Abstract
Background
REGEN-COV (previously known as REGN-COV2), a combination of the monoclonal antibodies casirivimab and imdevimab, has been shown to markedly reduce the risk of hospitalization or death among high-risk persons with coronavirus disease 2019 (Covid-19). Whether subcutaneous REGEN-COV prevents severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and subsequent Covid-19 in persons at high risk for infection because of household exposure to a person with SARS-CoV-2 infection is unknown.
Methods
We randomly assigned, in a 1:1 ratio, participants (≥12 years of age) who were enrolled within 96 hours after a household contact received a diagnosis of SARS-CoV-2 infection to receive a total dose of 1200 mg of REGEN-COV or matching placebo administered by means of subcutaneous injection. At the time of randomization, participants were stratified according to the results of the local diagnostic assay for SARS-CoV-2 and according to age. The primary efficacy end point was the development of symptomatic SARS-CoV-2 infection through day 28 in participants who did not have SARS-COV-2 infection (as measured by reverse-transcriptase–quantitative polymerase-chain-reaction assay) or previous immunity (seronegativity).
Results
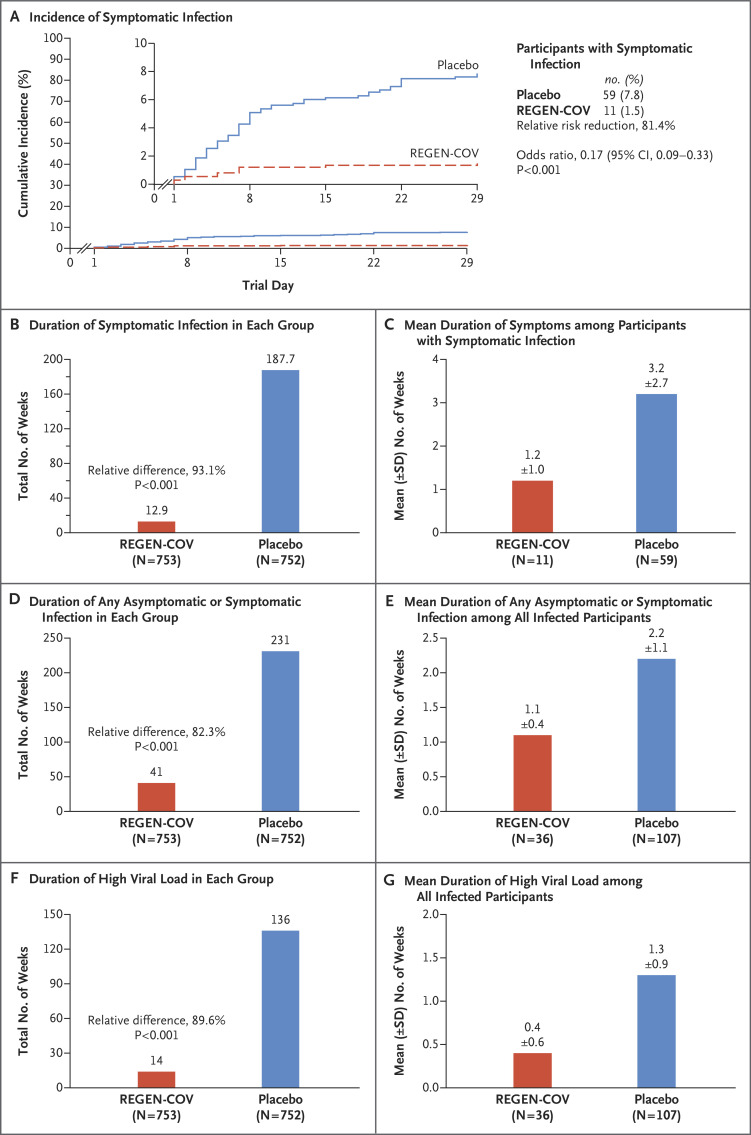
Symptomatic SARS-CoV-2 infection developed in 11 of 753 participants in the REGEN-COV group (1.5%) and in 59 of 752 participants in the placebo group (7.8%) (relative risk reduction [1 minus the relative risk], 81.4%; P<0.001). In weeks 2 to 4, a total of 2 of 753 participants in the REGEN-COV group (0.3%) and 27 of 752 participants in the placebo group (3.6%) had symptomatic SARS-CoV-2 infection (relative risk reduction, 92.6%). REGEN-COV also prevented symptomatic and asymptomatic infections overall (relative risk reduction, 66.4%). Among symptomatic infected participants, the median time to resolution of symptoms was 2 weeks shorter with REGEN-COV than with placebo (1.2 weeks and 3.2 weeks, respectively), and the duration of a high viral load (>104 copies per milliliter) was shorter (0.4 weeks and 1.3 weeks, respectively). No dose-limiting toxic effects of REGEN-COV were noted.
Conclusions
Subcutaneous REGEN-COV prevented symptomatic Covid-19 and asymptomatic SARS-CoV-2 infection in previously uninfected household contacts of infected persons. Among the participants who became infected, REGEN-COV reduced the duration of symptomatic disease and the duration of a high viral load. (Funded by Regeneron Pharmaceuticals and others; ClinicalTrials.gov number, NCT04452318.)
Coronavirus disease 2019 (Covid-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first detected in December 2019 and was declared a global pandemic in March 2020.1-3 REGEN-COV (previously known as REGN-COV2), a combination of two neutralizing monoclonal antibodies (casirivimab and imdevimab, administered together), binds noncompeting epitopes of the receptor-binding domain of the SARS-CoV-2 spike protein. This combination therapy retains neutralization potency against circulating SARS-CoV-2 variants of concern, including B.1.1.7 (or alpha), B.1.351 (or beta), B.1.617.2 (or delta), B.1.429 (or epsilon), and P.1 (or gamma), in vitro and in vivo and may protect against the selection of resistant variants.4-6 In outpatients with Covid-19, the use of REGEN-COV has been shown to reduce the incidence of hospitalization or death from any cause by approximately 70%, rapidly reduce the viral load, and shorten the duration of symptoms.7,8
We conducted a trial to evaluate whether subcutaneously administered REGEN-COV could be used to prevent Covid-19 among persons with ongoing exposure to a person infected with SARS-CoV-2. We used a household-contact trial design to assess whether REGEN-COV could prevent SARS-CoV-2 infection in a scenario with a high risk of lateral transmission. Nasopharyngeal swabs for reverse-transcriptase–quantitative polymerase-chain-reaction (RT-qPCR) testing for SARS-CoV-2 were obtained before randomization and were used to assign the participants to two analysis cohorts (referred to as Part A and Part B). Part A involved participants with negative test results, and Part B involved those with positive results. Here, we report the results in uninfected household contacts (Part A) from this phase 3 trial involving adolescents and adults.
Methods
Trial Design
In this two-part, randomized, double-blind, placebo-controlled trial, we assessed the efficacy and safety of subcutaneous REGEN-COV in preventing SARS-CoV-2 infection among previously uninfected household contacts of infected persons (Part A) and in treating recently infected asymptomatic persons (Part B). The trial was conducted at 112 sites in the United States, Romania, and Moldova.
Nasopharyngeal and serum samples were obtained from the trial participants at the screening (baseline) visit for RT-qPCR testing and serum antibody testing at a central laboratory. RT-qPCR testing was used to detect ongoing infection with SARS-CoV-2, whereas one or more positive results on serologic testing (anti-spike [S1] IgA, anti-spike [S1] IgG, or anti-nucleocapsid IgG) was used to detect a previous or ongoing infection in which an innate antibody immune response had already occurred (i.e., this test was used to determine whether the participant was seropositive or seronegative). Part A of the trial involved participants who were RT-qPCR–negative, and Part B involved those who were RT-qPCR–positive. The populations for Parts A and B were mutually exclusive, and the data were analyzed separately with different hierarchies and separate alpha allocations. Here, we describe the results of Part A.
The trial participants were randomly assigned (in a 1:1 ratio) to receive subcutaneous REGEN-COV at a total dose of 1200 mg (600 mg each of casirivimab and imdevimab) or placebo and were stratified according to age and to the results of local diagnostic tests to detect SARS-CoV-2. The trial consisted of a 1-day screening (baseline) period, a 28-day efficacy assessment period, and a 7-month follow-up period (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). After the review of safety data for the sentinel participants (i.e., the first 12 adolescents and 30 adults), we proceeded to full enrollment. The protocol is available at NEJM.org.
Trial Oversight
The trial was managed jointly by Regeneron Pharmaceuticals, the Covid-19 Prevention Network, and the National Institute of Allergy and Infectious Diseases. The authors vouch for the completeness and accuracy of the data, the comprehensive reporting of adverse events, and the fidelity of the trial to the protocol. All the participants provided written informed consent. Additional details are provided in the Methods section in the Supplementary Appendix.
Trial Participants
Asymptomatic, heathy adolescents (12 to 17 years of age) and adults (≥18 years of age) who were household contacts of an index patient (defined as the first person with a diagnosis of SARS-CoV-2 infection in the household) were eligible to participate if they anticipated living with the index patient for at least 28 days. Participants underwent randomization within 96 hours after collection of the index patient’s positive SARS-CoV-2 diagnostic test sample, and persons with previous SARS-CoV-2 infection were excluded. Full inclusion and exclusion criteria are provided in the Supplementary Appendix.
Intervention and Assessments
At baseline (day 1), the participants received a total dose of 1200 mg of REGEN-COV or placebo, administered by means of subcutaneous injection. The investigators conducted weekly interviews to assess for signs and symptoms of Covid-19 and adverse events since the last visit. If a participant tested positive for SARS-CoV-2 by RT-qPCR, data on the type and severity of signs and symptoms were collected weekly until resolution.
Nasopharyngeal swabs were obtained from the trial participants to test for SARS-CoV-2 by means of RT-qPCR at baseline and weekly during the efficacy assessment period. Weekly swabs were obtained from participants who tested positive until they tested negative twice. The analytic methods have been described previously.7
Some participants were referred to this trial through the index patient’s participation in a sister trial (COV-2067) of intravenous REGEN-COV or placebo in outpatients with Covid-19.7-9 The participants were interviewed at baseline to collect information on household members, including the index patient, and participant identification numbers in the two trials were linked in order to determine whether treatment with REGEN-COV in an index patient affected SARS-CoV-2 transmission among household contacts.
End Points
The prespecified primary efficacy analysis population in Part A of the trial consisted of participants who did not have evidence of previous infection (i.e., they were RT-qPCR–negative and seronegative) and who underwent randomization by January 28, 2021; this population excluded participants in the initial descriptive assessment (described in the Results section in the Supplementary Appendix). The primary efficacy end point was the percentage of participants in whom symptomatic, RT-qPCR–confirmed SARS-CoV-2 infection developed during the 28-day efficacy assessment period. A broad-term definition of symptomatic Covid-19 was used for this analysis. Alternative definitions of symptomatic disease (a strict-term definition and the Centers for Disease Control and Prevention [CDC] definition) were also used for secondary analyses and are detailed in the Methods section in the Supplementary Appendix. The primary and key secondary end points were tested hierarchically (Table S1). Full lists of secondary efficacy and exploratory end points are provided in the statistical analysis plan, available with the protocol at NEJM.org.
Safety end points included adverse events and adverse events of special interest (hypersensitivity and injection-site reactions, both grade ≥3). Safety end points are reported for participants who underwent randomization through January 28, 2021, until the data-cutoff date of March 11, 2021; the safety population included participants in the initial descriptive assessment.
Statistical Analysis
The statistical analysis plan for Part A of the trial was finalized before database lock and unblinding. The seronegative-modified full analysis set included all participants who were at least 12 years of age, underwent randomization, and were confirmed by RT-qPCR serologic testing at a central laboratory to be negative for SARS-CoV-2 at baseline; this analysis set excluded participants in the initial descriptive assessment. All efficacy end points are reported through the 28-day efficacy assessment period. Safety data are reported for all participants who received REGEN-COV or placebo, including those in the initial descriptive assessment.
We calculated that approximately 1248 seronegative participants from 430 households (assuming an average household size of 2.9 participants) would provide greater than 90% power to detect a 50% relative difference in the risk of symptomatic Covid-19 infection (with an assumed 10% attack rate in the placebo group) over the 28-day efficacy assessment period at a two-sided alpha level of 0.05. With a 5% attack rate in the REGEN-COV group (i.e., a 50% relative risk reduction), this between-group difference is equivalent to an odds ratio of 0.47.
In the primary analysis population, more than 80% of the households had only a single trial participant, so the primary efficacy end point was analyzed with the use of logistic regression. The model included the fixed category effects of trial group (REGEN-COV or placebo), region (United States or another country), and age (12 to 49 years or ≥50 years). The adjusted odds ratio and observed relative risk reduction (1 minus the relative risk) are reported. A statistical hierarchy was used to control for type I error on the basis of a two-sided alpha level of 0.05 to test the primary and key secondary end points. Analyses of the key secondary efficacy end points, details regarding the imputation of missing data, and the population and methods for the pharmacokinetic analyses are provided in the Supplementary Methods section in the Supplementary Appendix and the statistical analysis plan.
Results
Participants
This trial included 2475 participants, not including those in the initial descriptive assessment. A total of 2067 of these participants (83.5%) had confirmed SARS-CoV-2–negative RT-qPCR test results and were included in the Part A analysis; of these participants, 1505 (72.8%) also had no evidence of previous SARS-CoV-2 infection on serologic testing (i.e., they were seronegative at baseline). These 1505 participants for whom there was no evidence of previous or ongoing infection (the primary efficacy analysis population) were assigned to receive REGEN-COV (753 participants) or placebo (752 participants) (Fig. S2).
The mean age of the participants was 42.9 years, 45.9% were adolescent boys or men, 9.3% identified as Black, and 40.5% identified as Hispanic or Latinx. The median household size, including the index patient and other household members who did not participate in the trial, was 3 persons (interquartile range, 2 to 4). A total of 81.8% of the households consisted of only 1 RT-qPCR–negative, seronegative participant (Table 1). Baseline characteristics of the seropositive participants are presented in Table S3.
Table 1. Demographic and Clinical Characteristics of the Seronegative Population at Baseline.*.
| Characteristic | REGEN-COV (N=753) |
Placebo (N=752) |
Total (N=1505) |
|---|---|---|---|
| Age | |||
| Mean (range) — yr | 43.2 (12–87) | 42.7 (12–92) | 42.9 (12–92) |
| ≥50 yr — no. (%) | 294 (39.0) | 280 (37.2) | 574 (38.1) |
| Male sex — no. (%) | 333 (44.2) | 358 (47.6) | 691 (45.9) |
| Race or ethnic group — no. (%)† | |||
| White race | 653 (86.7) | 635 (84.4) | 1288 (85.6) |
| Black race | 62 (8.2) | 78 (10.4) | 140 (9.3) |
| Asian race | 23 (3.1) | 19 (2.5) | 42 (2.8) |
| American Indian or Alaska Native ethnic group | 3 (0.4) | 4 (0.5) | 7 (0.5) |
| Native Hawaiian or Pacific Islander ethnic group | 1 (0.1) | 2 (0.3) | 3 (0.2) |
| Other‡ | 11 (1.5) | 14 (1.9) | 25 (1.7) |
| Hispanic or Latinx ethnic group — no. (%)† | |||
| Yes | 291 (38.6) | 319 (42.4) | 610 (40.5) |
| No | 459 (61.0) | 428 (56.9) | 887 (58.9) |
| Other§ | 3 (0.4) | 5 (0.7) | 8 (0.5) |
| Mean weight — kg | 81.3±19.9 | 81.2±19.7 | 81.3±19.8 |
| BMI¶ | |||
| Mean | 28.9±12.4 | 28.5±6.3 | 28.7±9.8 |
| ≥30 — no. (%) | 261 (34.7) | 250 (33.2) | 511 (34.0) |
| High risk of Covid-19 — no. (%) | |||
| Any high-risk factor | 238 (31.6) | 221 (29.4) | 459 (30.5) |
| ≥65 yr of age | 76 (10.1) | 55 (7.3) | 131 (8.7) |
| BMI ≥35 | 99 (13.1) | 104 (13.8) | 203 (13.5) |
| Chronic kidney disease | 17 (2.3) | 11 (1.5) | 28 (1.9) |
| Diabetes | 58 (7.7) | 45 (6.0) | 103 (6.8) |
| Immunosuppressive disease | 5 (0.7) | 2 (0.3) | 7 (0.5) |
| Receipt of immunosuppressive therapy | 4 (0.5) | 11 (1.5) | 15 (1.0) |
| ≥55 yr of age with cardiovascular disease, hypertension, or COPD | 99 (13.1) | 90 (12.0) | 189 (12.6) |
| Total no. of households | 679 | 686 | 1209 |
| Household size — no. of participants/total no. of households (%) | |||
| 1 person | 486/679 (71.6) | 503/686 (73.3) | 989/1209 (81.8) |
| 2 persons | 146/679 (21.5) | 136/686 (19.8) | 172/1209 (14.2) |
| 3 persons | 30/679 (4.4) | 30/686 (4.4) | 31/1209 (2.6) |
| 4 persons | 13/679 (1.9) | 13/686 (1.9) | 13/1209 (1.1) |
| >4 persons | 4/679 (0.6) | 4/686 (0.6) | 4/1209 (0.3) |
| Participants with an index patient participating in COV-2067 trial — no. (%) | 187 (24.8) | 186 (24.7) | 373 (24.8) |
Plus–minus values are means ±SD. COPD denotes chronic obstructive pulmonary disease, and Covid-19 coronavirus disease 2019.
Race and ethnic group were reported by the participants. Data on Hispanic or Latinx ethnic group were collected separately from data on other ethnic groups.
“Other” may include not reported, unknown, or multiple races or ethnic groups.
“Other” may include not reported or unknown.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
A total of 459 of 1505 seronegative participants (30.5%) were at high risk for severe Covid-19 if they became infected with SARS-CoV-2 (Table 1). On June 3, 2021, in an Emergency Use Authorization (EUA) fact sheet, the Food and Drug Administration updated the criteria for persons who are considered to be at high risk for severe Covid-19 if they became infected.9 According to the updated criteria, in which the criteria for the body-mass index (the weight in kilograms divided by the square of the height in meters) changed from 35 or more to more than 25, a total of 1137 participants (75.5%) in this trial were at high risk for severe Covid-19 if they became infected (Table S2).
Approximately 25% of the participants lived with an index patient who was receiving REGEN-COV or placebo in the COV-2067 trial (Table 1). Treatment with REGEN-COV in index patients in that trial had no effect on the incidence of infection in this trial; these results are described in the Supplementary Appendix.
Prevention of SARS-CoV-2 Infection
Overall, symptomatic SARS-CoV-2 infection developed in 11 of 753 participants in the REGEN-COV group (1.5%) and in 59 of 752 participants in the placebo group (7.8%) (relative risk reduction, 81.4%; odds ratio, 0.17; P<0.001) (Table 2). Efficacy was apparent within days after the initiation of REGEN-COV (Figure 1A). Within the first week after administration of REGEN-COV or placebo, 9 of 753 participants in the REGEN-COV group (1.2%) and 32 of 752 participants in the placebo group (4.3%) had symptomatic SARS-CoV-2 infection (relative risk reduction, 71.9%); in weeks 2 to 4, a total of 2 of 753 (0.3%) and 27 of 752 (3.6%), respectively, had symptomatic SARS-CoV-2 infection (relative risk reduction, 92.6%; post hoc analysis) (Table S4). The findings were similar with the use of broad-term, strict-term, and CDC definitions of symptomatic SARS-CoV-2 infection (Table S5); in participants who were considered to be at high risk for progression to severe Covid-19 according to the updated EUA fact sheet (post hoc analysis) (Table S6)10; and regardless of baseline serologic status (Table S7).
Table 2. Primary and Key Secondary Efficacy End Points.*.
| End Point | REGEN-COV (N=753) |
Placebo (N=752) |
|---|---|---|
| Primary end point: symptomatic RT-qPCR–confirmed SARS-CoV-2 infection, broad-term definition† | ||
| No. of participants (%) | 11 (1.5) | 59 (7.8) |
| Relative risk reduction — % | 81.4 | — |
| Odds ratio (95% CI) | 0.17 (0.09–0.33) | — |
| P value‡ | <0.001 | — |
| Viral load >104 copies/ml§ | ||
| No. of participants/total no. (%) | 12/745 (1.6) | 85/749 (11.3) |
| Relative risk reduction — % | 85.8 | — |
| Odds ratio (95% CI) | 0.13 (0.07–0.24) | — |
| P value‡ | <0.001 | — |
| Duration of symptomatic RT-qPCR–confirmed SARS-CoV-2 infection, broad-term definition | ||
| Total no. of wk | 12.9 | 187.7 |
| Total duration/1000 participants — wk | 17.1 | 249.6 |
| Relative difference vs. placebo — %¶ | 93.1 | — |
| P value‖ | <0.001 | — |
| Mean duration of symptoms/participant with symptomatic infection — wk | 1.2±1.0 | 3.2±2.7 |
| Duration of high viral load (>104 copies/ml) among all participants§ | ||
| Total no. of wk | 14.0 | 136.0 |
| Total duration/1000 participants — wk | 18.8 | 181.6 |
| Relative difference vs. placebo — %¶ | 89.6 | — |
| P value‖ | <0.001 | — |
| Mean duration of high viral load/infected participant — wk | 0.4±0.6 | 1.3±0.9 |
| Duration of any RT-qPCR–confirmed symptomatic or asymptomatic SARS-CoV-2 infection | ||
| Total no. of wk | 41.0 | 231.0 |
| Total duration/1000 participants — wk | 54.4 | 307.2 |
| Relative difference vs. placebo — %¶ | 82.3 | — |
| P value‖ | <0.001 | — |
| Mean duration of overall infection/infected participant — wk | 1.1±0.4 | 2.2±1.1 |
| Any RT-qPCR–confirmed symptomatic or asymptomatic SARS-CoV-2 infection | ||
| No. of participants (%) | 36 (4.8) | 107 (14.2) |
| Relative risk reduction — % | 66.4 | — |
| Odds ratio (95% CI) | 0.31 (0.21–0.46) | — |
| P value** | <0.001 | — |
Plus–minus values are means ±SD. Key secondary end points are presented in order of the hierarchical testing sequence. CI denotes confidence interval, RT-qPCR reverse-transcriptase–quantitative polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
A sensitivity analysis with the use of a generalized estimation equation showed similar results.
The P value was based on a logistic-regression model adjusted for region (United States or other country) and age group (12 to 49 years or ≥50 years).
For the viral-load end points, only participants with at least one viral-load assessment during the 28-day efficacy assessment period were included in the analysis.
The calculation of the relative difference between REGEN-COV and placebo was based on the normalized weeks per 1000 participants.
The P value was based on a stratified Wilcoxon rank-sum test (van Elteren test) with region (United States or other country) and age group (12 to 49 years or ≥50 years) as strata.
The P value was based on multiple imputation with the use of fully conditional specification followed by a logistic-regression model including the trial group, region (United States or other country), and age group (12 to 49 years or ≥50 years).
Figure 1. SARS-CoV-2 Infection in the REGEN-COV and Placebo Groups.
Panel A shows the cumulative incidence of symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after administration of REGEN-COV or placebo during the 28-day efficacy assessment period. The relative risk reduction was calculated as 1 minus the relative risk. The inset shows the same data on an enlarged y axis. The P value is based on a logistic-regression model including the fixed category effects of trial group (REGEN-COV or placebo), region (United States or other country), and participant age (12 to 49 years or ≥50 years). Panel B shows the aggregate total weeks of symptomatic SARS-CoV-2 infection in each trial group. In Panels B, D, and F, the calculation of the relative difference is based on the normalized weeks per 1000 participants, and the P value is based on a stratified Wilcoxon rank-sum test (van Elteren test) with region (United States or other country) and age group (12 to 49 years or ≥50 years) as strata. Panel C shows the mean duration of symptoms. Panel D shows the aggregate total weeks of any asymptomatic or symptomatic SARS-CoV-2 infection in each trial group. Panel E shows the mean duration of overall infection. Panel F shows the aggregate total weeks of a high SARS-CoV-2 viral load (>104 copies per milliliter) in each trial group. Panel G shows the mean duration of a high SARS-CoV-2 viral load. In Panels F and G, if viral-load data were missing at a visit, that visit was not included in the analysis, and only participants with at least one nasopharyngeal swab sample to detect the viral load after baseline were included. CI denotes confidence interval.
The aggregate total number of weeks in which participants had symptoms was 12.9 weeks in the REGEN-COV group and 187.7 weeks in the placebo group (relative difference, 93.1%; P<0.001) (Figure 1B and Table 2). This outcome corresponded to a 2-week difference in the mean duration of symptomatic infection, from 1.2 weeks in the REGEN-COV group to 3.2 weeks in the placebo group (Figure 1C and Table 2).
Overall, asymptomatic or symptomatic SARS-CoV-2 infection developed in 36 of 753 participants in the REGEN-COV group (4.8%) and in 107 of 752 participants in the placebo group (14.2%) (relative risk reduction, 66.4%; odds ratio, 0.31; P<0.001) (Table 2). Consistent with this finding, the aggregate total number of weeks of any asymptomatic or symptomatic RT-qPCR–detectable SARS-CoV-2 infection was 41.0 weeks in the REGEN-COV group and 231.0 weeks in the placebo group, a relative difference of 82.3% (P<0.001) (Figure 1D and Table 2); this finding corresponded to an approximate 1-week difference in the mean duration of overall infection, from 1.1 weeks in the REGEN-COV group to 2.2 weeks in the placebo group (Figure 1E and Table 2).
In addition, 12 of 745 participants in the REGEN-COV group (1.6%) and 85 of 749 participants in the placebo group (11.3%) had a high SARS-CoV-2 viral load, defined as more than 104 copies per milliliter on nasopharyngeal RT-qPCR (relative risk reduction, 85.8%; odds ratio, 0.13; P<0.001) (Table 2). Of the participants who became infected after receiving REGEN-COV, the majority had a low viral load (Table S8). In a result consistent with this finding, the aggregate total number of weeks of a high SARS-CoV-2 viral load was 14.0 weeks in the REGEN-COV group and 136.0 weeks in the placebo group, an 89.6% relative difference (P<0.001) (Figure 1F and Table 2). This finding corresponded to an approximate 0.9-week difference in the mean duration of high-viral-load infection, from 0.4 weeks in the REGEN-COV group to 1.3 weeks in the placebo group (Figure 1G and Table 2).
Participants who became infected despite receipt of REGEN-COV also had a lower peak viral load than infected participants in the placebo group (Figure 2A), and the duration of high-viral-load infections (>104 copies per milliliter) was shorter (Fig. S3). REGEN-COV prevented high viral-load levels in both symptomatic and asymptomatic participants (Figure 2B and 2C). Additional data on the viral load are provided in Table S9.
Figure 2. Viral Load in Participants with Asymptomatic and Symptomatic Infection.
Panel A shows the peak viral load according to symptom status. Data points represent individual participants. Panel B shows the viral load at the first positive reverse-transcriptase–quantitative polymerase-chain-reaction (RT-qPCR) test in all participants. Panel C shows the viral load at the first positive RT-qPCR test in all infected participants, according to symptom status. The boxes represent interquartile ranges, with the horizontal line in each box representing the median and the whiskers showing the values that were 1.5 times the values represented at each end of the box. The large diamonds in the boxes represent the mean.
Subanalyses According to Age
Among the adolescent participants (12 to 17 years of age), a prespecified subanalysis involving seronegative participants showed that the incidence of symptomatic SARS-CoV-2 infection was 0% (0 of 34 participants) in the REGEN-COV group as compared with 12% (4 of 34 participants) in the placebo group, corresponding to a relative risk reduction of 100% (Table S10). Regardless of serologic status, symptomatic infection developed in 0 of 46 adolescent participants in the REGEN-COV group (0%) and in 4 of 43 adolescent participants (9%) in the placebo group (relative risk reduction, 100%).
Furthermore, prespecified subanalyses involving adults who were at least 50 years of age showed that the incidence of symptomatic SARS-CoV-2 infection was 2.0% (6 of 295 participants) in the REGEN-COV group and 9.3% (26 of 280 participants) in the placebo group, corresponding to a relative risk reduction of 78.1%. Post hoc efficacy analyses involving adults who were at least 65 years of age showed that the incidence of symptomatic SARS-CoV-2 infection was 1% (1 of 76 participants) in the REGEN-COV group and 13% (7 of 55 participants) in the placebo group, corresponding to a relative risk reduction of 89.7%.
Safety
A total of 20.2% of the participants in the REGEN-COV group and 29.0% of those in the placebo group had at least one adverse event, and 16.0% and 16.5%, respectively, had non–Covid-19 adverse events (Table S11). Adverse events that occurred in at least 2% of the participants included symptomatic Covid-19, asymptomatic Covid-19, headache, and injection-site reaction (Table 3). No adverse events of special interest were reported during the trial, and no participants withdrew from the trial because of an adverse event.
Table 3. Adverse Events.*.
| Event | REGEN-COV (N=1311) |
Placebo (N=1306) |
|---|---|---|
| number of participants (percent) | ||
| Symptomatic Covid-19 | 15 (1.1) | 112 (8.6) |
| Asymptomatic Covid-19 | 54 (4.1) | 108 (8.3) |
| Headache | 24 (1.8) | 46 (3.5) |
| Injection-site reaction | 55 (4.2) | 19 (1.5) |
Shown are the adverse events that occurred in at least 2% of the participants, regardless of the participants’ SARS-CoV-2 serologic status at baseline.
A total of 0.8% of the participants in the REGEN-COV group and 1.1% of those in the placebo group had at least one serious adverse event (Table S12). None of the serious adverse events in the REGEN-COV group were considered by the investigators to be related to Covid-19, REGEN-COV, or placebo. None of the participants in the REGEN-COV group had emergency department visits or hospitalizations due to Covid-19, whereas four participants in the placebo group visited an emergency department or were admitted to the hospital. Two deaths occurred outside the efficacy assessment period in the safety population of each trial group (in 2 of 1311 participants in the REGEN-COV group [0.2] and in 2 of 1306 participants in the placebo group [0.2]); none of these deaths were attributed by the investigators to Covid-19 (Table S13). In the REGEN-COV group, one participant died of congestive cardiac failure, and one participant with multiple coexisting conditions had sudden death that was not considered by the investigators to be related to Covid-19. In the placebo group, one participant died of a gunshot wound, and one participant died of cardiac arrest that was not considered by the investigators to be related to Covid-19.
Pharmacokinetics
Casirivimab and imdevimab were rapidly absorbed (Fig. S4); the mean concentrations in serum 1 day after administration were 22.1 mg per liter and 25.8 mg per liter, respectively. The antibodies reached maximal concentrations in serum at a median of 7 to 8 days. Casirivimab and imdevimab had linear elimination and had mean half-lives of 32.4 days and 27.0 days, respectively. At 28 days after administration, the mean concentrations of casirivimab and imdevimab in serum were 30.4 mg per liter and 24.6 mg per liter, respectively; these levels were above the estimated target dose for neutralization of SARS-CoV-2 (20 mg per liter). A summary of pharmacokinetic measures is provided in Table S14.
Discussion
More than 191 million persons have been infected with SARS-CoV-2 worldwide, and more than 4 million have died from complications of Covid-19.11 When we initiated the REGEN-COV program, we hypothesized that a combination of neutralizing, noncompeting monoclonal antibodies could have activity in both treatment and prevention of SARS-CoV-2 infection while retaining activity against the inevitable emergence of viral variants. In outpatients, intravenous REGEN-COV reduced the incidence of Covid-19–related hospitalization and death from any cause, rapidly resolved symptoms, and reduced the viral load.7,8
In designing this phase 3 clinical trial, we anticipated a high background incidence of lateral transmission in households with a person who had documented SARS-CoV-2 infection quarantining alongside other household members; this high incidence has subsequently been confirmed.12 This scenario is typical of many other scenarios in which uninfected persons are in close proximity to infected persons and would be at high risk for infection. These settings include hospitals, nursing homes, dense housing complexes, prisons, and schools. Prevention in these high-risk settings would also correlate with prevention in lower-risk settings.
Among household members who were confirmed to be both uninfected and without a recent history of infection, subcutaneous REGEN-COV prevented both symptomatic and overall SARS-CoV-2 infections, and the majority of participants who received this agent had minor or no adverse events. The relative risk reduction between the REGEN-COV group and the placebo group in the incidence of symptomatic SARS-CoV-2 infection was approximately 81%. Within 1 week after the initiation of REGEN-COV or placebo, the relative risk reduction was approximately 72%, which increased to approximately 93% after the first week. The relative risk reduction in high-viral-load infections (>104 copies per milliliter) was approximately 86%, and the relative risk reduction in all infections (symptomatic and asymptomatic) was approximately 66%. In participants in whom SARS-CoV-2 infection developed, those in the REGEN-COV group had a lower likelihood of symptoms than those in the placebo group. In the participants who became infected after receiving REGEN-COV or placebo and in whom symptomatic infection was confirmed, the duration of symptoms was 2 weeks shorter in the REGEN-COV group (in whom laboratory-confirmed symptomatic infection developed in 1.5%) than in the placebo group. Moreover, in participants in whom either symptomatic or asymptomatic infection developed, the magnitude and duration of detectable RNA (i.e., the peak viral load and weeks of RT-qPCR positivity) were markedly lower in the REGEN-COV group than in the placebo group.
The incidence of adverse events was numerically higher in the placebo group than in the REGEN-COV group, with the difference attributed to the higher number of Covid-19 infections observed in the placebo group. No injection-site or hypersensitivity reactions of grade 3 or higher occurred in either group. None of the participants in the REGEN-COV group, as compared with four participants in the placebo group, had an emergency department visit or a hospitalization during the efficacy assessment period. After subcutaneous administration of REGEN-COV, the concentrations of each antibody in serum were well above the predicted neutralization target concentration (according to preclinical data) as early as the first day after initiation and throughout the 28-day efficacy assessment period.
Although the primary efficacy analysis involved seronegative participants, analyses that were conducted irrespective of baseline serologic status showed that REGEN-COV prevented symptomatic infection. Combined with the observed safety profile of REGEN-COV, this result suggests that point-of-care serologic data will not be needed for treatment decisions in the clinic.
These data provide support for the potential use of REGEN-COV to prevent SARS-CoV-2 infection and symptomatic disease in persons in whom immediate protection is warranted; the use of REGEN-COV in such persons could decrease further spread and transmissibility of SARS-CoV-2 infection. Recent studies have shown that REGEN-COV has maintained activity against emerging variants of concern.4-6 Our trial also showed that subcutaneous administration of REGEN-COV was efficacious and had an acceptable safety profile; thus, it may provide substantial benefits because the health care resources necessary for an intravenous infusion may be avoided.
Despite the increasing use of highly effective vaccines, SARS-CoV-2 has not been eradicated. Moreover, it is unclear how many persons will ultimately choose to become vaccinated, how vaccine efficacy will wane over time, and how great a problem emerging variants of concern will be. For these reasons, a need will persist for a complementary approach to prevent the spread of SARS-CoV-2 infection in persons who are not vaccinated, who have waning vaccine-mediated protection over time or because of the emergence of variants, or who are immunocompromised and cannot mount an antibody-mediated antiviral response.
This trial showed that, throughout the 28-day observation period, the achieved concentrations of a single subcutaneous dose of REGEN-COV prevented symptomatic infection; thus, REGEN-COV has potential use as long-term prophylaxis in persons at risk for SARS-CoV-2 infection. Over the same period, the incidence of asymptomatic infection was also lower among participants who received REGEN-COV than among those who received placebo.
Acknowledgments
We thank the trial participants and their families; the members of the data and safety monitoring board; Caryn Trbovic, Ph.D., Brian Head, Ph.D., and S. Balachandra Dass, Ph.D., from Regeneron Pharmaceuticals for assistance with development of an earlier version of the manuscript; and Prime for formatting and copy editing suggestions for an earlier version of the manuscript.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
This article was published on August 4, 2021.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, cooperative agreement awards from the National Institute of Allergy and Infectious Diseases, NIH, and a grant (UM1AI068619) from the Covid-19 Prevention Network.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020. 2020. (https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020).
- 3.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baum A, Fulton BO, Wloga E, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020;369:1014-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copin R, Baum A, Wloga E, et al. The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies. Cell 2021. June 5 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021;593:130-135. [DOI] [PubMed] [Google Scholar]
- 7.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021;384:238-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody cocktail clinical outcomes study in Covid-19 outpatients. June 6, 2020. (https://www.medrxiv.org/content/10.1101/2021.05.19.21257469v2). preprint.
- 9.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody cocktail in outpatients with Covid-19. June 12, 2020. (https://www.medrxiv.org/content/10.1101/2021.06.09.21257915v1). preprint.
- 10.Fact sheet for health care providers: Emergency Use Authorization (EUA) of REGEN-COV (casirivimab and imdevimab). Tarrytown, NY: Regeneron, 2021. (https://www.regeneron.com/downloads/treatment-covid19-eua-fact-sheet-for-hcp.pdf). [Google Scholar]
- 11.World Health Organization. WHO coronavirus (COVID-19) dashboard. 2021. (https://covid19.who.int/).
- 12.Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open 2020;3(12):e2031756-e2031756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.