Abstract
Background
Thrombosis and inflammation may contribute to morbidity and mortality among patients with coronavirus disease 2019 (Covid-19). We hypothesized that therapeutic-dose anticoagulation would improve outcomes in critically ill patients with Covid-19.
Methods
In an open-label, adaptive, multiplatform, randomized clinical trial, critically ill patients with severe Covid-19 were randomly assigned to a pragmatically defined regimen of either therapeutic-dose anticoagulation with heparin or pharmacologic thromboprophylaxis in accordance with local usual care. The primary outcome was organ support–free days, evaluated on an ordinal scale that combined in-hospital death (assigned a value of −1) and the number of days free of cardiovascular or respiratory organ support up to day 21 among patients who survived to hospital discharge.
Results
The trial was stopped when the prespecified criterion for futility was met for therapeutic-dose anticoagulation. Data on the primary outcome were available for 1098 patients (534 assigned to therapeutic-dose anticoagulation and 564 assigned to usual-care thromboprophylaxis). The median value for organ support–free days was 1 (interquartile range, −1 to 16) among the patients assigned to therapeutic-dose anticoagulation and was 4 (interquartile range, −1 to 16) among the patients assigned to usual-care thromboprophylaxis (adjusted proportional odds ratio, 0.83; 95% credible interval, 0.67 to 1.03; posterior probability of futility [defined as an odds ratio <1.2], 99.9%). The percentage of patients who survived to hospital discharge was similar in the two groups (62.7% and 64.5%, respectively; adjusted odds ratio, 0.84; 95% credible interval, 0.64 to 1.11). Major bleeding occurred in 3.8% of the patients assigned to therapeutic-dose anticoagulation and in 2.3% of those assigned to usual-care pharmacologic thromboprophylaxis.
Conclusions
In critically ill patients with Covid-19, an initial strategy of therapeutic-dose anticoagulation with heparin did not result in a greater probability of survival to hospital discharge or a greater number of days free of cardiovascular or respiratory organ support than did usual-care pharmacologic thromboprophylaxis. (REMAP-CAP, ACTIV-4a, and ATTACC ClinicalTrials.gov numbers, NCT02735707, NCT04505774, NCT04359277, and NCT04372589.)
Coronavirus disease 2019 (Covid-19) is associated with inflammation and thrombosis.1-4 Critically ill patients with Covid-19 are at high risk for thrombosis despite receiving standard-dose pharmacologic thromboprophylaxis.5-8 Circulating biomarkers reflecting systemic inflammation and coagulation activation (e.g., d-dimer and C-reactive protein) are independently associated with a greater risk of respiratory failure, thrombosis, and death in patients with Covid-19.2,9,10 Inflammation and thrombosis may therefore be important contributors to poor outcomes.
Unfractionated and low-molecular-weight heparins are parenteral anticoagulants with antiinflammatory properties and possible antiviral properties.11,12 Given the reports of excess thrombotic risk, enhanced-dose anticoagulation strategies have been incorporated into some Covid-19 guidance statements, especially for critically ill patients.13,14 However, the effectiveness and safety of therapeutic-dose anticoagulation given to improve outcomes in Covid-19 are uncertain.
We conducted an international, adaptive, multiplatform, randomized, controlled trial to determine whether an initial strategy of therapeutic-dose anticoagulation with unfractionated or low-molecular-weight heparin improves in-hospital survival and reduces the duration of intensive care unit (ICU)–level cardiovascular or respiratory organ support in critically ill patients with Covid-19.
Methods
Trial Design and Oversight
Early in the Covid-19 pandemic, the lead investigators of three international adaptive platform trials harmonized their protocols and statistical analysis plans (available with the full text of this article at NEJM.org) to study the effect of therapeutic-dose anticoagulation in patients who were hospitalized for Covid-19 in one integrated, multiplatform, randomized clinical trial to accelerate the generation of evidence and maximize the external validity of the results (see the Supplementary Appendix, available at NEJM.org). The platforms included the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP),15 A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a), and the Antithrombotic Therapy to Ameliorate Complications of Covid-19 (ATTACC) trial.16 The platforms aligned the trial design, eligibility criteria, interventions, outcome measures, and statistical analysis plan (comparisons of the three platforms are provided in the Supplementary Appendix). Each platform was overseen by independent data and safety monitoring boards following a collaborative cross-platform interaction plan. The members of the writing committees vouch for the accuracy and completeness of the data and for the fidelity of the trials to the protocols.
The multiplatform trial was conducted in accordance with the principles of the Good Clinical Practice guidelines of the International Council for Harmonisation. Ethics and regulatory approval were obtained at each participating center. Written or oral informed consent, in accordance with regional regulations, was obtained from all patients or their surrogates. The trial was supported by multiple international funding organizations that had no role in the design, analysis, or reporting of the trial results, with the exception of the ACTIV-4a protocol, which received input on design from professional staff members at the National Institutes of Health and from peer reviewers.
Patients
All three platforms enrolled patients who were hospitalized for Covid-19. Although REMAP-CAP enrolled patients with suspected or confirmed Covid-19, only patients with infection confirmed by laboratory testing were included in the primary analysis of the multiplatform trial. The trial was designed to evaluate the effect of therapeutic-dose anticoagulation in patients with severe Covid-19 and in those with moderate Covid-19 stratified according to d-dimer level (high, low, or unknown). This report describes the results of the analyses involving patients with severe Covid-19; the results of analyses involving patients with moderate Covid-19 are reported separately.17
Severe Covid-19 was defined as Covid-19 that led to receipt of ICU-level respiratory or cardiovascular organ support (oxygen through a high-flow nasal cannula, noninvasive or invasive mechanical ventilation, extracorporeal life support, vasopressors, or inotropes) in an ICU. In ACTIV-4a, in which definitions of an ICU were thought to be challenging to operationalize during the pandemic, receipt of ICU-level organ support, irrespective of hospital setting, was used to define ICU-level care. Patients were ineligible if they had been admitted to the ICU with Covid-19 for 48 hours or longer (in REMAP-CAP) or to a hospital for 72 hours or longer (in ACTIV-4a and ATTACC) before randomization. They were also ineligible if they were at imminent risk for death and there was no ongoing commitment to full organ support, or if they were at high risk for bleeding, were receiving dual antiplatelet therapy, had a separate clinical indication for therapeutic-dose anticoagulation, or had a history of heparin sensitivity, including heparin-induced thrombocytopenia. Detailed exclusion criteria for the platforms are provided in the Supplementary Appendix.
Randomization
Randomization was performed with the use of separate central Web-based systems for each platform. Patients were randomly assigned to receive therapeutic-dose anticoagulation with unfractionated or low-molecular-weight heparin or to receive usual-care pharmacologic thromboprophylaxis in an open-label fashion. Patients in ACTIV-4a underwent randomization in a 1:1 ratio. The other two platforms specified response-adaptive randomization; randomization probabilities could be updated for those platforms during the period from each monthly adaptive interim analysis in the multiplatform trial to the end of enrollment (as described in the Supplementary Appendix).
Therapeutic-dose anticoagulation was administered according to local site protocols for the treatment of acute venous thromboembolism for up to 14 days or until recovery (defined as either hospital discharge or discontinuation of supplemental oxygen for at least 24 hours). Usual-care thromboprophylaxis was administered at a dose and duration determined by the treating clinician according to local practice, which included either standard low-dose thromboprophylaxis or enhanced intermediate-dose thromboprophylaxis. The anticoagulation and thromboprophylaxis regimens that were specified by each platform are detailed in the Supplementary Appendix. Some of the patients who were enrolled in REMAP-CAP also underwent randomization in the antiplatelet-agent domain and in other domains of that trial. There were no additional active domains in ACTIV-4a and ATTACC.
Outcome Measures
The primary outcome, organ support–free days, was evaluated on an ordinal scale indicating the number of days free of cardiovascular or respiratory organ support up to day 21 among patients who survived to hospital discharge; patients who died in the hospital by day 90 were assigned a value of –1. Among the patients who survived to hospital discharge, the number of days free of respiratory organ support (high-flow nasal cannula, noninvasive or invasive ventilation, or extracorporeal life support) and cardiovascular organ support (vasopressors or inotropes) through day 21 was recorded. A higher number of organ support–free days indicates a better outcome. Patients who were discharged from the hospital before day 21 were assumed to be alive and free of organ support through day 21.
Prespecified secondary outcomes included survival to hospital discharge, major thrombotic events or death (a composite of myocardial infarction, pulmonary embolism, ischemic stroke, systemic arterial embolism, or in-hospital death), and any thrombotic events (major thrombotic events or deep-vein thrombosis) or death. The outcomes of major thrombotic events or death and any thrombotic events or death were assessed through 28 days (in ACTIV-4a and ATTACC) or through hospital discharge (REMAP-CAP). Safety outcomes included major bleeding during the treatment period, as defined by the International Society of Thrombosis and Hemostasis for nonsurgical patients,18 and laboratory-confirmed heparin-induced thrombocytopenia. Thrombotic and bleeding events were adjudicated by independent platform-specific adjudication committees, the members of which were unaware of the treatment assignments. Definitions of all the outcomes are provided in the Supplementary Appendix.
Statistical Analysis
The multiplatform trial analyzed combined individual patient data from all platforms with the use of a single overarching Bayesian model (as described in the Supplementary Appendix and in the protocol). Monthly interim analyses of combined data from all platforms were planned within each of the prespecified patient cohorts. Randomization continued within each cohort until a statistical conclusion of superiority (defined as >99% posterior probability of a proportional odds ratio of >1) or futility (>95% posterior probability of a proportional odds ratio of <1.2) was made for a cohort. The stopping criteria for a statistical conclusion applied independently to each cohort, with the exception of the cohort with unknown d-dimer levels.
The primary analysis involved a Bayesian cumulative logistic-regression model (shown in the Supplementary Appendix) that was used to calculate the posterior distribution for the proportional odds ratio for organ support–free days. The primary model was adjusted for age, sex, trial site, and enrollment time interval (in 2-week intervals). Patients in the severe-disease and moderate-disease cohorts were included in the model. Weakly informative Dirichlet prior probability distributions were specified to model the baseline probabilities for each value for organ support–free days in the severe-disease and moderate-disease cohorts. The model was used to estimate treatment effects in each of the cohorts (the severe-disease cohort and the moderate-disease cohort stratified according to d-dimer level), with the use of a Bayesian hierarchical approach.19 The treatment effects of anticoagulation in the severe-disease and moderate-disease cohorts were nested in a hierarchical prior distribution centered on an overall intervention effect that had been estimated with a neutral prior distribution, but distinct cohort-specific effects were estimated. When consistent effects were observed between the cohorts, the posterior distribution for the intervention effect in each cohort was shrunk toward the overall estimate. For the purposes of this report, the primary analysis involved all the patients enrolled in the multiplatform trial (including the severe-disease and moderate-disease cohorts) for whom data on the primary outcome were available as of April 8, 2021. The analysis of this data set was prespecified in the statistical analysis plan.
The primary model was fit with the use of a Markov chain Monte Carlo algorithm with 100,000 samples from the joint posterior distribution, which allowed calculation of the posterior distributions for the odds ratios, including medians and 95% credible intervals, and the posterior probabilities of superiority (indicated by an odds ratio of >1), futility (indicated by an odds ratio of <1.2), or inferiority (indicated by an odds ratio of <1). A similar model was run for survival to hospital discharge. Prespecified sensitivity analyses of the primary model are described in the Supplementary Appendix. To assess the influence of potential prior enthusiasm for therapeutic-dose anticoagulation (i.e., a prior distribution expressing a higher probability of success with therapeutic-dose anticoagulation than with usual-care thromboprophylaxis), a sensitivity analysis was conducted with the use of an enthusiastic prior distribution (prior mean odds ratio, 1.75; 95% credible interval, 0.74 to 4.15; prior probability of superiority, 90%).
For the key secondary end points, similar models were restricted to the severe-disease cohort without borrowing information from the moderate-disease cohort. Subgroup analyses assessed whether the treatment effect varied according to age, sex, receipt of mechanical ventilation at baseline, and intensity of thromboprophylaxis dosing in the group that received usual-care thromboprophylaxis (defined on the basis of the pattern of practice at each site, as described in the Supplementary Appendix). In a post hoc exploratory analysis, a possible interaction between assignment to therapeutic-dose anticoagulation or usual-care thromboprophylaxis and assignment to receive an interleukin-6 receptor antagonist or standard care (control) in REMAP-CAP was evaluated.
Results
Characteristics of the Patients
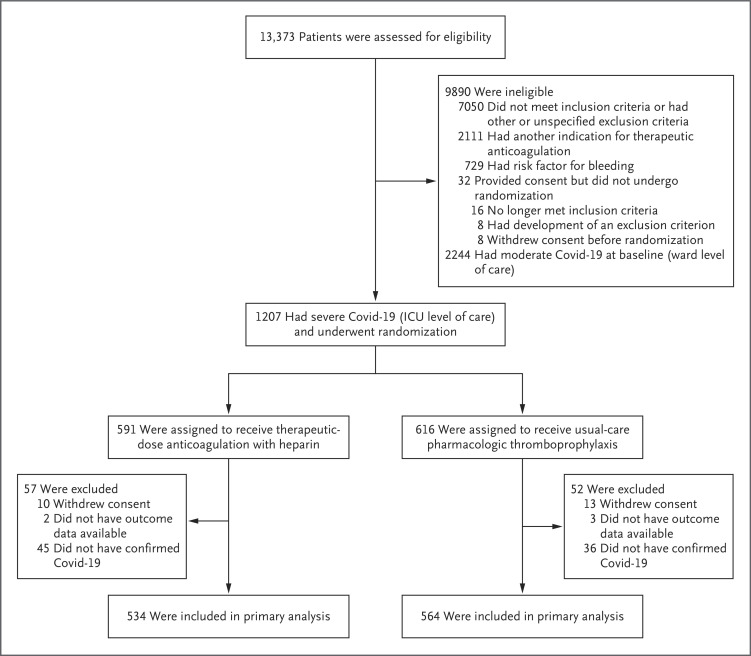
The first patient underwent randomization on April 21, 2020. During the trial, randomization proportions were modified in the REMAP-CAP platform to 0.388 for therapeutic-dose anticoagulation and 0.612 for usual-care pharmacologic thromboprophylaxis on the basis of an adaptive interim analysis on November 20, 2020 (see the Supplementary Appendix). Enrollment was discontinued in the severe-disease cohort on December 19, 2020, after an adaptive interim analysis showed that the statistical criterion for futility had been met. At that time, a total of 1207 patients with severe suspected or confirmed Covid-19 had undergone randomization at 393 sites in 10 countries (with 591 assigned to receive therapeutic-dose anticoagulation and 616 assigned to receive usual-care thromboprophylaxis) (Figure 1). Of these patients, 23 withdrew consent and 81 did not have laboratory-confirmed Covid-19; data on the primary outcome were not available for an additional 5 patients as of April 8, 2021. The current report presents the results of the primary analysis involving 1103 patients with severe confirmed Covid-19; data on the primary outcome were available for 1098 of these patients.
Figure 1. Screening, Enrollment, Randomization, and Inclusion in Analysis.
Sites used varying screening and documentation practices during the pandemic to identify eligible patients (shown in the protocol); as reported, 3799 were assessed for eligibility in ACTIV-4a, 7202 in ATTACC, and 2372 in REMAP-CAP. “Other” exclusion criteria included an absence of a diagnosis of coronavirus disease 2019 (Covid-19) and a duration of hospital stay anticipated to be less than 72 hours. Patients who had moderate Covid-19 at baseline may have been included in calculations for covariate adjustment and dynamic borrowing.
The baseline characteristics of the patients were similar in the two intervention groups (Table 1). The majority of patients were enrolled through REMAP-CAP (929 of 1103 enrolled patients, 84%). The pattern of anticoagulant administration in the intervention groups is described in Table S1 in the Supplementary Appendix. Among the patients who were assigned to receive usual-care thromboprophylaxis and for whom data were available, the initial postrandomization dose equivalent corresponded to standard low-dose thromboprophylaxis in 41% and to enhanced intermediate-dose thromboprophylaxis in 51%.
Table 1. Demographic and Clinical Characteristics of the Patients at Baseline.*.
| Characteristic | Therapeutic-Dose Anticoagulation (N=536) |
Usual-Care Thromboprophylaxis (N=567) |
|---|---|---|
| Age — yr | 60.4±13.1 | 61.7±12.5 |
| Male sex — no. (%) | 387 (72.2) | 385 (67.9) |
| Race — no./total no. (%)† | ||
| White | 316/427 (74.0) | 332/449 (73.9) |
| Asian | 69/427 (16.2) | 71/449 (15.8) |
| Black | 25/427 (5.9) | 20/449 (4.5) |
| Other | 17/427 (4.0) | 26/449 (5.8) |
| Country of enrollment — no. (%) | ||
| United Kingdom | 389 (72.6) | 395 (69.7) |
| United States | 79 (14.7) | 97 (17.1) |
| Canada | 40 (7.5) | 54 (9.5) |
| Brazil | 12 (2.2) | 6 (1.1) |
| Other‡ | 16 (3.0) | 15 (2.6) |
| Platform of enrollment — no. (%) | ||
| REMAP-CAP§ | 454 (84.7) | 475 (83.8) |
| ATTACC | 19 (3.5) | 21 (3.7) |
| ACTIV-4a | 63 (11.8) | 71 (12.5) |
| Median body-mass index (IQR)¶ | 30.4 (26.9–36.1) | 30.2 (26.4–34.9) |
| No. of patients with data | 470 | 488 |
| Median APACHE II score (IQR)‖ | 14 (8–21) | 13 (8–19) |
| No. of patients with data | 429 | 443 |
| Preexisting conditions — no./total no. (%) | ||
| Diabetes mellitus (type 1 or 2) | 171/536 (31.9) | 191/567 (33.7) |
| Severe cardiovascular disease** | 44/524 (8.4) | 45/558 (8.1) |
| Chronic kidney disease | 58/509 (11.4) | 43/521 (8.3) |
| Chronic respiratory disease†† | 129/517 (25.0) | 129/537 (24) |
| Chronic liver disease | 6/516 (1.2) | 3/548 (0.5) |
| Treatments at baseline — no./total no. (%)‡‡ | ||
| Antiplatelet agent§§ | 37/485 (7.6) | 38/494 (7.7) |
| Remdesivir | 174/532 (32.7) | 172/564 (30.5) |
| Glucocorticoids | 426/522 (81.6) | 458/555 (82.5) |
| Tocilizumab¶¶ | 11/532 (2.1) | 9/564 (1.6) |
| Baseline organ support — no. (%) | ||
| Low-flow nasal cannula or face mask or no supplemental oxygen | 8 (1.5) | 7 (1.2) |
| High-flow nasal cannula | 170 (31.7) | 188 (33.2) |
| Noninvasive ventilation | 215 (40.1) | 200 (35.3) |
| Invasive mechanical ventilation | 143 (26.7) | 172 (30.3) |
| Vasopressors or inotropes | 94 (17.5) | 109 (19.2) |
| Median Pao2:Fio2 ratio (IQR)‖ | 118 (88.5–159.5) | 118.5 (90.2–160.8) |
| No. of patients with data | 391 | 406 |
| d-dimer level ≥2 times ULN at site — no./total no. (%) | 100/210 (47.6) | 107/223 (48) |
| Median laboratory values (IQR) | ||
| d-dimer level — ng/ml | 823 (433–1740) | 890 (386.2–1844.2) |
| No. of patients with data | 189 | 196 |
| International normalized ratio | 1.1 (1–1.2) | 1.1 (1–1.2) |
| No. of patients with data | 327 | 324 |
| Neutrophil count — per mm3 | 7900 (5500–10,600) | 7800 (5600–10,700) |
| No. of patients with data | 446 | 478 |
| Lymphocyte count — per mm3 | 700 (500–1000) | 700 (500–900) |
| No. of patients with data | 447 | 482 |
| Platelet count — per mm3 | 247,000 (190,200–316,500) | 244,000 (182,000–312,000) |
| No. of patients with data | 530 | 561 |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. IQR denotes interquartile range, and ULN upper limit of the normal range.
Race was reported by the patients.
The other countries were Ireland, the Netherlands, Australia, Nepal, Saudi Arabia, and Mexico.
REMAP-CAP also enrolled patients with suspected but not confirmed coronavirus disease 2019 (Covid-19) (45 of those assigned to receive therapeutic-dose anticoagulation and 36 of those assigned to receive usual-care pharmacologic thromboprophylaxis).
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Scores on the Acute Physiology and Chronic Health Evaluation (APACHE) II and the ratio of the partial pressure of oxygen (Pao2) to the fraction of inspired oxygen (Fio2) were available only in REMAP-CAP. APACHE II scores range from 0 to 71, with higher scores indicating a greater severity of illness.
Severe cardiovascular disease was defined in REMAP-CAP as a baseline history of New York Heart Association class IV symptoms and was defined in ACTIV-4a and ATTACC as a baseline history of heart failure, myocardial Infarction, coronary artery disease, peripheral artery disease, or cerebrovascular disease (stroke or transient ischemic attack).
Chronic respiratory disease was defined as a baseline history of asthma, chronic obstructive pulmonary disease, bronchiectasis, interstitial lung disease, primary lung cancer, pulmonary hypertension, active tuberculosis, or the receipt of home oxygen therapy.
Treatments used recently or in the long term are included.
Patients who underwent concurrent randomization in the REMAP-CAP antiplatelet domain are not included here (47 of those assigned to therapeutic-dose anticoagulation and 66 of those assigned to usual-care pharmacologic thromboprophylaxis).
Patients who underwent concurrent randomization in the REMAP-CAP immunomodulation domain are not included here (150 of those assigned to therapeutic-dose anticoagulation and 123 of those assigned to usual-care pharmacologic thromboprophylaxis).
Primary Outcome
Among the patients assigned to receive therapeutic-dose anticoagulation, the median value for organ support–free days was 1 (interquartile range, –1 to 16); among the patients assigned to usual-care pharmacologic thromboprophylaxis, the median value was 4 (interquartile range, –1 to 16). The median adjusted proportional odds ratio for the effect of therapeutic-dose anticoagulation on organ support–free days was 0.83 (95% credible interval, 0.67 to 1.03), yielding a posterior probability of futility of 99.9% and a posterior probability of inferiority of 95.0% (Table 2 and Figure 2). A total of 335 of 534 patients (62.7%) assigned to receive therapeutic-dose anticoagulation and 364 of 564 patients (64.5%) assigned to receive usual-care thromboprophylaxis survived to hospital discharge. The median adjusted proportional odds ratio for survival to hospital discharge was 0.84 (95% credible interval, 0.64 to 1.11; posterior probability of inferiority, 89.2%). The median adjusted absolute difference in the percentage of patients who survived to hospital discharge (therapeutic-dose anticoagulation minus usual-care thromboprophylaxis) was –4.1 percentage points (95% credible interval, –10.7 to 2.4).
Table 2. Primary and Secondary Outcomes.
| Outcome | Therapeutic-Dose Anticoagulation (N=536) |
Usual-Care Thromboprophylaxis (N=567) |
Adjusted Difference in Risk (95% Credible Interval) | Adjusted Odds Ratio (95% Credible Interval)* | Probability of Superiority | Probability of Futility | Probability of Inferiority |
|---|---|---|---|---|---|---|---|
| median no. (IQR) | percentage points | % | % | % | |||
| Organ support–free days up to day 21†‡ | 1 (–1 to 16) | 4 (–1 to 16) | — | 0.83 (0.67 to 1.03) | 5.0 | 99.9 | 95.0 |
| no. of patients/total no. (%) | |||||||
| Survival to hospital discharge‡ | 335/534 (62.7) | 364/564 (64.5) | –4.1 (–10.7 to 2.4) | 0.84 (0.64 to 1.11) | 10.8 | 99.6 | 89.2 |
| Major thrombotic events or death§ | 213/531 (40.1) | 230/560 (41.1) | 1.0 (–5.6 to 7.4) | 1.04 (0.79 to 1.35) | 40.3 | — | 59.7 |
| Major thrombotic events¶ | 34/530 (6.4) | 58/559 (10.4) | — | — | — | — | — |
| Death in hospital | 199/534 (37.3) | 200/564 (35.5) | — | — | — | — | — |
| Any thrombotic events or death§ | 217/531 (40.9) | 232/560 (41.4) | 1.5 (–4.9 to 8.0) | 1.06 (0.81 to 1.38) | 33.4 | — | 66.6 |
| Any thrombotic events‖ | 38/530 (7.2) | 62/559 (11.1) | — | — | — | — | — |
| Death in hospital | 199/534 (37.3) | 200/564 (35.5) | — | — | — | — | — |
| Major bleeding§ | 20/529 (3.8) | 13/562 (2.3) | 1.1 (–0.6 to 4.4) | 1.48 (0.75 to 3.04) | 12.8 | — | 87.2 |
Odds ratios were adjusted for age, sex, trial site, and enrollment time interval.
Days free of cardiovascular or respiratory organ support was evaluated on an ordinal scale that combined in-hospital death (assigned a value of −1) and the number of days free of organ support up to day 21 among patients who survived to hospital discharge. Outcomes were known for 534 patients assigned to therapeutic-dose anticoagulation and for 564 patients assigned to usual-care pharmacologic thromboprophylaxis. The odds ratio is an adjusted proportional odds ratio.
The probabilities of superiority (odds ratio, >1), inferiority (odds ratio, <1), and futility (odds ratio, <1.2) of therapeutic-dose anticoagulation were computed from the posterior distribution.
The probabilities of superiority (odds ratio, <1) and inferiority (odds ratio, >1) of therapeutic-dose anticoagulation were computed from the posterior distribution.
Major thrombotic events include pulmonary embolism, myocardial infarction, ischemic cerebrovascular event, and systemic arterial thromboembolism.
Any thrombotic events include major thrombotic events or deep-vein thrombosis.
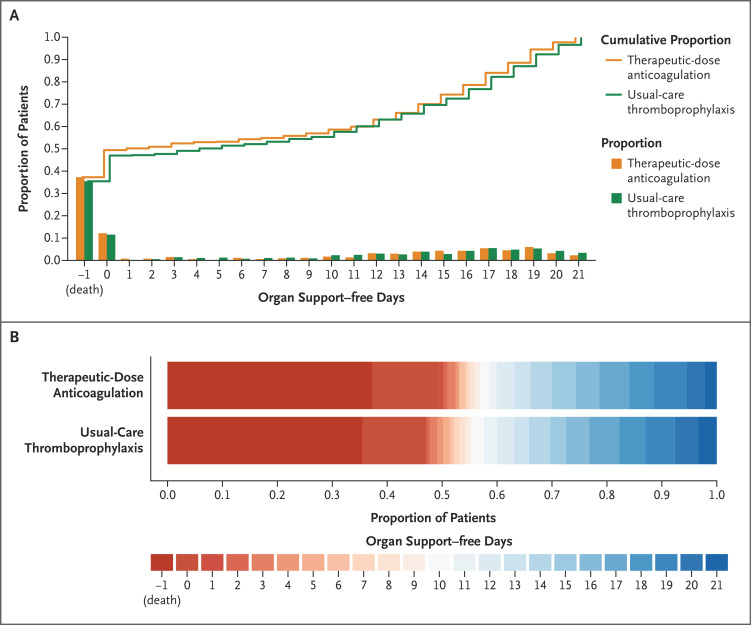
Figure 2. Organ Support–free Days Up to Day 21.
Panel A shows the proportions of patients in each intervention group with each value for organ support–free days, with death listed first on the x axis (−1). Curves that rise more slowly indicate a more favorable distribution in the number of days alive and free of organ support. The height of each curve at −1 indicates the in-hospital mortality associated with each intervention. The height of each curve at any point from 0 to 21 days indicates the proportion of patients with that number of organ support–free days or fewer (e.g., at 10 days, the curve indicates the proportion of patients with ≤10 organ support–free days). The difference in height between the two curves at any point represents the difference in the cumulative probability of having a number of organ support–free days less than or equal to that number on the x axis. Panel B shows the values for organ support–free days as horizontally stacked proportions for each intervention group. Red represents worse outcomes and blue better outcomes. The median adjusted odds ratio in the primary analysis was 0.83 (95% credible interval, 0.67 to 1.03; posterior probability of futility, 99.9%). Among the patients in REMAP-CAP, 12 patients assigned to receive therapeutic-dose anticoagulation and 19 patients assigned to receive usual-care pharmacologic thromboprophylaxis had 21 organ support–free days; the cardiovascular or respiratory organ support these patients had been receiving at the time of randomization was discontinued within 12 hours after randomization.
Sensitivity and Subgroup Analyses
In sensitivity analyses of the primary outcome (Table S2), incorporation of prior enthusiasm for therapeutic-dose anticoagulation did not modify the conclusion (median adjusted proportional odds ratio, 0.86; 95% credible interval, 0.70 to 1.07). The inclusion of patients with suspected Covid-19 or exclusion of patients who were concomitantly receiving an antiplatelet agent at baseline or those who underwent concomitant randomization in the REMAP-CAP antiplatelet-agent domain also yielded similar results. Among the 273 patients with severe confirmed Covid-19 who had also been randomly assigned to receive either an interleukin-6 receptor antagonist or no immunomodulation in REMAP-CAP, there was no evidence of a meaningful interaction between the anticoagulation and immunomodulation domains (Table S3 and Fig. S1). In prespecified subgroup analyses, the estimated effect did not vary meaningfully according to age, sex, baseline receipt of invasive mechanical ventilation, or the site-specific dosing pattern for usual-care pharmacologic thromboprophylaxis (intermediate vs. low dose) (Fig. S2).
Secondary Outcomes
Although fewer patients had major thrombotic events in the group assigned to receive therapeutic-dose anticoagulation than in the group assigned to receive usual-care pharmacologic thromboprophylaxis (6.4% vs. 10.4%), the incidence of the secondary efficacy outcome of major thrombotic events or death was similar in the two groups (40.1% and 41.1%, respectively; median adjusted odds ratio, 1.04; 95% credible interval, 0.79 to 1.35) (Table 2). An analysis incorporating deep-vein thrombosis showed similar results. A breakdown of the thrombotic events is provided in Table S4. A major bleeding event occurred during the treatment period in 3.8% of the patients assigned to receive therapeutic-dose anticoagulation and in 2.3% of those assigned to receive usual-care thromboprophylaxis (Table 2).
Discussion
In this multiplatform, randomized trial involving more than 1000 critically ill patients with confirmed Covid-19, therapeutic-dose anticoagulation did not increase the probability of survival to hospital discharge or the number of days free of cardiovascular or respiratory organ support and had a 95% probability of being inferior to usual-care pharmacologic thromboprophylaxis. There was an 89% probability that therapeutic-dose anticoagulation led to a lower probability of survival to hospital discharge than usual-care thromboprophylaxis. Bleeding complications were infrequent in both intervention groups.
Our results refute the hypothesis that routine therapeutic-dose anticoagulation benefits critically ill patients with Covid-19. This hypothesis was based in part on observational studies that reported an association between therapeutic-dose anticoagulation and improved outcomes.14,20,21 Multiple small and moderate-size randomized trials continue to evaluate different anticoagulation strategies in Covid-19.22
The net effect of anticoagulation on clinical outcomes in patients with Covid-19 may depend on the timing of initiation in relation to disease course and may vary with the severity of illness (and the degree of coagulation or inflammation) at the time that therapy is commenced.23-25 Despite demonstrable activation of coagulation in multiple organ systems in patients with severe Covid-19, it is possible that initiation of therapeutic-dose anticoagulation after severe Covid-19 has developed may be too late to alter the consequences of established disease processes.
In this trial, the probability of inferiority of therapeutic-dose anticoagulation with respect to the primary outcome was 95%. Mechanisms accounting for likely harm are uncertain. Although the incidence of major bleeding was numerically higher with therapeutic-dose anticoagulation than with usual-care thromboprophylaxis, it was still low (3.8%). Autopsy findings in patients with Covid-19 and severe acute respiratory distress syndrome have included microthrombosis but also alveolar hemorrhage.26 It is possible that in the presence of marked pulmonary inflammation, therapeutic-dose anticoagulation might exacerbate alveolar hemorrhage, leading to worse outcomes.
In this multiplatform trial, a harmonized pragmatic trial protocol was implemented by three platform networks spanning five continents. The interventions that were evaluated are familiar and widely available, rendering the findings broadly applicable to critically ill patients with severe Covid-19. The collaboration allowed us to reach a conclusion of futility with probable harm much more quickly than would have been possible as independent platforms.
One limitation of our trial is the open-label design, which may have introduced bias in the ascertainment of thrombotic events. A second possible limitation is that a substantial majority of the patients who were enrolled in the severe-disease cohort were in the United Kingdom, where national practice guidelines changed during the trial to recommend that patients with Covid-19 who were admitted to an ICU receive intermediate-dose anticoagulation for thromboprophylaxis.13 Many patients in the usual-care thromboprophylaxis group therefore received intermediate-dose thromboprophylaxis. It is possible that the effect of therapeutic-dose anticoagulation in patients with severe Covid-19 varies according to the type of treatment given to the comparator group, although we did not find evidence of meaningful differences in treatment effect according to site proclivity for low-dose or intermediate-dose thromboprophylaxis. Recent data also suggest that intermediate-dose thromboprophylaxis is not superior to standard or low-dose thromboprophylaxis for the treatment of critically ill patients.27
In critically ill patients with Covid-19, an initial strategy of therapeutic-dose anticoagulation with unfractionated or low-molecular-weight heparin was not associated with a greater probability of survival to hospital discharge or a greater number of days free of cardiovascular or respiratory organ support than was usual-care pharmacologic thromboprophylaxis. The probability that therapeutic-dose anticoagulation was inferior to usual-care thromboprophylaxis with respect to these outcomes was high.
Acknowledgments
We thank the patients and their families who participated in this trial and the members of the data and safety monitoring boards of each platform.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
The members of the writing committee are as follows: Ewan C. Goligher, M.D., Ph.D., Charlotte A. Bradbury, M.D., Ph.D., Bryan J. McVerry, M.D., Patrick R. Lawler, M.D., M.P.H., Jeffrey S. Berger, M.D., Michelle N. Gong, M.D., Marc Carrier, M.D., Harmony R. Reynolds, M.D., Anand Kumar, M.D., Alexis F. Turgeon, M.D., Lucy Z. Kornblith, M.D., Susan R. Kahn, M.D., John C. Marshall, M.D., Keri S. Kim, Pharm.D., Brett L. Houston, M.D., Lennie P.G. Derde, M.D., Ph.D., Mary Cushman, M.D., Tobias Tritschler, M.D., Derek C. Angus, M.D., M.P.H., Lucas C. Godoy, M.D., Zoe McQuilten, Ph.D., Bridget-Anne Kirwan, Ph.D., Michael E. Farkouh, M.D., Maria M. Brooks, Ph.D., Roger J. Lewis, M.D., Ph.D., Lindsay R. Berry, Ph.D., Elizabeth Lorenzi, Ph.D., Anthony C. Gordon, M.B., B.S., M.D., Tania Ahuja, Pharm.D., Farah Al-Beidh, Ph.D., Djillali Annane, M.D., Ph.D., Yaseen M. Arabi, M.D., Diptesh Aryal, M.D., Lisa Baumann Kreuziger, M.D., Abi Beane, Ph.D., Zahra Bhimani, M.P.H., Shailesh Bihari, Ph.D., Henny H. Billett, M.D., Lindsay Bond, H.B.Sc., Marc Bonten, Ph.D., Frank Brunkhorst, M.D., Meredith Buxton, Ph.D., Adrian Buzgau, B.A.S., Lana A. Castellucci, M.D., Sweta Chekuri, M.D., Jen-Ting Chen, M.D., Allen C. Cheng, Ph.D., Tamta Chkhikvadze, M.D., Benjamin Coiffard, M.D., Aira Contreras, M.A., Todd W. Costantini, M.D., Sophie de Brouwer, Ph.D., Michelle A. Detry, Ph.D., Abhijit Duggal, M.D., M.P.H., Vladimír Džavík, M.D., Mark B. Effron, M.D., Heather F. Eng, B.A., Jorge Escobedo, M.D., Lise J. Estcourt, M.B., B.Chir., D.Phil., Brendan M. Everett, M.D., M.P.H., Dean A. Fergusson, Ph.D., Mark Fitzgerald, Ph.D., Robert A. Fowler, M.D., Joshua D. Froess, M.S., Zhuxuan Fu, M.S., M.P.H., Jean P. Galanaud, M.D., Benjamin T. Galen, M.D., Sheetal Gandotra, M.D., Timothy D. Girard, M.D., M.S.C.I., Andrew L. Goodman, M.D., Herman Goossens, M.D., Cameron Green, M.Sc., Yonatan Y. Greenstein, M.D., Peter L. Gross, M.D., Rashan Haniffa, Ph.D., Sheila M. Hegde, M.D., M.P.H., Carolyn M. Hendrickson, M.D., Alisa M. Higgins, Ph.D., Alexander A. Hindenburg, M.D., Aluko A. Hope, M.D., M.S.C.E., James M. Horowitz, M.D., Christopher M. Horvat, M.D., M.H.A., David T. Huang, M.D., M.P.H., Kristin Hudock, M.D., M.S.T.R., Beverley J. Hunt, M.D., Mansoor Husain, M.D., Robert C. Hyzy, M.D., Jeffrey R. Jacobson, M.D., Devachandran Jayakumar, M.D., Norma M. Keller, M.D., Akram Khan, M.D., Yuri Kim, M.D., Ph.D., Andrei Kindzelski, M.D., Ph.D., Andrew J. King, Ph.D., M. Margaret Knudson, M.D., Aaron E. Kornblith, M.D., Matthew E. Kutcher, M.D., Michael A. Laffan, D.M., Francois Lamontagne, M.D., Grégoire Le Gal, M.D., Ph.D., Christine M. Leeper, M.D., Eric S. Leifer, Ph.D., George Lim, M.D., Felipe Gallego Lima, M.D., Kelsey Linstrum, M.S., Edward Litton, Ph.D., Jose Lopez-Sendon, Ph.D., Sylvain A. Lother, M.D., Nicole Marten, R.N., Andréa Saud Marinez, Pharm.D., Mary Martinez, M.S., Eduardo Mateos Garcia, M.D., Stavroula Mavromichalis, M.A., Daniel F. McAuley, M.D., Emily G. McDonald, M.D., Anna McGlothlin, Ph.D., Shay P. McGuinness, M.B., Ch.B., Saskia Middeldorp, M.D., Ph.D., Stephanie K. Montgomery, M.Sc., Paul R. Mouncey, M.Sc., Srinivas Murthy, M.D., Girish B. Nair, M.D., Rahul Nair, M.D., Alistair D. Nichol, M.B., Ph.D., Jose C. Nicolau, M.D., Ph.D., Brenda Nunez-Garcia, B.A., John J. Park, B.S., Pauline K. Park, M.D., Rachael L. Parke, Ph.D., Jane C. Parker, B.N., Sam Parnia, M.D., Ph.D., Jonathan D. Paul, M.D., Mauricio Pompilio, Ph.D., John G. Quigley, M.D., Robert S. Rosenson, M.D., Natalia S. Rost, M.D., Kathryn Rowan, Ph.D., Fernanda O. Santos, M.D., Marlene Santos, M.D., Mayler O. Santos, M.Sc., Lewis Satterwhite, M.D., Christina T. Saunders, Ph.D., Jake Schreiber, M.P.H., Roger E.G. Schutgens, M.D., Ph.D., Christopher W. Seymour, M.D., Deborah M. Siegal, M.D., Delcio G. Silva, Jr., M.Med., Aneesh B. Singhal, M.D., Arthur S. Slutsky, M.D., Dayna Solvason, Simon J. Stanworth, F.R.C.P., D.Phil., Anne M. Turner, M.P.H., Wilma van Bentum-Puijk, M.Sc., Frank L. van de Veerdonk, M.D., Ph.D., Sean van Diepen, M.D., Gloria Vazquez-Grande, M.D., Lana Wahid, M.D., Vanessa Wareham, H.B.Sc., R. Jay Widmer, M.D., Ph.D., Jennifer G. Wilson, M.D., Eugene Yuriditsky, M.D., Yongqi Zhong, M.B., M.P.H., Scott M. Berry, Ph.D., Colin J. McArthur, M.B., Ch.B., Matthew D. Neal, M.D., Judith S. Hochman, M.D., Steven A. Webb, M.P.H., Ph.D., and Ryan Zarychanski, M.D.
This article was published on August 4, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
REMAP-CAP was supported by the European Union through FP7-HEALTH-2013-INNOVATION: the Platform for European Preparedness Against (Re-)emerging Epidemics (PREPARE) consortium (grant 602525) and the Horizon 2020 research and innovation program: the Rapid European Covid-19 Emergency Research response (RECOVER) consortium (grant 101003589) and by grants from the Australian National Health and Medical Research Council (APP1101719 and APP1116530), the Health Research Council of New Zealand (16/631), the Canadian Institutes of Health Research (Strategy for Patient-Oriented Research Innovative Clinical Trials Program Grant 158584 and COVID-19 Rapid Research Operating Grant 447335), the U.K. National Institute for Health Research (NIHR) and the NIHR Imperial Biomedical Research Centre, the Health Research Board of Ireland (CTN 2014-012), the UPMC Learning While Doing Program, the Translational Breast Cancer Research Consortium, the French Ministry of Health (PHRC-20-0147), the Minderoo Foundation, Amgen, Eisai, the Global Coalition for Adaptive Research, and the Wellcome Trust Innovations Project (215522). The ATTACC platform was supported by grants from the Canadian Institutes of Health Research, LifeArc, Thistledown Foundation, Research Manitoba, CancerCare Manitoba Foundation, Victoria General Hospital Foundation, Ontario Ministry of Health, and the Peter Munk Cardiac Centre. The ACTIV-4a platform was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and administered through OTA-20-011 and was supported in part by NIH agreement 1OT2HL156812-01. Dr. Goligher is the recipient of an Early Career Investigator award from the Canadian Institutes of Health Research (grant AR7-162822). Dr. Gordon is funded by an NIHR Research Professorship (RP-2015-06-18). Dr. Turgeon is funded by a Canada Research Chair–Tier 2. Dr. Zarychanski is the recipient of the Lyonel G. Israels Research Chair in Hematology (University of Manitoba).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020;191:148-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18:1995-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J 2021;42:2270-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost 2020. September 25 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation 2020;142:184-186. [DOI] [PubMed] [Google Scholar]
- 6.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godoy LC, Goligher EC, Lawler PR, Slutsky AS, Zarychanski R. Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19. CMAJ 2020;192(40):E1156-E1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 2020;324:799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;136:489-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poterucha TJ, Libby P, Goldhaber SZ. More than an anticoagulant: do heparins have direct anti-inflammatory effects? Thromb Haemost 2017;117:437-444. [DOI] [PubMed] [Google Scholar]
- 12.Hippensteel JA, LaRiviere WB, Colbert JF, Langouët-Astrié CJ, Schmidt EP. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol 2020;319:L211-L217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. (2020). COVID-19 rapid guideline: reducing the risk of venous thromboembolism in over 16s with COVID-19. NICE guideline 186. November 20, 2020. (https://www.nice.org.uk/guidance/ng186/). [PubMed]
- 14.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol 2020;76:1815-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angus DC, Berry S, Lewis RJ, et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) study: rationale and design. Ann Am Thorac Soc 2020;17:879-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houston BL, Lawler PR, Goligher EC, et al. Anti-thrombotic therapy to ameliorate complications of COVID-19 (ATTACC): study design and methodology for an international, adaptive Bayesian randomized controlled trial. Clin Trials 2020;17:491-500. [DOI] [PubMed] [Google Scholar]
- 17.ATTACC, ACTIV-4a, and REMAP-CAP Investigators. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. DOI: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-694. [DOI] [PubMed] [Google Scholar]
- 19.McGlothlin AE, Viele K. Bayesian hierarchical models. JAMA 2018;320:2365-2366. [DOI] [PubMed] [Google Scholar]
- 20.Wijaya I, Andhika R, Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19: a systematic review. Clin Appl Thromb Hemost 2020;26:1076029620960797-1076029620960797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ionescu F, Jaiyesimi I, Petrescu I, et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: a retrospective propensity score-weighted analysis. Eur J Haematol 2021;106:165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tritschler T, Mathieu M-E, Skeith L, et al. Anticoagulant interventions in hospitalized patients with COVID-19: a scoping review of randomized controlled trials and call for international collaboration. J Thromb Haemost 2020;18:2958-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 2021;384:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;384:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020;173:268-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA 2021;325:1620-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.