Most persons who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are asymptomatic or have only mild-to-moderate symptoms, whereas others have progression to severe disease, characterized by acute respiratory distress encompassing pulmonary hyperinflammation and endothelial and broad multisystem dysfunction. A number of drugs have been developed or repurposed to prevent clinical deterioration. Mechanistically, therapeutic agents can be categorized as those that aim to target the viral life cycle, such as remdesivir or lopinavir–ritonavir; SARS-CoV-2–targeted antibody therapies1; and those that are focused on the host response, such as glucocorticoids and other immunomodulators.
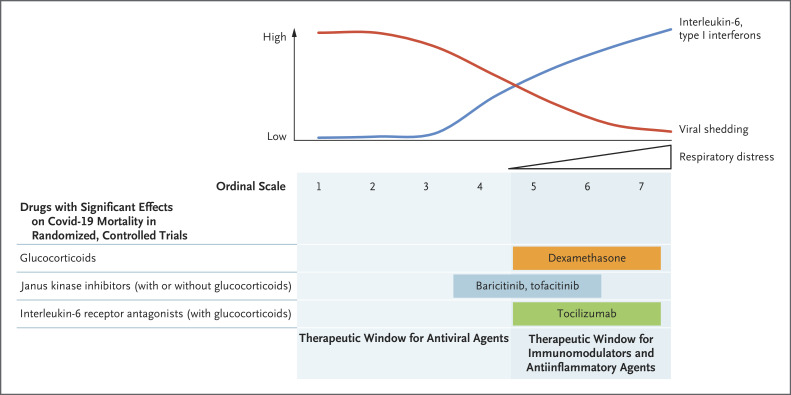
Immunomodulatory treatments have shown promise in reducing mortality among patients with coronavirus disease 2019 (Covid-19). In a randomized, open-label trial, treatment with dexamethasone plus usual care resulted in significantly lower 28-day mortality than usual care alone among patients receiving invasive mechanical ventilation (rate ratio, 0.64; 95% confidence interval [CI], 0.51 to 0.81) or supplemental oxygen without invasive mechanical ventilation (rate ratio, 0.82; 95% CI, 0.72 to 0.94), conditions that correspond to ordinal scores of 5 to 7 on the National Institute of Allergy and Infectious Diseases scale (the scale ranges from 1 to 8, with higher scores indicating a worse condition) (Figure 1).2 However, survival was not prolonged with dexamethasone therapy among patients who were not receiving supplemental oxygen, a condition corresponding to an ordinal score of 4 (rate ratio, 1.19; 95% CI, 0.92 to 1.55).
Figure 1. Overview of Current Therapies for Covid-19 with Effects on Mortality.
Early stages of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are characterized by high viral loads in the respiratory tract, which then decrease with progressing disease (red line). Inversely, the levels of inflammatory markers, including the proinflammatory cytokine interleukin-6 and type I interferons, increase with disease severity and respiratory distress (blue line). These observations suggest that antiviral agents are likely to be most effective in early stages of infection during the first week after symptom onset when disease is still mild to moderate. In contrast, immunomodulatory and antiinflammatory treatment is most effective in later stages of disease (ordinal scale score of 4 to 7; see below). Thus far, randomized, controlled trials have shown that treatment with glucocorticoids, Janus kinase inhibitors, and perhaps interleukin-6 receptor antagonists reduces mortality among patients with severe coronavirus disease 2019 (Covid-19). In contrast, antiviral agents have not been shown to have beneficial effects on survival. The ordinal scale of Covid-19 severity refers to definitions from the National Institute of Allergy and Infectious Diseases. The categories of the ordinal scale are as follows: a score of 1 indicates that the patient was not hospitalized, with no limitations on activities; 2, was not hospitalized but had limitation on activities or was receiving supplemental oxygen at home; 3, was hospitalized, without use of supplemental oxygen and no ongoing medical care; 4, was hospitalized and not receiving supplemental oxygen but was receiving ongoing medical care; 5, was hospitalized and receiving supplemental oxygen through low-flow devices; 6, was hospitalized and receiving oxygen through noninvasive ventilation or high-flow oxygen devices; 7, was hospitalized and receiving invasive mechanical ventilation or extracorporeal membrane oxygenation; and 8, died. Category 8 is not shown here.
Janus kinase (JAK) inhibitors constitute further weapons in the armamentarium here. Two randomized trials have evaluated the efficacy of the JAK inhibitors ruxolitinib and baricitinib in patients with Covid-19. In a small study, patients receiving ruxolitinib had significantly decreased levels, as compared with controls, of interleukin-6 and other cytokines.3 There were three deaths in the control group and none in the ruxolitinib group. However, the trial was underpowered to allow further conclusions. In the larger Adaptive Covid-19 Treatment Trial 2 (ACTT-2), patients were randomly assigned in a 1:1 ratio to receive either remdesivir plus baricitinib or remdesivir plus placebo.4 The inclusion of baricitinib reduced the recovery time (rate ratio for recovery, 1.16; 95% CI, 1.01 to 1.32; P=0.03) and improved clinical status at day 15 (odds ratio, 1.3; 95% CI, 1.0 to 1.6), particularly in patients with an ordinal score of 5 (receiving supplemental oxygen) or 6 (receiving high-flow oxygen therapy or noninvasive ventilation).
In this issue of the Journal, Guimarães et al. present the results of the Study of Tofacitinib in Hospitalized Patients with Covid-19 Pneumonia (STOP-COVID), a multicenter, randomized trial of the JAK inhibitor tofacitinib.5 At enrollment, 289 hospitalized patients were randomly assigned in a 1:1 ratio to receive either tofacitinib at a dose of 10 mg or placebo twice daily. The majority of patients (79% at baseline and 89% during hospitalization) received glucocorticoids, whereas this was true for only 22% of the patients in ACTT-2. It is notable that despite the relatively small size of the trial, the investigators found a significantly lower risk of death or respiratory failure with tofacitinib than with placebo among patients with ordinal scale scores of 4 to 6 (risk ratio, 0.63; 95% CI, 0.41 to 0.97; P=0.04). Whereas previous studies2,4 of glucocorticoids alone or of JAK inhibitors plus remdesivir have shown efficacy only in patients with ordinal scores of 5 to 7 and of 5 or 6, respectively, the results of the present trial suggest that a combination regimen of JAK inhibitors and glucocorticoids might widen the window of therapeutic benefit (Figure 1).
The idea that the combination of selective cytokine inhibitors with dexamethasone may have additive or even synergistic effects may explain some of the apparently conflicting results in trials of the interleukin-6 receptor antagonist tocilizumab in patients with Covid-19. Treatment with tocilizumab reduced mortality in both the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP; hazard ratio for survival vs. control, 1.61; 95% credible interval, 1.25 to 2.08) and the Randomised Evaluation of Covid-19 Therapy (RECOVERY) trial (rate ratio for death vs. usual care, 0.85; 95% CI, 0.76 to 0.94; P=0.003) among patients with ordinal scores of 5 to 7.6,7 In these trials, most patients (93% in REMAP-CAP and 82% in RECOVERY) were also treated with glucocorticoids, and there appeared to be an interaction between the effects of interleukin-6 receptor antagonists and those of glucocorticoids. In contrast, the COVACTA trial showed no significant difference in mortality with tocilizumab as compared with placebo (19.7% vs. 19.4%; difference, 0.3 percentage points; 95% CI, −7.6 to 8.2; P=0.94) in a patient population in which the percentage of patients receiving glucocorticoids during the trial was lower in the tocilizumab group than in the placebo group (33.7% vs. 52.1%).8
Combined, these data indicate that antiinflammatory therapy in hospitalized patients with Covid-19 who are receiving supplemental oxygen therapy or ventilation results in reduced overall mortality and that treatment with glucocorticoids and mechanistically distinct antiinflammatory agents, notably JAK inhibitors and perhaps interleukin-6 receptor antagonists, provides additive benefits. Among the antiinflammatory agents, JAK inhibitors have the distinct advantage that their low cost and oral administration allows for scalable use in low- and middle-income countries in regimens with or without concomitant glucocorticoids. Furthermore, some JAK inhibitors provide dosing flexibility, with an absence of drug–drug interactions, usefulness at lower glomerular filtration rates, a short half-life, and an established safety profile in highly vulnerable populations, notably the elderly.9
Disclosure Forms
Footnotes
Disclosure forms provided by the authors are available with the full text of this editorial at NEJM.org.
References
- 1.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325:632-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020;146:137-146.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021;384:795-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;385:406-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021;384:1491-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021;397:1637-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 2021;384:1503-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stebbing J, Sánchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv 2021;7:eabe4724-eabe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.