Abstract
A 61-year-old man was admitted to the medical intensive care unit following a 2-week history of weakness, lightheadedness and melena resulting in an acute anaemia. Upper endoscopy revealed multiple large gastric masses without evidence of active bleeding. CT of the chest revealed a large right upper lobe mass with bony destruction of the third rib and invasion into the anterior chest wall and mediastinum, as well as a soft-tissue density in the left kidney. Biopsy and histopathological review of both pulmonary and gastric masses revealed two distinct sarcomatous malignancies that, while both from a primary lung source, differed in their morphology. Natural history and behaviour are not well understood in sarcomas due to their rarity, but abdominal metastasis is considered an uncommon event in the progression of the disease. Gastrointestinal bleeding as the presenting symptom of a primary lung sarcoma is an atypical finding with no previously reported cases.
Keywords: GI bleeding, gastroenterology, stomach and duodenum, oncology, lung cancer (oncology)
Background
Lung cancers generally metastasise to the brain, liver, adrenal glands or contralateral lung, with the gastrointestinal tract being an uncommon site.1 Primary lung sarcoma (PLS) is a rare malignancy, accounting for less than 0.5% of all lung tumours.2 Herein, we report a case of PLS with gastric metastasis with divergent morphological characteristics. This unusual clinical progression and presentation of an already rare disease entity has not been previously reported.
Case presentation
The patient was a 61-year-old African American male who developed weakness, lightheadedness and melaena over a 2-week period. He denied vomiting, haematochezia, and abdominal pain as well as cough, haemoptysis and chest pain. His only significant medical history was chronic back pain, for which he took ibuprofen daily, and tobacco use disorder with 40-pack year history. He had never established with a primary care physician prior to hospitalisation and no other diagnosed medical conditions. Admission vital signs were significant for a blood pressure of 98/62, heart rate of 124 beats/min and respiratory rate of 22 breaths/min. Physical examination revealed cachexia with temporal wasting, pallor and global weakness. Digital rectal examination was negative for gross blood or melaena. No jaundice scleral icterus, or abdominal tenderness were appreciated.
Initial laboratory tests revealed a haemoglobin of 3.4 g/dL (13.7–17.5), an mean corpuscular volume (MCV) of 94.6 fL (79–92.0), a ferritin of 146 ng/mL (7–350), transferrin saturation of 6%, a blood urea nitrogen level of 16 mg/dL (6–20), and creatinine of 0.98 mg/dL (0.64–1.27). There were no prior encounters to compare these values to patient’s previous baseline.
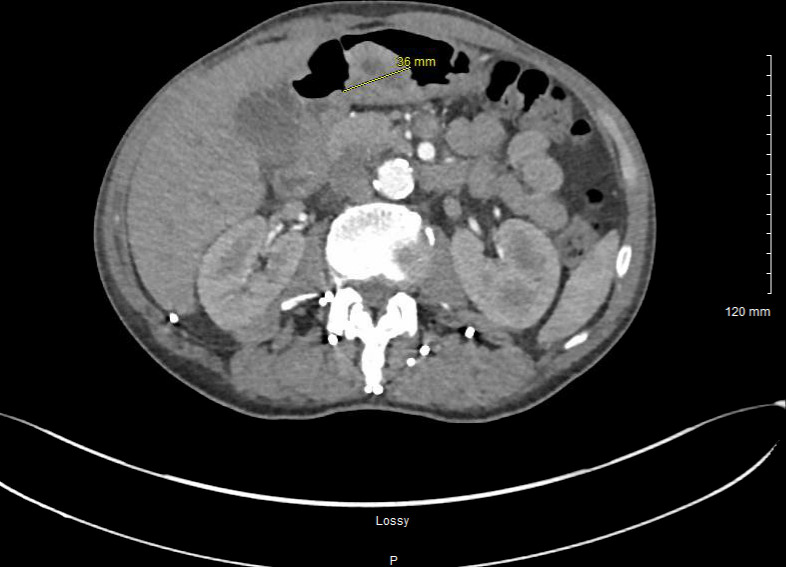
Chest radiography and contrasted CT both demonstrated a well-circumscribed mass in the right upper chest that measured 9.1×7.6 × 9.6 cm with invasion of anterior chest wall and mediastinum, concerning for a primary malignancy (figure 1). Bony destruction and scalloping of the third right rib was noted. Pleural-based masses in the posterior medial right upper lobe with mediastinal invasion were also found. A mass in the gastric antrum and diffuse nodularity in the fundus of the stomach was also noted on CT of the chest, concerning for primary malignancy of the stomach (figure 2).
Figure 1.
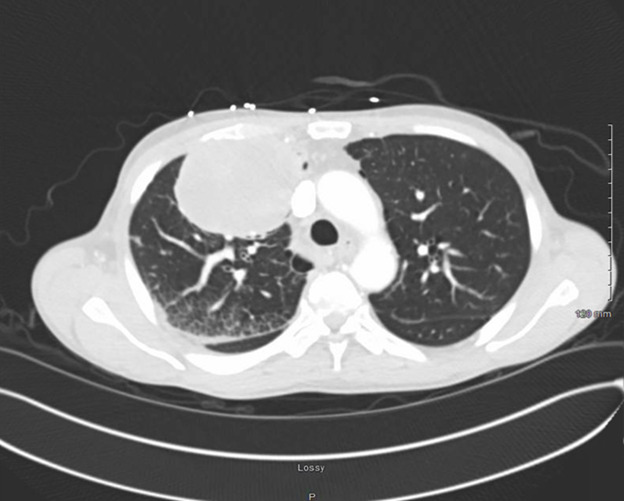
Contrast-enhanced CT scan of chest depicting a mass measuring 9.1×7.6 × 9.6 cm in the right upper lobe.
Figure 2.
Contrast-enhanced CT scan of the upper abdomen depicting a mass in the gastric antrum and nodularity of the fundus of the stomach.
Oesophagogastroduodenoscopy (EGD) was performed. Normal oesophageal mucosa was appreciated throughout entire oesophagus. In the stomach, a protruding, non-bleeding 2 cm mass of malignant gross appearance was found in the antrum and was partially obstructing the prepyloric area. A non-bleeding 3 cm mass of malignant appearance was found in the body (figure 3). Both masses had friable tissue and cold forceps biopsies were obtained from both. Duodenal tissue was normal in the entirety of the examined duodenum. Interventional radiology performed an ultrasound-guided core biopsy of the lung mass and no bronchoscopy was performed.
Figure 3.
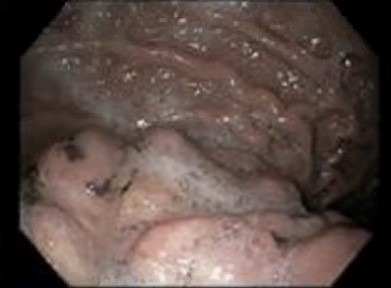
Endoscopic image from initial presentation. A non-bleeding 3 cm mass of malignant appearance in the gastric body.
Histopathological characterisation of the gastric mass demonstrated a proliferation of spindle cells with extensive mitoses as well as tumour necrosis while the chest mass was significant for an epithelioid differentiation pattern and pleomorphic nuclei. By immunohistochemistry, the gastric masses demonstrated focal and weak positivity for cytokeratin AE1/3 and S100, patchy positivity for CAM 5.2 and negativity for CD 3, 4 and 117, cytokeratin 5/6, desmin, DOG 1, EMA, HMB45, MART1 and SOX10. The chest mass was positive for PAX8 and vimentin, with weak and focal positivity for CA9 and cytokeratin AE1/3 and negative for B72.3, BerEp, calretinin, CEA, CD5/6, MOC31, napsin A, RCC, TTF-1 and WT11. Additional specialised stains were remarkable for cytokeratin osteoclast-associated receptor (OSCAR) positivity and calretinin, Erythroblast transformation-specific Related Gene (ERG), Periodic acid-Schiff (PAS), Paired Box 8 (PAX8) and Wilms Tumor 1 (WT-1) negativity in both tissues. The chest mass was strongly positive for C2-40, compared with only focal positivity in the gastric mass (a complete summary of the immunohistochemical findings is featured in tables 1–3). It was determined that each mass was morphologically unique but with a similar immunohistochemical profile that favoured a sarcomatoid carcinoma of lung primary with extension to the antrum and fundus of the stomach. The morphological discordance observed was attributed to divergent local differentiation.
Table 1.
Summary of the comparative immunohistological profile of masses from the chest and stomach
| Immunohistological study | Chest | Stomach |
| CK-OSCAR | + | + |
| D2-40 | + | + |
| Cytokeratin AE 1/3 | Weakly + | + |
| WT-1 | – | – |
| Calretinin | – | – |
| PAS | – | – |
| ER6 | – | – |
| CAM 5.2 | Patchy+staining | |
| S100 | Weakly + |
Table 2.
Immunohistological profile of stomach masses
| Positive | Negative |
| CAM 5.2 | EMA |
| Cytokeratin AE 1/3 | Cytokeratin 5/6 |
| S100 | DOG-1 |
| CD 117 | |
| CD3 | |
| 4 Desmin | |
| SMA | |
| MART 1 | |
| HMB 45 | |
| SOX 10 |
Table 3.
Immunohistological profile of chest masses
| Positive | Negative |
| Vimentin | RCC |
| Cytokeratin AE 1/3 | WT11 |
| CA 9 | TTF-1 |
| Napsin A | |
| CD 5/6 | |
| Calretinin | |
| CEA | |
| P63 | |
| B72-3 | |
| BerEp | |
| MOC31 |
Outcome and follow-up
One month after initial presentation, the patient returned to the hospital with a similar presentation. EGD at that time demonstrated increased size of previously known masses as well as new masses in the duodenum. At 6 weeks after the initial presentation, the patient decided to pursue comfort-directed care. Outpatient follow-up with haematology and oncology and a PET scan was deferred at this time, he declined palliative chemotherapy and radiation and was enrolled in hospice services. The patient ultimately passed away while enrolled in outpatient hospice services.
Discussion
Sarcomas are of mesenchymal origin and have a broad histopathological spectrum, with over 100 different classified histological subtypes, the most common of which are liposarcoma, leiomyosarcoma, undifferentiated sarcomas and gastrointestinal stromal tumours.3 4 Sarcomas are thought to arise from de novo mutations and not from pre-existing benign lesions. While most cases do not have a well-defined aetiology, there have been a number of identified predisposing factors such as genetic conditions, specific gene mutations/chromosomal translocations, radiotherapy, chemotherapy, chronic irritation and certain industrial chemicals.5–7
PLS are a rare malignancy and there are limited data on the natural history and propensity for metastasis. Within this tumour subtype, pulmonary leiomyosarcoma is also rare, representing 9% of all lung sarcomas.8 Originating from the neoplastic transformation of the peribronchial smooth muscle fibres, pulmonary leiomyosarcoma most frequently involve the larger bronchi of the left lower lobe.9 Most PLS occur during middle age, with a predominance towards males, with a reported median survival of approximately 24 months in those that underwent radical operations and less than 18 months in those without radical operations.10 The mechanism of metastasis in PLS is not completely understood. To date, there have not been any recorded infections, bacterial or viral, that predispose a patient to develop this type of malignancy. Patients who present with this subset of sarcoma show a chromosomal translocation of t(x;18) (p11;q11). This mutation causes the transcription of the genes SS18-SSX1, -SSX2, or SSX4, fusion genes that encode chimeric transcription factors.11 Additionally, in a different subset of pulmonary sarcomas called alveolar soft part sarcomas, the alveolar soft part sarcoma critical region 1 gene (ASPSCR1) fuses with the transcription factor E3 gene, which results in the production of abnormal proteins. Treatment of choice in PLS is surgical resection although radiotherapy can be used in cases of incomplete resection. Adjuvant chemotherapy can also play a therapeutic role based on tumour size and differentiation.12
Metastasis of pulmonary malignancies to the stomach or duodenum is rare but has been described across different cell types with differing clinical manifestations.13–16 Yang et al, for instance, found that only 2% of primary lung malignancies presented with symptomatic gastric metastasis.17 Such symptoms/clinical features include abdominal pain, bleeding, obstruction and perforation.18 In one recent case series of gastrointestinal complications in the setting of lung cancer, a total of 64 cases were reviewed and identified. Of these, 22 patients had perforation, 19 had obstruction, 10 had haemorrhage and 8 had intussusception. Metastatic sites were also documented in 57 of the 64 total cases, the most common of which was the jejunum with 29, followed by the ileum with 19, and then duodenum with 15.19 Multiple mechanisms as to metastases have been suggested and include hematogenous extension through spinal veins and lymphatic spread from the mediastinum through the retroperitoneum and mesentery.20
There has only been one previous case report of a PLS with the initial presentation as a gastrointestinal haemorrhage.21 In this case, a primary lung leiomyosarcoma was discovered as an incidental finding during an admission for abdominal pain and cholelithiasis. The lung malignancy was excised and, after 2 years, the patient again presented with gastrointestinal bleeding and was subsequently found to have metastasis to the small bowel. Surgical pathology of the abdominal metastasis was consistent with the patient’s previously identified primary lung malignancy.
The presented patient’s gastric lesion having divergent morphology makes this case unique in the available literature. There is no known mechanism that can easily explain this phenomenon. One theory would be that the acidic microenvironment in the stomach, which is known to include metaplasia of oesophageal squamous to gastric goblet cells in Barrett’s oesophagus, exerted a similar effect on the newly metastasised lung lesions. This process would need to be replicated in vitro but certainly is worth exploring should this become a pattern in reported patients.
Learning points.
This is an unusual presentation of a rare malignancy. Lung cancers rarely metastasise to the stomach and when they do, it is even more rare for patients to present with gastrointestinal symptoms. There is only one prior reported case of a primary lung sarcoma presenting with a gastrointestinal manifestation of disease.
When presented with an already rare diagnosis of primary lung malignancy that has extended into the gastrointestinal system, physicians should first confirm the tumour subtype through biopsy and immunohistochemical staining. If both primary and distant disease appear to be of sarcomatous origin, therapy should centre on treating metastatic sarcoma.
Management of sarcomatous lung cancer that metastasises to the gastrointestinal tract is challenging. It requires the coordination of multiple subspecialists and a vast range of expertise to provide quality care. Endoscopists should be involved and intervene emergently if there is concern for an acute gastrointestinal haemorrhage before obtaining biopsies and planning potential systemic or radiotherapy.
Acknowledgments
University of Louisville Internal Medicine Residency Program.
Footnotes
Contributors: As the primary author on this manuscript, I MJE, prepared the majority of the text, performed rough drafts and revisions. I collected all pertinent imaging, created the figures, obtained patient consent, and formatted the citations. As contributing authors, AP, SBR, and JG all read and reviewed the drafts, offered feedbacks and made edits on their own accord. JG was the attending on this case and we took care of the patient during his hospital course. JG also provided me feedback and direction on the direction of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Hillers TK, Sauve MD, Guyatt GH. Analysis of published studies on the detection of extrathoracic metastases in patients presumed to have operable non-small cell lung cancer. Thorax 1994;49:14–19. 10.1136/thx.49.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belbaraka R, Ismaili N. Primary sarcoma of the lung: a very rare diagnosis and poor prognosis. Clin Cancer Investig J 2014;3:176. 10.4103/2278-0513.130221 [DOI] [Google Scholar]
- 3.Bridge JA, Hogendoorn P, International Agency for Research on Cancer, World Health Organization(WHO), International Academy of Pathology . WHO classification of tumours of soft tissue and bone. 4th ed. Fletcher CDM, editor. IARC, 2013. [Google Scholar]
- 4.Zagars GK, Ballo MT, Pisters PWT, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43. 10.1002/cncr.11365 [DOI] [PubMed] [Google Scholar]
- 5.Farid M, Ngeow J. Sarcomas associated with genetic cancer predisposition syndromes: a review. Oncologist 2016;21:1002–13. 10.1634/theoncologist.2016-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radons J. Inflammatory stress and sarcomagenesis: a vicious interplay. Cell Stress Chaperones 2014;19:1–13. 10.1007/s12192-013-0449-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zambon P, Ricci P, Bovo E, et al. Sarcoma risk and dioxin emissions from incinerators and industrial plants: a population-based case-control study (Italy). Environ Health 2007;6:19. 10.1186/1476-069X-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollock RE, Karnell LH, Menck HR, et al. The National cancer data base report on soft tissue sarcoma. Cancer 1996;78:2247–57. [DOI] [PubMed] [Google Scholar]
- 9.Arnold LM, Burman SD, O-Yurvati AH. Diagnosis and management of primary pulmonary leiomyosarcoma. J Am Osteopath Assoc 2010;110:244–6. [PubMed] [Google Scholar]
- 10.Janssen JP, Mulder JJ, Wagenaar SS, et al. Primary sarcoma of the lung: a clinical study with long-term follow-up. Ann Thorac Surg 1994;58:1151–5. 10.1016/0003-4975(94)90476-6 [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, Abbas AK, Fausto N. Robbins and cotran pathologic basis of disease, professional edition E-book [Internet]. 9th ed. Saunders, 2014. Available: https://play.google.com/store/books/details?id=jJllBAAAQBAJ
- 12.Movsas B. Lung cancers. In: Pazdur R, Wagman LD, Camphausen KA, eds. Cancer management: a multidisciplinary approach. 5th ed. Huntingdon, NY: PRR, Inc, 2001: 487–506. [Google Scholar]
- 13.Yoshimoto A, Kasahara K, Kawashima A. Gastrointestinal metastases from primary lung cancer. Eur J Cancer 2006;42:3157–60. 10.1016/j.ejca.2006.08.030 [DOI] [PubMed] [Google Scholar]
- 14.Casella G, Di Bella C, Cambareri AR, et al. Gastric metastasis by lung small cell carcinoma. World J Gastroenterol 2006;12:4096–7. 10.3748/wjg.v12.i25.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzaki N, Hiraki A, Ueoka H, et al. Gastric perforation due to metastasis from adenocarcinoma of the lung. Anticancer Res 2002;22:1209–12. [PubMed] [Google Scholar]
- 16.Fletcher MS. Gastric perforation secondary to metastatic carcinoma of the lung: a case report. Cancer 1980;46:1879–82. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Feit N, Huang Y. Gastrointestinal metastasis of primary lung cancer: an analysis of 366 cases. Oncol Lett 2018;15:9766–76. 10.3892/ol.2018.8575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P-C, Lo C, Lin M-T, et al. Role of surgical intervention in managing gastrointestinal metastases from lung cancer. World J Gastroenterol 2011;17:4314–20. 10.3748/wjg.v17.i38.4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Zhou W, Qi W-L, et al. Gastrointestinal hemorrhage due to ileal metastasis from primary lung cancer. World J Gastroenterol 2015;21:3435–40. 10.3748/wjg.v21.i11.3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada H, Akahane T, Horiuchi A, et al. A case of lung squamous cell carcinoma with metastases to the duodenum and small intestine. Int Surg 2011;96:176–81. 10.9738/1380.1 [DOI] [PubMed] [Google Scholar]
- 21.Tamburini N, Quarantotto F, Maniscalco P, et al. Gastrointestinal bleeding in lung leiomyosarcoma history: report of a case. J Thorac Dis 2014;6:E163–5. 10.3978/j.issn.2072-1439.2014.07.21 [DOI] [PMC free article] [PubMed] [Google Scholar]