses), and thromboses in the right internal carotid artery, right internal jugular vein, hepatic vein (main and left branch), and distal lower-limb vein (one branch of the left trifurcation), along with a diagnosis of pulmonary embolism. According to the “dosing weight” on the Ontario dose calculator, for a male patient weighing 140 kg with a height 185 cm, the IVIG dose would be 105 g; the actual dose the patient received was 100 g. A third dose of IVIG was given on day 24 because of concern regarding a partial loss of the IVIG effect, with possible exacerbation of VITT, since the patient’s platelet count fell from 125,000 to 106,000 per cubic millimeter and the d-dimer level increased from 14.8 to more than 20 mg per liter. After the third dose of IVIG, the platelet count rose to 165,000 per cubic millimeter, and the d-dimer level fell to 13.1 mg per liter. SC denotes subcutaneous, and UFH unfractionated heparin (which is shown in units per kilogram per hour in Patient 2; details regarding heparin dosing were not available for Patient 1).
In Patient 1, the platelet count rose from 39,000 to 77,000 per cubic millimeter during treatment with intravenous heparin, which was stopped before surgery. The platelet count did not change postoperatively during the 5-day administration of argatroban. However, after the administration of IVIG, the platelet count rose from 74,000 to 114,000 per cubic millimeter during a 2-day period, at which time the patient was discharged while receiving oral apixaban. At a follow-up visit 9 days later, the platelet count had normalized at 166,000 per cubic millimeter. Although mild thrombocytopenia recurred during the next 3 weeks, the d-dimer levels normalized.
In Patient 2, the platelet count initially rose from 36,000 to 77,000 per cubic millimeter after the administration of intravenous heparin. Subsequently, the platelet count fell, and heparin was switched to fondaparinux. After treatment with IVIG, the platelet count rose from 27,000 to 124,000 per cubic millimeter during a 3-day period; 7 days after the initiation of IVIG, the platelet count was 640,000 per cubic millimeter.
In Patient 3, no initial heparin was given. After VITT was diagnosed, IVIG and fondaparinux were administered, which resulted in an increase in the platelet count from 35,000 to 125,000 per cubic millimeter during a 3-day period, followed by a decrease to 106,000 per cubic millimeter and an increase in the d-dimer level; after a third dose of IVIG (as shown in the fourth blood sample), the platelet count rose to 165,000 per cubic millimeter, and the d-dimer level fell once again.
None of the three patients had clinical evidence of new or progressive thrombosis after IVIG treatment.
Laboratory Testing
Two of the three patients (Patients 2 and 3) had evidence of disseminated intravascular coagulation, including elevated d-dimer levels (>10 and >20 mg per liter, respectively [reference range, <0.50]), low-normal fibrinogen levels (140 and 200 mg per deciliter, respectively [reference range, 160 to 420]), and a mildly increased international normalized ratio (peak, 1.3 and 1.4, respectively [reference range, <1.2]). These results met the criteria for overt disseminated intravascular coagulation.15 After treatment with IVIG, the two patients had a reduction in serial d-dimer levels and an increase in serial fibrinogen levels, findings that were consistent with decreased hypercoagulability.
Platelet Immunologic Analyses
All three patients tested strongly positive for antibodies against PF4–polyanion complexes on ELISA (Table 1). No consistent reduction in ELISA reactivity was seen after treatment with IVIG, which indicated that IVIG did not inhibit VITT antibody binding to PF4. Patient 2 tested negative on a latex-based immunoturbidimetric assay (HemosIL HIT-Ab(PF4-H), Instrumentation Laboratory), a local rapid-screening test for HIT antibodies. According to a recent report,4 this screening test has shown negative results for VITT antibodies.
Table 1. ELISA Reactivity before and after Treatment with IVIG.*.
| Patient No. | ELISA Results | ||||
|---|---|---|---|---|---|
| Before IVIG | After First IVIG Dose | After Second IVIG Dose | After Third IVIG Dose | After Fourth IVIG Dose | |
| OD units | |||||
| Patient 1 | 2.70 | 2.80 | 2.86 | 2.91 | NA |
| Patient 2 | 1.78 | 2.38 | 2.39 | NA | NA |
| Patient 3 | 2.69 | 2.72 | 2.76 | 2.70 | 2.80 |
Results are shown in optical density (OD) units on enzyme-linked immunosorbent assay (ELISA) for IgG, IgA, and IgM antibodies against platelet factor 4 (PF4)–polyanion complexes (reference value, 0.40 OD units) before and after the administration of intravenous immune globulin (IVIG) in the three study patients. No consistent reduction in ELISA reactivity was seen after treatment with IVIG, which indicates that IVIG did not inhibit VITT antibody binding to PF4. The addition of a high level of heparin (100 U per milliliter) inhibited reactivity by more than 90% in all 12 samples tested (not shown in the table). NA denotes not applicable.
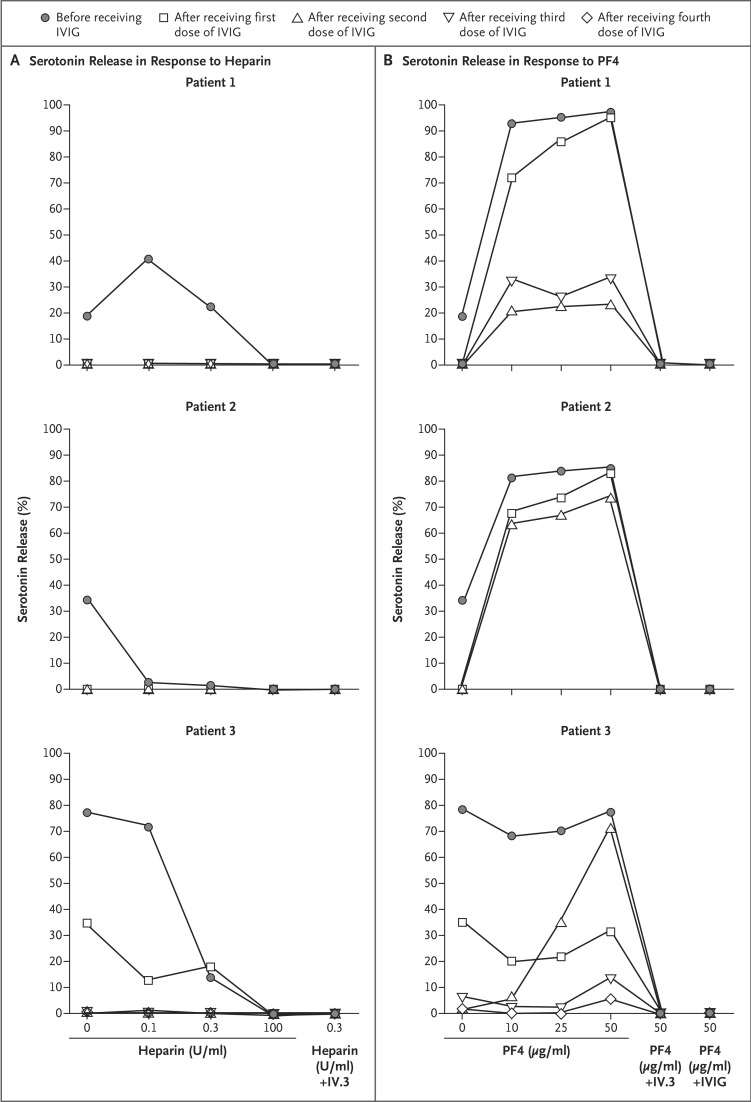
Serum obtained before IVIG administration (baseline) in the three patients showed three different reaction patterns on the serotonin-release assay, the standard platelet-activation assay for HIT. Patient 1 tested weakly positive for HIT, with serum producing 19% serotonin release with heparin at a concentration of 0 U per milliliter, 41% at 0.1 U per milliliter, 23% at 0.3 U per milliliter, and 0% at 100 U per milliliter (Figure 2A). (On this assay, a positive result is a release of >20% with heparin at a concentration of 0.1 U per milliliter or at a concentration of 0.3 U per milliliter that is inhibited at 100 U per milliliter.) Testing of serum from Patient 2 showed an atypical result, with 35% serotonin release observed in the absence of heparin that was inhibited to less than 5% with the addition of heparin at a concentration of 0.1 U per milliliter and 0.3 U per milliliter. Testing of serum from Patient 3 also showed an atypical result, with serotonin release of 78% with heparin at a concentration of 0 U per milliliter and 72% at 0.1 U per milliliter. For all three patients, serum-induced serotonin release was not observed after one or two doses of IVIG.
Figure 2. Results of Platelet-Activation Assays.
Panel A shows the results of a conventional platelet-activation assay for heparin-induced thrombocytopenia (a serotonin-release assay) in the three study patients. Platelet activation was inhibited in serum obtained from the three patients after treatment with IVIG. Panel B shows the results of a modified platelet-activation assay to detect VITT antibodies reactive against platelet factor 4 (PF4) in the three patients. Variable levels of inhibition of PF4-enhanced serotonin release were seen in patients’ serum obtained after treatment with IVIG. Complete inhibition was seen with the addition of FcγIIa receptor–blocking monoclonal antibody (IV.3) or the addition of IVIG at a concentration of 10 mg per milliliter.
In Patients 1 and 2, the addition of PF4 (10 μg per milliliter) to serum obtained at baseline showed strong (>80%) serotonin release (Figure 2B). No effect of PF4 was seen in the baseline serum from Patient 3, which showed a 78% serotonin release in the absence of PF4. In all three patients, serum that was obtained after IVIG treatment showed a reduction in reactivity in the presence of PF4; these reductions ranged from marked (in Patient 3) to minor (in Patient 2). Patient 2, whose serum showed the least reduction in serotonin release in the presence of PF4 after IVIG administration, had the greatest increase in the platelet count (from 27,000 to 640,000 per cubic millimeter during a 7-day period).
Discussion
The use of high-dose IVIG to treat thrombosis is unusual, especially considering that thrombotic events have been well documented after IVIG administration in patients with immune thrombocytopenic purpura, a hemorrhagic disorder.16,17 However, in the case of patients with autoimmune HIT9-11 and in our three study patients with VITT, the inhibition of serum-induced platelet-activating properties by IVIG was associated with increased platelet counts.
These findings probably reflect in vivo inhibition of antibody-induced platelet activation and reduced hypercoagulability, as shown by an increase in fibrinogen levels and a decrease in d-dimer levels in Patients 2 and 3. Increasing the platelet count is especially important when patients have severe thrombocytopenia and multiple unusual thromboses that require therapeutic-dose anticoagulation, especially in the context of hemorrhagic transformation after cerebral infarction. Moreover, antibodies that have been implicated in HIT also activate monocytes18 and neutrophils19 through membrane Fcγ receptors. Since patients with VITT can have severe thrombocytopenia that potentially lasts for several weeks, early administration of IVIG may be an important adjunct therapy to anticoagulation for the management of VITT. Currently, several VITT treatment guidelines recommend up-front administration of high-dose IVIG when VITT is strongly suspected and thrombosis is present.20-22 The dosing recommendation of 1 g per kilogram of body weight20-22 on two consecutive days (i.e., a total of 2 g per kilogram) can be ambiguous, since the applicable body weight can range from “ideal” to “actual” weight or an intermediate value called “dosing” weight.14 We suggest the use of dosing weight at the very least and preferably the actual body weight in making these calculations,20,22 given the dose-dependent effects of IVIG in decreasing antibody-induced platelet activation.9,23
Our study also shows how the platelet-activation test used in North American reference laboratories — the serotonin-release assay — can be adapted to detect VITT antibodies by including a reaction well for PF4. Recently, Greinacher and colleagues1 found that supplementation of PF4 (10 μg per milliliter) was helpful in diagnosing VITT with the use of a washed-platelet activation test based on visual detection of platelet aggregation. We found that the reactivity patterns of the serum obtained from our study patients with VITT were heterogeneous, since two samples tested positive (one weakly) on the conventional test for HIT, although the reaction pattern in serum obtained from Patient 3 was atypical. In Patients 1 and 2, who had weak and negative results, respectively, on the conventional test for heparin-dependent antibodies, the addition of PF4 resulted in strong serotonin release. Thus, we suggest that testing for HIT and VITT antibodies can be readily accomplished by performing the standard platelet-activation assay at the usual conditions, with the addition of PF4 (≥10 μg per milliliter) without heparin. We also recommend testing at 0 U per milliliter as a buffer control, since serum-induced serotonin release in the absence of heparin is a feature of autoimmune HIT.6,7 Our observations support the recommendation to test for VITT antibodies in serum before IVIG administration to avoid false negative results on the serotonin-release assay.24 In contrast, ELISA reactivity was not inhibited by IVIG treatment, as shown in Table 1 and as reported in patients with HIT.9,23
In our study, we describe three of the first patients in Canada in whom VITT was diagnosed after ChAdOx1 nCoV-19 vaccination. All three patients received doses of vaccine that were produced by the Serum Institute of India, which at the time of this study had supplied approximately two thirds of the ChAdOx1 nCoV-19 doses administered in Canada.25 After the initial cases of VITT were reported in Europe (beginning in late March 2021), the rollout of the AstraZeneca vaccine program in Canada was restricted to persons who were 55 years of age or older. This restriction accounted for the older ages of our three patients (72, 63, and 69 years of age). Of note, all three patients had one or more arterial thrombotic events. In addition, two patients had venous thrombosis. Of the 41 patients with VITT who had been described at the time of this report, arterial thrombosis had been diagnosed in only 5.1-5 However, the patients who have been described in other studies were mostly younger than those in our report. We suspect that older persons with VITT may be more likely to present with arterial thrombotic events.
Acknowledgments
We thank Jane C. Moore, B.Sc., for technical assistance; Jo-Ann I. Sheppard, B.Sc., for preparing earlier versions of the figures; Genevieve Huynh-Trudeau, M.D., Renaud Allen-Lefebvre, M.D., and the entire team involved in the care of Patient 1; and Elizabeth A. MacKay, M.D., Leslie Skeith, M.D., Paul F. Petrasek, M.D., Melissa Jones, M.D., Annie Boisvert, M.D., and the entire teams involved in the care of Patients 2 and 3.
Disclosure Forms
Editor’s note: After the submission of this manuscript, an independent group of authors submitted a summary of the case of Patient 2 reported here to the Canadian Medical Association Journal, and that report has been published (CMAJ 2021 June 14;193:E906-910).
This article was published on June 9, 2021, and last updated on August 19, 2021, at NEJM.org.
Footnotes
Supported by a grant (CIHR 452655, to Dr. Nazy) from the Canadian Institutes of Health Research.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. DOI: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muir K-L, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med 2021;384:1964-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J, Davis K, Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination — response from the manufacturer. N Engl J Med 2021;384:1965-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost 2017;15:2099-2114. [DOI] [PubMed] [Google Scholar]
- 7.Warkentin TE, Makris M, Jay RM, Kelton JG. A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am J Med 2008;121:632-636. [DOI] [PubMed] [Google Scholar]
- 8.Vayne C, Nguyen T-H, Rollin J, et al. Characterization of new monoclonal PF4-specific antibodies as useful tools for studies on typical and autoimmune heparin-induced thrombocytopenia. Thromb Haemost 2021;121:322-331. [DOI] [PubMed] [Google Scholar]
- 9.Warkentin TE. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: a review. Expert Rev Hematol 2019;12:685-698. [DOI] [PubMed] [Google Scholar]
- 10.Park BD, Kumar M, Nagalla S, et al. Intravenous immunoglobulin as an adjunct therapy in persisting heparin-induced thrombocytopenia. Transfus Apher Sci 2018;57:561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang SR, Wang Y, Weil EL, Padmanabhan A, Warkentin TE, Pruthi RK. Cerebral venous sinus thrombosis associated with spontaneous heparin-induced thrombocytopenia syndrome after total knee arthroplasty. Platelets 2020. October 1 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 12.Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood 1986;67:27-30. [PubMed] [Google Scholar]
- 13.Nazi I, Arnold DM, Warkentin TE, Smith JW, Staibano P, Kelton JG. Distinguishing between anti-platelet factor 4/heparin antibodies that can and cannot cause heparin-induced thrombocytopenia. J Thromb Haemost 2015;13:1900-1907. [DOI] [PubMed] [Google Scholar]
- 14.Ontario Regional Blood Coordinating Network. Ideal body weight calculator with IVIg dosing. 2021. (https://ivig.transfusionontario.org/dose/).
- 15.Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001;86:1327-1330. [PubMed] [Google Scholar]
- 16.Woodruff RK, Grigg AP, Firkin FC, Smith IL. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet 1986;2:217-218. [DOI] [PubMed] [Google Scholar]
- 17.Daniel GW, Menis M, Sridhar G, et al. Immune globulins and thrombotic adverse events as recorded in a large administrative database in 2008 through 2010. Transfusion 2012;52:2113-2121. [DOI] [PubMed] [Google Scholar]
- 18.Arepally GM, Mayer IM. Antibodies from patients with heparin-induced thrombocytopenia stimulate monocytic cells to express tissue factor and secrete interleukin-8. Blood 2001;98:1252-1254. [DOI] [PubMed] [Google Scholar]
- 19.Gollomp K, Kim M, Johnston I, et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight 2018;3(18):e99445-e99445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pai M, Chan B, Stall NM, et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT) following adenovirus vector COVID-19 vaccination. Science briefs of the Ontario COVID-19 Science Advisory Table. May 7, 2021. ( 10.47326/ocsat.2021.02.17.2.0). [DOI]
- 21.Guidance from the Expert Haematology Panel (EHP) on Covid-19 vaccine-induced immune thrombocytopenia and thrombosis (VITT). Updated guidance on management, version 1.7. April 20, 2021. (https://b-s-h.org.uk/media/19590/guidance-version-17-on-mngmt-of-vitt-20210420.pdf).
- 22.Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie 2021. April 1 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 23.Warkentin TE, Climans TH, Morin P-A. Intravenous immune globulin to prevent heparin-induced thrombocytopenia. N Engl J Med 2018;378:1845-1848. [DOI] [PubMed] [Google Scholar]
- 24.Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost 2021;19:1585-1588.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health Canada. Covid-19 vaccination in Canada. 2021. (https://health-infobase.canada.ca/covid-19/vaccination-coverage/).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.