Abstract
Objectives
Previous research supports that subjective views on aging (VoA), such as older subjective age (SA) and negative attitudes toward own aging (ATOA), go along with negative outcomes. A differentiated treatment of health and disease as antecedents of VoA is largely lacking. Therefore, our objective was to estimate the relationship between generally framed physical, affective, and cognitive health as well as specific diseases and VoA, operationalized both as SA and ATOA.
Methods
Data were drawn from the ActiFE Ulm study for which a representative sample of community-dwelling older people (65–90 years) was recruited at baseline. Follow-ups were conducted 7.7 years (median) after recruitment (N = 526). Health- and disease-related data at baseline, based on established assessment procedures for epidemiological studies, were regressed on VoA (1-item SA indicator, 5-item ATOA scale) measures at follow-up.
Results
Reported severity of affective health problems such as depression was the strongest general risk factor for both older SA and negative ATOA. Also, some but not all major diseases considered were associated with VoA. Notably, back pain predicted negative ATOA, while cancer was associated with older SA. Rheumatism was linked with more negative ATOA along with higher SA. Throughout analyses, explained variance in ATOA was considerably higher than in SA.
Discussion
Affective health problems, such as depression, should be regarded as a major correlate of subjective aging views. Interestingly, diseases do not have to be life-threatening to be associated with older SA or negative ATOA.
Keywords: Attitudes toward own aging, Depression, Subjective age
The concept of subjective views on aging (VoA) has received considerable attention in gerontological research. In essence, and as opposed to chronological age, VoA aim to capture an intuitive evaluation of a person’s aging process and can be expected to gain significantly in importance as people age (Diehl et al., 2014). In particular, studies on VoA have shown them to be a solid predictor of key outcomes, such as reduced functional status (Sargent-Cox et al., 2012a), frailty (Gale & Cooper, 2018), cognitive impairment (Siebert et al., 2018), depression (Keyes & Westerhof, 2012), active health behavior (Wienert et al., 2015), and longevity (Westerhof et al., 2014), even when controlling for a broad range of confounders. Indeed, VoA have been discussed as having the potential to serve as a biopsychosocial marker of aging, given their relation with bioindicators such as C-reactive protein and cystatin-C (Diehl et al., in press; Stephan et al., 2018).
Numerous approaches for the operationalization of VoA with varying degrees of complexity exist (Diehl et al., 2014). Due to its simplicity, the widespread approach is to use a single-item question inquiring about how old a person feels, from which a proportional-difference score can be calculated (Rubin & Berntsen, 2006). A large body of research has shown that the difference score between a person’s subjective age (SA) and actual chronological age is a reliable predictor of a variety of outcomes, including subjective well-being (Westerhof & Barrett, 2005), physical, mental, self-rated health (Spuling et al., 2013), and mortality (Kotter-Grühn et al., 2009; see also Westerhof et al., 2014). Another well-established measure is the unidimensional attitude toward own aging (ATOA) scale, where participants rate how much stereotypically presented age-related changes apply to themselves (Lawton, 1975; Miche et al., 2014). As reported in quite a body of previous work, more negative ATOA is related longitudinally with lowered health, physical functioning, and longevity (e.g., Kotter-Grühn et al., 2009; Levy et al., 2002; Sargent-Cox et al., 2012b; see also Westerhof et al., 2014).
Both measures are established indicators of VoA, and can be placed within the wider theoretical framework of life-span developmental psychology characterizing aging as a changing pattern of gains and losses (Baltes, 1987). However, they cover different aspects and follow a different logic. SA represents a general subjective interpretation of one’s age along the young–old continuum without referring to any information regarding actual aging experiences or valences attributed to such experiences (Diehl et al., 2014; Montepare, 2009). Somewhat differently, ATOA strives to capture evaluative components of behavior toward older adults as a group as well as toward the process of aging as a personal experience and thus offers a combination of societal and individual attitudes (Hess, 2006). Therefore, using both measures (as we will do in this study) is complementary and broadens the VoA picture.
Antecedents of Subjective VoA: The Role of Health
While there is substantial work on the consequences of positive and negative VoA on health outcomes, research is less clear when it comes to health variables as antecedents of VoA. Respective insights are crucial to counteract a vicious circle: lowered health might create more negative VoA and in turn, negative VoA might then, for example, through the pathway of reduced health behavior, lead to even worse health. Research points to the existence of such bidirectionality at the general health as well as mental health level, although the direction from VoA to health consequences seems stronger than from health problems to VoA consequences (Dutt et al., 2018; Spuling et al., 2013; Wurm et al., 2008).
Infurna et al. (2010), Miche et al. (2014), and Stephan et al. (2018) report that various subjective and objective health markers indicating lowered health are linked with older SA and negative ATOA. Also waist circumference and grip strength, both global markers of health in older age (Wagner et al., 2016), have been tied to VoA (Stephan et al., 2015). Concluding, while general health effects on both SA and ATOA are established in the literature, the picture has remained incomplete due to at least three primary reasons. First, the effects of specific morbidities on VoA deserve more attention, given that they may differentially affect VoA. For example, diseases such as arthritis with direct negative consequences (motor behavior, pain occurrences) may affect VoA more strongly as compared to “silent” diseases such as hypertension or elevated cholesterol. Indeed, Stephan et al. (2015) reported that “perceptible” biomarkers (e.g., lower peak expiratory flow, grip strength) were associated with higher SA, whereas imperceptible ones (i.e., blood pressure, telomere length) were not. Similarly, everyday body problems appeared to have negative effects on SA (Barrett & Gumber, 2020).
Second, mental health problems such as depression may generally impair a person’s day-to-day functioning in multiple facets (Kennedy et al., 2007; Stuck et al., 1999) and therefore likely provide a similar or even stronger threat to positive aging expectations and VoA when compared to physical disease. Based on the cognitive theory of depression by Beck (1979), depression is characterized by automatic negative thoughts and evaluations regarding oneself, the future, and the environment. Cognitive biases such as overgeneralizations of the meaning of negative events involving age-related losses would therefore be expected to markedly manifest themselves in a threat to positive aging expectations.
Also note in this context that physical disease seems to underlie the health paradox, meaning that objectively diagnosed illnesses are subjectively not necessarily perceived as a decreased health situation (Staats et al., 1993; Wettstein et al., 2016).
Third, cognitive functioning is an important health issue in old age, but respective empirical work has remained inconsistent. Hughes and Lachman (2018) reported a meaningful link between better memory performance and younger SA while other studies did not find consistent support for a cognitive functioning and VoA interrelation (Gabrian & Wahl, 2017; Jaconelli et al., 2017).
We are not aware of any study that has systematically investigated, beyond general health associations at the physical, mental, and cognitive level, the potentially differential association of specific diseases with VoA.
Research Aims and Hypotheses
Drawing from data of an established population-based epidemiological study that targeted a range of health, motor functioning, and disease markers across time (ActiFE Ulm study, Germany; see Denkinger et al., 2010), our first goal has been to estimate the association between generally framed health variables (representing physical, mental, and cognitive health/impairment) and VoA (SA, ATOA). We expect that better health generally goes along with feeling younger and more positive ATOA. However, given the potentially broad-range role of affective health on daily quality of life as contrasted with the frequently more circumscribed and limited effects of cognitive health, we expect that lowered affective health will reveal a closer association with more negative ATOA and older SA as compared to cognitive health, and will play, together with physical health, the relatively most important role to explain variance in SA and ATOA. Based on theoretical considerations about the sensitivity of VoA regarding experienced losses (Baltes, 1987), we generally expect that change-scores indicating stronger impairments of health-related variables will be negatively related to VoA. This is based on the logic that decline in physical, mental, or cognitive health over a considerable time span may indicate a necessary shift of resources away from growth and toward maintenance or regulation of the loss of existing capabilities (Baltes, 1997; Smith, 2003). This would in turn negatively affect the desirable balance of gains and losses, making it therefore difficult to retain positive ATOA or to identify with a younger age, where gains are typically expected to outnumber losses (Diehl et al., 2014).
Our second goal has been to estimate the potentially differential association between a range of diseases in old age and VoA. Here, we expect that within the range of illnesses, those affecting daily quality of life (e.g., through impaired motor functioning, enduring pain, undermining of a positively toned daily mood) will be associated more strongly to VoA as compared to those diseases without such directly perceptible consequences. Further, following the reasoning of our first hypothesis, we expect that affective diseases such as depression, which greatly affect quality of life, will show some of the strongest negative associations with VoA.
Third, we expect similar effects in both VoA indicators considered, that is, SA and ATOA as outcome variables. However, given the closer to everyday life approach inherent in the ATOA scale representing different areas of well-being and quality of life (e.g., feeling useful, things are getting worse), we expect that health indicators may be more strongly linked with the ATOA indicator, thus explaining more variance as compared to SA.
Method
Study Design and Setting
This study is based on data from the epidemiological ActiFE Ulm study, for which a detailed study protocol is available (Denkinger et al., 2010). The ActiFE Ulm study was designed as a prospective, observational cohort study of community-dwelling older adults, with the general aim of estimating the longitudinal link of accelerometer-based physical activity with numerous health-related outcomes as well as providing data on health and disease status of older adults at large assessed by trained study staff. Baseline data (2009–2010) were collected from a representative population-based random sample (greater Ulm, Neu-Ulm, and Alb-Donau Kreis areas, located in the South of Germany, initial response 20%). The follow-up for the data used in this article was conducted after 7.7 years (median, 2017–2018). Data on VoA were collected during this follow-up using a questionnaire. The ethics committee of Ulm University approved of the study’s respective protocol in 2009 (Application No. 318/08).
Participants
Inclusion criteria of the ActiFE Ulm study at baseline were as follows: age (65–90 years), informed consent, the ability to, with or without assisting devices, walk through one’s own room. Potential participants with severe visual, auditory, or cognitive impairment were excluded. Those with serious difficulties understanding the German language were excluded from the interviews and therefore did not provide questionnaire data.
Overall N = 526 older adults participated in this follow-up (7.7 years, after baseline) of the ActiFE Ulm study, representing about 35% of the study population at baseline (n = 1,506). There were slightly more male (57% or 300/526) than female (43% or 226/526) participants (biological sex). Concerning participants’ education, about half of the sample (50% or 259/526) had more than 9 years of school education. Nine years of school education or more are seen as the milestone for completing the basic education in the German system. With the recruitment area being part of South Germany the participants were from a region that can be considered economically prosperous.
Assessment of Subjective VoA
The VoA used in this article were solely assessed at follow-up. ATOA was measured using the subscale of the Philadelphia Geriatric Center Morale Scale (Lawton, 1975). This scale consists of five items addressing the subjective evaluation of one’s aging process, for example, “I have as much pep as I had last year,” “As you get older, you are less useful,” and “I am as happy now as when I was younger,” dichotomously rated as “Yes” or “No” and condensed into a summary score. This ATOA scale is an established instrument and was reported to have acceptable internal consistency in previous studies (Kornadt et al., 2019; Siebert et al., 2016). In our sample internal consistency was, considering the small number of items, acceptable (Cronbach’s α = 0.66).
SA was measured by participants’ response on the single-item scale (“How old have you felt in the past few days?”). Participants were asked to provide their SA in years in an open format. As suggested by Rubin and Berntsen (2006), a proportional score was calculated by subtracting the chronological age from the felt age and by dividing this by the chronological age. The resulting score reflects how much older or younger a person feels relative to their chronological age. A proportional score of −0.20, for example, indicates that the person feels approximately 20% younger than their actual age (Diehl et al., in press; Rubin & Berntsen, 2006).
Assessment of Predictor Variables
Cognitive health was assessed with the Mini-Mental State Examination (MMSE; Folstein et al., 1975). The scale ranges from 0 to 30 points and is performance-based. Higher values indicate better cognitive health.
General affective health problems were measured with the German version of the Hospital Anxiety and Depression Scale (HADS-D; Petermann, 2015). The HADS consists of 14 items which are rated on a 4-level Likert scale referring to a participant’s subjective self-evaluation with higher scores indicating stronger symptoms. Seven items address anxiety (e.g., “Worrying thoughts go through my mind”) while the other seven focus on depression (e.g., “I feel slowed down”). A review that examined the properties of the HADS found the Cronbach’s α for the subscales to be on average about 0.82 (anxiety) and 0.83 (depression) (Bjelland et al., 2002).
Participants’ activities of daily living (ADL) were assessed with an established questionnaire (for details, see Denkinger et al., 2010; Katz et al., 1970). Items were answered on a 4-point Likert scale (“no difficulties performing the task” to “major difficulties performing the task”). Participants therefore give subjective evaluations describing their performance. Lower scores are to be interpreted as better functioning.
Beyond these general health indicators, the ActiFE Ulm study also provides a range of disease diagnoses at baseline as part of its medical assessment, which we used to examine their association with VoA. Disease diagnoses were assessed using structured interview questions and strived to rely on objective health information (e.g., “Has your physician ever told you that you have had a heart attack?”). Accordingly, responses were dummy-coded (yes/no) group variables. Hereafter, these variables are referred to as “disease groups.” As these disease groups originally included a combined category for depression or anxiety, we supplemented them by creating separate categories for depression and anxiety based on the recommended cutoffs (score >8) of the respective HADS subscales.
Further, the total number of each participants’ diseases at baseline was calculated to function as an overall measure of comorbidity.
Statistical Methods
For the quantitative description of the cohort means, standard deviations, medians, interquartile ranges, and ranges were calculated for continuous variables. For categorical variables absolute and relative frequencies were calculated. Further, for a descriptive display of associations between the central study variables, zero-order correlations were calculated using Pearson’s r. The sample was also grouped in participants with relatively positive versus negative ATOA and relatively young versus old SA based on median splits of the respective variables. Standardized differences for these groups were calculated across the central study variables.
The main analyses were based on linear regression models: Hierarchical multiple linear regression was used to model the predictor–outcome relationship between general risk factors and VoA. The same pattern of block-wise predictor-inclusion was used when modeling SA and ATOA: (a) demographic factors (age and sex), (b) functional health (ADL) and overall comorbidity (number of diagnoses), (c) affective health (HADS), (d) cognitive health (MMSE). For the scales change-scores (follow-up minus baseline) were included into the regression models alongside their baseline values.
A series of simple and multiple regression models were used to examine the association between specific disease groups and VoA. For each of the disease groups with at least n = 30 cases in the sample, the crude and adjusted effects on VoA were calculated. Adjusted effects controlled for the same set of covariates (chronological age, sex, comorbidity as number of additional reported diagnoses, baseline scores of MMSE, HADS, and ADL) except for the disease group depression/anxiety categories where HADS baseline scores were not included due to the direct theoretical relationship and multicollinearity between the variables. From these regression models estimates for the effect sizes on the original scale (unstandardized betas) were displayed in a forest-plot figure. As a standardized effect size Cohen’s d was calculated for the adjusted effects. In these analyses the adjusted effects did not include longitudinal ADL, MMSE, and HADS change-scores to avoid controlling the disease effect for, for example, potentially mediating variables.
Analyses involving SA were conducted based on a reduced sample that excluded SA outliers, defined as further than ±2 SDs from the mean. This definition of outliers was chosen after exploratory analyses, as unrestricted items such as SA are more prone to produce extreme results. Consequently, in analyses involving SA, the lower and the upper 2.5% of cases had to be excluded.
For linear modeling, missing values in the data set were handled by a full information maximum likelihood approach (Allison, 2012). MMSE change-scores had the most missing values with 16%. Other variables did not exceed 10% missing values (Supplementary Table 1). Throughout the analyses 95% confidence intervals (CIs) were used to quantify uncertainty. Cohen’s d effect sizes are interpreted based on the reference frame provided within this and similar studies, and further, with respect to existing classifications (Cohen, 2013). Statistical analyses were conducted using SAS version 9.4, and R version 3.5.1.
Results
Descriptive Data
Descriptive statistics of the total sample (N = 526) are reported in Table 1 (SA outlier excluded sample: Supplementary Table 2). For reference, characteristics of the ActiFE Ulm participants that were alive during the follow-up period but did not participate are given in Supplementary Table 3. Nonparticipants appeared, on average, more vulnerable than completers with, for example, older age, lower MMSE, and more comorbidities. Zero-order correlations of the study variables, and their associated standardized differences between the VoA groups when a median split was applied, are reported in Supplementary Tables 4 and 5, respectively. As can be taken from Supplementary Table 4, a calculated Pearson correlation between ATOA and SA found only a moderate effect (r = −0.30; 95% CI [−0.37, −0.21]). Most of the participants reported to feel younger (76% or 384/502), some reported to feel exactly as old (19% or 93/502), and only a minority reported to feel older (5% or 25/502) than their chronological age. After exclusion of outliers, participants reported feeling about 7% younger (95% CI [−0.08, −0.06]) than their chronological age. Cross-sectional descriptive data on VoA across different ages are displayed in Supplementary Figure 1. Relative to the median, there were fewer participants reporting negative than positive ATOA (40% or 208/526) and similarly, fewer participants that reported a relatively older than younger SA (24% or 118/502). Negative ATOA or older SA appeared to identify a more vulnerable subpopulation with, for example, higher age, stronger reported symptoms of affective health problems, lower cognitive functioning, and worse reported performance regarding ADL (see Supplementary Table 5).
Table 1.
Descriptive Statistics for the Total Sample (N = 526) Used in the Analyses
| Variables | n (%) | Mean (SD) | Median | Interquartile range (Q1, Q3) | Range (min, max) |
|---|---|---|---|---|---|
| Age | 72.72 (5.1) | 71.65 | (68.70, 75.20) | (65.30, 88.00) | |
| Sex male | 300 (57) | ||||
| Sex female | 226 (43) | ||||
| Education ≤ 9 years | 261 (50) | ||||
| Education > 9 years | 259 (50) | ||||
| BMI | 27.25 (3.6) | 26.90 | (24.80; 29.50) | (17.30; 42.80) | |
| MMSE | 28.63 (1.5) | 29 | (28, 30) | (23, 30) | |
| ΔMMSE | −0.48 (2.0) | 0 | (−1, 2) | (−5, 7) | |
| HADS | 7.21 (4.7) | 7 | (4, 10) | (0, 33) | |
| ΔHADS | 1.04 (4.3) | 1 | (−2, 4) | (−17, 17) | |
| ADL | 0.51 (1.4) | 0 | (0, 0) | (0, 14) | |
| ΔADL | 1.05 (2.5) | 0 | (0, 1) | (−8, 18) | |
| Diagnoses count | 3.51 (2.0) | 3 | (2, 4) | (0, 10) | |
| ATOA | 2.90 (1.6) | 3 | (2, 4) | (0, 5) | |
| Subj. age | −0.08 (0.1) | −0.06 | (−0.12, −0.02) | (−0.58, 0.06) |
Notes: Δ denotes change-scores: follow-up − baseline. ADL = activities of daily living; ATOA = attitudes toward own aging; BMI = body mass index; Diagnoses count = number of self-reported diagnoses; HADS = total score of the Hospital Anxiety and Depression Scale; MMSE = Mini-Mental State Examination; SD = standard deviation; Subj. age = subjective age as proportional score.
Associations Between Global Health Indicators and VoA
To examine the association between general health indicators and ATOA, hierarchical multiple regression was conducted and the results are reported in Table 2. While in the first model with sex and chronological age as predictors of ATOA no significant sex association was found, ATOA was negatively associated with chronological age. The effect of chronological age was reduced when, in a second step, functional status and overall comorbidity were added to the model. Both a reduced functional status as measured by ADL (baseline and change-scores) and a higher overall comorbidity were negatively related to ATOA. Longitudinal change in ADL appeared to be the strongest variable within this model, with stronger decline in ADL indicating more negative ATOA. When, in a third model, participants’ affective health as HADS scores were added, these variables (HADS baseline and change from baseline) showed the strongest relationship with ATOA, with stronger affective health problems at baseline as well as longitudinally increasing affective health problems being associated to more negative ATOA. In a fourth and last model, cognitive health as MMSE scores was added. MMSE scores did not seem to notably improve the model beyond the already existent variables, and while the general direction of the effect pointed toward more negative ATOA with increasing cognitive deficiencies, this effect was quite limited in strength.
Table 2.
Summary of Hierarchical Regression With ATOA as Outcome (N = 526)
| Model 1: demographics | Model 2: + functional status and overall comorbidity | Model 3: + affective health | Model 4: + cognitive health | |||||
|---|---|---|---|---|---|---|---|---|
| Predictor | B | β [95% CI: β] | B | β [95% CI: β] | B | β [95% CI: β] | B | β [95% CI: β] |
| Age | −0.08* | −0.27 [−0.35, −0.19] | −0.05* | −0.15 [−0.24, −0.06] | −0.04* | −0.14 [−0.22, −0.06] | −0.04* | −0.14 [−0.22, −0.06] |
| Sex | −0.18 | −0.06 [−0.14, 0.02] | −0.04 | −0.01 [−0.09, 0.07] | −0.04 | −0.01 [−0.09, 0.06] | −0.03 | −0.01 [−0.09, 0.06] |
| ADL | −0.14* | −0.13 [−0.21, −0.04] | −0.10* | −0.10 [−0.17, −0.02] | −0.10* | −0.10 [−0.17, −0.02] | ||
| ΔADL | −0.13* | −0.21 [−0.30, −0.13] | −0.09* | −0.14 [−0.22, −0.06] | −0.09* | −0.15 [−0.22, −0.06] | ||
| Diagnoses count | −0.10* | −0.12 [−0.22, −0.04] | −0.02 | −0.03 [−0.11, 0.05] | −0.02 | −0.03 [−0.12, 0.05] | ||
| HADS | −0.14* | −0.43 [−0.51, −0.35] | −0.14* | −0.43 [−0.51, −0.34] | ||||
| ΔHADS | −0.11* | −0.31 [−0.39, −0.23] | −0.11* | −0.31 [−0.39, −0.23] | ||||
| MMSE | 0.03 | 0.03 [−0.06, 0.12] | ||||||
| ΔMMSE | −0.04 | −0.05 [−0.14, 0.05] | ||||||
| R 2 | 0.08 | 0.15 | 0.30 | 0.31 | ||||
| ΔR2 | 0.08* | 0.15* | 0.00 | |||||
Notes: Δ denotes change-scores: follow-up − baseline. ADL = activities of daily living; CI = confidence interval; Diagnoses count = number of self-reported diagnoses; HADS = total score of the Hospital Anxiety and Depression Scale; MMSE = Mini-Mental State Examination. The outcome is attitudes toward own aging (ATOA).
*p < .05.
When the same hierarchical multiple regression approach was used to model SA as an outcome (reported in Table 3), a comparable pattern emerged in which affective health as HADS scores defined the dominant factor. Similar to the ATOA models, stronger affective health problems at baseline as well as longitudinally increasing affective health problems were associated to older SA at follow-up. However, the overall amount of explained variance differed markedly in each step, with the full ATOA model explaining roughly 31% of variance, while the full SA model explained only about 9% of variance.
Table 3.
Summary of Hierarchical Regression With Subjective Age (Proportional Score) as Outcome, Subjective Age Outlier Excluded Data Set (n = 502)
| Model 1: demographics | Model 2: + functional status and overall comorbidity | Model 3: + affective health | Model 4: + cognitive health | |||||
|---|---|---|---|---|---|---|---|---|
| Predictor | B | β [95% CI: β] | B | β [95% CI: β] | B | β [95% CI: β] | B | β [95% CI: β] |
| Age | 0.001* | 0.10 [0.01, 0.19] | 0.000 | 0.03 [−0.06, 0.13] | 0.000 | 0.02 [−0.07, 0.12] | 0.000 | 0.02 [−0.08, 0.11] |
| Sex | 0.002 | 0.02 [−0.07, 0.10] | −0.001 | −0.01 [−0.10, 0.08] | −0.001 | −0.01 [−0.10, 0.08] | −0.001 | −0.01 [−0.09, 0.08] |
| ADL | 0.003 | 0.07 [−0.02, 0.17] | 0.003 | 0.07 [−0.02, 0.16] | 0.003 | 0.07 [−0.02, 0.16] | ||
| ΔADL | 0.003* | 0.12 [0.02, 0.21] | 0.002 | 0.08 [−0.01, 0.18] | 0.003* | 0.10 [0.00, 0.19] | ||
| Diagnoses count | 0.002 | 0.07 [−0.02, 0.17] | 0.001 | 0.03 [−0.07, 0.12] | 0.001 | 0.03 [−0.07, 0.13] | ||
| HADS | 0.003* | 0.19 [0.09, 0.29] | 0.002* | 0.18 [0.08, 0.28] | ||||
| ΔHADS | 0.003* | 0.20 [0.11, 0.30] | 0.003* | 0.20 [0.10, 0.30] | ||||
| MMSE | −0.003 | −0.07 [−0.18, 0.04] | ||||||
| ΔMMSE | 0.001 | 0.03 [−0.09, 0.14] | ||||||
| R 2 | 0.01 | 0.04 | 0.08 | 0.09 | ||||
| ΔR2 | 0.03 | 0.04* | 0.01 | |||||
Notes: Unstandardized betas (B) are presented with an extra decimal to make the effects displayable on their original scale. Δ denotes change-scores: follow-up − baseline. ADL = activities of daily living; CI = confidence interval; Diagnoses count = number of self-reported diagnoses; HADS = total score of the Hospital Anxiety and Depression Scale; MMSE = Mini-Mental State Examination. The outcome is subjective age as proportional score.
*p < .05.
Also, as indicated by the standardized coefficients in both modeling approaches, longitudinal change-scores of the respective health-related variables explained similar proportions of overall variance within a model as their associated baseline scores, and thus appeared to be of comparable importance.
Associations Between Specific Diseases and VoA
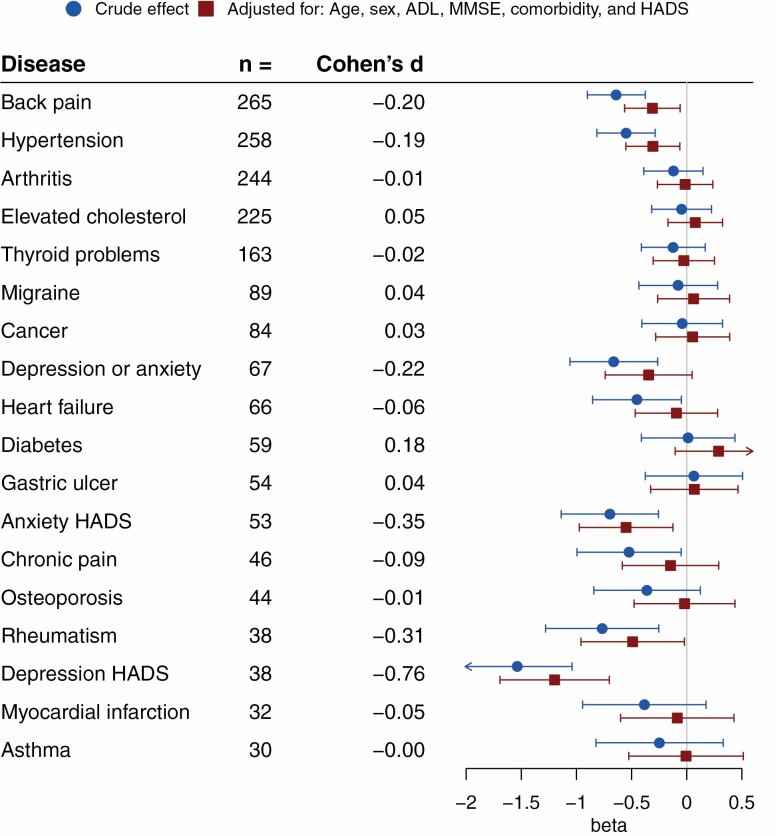
Finally, we used the collected data on participant’s self-reported diseases to estimate the association of specific disease groups with VoA. For this a crude estimate using only the dichotomous variable for the specific disease, and controlled estimates controlling for chronological age, sex, number of other comorbidities, MMSE, ADL, and HADS were calculated (exception: depression or anxiety categories as predictors were not HADS controlled). Results are summarized in Figure 1. While none of the diseases showed a discernible positive association with ATOA, with uncontrolled estimates negative association to ATOA existed for back pain, hypertension, all depression/anxiety categories (reported diagnosis of depression or anxiety, depression as rated by the HADS depression-subscale, anxiety as rated by the HADS anxiety-subscale), heart failure, chronic pain, and rheumatism. With the controlled approach, effects remained solid for back pain, hypertension, HADS depression/anxiety categories, and rheumatism. Therefore, participants within these categories reported more negative ATOA than their respective counterparts. Standardized effect sizes were mostly small, though depression, as classified by the HADS subscale, showing the most pronounced effect size equates to a medium to large effect by Cohen’s classification. This averages to roughly 1.2 points less on the ATOA scale in the depressed (HADS) versus the nondepressed (HADS) group of participants.
Figure 1.
Disease groups and attitudes toward own aging (ATOA). Crude and adjusted estimates of the unstandardized beta from linear regression with 95% confidence intervals for the association of disease groups with ATOA. Depression/anxiety categories were not HADS controlled. Number of cases for the respective disease groups and point estimates of Cohen’s d (rounded to two decimals) for the adjusted effect are also presented. Lower betas or Cohen’s d indicate more negative ATOA (N = 526). ADL = activities of daily living; HADS = Hospital Anxiety and Depression Scale; MMSE = Mini-Mental State Examination.
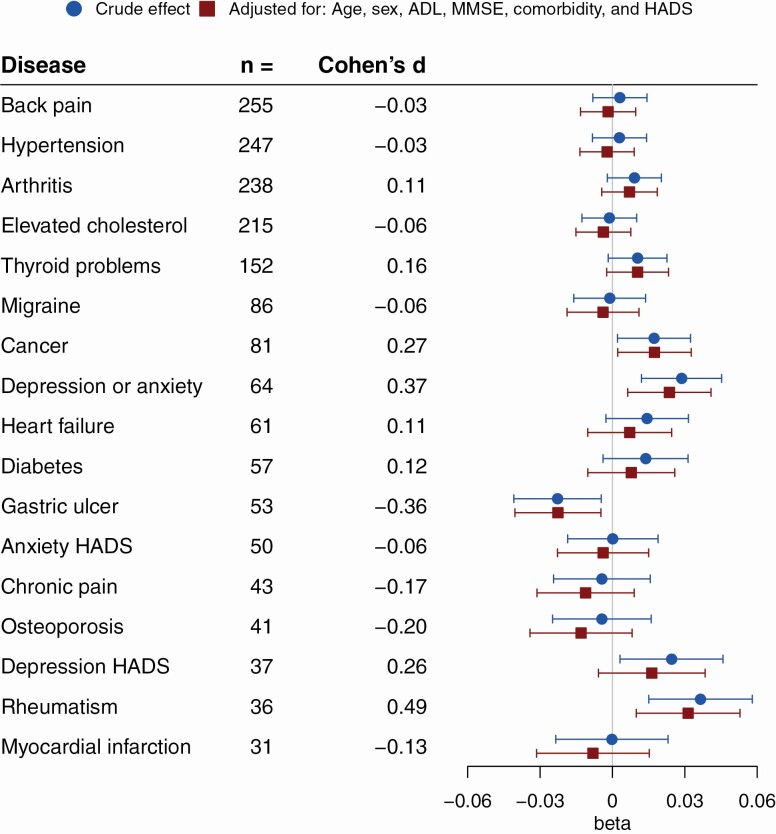
Comparable to ATOA, when disease effects were examined with SA as outcome (see Figure 2), there was an overall tendency toward reporting older SA for most disease groups. Gastric ulcer patients were an exception to this, reporting younger SA even when confounders were controlled for. As was the case with ATOA, when controlled for confounders, participants with rheumatism reported an older SA. A self-reported diagnosis of either depression or anxiety was also associated to older SA. While the HADS depression category showed a tendency toward an association with an older SA, this effect did not remain solid when controlled for confounders. Participants with cancer reported an older SA, but not a more negative ATOA. In the modeling of SA rheumatism showed the strongest effect size, amounting to participants with rheumatism feeling roughly 3% older than their actual age or a moderate effect size following Cohen’s classification of standardized effect sizes.
Figure 2.
Disease groups and subjective age (proportional score). Crude and adjusted estimates of the unstandardized beta from linear regression with 95% confidence intervals for the association of disease groups with subjective age (proportional score). Depression/anxiety categories were not HADS controlled. Number of cases for the respective disease groups and point estimates of Cohen’s d (rounded to two decimals) for the adjusted effect are also presented. Higher betas or Cohen’s d indicate a higher subjective age, that is, participants feeling older (n = 502). ADL = activities of daily living; HADS = Hospital Anxiety and Depression Scale; MMSE = Mini-Mental State Examination.
Discussion
As was to be expected in light of the existing literature (Westerhof et al., 2003), this cohort of community-dwelling older adults reported mostly positive VoA. Our result that older participants felt on average about 7% younger than their chronological age is considerably less than what Rubin and Berntsen (2006) found, but similar to what Kleinspehn-Ammerlahn et al. (2008) reported. To the best of our knowledge, this is the first study that, besides general health-related factors, examined the effects of a range of diseases on VoA.
Major findings can be summarized as follows: First, considering general health factors as longitudinal correlates of VoA, a magnitude of effects can be explained by mental health issues of the affective type, such as depressive symptoms. Second, VoA appear to be differentially associated with specific disease groups, for instance, hypertension, rheumatism, and cancer. In addition, we found that morbidities do not need to be life-threatening to be associated with VoA.
Regarding general health factors as longitudinal correlates of VoA, self-reported symptoms of affective health emerged as a dominant factor, with stronger reported symptoms of affective health problems at baseline and longitudinally increasing affective health problems being associated to more negative ATOA as well as to older SA. This result supports numerous findings tying mental health and specifically depression to VoA (Bergland et al., 2014; Dutt & Wahl, 2017; Keyes & Westerhof, 2012). Our results also complement findings based on a personality oriented approach: Kornadt et al. (2019) reported that neuroticistic traits, a major risk factor for affective problems (Zinbarg et al., 2016), predicted negative ATOA longitudinally. Importantly, the authors found no evidence of this being a bidirectional effect, therefore hinting at a possibly causal relationship. Further, our results substantiate findings from a different culture, as comparable results were shown in a sample of community-dwelling older adults in Korea (Hwang & Hong, 2019). As in previous research, we could also not support a strong role of cognitive health in VoA when other factors were accounted for (Gabrian & Wahl, 2017; Jaconelli et al., 2017). However, in this context the limitations of the MMSE as a measure for cognitive health in community-dwellers (i.e., ceiling effects as also observed in this study) should be taken into account, and may be an opportunity for future research. The dominance of the more subjective health indicators as opposed to the objective- or performance-based parameters can be seen as partial support for the health paradox in older age (Wettstein et al., 2016), whereas the general finding that decline in some health variables over a longer period of time was associated with more negative ATOA and older SA supports conceptual reasoning that connects VoA to a gain/loss dynamic of life-span development (Diehl et al., 2014). Our finding that models with ATOA as an outcome explained considerably more variance than those with SA is likewise in accordance with theoretical considerations and empirical observations by Diehl et al. (2014), stating that VoA constitute complex constructs which benefit from more sophisticated approaches than mere 1-item measures.
While numerous studies have investigated selected disease groups such as, for example, cancer (Martin et al., 2019) or cardiovascular events on VoA (Wurm et al., 2020), this is the first study that, in a population-based approach, examined the effects in parallel based on a range of diseases that were based on a structured interview by trained study personnel. From a theoretical point of view, diseases should be expected to mostly influence VoA in a negative way (Stephan et al., 2018), which as a general tendency with only one exception across a wide range of diseases, was clearly present in our results. The single counterintuitive result that gastric ulcer was associated to younger SA could be explained by the fact that from a medical perspective efficient treatments are available, and that longitudinal effects over more than 7 years are improbable after recovery (Kuna et al., 2019). It should further be noted that according to given general conventions about standardized effect sizes the effects found in our analyses were within the boundaries of moderate to small effects (Cohen, 2013). While there were parallels as to which disease groups were associated with VoA across the two operationalizations, there were also discrepancies. Beyond different depression categories, rheumatism predicted negative VoA in both constructs. Clinical symptoms of rheumatic disease, such as pain, stiffness, swelling and an associated progressing course affect a variety of facets of quality of life (Walker & Littlejohn, 2007), and may consequently, due to their strong salience in everyday life, lead to pessimistic evaluations of the future aging process. A history of back pain or hypertension was tied to ATOA. As qualitative research on back pain suggests, the former finding could be explained by people perceiving restrictive back pain as an indicator of inevitable age-related problems (Makris et al., 2015). The latter finding on hypertension, however, appears surprising considering that no solid effect for myocardial infarction could be shown, and that cardiovascular events of larger magnitude were previously shown to be associated with VoA in different studies (Diehr et al., 2001; Wurm et al., 2020). In our study, cancer was tied to SA but no solid association to ATOA as reported in recent findings (Martin et al., 2019) could be shown. In general, disease groups examined in this study are heterogeneous, and consequently produced heterogeneous results: Although mental illness generally showed a stronger association to both SA and ATOA, some physical illnesses such as rheumatism stand out and achieved similarly strong effects as affective disorders. A single overarching theoretical framework to explain differential findings cannot be provided and would be in contradiction of the exploratory nature of these analyses.
Limitations
Though this study is based on high-quality data, several limitations must be considered. First, although we rely on a longitudinal design, we refrain from causal inferences. The mere examination of predictor–outcome relationships between diseases and VoA did not permit us to examine the likely entangled pathways through which the effects might appear. The possibility that these might differ across the heterogeneous disease groups must be considered. Further, the follow-up period of more than 7 years targets long-term changes and might introduce potential bias through selective survival and participation.
It should also be noted that the scale used to assess ATOA showed a limited reliability as measured by Cronbach’s α.
Testing many disease groups introduces a problem of multiplicity. Because, however, the aim of these analyses was not of confirmatory nature, but more to explore several disease groups to produce a selection of potential high-risk groups, this is a common limitation of studies following similar research targets.
Given the imbalances of the diseased versus nondiseased groups in the sample which are introduced by natural differences in the prevalence, loss of power in the smaller disease groups has to be considered. The deliberate decision to analyze only disease groups with at least 30 cases was made to restrict further problems that might arise from these imbalances.
Finally, all known biases and problems of (interviewer-led) self-report measures must be considered. While the data on history of diseases were collected by trained professionals, it was not based on a physical examination by a physician itself or validated by other means.
Conclusion
Affective mental health factors such as depression appear to be a major component associated to more negative ATOA as well as older SA and were found in the present study to be consistently stronger as compared to physical and cognitive health indicators. Further, we were able to show that age-related diseases are differentially associated with both SA and ATOA. Therefore, considering only general health indicators may be a too limited view. As we also found, diseases do not necessarily have to be life-threatening to be associated with negative ATOA or older SA. Instead, their phenomenology of putting strain on to day-to-day life in terms of undermining well-being as well as direct behavioral restrictions might be the major factor. Although further data and confirmatory approaches are needed, our findings suggest that VoA may be an important component that so far has only found limited attention in health-related quality of life models and assessments targeting older adults.
Supplementary Material
Acknowledgments
The data that support the findings of this study (not preregistered) are from the ActiFE Ulm study. Restrictions apply to the availability of these data, which were used under license. We thank the entire team of the ActiFE Ulm study for their work involving the data collection.
Funding
A. Schönstein is a member of the Graduate Program “People with Dementia in General Hospitals,” located at the Network Aging Research (NAR), Heidelberg University, Germany, and received a Doctoral Fellowship funded by the Robert Bosch Foundation, Stuttgart, Germany. This study was also partly funded by the German Research Foundation (DFG, grant agreements RO2606/14-1 and DE2674/1-1).
Conflict of Interest
None declared.
Author Contributions
H.-W. Wahl and A. Bahrmann contributed equally and share the last authorship.
References
- Allison, P. D. (2012). Handling missing data by maximum likelihood. Paper presented at the SAS global forum.
- Baltes, P. B. (1987). Theoretical propositions of life-span developmental psychology: On the dynamics between growth and decline. Developmental Psychology, 23(5), 611. doi: 10.1037/0012-1649.23.5.611 [DOI] [Google Scholar]
- Baltes, P. B. (1997). On the incomplete architecture of human ontogeny: Selection, optimization, and compensation as foundation of developmental theory. American Psychologist, 52(4), 366. doi: 10.1037/0003-066X.52.4.366 [DOI] [PubMed] [Google Scholar]
- Barrett, A. E., & Gumber, C. (2020). Feeling old, body and soul: The effect of aging body reminders on age identity. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(3), 625–629. doi: 10.1093/geronb/gby085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. (1979). Cognitive therapy and the emotional disorders. Penguin. [Google Scholar]
- Bergland, A., Nicolaisen, M., & Thorsen, K. (2014). Predictors of subjective age in people aged 40–79 years: A five-year follow-up study. The impact of mastery, mental and physical health. Aging & Mental Health, 18(5), 653–661. doi: 10.1080/13607863.2013.869545 [DOI] [PubMed] [Google Scholar]
- Bjelland, I., Dahl, A. A., Haug, T. T., & Neckelmann, D. (2002). The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research, 52(2), 69–77. doi: 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- Cohen, J. (2013). Statistical power analysis for the behavioral sciences. Academic Press. [Google Scholar]
- Denkinger, M. D., Franke, S., Rapp, K., Weinmayr, G., Duran-Tauleria, E., Nikolaus, T., Peter, R., & ActiFE Ulm Study Group. (2010). Accelerometer-based physical activity in a large observational cohort—Study protocol and design of the activity and function of the elderly in Ulm (ActiFE Ulm) study. BMC Geriatrics, 10, 50. doi: 10.1186/1471-2318-10-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl, M., Brothers, A. F., & Wahl, H.-W. (In press). Self-perceptions and awareness of aging: Past, present, and future. In Schaie K. W. & Willis S. L. (Eds.), Handbook of psychology of aging (9th ed.). Academic Press. [Google Scholar]
- Diehl, M., Wahl, H. W., Barrett, A. E., Brothers, A. F., Miche, M., Montepare, J. M., Westerhof, G. J., & Wurm, S. (2014). Awareness of aging: Theoretical considerations on an emerging concept. Developmental Review, 34(2), 93–113. doi: 10.1016/j.dr.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehr, P., Williamson, J., Patrick, D. L., Bild, D. E., & Burke, G. L. (2001). Patterns of self-rated health in older adults before and after sentinel health events. Journal of the American Geriatrics Society, 49(1), 36–44. doi: 10.1046/j.1532-5415.2001.49007.x [DOI] [PubMed] [Google Scholar]
- Dutt, A. J., Gabrian, M., & Wahl, H. W. (2018). Awareness of age-related change and depressive symptoms in middle and late adulthood: Longitudinal associations and the role of self-regulation and calendar age. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(6), 944–953. doi: 10.1093/geronb/gbw095 [DOI] [PubMed] [Google Scholar]
- Dutt, A. J., & Wahl, H. W. (2017). Feeling sad makes us feel older: Effects of a sad-mood induction on subjective age. Psychology and Aging, 32(5), 412–418. doi: 10.1037/pag0000179 [DOI] [PubMed] [Google Scholar]
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gabrian, M., & Wahl, H. W. (2017). Being slower, feeling older? Experimentally induced cognitive aging experiences have limited impact on subjective age. European Journal of Ageing, 14(2), 179–188. doi: 10.1007/s10433-016-0400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, C. R., & Cooper, C. (2018). Attitudes to ageing and change in frailty status: The English Longitudinal Study of Ageing. Gerontology, 64(1), 58–66. doi: 10.1159/000477169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, T. M. (2006). Attitudes toward aging and their effects on behavior. In J. E. Birren & K. W. Schaire (Eds.), Handbook of the psychology of aging (pp. 379–406). Elsevier. doi: 10.1016/B978-012101264-9/50020-3 [DOI] [Google Scholar]
- Hughes, M. L., & Lachman, M. E. (2018). Social comparisons of health and cognitive functioning contribute to changes in subjective age. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(5), 816–824. doi: 10.1093/geronb/gbw044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, Y., & Hong, G. S. (2019). Predictors of subjective age in community-dwelling older adults in Korea. Geriatric Nursing (New York, N.Y.), 40(3), 314–319. doi: 10.1016/j.gerinurse.2018.11.008 [DOI] [PubMed] [Google Scholar]
- Infurna, F. J., Gerstorf, D., Robertson, S., Berg, S., & Zarit, S. H. (2010). The nature and cross-domain correlates of subjective age in the oldest old: Evidence from the OCTO Study. Psychology and Aging, 25(2), 470–476. doi: 10.1037/a0017979 [DOI] [PubMed] [Google Scholar]
- Jaconelli, A., Terracciano, A., Sutin, A. R., Sarrazin, P., Raffard, S., & Stephan, Y. (2017). Subjective age and dementia. Clinical Gerontologist, 40(2), 106–113. doi: 10.1080/07317115.2016.1187695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, S., Downs, T. D., Cash, H. R., & Grotz, R. C. (1970). Progress in development of the index of ADL. The Gerontologist, 10(1), 20–30. doi: 10.1093/geront/10.1_part_1.20 [DOI] [PubMed] [Google Scholar]
- Kennedy, N., Foy, K., Sherazi, R., McDonough, M., & McKeon, P. (2007). Long-term social functioning after depression treated by psychiatrists: A review. Bipolar Disorders, 9(1–2), 25–37. doi: 10.1111/j.1399-5618.2007.00326.x [DOI] [PubMed] [Google Scholar]
- Keyes, C. L., & Westerhof, G. J. (2012). Chronological and subjective age differences in flourishing mental health and major depressive episode. Aging & Mental Health, 16(1), 67–74. doi: 10.1080/13607863.2011.596811 [DOI] [PubMed] [Google Scholar]
- Kleinspehn-Ammerlahn, A., Kotter-Grühn, D., & Smith, J. (2008). Self-perceptions of aging: Do subjective age and satisfaction with aging change during old age? The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63(6), P377–P385. doi: 10.1093/geronb/63.6.p377 [DOI] [PubMed] [Google Scholar]
- Kornadt, A. E., Siebert, J. S., & Wahl, H. W. (2019). The interplay of personality and attitudes toward own aging across two decades of later life. PLoS One, 14(10), e0223622. doi: 10.1371/journal.pone.0223622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter-Grühn, D., Kleinspehn-Ammerlahn, A., Gerstorf, D., & Smith, J. (2009). Self-perceptions of aging predict mortality and change with approaching death: 16-year longitudinal results from the Berlin Aging Study. Psychology and Aging, 24(3), 654–667. doi: 10.1037/a0016510 [DOI] [PubMed] [Google Scholar]
- Kuna, L., Jakab, J., Smolic, R., Raguz-Lucic, N., Vcev, A., & Smolic, M. (2019). Peptic ulcer disease: A brief review of conventional therapy and herbal treatment options. Journal of Clinical Medicine, 8(2), 179. doi: 10.3390/jcm8020179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton, M. P. (1975). The Philadelphia geriatric center morale scale: A revision. Journal of gerontology, 30(1), 85–89. doi: 10.1093/geronj/30.1.85 [DOI] [PubMed] [Google Scholar]
- Levy, B. R., Slade, M. D., Kunkel, S. R., & Kasl, S. V. (2002). Longevity increased by positive self-perceptions of aging. Journal of Personality and Social Psychology, 83(2), 261–270. doi: 10.1037//0022-3514.83.2.261 [DOI] [PubMed] [Google Scholar]
- Makris, U. E., Higashi, R. T., Marks, E. G., Fraenkel, L., Sale, J. E., Gill, T. M., & Reid, M. C. (2015). Ageism, negative attitudes, and competing co-morbidities—Why older adults may not seek care for restricting back pain: A qualitative study. BMC Geriatrics, 15, 39. doi: 10.1186/s12877-015-0042-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A. V., Eglit, G. M., Maldonado, Y., Daly, R., Liu, J., Tu, X., & Jeste, D. V. (2019). Attitude toward own aging among older adults: Implications for cancer prevention. The Gerontologist, 59(Supplement_1), S38–S49. doi: 10.1093/geront/gnz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miche, M., Elsässer, V. C., Schilling, O. K., & Wahl, H. W. (2014). Attitude toward own aging in midlife and early old age over a 12-year period: Examination of measurement equivalence and developmental trajectories. Psychology and Aging, 29(3), 588–600. doi: 10.1037/a0037259 [DOI] [PubMed] [Google Scholar]
- Montepare, J. M. (2009). Subjective age: Toward a guiding lifespan framework. International Journal of Behavioral Development, 33(1), 42–46. doi: 10.1177/0165025408095551 [DOI] [Google Scholar]
- Petermann, F. (2015). Hospital anxiety and depression scale, deutsche version (HADS-D). Zeitschrift für Psychiatrie, Psychologie und Psychotherapie, 59(3), 251–253. doi: 10.1024/1661-4747/a000077 [DOI] [Google Scholar]
- Rubin, D. C., & Berntsen, D. (2006). People over forty feel 20% younger than their age: Subjective age across the lifespan. Psychonomic Bulletin & Review, 13(5), 776–780. doi: 10.3758/bf03193996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent-Cox, K. A., Anstey, K. J., & Luszcz, M. A. (2012a). Change in health and self-perceptions of aging over 16 years: The role of psychological resources. Health Psychology, 31(4), 423–432. doi: 10.1037/a0027464 [DOI] [PubMed] [Google Scholar]
- Sargent-Cox, K. A., Anstey, K. J., & Luszcz, M. A. (2012b). The relationship between change in self-perceptions of aging and physical functioning in older adults. Psychology and Aging, 27(3), 750–760. doi: 10.1037/a0027578 [DOI] [PubMed] [Google Scholar]
- Siebert, J. S., Wahl, H. W., Degen, C., & Schröder, J. (2018). Attitude toward own aging as a risk factor for cognitive disorder in old age: 12-year evidence from the ILSE study. Psychology and Aging, 33(3), 461–472. doi: 10.1037/pag0000252 [DOI] [PubMed] [Google Scholar]
- Siebert, J. S., Wahl, H.-W., & Schröder, J. (2016). The role of attitude toward own aging for fluid and crystallized functioning: 12-year evidence from the ILSE study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(5), 836–845. doi: 10.1093/geronb/gbw050 [DOI] [PubMed] [Google Scholar]
- Smith, J. (2003). The gain–loss dynamic in lifespan development: Implications for change in self and personality during old and very old age. In U. M. Staudinger & U. Lindenberger (Eds.), Understanding human development: Dialogues with lifespan psychology (pp. 215–241). Kluwer Academic Publishers. doi: 10.1007/978-1-4615-0357-6_10 [DOI] [Google Scholar]
- Spuling, S. M., Miche, M., Wurm, S., & Wahl, H.-W. (2013). Exploring the causal interplay of subjective age and health dimensions in the second half of life. Zeitschrift für Gesundheitspsychologie, 21(1), 5–15. doi: 10.1026/0943-8149/a000084 [DOI] [Google Scholar]
- Staats, S., Heaphey, K., Miller, D., Partlo, C., Romine, N., & Stubbs, K. (1993). Subjective age and health perceptions of older persons: Maintaining the youthful bias in sickness and in health. International Journal of Aging & Human Development, 37(3), 191–203. doi: 10.2190/373B-PJ6U-DWAA-4K03 [DOI] [PubMed] [Google Scholar]
- Stephan, Y., Sutin, A. R., & Terracciano, A. (2015). How old do you feel? The role of age discrimination and biological aging in subjective age. PLoS One, 10(3), e0119293. doi: 10.1371/journal.pone.0119293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, Y., Sutin, A. R., Terracciano, A., Ryff, C., & Krueger, R. F. (2018). Determinants and implications of subjective age across adulthood and old age. In C. D. Ryff & R. F. Krueger (Eds.), The Oxford handbook of integrative health science (pp. 87–96). Oxford University Press. doi: 10.1093/oxfordhb/9780190676384.013.7 [DOI] [Google Scholar]
- Stuck, A. E., Walthert, J. M., Nikolaus, T., Büla, C. J., Hohmann, C., & Beck, J. C. (1999). Risk factors for functional status decline in community-living elderly people: A systematic literature review. Social Science & Medicine (1982), 48(4), 445–469. doi: 10.1016/s0277-9536(98)00370-0 [DOI] [PubMed] [Google Scholar]
- Wagner, K.-H., Cameron-Smith, D., Wessner, B., & Franzke, B. (2016). Biomarkers of aging: From function to molecular biology. Nutrients, 8(6), 338. doi: 10.3390/nu8060338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, J. G., & Littlejohn, G. O. (2007). Measuring quality of life in rheumatic conditions. Clinical Rheumatology, 26(5), 671–673. doi: 10.1007/s10067-006-0450-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhof, G. J., & Barrett, A. E. (2005). Age identity and subjective well-being: A comparison of the United States and Germany. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60(3), S129–S136. doi: 10.1093/geronb/60.3.s129 [DOI] [PubMed] [Google Scholar]
- Westerhof, G. J., Barrett, A. E., & Steverink, N. (2003). Forever young? A comparison of age identities in the United States and Germany. Research on Aging, 25(4), 366–383. doi: 10.1177/0164027503025004002 [DOI] [Google Scholar]
- Westerhof, G. J., Miche, M., Brothers, A. F., Barrett, A. E., Diehl, M., Montepare, J. M., Wahl, H. W., & Wurm, S. (2014). The influence of subjective aging on health and longevity: A meta-analysis of longitudinal data. Psychology and Aging, 29(4), 793–802. doi: 10.1037/a0038016 [DOI] [PubMed] [Google Scholar]
- Wettstein, M., Schilling, O. K., & Wahl, H. W. (2016). “Still feeling healthy after all these years”: The paradox of subjective stability versus objective decline in very old adults’ health and functioning across five years. Psychology and Aging, 31(8), 815–830. doi: 10.1037/pag0000137 [DOI] [PubMed] [Google Scholar]
- Wienert, J., Kuhlmann, T., & Lippke, S. (2015). Direct effects of a domain-specific subjective age measure on self-reported physical activity—Is it more important how old you are or how old you feel? Health Psychology Report, 3(2), 131–139. doi: 10.5114/hpr.2015.51450 [DOI] [Google Scholar]
- Wurm, S., Tomasik, M. J., & Tesch-Römer, C. (2008). Serious health events and their impact on changes in subjective health and life satisfaction: The role of age and a positive view on ageing. European Journal of Ageing, 5(2), 117–127. doi: 10.1007/s10433-008-0077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm, S., Wiest, M., Wolff, J. K., Beyer, A. K., & Spuling, S. M. (2020). Changes in views on aging in later adulthood: The role of cardiovascular events. European Journal of Ageing, 17(4), 457–467. doi: 10.1007/s10433-019-00547-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinbarg, R. E., Mineka, S., Bobova, L., Craske, M. G., Vrshek-Schallhorn, S., Griffith, J. W., Wolitzky-Taylor, K., Waters, A. M., Sumner, J. A., & Anand, D. (2016). Testing a hierarchical model of neuroticism and its cognitive facets: Latent structure and prospective prediction of first onsets of anxiety and unipolar mood disorders during 3 years in late adolescence. Clinical Psychological Science, 4(5), 805–824. doi: 10.1177/2167702615618162 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.