Abstract
Objective:
The objective of the study was to evaluate the validity of the Beck Depression Inventory-II (BDI-II) when used to measure depression in patients with hepatitis C virus (HCV).
Method:
Factor analysis was utilized to validate the BDI-II in a sample of 671 patients with HCV recruited from a large Veterans Affairs medical center. The data were split randomly: the first half was subjected to exploratory factor analysis, and confirmatory factor analysis was used with the second half to confirm the model. Diagnostic data were retrieved from the electronic medical records.
Results:
Subjects were 97.0% male, average age was 52.8 years, 16.1% had a cirrhosis diagnosis, 62.9% had a current major depressive disorder diagnosis, and 42.3% endorsed significant depressive symptoms on the BDI-II. A two-factor model was an excellent fit for the data; the factors were labeled Cognitive–Affective and Somatic. Patients scored significantly higher on the Somatic factor than on the Cognitive–Affective factor (P<.001), and this discrepancy increased when comparing patients based on whether they had a diagnosis of cirrhosis.
Conclusions:
When screening for depression in HCV patients, questions targeting cognitive and affective symptoms of depression may provide a more valid measurement of depression than questions targeting somatic symptoms of depression, particularly for patients with more advanced liver disease.
Keywords: Hepatitis C virus, Depression, Factor analysis, Liver disease, Beck Depression Inventory
1. Introduction
Chronic hepatitis C virus (HCV) infection is estimated to affect 1.3% of the US population, approximately 3.2 million individuals [1]. There is a higher prevalence of HCV among specific segments of the population, including men, African–Americans, lower income and lower education groups, psychiatric patients and injection drug users [1,2]. Among veterans seeking care at Veterans Affairs (VA) medical centers, the prevalence rate is estimated to be 5.4% [3]. HCV infection is a major cause of cirrhosis, hepatocellular carcinoma and the primary indication for liver transplantation [4,5].
Approximately 20% to 40% of patients with HCV experience clinically significant symptoms of depression [6–11]. Depressive symptoms are important contributors to fatigue, functional disability and decreased health-related quality of life in patients with HCV [7,12,13]. Depressive symptoms prior to initiating antiviral treatment for HCV are associated with greater likelihood of developing a major depressive disorder (MDD) during treatment [6,14,15], which may inhibit treatment effectiveness [16,17]. Moderate to severe depressive symptoms are also a common reason for postponing or excluding patients from antiviral therapy [18], although if symptoms are detected early and treated appropriately, success rates for antiviral therapy are comparable with those for individuals without depression [19]. Therefore, accurate screening and timely intervention for depressive symptoms are important for patients with HCV.
It has been suggested that depression screening instruments commonly used in psychiatric settings tend to overestimate depressive symptoms when applied in medical populations because the somatic symptoms of depression are also common symptoms of physical illness [20,21]. A commonly used screening instrument in medical settings is the Beck Depression Inventory-Second Edition (BDI-II); however, several symptoms measured by the BDI-II are also common symptoms of chronic illness, including fatigue, sleep difficulty and appetite changes. This appears to contribute to a high rate of false positives for depression when the BDI-II is used with patients with chronic illness [20–23]. Since significant depression symptoms can sometimes prohibit patients from important medical treatments, some investigators have suggested that patients with chronic illness should only be assessed with instruments that exclude somatic symptoms [21].
Factor analysis has often been used to clarify the validity of the BDI-II when used with specific subpopulations. In psychiatric samples, the majority of studies have found either a two- or three-factor model consisting of cognitive, somatic and affective symptoms [24]. Among studies that found a two-factor model (most common), it has been suggested that the affective items tend to shift between the cognitive and somatic factors depending on the population under study [25,26]. Studies in medical populations have also found both two- and three-factor models; however, the somatic symptoms consistently form a separate, distinct factor (e.g., in populations with obesity [27], Parkinson’s disease [28], cardiac disease [29], human immunodeficiency virus [30] and chronic pain [31,32]). It has been difficult to determine if the somatic factor is measuring a different construct (i.e., physical illness) or if individuals with chronic illness tend to express depression symptoms primarily through somatic complaints [20].
The only factor analysis study to date of HCV patients and the BDI-II was conducted with a population of 193 injection drug users [33]. This study used principal components analysis to isolate a three-factor solution labeled Negative Affect, Somatic/Negative Affect and Irritability. However, it is difficult to generalize these findings to HCV patients seeking treatment due to the potentially confounding characteristics of the population of active injection drug users.
The purpose of this study was to conduct an exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) on the BDI-II scores of patients with HCV. The process of factor analysis separates out random variation from common variation for each observed item. The researcher can then identify the true common variance among sets of items and isolate them into factors. Exploratory factor analysis allows the researcher to determine how many factors may exist within a set of items. Confirmatory factor analysis allows the researcher to specify the number and composition of the factors and ascertain whether the model fits the observed data [34].
This is the first factor analytic study of a depression screening instrument in a large sample of treatment-seeking HCV patients. Untreated depression has been shown to impede treatment for HCV and is a common reason for postponing or excluding patients from antiviral therapy. Since depressive symptoms may be overestimated through use of screening measures such as the BDI-II, the results of this study can provide information that is useful for improving the validity of this specific measure, thus reducing the number of false positives for depression and inappropriate treatment exclusion.
2. Methods
2.1. Participants
Participants were recruited between May 2002 and November 2006 from an optional, single-session education class conducted through the Northwest Hepatitis C Resource Center at the Portland VA Medical Center. A total of 927 HCV-positive veterans provided consent for use of their data. Of these, 803 patients completed the full study questionnaires. Due to the confounding nature of the psychiatric side effects of Interferon/Ribavirin (IFN/RBV) therapy, 126 subjects who were receiving IFN/RBV at the time they filled out their BDI-II were excluded. An additional six subjects were excluded due to missing data on the BDI-II, leaving 671 participants with complete data for analysis. This study was approved by the Institutional Review Board at the Portland VA Medical Center, and all participants provided written informed consent.
Participants provided information on demographic characteristics, including age, gender, marital status and race. Individuals were considered HCV positive if they had evidence in their medical record of a detectable HCV viral load based on polymerase chain reaction tests. Electronic medical records were also reviewed for each participant to obtain key medical information including data on diagnoses of liver cirrhosis, HCV genotype (when available) and history of receiving antiviral therapy. Information on history of psychiatric diagnoses was obtained by extracting ICD-9-CM codes from the electronic medical record with the assistance of the Veterans Integrated Service Network 20 Data Warehouse. Psychiatric diagnoses were identified using ICD-9-CM codes recorded in the database and from individual patient visits during the 12 months prior to assessment with the BDI-II. The Data Warehouse is a centralized database that retrieves clinical data from several regional VA medical facilities and two national VA databases. During the study period the database was updated on a daily basis, and studies of the VA databases have found good reliability for diagnostic information [35]. The use of administrative databases to acquire diagnostic information is typically characterized by high specificity but variable sensitivity as compared with manual chart review by a trained clinician [36].
2.2. Beck Depression Inventory–Second Edition
The BDI-II [27] is a 21-item self-report questionnaire designed to assess depressive symptomatology. The second edition was developed to make the measure better correspond with the diagnostic criteria for depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. The BDI-II provides cutoff scores for symptom severity: 0–13=minimal, 14–19=mild, 20–28=moderate and 29–63=severe. A “conservative cutoff” score of ≥17 indicates significant depressive symptoms. Many studies have supported the reliability and validity of the BDI-II, and it is one of the most common measurements of depression utilized in a variety of inpatient and outpatient settings [37,38].
2.3. Data analysis
Descriptive data were analyzed using the Statistical Package for the Social Sciences (SPSS, version 17.0). A series of χ2 and t tests were used to analyze relationships between BDI-II total scores, demographic data, depressive disorder diagnosis and cirrhosis diagnosis. The factor analysis followed the common procedure for establishing factor structure when the relationship between the observed data and underlying variables is uncertain [39]. A random number generator assigned each case a value of either 0 or 1, and the sample was split according to the assigned values, resulting in two samples of 341 and 330. The first sample of 341 was subjected to EFA to establish the initial model solution, and the second sample of 330 was subjected to CFA to confirm the model. In choosing the type of analysis for EFA, our goal was to derive a factor structure that provides a theoretically meaningful explanation of the observed data from which we could form a hypothesis and test it using CFA. Therefore, instead of a principal components analysis — which provides a useful but theoretically problematic set of composite variables — we chose a maximum likelihood analysis to extract an initial unrotated solution. Because the factors were derived from a single validated instrument measuring depression, we used a Promax (oblique) rotation that assumes the factors are correlated. We created a parsimonious factor structure by conducting a second CFA using items from the first CFA with high factor loadings. Last, a series of χ2 and t tests were used to analyze relationships between the factor scores, demographic data, depressive disorder diagnosis and cirrhosis diagnosis. A significance level of .05 was used for all statistical tests. Factor analyses were conducted with the MPlus (v. 4.21) program [40].
3. Results
3.1. Participants
The sample was 97.0% male, with an average age of 52.8 years (S.D.=6.25). The majority (62.9%) had a current MDD diagnosis and a current substance use disorder diagnosis (68.7%). Individuals with current MDD scored significantly higher on the BDI-II total score (M=30.14, S.D.=19.49) than those without MDD (M=12.07, S.D.=11.53) [t(669)=13.31, P<.001]. In our sample, 108 participants (16.1%) had a diagnosis of liver cirrhosis. Patients with a cirrhosis diagnosis scored significantly higher on the BDI-II total score (M=18.26, S.D.=13.11) than patients without a cirrhosis diagnosis (M=15.67, S.D.=11.94) [t(669)=−2.04, P=.042]. Patients with a cirrhosis diagnoses were equally likely as patients without a cirrhosis diagnosis to have a current MDD diagnosis (χ2=.204, df=1, P=.651). Demographic characteristics, including marital status, HCV genotype, cirrhosis diagnosis, other mental health diagnoses and ethnicity, are displayed in Table 1.
Table 1.
Demographic characteristics of 671 patients with HCV
| Variable | Mean | Standard deviation |
|---|---|---|
| Age | 52.8 | 6.3 |
| N | % | |
|
|
||
| Male gender | 650 | 97.0 |
| Marital status | ||
| Single | 105 | 15.6 |
| Married | 215 | 32.0 |
| Separated/divorced | 316 | 47.1 |
| Widowed | 28 | 4.2 |
| Race | ||
| Caucasian/white | 431 | 64.2 |
| African American | 39 | 5.8 |
| Native American | 15 | 2.2 |
| Latino | 15 | 2.2 |
| Asian | 8 | 1.2 |
| No response/unknown | 170 | 25.3 |
| HCV genotype | ||
| 1 | 281 | 70.6 |
| 2 | 71 | 17.8 |
| 3 | 38 | 10.0 |
| 4 | 7 | 1.8 |
| Unknown | 273 | 40.7 |
| Cirrhosis diagnosis | 108 | 16.1 |
| Mental health diagnoses | ||
| MDD | 422 | 62.9 |
| Posttraumatic stress disorder | 241 | 35.9 |
| Bipolar disorder | 52 | 7.8 |
| Psychotic disorder | 105 | 15.6 |
| Alcohol or substance use disorder | 461 | 68.7 |
3.2. BDI-II Scores
Mean item and total BDI-II scores for the entire sample and the percentage of the sample endorsing symptoms on a given item are presented in Table 2. Regarding general symptom severity, as indicated by total BDI-II score, 48.7% (n=327) of the sample endorsed minimal symptoms, 17.9% (n=120) mild, 16.1% (n=108) moderate and 17.3% (n=116) severe. With the use of the conservative cutoff of BDI-II total score ≥17, 42.3% (n=284) of the sample endorsed clinically significant depressive symptoms.
Table 2.
Descriptive data for the BDI-II in 671 patients with hepatitis C
| Item | Mean | Standard deviation | % Endorsinga |
|---|---|---|---|
| 1. Sadness | 0.51 | 0.71 | 41.4 |
| 2. Pessimism | 0.73 | 0.82 | 55.0 |
| 3. Past failure | 0.87 | 0.91 | 55.3 |
| 4. Loss of pleasure | 0.90 | 0.82 | 64.8 |
| 5. Guilty feelings | 0.62 | 0.74 | 49.2 |
| 6. Punishment feelings | 0.43 | 0.91 | 23.0 |
| 7. Self-dislike | 0.78 | 0.90 | 49.0 |
| 8. Self-criticalness | 0.65 | 0.85 | 45.5 |
| 9. Suicidal thoughts | 0.30 | 0.50 | 27.9 |
| 10. Crying | 0.56 | 0.96 | 33.1 |
| 11. Agitation | 0.64 | 0.82 | 46.6 |
| 12. Loss of interest | 0.89 | 0.97 | 56.8 |
| 13. Indecisiveness | 0.63 | 0.86 | 43.1 |
| 14. Worthlessness | 0.57 | 0.78 | 40.8 |
| 15. Loss of energy | 1.17 | 0.74 | 83.8 |
| 16. Changes in sleeping pattern | 1.32 | 1.01 | 76.2 |
| 17. Irritability | 0.71 | 0.82 | 41.4 |
| 18. Changes in appetite | 0.88 | 0.87 | 61.4 |
| 19. Concentration difficulty | 0.87 | 0.85 | 59.5 |
| 20. Tiredness or fatigue | 1.15 | 0.87 | 76.9 |
| 21. Loss of interest in sex | 0.97 | 0.96 | 61.1 |
| Total score | 16.15 | 12.18 | 42.3b |
Item score ≥1.
Total score ≥17.
3.3. Exploratory factor analysis
Two factors were present in the data as indicated by two eigenvalues over 1.00 (9.932, 1.463). The two-factor solution produced a good model fit as indicated by a χ2 value that was no more than twice the degrees of freedom (χ2=356.81, df=169). On the basis of this analysis, 11 items were retained in Factor 1 and 7 were retained in Factor 2. Three items were eliminated due to significant cross loading. Data on the items included in each factor and their loadings are displayed in Table 3. The internal consistencies (coefficient alpha) for the two factors were .909 and .840, respectively.
Table 3.
Results of the EFA of the BDI-II in 341 patients with hepatitis C
| Item | Factor 1 | Factor 2 | Retained |
|---|---|---|---|
| 1. Sadness | .509 | .204 | Factor 1 |
| 2. Pessimism | .518 | .197 | Factor 1 |
| 3. Past failure | .782 | −.050 | Factor 1 |
| 4. Loss of pleasure | .523 | .353 | Eliminated |
| 5. Guilty feelings | .650 | .067 | Factor 1 |
| 6. Punishment feelings | .723 | −.115 | Factor 1 |
| 7. Self-dislike | .733 | .049 | Factor 1 |
| 8. Self-criticalness | .747 | .037 | Factor 1 |
| 9. Suicidal thoughts | .599 | .036 | Factor 1 |
| 10. Crying | .629 | −.003 | Factor 1 |
| 11. Agitation | .499 | .086 | Factor 1 |
| 12. Loss of interest | .453 | .357 | Eliminated |
| 13. Indecisiveness | .476 | .349 | Eliminated |
| 14. Worthlessness | .745 | .118 | Factor 1 |
| 15. Loss of energy | −.007 | .775 | Factor 2 |
| 16. Changes in sleeping pattern | .103 | .505 | Factor 2 |
| 17. Irritability | .256 | .509 | Factor 2 |
| 18. Changes in appetite | .150 | .488 | Factor 2 |
| 19. Concentration difficulty | .274 | .512 | Factor 2 |
| 20. Tiredness or fatigue | −.123 | .924 | Factor 2 |
| 21. Loss of interest in sex | .043 | .507 | Factor 2 |
3.4. Confirmatory factor analysis
The CFA was specified according to the EFA. Model fit is evaluated with the comparative fit index (CFI) and χ2; the CFI measures the proportion of variance accounted for by the model and should be close to 1.00, while the χ2 should be less than twice the degrees of freedom. The solution fit the data reasonably well without modification (χ2=336.54, df=134, CFI=0.93). The model was modified with the inclusion of four covariances (only one that was a cross factor). This was done in order to model significant covariance between variables that cannot be accounted for by the model solution and is common practice in fitting CFA models. The final fit functions of this model were superb (χ2=235.54, df=130, CFI=0.97). The final solution is shown in Fig. 1. All factor loadings are standardized, and the four error covariances are shown. Error variances were left out of the figure for parsimony.
Fig. 1.
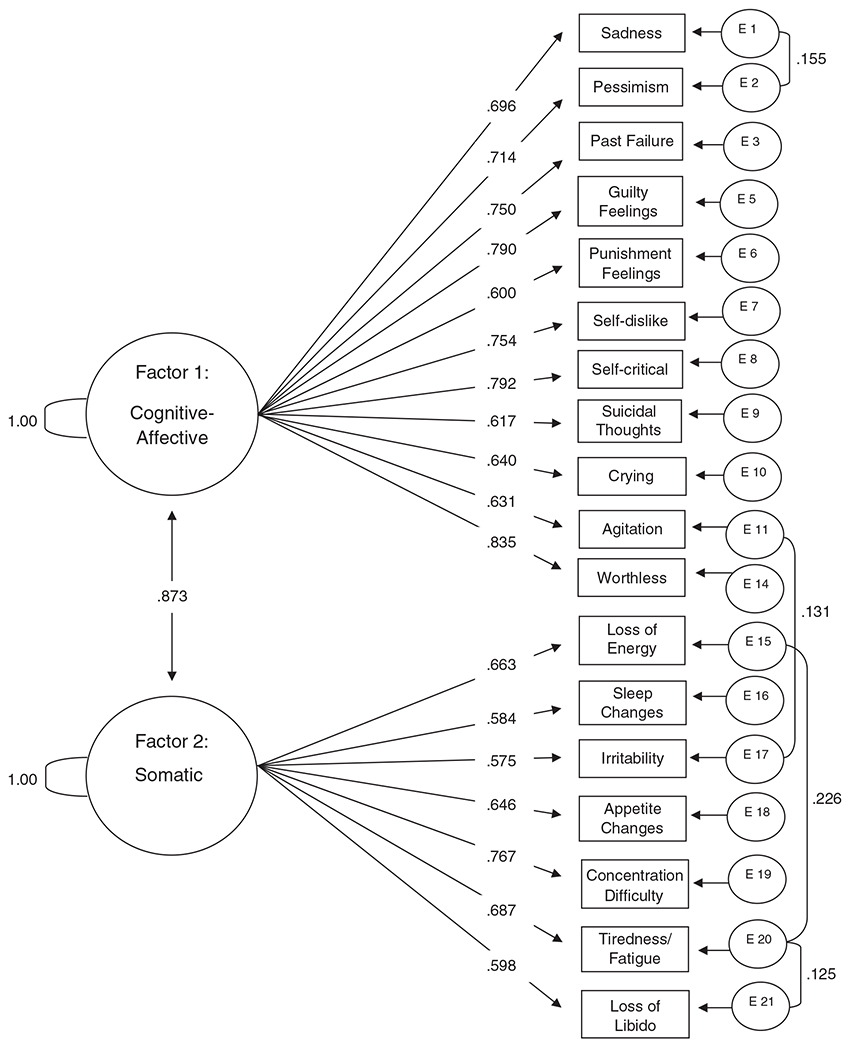
Confirmatory factor analysis of the items retained in the EFA of the BDI-II (N=330).
3.5. Interpretation of factors
The items that comprised the first factor were largely a measure of cognitive and affective symptoms. The highest-loading items in the EFA were past failure (.782), self-criticalness (.747), worthlessness (.745) and self-dislike (.733). In the CFA, the items comprising the final model solution, in order of the strength of their factor loadings, were worthlessness (.835), self-criticalness (.792), guilty feelings (.790), self-dislike (.754), past failure (.750), pessimism (.714), sadness (.696), crying (.640), agitation (.631), suicidal thoughts (.617) and punishment feelings (.600). This factor was labeled “Cognitive–Affective.”
The items that comprised the second factor were largely a measure of somatic symptoms. In the EFA, the highest-loading items were tiredness or fatigue (.924), loss of energy (.775) and irritability (.509). In the CFA, the items comprising the final model solution, in order of the strength of their factor loading, were concentration difficulty (.767), tiredness or fatigue (.687), loss of energy (.663), changes in appetite (.646), loss of interest in sex (.598), changes in sleeping pattern (.584) and irritability (.575). This factor was labeled “Somatic.”
3.6. Parsimonious factor structure
In order to shorten the factors to smaller parsimonious scales, a second CFA was conducted to model the six items from the first CFA with factor loadings above .70 and the four items from the second factor with factor loadings above .65. This new CFA model was run on the second half of the data set, and the model fit the data quite well (χ2=78.42, df=32, CFI=0.97). The factors showed good reliability estimates (.91 and .81, respectively). This shortened solution is shown in Fig. 2.
Fig. 2.
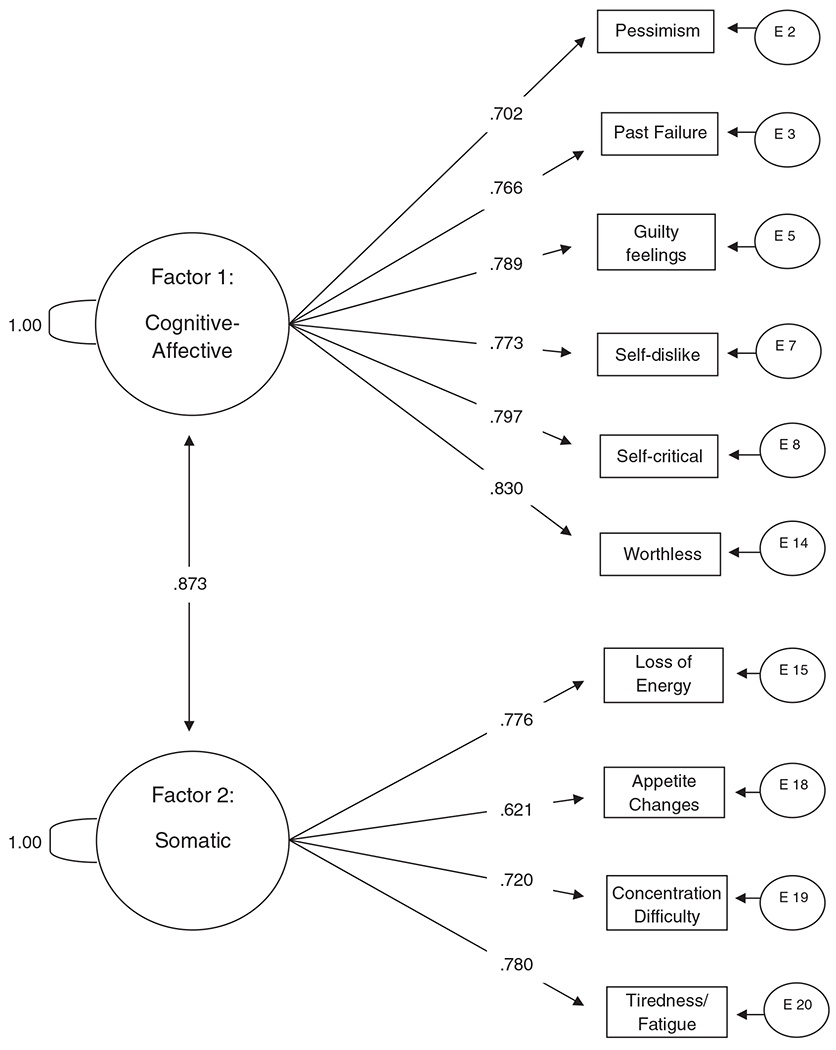
Confirmatory factor analysis for a reduced set of items from the BDI-II (N=330).
3.7. Factor scale scores and their relationship with psychiatric and medical data
The mean item score on the Somatic factor (M=1.01, S.D.=.63) was significantly higher than the mean item score on the Cognitive–Affective factor (M=.61, S.D.=.59) [t(670)=−22.64, P<.001]. Patients with a current MDD diagnosis scored significantly higher on the mean item scores of both the Cognitive–Affective factor (M=.81, S.D.=.62) [t(669)=12.62, P<.001], and the Somatic factor (M=1.20, S.D.=.61) [t(669)=7.50, P<.001], compared with individuals without an MDD diagnosis. Individuals with a cirrhosis diagnosis scored significantly higher than individuals without a cirrhosis diagnosis on the mean item score of the Somatic factor (M=1.14, S.D.=.67) [t(669)=−2.42, P=.016], but not of the Cognitive–Affective factor (M=.68, S.D.=.63) [t(669)=−1.34, P=.18].
4. Discussion
Major depressive disorder is prevalent in patients with HCV, and it has been suggested that the occurrence of depression during the course of antiviral treatment for HCV can reduce treatment effectiveness [16,17]. Because pretreatment depressive symptoms may predict the onset of MDD during treatment [6,14,15], moderate to severe depressive symptoms are a common reason for postponing or excluding patients from treatment [18]. Several new antiviral medications for HCV are currently in development, and two are awaiting FDA approval for widespread use. While these medications show promise of increased effectiveness for reducing HCV viral load, they still require combination with traditional antiviral medications that are associated with psychiatric side effects [41]. Additionally, since the number of individuals infected with HCV for 20 years or longer is estimated to increase until approximately 2015 [42], the demand for effective antiviral treatments will continue to intensify.
The BDI-II is frequently used to assess depressive symptomatology in medical populations despite the fact that its validity may be compromised due to the overlap of somatic symptoms common to both depression and chronic illness. This overlap may cause depression severity to be overestimated in patients with HCV, which in turn may contribute to the exclusion of patients from antiviral treatment who would otherwise benefit from a potentially lifesaving treatment. With the aim of improving the validity of the BDI-II for patients with HCV and shedding light on the unique nature of the depressive syndrome in HCV-positive individuals as measured by the BDI-II, this article described an EFA and CFA conducted on the BDI-II scores of 671 patients with HCV.
Our initial EFA suggested a two-factor structure that was confirmed by subsequent CFA. We interpreted the first factor as measuring the cognitive and affective aspects of depression, and the second factor as measuring the somatic aspects of depression. This two-factor structure has similarities with prior factor analytic studies of medical populations [28,43]. Our findings suggest that when depression is measured with the BDI-II in patients with HCV, the cognitive and affective symptoms of depression form a distinct cluster separate from the somatic symptoms of depression.
The average item score on the Somatic factor was significantly higher than the average item score on the Cognitive–Affective factor for the entire sample. This finding was expected considering that we utilized a sample of individuals with a chronic medical illness sharing many of the same somatic complaints common among individuals with depression. Low energy and fatigue are prominent symptoms of chronic HCV infection; as expected, 84% and 77% of our sample endorsed these symptoms on the BDI-II, respectively. Additionally, individuals with a current MDD diagnosis scored significantly higher on both factors than did individuals without an MDD diagnosis, suggesting that both factors are sensitive to a history of depression.
It is possible that the factors found in our analysis are illustrative of two depressive symptom dimensions that are correlated with distinct biological mechanisms, as we and others have shown in patients with chronic medical illness [44,45]. On the other hand, considering that the majority of the symptoms measured by the Somatic factor are also common symptoms of chronic HCV infection, it is also possible this factor represents a set of symptoms that, in the HCV-positive population, may not be directly related to depression. For example, patients in our sample with cirrhosis scored significantly higher on the Somatic factor and the BDI-II total score than did individuals with less severe liver disease, despite the fact that they were equally likely to have a current diagnosis of MDD. This finding indicates that increasing physical problems as the result of worsening liver disease may cause inflation of the Somatic factor score, which potentially compromises the validity of both the Somatic factor score and the BDI-II total score. Therefore, the Cognitive–Affective factor may be a more valid measure of depression as liver disease progresses. In a prior study of 89 patients with chronic medical illness, a Cognitive–Affective subscale on the BDI detected clinical depression as well as a common specialized self-report measure designed for medical patients [46]. While additional studies are needed to confirm the specificity of the Cognitive–Affective factor as a valid standalone measure of depression in patients with HCV, at a minimum, it would be helpful in clinical practice to analyze the Cognitive–Affective and Somatic subscales in order to draw attention to those individuals whose depression symptom profile is in need of further clarification. Individuals who score high on the BDI-II primarily due to somatic symptoms would benefit from a more rigorous assessment of their depressive symptoms. It is also important to use caution when utilizing other depression screening measures that employ somatic symptoms in their overall measurement of depression in patients with HCV. Similar suggestions have been made in prior studies with patients with other chronic illnesses [21,47].
This study has several limitations. Generalizability is limited by the fact that we recruited patients from a one-time HCV educational class. It is possible that HCV patients who attended this class were inherently different from the HCV patients who chose not to attend. Furthermore, our sample was a predominantly Caucasian, male sample of veterans seeking medical care at one VA medical center. Studies that utilize a more representative sample of HCV-positive patients would reinforce the external validity of our findings. The sample was recruited from a population of individuals seeking HCV treatment at an outpatient clinic. This sample may have different characteristics from the general population of individuals with HCV, particularly those who are unaware of their HCV-positive status. We also did not control for use of antidepressant medications in our sample. While it is unlikely this would change the overall factor model, it is possible that use of antidepressants could alter specific clusters of symptoms over time and attenuate the BDI-II total score. Finally, we used medical records to document diagnoses of MDD, which may result in under- or overreporting of diagnoses. A structured clinical interview may provide more reliable assessment.
Despite these limitations, this study has several significant strengths. It is the first to investigate the factor structure of a depression screening instrument in a large, treatment-seeking sample of patients with HCV; thus, the results have important implications for clinical practice and future research. A two-factor structure of the BDI-II with separate underlying variables representing Cognitive–Affective and Somatic symptoms was an excellent fit for our data. While both factors were sensitive to individuals with a current MDD diagnosis, our sample tended to score significantly higher on the Somatic factor than on the Cognitive–Affective factor, and individuals with a cirrhosis diagnosis scored significantly higher on the Somatic factor and BDI-II total score than individuals without a cirrhosis diagnosis, despite being equally likely to have a current MDD diagnosis. Therefore, when screening for depression in patients with HCV, items targeting the cognitive and affective symptoms of depression may provide a more valid measure of depression than items targeting the somatic symptoms of depression.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Portland VA Medical Center. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs. We would also like to acknowledge the time and effort of the members of the Northwest Hepatitis C Resource Center who worked on this project, including Julie Nelligan, Alex Linke, Brad Witke, Matthew McQuesten, Daniele Bjornson and Emily Kizer.
References
- [1].Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the Unites States, 1999 through 2002. Ann Intern Med 2006;144:705–15. [DOI] [PubMed] [Google Scholar]
- [2].Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry 2003;160:172–4. [DOI] [PubMed] [Google Scholar]
- [3].Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology 2005;41:88–96. [DOI] [PubMed] [Google Scholar]
- [4].Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci 2006;3:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marcellin P Hepatitis C: the clinical spectrum of the disease. J Hepatol 1995;31:9–16. [DOI] [PubMed] [Google Scholar]
- [6].Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry 2000;157:867–76. [DOI] [PubMed] [Google Scholar]
- [7].Dwight MM, Kowdley KV, Russo JE, et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res 2000;49:311–7. [DOI] [PubMed] [Google Scholar]
- [8].Fireman M, Indest D, Blackwell AD, Whitehead AJ, Hauser P. Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): the importance of routine mental health screening as a component of comanagement model of care. Clin Infect Dis 2005;40:286–91. [DOI] [PubMed] [Google Scholar]
- [9].Golden J, Conroy RM, O’Dwyer AM, Golden D, Hardouin J. Illness-related stigma, mood and adjustment to illness in persons with hepatitis C. Soc Sci Med 2006;63:3188–98. [DOI] [PubMed] [Google Scholar]
- [10].Golden J, O’Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen Hosp Psychiatry 2005;27:431–8. [DOI] [PubMed] [Google Scholar]
- [11].Nelligan J, Loftis JM, Matthews AM, et al. Depression co-morbidity and antidepressant use in veterans with chronic hepatitis C. J Clin Psychiatry 2008;69:810–6. [DOI] [PubMed] [Google Scholar]
- [12].Dan AA, Martin LM, Crone C. Depression, anemia and health-related quality of life in chronic hepatitis C. J Hepatol 2006;44:491–8. [DOI] [PubMed] [Google Scholar]
- [13].Rowan PJ, Al-Jurdi R, Tavakoli-Tabasi S, et al. Physical and psychosocial contributors to quality of life in veterans with hepatitis C not on antiviral therapy. J Clin Gastroenterol 2005;39:731–6. [DOI] [PubMed] [Google Scholar]
- [14].Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state. N Engl J Med 1999;340:1370. [DOI] [PubMed] [Google Scholar]
- [15].Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry 2002;7:942–7. [DOI] [PubMed] [Google Scholar]
- [16].Leutscher PD, Lagging M, Buhl MR, et al. Evaluation of depression as a risk factor for treatment failure in chronic hepatitis C. Hepatology 2010;52:430–5. [DOI] [PubMed] [Google Scholar]
- [17].Renault PF, Hoofnagle JH, Park Y, et al. Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med 1987;147:1577–80. [PubMed] [Google Scholar]
- [18].Rowan PJ, Tabasi S, Abdul-latif M, Kunik ME, El-Serag HB. Psychosocial factors are the most common contraindications for antiviral therapy at initial evaluation in veterans with chronic hepatitis C. J Clin Gastroenterol 2004;38:530–4. [DOI] [PubMed] [Google Scholar]
- [19].Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patients with and without preexisting major depressive disorder. Psychosomatics 2009;50:500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brown-DeGagne A, McGlone J, Santor D. Somatic complaints disproportionately contribute to Beck Depression Inventory estimates of depression severity in individuals with multiple chemical sensitivity. J Occup Environ Med 1998;40:862–9. [DOI] [PubMed] [Google Scholar]
- [21].Kalichman SC, Sikkema KJ, Somlai A. Assessing persons with human immunodeficiency virus (HIV) infection using the Beck Depression Inventory: disease processes and other potential confounds. J Pers Assess 1995;64:86–100. [DOI] [PubMed] [Google Scholar]
- [22].Golden J, Conroy RM, O’Dwyer AM. Reliability and validity of the Hospital Anxiety and Depression Scale and the Beck Depression Inventory (Full and FastScreen scales) in detecting depression in persons with hepatitis C. J Affect Disord 2007;100:265–9. [DOI] [PubMed] [Google Scholar]
- [23].Holtzheimer PE, Veitengruber J, Wang CC, et al. Utility of the Beck Depression Inventory to screen for and track depression in injection drug users seeking hepatitis C treatment. Gen Hosp Psychiatry 2010;32:426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vanheule S, Desmet M, Groenvynck H, Rosseel Y, Fontaine J. The factor structure of the Beck Depression Inventory-II. Assessment 2008;15:177–87. [DOI] [PubMed] [Google Scholar]
- [25].Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio (TX): Psychological Corp; 1996. [Google Scholar]
- [26].Steer RA, Ball R, Ranieri WF, Beck AT. Dimensions of the Beck Depression Inventory-II in clinically depressed outpatients. J Clin Psychol 1999;55:117–28. [DOI] [PubMed] [Google Scholar]
- [27].Hayden MJ, Dixon JB, Dixon ME, O’Brien PE. Confirmatory factor analysis of the Beck Depression Inventory in obese individuals seeking surgery. Obes Surg 2010;20:432–9. [DOI] [PubMed] [Google Scholar]
- [28].Visser M, Leentjens AF, Marinus J, Stiggelbout AM, Van Hilten JJ. Reliability and validity of the Beck Depression Inventory in patients with Parkinson’s disease. Mov Disord 2006;21:668–72. [DOI] [PubMed] [Google Scholar]
- [29].Thombs BD, Ziegelstein RC, Beck CA, Pilote L. A general factor model for the Beck Depression Inventory-II: validation in a sample of patients hospitalized with acute myocardial infarction. J Psychosom Res 2008;65:115–21. [DOI] [PubMed] [Google Scholar]
- [30].Castellon SA, Hardy DJ, Hinkin CH, et al. Components of depression in HIV-1 infection: their differential relationship to neurocognitive performance. J Clin Exp Neuropsychol 2006;28:420–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Harris CA, D’Eon JL. Psychometric properties of the Beck Depression Inventory-Second Edition (BDI-II) in individuals with chronic pain. Pain 2008;137:609–22. [DOI] [PubMed] [Google Scholar]
- [32].Morely S, Williams AC, Black S. A confirmatory factor analysis of the Beck Depression Inventory in chronic pain. Pain 2002;99:289–98. [DOI] [PubMed] [Google Scholar]
- [33].Golub ET, Latka M, Hagan H, et al. Screening for depressive symptoms among HCV-infected injection drug users: examination of the utility of the CES-D and the Beck Depression Inventory. J Urban Health 2004;81:278–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nunnally JC, Bernstein IH. Psychometric theory. 3rd Ed.New York: McGraw-Hill; 1994. [Google Scholar]
- [35].Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med 1999;14:555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care 2002;40:675–85. [DOI] [PubMed] [Google Scholar]
- [37].Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess 1996;67:588–97. [DOI] [PubMed] [Google Scholar]
- [38].Steer RA, Ball R, Ranieri WF. Further evidence for the construct validity of the Beck Depression Inventory-II with psychiatric outpatients. Psychol Rep 1997;80:443–6. [DOI] [PubMed] [Google Scholar]
- [39].Byrne BA. Factor analytic models: viewing the structure of an assessment instrument from three perspectives. J Pers Assess 2005;85:17–32. [DOI] [PubMed] [Google Scholar]
- [40].Muthén LK, Muthén BO. Mplus user’s guide. Los Angeles (CA): Muthén & Muthén; 1998–2007. [Google Scholar]
- [41].Gentile I, Viola C, Borgia F, Castaldo G, Borgia G. Telaprevir: a promising protease inhibitor for the treatment of hepatitis C virus infection. Curr Med Chem 2009;16:1115–21. [DOI] [PubMed] [Google Scholar]
- [42].Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31:777–82. [DOI] [PubMed] [Google Scholar]
- [43].Siegert RJ, Walkey FH, Turner-Stokes L. An examination of the factor structure of the Beck Depression Inventory-II in a neurorehabilitation inpatient sample. J Int Neuropsychol Soc 2009;15:142–7. [DOI] [PubMed] [Google Scholar]
- [44].Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. Neurosci Lett 2008;430:264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptoms dimensions. Neuropsychopharmacology 2002;26:643–52. [DOI] [PubMed] [Google Scholar]
- [46].Clark DA, Steer RA. Use of nonsomatic symptoms to differentiate clinically depressed and nondepressed hospitalized patients with chronic medical illnesses. Psychol Rep 1994;75:1089–90. [DOI] [PubMed] [Google Scholar]
- [47].Poole H, Bramwell R, Murphy P. Factor structure of the Beck Depression Inventory-II in patients with chronic pain. Clin J Pain 2006;22:790–8. [DOI] [PubMed] [Google Scholar]


