Abstract
Purpose
Mesenchymal stromal cells (MSCs) have been shown to enhance tissue repair as a cell-based therapy. In preparation for a phase I clinical study, we evaluated the safety, dosing, and efficacy of bone marrow–derived MSCs after subconjunctival injection in preclinical animal models of mice, rats, and rabbits.
Methods
Human bone marrow–derived MSCs were expanded to passage 4 and cryopreserved. Viability of MSCs after thawing and injection through small-gauge needles was evaluated by vital dye staining. The in vivo safety of human and rabbit MSCs was studied by subconjunctivally injecting MSCs in rabbits with follow-up to 90 days. The potency of MSCs on accelerating wound healing was evaluated in vitro using a scratch assay and in vivo using 2-mm corneal epithelial debridement wounds in mice. Human MSCs were tracked after subconjunctival injection in rat and rabbit eyes.
Results
The viability of MSCs after thawing and immediate injection through 27- and 30-gauge needles was 93.1% ± 2.1% and 94.9% ± 1.3%, respectively. Rabbit eyes demonstrated mild self-limiting conjunctival inflammation at the site of injection with human but not rabbit MSCs. In scratch assay, the mean wound healing area was 93.5% ± 12.1% in epithelial cells co-cultured with MSCs compared with 40.8% ± 23.1% in controls. At 24 hours after wounding, all MSC-injected murine eyes had 100% corneal wound closure compared with 79.9% ± 5.5% in controls. Human MSCs were detectable in the subconjunctival area and peripheral cornea at 14 days after injection.
Conclusions
Subconjunctival administration of MSCs is safe and effective in promoting corneal epithelial wound healing in animal models.
Translational Relevance
These results provide preclinical data to support a phase I clinical study.
Keywords: mesenchymal stromal cells, bone marrow, cornea, clinical translation
Introduction
An intact corneal epithelium provides a critical defensive barrier and is essential for clear vision. After nonpenetrating trauma, the cornea typically re-epithelializes promptly, minimizing the risk of infection, opacification, and perforation. Epithelial defects can persist in the presence of certain pathologic conditions, such as limbal stem cell deficiency, exposure keratopathy, and neurotrophic keratitis. Management of patients with nonhealing corneal epithelial wounds can be challenging. Several strategies are currently available in treatment of these conditions including limbal stem cell transplantation, amniotic membrane transplantation, and soft contact lenses. Despite many advances, there is still an unmet need for effective clinical strategies to promote corneal repair in these patients in which the outcomes of current therapies are suboptimal.1 For instance, in the setting of severe chemical injuries in which there are very few to no live cells remaining in the cornea, none of the standard treatments can alter the disease course. The emergent cell-based therapies using mesenchymal stromal cells (MSCs) may potentially support the corneal structure and prove useful in addressing these unmet clinical needs in severe ocular surface disease.2
MSCs are found in ubiquitous niches in most adult tissues such as bone marrow, fat, heart, skin, umbilical cord, dental pulp, and the cornea.3–5 MSCs have been under investigation as a cell-based therapy for treating a wide range of human diseases because of their anti-inflammatory, antifibrotic, and regenerative properties.6,7,9–11 MSCs have been shown to exert wound healing effects primarily through paracrine activity of secreted factors, which includes immunomodulatory factors that dampen inflammation, as well as extracellular matrix factors that contribute to tissue repair.9,12,13 In the eye, animal studies have shown that MSCs are able to reduce scarring, neovascularization, and inflammation, while promoting epithelialization in the cornea via the release of trophic and growth factors.3,8,14–18 These properties, together with the fact that MSCs can be readily obtained, expanded in vitro, and cryopreserved, make them a promising therapeutic candidate for healing human corneal wounds. It is hard to tell whether MSCs will be more or less effective compared with other methods without randomized clinical trials.
Subconjunctival injection is a standard technique in numerous ocular procedures with a relatively low complication rate.19 Considering its high reproducibility and consistency, it provides a suitable delivery mechanism to the surface of the eye. Subconjunctival injection of MSCs has previously been reported in several animal studies.20–22 In a recent study in a murine model of graft versus host disease (GVHD), treatment with human MSCs by subconjunctival injection was effective in reducing corneal inflammation and squamous metaplasia on the ocular surface.21 In another study in rats, a single subconjunctival injection appeared more effective than transplantation of MSCs grown over amniotic membrane in enhancing corneal wound healing and decreasing neovascularization after chemical injury.23 Several studies have evaluated direct intrastromal injection of MSCs and demonstrated production of collagen and extracellular matrix in the area of injection.24
To date, the clinical application of MSCs to the cornea has been limited to a few clinical studies.25,26 In particular, the use of bone marrow–derived MSCs in the cornea has only been reported in one clinical study by Calonge et al.,26 who compared the safety and efficacy of allogenic bone marrow–derived MSCs transplanted on amniotic membrane with cultivated limbal epithelial transplantation (CLET) in patients with limbal stem cell deficiency. They showed that MSCs were as safe and efficacious as CLET.26 Most recently, a clinical study has reported the safety of transconjunctival injection of MSCs into the lacrimal gland.27 Currently, there are insufficient data on the long-term in vivo safety of MSCs administered locally to the eye by subconjunctival injection. Therefore in this study, in preparation for a phase I human clinical trial, we evaluated MSCs in meeting quality control measures including viability and potency, as well as in vivo safety and efficacy in preclinical models.
Material and Methods
Preparation of the MSCs
Human bone marrow–derived MSCs were harvested from healthy donors under institutional review board approval from two sources: University of Wisconsin, Madison (Peiman Hematti, MD) and Rooster Bio (Frederick, MD). Human MSCs had previously been characterized according to the International Society for Cellular Therapy (ISCT) guidelines. MSCs were cultured in either serum containing media (MEM-Alpha plus 10% fetal bovine serum, 1X L-Glutamine, 1X NEAA, all from Corning, Manassas, VA) or a MSC-specific serum and xeno-free media (Rooster Nourish-MSC-XF; Rooster Bio). For rabbit safety studies, rabbit MSCs were purchased from Cyagen Biosciences (Santa Clara, CA), which provided a certificate of analysis of characterization by the tri-lineage differentiation. Rabbit MSCs were similarly cultured in xeno-free Rooster MSC media. On reaching 80% confluency, MSCs were trypsinized with TrypLE Express (Thermo Fisher Scientific, Waltham, MA), counted using a cell counter, and cryopreserved in a xeno-free storage medium containing 5% DMSO (CryoStor CS5; Biolife Solutions, Bothell, WA).
For cryopreservation, MSCs were aliquoted into sterile 2 mL-sized vials (Afton Scientific, Charlottesville, VA and West Pharmaceutical, Exton, PA) at concentrations of 1 × 107 and 2 × 107 cells per mL. Sterile stopper and flip-top cap were placed on the vials, which were manually closed using a hand crimper. The vials were placed in Mr. Frosty freezing container (Thermo Fisher Scientific) with cooling rate of −1°C per minute and were stored at −80°C overnight. The following day, the vials were transferred to a liquid nitrogen tank and were stored in the vapor phase. The MSCs were cryopreserved for only a couple of weeks to a month before we used on the animal, which do not affect the cells morphology and biologic functions.28
Animals and Housing
All animal experiments were conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol was approved by the Committee on the Ethics of Animal Experiments of University of Illinois at Chicago (UIC).
Mice (C57BL/6) that were 4 to 8 weeks old were used for experiments and were bred in-house at the Biological Resource Laboratory, UIC. Sprague Dawley rats (0.200–0.250 kg) and New Zealand White Rabbits (2.5–2.8 kg) were purchased from Charles River Laboratories (Wilmington, MA). All animals were kept on diurnal cycles of 12 h/light and 12 h/dark with ad libitum access to food. Intraperitoneal 100 mg/kg of ketamine (Hospira, Inc., Lake Forest, IL) and 50 mg/kg of xylazine (Lloyd Laboratories, Shenandoah, IA) were used to anesthetize mice and rats, whereas subcutaneous 50 mg/Kg of ketamine and 10 mg/Kg of xylazine were used for rabbits. Topical 1% proparacaine eye drops (Sandoz, Inc., Princeton, NJ) were instilled as a local anesthetic prior to every procedure. Postoperative analgesic with subcutaneous buprenorphine 0.01 to 0.05 mg/kg (Buprenex; Reckitt Benckiser Healthcare, Richmond, VA) was administered after surgery and every 72 hours until complete wound closure. Topical erythromycin ointment was administered in the injected eye once directly after subconjunctival injection.
The current study included 20 mice (10 in the treated group and 10 in the control group), 10 rats (5 in the treated group and 5 in the control group), and 18 rabbits (6 rabbits injected with 1 × 106, 6 injected with 3 × 106, and 6 injected with 6 × 106 MSCs) (Table 1). All the rabbit studies were done with the same batch of cells. Mouse and rat studies were done with different batches. Overall, three batches were used.
Table 1.
Summary of Animal Studies
| Mouse Model | Rat Model | Rabbit Model | |
|---|---|---|---|
| Animal numbers | 20 mice (10 treated and 10 control) | 10 rats (5 treated and 5 control) | 18 rabbits (6 injected with 1×106, 6 injected with 3×106, and 6 injected with 6×106 MSCs) |
| Damage model | In vivo damage model: a 2-mm area of the central epithelium was demarcated and removed by an AlgerBrush II | N/A | N/A |
| Read outs | Potency of MSCs on accelerating wound healing | Tracking of labeled human MSCs after subconjunctival injection | In vivo safety of human and rabbit MSCs |
Flow Cytometry
Both fresh MSCs (from culture plate) and thawed MSCs (from cryopreserved vials) were subjected to flow cytometry. Cryopreserved MSCs immediately after thawing (in 37°C water bath) and trypsinized fresh MSCs were collected separately with phosphate-buffered saline (PBS) then transferred into 50-mL tubes. They were centrifuged and washed with PBS two times. After final centrifugation supernatant was discarded, the cells were then incubated with Fc block (BD Pharmingen, Franklin Lakes, New Jersey; cat#: 564220 for human). The 1 × 106 MSCs were then aliquot into 1-mL polystyrene round-bottom tubes and stained with the primary antibodies diluted in PBS with 10% fetal calf serum at 4°C for 1 hour. Anti-human CD90, CD73, HLA- DR, CD14, CD11b, and CD45 FITC conjugated antibodies (Biolegend, San Diego, California) were used as the primary antibodies with 1:100 dilution. After primary antibodies incubation, MSCs were centrifuged and washed with 10% fetal calf serum on PBS three times. Flow cytometry data were acquired on CytoFLEX (Beckman Coulter, Brea, California). Data were analyzed using FlowJo software (FlowJo, Ashland, OR, Becton, Dickinson and Company; 2019). Human MSCs were identified being small fibroblast-like cells that were spindle-shaped under 2D culture conditions. Using flowcytometry, they were identified as CD73-, CD90-, and CD105-positive and CD11b-, CD14-, and CD45-negative.
Release criteria for MSC product included positive rate of more than 75% for CD73, CD90, and CD105 and less than 3% for negative markers CD11b, CD14, and CD45.
For rabbit MSCs, the main difference was that the cell surface markers are not well-defined like human MSCs, therefore flow cytometry was not performed (the morphology and tri-lineage differentiation of rabbit MSCs was mainly used).
Viability
Cryopreserved MSCs were thawed in a 37°C water bath for 5 minutes, after which the flip top cap was removed, and the cell suspension was aspirated into a 1-cc syringe using a 16-gauge needle. The needle was changed to either a 27- or 30-gauge needle, and the cells were injected into 1.5-mL microcentrifuge tubes. As control, thawed MSC suspension were drawn up and injected through a 200-µL pipette tip.
The viability of the injected MSCs was tested using two different assays. Trypan blue exclusion assay was used to count the total number of live (nonstaining) and dead (blue) cells under bright field microscopy (Leica, Wetzlar, Germany, DMi1). In addition to trypan blue, the fluorescent dyes Calcein AM (for viable cells) and propidium iodide (PI, for nonviable cells, both from Thermo Fisher Scientific) were also used to stain the cells according to the manufacturer's protocol. Following 15 minutes of incubation, the cells were imaged using a spinning-disc confocal microscope (Carl Zeiss, Jena, Germany). The images were analyzed with MetaMorph microscopy automation and image analysis software (Molecular Devices, San Jose, CA). Viability tests were performed on MSC suspensions immediately after thawing and hourly for 4 hours on thawed MSCs kept in the vial at room temperature (to determine how long MSCs remain viable in the vial after thawing).
In Vitro Wound Healing (Potency) Assay
Immortalized human corneal-limbal epithelial cells (HCLE cells, kindly donated by Dr. Ilene Gipson, Schepen Eye Research Institute, Massachusetts Eye and Ear Infirmary) were grown to confluency in 6-well plates in keratinocyte serum free media (KSFM; Thermo Fisher).29,30 The monolayer of cells was scratched using a 200-µL sterile pipette tip and washed twice with PBS to remove the floating cells. A day before the experiment, 3 × 105 MSCs were thawed and plated on 3-µm cell culture inserts (Corning, Manassas, VA). Both MSCs-plated and control insert (no cells) were supplemented with MEM-alpha with serum for 2 hours, then washed with PBS twice and replaced with MEM-alpha without serum. After making the scratches, the inserts were transferred to the 6-well plates containing scratched HCLE cells. The scratch wounds were photographed hourly for 18 hours using a spinning-disc confocal microscope (Carl Zeiss). The remaining wound area at 18 hours was quantified using ImageJ software version 1.8.0-112 (National Institutes of Health, Bethesda, MA).
In Vivo Safety of Subconjunctival Injection of MSCs
The in vivo safety of subconjunctival injection of MSCs was evaluated in both mice and rabbits. For the mouse model, once the animals were fully anesthetized, cryopreserved human bone marrow MSCs were thawed and 4 × 104 MSCs (2 uL) were subconjunctivally injected. Cryopreserved media was injected as a control with the same volume. For the rabbit model, once the rabbits were fully anesthetized, eyelid and surrounding orbital area were prepped with povidone iodine, and a sterile field area was created using a sterile drape. Cryopreserved human or rabbit bone marrow MSCs were thawed and drawn up into a syringe. One eye of each rabbit was subconjunctivally injected near the limbus with either 1 × 106 (50 uL), 3 × 106 (150 uL), or 6 × 106 MSCs (two injections of 3 × 106). In both mouse and rabbit models, injection was performed over 1 to 2 seconds. Rabbit eye (2.5–3 mL volume) is approximately 100 to 120 times the size of a mouse eye (25 uL). The dose injected in mice (4 × 104) is approximately 125 times less than the highest dose injected in rabbits 6 × 106, therefore doses per eye volume are roughly equivalent in both rabbit and mouse eyes. Subconjunctival injection was performed superiorly at 12 o'clock and inferiorly at 6 o'clock. In this experiment the animals did not receive steroid medication. They were assessed and photographed using a camera-equipped Nikon FS-2 slit lamp biomicroscope (Haag-Streit AG, Koeniz, Switzerland). The mice were followed until 2 weeks postinjection, and rabbits were followed weekly up to 3 months, for any pathological changes including corneal and conjunctival defects, inflammation, scarring, infection, and other signs of ocular toxicity.31 At the end of the experiment, all eyes were enucleated, paraffin embedded, cross sectioned, stained with H&E, and the sections were analyzed by an ocular pathologist.
In Vivo Wound Healing
C57BL/6J mice were anesthetized as noted earlier, after which a 2-mm area of the central epithelium was demarcated and removed by an AlgerBrush II (The Alger Company, Lago Vista, TX) as previously described.17 The wounded eyes were then given a subconjunctival injection of 4 × 104 (2 µL) human MSCs, or equal volume of vehicle control (cryopreservation media). Wound closure was monitored at 0 and 24 hours using fluorescein staining and photographed using a camera-equipped Nikon FS-2 slit lamp biomicroscope. The percentages of wound closure was compared with baseline for each eye, using ImageJ software.17 The timepoint was chosen based on the fact that the control wound would close within the next 12 hours.
MSC Labeling and Tracking
To track the injected MSCs in vivo, cell suspensions of human MSCs were labeled with live cell tracker CM-Dil (Cat: C7000; Thermo Fisher Scientific) and incubated in 1 µL of labeling solution per mL of Hank Balanced-Salt Solution (Sciencell, Carlsbad, CA) for 5 minutes at 37°C followed by incubation at 4°C for 15 minutes. After rinsing three times with PBS, cells were plated into a flask and left overnight in the 37°C incubator. This procedure was repeated daily for three consecutive days. A total of 1 × 105 labeled MSCs were subconjuctivally injected into the right eye of rats and 1 × 106 labeled MSCs for rabbits at two sites. An in vivo fluorescent microscope (Axiozoom; Carl Zeiss) was used to visualize the MSCs immediately after injection and weekly up to 12 weeks for rats and up to 2 weeks for rabbits. Enucleated MSC-injected rabbit eyes were cryosectioned, fixed with 4% paraformaldehyde, mounted on a slide with Vectashield mounting medium (Vector Laboratories, Burlingame, California) and imaged using a confocal microscope (LSM 710, Carl Zeiss). Additional tracking of the human MSCs was performed by immunostaining of the rabbit cornea with anti-human CD90 (from Biolegend; cat#328107, FITC antihuman CD90 [Thy1]).
Statistical Analysis
All the animal surgeries were done by one of the authors, and all in vitro studies had at least three replicates. Results are presented as mean ± standard deviation (SD) of three independent experiments. Unpaired 2-tailed Student's t-test or 1-way ANOVA followed by Tukey multiple comparison test were used when appropriate using GraphPad Prism software (GraphPad Software, San Diego, CA) and Microsoft Excel (Microsoft Corp, Redmond, WA). Differences were considered significant when P < 0.05. Error bars show SD of the mean.
Results
MSC Characterization
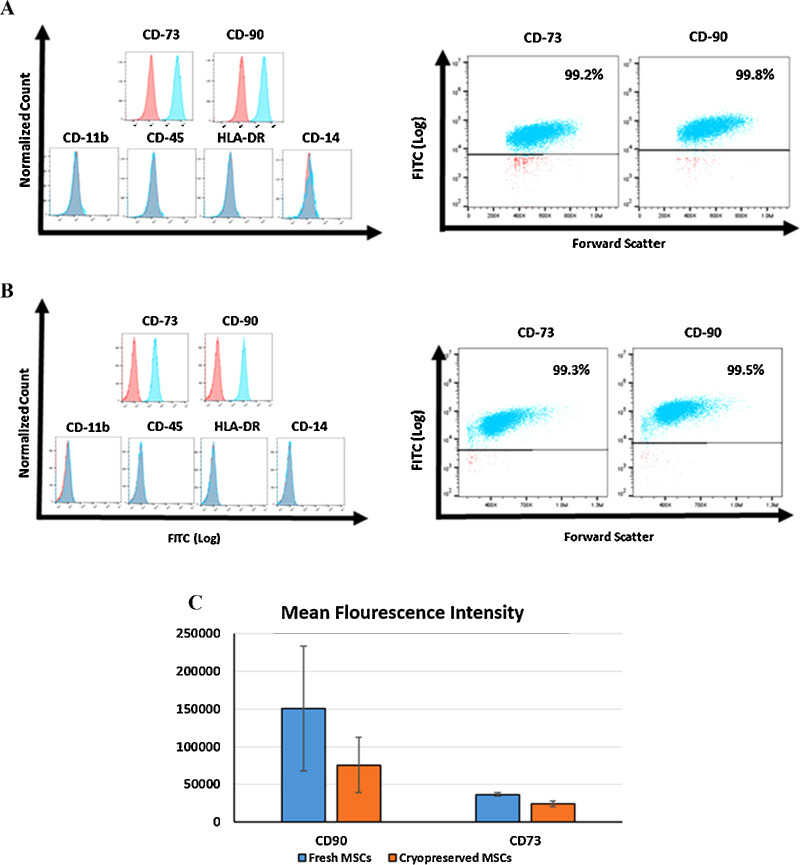
To confirm the identity of the human bone marrow MSC, the cells were subjected to flow cytometry for the detection of established MSC cell surface markers. In addition, we sought to determine if cryopreserved MSCs could be assessed by flow cytometry immediately after thawing. As shown in Figure 1, both cryopreserved (i.e., immediately after thawing) cells (A) and fresh (i.e., harvested from cell culture) cells (B) were more than 90% positive for CD73 and CD90. They were negative for HLA-DR, CD11b, CD14, and CD45. The mean fluorescence intensity for both cryopreserved and fresh MSCs are demonstrated in Figure 1C.
Figure 1.
Flow cytometry confirming the identity of human bone marrow–derived MSCs. (A) MSC vials were thawed and immediately stained and subjected to flow cytometry for positive and negative MSC surface markers. These were compared with the results from freshly harvested MSCs (B) (prior to cryopreservation). (C) Mean fluorescence intensity in both fresh (i.e., harvested from cell culture) cells and cryopreserved (i.e., immediately after thawing) cells.
Viability
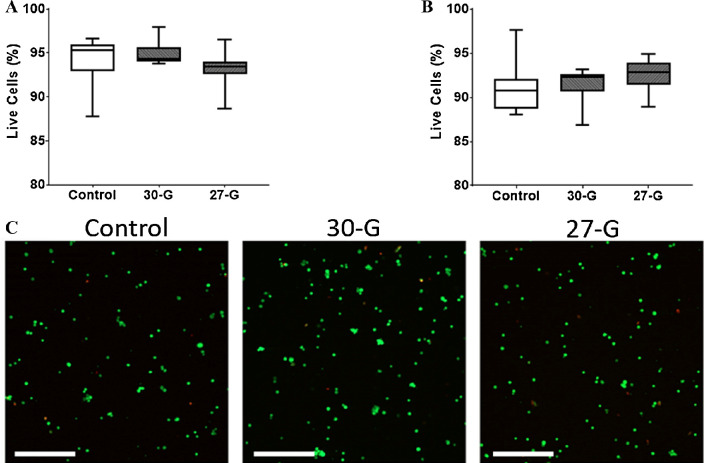
We evaluated the viability of human MSCs after thawing and injection through small-gauge needles used for subconjunctival injection. Thawed MSCs injected through 27- and 30-gauge needles demonstrated 93.1% ± 2.1% and 94.9% ± 1.3% viability by trypan blue staining, respectively, which were similar to control at 94.1% ± 2.7% (P = 0.51). Similar results were found by calcein/PI staining, which demonstrated no significant difference between the needle-injected cells and the control, with viability of 92.5% ± 1.9% for 27-gauge and 91.5% ± 1.9% for 30-gauge needle compared with 91.1% ± 2.7% (P = 0.65) for control (Fig. 2). The viability of rabbit MSCs was 92%.
Figure 2.
Viability of MSCs after injection through different sized needles. Cryopreserved MSCs were thawed then immediately injected through 27G and 30G needles. Viability was assessed using the standard trypan blue exclusion assay (A) and calcein/PI staining (B). Representative confocal microscopy images of the calcein/PI staining of MSCs (C). Green indicates viable cells, whereas red indicates dead cells. Boxes show the interquartile range (25%–75%), whiskers encompass the range (minimum–maximum), and horizontal lines represent the mean. Data shown are representative of three independent experiments. G: gauge. Scale bars: 500 µm.
To assess whether the concentration of MSCs affected their viability, we injected either 1 × 107 or 2 × 107 MSCs per mL through 27- and 30-gauge needles. There was no significant difference in viability between the two different cell concentrations with either needle size (Supplementary Fig. S1). Finally, the effect of temperature on the viability of MSCs after thawing was evaluated. There was no notable difference in their viability when MSCs were left in the storage medium at room temperature or on ice for up to 4 hours. After 4 hours, the MSC viability started to decline in both temperatures (Supplementary Fig. S1).
Safety of Subconjunctivally Injected MSCs
Given that human MSCs could induce a xenogenic immune response in rabbits, to better assess safety, both human and rabbit MSCs were studied separately. MSCs were subconjunctivally injected at three different doses (1, 3, and 6 × 106 cells) into rabbit eyes.
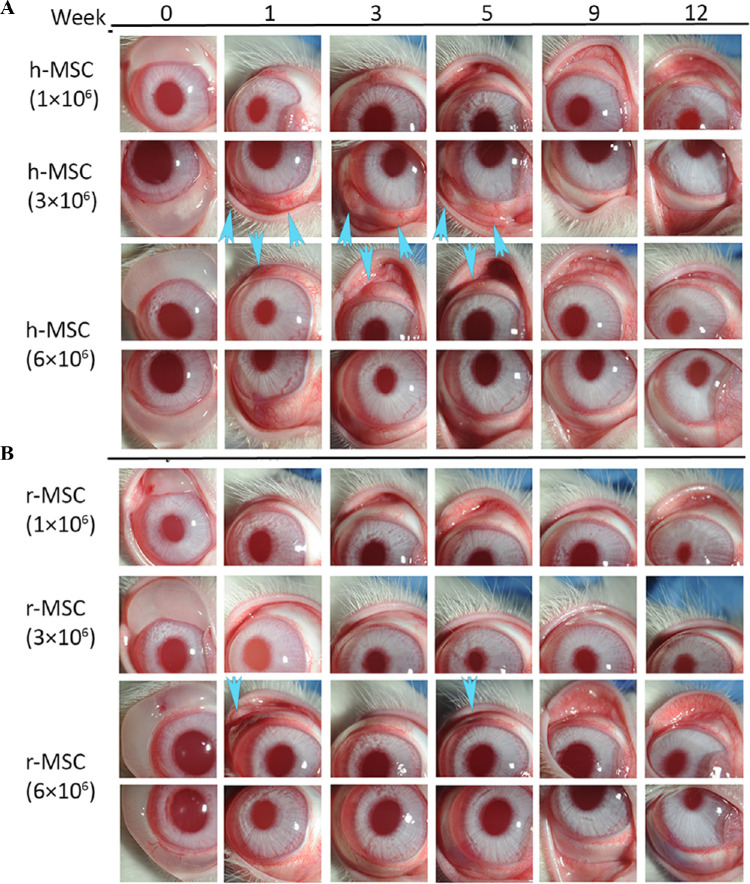
On slit lamp examination, rabbits injected with human and rabbit MSCs showed normal corneas without any opacity, scarring, neovascularization, or epithelial defects. Four out of 9 rabbits injected with human MSCs showed conjunctival injection at or within 2 clock hours of the original site of subconjunctival injection, which resolved after 6 weeks without any additional treatment (Fig. 3A). In rabbits injected with rabbit MSCs, only 1/9 rabbits injected with 6 × 106 rabbit MSCs (P = 0.2) showed conjunctival redness and swelling on the first week after injection (Fig. 3B), albeit more attenuated (compared with the higher dose of human MSCs) and with faster resolution. Some 66.6% (2/3) of rabbits injected with 1 × 106 human MSCs showed injection, compared with 66.6% (2/3 rabbits) in 3 × 106 group and 0% (0/3 rabbits) in 6 × 106 group. Histopathology at 12 weeks showed normal corneal structure, with mild conjunctival inflammation in human MSC injected eyes (ranging from 0 to 1/2 + on a scale of 0 to 4). The posterior segment and all other ocular structures were normal in both MSC-injected groups (Supplementary Fig. S2A). The individual results for each rabbit are summarized in Table 2. Histology images of the conjunctival area are provided in Supplementary Figures S2B–D to assess the inflammatory cells infiltration.
Figure 3.
Subconjunctival injection of human or rabbit bone marrow MSCs into uninjured rabbit eyes. Human (A) or rabbit (B) bone marrow–derived MSCs were subconjunctivally injected in rabbits at different doses and were followed up to 12 weeks to evaluate the safety. Some rabbit eyes injected with higher doses of human MSCs showed mild redness and swelling in the conjunctival injection area until week 6 (arrows), which resolved by week 12. This pattern was also observed on the first week when rabbit corneas were injected with higher concentration (6 × 106) of rabbit MSCs. In weekly follow-ups, there was no abnormal finding including corneal epithelial defects, corneal neovascularization and haze formation, conjunctival scarring, or any other ocular toxicity in any groups. h-MSC: human MSC; r-MSC: rabbit MSC.
Table 2.
Summary of Clinical Findings and Histopathology in Rabbit Eyes Subconjunctivally Injected with Varying Doses of Human and Rabbit MSCs
| Clinical Findings | Histopathology | |||||||
|---|---|---|---|---|---|---|---|---|
| Week 1 to 6 | Week 7 to 12 | |||||||
| Rabbit # | Cornea (Epithelial Defect, Scarring, Opacity/Neovascularization) | Conjunctiva (Scarring, Redness/Swelling) | Cornea (Epithelial Defect, Scarring, Opacity/Neovascularization) | Conjunctiva (Scarring, Redness/Swelling) | Cornea | Conjunctiva | Post. Segment and Other Structures | |
| 1 million rabbit BM-MSC | 1902 | Normal | Normal | Normal | Normal | Normal | + 1/2 Inflammation | Normal |
| 1903 | Normal | Normal | Normal | Normal | Normal | Normal | Normal | |
| 1905 | Normal | Normal | Normal | Normal | Normal | Normal | Normal | |
| 3 million rabbit BM-MSC | 1907 | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| 1908 | Normal | Normal | Normal | Normal | Normal | Normal | Normal | |
| 4441 | Normal | Normal | Normal | Normal | Normal | Normal | Normal | |
| 6 million rabbit BM-MSC | 4442 | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| 4443 | Normal | Mild redness | Normal | Normal | Normal | Normal | Normal | |
| 4444 | Normal | Mild swelling/redness | Normal | Normal | Normal | Normal | Normal | |
| 1 million human BM-MSC | 1904 | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| 1906 | Normal | Normal | Normal | Normal | Normal | +1 Inflammation | Normal | |
| 1909 | Normal | Mild redness | Normal | Normal | Normal | + 1/2 Inflammation | Normal | |
| 3 million human BM-MSC | 1910 | Normal | Mild swelling/redness | Normal | Normal | Normal | + 1/2 Inflammation | Normal |
| 1911 | Normal | Mild swelling/redness | Normal | Normal | Normal | Normal | Normal | |
| 4437 | Normal | Mild swelling/redness | Normal | Normal | Normal | +1/4 Inflammation | Normal | |
| 6 million human BM-MSC | 4436 | Normal | Mild swelling/redness | Normal | Normal | Normal | Normal | Normal |
| 4439 | Normal | Mild swelling/redness | Normal | Normal | Normal | Normal | Normal | |
| 4440 | Normal | Normal | Normal | Normal | Normal | Normal | Normal | |
BM, bone marrow.
Mice injected with cryopreserved human MSCs or cryopreservation solution were followed until day 14. Slit lamp examination and histopathology results did not show any signs of toxicity in the conjunctiva area, including no inflammation or scar formation at the site of injection, and no sign of corneal haziness, scarring, or neovascularization (Supplementary Fig. S3).
Cryopreserved MSCs Accelerate Wound Healing Both In Vitro and In Vivo
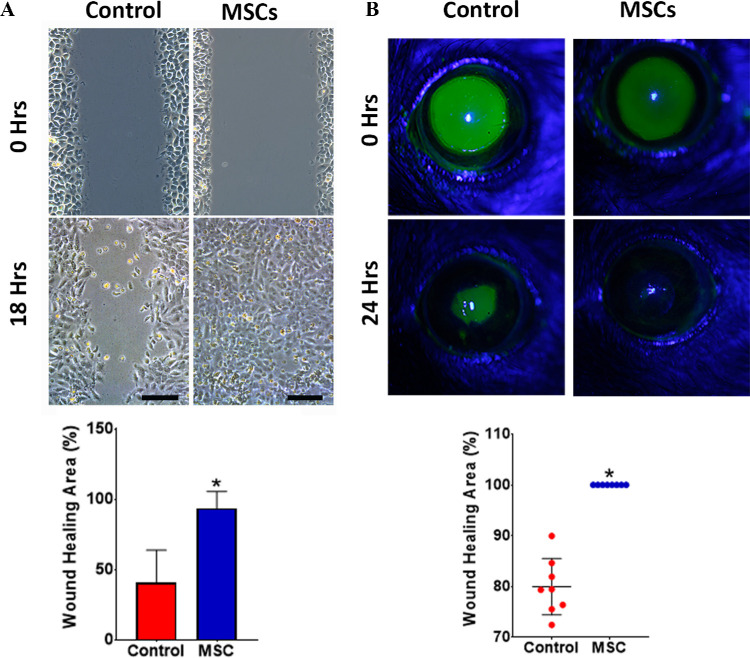
The potency of thawed human MSCs was assessed by evaluating their effect on corneal epithelial scratch wound closure. Epithelial cells co-cultured with human MSCs (via cell-culture insert) closed scratch wounds significantly faster with 93.5% ± 12.1% mean wound healing area at 18 hours compared with that of the control with 40.8% ± 23.1% (P < 0.001) (Fig. 4A).
Figure 4.
Efficacy of MSCs in promoting corneal epithelial wound healing in vitro and in vivo. (A) Scratch wounded human corneal epithelial cells were co-cultured with freshly thawed MSCs (i.e., cell culture insert) showing significantly greater wound closure at 18 hours compared with control. (B) The effect of MSCs in vivo after 2-mm central corneal epithelial debridement wounds in mice showing significantly greater wound healing in all the treated eyes compared with control. Error bars: standard deviation. *P < 0.001. Scale bars: 200 µm.
In vivo, the effect of human MSC subconjunctival injection was evaluated using an epithelial mechanical injury model in mice. At 24 hours, all the human MSC injected eyes demonstrated complete (100%) wound closure, whereas the vehicle control group had a mean wound healing area of 79.9% ± 5.5% (P < 0.001) (Fig. 4B).
Tracking of Labeled MSCs
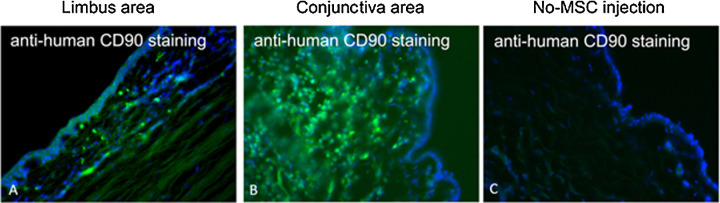
To track the MSCs in the conjunctiva and cornea after injection, labeled MSCs were cryopreserved, then later thawed and subconjunctivally injected into rat and rabbit eyes. Using in vivo fluorescence microscopy, red fluorescence was detectable at the site of injection up to 7 weeks in rat eyes (Fig. 5). Histologically, human MSCs were detectable in both the limbus and central cornea in the rabbit eyes at day 14 by CD90 staining (last time point examined) (Fig. 6).
Figure 5.
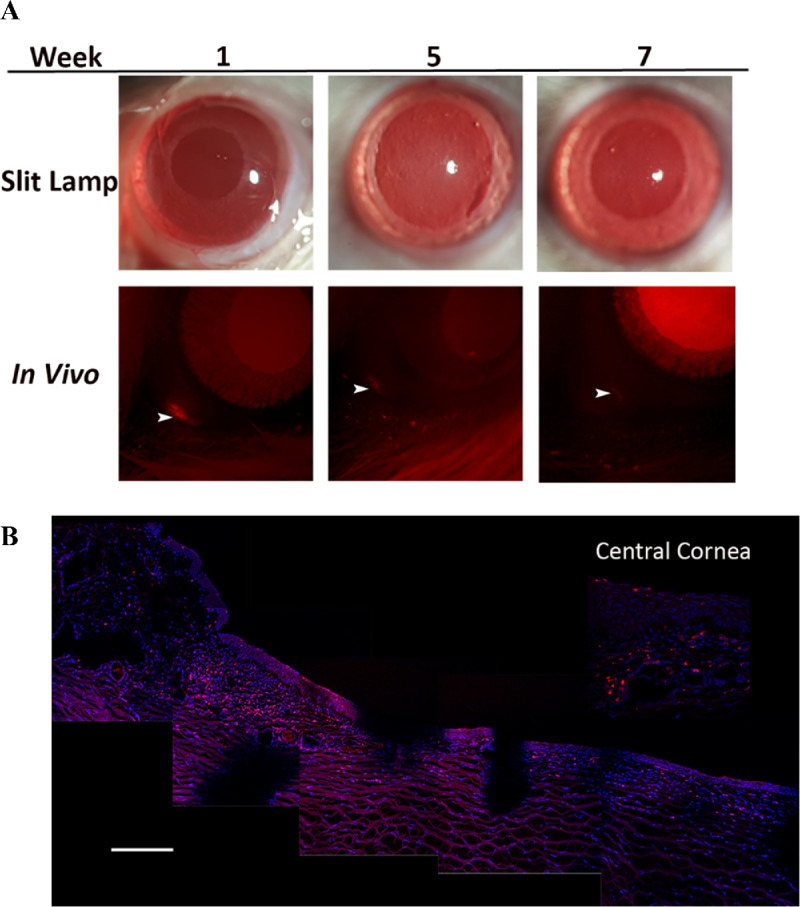
Subconjunctival injection of labeled MSCs into rat and rabbit eyes. (A) Labeled human MSCs were thawed then subconjunctivally injected into the rat eyes and followed serially by in vivo imaging up to 12 weeks. Red fluorescence at the site of injection progressively declined and could be visualized in the conjunctiva up to week 7 (arrowhead). (B) Histopathology of rabbit corneas at day 14 showing the presence of labeled cells (red) in the limbus and central cornea (gaps in the tissue are an artifact from cutting). Scale bar: 100 µm.
Figure 6.
Rabbit section, anti-human CD90 staining to identify the human MSCs after subconjunctival injection. (A) Limbal area of rabbit eye, which was injected with human MSCs. (B) Conjunctival area of rabbit eye injected with human MSCs. (C) Conjunctival area of rabbit eye without MSC injection (control).
Discussion
MSCs have been reported to be effective in promoting corneal wound repair in numerous experimental studies.15–17,26,32–35 MSCs have the ability to differentiate into different various types of mesenchymal lineages, proliferate, and secrete anti-inflammatory and growth factors promoting wound healing. The purpose of the current study was to evaluate the safety, viability, and dosing of subconjunctival injection of cryopreserved MSCs, and the efficacy of this mode of delivery in promoting corneal epithelial wound healing as a proof of concept in preparation for a phase I human clinical trial evaluating the efficacy of subconjunctival MSCs as a possible treatment of nonhealing corneal epithelial wounds. In particular, it is hoped that allogeneic MSCs will engraft temporarily on the ocular surface and secrete factors that enhance the function of the epithelium while modulating inflammation. This study has focused specifically on subconjunctival delivery, however, other modes of delivery or application on top of the cornea (e.g., embedded in fibrin gel) may also be considered.
We chose the rabbit eye mainly because of its similar size to human eye, which allowed us to assess dosing. The rabbit conjunctival histology is similar to human, and rabbit eyes have been used extensively to study subconjunctival/Tenon's fibrosis (for glaucoma filtration surgery).
The results from the viability studies showed that cryopreserved MSCs maintain greater than 90% viability after thawing and injection through 27- and 30-gauge small needles. This confirms the feasibility of cryopreserving MSCs and thawing just prior to injection. Of note, we purposely did not include a washing step prior to administration. The main reason is that a washing step would require additional procedures and equipment prior to injection and our preference was to have an MSC vial as a ready-to-inject product. As a result of not having a washing step, some of the dimethyl sulfoxide (DMSO) in the cryopreservation solution will also be present and hence could affect viability. However, this was not found to affect viability up to 3 to 4 hours at room temperature. Although the toxicity of DMSO has not been evaluated in vivo in our studies, it is unlikely that DMSO was the cause of the redness in the rabbits because the redness was mainly noted in eyes with human MSCs injection and otherwise the eyes with rabbit MSC injection at the highest dose had very little to no redness. This is further confirmed by the mouse studies, which did include a group with DMSO storage media alone (no cells) and there were no adverse effects observed.
This study also confirmed the preclinical safety and explored possible doses of MSCs that can be delivered by subconjunctival injection. A previous study found subconjunctival injection to be superior to topical and systemic administration for delivering cells to the cornea.36 The same study also reported better corneal wound healing in terms of reduced opacity, inflammation, and fibrosis from systemic and subconjunctival delivery methods compared with topical and intraperitoneal delivery methods.36 Subconjunctival injection has several distinct advantages, including higher MSC concentration at the site of corneal injury,34,37–39 better retention,23,34 ease of performing the procedure in an outpatient setting owing to its less invasive nature, and ease of repeatability should the need arise. When formulated at 20 million MSCs per mL, an injection of 3 million cells would require a volume of 150 µL, which is acceptable for subconjunctival injection while maintaining the subconjunctival bleb close to the limbus. For 6 million cells, we prefer 2 injections of 150 µL on opposite sides of the cornea instead of a single injection because larger volumes of injection would involve more ballooning of the posterior conjunctiva. The results from this study demonstrate the retention of subconjunctivally injected MSCs at the site of injection close to the limbus with migration of the MSCs toward the central cornea.
Our preclinical results confirm the safety of MSCs without any adverse effects in any of the tested animals up of 90 days (Fig. 3). There was mild conjunctival redness and swelling at the site of injection in rabbits that were injected with higher doses of human MSCs, possibly owing to cross species immunologic reactions. The xenogenic nature of the inflammation is supported by the fact that rabbit MSCs did not induce any inflammation beyond the first week (Fig. 3). Schmuck et al.40 has shown that even treatment of intravenous MSCs derived from the same species induced a local lymphoproliferative response with no evidence of systemic inflammatory signs.
Often, a combination of multiple methods comprising in vitro and in vivo assays is used to evaluate the potency of any cell therapy,41,42 and according to the International Conference on Harmonization (ICH) guideline, potency assays should be customized to the intended clinical use.43 Given that our intended therapeutic application of MSCs is for promoting corneal epithelial wound healing, we developed our potency assay to assess epithelial wound closure using an in vitro scratch assay. Likewise, we established its in vivo efficacy in a murine corneal epithelial wound healing model. These results support the clinical application of subconjunctivally delivered MSCs for promoting corneal regeneration.
We were limited by the volume that can be subconjunctivally injected and accordingly we were not able to evaluate the dose of subconjunctival MSCs injection that would cause ocular side effects such as scarring or inflammation.
Conclusions
This study provides a safety evaluation of freshly thawed cryopreserved MSCs along with dosing information for subconjunctival injection in a rabbit eye, which is comparable in size to the human eye. We also established an in vitro potency assay to assess the wound healing properties of MSCs. These results provide preclinical safety data to support a phase I safety study in humans.
Supplementary Material
Acknowledgments
The authors thank Ruth Zelkha for assistance with imaging, Tara Nguyen for assistance with animal experiments, and Lauren Kalinoski for assistance in creating a graphic illustrations.
Supported by R01 EY024349 (ARD) and Core grant EY01792 from NEI/NIH; MR130543 and VR170180 (ARD) from US Department of Defense, US ARMY, University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520 (PH), unrestricted grant to the department from RPB; and UW-Madison Don Anderson fund for GVHD research, and Crystal Carney Fund for Leukemia Research. The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Disclosure: I. Putra, None; X. Shen, None; K.N. Anwar, None; B. Rabiee, None; R. Samaeekia, None; E. Almazyad, None; P. Giri, None; S. Jabbehdari, None; M.R. Hayat, None; A.M. Elhusseiny, None; M. Ghassemi, None; N. Mahmud, None; D.P. Edward, None; C.E. Joslin, None; M.I. Rosenblatt, None; R. Dana, None; M. Eslani, None; P. Hematti, None; A.R. Djalilian, None
References
- 1.Ziaei M, Greene C, Green CR.. Wound healing in the eye: therapeutic prospects. Adv Drug Deliv Rev. 2018; 126: 162–176. [DOI] [PubMed] [Google Scholar]
- 2.Yazdanpanah G, Haq Z, Kang K, Jabbehdari S, Rosenblatt ML, Djalilian AR.. Strategies for reconstructing the limbal stem cell niche. Ocul Surf. 2019; 17(2): 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eslani M, Putra I, Shen X, et al.. Cornea-derived mesenchymal stromal cells therapeutically modulate macrophage immunophenotype and angiogenic function. Stem Cells. 2018; 36(5): 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinnamaneni N, Funderburgh JL.. Concise review: stem cells in the corneal stroma. Stem Cells. 2012; 30(6): 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galindo S, Herreras JM, López-Paniagua M, et al.. Therapeutic effect of human adipose tissue-derived mesenchymal stem cells in experimental corneal failure due to limbal stem cell niche damage. Stem Cells. 2017; 35(10): 2160–2174. [DOI] [PubMed] [Google Scholar]
- 6.Oh JY, Kim MK, Shin MS, et al.. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008; 26(4): 1047–1055. [DOI] [PubMed] [Google Scholar]
- 7.Amirjamshidi H, Milani B, Sagha H, et al.. Limbal fibroblast conditioned media: a non-invasive treatment for limbal stem cell deficiency. Mol Vis. 2011; 17: 658. [PMC free article] [PubMed] [Google Scholar]
- 8.Eslani M, Putra I, Shen X, et al.. Corneal mesenchymal stromal cells are directly antiangiogenic via PEDF and sFLT-1. Invest Ophthalmol Vis Sci. 2017; 58(12): 5507–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spees JL, Lee RH, Gregory CA.. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016; 7(1): 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson SE, Bentz ML, Hematti P.. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg. 2010; 125(2): 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battiwalla M, Hematti P.. Mesenchymal Stem Cells in Hematopoietic Stem Cell Transplantation. Philadelphia, PA: Taylor & Francis; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phinney DG, Pittenger MF.. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017; 35(4): 851–858. [DOI] [PubMed] [Google Scholar]
- 13.Ward MR, Abadeh A, Connelly KA.. Concise review: rational use of mesenchymal stem cells in the treatment of ischemic heart disease. Stem Cells Transl Med. 2018; 7(7): 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahu A, Foulsham W, Amouzegar A, Mittal SK, Chauhan SK.. The therapeutic application of mesenchymal stem cells at the ocular surface. Ocul Surf. 2019; 17(2): 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes-Cunha GM, Na KS, Putra I, et al.. Corneal wound healing effects of mesenchymal stem cell secretome delivered within a viscoelastic gel carrier. Stem Cells Transl Med. 2019; 8(5): 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saghizadeh M, Kramerov AA, Svendsen CN, Ljubimov AV.. Concise review: stem cells for corneal wound healing. Stem Cells. 2017; 35(10): 2105–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samaeekia R, Rabiee B, Putra I, et al.. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2018; 59(12): 5194–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM.. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018; 9: 2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zatezalo CC, Tavakoli M, Ayala-Haedo J, et al.. A prospective randomized comparative clinical trial to analyze pain and surgical outcomes between frontal nerve blocks and subconjunctival anesthesia for conjunctival mullerectomy resection. Ophthalmic Plast Reconstr Surg. 2018; 34(6): 575–578. [DOI] [PubMed] [Google Scholar]
- 20.Davis AB, Schnabel LV, Gilger BC.. Subconjunctival bone marrow-derived mesenchymal stem cell therapy as a novel treatment alternative for equine immune-mediated keratitis: a case series. Vet Ophthalmol. 2019; 22(5): 674–682. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Carrasco R, Sánchez-Abarca LI, Nieto-Gómez C, et al.. Subconjunctival injection of mesenchymal stromal cells protects the cornea in an experimental model of GVHD. Ocul Surf. 2019; 17(2): 285–294. [DOI] [PubMed] [Google Scholar]
- 22.Jia Z, Li F, Zeng X, Lv Y, Zhao S.. The effects of local administration of mesenchymal stem cells on rat corneal allograft rejection. BMC Ophthalmol. 2018; 18(1): 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghazaryan E, Zhang Y, He Y, et al.. Mesenchymal stem cells in corneal neovascularization: comparison of different application routes. Mol Med Rep. 2016; 14(4): 3104–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almaliotis D, Koliakos G, Papakonstantinou E, et al.. Mesenchymal stem cells improve healing of the cornea after alkali injury. Graefes Arch Clin Exp Ophthalmol. 2015; 253(7): 1121–1135. [DOI] [PubMed] [Google Scholar]
- 25.Bandeira F, Goh T-W, Setiawan M, Yam GH-F, Mehta JS.. Cellular therapy of corneal epithelial defect by adipose mesenchymal stem cell-derived epithelial progenitors. Stem Cell Res Ther. 2020; 11(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calonge M, Perez I, Galindo S, et al.. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl Res. 2019; 206: 18–40. [DOI] [PubMed] [Google Scholar]
- 27.Møller-Hansen M, Larsen AC, Toft PB, et al.. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul Surf. 2020; 19: 43–52. [DOI] [PubMed] [Google Scholar]
- 28.Bahsoun S, Coopman K, Akam EC.. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: a systematic review. J Transl Med. 2019; 17(1): 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baradaran-Rafii A, Eslani M, Haq Z, Shirzadeh E, Huvard MJ, Djalilian AR.. Current and upcoming therapies for ocular surface chemical injuries. Ocul Surf. 2017; 15(1): 48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gidfar S, Milani FY, Milani BY, et al.. Rapamycin prolongs the survival of corneal epithelial cells in culture. Sci Rep. 2017; 7: 40308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichel MB, Cordeiro MF, Alexander RA, Cree IA, Bhattacharya SS, Khaw PT.. New model of conjunctival scarring in the mouse eye. Br J Ophthalmol. 1998; 82(9): 1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di G, Du X, Qi X, et al.. Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG-6-dependent stem cell activation and macrophage switch. Invest Ophthalmol Vis Sci. 2017; 58(10): 4344–4354. [DOI] [PubMed] [Google Scholar]
- 33.Ye J, Lee SY, Kook KH, Yao K.. Bone marrow-derived progenitor cells promote corneal wound healing following alkali injury. Graefes Arch Clin Exper Ophthalmol. 2008; 246(2): 217–222. [DOI] [PubMed] [Google Scholar]
- 34.Yao L, Li Z-r, Su W-r, et al.. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012; 7(2): e30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phinney DG, Prockop DJ.. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007; 25(11): 2896–2902. [DOI] [PubMed] [Google Scholar]
- 36.Shukla S, Mittal SK, Foulsham W, et al.. Therapeutic efficacy of different routes of mesenchymal stem cell administration in corneal injury. Ocul Surf. 2019; 17(4): 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang T-S, Cai L, Ji W-Y, et al.. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis. 2010; 16: 1304. [PMC free article] [PubMed] [Google Scholar]
- 38.Reinshagen H, Auw-Haedrich C, Sorg RV, et al.. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011; 89(8): 741–748. [DOI] [PubMed] [Google Scholar]
- 39.Coulson-Thomas VJ, Caterson B, Kao WWY.. Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells. 2013; 31(10): 2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmuck EG, Koch JM, Hacker TA, et al.. Intravenous followed by x-ray fused with MRI-guided transendocardial mesenchymal stem cell injection improves contractility reserve in a swine model of myocardial infarction. J Cardiovasc Transl Res. 2015; 8(7): 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Food and Drug Administration. Guidance for industry: potency tests for cellular and gene therapy products. 2011. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/potency-tests-cellular-and-gene-therapy-products. Accessed Janurary, 2021.
- 42.Chinnadurai R, Rajakumar A, Schneider AJ, Bushman WA, Hematti P, Galipeau J.. Potency analysis of mesenchymal stromal cells using a phospho-STAT matrix loop analytical approach. Stem Cells. 2019; 37: 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.International Conference on Harmonisation; guidance on specifications: test procedures and acceptance criteria for biotechnological/biological products. Notice. Food and Drug Administration, HHS. Fed Regist. 1999; 64(159): 44928–449235. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.