Abstract
Background
Despite the high efficacy of the BNT162b2 messenger RNA vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rare breakthrough infections have been reported, including infections among health care workers. Data are needed to characterize these infections and define correlates of breakthrough and infectivity.
Methods
At the largest medical center in Israel, we identified breakthrough infections by performing extensive evaluations of health care workers who were symptomatic (including mild symptoms) or had known infection exposure. These evaluations included epidemiologic investigations, repeat reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assays, antigen-detecting rapid diagnostic testing (Ag-RDT), serologic assays, and genomic sequencing. Correlates of breakthrough infection were assessed in a case–control analysis. We matched patients with breakthrough infection who had antibody titers obtained within a week before SARS-CoV-2 detection (peri-infection period) with four to five uninfected controls and used generalized estimating equations to predict the geometric mean titers among cases and controls and the ratio between the titers in the two groups. We also assessed the correlation between neutralizing antibody titers and N gene cycle threshold (Ct) values with respect to infectivity.
Results
Among 1497 fully vaccinated health care workers for whom RT-PCR data were available, 39 SARS-CoV-2 breakthrough infections were documented. Neutralizing antibody titers in case patients during the peri-infection period were lower than those in matched uninfected controls (case-to-control ratio, 0.361; 95% confidence interval, 0.165 to 0.787). Higher peri-infection neutralizing antibody titers were associated with lower infectivity (higher Ct values). Most breakthrough cases were mild or asymptomatic, although 19% had persistent symptoms (>6 weeks). The B.1.1.7 (alpha) variant was found in 85% of samples tested. A total of 74% of case patients had a high viral load (Ct value, <30) at some point during their infection; however, of these patients, only 17 (59%) had a positive result on concurrent Ag-RDT. No secondary infections were documented.
Conclusions
Among fully vaccinated health care workers, the occurrence of breakthrough infections with SARS-CoV-2 was correlated with neutralizing antibody titers during the peri-infection period. Most breakthrough infections were mild or asymptomatic, although persistent symptoms did occur.
Since its rollout in late 2020 in Israel, the BNT162b2 messenger RNA vaccine (Pfizer–BioNTech) has been highly effective in preventing clinically significant coronavirus disease 2019 (Covid-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1-3 The vaccine has also been shown to reduce the incidence of asymptomatic infection and the associated infectivity.4,5 However, breakthrough infections have emerged in a small percentage of vaccine recipients, a phenomenon that has been described in other countries and health care institutions.6-8 To date, no correlate of protection from breakthrough infection has been reported.9
At the Sheba Medical Center in Ramat Gan, we conducted a prospective cohort study to assess the effectiveness of the BNT162b2 vaccine among health care workers and to examine possible correlates of protection and infectivity in this population.
Methods
Study Setting
Sheba Medical Center is the largest medical center in Israel and is staffed by 12,586 health care workers, including employees, students, and volunteers. From December 19, 2020, to April 28, 2021, a total of 91% of the center personnel received two doses of the BNT162b2 vaccine. This period was followed by a rapid decrease in newly detected cases.4,10 Simultaneously, efforts were extended to identify new cases with the use of daily health questionnaires, a telephone hotline, extensive epidemiologic investigations of exposure events, and contact tracing of infected patients and personnel. Testing for the presence of SARS-CoV-2 by means of reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay remained readily available for fully vaccinated staff members who were symptomatic or had been exposed to an infected person, regardless of symptoms. Antigen-detecting rapid diagnostic testing (Ag-RDT) was available as an initial screening tool in the personnel clinic in combination with RT-PCR testing. The study was approved by the institutional review board at Sheba Medical Center.
Study Design and Population
On January 20, 2021, we initiated the study among health care workers at Sheba Medical Center, 11 days after the first staff members had received a second dose of the BNT162b2 vaccine. Data were collected for 14 weeks, until April 28. Concurrently, the third and largest Covid-19 pandemic surge emerged in Israel and reached its peak on January 14, 2021, with reports of an average of 8424 daily cases.
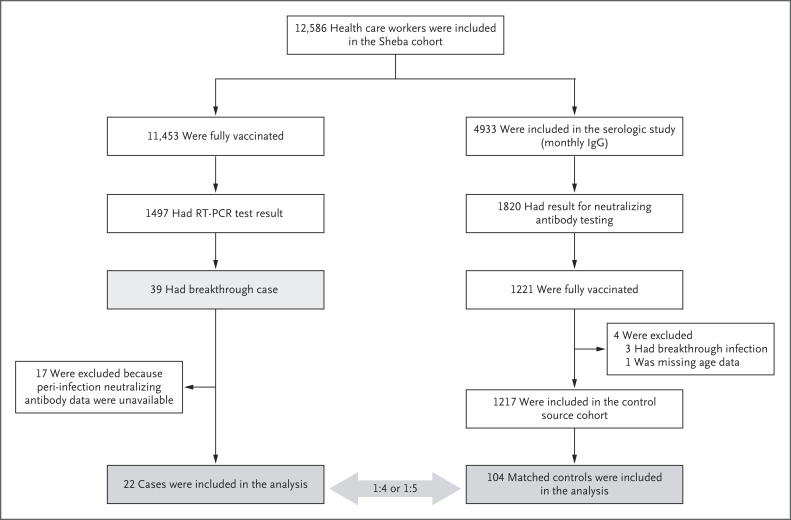
The study goal was to identify every breakthrough infection, including asymptomatic infections, that occurred during the study period among the health care workers at the center. A breakthrough infection was defined as the detection of SARS-CoV-2 on RT-PCR assay performed 11 or more days after receipt of a second dose of BNT162b2 if no explicit exposure or symptoms had been reported during the first 6 days. In this study, we characterized all breakthrough infections among fully vaccinated health care workers and conducted a matched case–control analysis to identify possible correlates of breakthrough infection. For the case–control analysis, we selected control serum samples that had been obtained during a prospective cohort study to analyze vaccine-induced immune responses and dynamics at the Sheba Medical Center.11 (Details regarding the serologic study are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.) Among the health care workers who had participated in the serologic study, those who had results of neutralizing antibody testing and complete data were eligible as a basis for selecting controls (Figure 1).
Figure 1. Cases and Controls in Study Design.
Shown is the chronologic sequence of events for health care workers who were included in the case–control study. There was some overlap between the cases and controls, since some of the workers with breakthrough cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection had also been included in the earlier serologic analysis from which control samples were selected. A case–control ratio of 1:4 or 1:5 was selected to maximize the statistical power of the study. RT-PCR denotes reverse transcriptase–polymerase chain reaction.
For each breakthrough case, we matched samples that had been obtained from four or five uninfected controls according to the following variables: sex, age, the interval between the second dose of BNT162b2 vaccine and serologic testing, and immunosuppression status. We compared neutralizing antibody titers obtained within a week before SARS-CoV-2 detection on RT-PCR testing, including the day of diagnosis (peri-infection period); peak neutralizing antibody titers obtained during the initial postvaccination period; and S-specific IgG antibodies against SARS-CoV-2 obtained at both time points. Breakthrough cases for which serologic samples were not available were excluded from this analysis.
Data and Sample Collection
All health care workers with breakthrough infection were immediately contacted, and an epidemiologic investigation was conducted by the hospital Infection Prevention and Control Unit. All health care workers with positive test results were requested to undergo several additional tests, including viral genome sequencing, repeat RT-PCR testing, Ag-RDT, and SARS-CoV-2 serologic testing. Testing for the presence of neutralizing and anti-S IgG antibodies was performed on the day of detection of infection unless the health care worker had participated in the Sheba serologic study,11 and these results were available from the week preceding detection. After recovery from infection, all health care workers were asked to provide a second blood sample for the measurement of N-specific IgG antibodies. In line with hospital protocol for the detection of secondary infections, close in-hospital contacts of infected health care workers were asked to undergo RT-PCR testing 5 days after their last known exposure. Infected persons were also urged to advise their household members and other close community contacts to undergo RT-PCR testing.
Nasopharyngeal swabs were collected by trained personnel, and RT-PCR testing was performed with the use of the Allplex 2019-nCoV assay (Seegene), with findings expressed as the cycle threshold (Ct) for the gene encoding the nucleocapsid protein (N gene). A Ct value of less than 30, which indicated an increased viral load, was used to determine infectivity.12,13 We performed Ag-RDT using the NowCheck COVID-19 Ag test (Bionote).
To identify variants of concern, we performed multiplex real-time one-step RT-PCR assays to detect mutations in the spike (S) protein (E484K, N501Y, and HV69/70). To verify the results of this testing, whole-genome sequencing was performed with the use of the COVIDSeq library preparation kit (Illumina), as described previously.14
We used three different measures to assess antibody-mediated immune responses: serologic testing for S1 IgG antibodies (Beckman Coulter), SARS-CoV-2 pseudovirus neutralization assay,15 and Elecsys Anti-SARS-CoV-2 Immunoassay (Roche) to test for anti-N antigen. We assessed two outcome measures — neutralizing antibodies and IgG antibodies — and obtained titers at two time points: the peri-infection period (within 1 week before infection) and the peak period (within the first month after the second dose of vaccine).
Statistical Analysis
For the case–control analyses, we included data from all breakthrough case patients for whom peri-infection neutralizing antibody titers were available. A case–control ratio of 1:4 or 1:5 was selected to maximize the statistical power of the study. We matched the control samples with the case samples using the algorithm that is described in detail in the text and in Figure S1 in the Supplementary Appendix. On the basis of this algorithm, we first performed case–control matching according to the interval between the second vaccine dose and serologic testing, followed by categorization according to sex, age, and immunosuppression status. If this pool yielded more than five controls, we selected five at random. For a single case patient with immunosuppression, only three of four controls were sex-matched. In an additional subgroup analysis, we excluded three asymptomatic case patients with borderline results (repeat Ct, >35). To further confirm the robustness of our matching criteria, we performed a sensitivity analysis with a different order of covariates in the matching algorithm that used a uniform age criterion and did not match for sex. (Details regarding the sensitivity analysis are provided in the Supplementary Appendix.)
To measure the antibody-mediated immune response, we compared log-transformed antibody titers between cases and matched controls using a generalized estimating equation (GEE) with the group assignment (case or control) used as the predictor. For the analyses of the full cohort and the subgroup that excluded the borderline cases, we report the observed geometric mean titer (GMT) and its 95% confidence interval, the GMT predicted by the GEE model, and the ratio of cases to controls (the GMT of the cases divided by the GMT of the controls).
To assess the correlation between the lowest Ct value and the neutralizing antibody level during the peri-infection period, we applied a linear regression model to estimate the slope of the regression line and its 95% confidence interval. We used the chi-square test or Fisher’s exact test to compare demographic and clinical characteristics of the case patients who were included in the case–control study with those for whom peri-infection results of neutralizing antibody testing were not available.
Results
Breakthrough Infections
Among 11,453 fully vaccinated health care workers, 1497 (13.1%) underwent RT-PCR testing during the study period. Of the tested workers, 39 breakthrough cases were detected. More than 38 persons were tested for every positive case that was detected, for a test positivity of 2.6%. Thus, this percentage was much lower than the test positivity rate in Israel at the time, since the ratio between positive results and the extensive number of tests that were administered in our study was much smaller than that in the national population.
Of the 39 breakthrough case patients, 18 (46%) were nursing staff members, 10 (26%) were administration or maintenance workers, 6 (15%) were allied health professionals, and 5 (13%) were physicians. The average age of the 39 infected workers was 42 years, and the majority were women (64%). The median interval from the second vaccine dose to SARS-CoV-2 detection was 39 days (range, 11 to 102). Only one infected person (3%) had immunosuppression. Other coexisting illnesses are detailed in Table S1.
In all 37 case patients for whom data were available regarding the source of infection, the suspected source was an unvaccinated person; in 21 patients (57%), this person was a household member. Among these case patients were two married couples, in which both sets of spouses worked at Sheba Medical Center and had an unvaccinated child who had tested positive for Covid-19 and was assumed to be the source. In 11 of 37 case patients (30%), the suspected source was an unvaccinated fellow health care worker or patient; in 7 of the 11 case patients, the infection was caused by a nosocomial outbreak of the B.1.1.7 (alpha) variant. These 7 patients, who worked in different hospital sectors and wards, were all found to be linked to the same suspected unvaccinated index patient who had been receiving noninvasive positive-pressure ventilation before her infection had been detected.
Of the 39 cases of infection, 27 occurred in workers who were tested solely because of exposure to a person with known SARS-CoV-2 infection. Of all the workers with breakthrough infection, 26 (67%) had mild symptoms at some stage, and none required hospitalization. The remaining 13 workers (33% of all cases) were asymptomatic during the duration of infection; of these workers, 6 were defined as borderline cases, since they had an N gene Ct value of more than 35 on repeat testing.
The most common symptom that was reported was upper respiratory congestion (36% of all cases), followed by myalgia (28%) and loss of smell or taste (28%); fever or rigors were reported in 21% (Table S1). On follow-up questioning, 31% of all infected workers reported having residual symptoms 14 days after their diagnosis. At 6 weeks after their diagnosis, 19% reported having “long Covid-19” symptoms, which included a prolonged loss of smell, persistent cough, fatigue, weakness, dyspnea, or myalgia. Nine workers (23%) took a leave of absence from work beyond the 10 days of required quarantine; of these workers, 4 returned to work within 2 weeks. One worker had not yet returned after 6 weeks.
Verification Testing and Secondary Infections
Repeat RT-PCR assays were performed on samples obtained from most of the infected workers and for all case patients with an initial N gene Ct value of more than 30 to verify that the initial test was not taken too early, before the worker had become infectious. A total of 29 case patients (74%) had a Ct value of less than 30 at some point during their infection. However, of these workers, only 17 (59%) had positive results on a concurrent Ag-RDT. Ten workers (26%) had an N gene Ct value of more than 30 throughout the entire period; 6 of these workers had values of more than 35 and probably had never been infectious.
Of the 33 isolates that were tested for a variant of concern, 28 (85%) were identified as the B.1.1.7 variant, by either multiplex PCR assay or genomic sequencing. At the time of this study, the B.1.1.7 variant was the most widespread variant in Israel and accounted for up to 94.5% of SARS-CoV-2 isolates.1,16 Since the end of the study, the country has had a surge of cases caused by the delta variant, as have many other countries worldwide.
Thorough epidemiologic investigations of data regarding in-hospital contact tracing did not detect any cases of transmission from infected health care workers (secondary infections) among the 39 primary infections. Among the 31 cases for whom data regarding household transmission (including symptoms and RT-PCR results) were available, no secondary infections were detected, including 10 case patients and their 27 household members in whom the health care worker was the only index case patient.
Data regarding postinfection N-specific IgG antibodies were available for 22 of 39 case patients (56%) on days 8 to 72 after the first positive result on RT-PCR assay. Of these workers, 4 (18%) did not have an immune response, as detected by negative results on N-specific IgG antibody testing. Among these 4 workers were 2 who were asymptomatic (Ct values, 32 and 35), 1 who underwent serologic testing only on day 10 after diagnosis, and 1 who had immunosuppression.
Case–Control Analysis
The results of peri-infection neutralizing antibody tests were available for 22 breakthrough cases. Included in this group were 3 health care workers who had participated in the serologic study and had a test performed in the week preceding detection; in 19 other workers, neutralizing and S-specific IgG antibodies were assessed on detection day. Of these 19 case patients, 12 were asymptomatic at the time of detection. For each case, 4 to 5 controls were matched as described (Fig. S1). In total, 22 breakthrough cases and their 104 matched controls were included in the case–control analysis.
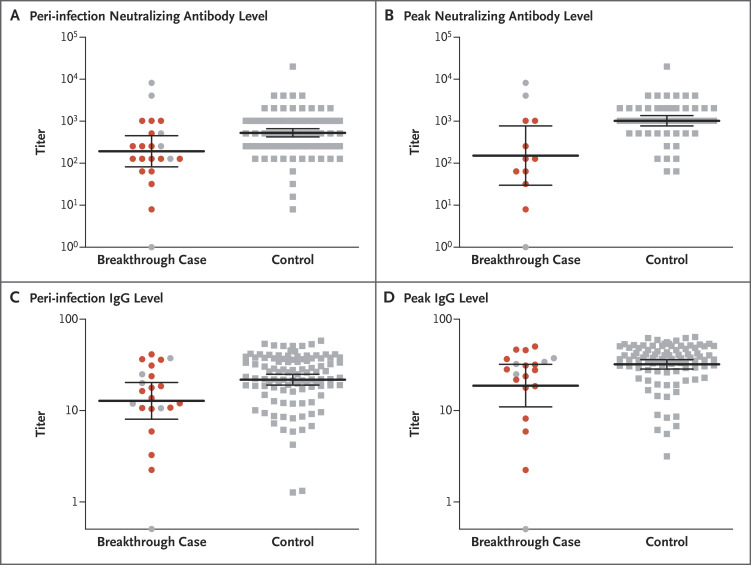
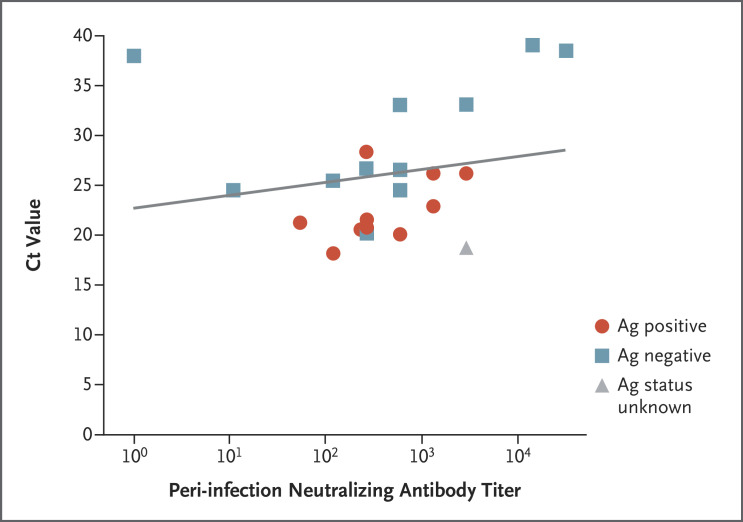
The predicted GMT of peri-infection neutralizing antibody titers was 192.8 (95% confidence interval [CI], 67.6 to 549.8) for cases and 533.7 (95% CI, 408.1 to 698.0) for controls, for a predicted case-to-control ratio of neutralizing antibody titers of 0.361 (95% CI, 0.165 to 0.787) (Table 1 and Figure 2A). In a subgroup analysis in which the borderline cases were excluded, the ratio was 0.353 (95% CI, 0.185 to 0.674). Peri-infection neutralizing antibody titers in the breakthrough cases were associated with higher N gene Ct values (i.e., a lower viral RNA copy number) (slope of regression line, 171.2; 95% CI, 62.9 to 279.4) (Figure 3).
Table 1. Population Characteristics and Outcomes in the Case–Control Study.
| Variable | Cases (N=22) |
Controls (N=104) |
Ratio of Cases to Controls* |
|---|---|---|---|
| Population characteristics | |||
| Demographic | |||
| Female sex — no. (%) | 14 (64) | 70 (67) | |
| Mean age — yr | 43 | 45 | |
| Coexisting illnesses — no. (%) | |||
| Immunosuppression | 1 (4.5) | 4 (3.8) | |
| Autoimmune disease | 0 | 1 (1) | |
| Body-mass index >30† | 0 | 1 (1) | |
| Median interval from second dose and antibody test — days | 36 | 35 | |
| Outcomes ‡ | |||
| Peri-infection neutralizing antibody | |||
| No. of participants | 22 | 104 | |
| Observed GMT (95% CI) | 192.8 (81.8–454.3) |
530.4 (424.4–662.8) |
|
| Predicted GMT by GEE model (95% CI) | 192.8 (67.6–549.8) |
533.7 (408.1–698.0) |
0.361 (0.165–0.787) |
| Peri-infection neutralizing antibody without borderline results | |||
| No. of participants | 19 | 89 | |
| Observed GMT (95% CI) | 177.7 (98.2–321.7) |
501.3 (395.1–636.0) |
|
| Predicted GMT by GEE model (95% CI) | 178.2 (70.6–449.8) |
505.4 (382.5–667.8) |
0.353 (0.185–0.674) |
| Peri-infection anti-S IgG | |||
| No. of participants | 22 | 103 | |
| Observed GMT (95% CI) | 11.2 (5.7–22.0) |
21.8 (18.9–25.0) |
|
| Predicted GMT by GEE model (95% CI) | 11.2 (5.3–23.9) |
21.8 (18.6–25.5) |
0.514 (0.282–0.937) |
| Peri-infection anti-S IgG without borderline results | |||
| No. of participants | 19 | 88 | |
| Observed GMT (95% CI) | 13.8 (9.5–20.0) |
21.3 (18.5–24.5) |
|
| Predicted GMT by GEE model (95% CI) | 13.8 (7.9–23.9) |
21.4 (18.2–25.1) |
0.646 (0.437–0.954) |
| Peak neutralizing antibody§ | |||
| No. of participants | 12 | 56 | |
| Observed GMT (95% CI) | 152.2 (29.9–775.1) |
1028.0 (772.2–1368.0) |
|
| Predicted GMT by GEE model (95% CI) | 152.2 (30.5–759.3) |
1027.5 (761.6–1386.2) |
0.148 (0.040–0.548) |
| Peak neutralizing antibody without borderline results | |||
| No. of participants | 9 | 41 | |
| Observed GMT (95% CI) | 118.5 (35.5–395.3) |
1029.0 (735.3–1440.0) |
|
| Predicted GMT by GEE model (95% CI) | 119.2 (30.4–467.5) |
1043.4 (721.0–1509.9) |
0.114 (0.042–0.309) |
| Peak anti-S IgG | |||
| No. of participants | 20 | 92 | |
| Observed GMT (95% CI) | 16.3 (7.4–35.5) |
32.1 (28.5–36.2) |
|
| Predicted GMT by GEE model (95% CI) | 16.3 (7.4–35.8) |
32.2 (28.6–36.2) |
0.507 (0.260–0.989) |
| Peak anti-S IgG without borderline results | |||
| No. of participants | 17 | 77 | |
| Observed GMT (95% CI) | 21.9 (14.3–33.4) |
32.6 (28.7–36.9) |
|
| Predicted GMT by GEE model (95% CI) | 22.0 (13.4–36.0) |
32.6 (28.8–37.0) |
0.021 (0.016–0.026) |
No case-to-control ratios were calculated for the population characteristics.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Shown is the observed log-transformed geometric mean titer (GMT) of antibody in cases and matched controls, as well as results predicted with the use of a generalized estimating equation (GEE). For the analyses of the full cohort and the subgroup that excluded borderline cases (i.e., participants who were asymptomatic with a repeat cycle threshold of >35), shown is the observed GMT and 95% confidence interval, as well as results predicted by the GEE model, along with the predicted ratio between cases and controls. The peri-infection period was the week before the detection of SARS-CoV-2, including the day of diagnosis.
The peak titers are the highest values obtained within a month after the second dose of vaccine.
Figure 2. Neutralizing Antibody and IgG Titers among Cases and Controls, According to Timing.
Among the 39 fully vaccinated health care workers who had breakthrough infection with SARS-CoV-2, shown are the neutralizing antibody titers during the peri-infection period (within a week before SARS-CoV-2 detection) (Panel A) and the peak titers within 1 month after the second dose (Panel B), as compared with matched controls. Also shown are IgG titers during the peri-infection period (Panel C) and peak titers (Panel D) in the two groups. Each case of breakthrough infection was matched with 4 to 5 controls according to sex, age, immunosuppression status, and timing of serologic testing after the second vaccine dose. In each panel, the horizontal bars indicate the mean geometric titers and the 𝙸 bars indicate 95% confidence intervals. Symptomatic cases, which were all mild and did not require hospitalization, are indicated in red.
Figure 3. Correlation between Neutralizing Antibody Titer and N Gene Cycle Threshold as Indication of Infectivity.
The results of antigen-detecting (Ag) rapid diagnostic testing for the presence of SARS-CoV-2 are shown, along with neutralizing antibody titers and N gene cycle threshold (Ct) values in 22 fully vaccinated health care workers with breakthrough infection for whom data were available (slope of regression line, 171.2; 95% CI, 62.9 to 279.4).
A peak neutralizing antibody titer within the first month after the second vaccine dose was available for only 12 of the breakthrough cases; the GEE predicted peak neutralizing antibody titer was 152.2 (95% CI, 30.5 to 759.3) in 12 cases and 1027.5 (95% CI, 761.6 to 1386.2) in 56 controls, for a ratio of 0.148 (95% CI, 0.040 to 0.548) (Figure 2B). In the subgroup analysis in which borderline cases were excluded, the ratio was 0.114 (95% CI, 0.042 to 0.309).
The observed and predicted GMTs of peri-infection S-specific IgG antibody levels in breakthrough infection cases were lower than that in controls, with a predicted ratio of 0.514 (95% CI, 0.282 to 0.937) (Figure 2C). The observed and predicted peak IgG GMTs in cases were also somewhat lower than those in controls (0.507; 95% CI, 0.260 to 0.989) (Figure 2D).
To assess whether our practice of measuring antibodies on the day of diagnosis created bias by capturing anamnestic responses to the current infection, we plotted peak (first-month) IgG titers against peri-infection titers on the day of diagnosis in 13 case patients for whom both values were available. In all cases, peri-infection titers were lower than the previous peak titers, indicating that the titers that were obtained on the day of diagnosis were probably representative of peri-infection titers (Fig. S2).
Discussion
In this study, we characterized all Covid-19 breakthrough infections among 39 fully vaccinated health care workers during the 4-month period after the second vaccine dose and compared the peri-infection humoral response in these workers with the response in matched controls. We found a low rate of breakthrough infection (0.4%). Among the 39 workers who tested positive for Covid-19, most had few symptoms, yet 19% had long Covid-19 symptoms (>6 weeks).
Most of the infected health care workers had N gene Ct values that suggested they had been infectious at some point. These workers included some who had been asymptomatic and thus who had infections that would not have been detected without the rigorous screening that followed any minor known exposure. This factor suggests that at least in some cases, the vaccine protected against symptomatic disease but not against infection. However, no secondary infections were traced back to any of the breakthrough cases, which supports the inference that these workers were less contagious than unvaccinated persons, as has been reported previously.4,5,17,18 Mandated isolation after positive results on RT-PCR assay regardless of vaccination status could have contributed to this observation. Most important, we found that low titers of neutralizing antibody and S-specific IgG antibody may serve as markers of breakthrough infection.
Identifying immune correlates of protection (or lack thereof) from SARS-CoV-2 is critical to predicting how the expected antibody decay will affect clinical outcomes, if and when a booster dose will be needed, and whether vaccinated persons are protected. Such capacity for prediction is particularly important for new vaccine development. The assumption that the presence of neutralizing antibodies would correlate with protection from reinfection with SARS-CoV-2 has been supported by studies comparing the incidence of infection between seropositive and seronegative persons.9,19 Recently, Khoury et al.20 and Earle et al.21 determined that the neutralization level is highly predictive of immune protection in comparing population values from vaccine efficacy and immunogenicity trials. Here, we report data on persons in a vaccinated population that support this correlate of protection.
Neutralizing antibody titers are typically not readily available, and a more practical immune correlate of protection is required, such as the anti-S IgG titer. We and others have previously found a significant correlation between neutralizing antibody titers and anti-S or anti–receptor binding domain IgG antibody titers.11,22 In this study, the correlation between levels of neutralizing antibodies and breakthrough infections was stronger than that for IgG antibodies.
We found that the difference in the peak titers of neutralizing and IgG antibodies between cases and controls was more strongly associated with the risk of infection than the difference in the peri-infection titers. This finding was consistent with the hypothesis that the neutralizing antibody titer after vaccination is a marker of overall immune response and suggested a possible role for the IgG titer. Thus, a decrease in the titer of either of these antibodies (rather than in the peak titer) may not accurately predict a decrease in protection. Moreover, we found that the peri-infection neutralizing antibody titers correlated with the viral load and thus with the infectivity of breakthrough cases. This result may eventually be even more important, since vaccine-induced immunity has been shown to be greatly protective against clinical disease but somewhat less protective against both infection and infectivity.4 Yet in this relatively small cohort, we could not determine a specific protective titer for either serologic measure that was tested. Furthermore, our cohort included health care workers who were mostly young and healthy, and all breakthrough cases were mild. We have previously reported that 95% of vaccinated health care workers were found to have a neutralizing antibody titer of more than 256 within 2 weeks after the second BNT162b2 vaccine dose.11 However, it remains to be determined whether the decay of serum antibody levels is a good indicator for the timing of booster administration. The degree of protection may depend more on the initial immune response than on the decay of antibody levels, since memory cells are expected to respond to future exposures. Our results suggest that the peak antibody titers also correlated with protection, despite the low number of cases in our study.
We identified the B.1.1.7 variant in 85% of cases, similar to its prevalence in the community.1,16 This finding is in line with reports from California, New York, and Massachusetts23-25 showing that the distribution of variants of concern in breakthrough infections was similar to that in the general unvaccinated population. These findings suggest that breakthrough isolates do not reflect selection pressure toward particular immunity-evading variants. In contrast, reports in which certain variants of concern were more prevalent in breakthrough infections have been published as well.8,16,26 Our study was not designed to address this question regarding variants of concern in breakthrough infections.
Our study has several limitations. First, even though we provide extensive documentation of a cohort of breakthrough infections, the numbers of cases were relatively small. Second, this cohort represents mostly young and healthy persons, and all breakthrough infections were mild and did not require hospitalization. Thus, we could not determine the correlate of protection from severe infection or infection in vulnerable populations of older persons with coexisting illnesses. Third, we may have missed asymptomatic cases despite the intensive effort to test all exposed health care workers, since we did not conduct surveillance testing. Fourth, the controls were not matched according to testing or exposure but only according to the timing of serologic testing in vaccinated, uninfected health care workers. Thus, we could not control for differences in the risk of exposure to Covid-19. This factor may have led to an underestimation of the difference in protection between cases and controls. Finally, in many case patients, the peri-infection antibody titer that was available had been obtained on the day of detection of the infection (which in some cases could have been a few days into the infection period) and therefore was possibly already elevated because of the infection. However, since most cases were detected in the presymptomatic stage, we expect that such contamination of results was minor. Moreover, we found that among the case patients in whom both peri-infection and earlier neutralizing antibody results were available, the majority of titers were lower during the peri-infection period than during the earlier period, which also suggests that this contamination was negligible. If such contamination were substantial, the result would likely be biased toward the null hypothesis of no relationship between antibody titers and breakthrough infection.
In this study, we found that although the BNT162b2 vaccine is extremely effective, rare breakthrough infections carry an infectious potential and create a special challenge, since such infections are often asymptomatic and may pose a risk to vulnerable populations.
Acknowledgments
We thank the members of the nursing staff in the Infection Prevention and Control Unit and the Epidemiologic Investigation team for their thorough and repeated questioning and sampling; Miki Goldenfeld and Lilac Meltzer for sample collection; Yael Beker-Ilani, Maayan Atias, and Hanaa Jaber for performing laboratory analyses; and Laurence Freedman and Havi Morad for statistical advice.
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on July 28, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Dr. Lipsitch was supported by the Morris–Singer Foundation and by a cooperative agreement (U01CA261277) with the National Cancer Institute.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regev-Yochay G, Amit S, Bergwerk M, et al. Decreased infectivity following BNT162b2 vaccination: a prospective cohort study in Israel. Lancet Reg Health Eur 2021;7:100150-100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petter E, Mor O, Zuckerman N, et al. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. February 8, 2021. (https://www.medrxiv.org/content/10.1101/2021.02.08.21251329v1). preprint.
- 6.Stephenson J. COVID-19 vaccinations in nursing home residents and staff give robust protection, though breakthrough infections still possible. JAMA Health Forum 2021;2(4):e211195-e211195 (https://jamanetwork.com/channels/health-forum/fullarticle/2779436). [DOI] [PubMed] [Google Scholar]
- 7.Tyagi K, Ghosh A, Nair D, et al. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in New Delhi, India. Diabetes Metab Syndr 2021;15:1007-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med 2021;384:2212-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krammer F. Correlates of protection from SARS-CoV-2 infection. Lancet 2021;397:1421-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021;397:875-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 vaccine-induced immune responses and dynamics vary among age groups, sex and co-morbidities: a longitudinal prospective cohort study. March 4, 2021. (https://ssrn.com/abstract=3790408). preprint.
- 12.Jefferson T, Spencer EA, Brassey J, Heneghan C. Viral cultures for COVID-19 infectious potential assessment — a systematic review. Clin Infect Dis 2020. December 3 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020;25:2001483-2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuckerman NS, Pando R, Bucris E, et al. Comprehensive analyses of SARS-CoV-2 transmission in a public health virology laboratory. Viruses 2020;12:854-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieterle ME, Haslwanter D, Bortz RH III, et al. A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition. Cell Host Microbe 2020;28(3):486-496.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med 2021. June 14 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratò S, Paladino ME, Riva MA, Deni M, Belingheri M. SARS-CoV-2 transmission risk to household and family contacts by vaccinated healthcare workers. J Occup Environ Med 2021;63(7):e474-e476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah ASV, Gribben C, Bishop J, et al. Effect of vaccination on transmission of COVID-19: an observational study in healthcare workers and their households. March 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.03.11.21253275v1). preprint.
- 19.Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 2021;397:1459-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021. May 17 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 21.Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021;39:4423-4428.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Criscuolo E, Diotti RA, Strollo M, et al. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J Med Virol 2021;93:2160-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson KB, Pinsky BA, Rath MEM, et al. Post-vaccination SARS-CoV-2 infections and incidence of the B.1.427/B.1.429 variant among healthcare personnel at a northern California academic medical center. April 24, 2021. (https://www.medrxiv.org/content/10.1101/2021.04.14.21255431v2). preprint. [DOI] [PMC free article] [PubMed]
- 24.Thompson CN, Hughes S, Ngai S, et al. Rapid emergence and epidemiologic characteristics of the SARS-CoV-2 B.1.526 variant — New York City, New York, January 1–April 5, 2021. MMWR Morb Mortal Wkly Rep 2021;70:712-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouton TC, Lodi S, Turcinovic J, et al. COVID-19 vaccine impact on rates of SARS-CoV-2 cases and post vaccination strain sequences among healthcare workers at an urban academic medical center: a prospective cohort study. April 27, 2021. (https://www.medrxiv.org/content/10.1101/2021.03.30.21254655v2). preprint. [DOI] [PMC free article] [PubMed]
- 26.Philomina J B, Jolly B, John N, et al. Genomic survey of SARS-CoV-2 vaccine breakthrough infections in healthcare workers from Kerala, India. J Infect 2021. May 25 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.