Abstract
Effective treatment of sexually transmitted infections (STIs) is limited by diagnostics that cannot deliver results rapidly while the patient is still in the clinic. The gold standard methods for identification of STIs are nucleic acid amplification tests (NAATs), which are too expensive for widespread use and have lengthy turnaround times. To address the need for fast and affordable diagnostics, we have developed a portable, rapid, on-cartridge magnetofluidic purification and testing (PROMPT) polymerase chain reaction (PCR) test. We show that it can detect Neisseria gonorrhoeae, the pathogen causing gonorrhea, with simultaneous genotyping of the pathogen for resistance to the antimicrobial drug ciprofloxacin in <15 min. The duplex test was integrated into a low-cost thermoplastic cartridge with automated processing of penile swab samples from patients using magnetic beads. A compact instrument conducted DNA extraction, PCR, and analysis of results while relaying data to the user via a smartphone app. This platform was tested on penile swab samples from sexual health clinics in Baltimore, MD, USA (n = 66) and Kampala, Uganda (n = 151) with an overall sensitivity and specificity of 97.7% (95% CI, 94.7 to 100%) and 97.6% (95% CI, 94.1 to 100%), respectively, for N. gonorrhoeae detection and 100% concordance with culture results for ciprofloxacin resistance. This study paves the way for delivering accessible PCR diagnostics for rapidly detecting STIs at the point of care, helping to guide treatment decisions and combat the rise of antimicrobial resistant pathogens.
INTRODUCTION
The rates of sexually transmitted infections (STIs) worldwide have increased markedly in the past decade, posing an enormous burden on global public health infrastructure (1). An estimated 1 million people worldwide acquire a curable STI daily (2). Neisseria gonorrhoeae, the etiological agent responsible for gonorrhea, is particularly concerning due to the rapid development of antimicrobial resistance (3, 4). With upward of 87 million annual N. gonorrhoeae infections worldwide, no available vaccine, a slow rate of antibiotic discovery, and a dearth of approved alternative antimicrobial treatments, the possibility of untreatable N. gonorrhoeae presages a looming global health care crisis (2, 4, 5).
Diagnosis of N. gonorrhoeae is the first step toward treatment and control. N. gonorrhoeae identification is routinely conducted using nucleic acid amplification tests (NAATs), such as polymerase chain reaction (PCR) (1, 6). Although NAATs have largely replaced culture-based methods as the gold standard for detection of N. gonorrhoeae, many NAATs take hours to complete, leading clinicians to prescribe antibiotic treatments empirically to avoid losing patients to follow-up. The cost of empiric treatment may be high, and a lack of diagnostic certainty can preclude use of more affordable oral medications for treating N. gonorrhoeae such as ciprofloxacin.
For low-income countries where testing options are absent or limited, particularly in sub-Saharan Africa, N. gonorrhoeae infections pose an especially heavy burden (2, 7). Limited STI surveillance and screening coupled with poor antimicrobial stewardship has led to a high prevalence of N. gonorrhoeae infections and antimicrobial resistance (7, 8). Rapid diagnostics at the point of care (POC) with antimicrobial resistance characterization have been highlighted as a viable solution to address this issue (9, 10). Fast, affordable, and accurate diagnostics that provide pathogen identification and antimicrobial resistance results are needed to shift the clinical workflow globally from syndromic treatment to precision therapy.
Commercialized rapid POC PCR technologies either are too slow, lack antimicrobial resistance data, or are too costly for widespread adoption in resource-limited settings (6, 11–16). We propose the use of magnetofluidic platforms coupled with inexpensive disposable assay cartridges to automate, accelerate, and simplify N. gonorrhoeae testing. Magnetofluidics leverages transport of functionalized magnetic beads to automate complex bioassays (17, 18). All manual sample handling steps typically required for a molecular assay can be accomplished with magnetic field gradients using electromagnetic coils or by moving permanent magnets (19–21). We previously reported a magnetofluidic mobile NAAT platform with sample-to-answer detection of chlamydia in vaginal swabs, although the assay was limited in speed (>1 hour) and tested only a single genomic target for identification of the pathogen (22).
Here, we introduce the next generation of magnetofluidic cartridges and instrumentation and demonstrate the utility of our platform with a 15-min PCR assay for identifying N. gonorrhoeae and determining susceptibility of this pathogen to ciprofloxacin directly in clinical samples. This portable, rapid, on-cartridge magnetofluidic purification and testing (PROMPT) platform enabled automated on-site diagnosis in settings with confined workspaces, minimal resources, and unreliable electrical supply. We validated the PROMPT platform cartridge and compared it to culture assays and commercial NAAT assays using 35 clinical isolates and 66 penile swab eluates from sexual health clinics in Baltimore, MD, USA, as well as 151 penile swabs from multiple clinics in Kampala, Uganda. To assess the potential use and impact of the PROMPT platform, we performed on-site testing in sexual health clinics in Baltimore, conducted field testing in Kampala, and surveyed clinicians and medical laboratory technicians about POC testing in Kampala.
RESULTS
PROMPT platform design and operation
The PROMPT platform was designed for automated testing at the POC, which would allow clinicians to prescribe targeted antibiotic treatments, reduce overtreatment, and mitigate the need for follow-up visits (Fig. 1A). To enable POC use, the PROMPT platform was engineered to have a small footprint (12.7 cm by 13.4 cm by 8.4 cm) and require minimal power consumption. The instrument uses a portable mobile phone charger (5 V, ~2 A, 10,000 mAh), which supplies power to perform more than 20 tests.
Fig. 1. PROMPT platform design and operation.
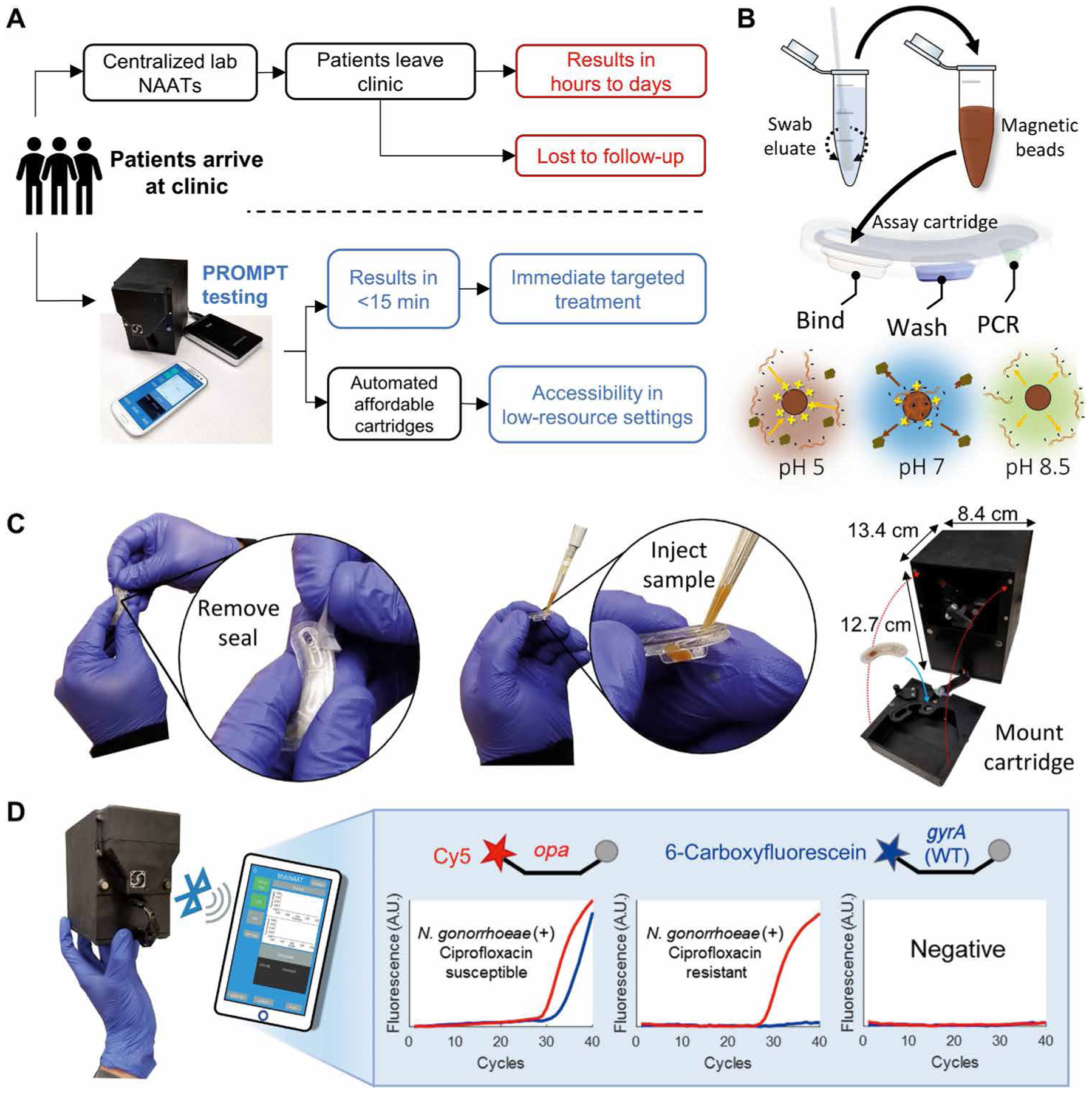
(A) Comparison of standard of care for N. gonorrhoeae diagnosis (top) versus proposed clinical workflow with the PROMPT platform (bottom). (B) Schematic shows processing of patient swab samples. Steps include swab elution, mixing eluate with magnetic beads, and injection into the assay cartridge, followed by pH-mediated automation of nucleic acid binding, purification, and elution using transfer of the beads through cartridge wells containing preloaded buffers. (C) Photographs of the PROMPT assay cartridge. Cartridges are sealed before use with an adhesive tape, which is removed before injection of the sample into the first well. The instrument’s faceplate detaches for loading the cartridge onto the PCR heat block. Remounting the faceplate with magnetic clasps aligns the cartridge with the instrument’s magnet arm and fluorescence detector. (D) Photograph and schematic showing the instrument interfacing with a smartphone or tablet using Bluetooth for real-time reporting of PCR amplification. PCR was used to determine whether N. gonorrhoeae was present in the clinical sample by opa amplification in the Cy5 channel (red) and whether the bacterial strain was susceptible to ciprofloxacin by wild-type (WT) gyrA amplification in the 6-carboxyfluorescein channel (blue). A.U., arbitrary units.
The PROMPT workflow streamlined N. gonorrhoeae testing to three manual steps: elution of a swab, combining the swab eluate with a magnetic bead solution, and loading the combined solution into the cartridge (Fig. 1B). The disposable cartridge was the key feature of the platform that enabled automated sample preparation, rapid turnaround time, and a low cost per test. After sample loading, the cartridge was mounted onto the faceplate of the instrument (Fig. 1C and fig. S1). Within the cartridge, capture and release of the negatively charged nucleic acids was mediated by electrostatic attraction to a pH-responsive cationic coating on the magnetic beads (Fig. 1B). An acidic (pH 5) binding solution induced a positive charge on the beads for DNA capture and facilitated bacterial lysis (fig. S2). Transfer of the beads from the binding buffer into a neutral pH wash buffer removed contaminants that might inhibit the assay while retaining bound nucleic acids for subsequent elution directly into the PCR buffer (Fig. 1B). The more basic (pH 8.5) PCR buffer neutralized the beads’ charge for release of the nucleic acids.
PCR-based detection of N. gonorrhoeae and determination of ciprofloxacin susceptibility were performed using an integrated two-color epifluorescence detector (23) for signal acquisition from duplexed hydrolysis probes targeting the multicopy opa gene (24) and wild-type gyrA gene (25) (Fig. 1D). Fluorescence signals were wirelessly transferred to a smartphone app for analysis (Fig. 1D and movie S1). In the presence of an S91F mutation in the gyrA gene, which is highly predictive of ciprofloxacin resistance (26), the 6-carboxyfluoroscein–labeled probe did not bind to the gyrA target sequence, resulting in absent signal amplification in the 6-carboxyfluoroscein fluorescence channel. N. gonorrhoeae–positive samples with Cy5-labeled opa amplification and an absent gyrA signal were classified as ciprofloxacin resistant.
Magnetofluidic sample preparation and rapid PCR
The PROMPT platform achieved sample-to-answer diagnosis in <15 min through a combination of automated magnetofluidic sample preparation and rapid PCR. Transfer of the magnetic beads through the cartridge reagents was conducted with biaxial actuation of opposing neodymium magnets attached to a servo motor assembly (Fig. 2, A and B). Elution of the beads into the final reagent well coupled the sample preparation directly to the rapid PCR assay. The short turnaround time of the PCR assay was enabled by both enhanced assay chemistry to maximize reaction kinetics and optimized heat block geometry to quickly cycle the temperature.
Fig. 2. Rapid PCR assay and instrumentation.
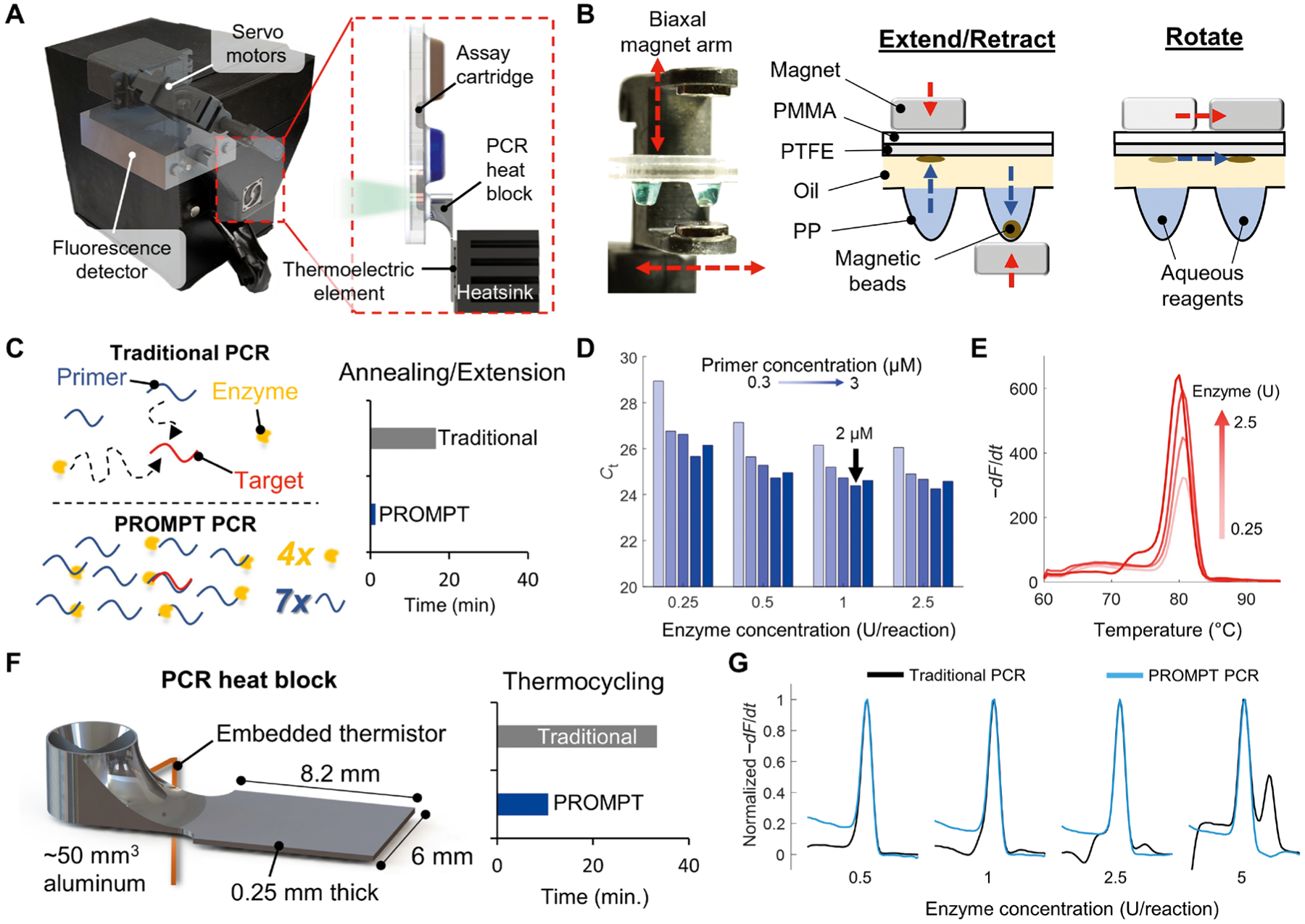
(A) Schematic of the internal components overlaid on a photograph of the PROMPT instrument. The assay cartridge mounts directly onto an aluminum PCR heat block, which is aligned with a fluorescence detector and servo-actuated magnet arm in the instrument. (B) Photograph of the magnet arm and schematic with cross-sectional view of the cartridge showing acrylic [polymethyl methacrylate (PMMA)], polytetrafluoroethylene (PTFE), and polypropylene (PP) plastic layers. The arm rotates and extends/retracts two opposing permanent magnets to transfer the magnetic beads between cartridge reagents stored in thermoformed wells. Red arrows indicate movement of the magnet arm, and blue arrows show the corresponding direction of bead transfer. (C) Comparison of traditional PCR and PROMPT PCR kinetics, with elevated primer and enzyme concentrations enabling less annealing and extension time of amplified products using the PROMPT platform. (D) Optimization of enzyme and primer conditions by cycle threshold (Ct) for PCR (n = 1 for each condition) with 1-s extension hold times using the opa assay. (E) Melt-curve analysis of opa amplicons using the 2 μM primer conditions. (F) Schematic shows the miniaturized aluminum heat block, which decreases thermocycling time compared to traditional benchtop thermocycler routines. (G) Melt-curve analysis of PCR amplicons from a traditional benchtop PCR routine is compared to that for the PROMPT cartridge rapid PCR assay (n = 1 for each condition).
Rapid PCR techniques overcome the necessity of long temperature hold times in traditional PCR routines by increasing the concentrations of primer, Mg2+, and enzyme (DNA polymerase) to reduce the combined annealing and extension times to <1 s per cycle (27–29). Increased concentrations of primer, Mg2+, and enzyme improved enzyme processivity and reduced the time needed for diffusion of reagents to anneal with target sequences such that primer extension occurred immediately upon reaching the primer annealing temperature (Fig. 2C). Denaturation of amplicons is a first-order process that occurs within 500 ms at sufficiently high temperatures (29). Previous studies on rapid PCR required specialized instruments impractical for clinical translation, overlooked sample preparation times, and used single-plex assays with intercalating dyes or electrophoresis for analysis (28–31). In contrast, our PROMPT cartridge integrated sample preparation with rapid PCR to form a multiplexed probe assay in a portable and user-friendly format. Compared to a traditional 40-cycle PCR assay with 20-s extension and 5-s denaturing hold times, the PROMPT assay’s 1-s hold times translated to an overall decrease in annealing and denaturing time from 16.7 min to a little over 1 min (Fig. 2C).
By increasing the opa primer and enzyme concentrations to about seven- and fourfold of their respective standard concentrations (2 μM primer, 1 U enzyme per 10-μl reaction), we maximized the efficiency of the assay with 1-s hold times for annealing and denaturation (Fig. 2D). The specificity of the opa target amplification, verified by consistent melt curve peaks at 80°C (Fig. 2E), indicated specific amplification of the opa target, with increasing final amplicon quantity (taller melt curve peaks) correlating with increasing enzyme concentration. The 1 U per reaction was selected for the final assay as nonspecific amplification (melt peak aberration at 75°C) was noted with the 2.5 U concentration (Fig. 2E).
Even with ideal assay kinetics, a PCR assay is only as fast as the thermocycler supporting it. Traditional microfluidic devices require large and slow flat-bed heaters to match their planar geometry (32), whereas our cartridge’s extruded well design allowed for spatial isolation of the PCR reagents for targeted thermal control. To determine optimal geometries of the heating block for maximizing thermal ramp rates, finite element heat transfer simulations were conducted with a parametric sweep of heat block dimensions (figs. S3 and S4). These simulations demonstrated that at this scale, heat block volume was the primary determining factor in thermal ramp rate, and the thermal conductivity of aluminum (~200 W m−1 K−1) was sufficient to rapidly heat the PCR assay even with geometric constrictions resulting in cross-sectional areas as small as 0.05 mm2.
The final heat block design (Fig. 2F) minimized thermal mass by conforming precisely to the dimensions of the thermoelectric element with the well cantilevered to allow magnetic arm accessibility. A minimum heat block thickness of 0.25 mm was chosen due to practical machining limitations and to provide sufficient mechanical strength to withstand 5 lbs of force (~22.2 N) applied to the end of the well without yielding given a 10-fold safety margin. In the final assembly, heating rates ranged from 10.4° to 4.2°C/s and cooling rates from 10.0° to 18.5°C/s between 60° and 100°C (fig. S5), resulting in a <12-min 40-cycle PCR compared to >50-min 40-cycle PCR by conventional thermocyclers.
The use of elevated enzyme concentrations in a traditional PCR benchtop assay produced aberrations in the melt curves (black lines in Fig. 2G). However, with the PROMPT thermocycler, consistent melt curves with singular peaks were obtained regardless of enzyme concentration (blue lines in Fig. 2G). This demonstrated that the shorter annealing times used in the PROMPT PCR platform resulted in more specific amplification by limiting formation of less thermodynamically stable nonspecific dimers (27).
Assay cartridge design and validation
The cartridge wells contained the assay reagents in static droplets stabilized by surface tension using a continuous layer of silicone oil enabling captured analytes to be transferred via magnetic beads (Fig. 2B). Sample preparation within the cartridge was completed in 2.5 min, and 40 cycles of PCR were completed within 12 min, resulting in a <15-min turnaround time (Fig. 3A). Traditional benchtop PCR thermocycler routines would complete <10 cycles in the same time period (Fig. 3B).
Fig. 3. PROMPT cartridge assay evaluation.
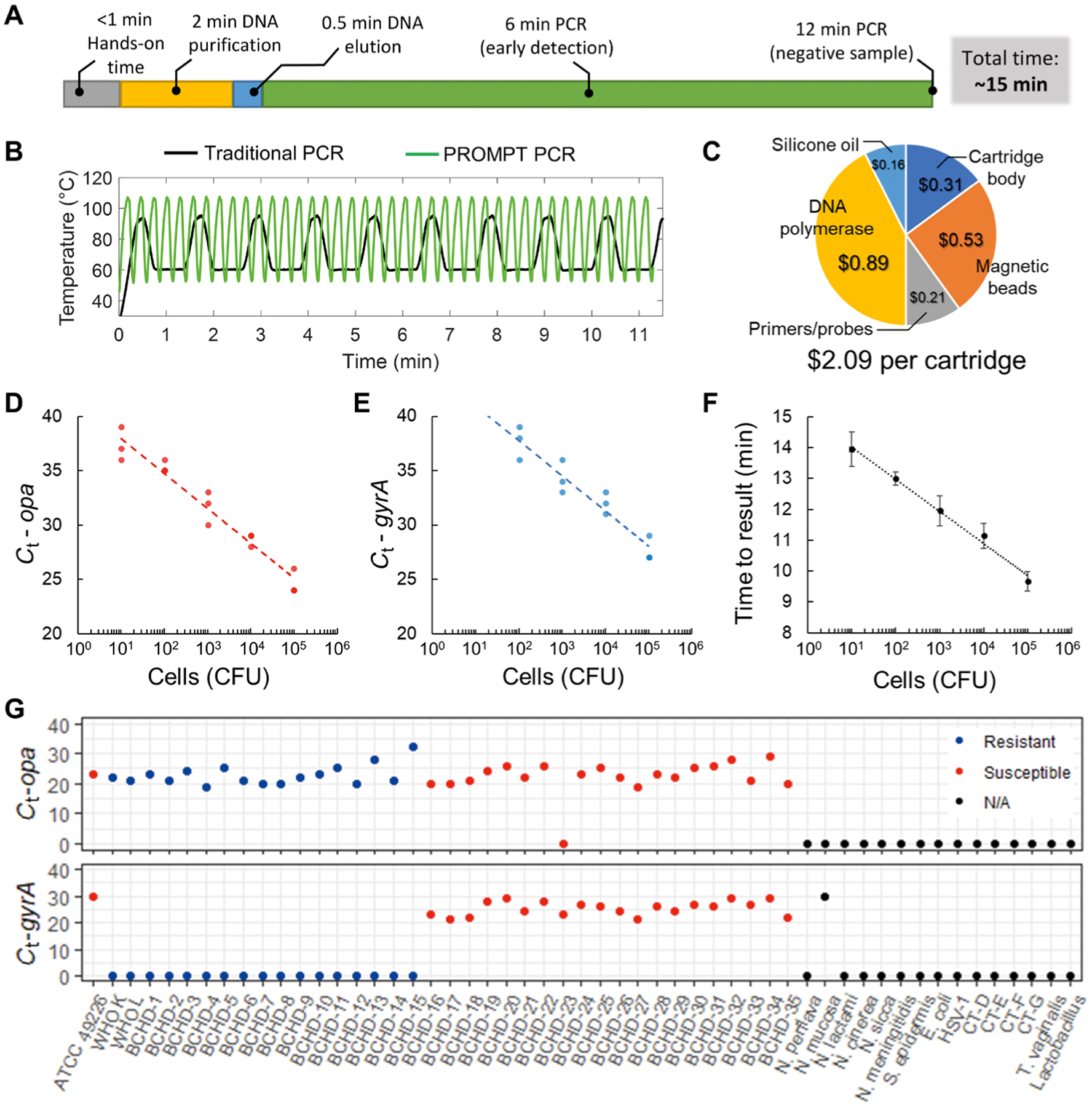
(A) Overall sample-to-answer workflow includes <1 min of hands-on time with DNA purification and with PCR amplification automated for a total time of 15 min. (B) Rapid thermocycling in the PROMPT instrument completes 40 cycles of PCR within 12 min during which a traditional thermocycling routine completes <10 cycles. (C) Cost breakdown for cartridge components. (D and E) Cartridge assay standard curves are shown using 10-fold serial dilutions run in triplicate (n = 3) from 1 to 105 CFU of N. gonorrhoeae input, with duplexed detection of opa (red) and wild-type gyrA (blue). (F) Time to result (minutes) using a live-detection algorithm versus N. gonorrhoeae CFU input to the cartridge. Error bars represent SD. (G) Cycle threshold (Ct) values for assay validation using reference strains of N. gonorrhoeae (ATCC, WHO), BCHD clinical isolates, related Neisseria species, and other organisms known to infect the urogenital tract. N. gonorrhoeae strains are color-coded by susceptibility to ciprofloxacin. No amplification is indicated by a Ct equal to 0.
The cartridge design builds upon previous magnetofluidic cartridges (22, 23) assembled from laser-cut and thermoformed components with preloaded reagents (fig. S6). Combining the cost of assay reagents and thermoplastics resulted in a cost of US$2.09 per cartridge (Fig. 3C). The use of polypropylene sheets for the thermoformed wells of the cartridge provided a chemically inert PCR-compatible scaffold, which allowed for 1 month of storage with consistent performance (fig. S7 and data file S1).
The limit of detection (LoD) of the cartridge assay was evaluated using serial dilutions of a ciprofloxacin-susceptible N. gonorrhoeae reference strain [American Type Culture Collection (ATCC) 49226]. The opa assay for detection of N. gonorrhoeae showed successful amplification of opa in all replicates down to 10 colony-forming units (CFUs); the gyrA assay for detection of ciprofloxacin resistance demonstrated a less sensitive LoD of 100 CFUs (Fig. 3, D and E). Real-time reporting of results using a custom regression algorithm (figs. S8 and S9) allowed for a turnaround time of <10 min for samples with a high bacterial load (Fig. 3F).
The performance of the opa and gyrA assays was evaluated against a panel of well-characterized N. gonorrhoeae reference strains and archived N. gonorrhoeae clinical isolates (Fig. 3G and table S1). Only one of the N. gonorrhoeae isolates produced a false-negative opa result (BCHD-23); the remaining 37/38 N. gonorrhoeae isolates were identified by the opa assay. All ciprofloxacin-susceptible isolates produced a detectable wild-type gyrA amplification signal.
Specificity of the cartridge assay was assessed with 15 bacterial and viral species known to infect the human urogenital tract, including six Neisseria species and four Chlamydia trachomatis serovars (Fig. 3G and table S2). None of the pathogens produced any false-positive amplification with the opa assay. Cross-reactivity of the gyrA assay was observed with the bacterial species Neisseria mucosa. The gyrA gene may also be present in other bacteria, particularly in Neisseria commensal species. In order for positive gyrA amplification to be considered valid for detection of ciprofloxacin-susceptible N. gonorrhoeae strains, the PROMPT assay must also be positive for opa gene amplification.
Validation of the PROMPT assay using clinical samples
Before blinded testing or field testing, the clinical sensitivity of the PROMPT cartridge assay was evaluated using 32 archived urethral swab eluates (33). All samples were correctly classified as N. gonorrhoeae positive (n = 18) or N. gonorrhoeae negative (n = 14) by the PROMPT assay in full concordance with comparator results from NAAT assays (table S3). Of the 18 N. gonorrhoeae–positive samples, 13 were identified as having gyrA wild-type sequences indicative of ciprofloxacin susceptibility. Phenotypic susceptibility data were not available for these samples.
After platform validation with archived clinical samples, field testing at the POC using a blinded approach was implemented at two Baltimore City Health Department (BCHD) sexual health clinics during May and June 2019. Penile-meatal clinical swab samples were prospectively collected after informed consent, and tested on the PROMPT platform. Of the 34 samples tested, two were identified as N. gonorrhoeae positive by the PROMPT cartridge assay, by culture, and by commercial NAAT assays. All N. gonorrhoeae–negative samples were correctly classified by the PROMPT cartridge assay. The two N. gonorrhoeae–positive samples were identified as having wild-type gyrA sequences. Phenotypic antimicrobial susceptibility testing confirmed that the isolates cultured from these two samples were susceptible to ciprofloxacin. Figure 4A illustrates the PROMPT cartridge sensitivity with the opa assay for N. gonorrhoeae detection using the Baltimore clinic swabs (orange and light gray circles). Figure 4B shows the error-free prediction of ciprofloxacin susceptibility with the gyrA assay in the Baltimore clinic strains isolated from swabs and based on antimicrobial resistance profiles determined by culture.
Fig. 4. Clinical validation of the PROMPT platform.
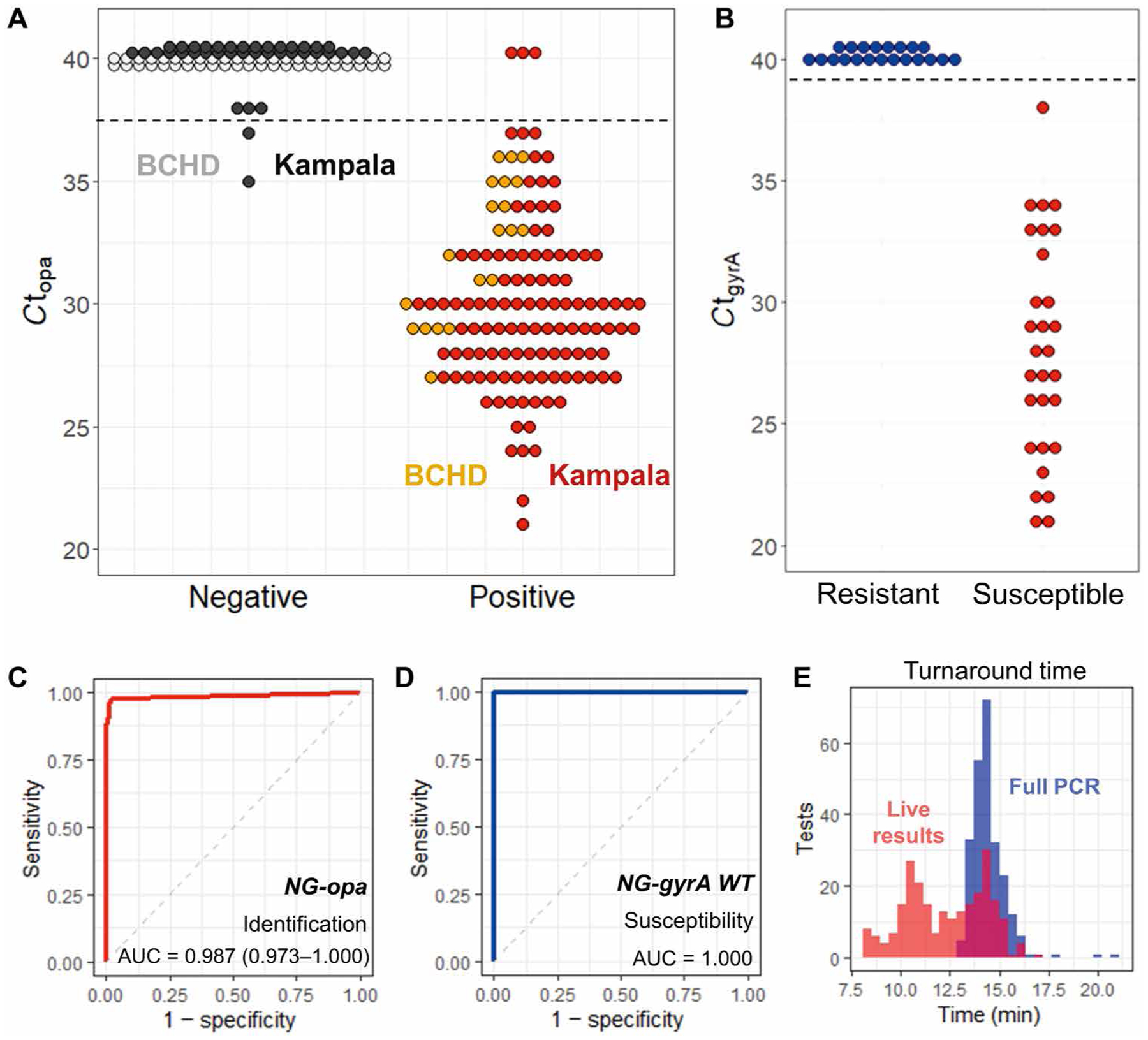
(A) Results of N. gonorrhoeae detection by the PROMPT cartridge assay show opa amplification cycle threshold (Ct) for BCHD swabs (n = 66) and swabs collected in Kampala, Uganda (n = 151). Samples with no amplification in this plot are given a Ct set at 40 (gray, black). Horizontal dashed lines indicate cutoff Ct values used to determine positive amplification by the PROMPT cartridge assay. (B) Ciprofloxacin susceptibility of N. gonorrhoeae in BCHD samples was predicted by amplification of wild-type gyrA and is compared to culture results for antimicrobial susceptibility testing. (C) ROC curve for N. gonorrhoeae (NG) identification by opa amplification in all swab samples. (D) ROC curve for N. gonorrhoeae ciprofloxacin susceptibility by gyrA amplification. (E) Turnaround times for all swab samples using the complete 40-cycle PCR (blue) or determined by the live-reporting algorithm for opa gene amplification (red).
Additional blinded testing of the PROMPT cartridge assay was performed using participant-collected penile-meatal swabs after informed consent, from six clinics in Kampala, Uganda between October 2019 and February 2020 (n = 151) (fig. S10). Swab eluates (n = 51) were tested on-site at the Infectious Diseases Institute (IDI) in Kampala with two PROMPT instruments and compared to culture and commercial NAAT results. Because of the coronavirus disease 2019 (COVID-19) pandemic, the remaining swabs (n = 100) were analyzed in the laboratory at Johns Hopkins University. Of the 151 samples collected in Kampala, 114 were N. gonorrhoeae positive by a NAAT assay. Two samples were negative by NAAT but confirmed as N. gonorrhoeae positive by culture, resulting in a prevalence of 76.8% (116 of 151). The PROMPT cartridge assay failed to detect three N. gonorrhoeae–positive samples, and two N. gonorrhoeae– negative samples were classified as false positives (Fig. 4A). All cultured N. gonorrhoeae isolates from the Kampala samples (n = 85) were determined to be ciprofloxacin resistant by antimicrobial susceptibility testing, which is consistent with published local antimicrobial resistance patterns (7), and in full concordance with results of the gyrA assay.
Receiver operating characteristic (ROC) curves for the identification of N. gonorrhoeae by opa genotyping and antimicrobial susceptibility testing by gyrA genotyping (Fig. 4, C and D) had area under the curve values of 0.987 [95% confidence interval (CI), 0.973 to 1.000] and 1.0, respectively. The PROMPT cartridge assay matched the NAAT and culture results with a high degree of concordance for an overall clinical sensitivity and specificity of 97.7% (95% CI, 94.7 to 100%) and 97.6% (95% CI, 94.1 to 100%), respectively. Results obtained using the PROMPT cartridge assay were compared to NAAT, culture, and phenotypic antimicrobial susceptibility testing results. (See data file S2 for compiled results from all cartridge runs and table S4 for a summary of discrepant results.) The average full assay turnaround time was 14.5 min (SD, 0.9 min; median, 14.4 min), starting with the cartridge mounted into the PROMPT instrument to completion of 40 PCR cycles (Fig. 4E). When using the live-reporting algorithm to determine when amplification was first evident, the turnaround time decreased to 11.1 min on average (SD, 1.5 min; median, 10.9 min).
End-user survey
To assess the potential utility of POC testing with the PROMPT platform, we conducted a survey of clinicians and laboratory staff (n = 30) in Kampala (figs. S11 and S12 and data file S3). Most (93%) of the respondents indicated that assay sensitivity was the most important characteristic of a diagnostic test for N. gonorrhoeae, followed by turnaround time (76.6%). For POC testing in general, participants preferred a test that was fast (24 of 30) and simple to use (22 of 30), with less than 50% (13 of 30) prioritizing cost or mobility of the test (12 of 30) (fig. S11B).
The survey also assessed general attitudes about POC testing, duration of typical patient visits, and desired test duration to fit clinical workflow (fig. S11C). POC tests were generally preferred to laboratory testing (24 of 30), and more than 50% (16 of 30) of respondents stated that a typical patient’s visit lasted <1 hour. To fit POC testing into the timeline of a patient’s visit, most respondents desired a turnaround time of <30 min (23 of 30); six participants preferred a turnaround time of <15 min.
After indicating preferences for desired testing characteristics, participants were shown a demonstration of the PROMPT platform operation (movie S1), after which they answered questions regarding their impressions of the test. At least 90% of survey participants selected “extremely easy” or “easy” when asked about ease of following instructions, running the test, and interpreting results. Participants commented positively that such a test would save them from “long waits for the cumbersome gram [sic] stain and culture tests”; resistance profiles were highly desired to replace their current tests “which may take…almost a week in our health centre to get results.” One respondent was concerned about the utility of ciprofloxacin resistance testing in Uganda due to the high prevalence of antimicrobial resistance, and suggested cephalosporin susceptibility testing instead. Other concerns pertained to the limited availability of smartphones, and that connection to a computer would improve patient data security and the ability to retrieve results. Despite the few concerns raised, nearly all of the participants (29 of 30) indicated that they “Definitely” or “Probably” would use the PROMPT platform if it was made available.
DISCUSSION
Rapid identification of the bacterial pathogen N. gonorrhoeae and associated antimicrobial susceptibility will help to promote better antimicrobial stewardship to reduce selection of antimicrobial resistant bacterial strains. Although ciprofloxacin is no longer recommended for the treatment of N. gonorrhoeae in the United States (34), up to 80% of N. gonorrhoeae infections are susceptible to ciprofloxacin (35). POC use of our PROMPT platform to rapidly identify ciprofloxacin-susceptible N. gonorrhoeae infections opens up opportunities for providing access to less expensive oral ciprofloxacin compared to the current first-line recommended injectable antibiotic ceftriaxone (36–39).
Recent POC diagnostics have trended toward isothermal techniques, whereas our work demonstrates that PCR-based assays can provide a fast turnaround time while maintaining high sensitivity and specificity in a cost-effective POC format. A major advantage of using a proven NAAT strategy like PCR over isothermal techniques is the opportunity for facile adoption of established clinical assays with the PROMPT cartridge. The opa and gyrA assays that we used in our PROMPT cartridge have been shown to be highly sensitive and specific, with <1% false-positive or false-negative results (24, 40–45).
Proponents of isothermal NAAT platforms as alternatives to PCR highlight the presumed advantages in instrument simplicity or “instrument-free” operation, although many fail to emphasize the necessity for nucleic acid purification before testing (46–48). Without integrated sample preparation, these techniques require a trained professional and laboratory equipment (heaters, centrifuges, etc.) to process clinical samples, which ultimately provides little benefit over traditional PCR assays. Our PROMPT platform addresses both sample preparation and rapid turnaround time in a format that requires minimal training to operate. Tests with instrumentation and connectivity capabilities may be preferable in clinical settings to remove risk of errors associated with user interpretation of test readouts or for interfacing with health care records (as noted by our survey respondents). By avoiding complex moving parts and precision labyrinthine microchannels traditionally found in POC diagnostic devices, the magnetofluidic cartridge is suitable for high-throughput manufacture at an affordable cost. Compared to the leading industry POC PCR diagnostic cartridges, our magnetofluidic cartridge design has potential to provide even faster results, contribute less plastic waste to the environment, and enable greater access to diagnostics in low-resource settings.
For translation of the PROMPT platform to patient care settings, there are limitations in our study that must be addressed. Performance of the test with vaginal and extragenital swabs, and urine samples needs to be validated. Future cartridges would benefit from higher multiplexing to include assay controls, other antimicrobial resistance markers, or additional STIs such as chlamydia. User workflow could be further simplified by incorporating swab elution into the magnetic bead mixture directly in the cartridge. Furthermore, cartridges in the current format are limited to cold chain storage (−20°C). For widespread adoption, it may be necessary to adapt the cartridges for dry reagents to ensure prolonged shelf-life at room temperature. Nonetheless, advances demonstrated so far in magnetofluidic cartridge technology show promise for delivering rapid gold standard infectious disease diagnostics in a scalable and accessible format that could improve clinical management of STIs.
MATERIALS AND METHODS
Study design
We performed a cross-sectional, nonrandomized diagnostic accuracy study to determine the analytical performance of our PROMPT platform for detection of N. gonorrhoeae and characterization of ciprofloxacin susceptibility, and compared its performance with that of laboratory-based NAAT and culture assays. Following test development and preliminary testing using archived specimens, male participants were offered enrollment to self-collect penile-meatal swabs to be tested on the PROMPT platform and in comparator gold standard tests. Following written informed consent, male participants were recruited in Baltimore, MD, USA and Kampala, Uganda. Whenever possible, testing of the new assay was performed on-site to evaluate the performance in clinical settings outside of the research laboratory. PROMPT operators were blinded to NAAT and culture results. A total of 34 participants in Baltimore and 250 participants in Kampala were recruited and provided samples for the study, but only 151 samples from Kampala were included in this study due to delays associated with the ongoing COVID-19 pandemic. Ethical oversight was provided by the Johns Hopkins Institutional Review Board (IRB) for both studies (IRB numbers 00215298 and 00199514). In addition, the Joint Clinical Research Center at Makerere University (protocol reference number JC0919) and the Ugandan National Council for Science and Technology (study number HS455ES) approved the study.
For the Baltimore study, we enrolled a prospective, convenience sample of participants to evaluate the performance of the PROMPT platform in a clinical setting with low prevalence of N. gonorrhoeae and ciprofloxacin resistance. This initial study was used to develop the protocol for testing prospectively collected samples in a clinical setting before the Uganda study. For the Uganda study, to obtain the minimal sample size, the area under the ROC curve (AUC) with the PROMPT assay was estimated. In these estimations, N. gonorrhoeae positivity in men presenting to clinics in Kampala with urethral discharge was assumed to be between 75 and 85%. Using a null hypothesis of AUC at 0.80, assuming a 0.10 increase in AUC with the PROMPT assay and with a type 1 error of 5% and 80% power, a total of 213 participants was required. However, anticipating that 15% or greater results would be uninterpretable (the test result was invalid or indeterminate), the sample size was adjusted to a total of 250 study participants.
Assay reagents
Magnetic particle binding buffer solutions for each cartridge were formulated using 10 μl of acetic acid–based binding buffer, 4 μl of ChargeSwitch magnetic particles (25 mg/ml) (ChargeSwitch gDNA Mini Bacteria Kit, Invitrogen), and 1 or 10 μl of 10% Tween 20 (fig. S13). For magnetic bead wash buffer, the wash buffer from the same ChargeSwitch kit was supplemented with a final concentration of 0.2% (v/v) Tween 20 to reduce surface tension between the buffer and the oil and permit maximum magnetic bead extraction. The PCR buffers and sequences of the primers and probes are described in tables S5 and S6. Direct elution of the magnetic beads into the PCR buffers was shown to remove primers and enzyme from the assay (fig. S14), but these effects were compensated with excess primer and enzyme in the PROMPT PCR.
PROMPT cartridge
The magnetofluidic cartridges were composed of three thermoplastic layers (fig. S6). The bottom layer was fabricated by thermoforming 0.2-mm-thick polypropylene sheet (AKAHA) over three-dimensional (3D)–printed molds (Form 2, USA) designed in Solidworks 2017 computer-aided design (CAD) software (Dassault Systèmes) to produce extruded wells. The middle layer was laser-cut from 1.5-mm-thick clear acrylic [polymethyl methacrylate (PMMA)] sheet (McMaster-Carr) with pressure-sensitive adhesive (PSA) (9472LE adhesive transfer tape, 3M) laminated on both sides. The top layer was laser-cut from 0.75-mm-thick acrylic (ePlastics) with polytetrafluoroethylene (PTFE) tape (McMaster-Carr) laminated to one side and patterned by laser-etching.
Assay reagents (10 μl of PCR solution and 50 μl of wash buffer) were loaded into the corresponding wells of the thermoformed section, followed by joining the thermoformed section with PSA, and loading the cartridge with 420 μl of silicone oil (50 c St, Millipore-Sigma) to cover the wells and remaining empty space. The cartridges were used immediately, or the sample injection port was sealed with adhesive tape (Scotch Magic Tape, 3M) and the cartridge was stored at −20°C until use. For testing, an aliquot of sample and magnetic bead binding buffer (acceptable volumes between 10 and 100 μl) was injected directly into the first well of the cartridge and the cartridge was mounted onto the PROMPT instrument faceplate with the PCR well (Fig. 1C).
Instrumentation design and assembly
The housing of the PROMPT instrument and magnetic arm was 3D-printed (Formlabs Form 2, black resin) and equipped with permanent neodymium magnets for magnetic clasping, alignment, and magnetofluidic transfer in the cartridges (fig. S1). A microcontroller (Arduino Uno R3) monitored and controlled the heat block temperature with current supplied by a motor shield (Arduino Motor Shield Rev3), and an additional custom printed circuit board shield provided wiring connections for the fluorescence detector (Fluo Sens Integrated, Qiagen) and magnetic arm motors. The magnetic arm motors consisted of a linear servo (PQ12-R, Actuonix), which was secured to the shaft of a rotary servo (HS-485HB Hitec RCD) for biaxial actuation. The fluorescence detection and heating module was assembled as previously described (23), with the exception of the design of the aluminum heat block that was optimized for rapid thermocycling. Finite element heat transfer simulations to characterize effects of heat block dimensions on temperature ramp rates were conducted using COMSOL Multiphysics software version 5.1.
The instrument communicated to our custom smartphone app through a wireless serial connection using a Bluetooth module (Bestgle HC-05) connected to the microcontroller. A switch in the back of the instrument controlled power to the Bluetooth module to switch the communication from smartphone/tablet mode to universal serial bus (USB) connection for programming with a computer. The instrument was powered through a barrel-plug connection to a portable 5-V power bank (Anker PowerCore, 10,000 mAh) or an alternating current (AC) adapter.
To control the instrument and automate data collection and analysis, a graphic user interface (GUI) was developed in Java using Processing (Processing 3.4, www.processing.org) for both PC and Android devices. During clinical testing, the devices were controlled with a Samsung Galaxy S4 smartphone or Android tablet (Asus ZenPad). The GUI displayed real-time fluorescence data and live updates if amplification for either target was detected (movie S1).
PCR real-time fluorescence analysis algorithm
A custom algorithm for identifying the presence of PCR amplification was implemented in the Processing GUI to automatically report the opa and gyrA results in real time. The algorithm is described in figs. S8 and S9.
Clinical isolates and archived swab testing
N. gonorrhoeae isolates from international repositories (ATCC 49226, WHO K, WHO L) or obtained from previous studies in the BCHD clinics (n = 35) (49) were cultured as previously described (48) and tested on the PROMPT cartridge to evaluate assay sensitivity. Phenotypic ciprofloxacin susceptibility data were available for all N. gonorrhoeae isolates (table S1). To evaluate cartridge functionality in a clinical matrix, archived eluates of well-characterized urethral swabs collected from BCHD clinics in 2015 (33) were also tested on the PROMPT platform.
Clinical enrollment and sample collection
After providing written informed consent, patients in the Baltimore, MD, study self-collected two penile-swabs; one of the swabs was eluted into 500 μl of phosphate-buffered saline (PBS). The PBS eluate was used for PROMPT analysis (50 μl), N. gonorrhoeae culture (100 μl), and NAAT assay (Hologic Aptima CT/NG) (200 μl), and the remainder was stored at −20°C for retesting or subsequent studies.
In the Kampala, Uganda study, after informed consent, participants self-collected two penile-meatal swabs at six local health clinics. Before study enrollment, participants provided a urethral swab for the World Health Organization (WHO) Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) (fig. S10), which was cultured for N. gonorrhoeae and tested for antimicrobial susceptibility. At the Makerere University IDI laboratory, the penile-meatal swabs were each eluted into 500 μl of PBS, and the eluates were combined and used for PROMPT testing and NAAT. Samples were either tested immediately on PROMPT cartridges or stored frozen until tested.
Statistical analysis
Data analysis of clinical results was conducted using R software version 3.6.2. Generation of ROC curves with sensitivity, specificity, and AUC calculations was done using the pROC package version 1.16.2.
Supplementary Material
Fig. S1. PROMPT instrument housing.
Fig. S2. N. gonorrhoeae lysis evaluation.
Fig. S3. Finite-element heat transfer simulations for aluminum well design.
Fig. S4. Finite-element heat transfer simulations for aluminum heat block dimensions.
Fig. S5. Heat block thermocycling characterization.
Fig. S6. Cartridge fabrication.
Fig. S7. Cartridge shelf-life.
Fig. S8. Linear regression algorithm for Ct determination.
Fig. S9. Ct determination visualization.
Fig. S10. Kampala clinical testing workflow.
Fig. S11. End-user survey responses.
Fig. S12. End-user survey respondent data.
Fig. S13. Binding buffer adjustment.
Fig. S14. Magnetic bead interactions with PCR reagents.
Table S1. Cartridge assay validation with N. gonorrhoeae isolates.
Table S2. Cartridge assay specificity evaluation.
Table S3. Archived Baltimore clinical sample validation.
Table S4. Discrepant PROMPT results in clinical validation.
Table S5. Duplexed PCR assay buffer.
Table S6. PCR primers and probes.
Data file S1. Shelf-life data.
Data file S2. N. gonorrhoeae PROMPT cartridge data.
Data file S3. Survey questions.
Movie S1. PROMPT platform operation.
Acknowledgments:
We thank M. Barnes and P. Barnes for help in setting up our study and on-site testing in the BCHD clinics. We also thank T. Hu and J. Chung for assistance in cartridge and instrument testing.
Funding:
This work was funded by the NIH (grants R01AI138978, U54EB007958, and R61AI154628 to T.-H.W.).
Footnotes
Competing interests:
A.Y.T. and T.-H.W. are co-inventors on patent PCT/US2019/029937 “A disposable reagent scaffold for biochemical process integration” that is associated with this study.
Data and materials availability:
The software for the mobile application programmed in Processing (version 3.4) including the algorithm for fluorescence signal analysis is available at https://doi.org/10.5281/zenodo.4613082. All other data associated with this study are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Hull S, Kelley S, Clarke JL, Sexually transmitted infections: Compelling case for an improved screening strategy. Popul. Health Manag 20, S-1–S-11 (2017). [PubMed] [Google Scholar]
- 2.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, Chico RM, Smolak A, Newman L, Gottlieb S, Thwin SS, Broutet N, Taylor MM, Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ 97, 548–562 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unemo M, Golparian D, Eyre DW, Antimicrobial resistance in Neisseria gonorrhoeae and treatment of gonorrhea, in Neisseria gonorrhoeae, Methods in Molecular Biology, Christodoulides M, Ed. (Humana, 2019), vol. 1997, pp. 37–58. [DOI] [PubMed] [Google Scholar]
- 4.Unemo M, Shafer WM, Antimicrobial resistance in Neisseria gonorrhoeae in the 21st Century: Past, evolution, and future. Clin. Microbiol. Rev 27, 587–613 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alirol E, Wi TE, Bala M, Bazzo ML, Chen XS, Deal C, Dillon JAR, Kularatne R, Heim J, Hooft van Huijsduijnen R, Hook EW, Lahra MM, Lewis DA, Ndowa F, Shafer WM, Tayler L, Workowski K, Unemo M, Balasegaram M, Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines. PLOS Med. 14, e1002366 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaydos CA, Melendez JH, Point-by-point progress: Gonorrhea point of care tests. Expert Rev. Mol. Diagn 20, 803–813 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Workneh M, Hamill MM, Kakooza F, Mande E, Wagner J, Mbabazi O, Mugasha R, Kajumbula H, Walwema R, Zenilman J, Musinguzi P, Kyambadde P, Lamorde M, Manabe YC, Antimicrobial resistance of Neisseria gonorrhoeae in a newly implemented surveillance program in Uganda: Surveillance report. JMIR Public Health Surveill. 6, e17009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fingerhuth SM, Bonhoeffer S, Low N, Althaus CL, Antibiotic-resistant Neisseria gonorrhoeae spread faster with more treatment, not more sexual partners. PLOS Pathog. 12, e1005611 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuite AR, Gift TL, Chesson HW, Hsu K, Salomon JA, Grad YH, Impact of rapid susceptibility testing and antibiotic selection strategy on the emergence and spread of antibiotic resistance in gonorrhea. J. Infect. Dis 216, 1141–1149 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadiq ST, Mazzaferri F, Unemo M, Rapid accurate point-of-care tests combining diagnostics and antimicrobial resistance prediction for Neisseria gonorrhoeae and Mycoplasma genitalium. Sex. Transm. Infect 93, S65–S68 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Gaydos CA, Review of use of a new rapid real-time PCR, the Cepheid GeneXpert ® (Xpert) CT/NG assay, for Chlamydia trachomatis and Neisseria gonorrhoeae: Results for patients while in a clinical setting. Expert Rev. Mol. Diagn 14, 135–137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Causer LM, Guy RJ, Tabrizi SN, Whiley DM, Speers DJ, Ward J, Tangey A, Badman SG, Hengel B, Natoli LJ, Anderson DA, Wand H, Wilson D, Regan DG, Shephard M, Donovan B, Fairley CK, Kaldor JM, Molecular test for chlamydia and gonorrhoea used at point of care in remote primary healthcare settings: A diagnostic test evaluation. Sex. Transm. Infect 94, 340–345 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Widdice LE, Hsieh Y-HH, Silver B, Barnes M, Barnes P, Gaydos CA, Performance of the atlas genetics rapid test for Chlamydia trachomatis and women’s attitudes toward point-of-care testing. Sex. Transm. Dis 45, 723–727 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low N, Unemo M, Molecular tests for the detection of antimicrobial resistant Neisseria gonorrhoeae: When, where, and how to use? Curr. Opin. Infect. Dis 29, 45–51 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, Marrazzo JM, Sonder GJB, Schwebke JR, Hoornenborg E, Peeling RW, Philip SS, Low N, Fairley CK, Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis 17, e235–e279 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Gaydos C, Hardick J, Point of care diagnostics for sexually transmitted infections: Perspectives and advances. Expert Rev. Anti Infect. Ther 12, 657–672 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin DJ, Wang TH, Magnetic droplet manipulation platforms for nucleic acid detection at the point of care. Ann. Biomed. Eng 42, 2289–2302 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Nguyen N-T, Magnetic digital microfluidics—A review. Lab Chip 17, 994–1008 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Lehmann U, Vandevyver C, Parashar VK, Gijs MAM, Droplet-based DNA purification in a magnetic lab-on-a-chip. Angew. Chem. Int. Ed. Engl 45, 3062–3067 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Wang TH, Full-range magnetic manipulation of droplets via surface energy traps enables complex bioassays. Adv. Mater 25, 2903–2908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiou C-H, Jin Shin D, Zhang Y, Wang T-H, Topography-assisted electromagnetic platform for blood-to-PCR in a droplet. Biosens. Bioelectron 50, 91–99 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin DJ, Athamanolap P, Chen L, Hardick J, Lewis M, Hsieh YH, Rothman RE, Gaydos CA, Wang TH, Mobile nucleic acid amplification testing (mobiNAAT) for Chlamydia trachomatis screening in hospital emergency department settings. Sci. Rep 7, 4495 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin DJ, Trick AY, Hsieh Y-H, Thomas DL, Wang T-H, Sample-to-answer droplet magnetofluidic platform for point-of-care hepatitis C viral load quantitation. Sci. Rep 8, 9793 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabrizi SN, Chen S, Tapsall J, Garland SM, Evaluation of opa-based real-time PCR for detection of Neisseria gonorrhoeae. Sex. Transm. Dis 32, 199–202 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Giles J, Hardick J, Yuenger J, Dan M, Reich K, Zenilman J, Use of applied biosystems 7900HT sequence detection system and taqman assay for detection of quinolone-resistant Neisseria gonorrhoeae. J. Clin. Microbiol 42, 3281–3283 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allan-Blitz LT, Wang X, Klausner JD, Wild-type gyrase a genotype of Neisseria gonorrhoeae predicts in vitro susceptibility to ciprofloxacin: A systematic review of the literature and meta-analysis. Sex. Transm. Dis 44, 261–265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittwer CT, Garling DJ, Rapid cycle DNA amplification: Time and temperature optimization. Biotechniques 10, 76–83 (1991). [PubMed] [Google Scholar]
- 28.Farrar JS, Wittwer CT, Extreme PCR: Efficient and specific DNA amplification in 15–60 seconds. Clin. Chem 61, 145–153 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Millington AL, Houskeeper JA, Quackenbush JF, Trauba JM, Wittwer CT, The kinetic requirements of extreme qPCR. Biomol. Detect. Quantif 17, 100081 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuzil P, Zhang C, Pipper J, Oh S, Zhuo L, Ultra fast miniaturized real-time PCR: 40 cycles in less than six minutes. Nucleic Acids Res. 34, e77 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler EK, Hara CA, Frank J, Deotte J, Hall SB, Benett W, Spadaccini C, Beer NR, Under-three minute PCR: Probing the limits of fast amplification. Analyst 136, 3707–3712 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Park S, Zhang Y, Lin S, Wang T-H, Yang S, Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnol. Adv 29, 830–839 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melendez JH, Hardick J, Barnes M, Barnes P, Geddes CD, Gaydos CA, Molecular characterization of markers associated with antimicrobial resistance in Neisseria gonorrhoeae identified from residual clinical samples. Sex. Transm. Dis 45, 312–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC), Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb. Mortal. Wkly Rep 56, 332–336 (2007). [PubMed] [Google Scholar]
- 35.Kirkcaldy RD, Harvey A, Papp JR, del Rio C, Soge OO, Holmes KK, Hook EW, Kubin G, Riedel S, Zenilman J, Pettus K, Sanders T, Sharpe S, Torrone E, Neisseria gonorrhoeae antimicrobial susceptibility surveillance—The gonococcal isolate surveillance project, 27 Sites, United States, 2014. MMWR Surveill. Summ 65, 1–19 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Allan-Blitz L-T, Hemarajata P, Humphries RM, Kimble M, Elias S, Klausner JD, Ciprofloxacin may be efficacious in treating wild-type gyrase a genotype Neisseria gonorrhoeae infections. Sex. Transm. Dis 45, e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allan-Blitz L-T, Humphries RM, Hemarajata P, Bhatti A, Pandori MW, Siedner MJ, Klausner JD, Implementation of a rapid genotypic assay to promote targeted ciprofloxacin therapy of Neisseria gonorrhoeae in a large health system. Clin. Infect. Dis 64, 1268–1270 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melendez JH, Hsieh Y-H, Barnes M, Hardick J, Gilliams EA, Gaydos CA, Can ciprofloxacin be used for precision treatment of gonorrhea in public STD clinics? Assessment of ciprofloxacin susceptibility and an opportunity for point-of-care testing. Pathogens 8, 189 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cyr SS, Barbee L, Workowski KA, Bachmann LH, Pham C, Schlanger K, Torrone E, Weinstock H, Kersh EN, Thorpe P, Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb. Mortal. Wkly Rep 69, 1911–1916 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goire N, Nissen MD, LeCornec GM, Sloots TP, Whiley DM, A duplex Neisseria gonorrhoeae real-time polymerase chain reaction assay targeting the gonococcal porA pseudogene and multicopy opa genes. Diagn. Microbiol. Infect. Dis 61, 6–12 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Geraats-Peters CWM, Brouwers M, Schneeberger PM, van der Zanden AGM, Bruisten SM, Weers-Pothoff G, Boel CHE, van den Brule AJC, Harmsen HG, Hermans MHA, Specific and sensitive detection of Neisseria gonorrhoeae in clinical specimens by real-time PCR. J. Clin. Microbiol 43, 5653–5659 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verma R, Sood S, Bala M, Mahjan N, Kapil A, Sharma VK, Pandey RM, Samantaray JC, Evaluation of an opa gene-based nucleic acid amplification test for detection of Neisseria gonorrhoeae in urogenital samples in North India. Epidemiol. Infect 140, 2110–2116 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Maze MJ, Young S, Creighton J, Anderson T, Werno A, Nucleic acid amplification of the opa gene for detection of Neisseria gonorrhoeae: Experience from a diagnostic laboratory. J. Clin. Microbiol 49, 1128–1129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckley C, Trembizki E, Donovan B, Chen M, Freeman K, Guy R, Kundu R, Lahra MM, Regan DG, Smith H, Whiley DM; GRAND Study Investigators, A real-time PCR assay for direct characterization of the Neisseria gonorrhoeae GyrA 91 locus associated with ciprofloxacin susceptibility. J. Antimicrob. Chemother 71, 353–356 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Gaydos CA, Cartwright CP, Colaninno P, Welsch J, Holden J, Ho SY, Webb EM, Anderson C, Bertuzis R, Zhang L, Miller T, Leckie G, Abravaya K, Robinson J, Performance of the abbott realtime CT/NG for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol 48, 3236–3243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoepp NG, Schlappi TS, Curtis MS, Butkovich SS, Miller S, Humphries RM, Ismagilov RF, Rapid pathogen-specific phenotypic antibiotic susceptibility testing using digital LAMP quantification in clinical samples. Sci. Transl. Med 9, eaal3693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snodgrass R, Gardner A, Semeere A, Kopparthy VL, Duru J, Maurer T, Martin J, Cesarman E, Erickson D, A portable device for nucleic acid quantification powered by sunlight, a flame or electricity. Nat. Biomed. Eng 2, 657–665 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F, Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a and Csm6. Science 360, 439–444 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melendez J, Hardick J, Barnes M, Page K, Gaydos C, Antimicrobial susceptibility of Neisseria gonorrhoeae isolates in Baltimore, Maryland, 2016: The importance of sentinel surveillance in the Era of multi-drug-resistant gonorrhea. Antibiotics 7, 77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. PROMPT instrument housing.
Fig. S2. N. gonorrhoeae lysis evaluation.
Fig. S3. Finite-element heat transfer simulations for aluminum well design.
Fig. S4. Finite-element heat transfer simulations for aluminum heat block dimensions.
Fig. S5. Heat block thermocycling characterization.
Fig. S6. Cartridge fabrication.
Fig. S7. Cartridge shelf-life.
Fig. S8. Linear regression algorithm for Ct determination.
Fig. S9. Ct determination visualization.
Fig. S10. Kampala clinical testing workflow.
Fig. S11. End-user survey responses.
Fig. S12. End-user survey respondent data.
Fig. S13. Binding buffer adjustment.
Fig. S14. Magnetic bead interactions with PCR reagents.
Table S1. Cartridge assay validation with N. gonorrhoeae isolates.
Table S2. Cartridge assay specificity evaluation.
Table S3. Archived Baltimore clinical sample validation.
Table S4. Discrepant PROMPT results in clinical validation.
Table S5. Duplexed PCR assay buffer.
Table S6. PCR primers and probes.
Data file S1. Shelf-life data.
Data file S2. N. gonorrhoeae PROMPT cartridge data.
Data file S3. Survey questions.
Movie S1. PROMPT platform operation.
Data Availability Statement
The software for the mobile application programmed in Processing (version 3.4) including the algorithm for fluorescence signal analysis is available at https://doi.org/10.5281/zenodo.4613082. All other data associated with this study are present in the paper or the Supplementary Materials.


