Abstract
Targeted alpha therapy (TAT) is an area of research with rapidly increasing importance as the emitted alpha particle has a significant effect on inducing cytotoxic effects on tumor cells while mitigating dose to normal tissues. Two significant isotopes of interest within the area of TAT are thorium-227 and actinium-225 due to their nuclear characteristics. Both isotopes have physical half-lives suitable for coordination with larger biomolecules, and additionally actinium-225 has potential to serve as an in vivo generator. In this review, the authors will discuss the production, purification, labeling reactions, and biological studies of actinium-225 and thorium-227 complexes and clinical studies.
Keywords: Thorium-227, Actinium-225, alpha therapy, radiolabeling
Introduction
Targeted Alpha Therapy (TAT) is a growing area of investigation in the field of radiopharmaceuticals. A review performed using the Web of Knowledge, showed that since the first treatment of a leukemia patient with an alpha emitter in 19951, publications in this area have increased approximately 1500%. There have been a few reviews and editorials that examine different aspects of TAT2–8. This review will present an update on actinium-225 (225Ac) and thorium-227 (227Th) chemistry, radiochemistry, chelators, production of isotopes and preclinical and clinical examples.
Pre-clinical and clinical investigations of alpha particle emitters have been driven in part by increased isotope availability, understanding and development of safe handling protocols, and development over the years of libraries of bifunctional chelators for conjugation of radionuclides to targeting vectors. The isotopes discussed herein show promise for TAT as they both emit multiple alpha particles in their decay chains.
The moderate pathlength (50 – 100 µm) and high linear energy transfer (LET: 80 keV/µm) of alpha particles appear to be suitable for disseminated disease, small neoplasms, micrometastases and elimination of single tumor cells. Thus, alpha particles have been considered complementary to the long pathlength (<12 mm) and low LET (∼ 0.2 keV/μm) of beta particles that are effective in large, heterogeneous tumors where the long β-range evenly distributes the radiation dose. The long range of beta particles can also result in irradiation of healthy tissue surrounding the tumor site. Recent clinical studies show that alpha emitters may be effective in controlling bulky disease9 and in overcoming treatment resistance to beta particle therapy
An alpha particle is a 4He+2 nucleus with an immense mass compared to electrons and is monoenergetic with initial kinetic energy between 5 and 9 MeV. Unlike beta particles, alpha particles maintain an almost linear path with destruction occurring all along the path and not just primarily at the end of the path. Interaction of alpha particles with biological tissue launches complex molecular pathways6. The primary target of the alpha particle’s high LET radiation is DNA, where double strand breaks result from a single alpha particle track resulting in cell sterilization10. In contrast, beta particle irradiation produces mainly ionization resulting in single strand breaks, and exhibits about 500 times lower cytotoxicity6.
Secondary effects may enhance the ability of alpha and beta particles for cell killing. The ability of a particle to damage neighboring cells is called the “crossfire effect”. The cross-fire effect is operative with beta emitters due to their longer range in tissue and is advantageous in heterogeneous tumors, Figure 1. However, recent clinical studies demonstrate that alpha particles possess noteworthy therapeutic effects on large tumors and large bulky disease although this may be due to other mechanisms9, 11–12. To wit, alpha emitters have been applied using DOTATOC13 for neuroendocrine tumors and a prostate-specific membrane antigen (PSMA) ligand for prostate cancer14. The short range of alpha particles in tissue may result in an irradiation that is truly targeted to tumor cells with minimal effects on normal tissues.
Figure 1.
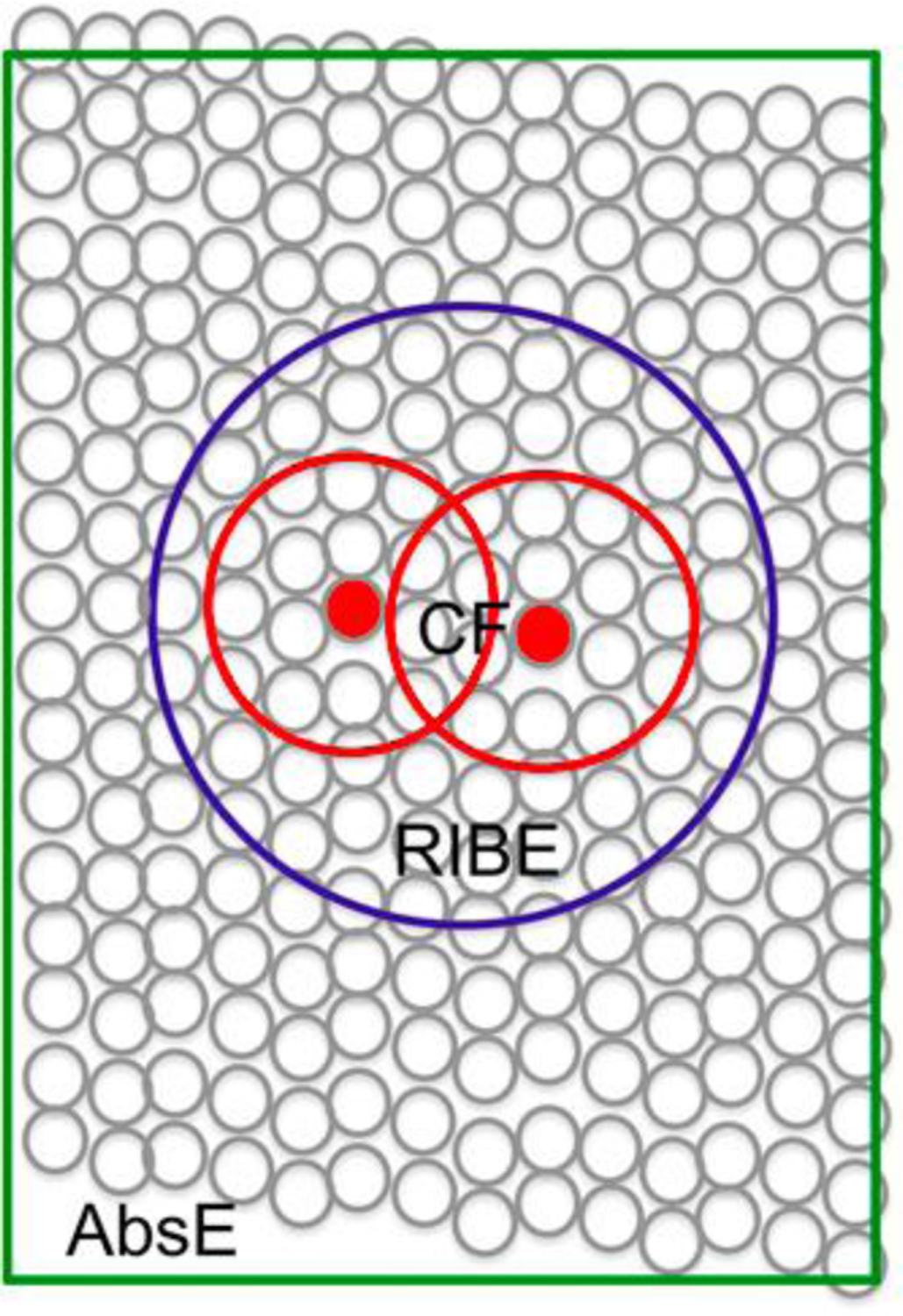
schematic describing the crossfire (CF) effect, radiation-induced bystander (RIBE) effect, and the abscopal (AbsE) effect (see text) this image was originally published in JNM. Haberkorn,U., Giesel, F., Morgenstern, A., Kratochwil, C The Future of Radioligand Therapy: alpha, beta or both? J Nucl Med. 2017;7:1017–1018. © SNMMI 9
Another mechanism operative in enhancing therapeutic effects is the “radiation-induced bystander effect” (RIBE), Figure 1. This effect results in cells that are in the proximity of cells exposed to ionizing radiation but themselves not directly exposed, behaving as if they have been exposed9. While the exact mechanism of RIBE is not known, it is likely that information is transmitted from irradiated cells to neighboring cells by stress signal factors. Finally, the “abscopal effect” is a phenomenon that has been observed in a variety of tumors and is attributed to irradiation-induced immune mechanisms that result in a therapeutic response in remote lesions.15 The cell-killing efficiency of alpha particles is independent of cellular oxygenation.16
For optimized therapeutic efficacy, the α-cytotoxic payload should accumulate selectively in diseased tissue and deliver a suitable radiation dose to tumor sites while sparing normal organs and surrounding healthy tissue. In order to achieve optimized therapeutic efficacy, it is important to match the physical half-life of the alpha particle, and relative biological effectiveness to tumor mass, size, and biological half-life of the vector. Unlike beta particles, alpha particles have high linear energy transfer (LET) with a mean energy deposition of 100 keV/μm. This can result in DNA double strand breaks without the use of oxygen, unlike beta particles, and thus are effective in hypoxic tissues. Cell death can result from only a single alpha particle, and can be dose dependent17. Additional advantages of alpha particles are severe chromosome damage even at mitosis, destruction of cells independent of cell cycle, and as demonstrated in the clinic, the potential to overcome resistance. While radium dichloride exhibits bone-targeting properties due to its similarity to calcium (vide infra), most alpha emitters require conjugation to targeting vectors to deliver the α-cytotoxic payload to cells expressing high target concentrations1–2. Certainly, 225Ac and 227Th, as members of the actinide series, require chelators for stabilization and attachment to targeting molecules. Targeting molecules are generally antibodies, peptides and small molecules. Antibodies are exquisitely selective for their antigens expressed on tumor cells resulting in high tumor uptake and low accumulation in healthy tissue. However, antibodies possess long blood circulation time (generally days) that increases the risk of hematotoxicity and myelotoxicity. Alternatively, small molecules and peptides can exhibit high tumor penetration and faster clearance than antibodies9.
While TAT is a growing area of interest and research, there is currently only one alpha emitting radioisotope which has been approved for treatment by the food and drug administration. Radium dichloride (223RaCl2) marketed under the name Xofigo, has been approved for the palliative treatment of castration resistant metastatic prostate cancer tumors in bone18. In this case, no bifunctional ligand nor targeting vector is required. Radium (Ra2+) is a calcium mimic, and as a metal ion binds to areas of increased bone turnover in skeletal metastases19; subsequently double strand DNA breaks induce apoptotic effects20. Here, the short range and high LET of the emitted alpha particles result in optimized targeting and mitigate adverse effects to surrounding normal tissue21–22.
Handling Alpha Emitting Radionuclides
Handling alpha emitting radionuclides is an important issue with the main concern being internalization and deposition in healthy tissues. Due to the short range of alpha emitters, they can be shielded by gloves, as they do not constitute an external radiation hazard. In handling of alpha emitters researchers need to consider the specific radionuclide as well as its progeny. While the Geiger-Muller survey meter is the usual probe in a radiochemistry lab, in the case of alpha emitters, researchers should additionally employ an alpha probe consisting of a ZnS(Ag) scintillator and a photo-multiplier (PM) tube 23. All meters should be properly calibrated and run at the optimal operating voltage of the PM tube for the specific radionuclide. Allowable removable contamination levels for alpha emitters is 50 times less than for beta emitters. Thus, it is critical to mitigate contamination as much as possible to achieve low levels of contamination to be compliant with regulations. In addition, alpha probes are generally less efficient than other detectors thus making the detection of alpha contamination somewhat challenging. Researchers should work with alpha emitters that possess low abundance and low energy gamma emission in a well-ventilated hood or glove box. Alternatively, handling alpha emitters that emit high energy γ-rays, should be performed in a hot cell or behind 6-inch lead bricks using remote handling techniques. Double gloving should be employed, and wipe tests should be performed with analysis using a gamma counter and/or liquid scintillation counter.
Actinium-225
General Chemistry and Radiochemistry
Actinium is a radioactive element with atomic number 89 and possesses 32 isotopes. Of the 32 isotopes, 227Ac and 228Ac are present in nature as a result of the naturally occurring 235U and 232Th decay chains. The chemistry of actinium is poorly developed owing to its limited supply and special facilities and handling required of all Ac isotopes.
Actinium usually exists as a +3 ion in aqueous solution and is considered to possess chemical properties similar to lanthanum +3. In fact, La3+ is often used as a nonradioactive surrogate for Ac3+. The 6-coordinate ionic radius of La3+ (1.03 Å) is smaller than Ac3+ (1.12 Å)24. The low charge density renders Ac3+ a very basic +3 ion. The first hydrolysis constant, pK1h, represents the ability of the metal to polarize coordinated water to favor release of a proton and formation of AcOH2+, and for Ac3+ this was measured by an ion exchange method and determined to be 9.4 ± 0.125. This study also measured pK1h of La(III) as 9.0 + 0.1 under similar conditions. Other studies show the first hydrolysis constant of La3+ to be 8.63 by different methods26. These studies suggest that the first hydrolysis constants are consistent with the charge densities of Ac3+ and La3+. This also suggests that Ac3+ is a “hard” metal ion. Practically speaking, this information suggests that basic conditions may be used for radiolabeling of Ac complexes.
Spectroscopically Ac3+ is invisible to many forms of routine spectroscopy, such as ultraviolet-visible, fluorescence, electron paramagnetic resonance, etc. due to its electronic configuration (5f0 6d0). Using the long-lived isotope 227Ac (t1/2: 21.772 y) Ferrier et. al. measured the L3-edge X-ray absorption near-edge structure (XANES) representing the first actinium XANES measurement. This study bodes well for study of Actinium via X-ray absorption spectroscopy (XAS)27. The interpretation of the extended X-ray absorption fine-structure (EXAFS) data from room temperature solutions containing Ac in HCl demonstrated that the Ac3+ was coordinated to ∼ 3 Cl− and ∼ 6 H2O inner -sphere ligands. The calculated coordination numbers agreed with experimental values, and this study demonstrated that Ac tends to possess more Cl− inner sphere ligands than americium consistent with the notion that Ac3+ is substantially less polarizing than the rest of the f-elements and confirming it as a hard acid. Later, the group reported a XAFS study wherein 10.9 + 0.5 water molecules were directly coordinated to the Ac3+ cation with an Ac-OH2O distance of 2.63 (1)Å28. This was in agreement with Molecular Dynamics- Density Functional Theory (MD-DFT) results. Having 11 inner sphere water molecules is reasonable for the large Ac3+ ion; this is consistent with coordination numbers determined by EXAFS for other +3 actinide and lanthanide aquo ions. The coordination number of 11 is consistent with the current ligands, discussed below, containing up to 12 donor atoms2. The long Ac-H2O distance is consistent with actinium as the largest +3 cation known.
225Ac decays via alpha emission (6 MeV) with a half-life of 9.92 d to six consecutive daughter isotopes to stable 209Bi (Figure 2). These isotopes include francium-221 (221Fr; t1/2= 4.9 min, α decay (100%)), astatine-217 (217At; t1/2= 32.2 ms, α decay (99.99%)), bismuth-213 (213Bi; t1/2= 60.5 min, α decay (35.94%), β− decay (64.06%)), thallium-209 (209Tl; t1/2= 2.162 min, β− decay (100%)), lead-209 (209Pb; t1/2= 3.23 h, β− decay (100%)), and stable bismuth-209 (209Bi)29. Since 225Ac itself cannot be detected directly with gamma spectroscopy, as it does not emit a detectable gamma ray, time must be allowed for the detectable daughter, 213Bi to grow in and be observed by gamma detection.
Figure 2.
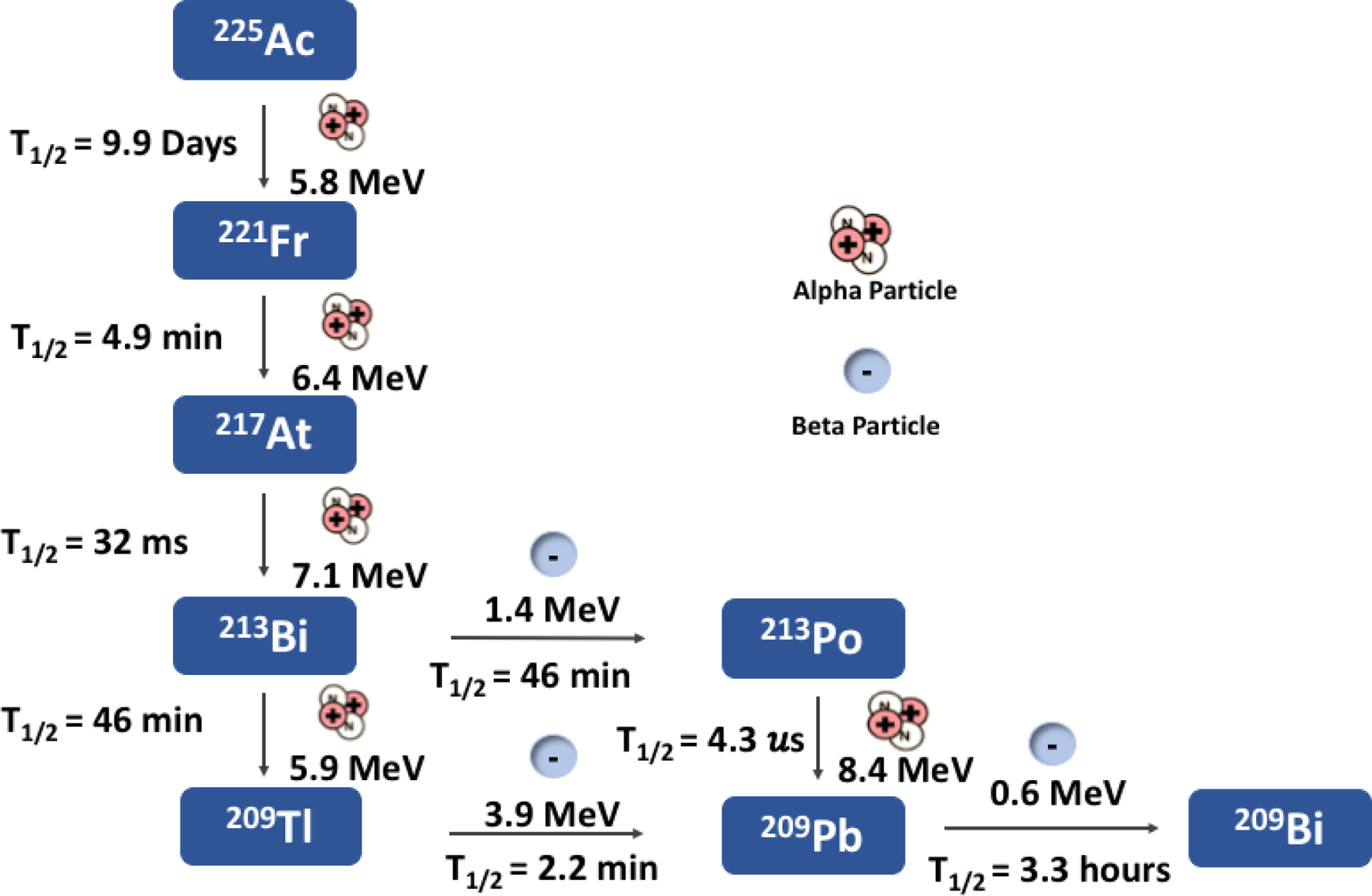
Ac-225 decay chain
Production of Actinium-225
One method of production for 225Ac is the separation from thorium-229 (229Th). The 229Th is obtained from the decay of uranium-233 typically from waste streams. As reported by Boll et. al. 30, to separate the actinium from 229Th, the stock solution is loaded onto a MP1 anion exchange resin in the chloride form preconditioned with 8 M nitric acid (HNO3) to remove the bulk thorium. The resulting solution containing radium and actinium is evaporated, reconstituted with 8 M HNO3, and subsequently passed through a second anion exchange column. The eluate is then evaporated and reconstituted in 10 M HCl which is then passed through a third anion exchange column to remove iron (Fe) as the +3 cation. The resulting solution containing radium and actinium is evaporated and reconstituted with 0.1 M HNO3 and loaded onto an AG50-X4 cation exchange column. The radium is eluted with 1.2 M HNO3, and followed by the actinium which is eluted with 8 M HNO3. The final purification step includes evaporation and reconstitution of the actinium with 0.1 M HNO3, loading onto a second cation exchange column, rinsing the column to remove residual radium, then eluting the final actinium product with 8 M HNO3. The purity of the final product is 99.6 ± 0.7% with the impurity contributions coming from radium isotopes (225Ra: 0 – 1.1%, 224Ra: 0.02 – 0.06%)30. This 5-step process takes approximately 60 d and yields around 3.7 GBq of 225Ac with an 80% yield. Unfortunately, while this method of production is providing material for preclinical and clinical trials, it does not produce a sufficient amount of material necessary for all clinical trials31.
Actinium can also be produced through the proton spallation of thorium-232 (232Th) as shown by Harvey et. al.32–34 which allows for the production of 225Ac without the use of heavily regulated special material such as uranium-233 (233U). Here thorium samples with a volume of 2.95 cm3 were encapsulated in a copper enclosure with a thickness of 0.127 cm. The samples were irradiated with an 8 GeV proton beam over a 4-day period. The spallation yields several different radioisotopes of interest in additional to actinium to include 225Ra, 223Ra, and 227Th. The major disadvantage to this production method is the coproduction of long-lived 227Ac (t1/2= 21.78 y), where the isotope is less than 0.2% of the final product mixture35. While the byproduct has no effect on labelling, it does complicate waste handling. This work has been advanced by the Department of Energy Isotope Program through its Tri-lab efforts brought together to advance the production of 225Ac by irradiations of 232Th. The effort involves using the high energy accelerators at Brookhaven National Laboratory (BNL) and Los Alamos National Laboratory (LANL) to irradiate 10– 100-gram targets of 232Th which is then shipped to Oak Ridge National Laboratory (ORNL) for subsequent processing and distribution. At this point they have distributed over 9.25 GBq of material. BNL is upgrading hot cell facilities to enable curie level production and LANL is building a new facility near their accelerator to allow for production. Recently a drug master file (DMF) was submitted for this material to the FDA to enable use in clinical trials. This work was undertaken to provide a robust, year-round, quality supply of 225Ac. TRIUMF is pursuing a similar production route using their 400 MeV accelerator and shipping targets to Canadian Nuclear Laboratories (CNL) for processing. Studies performed by Dadachova et. al. in which she evaluated the impact of the 227Ac content from high energy accelerator produced 225Ac showed that the impact of the 227Ac on biodistribution and dose is negligible32. However, its long half-life does pose concerns with licensing and waste.
225Ac can also be produced via the 226Ra(p,2n)225Ac nuclear reaction using low energy protons from a medical cyclotron; this is potentially a beneficial route as it appears to eliminate 227Ac. Apostolidis et. al. measured the cross-section of the proton induced reaction and reported that the maximum occurs at 16.8 MeV36. Simulations show that the production route does however produce actinium-226 (226Ac) where the activity contribution could be up to 11%. The half-life of 226Ac is relatively short, t1/2= 29 h, compared to 225Ac which allows for decay during the processing steps and a decrease in the 226Ac:225Ac ratio over time. Challenges in using 226Ra as the target material is the alpha decay to gaseous 222Rn and the need for recycling of the target material due to limited availability. The 222Rn (t1/2=3.82 d) is highly radioactive and due to its gaseous nature is difficult to prevent from spreading. Predictions show that in scaled up productions irradiating 1 g 226Ra targets with 20 MeV proton can produce 3.996 GBq of 225Ac per month36.
Another route that is being considered by a number of groups, including Argonne National Laboratory, is the photonuclear route on 226Ra(γ,n)225Ra→225Ac. A five-day irradiation can result in roughly 37000MBg of 225Ra which then can be milked every three days to give 3700–7400MBq-of 225Ac. This method also gives 225Ac free of 227Ac. Challenge with this route is the need to use and recycle the 226Ra target and complications with the 222Rn daughter as stated above.
Radiolabeling and chelator development
All clinical tests and most preclinical research have been conducted using 225Ac derived from the decay of 229Th and subsequent radiochemical extraction. Initial efforts in the chelation of 225Ac for in vivo purposes were implemented to reduce the toxicity of the radiometal to the liver and bone29. This was first evaluated by using citrate where Beyer et. al. compared the biodistribution of [225Ac]Ac-citrate to the distribution of [169Yb]Yb-citrate and determined that the actinium citrate complex overall had less than optimal whole-body clearance but had fast blood clearance, low bone uptake, and higher liver uptake compared to the ytterbium congener37. In this study, the 225Ac was produced by the irradiation U3O8 with 650 MeV protons, separation of Ra by BaSO4 precipitation, and isolation of the pure 225Ac by cation exchange chromatography. These studies were subsequently followed up by chelating 225Ac with conventional ligands such as ethylenediaminetetramethylenephosphonic acid (EDTMP), ethylenediaminetetraacetic acid (EDTA), and N-[(R)-2-amino-3-(4-nitrophenyl)propyl]-trans(S,S)-cyclohexane-1,2-diamine-N,N,N’,N”,N”-pentacetic acid (CHX-A-DTPA). In vitro studies wherein cation exchange columns were used to determine “free” Actinium (bound to the column) and complexed Ac shows that only DOTA (1,4,7,10-tetrazacyclododecane-N,N’,N”,N’”-tetraacetic acid) demonstrates around 1% free Ac, while the other chelators do not form stable complexes (almost all Ac bound to column). Intermediate behavior was exhibited by 225Ac DOTMP (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylenephosphonic acid) where 78% complex and 22% free actinium was observed38. The in vivo stability was evaluated in mouse models and tracked with in vitro studies. All Ac ligand complexes except DOTA exhibited significant liver uptake, while the Ac-DOTA complex exhibited 3.29+1.05 %ID/gram liver uptake39. Nevertheless, DOTMP has been labeled with 225Ac and evaluated by Henriksen et. al. to assess the biodistribution of bone-targeting molecules40. The biodistribution in BALB/c mice showed that the [225Ac]Ac-DOTMP complexes localized more readily in the bone versus soft tissues.
While DOTA is routinely used as a chelator for radiometals, including actinium, it is not the ideal chelator for 225Ac. The main challenge when complexing radiometals to the DOTA ligand is the heat necessary to deprotonate the nitrogen donor groups and bind the radiometal within the cyclic ring. Heating during the radiolabeling protocol is particularly problematic if the DOTA is conjugated to a biomolecule, such as an antibody, where heat would destroy the tertiary structure and affinity of the molecule. As of now, commercially available bifunctional DOTA ligands, such as p-SCN-Bn-DOTA, are commonly used. McDevitt discovered a “2 step” method for production of 225Ac antibody constructs38. In this approach, the p-SCN-Bn-DOTA was radiolabeled with 225Ac in the first step. The second step consists of conjugation of [225Ac] Ac--DOTA to the antibody. This approach suffers from hydrolysis of the SCN moiety resulting in loss of 90% of the actinium because this is conjugated to non-reactive forms of DOTA. A one-step method was reported by Maguire et. al41. that afforded products with 30-fold higher specific activities than typically achieved with the two-step method. However, this method requires conjugation of the IgG antibody with > 10 DOTA chelators per construct; this procedure may not be feasible with most antibodies and could reduce the affinity of the radioimmunoconjugate.
Poty et. al. reported utilizing inverse electron-demand Diels-Alder (IEDDA) reaction for the synthesis of 225Ac immunoconjugates42. In this approach, a DOTA-with pendent tetrazine (Tz) is radiolabeled with 225Ac at 37°C for one hour. Subsequently, the [225Ac]Ac-DOTA-Tz is reacted with the antibody conjugated to transcyclooctene (TCO). The Tz reacts rapidly and bioorthogonally with the antibody-TCO thus forming the 227Ac radioimmunoconjugate.
Additionally, even though the loss of Ac from DOTA is not as drastic as the chelators mentioned above, there is some release of Ac over time as demonstrated in in vivo and in vitro studies. A study by Horrocks demonstrates an inverse relationship of lanthanide DOTA stability constants with respect to ionic radius43. EXAFS studies, vide supra, suggest that Ac3+ can accommodate high coordination numbers. These suggest that new chelators should be developed for 225Ac perhaps with expanded cavities and possibly higher number of donor atoms to better match the large ionic radius of Ac3+.
The general instability of first-generation actinium chelators and the requirement of heat for complexation of DOTA gave rise to the development of new chelators. A novel chelate, 1,4,7,10,13,16-hexaazacyclohexadecane-N,N’,N”,N’”,N””,N’””-hexaacetic acid (HEHA) Figure 3 was developed with the goal to increase the in vivo stability of 225Ac complexes. The chelate is an 18 membered macrocyclic ring, consisting of 12 coordination sites which satisfies the actinium coordination sphere. To achieve a radiolabeling yield greater than 90%, 100 μL of an actinium stock (10 MBq in 0.1 M HNO3) was mixed for 30 min with 20 μL of the HEHA ligand (1 X 10−2M) at 40 °C while keeping the pH at 5 with 1.0 M ammonium acetate (NH4OAc). To biologically evaluate the complex the reaction mixture was purified on a Chelex column and diluted with 2-ethanesulfonic acid (MES) buffer. The resulting solution (92.5 kBq) was intravenously injected into normal female BALB/c mice and results showed the macrocyclic chelator labeled with 225Ac had increased stability and lower overall toxicity when compared to non-macrocyclic ligands. To further investigate the HEHA ligand for preclinical use, the ligand was bifunctionalized with a benzyl isothiocyanate group and conjugated to BL-3 monoclonal antibodies44. To radiolabel the HEHA-mAbs, an 225Ac stock (1.85 – 7.4 MBq) in 0.15 M NH4OAc with a pH of 7.0 was reacted for 30 min at 37 °C with the mAb conjugate (0.3 – 0.4 mg) in the same buffer. To assess the stability of the [225Ac]Ac-HEHA-mAb the construct was incubated in fetal bovine serum at 37 °C and aliquots were analyzed via radio-HPLC. This radiolabeling method resulted in a 60–85% yield and the stability of the radioimmunoconjugate in serum showed to be favorable at very early time points however the complex begins to disassociate after 1 hour.
Figure 3.
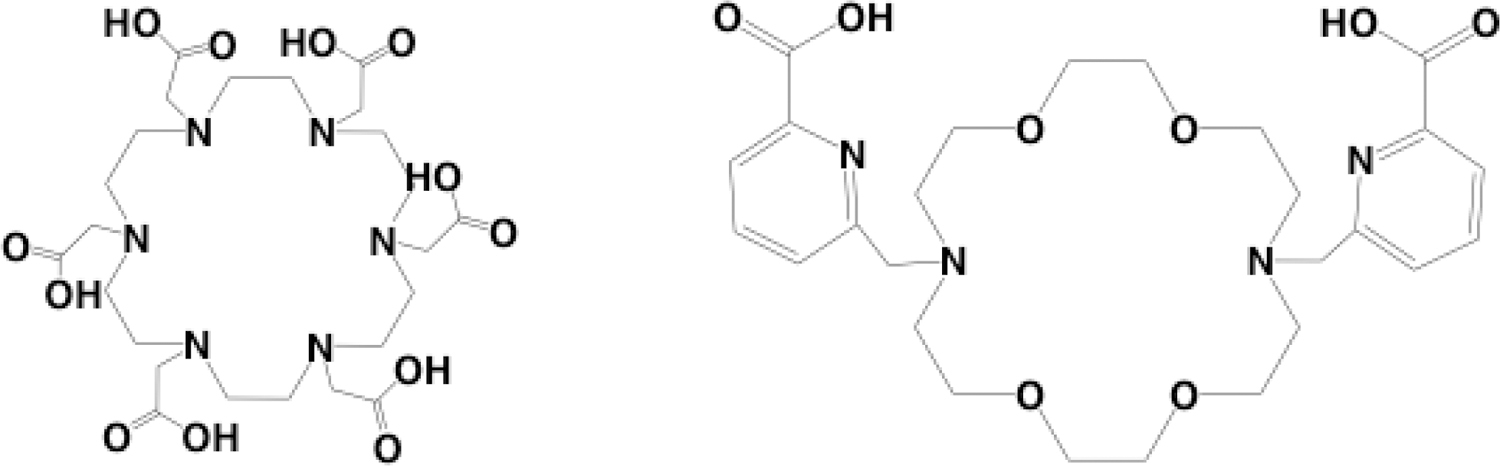
Structures of HEHA(left) and Macropa (Right)
Another 18 member macrocyclic ligand, N,N’-bis[(6-carboxy-2-pyridil)methyl]-4,13-diaza-18-crown-6 (H2macropa), Figure 3 was developed to further stabilize actinium in vivo45. In this study the 225Ac was produced by spallation of uranium carbide and separated by a mass separator using the Isotope Separator and Accelerator on-line facility at TRIUMF. Here, 225Ac was separated from 225Ra using a DGA column and isolated in 0.05M HNO3 for radiolabeling experiments. The H2Macropa ligand (59 μM) was mixed with 26 kBq of 225Ac in 0.15 M NH4OAc pH 5.5 – 6 and the radiochemical yield was evaluated by ITLC on silica gel plates. Remarkably a radiochemical yield of 98% was achieved within 5 min at room temperature. The ability to complex actinium at room temperature is very promising for the future of radiolabeling biomolecules with actinium. The biodistribution of the 225Ac-macropa complex was evaluated by intravenously injecting 10 – 15 kBq of the complex into normal female B57BL/6 mice (n= 3). The biodistribution of the complex was evaluated at 15 min, 1 hour, and 5 hours, and the results were compared to “free” actinium as [225Ac]Ac(NO3)3. The results showed that uptake trends for 225Ac-macropa closely resembled that of [225Ac]Ac-DOTA. The majority of the uptake was found in the urine, bladder, and kidneys where by 1-hour post injection 489.11, 6.23 and 0.40 %ID/g were found in the organs respectively Figure 4 (a–c). The authors found no disassociation of the actinium macropa complex, but they did note there was slower clearance of the complex compared to the DOTA complex, most likely due to the positive charge on the complex. Macropa was bifunctionalized and conjugated to a PSMA/albumin targeting molecule, RPS-070. Radiolabeling proceeded in the same manner and the resulting construct was evaluated in LNCaP tumor bearing mice. The biodistribution showed rapid clearance through the kidneys (52 ± 16% ID/g 4 hours post injection) and localization at the tumor site (13 ± 3% ID/g 4 hours post injection) with observable tumor washout over time45.
Figure 4 (a–c).
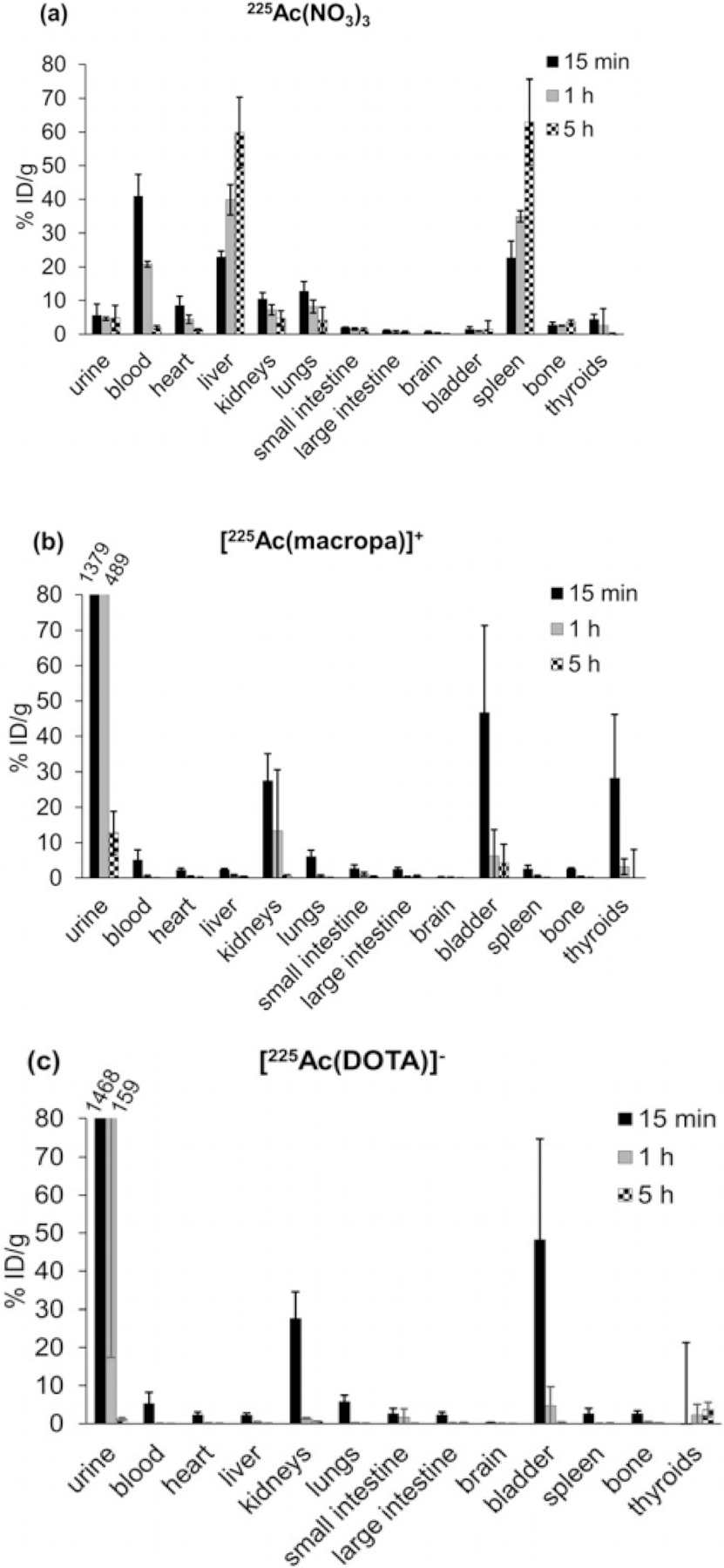
Biodistributions of Ac[NO3], Ac[Macropa], and Ac[DOTA]
Case Studies: Preclinical
Some of the earliest preclinical studies with 225Ac was performed by McDevitt et. al. using actinium-225 as an in vivo alpha generator coupled to internalizing monoclonal antibodies46. Their initial interest was in 213Bi but its short half-life limited its delivery. Solution they pioneered was what they termed the “atomic” generator; a single 225Ac atom conjugated to the delivery construct46. They showed in vitro, these constructs specifically killed leukemia, lymphoma, breast, ovarian, neuroblastoma, and prostate cancer cells at becquerel (picocurie) levels. Injection of single doses of the constructs at kilobecquerel (nanocurie) levels into mice bearing solid prostate carcinoma or disseminated human lymphoma induced tumor regression and prolonged survival, without toxicity, in a substantial fraction of animals46.
Anti-angiogenic therapy with 225Ac radiolabeled to the monoclonal antibody E4G10 that targets vascular endothelial cadherin was evaluated in a prostatic tumor model47. A two-step radiolabeling method was used in which 225Ac was first labeled to Meo-DOTA-NCS and then the radiolabeled conjugate was attached to the antibody as previously reported38. The 225Ac-E4G10 was evaluated in mice bearing LNCaP prostate tumors and compared to controls that consisted of 1% human serum albumin, unlabeled E4G10, and Ac-225 labeled IgG. Treatment with 50 nCi of 225Ac-E4G10 was administered on days 3,48 5, 7, and 10. Suppressed tumor growth, enhanced tumor cell apoptosis and prolonged animal survival were observed, without gross or histopathological toxicity in normal tissues or their vasculature. Synchronized administration of 225Ac-E4G10 and paclitaxel resulted in enhancement of the anti-tumor response47. Construct was further shown to eradicate bone marrow-derived endothelial progenitors.
These promising results were followed on by the evaluation of single wall carbon nanotube (SWCNT) constructs covalently appended with radiometal chelates of DOTA for 225Ac radiolabeling and desferrioxamine B (DFO) for Zr-89 radiolabeling and the tumor neovascular targeting antibody E4G1049. The SWCNT platform were chosen as the scaffold as they are nonimmunogenic, can be modified to incorporate multiple diagnostic and therapeutic modalities, rapidly clear the blood and are cleared via the kidneys. Thus, they offered the opportunity to improve the pharmacokinetics observed for antibodies i.e. slow clearance from the blood and the ability to image uptake. These SWNCT([225Ac] Ac-DOTA)EG410 constructs were evaluated in a tumor xenograft model of human adenocarcinoma (LS174T). PET imaging was performed with the SWCNT([89Zr]Zr-DFO)E4G10 which showed rapid blood clearance in less than an hour and specific tumor accumulation, demonstrating the enhanced pharmacokinetics offered by the SWCNT platform compared to the E4G10 antibody construct. Further, it offered enhanced cargo delivery and higher specific activity compared to the antibody alone, showed therapeutic efficacy, good image contrast and specificity for the target as well as being well tolerated with a safe profile. Therapy resulted in significant tumor volume reductions and improved survival rates as demonstrated by Kaplan Meier curves to the controls49.
It has been previously reported that during the treatment of prostate cancer as DNA damage occurs there is an upregulation of the androgen receptor associated with DNA repair genes50–51. McDevitt et. al. sought to take advantage of the over expression of the androgen receptor by attempting to elicit cell death through targeting human kallikrein peptidase 2 (hK2) whose expression is directly regulated by the androgen receptor52. The hu11B6 monoclonal antibody, developed as a diagnostic for catalytically active hK2, is internalized into the nucleus once bound to active hK2. Therefore, an 225Ac labeled hu11b6 would also internalize into the nucleus of the tumor cell inducing DNA double strand breaks leading to cell death. The cell death would in turn cause an increased expression of hK2 due to the DNA repair creating a loop of cell death and target expression. The hu11B6 antibody was conjugated with DOTA to prepare it for labeling as previously reported53 where the SCN-DOTA was radiolabeled with 225Ac (pH≈ 5.4, temp ≈ 56, time ≈ 42 min), prior to conjugation to the antibody (pH≈ 8.7 temp ≈ 36, time ≈ 52 min). It was reported that the radiochemical yield was 3.7 ± 2.1% while the radiochemical purity was 99.3 ± 0.5%, and the specific activity was determined to be 2.92 ± 2.03 kBq/g. Male athymic BALB/c nude mice were implanted with 1 – 5 X 106 LNCaP-AR cells subcutaneously injected into the right and left flanks. Mice were divided into two experimental groups and one control group. The first experimental group was treated with [225Ac]Ac-hu11B6, which recognizes hK2 and internalizes inside the cell. The second experimental group was treated with the specific, non-internalizing version of the drug, [225Ac]Ac- hu11B6-H435A, which recognizes hK2 but does not internalize. The control group was treated with non-specific [225Ac]Ac-huIgG1 antibody, and each group was administered 11.1 kBq of the labeled mAb. The results showed that mice treated with [225Ac]Ac-hu11B6 survived for a median of 108 days after treatment. While mice treated with [225Ac]Ac-hu11B6-H435A survived for a median of 22.5 days, and the control group survived 18 days after treatment. The cell death caused by [225Ac]Ac-hu11B6 resulted in increased hK2 expression which was confirmed by its observed increase of accumulation at the tumor site over time. This confirms the hypothesis of the alpha particle feed-forward oncoaddition mechanism.
225Ac has been incorporated into liposomes and nanoparticles to overcome some challenges in working with 225Ac or any alpha emitter. A major challenge is that the energy of the decay is sufficient to break chemical bonds resulting in the release of the daughter nuclides from the chelator. This release can then cause normal tissue toxicity. In order to circumvent this phenomenon many groups have investigated the use of alternative chelator systems that can efficiently house 225Ac and its daughters. Sofu, et. al. evaluated the use of liposomes to encapsulate multiple 225Ac and to retain its daughters at the tumor site thus reducing toxicity to normal tissues54. Liposomes had been used previously to selectively deliver radionuclides to tumors and sites of infection for diagnosis55–56. The 225Ac was passively trapped in the liposomes and at several time points the liposomes were separated from the mother liquor and measured by gamma spectroscopy to analyze for the gamma emission from 213Bi. Pegylated phophatydylcholine-cholesterol liposomes of various sizes and containing different charges were manufactured and used to trap more than 2 Ac atoms. Zwitterionic liposomes demonstrated the highest retention of upwards of 88% out to 30 days. Cationic liposomes exhibited lower retention. It was theoretically calculated that liposomes sized in excess of 650 nm in diameter were needed to retain greater than 50% of the 213Bi daughters. Experimental results indicated retention was 10% less than results predicted by the theoretical models. Studies indicated liposomes could result in delivery of multiple 225Ac but very large liposomes would be need which could limit the application to locoregional therapies.
Woodward48 developed 3–5 nm diameter monazite (LaPO4) nanoparticles (NP) as carriers for 225Ac. 225Ac[LaPO4] NP were synthesized and conjugated to one of two antibodies, mAb-201B or mAb-33. The NP only partially retained daughters with more than 50% of the 221Fr and 213Bi released from the NP lattice. Addition of two layers of LaPO4 reduced leaching of 221Fr to 20% 57. Robertson et. al 58. investigated the use of multi-shell nanoparticles to encapsulate 225Ac and its daughters. Nanoparticles with 4 GdPO4 shells followed by a gold coating demonstrated greatest retention of 225Ac (>99.99%) and its daughters, with up to 98% of 221Fr retained. Although multilayered NP exhibited higher retention properties, the multistep synthesis is very time consuming. Salvanou et. al.59 examined the use of 225Ac labeled macrocycle Au nano particles in brachytherapy. Gold NP were synthesized, reduced in the presence of TADOTAGA ligands to yield Au-TADOTAGA, then radiolabeled with 225Ac. A retardation of tumor growth was observed with a dose of 0.25 kBq/ml. Cedrowska et. al. 60 examined the use of 225Ac labeled titanium dioxide (TiO2) nanoparticles for TAT (REF). TiO2 nanoparticles modified with substance P, a peptide fragment that targets NK1 receptors on glioma cells trough the silane-PEG-NHS linker were labeled with 225Ac. The labeled bioconjugates displayed leaching of 221Fr, a first decay daughter of 225Ac, in cerebrospinal fluid after 10 days. 225Ac binds to the nanoparticles via exchange with a hydroxyl group on the surface, it is believed that the recoil energy of the alpha decay is distributed throughout the entire nanoparticle and is therefore reduced. The absorbed recoil energy minimizes the path of the daughters enabling their recapture by TiO2, a known cation exchanger for +3 ions. Cytotoxicity in vitro remained high in T98G glioma cells. The 225Ac labeled TiO2 reduced metabolic activity of the tumors while delivering smaller does to surrounding tissues.
Cornell prime dots (C’ Dots) are next generation ultra-small fluorescent core-shell silica nanoparticles that can be tracked due to dyes encapsulated in the core of the particle, which emit fluorescence in the near -infrared region. One advantage to using these ultra-small nanoparticles (<10 mm) for tumor targeting is their clearance by renal filtration. C’ dots were functionalized with the α melanocyte-stimulating hormone (αMSH) which targets the melanocortin-1 receptor (MC1R) and radiolabeled with 225Ac to evaluate the tumor microbiome environments after exposure to the immunotherapeutic. Urbanska et. al. reported the biodistribution of DOTA chelated [225Ac]Ac-αMSH-PEG- Cy5-C’in both healthy and tumor bearing C57BL/6J mice, where 11.1 kBq were administered in both studies61. In normal mice, high accumulation of the 225Ac nanoparticles was observed in the blood (25.37 ± 8.87 %ID/g, 1-hour post injection, n= 3) and were excreted through the renal track (149.9 ± 96.1 %ID/g, n= 3). Throughout all time points the organs with the highest accumulation of the nanoparticles were the liver (7.02 ± 0.35 %ID/g), spleen (6.58 ± 1.86 %ID/g), and kidney (6.52 ± 0.54 %ID/g). In the B16-F10 tumor bearing mice, there were similar trends at early time points, where significant accumulation was found in the blood (22.47 ± 10.39 %ID/g, 1-hour post injection, n= 5) and clearance through the kidneys (28.07 ± 42.15 %ID/g, 1 hour post injection, n= 5). By 1-hour post injection 5.30 ± 1.71 %ID/g (n= 5) was observed at the tumor site. Despite targeting the tumor, there was significant localization in the liver (4.79 ± 0.36 %ID/g), spleen (3.61 ± 1.38 %ID/g), and kidney (4.62 ± 1.38 %ID/g). A therapeutic study was also performed using C57BL/6J mice subcutaneously implanted with B16-F10 cells which grew 8 to 10 days prior to treatment. Mice were divided into three treatment groups, and administered either: 11.1 kBq of [225Ac]Ac-αMSH-PEG-Cy5-C’, 11.1 kBq of the non-targeting [225Ac]Ac-αNH2-PEG-Cy5-C’, or 1% human serum albumin. Mice treated with the targeted drug had a median survival time of 26 days, mice treated with the non-targeted version had a median survival time of 21 days, and the control group injected with the 1% human serum albumin solution had a median survival time of 14 days. The tumor microenvironment of mice administered [225Ac]Ac-αMSH-PEG-Cy5-C’ was evaluated and it was determined that the pharmacological consequence of the emitted alpha particles and the nanoparticle contribute to the cytotoxicity of the tumor cells. The authors note that there were changes to the macrophage, T-cell and NK cell populations and that ancillary immunotherapies should be used in conjunction with the 225Ac nanoparticles
In another study to investigate treatment of glioblastoma62, liposomes were modified by incorporation of a small-molecule integrin antagonist, that strongly binds to αVβ3 integrin that is highly expressed on glioblastoma cells. The αVβ3 integrin-specific liposomes modified with DOTA were labeled with actinium at 70 °C for 50 min. These 225Ac labeled αVβ3-specific liposomes (225Ac-IA-TLs) enhanced permeability of the blood-brain barrier (BBB) and newly formed tumor vasculature (blood tumor barrier, BTB). Moreover, the resultant complex resulted in double DNA strand breaks within the BBB and the within the BTB.
To test the efficacy of the labeled liposomes athymic nude mice implanted with U87 MG human glioblastoma cells. Ten days post implantation 37 kBq/ 5 μL of the [225Ac]Ac-IA-TLs were injected intravenously. The mice (n= 3) were sacrificed 2, 5, and 10 d post injection to assess the permeability induced by the actinium labeled liposome. Evans Blue dye, which does not permeate the BBB, was injected 6 hours prior to sacrifice to demonstrate the effect of permeability the labeled liposomes had on the BBB. In mice sacrificed 2 days after the injection of the liposomes, “modest” penetration was observed. However, “more extensive” penetration of the dye was observed in mice sacrificed after 5 days Figure 5. The results indicate that 225Ac-IA-TLs increased the potential for permeability across the BBB, and thus are a potential candidate for delivery of therapeutics across the BBB.
Figure 5.
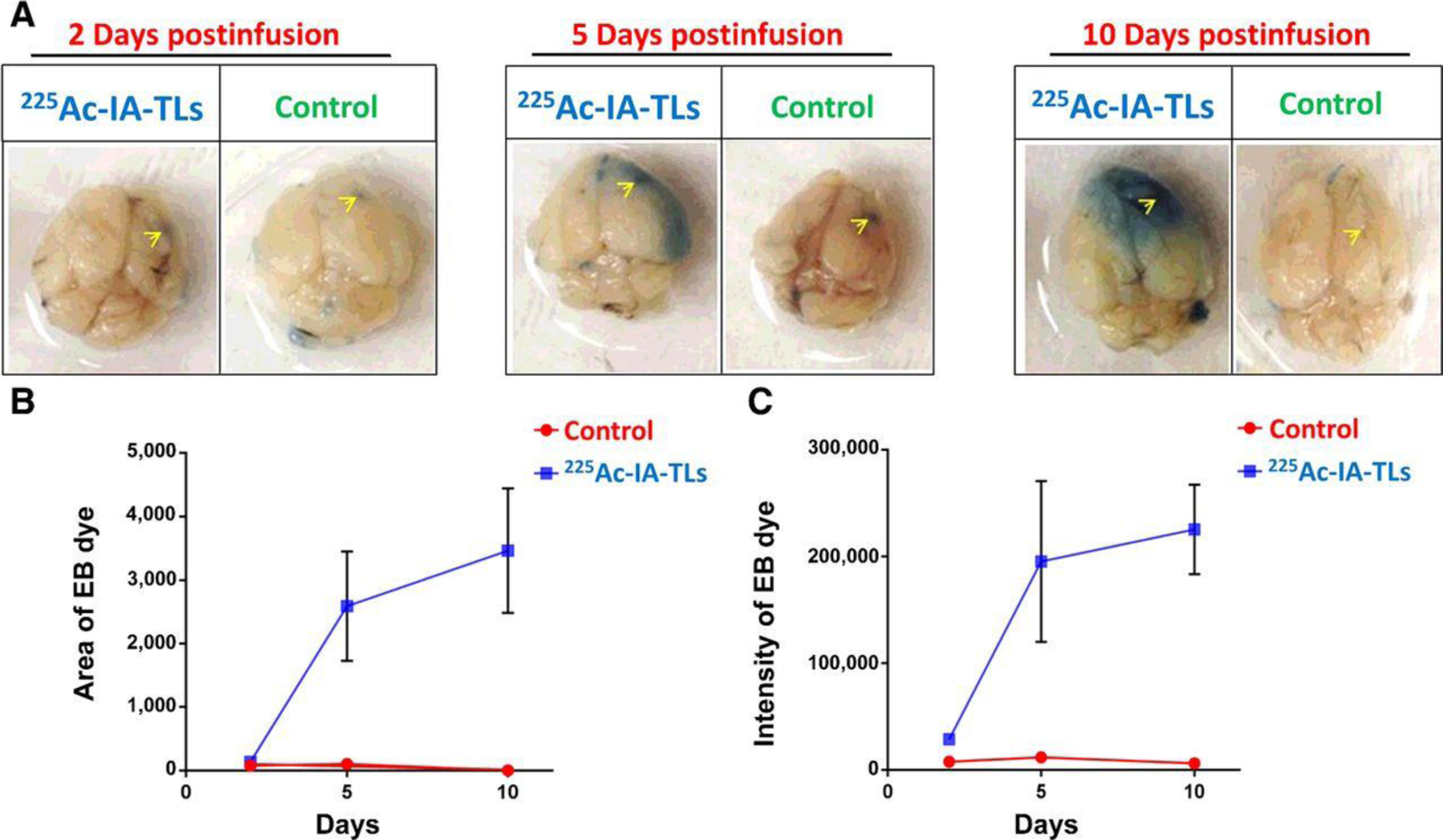
225Ac-IA-TLs result in BBB opening in immunocompetent mice. A. BBB opening in immunocompetent mice infused with 225Ac-IA-TLs 2 days, 5 days and 10 days post injection compared to saline infused immunocompetent mice as demonstrated by intraveneously injected Evans Blue dye. Site of intracranial injection marked by yellow arrows. B and C: Area and intensity of Evans Blue dye was found to be greater in mice that were intracranially infused with 225Ac-IA-TLs when compared with the saline control up to 10 days postinfusion62.
Clinical Experiences with 225Ac
225Ac-PMSA-617
The most extensive clinical experience to date has been gained with more than 300 prostate cancer patients with 225Ac-PMSA-617. PMSA-617 is a low molecular weight glu-urea-class of molecules that possesses high affinity to prostate-specific membrane antigen, glutamate carboxypeptidase II (PMSA), an enzyme overexpressed in most prostate tumors. The first study of the treatment of metastatic prostate cancer using [225Ac]AcPSMA-617 in humans was conducted on patients where β therapy ([177Lu]Lu-PMSA-617) was not effective 14. Two metastatic castrate-resistant-prostate cancer patients presented with late stage disease and widespread metastases and received [225Ac]Ac-PMSA-617 (100kBq/kg, bimonthly) as salvage therapy after the presence of PSMA-positive tumor phenotype had been validated by 68Ga-PMSA-11 PET/CT. After three doses of treatment, both patients showed a complete response, i.e. a dramatic decrease in the presence of the tumors and the concentration of prostate-specific antigen (PSA) in the patient’s blood14. One of the serious issues with [225Ac]Ac -PMSA-617 is xerostomia, that is, salivary gland toxicity.
Since that report, there have been various clinical experiences of [225Ac]Ac-PMSA- 617 with different treatment regimens. These are summarized in detail in reference63. Briefly, three approaches were explored; one approach (dynamic de-escalation) utilized a fixed dose of 8 MBq in the first cycle. At a certain point, the treatment activity is reduced by 2 MBq in the subsequent treatment cycle or increased in the case of prostate specific antigen (PSA) nonresponse. In a second approach, treatment activity was reduced to 6 MBq at the beginning. In a third regimen, cocktails of 4 MBq [225Ac]Ac-PMSA-617 and 4 MBq [177Lu]LuPMSA- 617 were administered, effectively reducing the dose to approximately 50% of the fist approach. Dynamic de-escalation and the cocktail approach improved tolerability without losing too much antitumor activity. Other studies employing the dynamic de-escalation and variations of the cocktail approach where [177Lu]Lu-PMSA-617 were co-administered with [225Ac]Ac- PMSA-617 were discussed63 and reported to reduce salivary gland toxicity.
225Ac-J591: NCT03276572
This ongoing clinical trial is for men with advanced prostate cancer64. The purpose is to find the highest dose level of 225Ac-J591 that can be administered without severe side effects. This phase I Dose-Escalation Trial of 225Ac-J591 in patients with metastatic castration-resistant prostate cancer is estimated to enroll 42 patients. J591 is a monoclonal antibody that recognizes PSMA. The treatment phase is comprised of eight visits over approximately 12 weeks. A single dose will be given on treatment day and subjects will subsequently undergo PET/CT using [68Ga]Ga-PSMA-HBED to document treatment response.
225Ac-Lintuzumab
Lintuzumab is a humanized monoclonal antibody, HuM195, that targets the cell surface antigen CD33 that is expressed on the vast majority of acute myeloid leukemia (AML) cells. [225Ac]Ac-lintuzumab clinical trials was discussed in detail in reference 65. An initial phase I dose-escalation trial demonstrated that for a single infusion of [225Ac]Ac-lintuzumab in patients with relapsed or refractory acute myeloid leukemia, the maximum tolerated dose (MTD) was determined to be 111 kBq/kg with antileukemic activity across all dose levels. No evidence of radiation-induced nephrotoxicity was seen. Peripheral blasts were eliminated in 63% of the patients at doses of > 37 kBq/kg. Bone marrow blast reduction was observed in 67% of patients 66. Subsequently, a multicenter phase I dose -escalation trial was conducted to define MTD, toxicity, and response rate of fractionated-dose [225Ac]Ac-lintuzumab when combined with low -dose cytarabine (LDAC) in older patients with untreated AML who were not candidates for intensive chemotherapy 67. Myelosuppression and infectious complications were common adverse events, and the MTD was not reached as responses were seen with patients receiving > 37 kBq/kg. A phase II trial of [225Ac]Ac-lintuzumab was conducted in patients age 60 years and older 68. This study resulted in a lower rate of myelosuppression for 22% of patients receiving two 55.5 kBq/kg fractions but a lower response rate than seen for 69% of patients receiving two fractions of 74 kBq/kg.
Patients receiving [225Ac]Ac-HuM195 have also been administered furosemide and spironolactone to prevent potential radiation-induced renal toxicity 69.
225Ac-DOTATOC for therapy of Neuroendocrine tumors
[225Ac]Ac-DOTATOC was investigated in vitro and in preclinical animal models 70. Therapy using [225Ac]Ac-DOTATOC was clinically tested with 39 patients with progressive neuroendocrine tumors71 the MTD of a single-cycle [225Ac]Ac-DOTATOC was considered to be 40 MBq while multiple fractions are tolerated with 25 MBq every 4 months or 18.5 MBq every two months.
225Ac substance P analogs for Glioma therapy
225Ac labeled DOTA-[Thi8, Met (O2)11]-substance P showed promising anti-tumor efficacy72. Clinical testing of intratumoral/intercavitary injection of 225Ac labeled DOTA-[Thi8, Met (O2)11]- substance P has been started at Medical University Warsaw in collaboration with JRC Karlsruhe. To date, >20 glioma patients have been treated with activities ranging from 10 to 42 MBq 225Ac labeled DOTA-[Thi8, Met (O2)11]- substance P. The treatment has been well tolerated, analysis of therapeutic efficacy, dose escalation and patient recruitment is ongoing 73.
Thorium-227
Chemical Properties and Decay
Thorium is an oxophilic metal with complicated coordination chemistry. At high pH (>7), thorium precipitates out of solution in the form of various water insoluble oxides thus making coordination difficult. Thorium is commonly found in the +4-oxidation state. The standard reduction potential of the Th(IV)/Th(III) couple is −3.7V74 which makes reduction difficult and makes the metal generally redox inactive. While thorium is a +4 metal, it can have coordination numbers from 4–15, the most common coordination number being eight75. Thorium-227 has a half-life of 18.7 days and decays via alpha particle emission to radium-223 (t1/2= 11.4 d), Radon-219 (t1/2= 3.96 s), polonium-215 (t1/2=1.78 ms), lead-211 (t1/2=36.1 min) , bismuth-211 (t1/2= 2.1 min), thallium-207 (t1/2= 4.77 min), and finally lead- 207 (Stable). In less than one half-life, 227Th and 223Ra exist in transient equilibrium. Two of the daughters of radium-223 decay are lead isotopes, 211Pb and 207Pb, which can compete with thorium for ligands in a labeling reaction. Both lead and thorium can exist as +4 metals and can be coordinated to the same ligand.
Production
Thorium-227 is obtained from the decay of 227Ac (t½= 21.8 years). The main source of 227Ac is neutron irradiation of 226Ra with thermal neutrons to produce 227Ra which decays via beta emission to 227Ac. The neutron irradiation can produce 227Ac in GBq quantities76 and be efficiently separated. The 227Th obtained from 227Ac, also contains 223Ra which is separated following the 227Th elution using ion chromatography resins. To perform this separation Boll et. al.30 used the MP1 anionic exchange resin, while McAlister et. al.77 used a combination of resins (UTEVA, DGA) and a prefilter resin.
To achieve optimal radiolabeling and subsequent analysis 227Th must be chelated immediately upon receipt or purified prior to radiolabeling. This eliminates interference with the daughters and ensures that only 227Th is available for labeling. Labeling with partially decayed thorium reduces the radiolabeling yields of 227Th, and more importantly, due to the unlabeled daughter isotopes, may increase the toxicity in vivo. 227Th can be purified using an anion exchange column. Thorium forms the [227Th][Th(NO3)6]2− complex at high concentrations of nitric acid (>7 M) 18, 30, 34, 78–80, and binds strongly to anion exchange resins. The daughters of thorium (radium, radon, polonium, lead, bismuth, thallium), do not form these anionic complexes in high concentrations of nitric acid, and elute from the column in the loading fraction as free metals. Thorium can be eluted by lowering the acid concentration and decomposing the [227Th][Th(NO3)6]2− complex. The employment of an anion exchange resin to purify or separate thorium is a convenient and important approach to ensure the purity of thorium.
The first decay daughter of 227Th is 223Ra, Figure 6, which will not radiolabel with conventional ligands, however, 223Ra contributes as a radioactive impurity that can lead to toxicity through bone localization. One of the daughters of 223Ra, 211Pb (t1/2= 36 minutes, β- 1.38 MeV), can compete with thorium for labeling with conventionally used chelators, especially DOTA. Lead can exist in either the +2 or +4 oxidation state. The ligand DOTA binds very well to Pb+4 (log ß = 24.3)81 and this will reduce yields of the Th+4 DOTA product. The presence of 211Pb can be a problem as it will compete for uptake with 227Th and therefore, reduce the amount of 227Th going to the target site. The other daughters of 223Ra will not bind DOTA efficiently and can redistribute and contribute to toxicity in vivo. One way to circumvent the presence of the 223Ra daughters in radioimmunotherapy (RIT) is to conduct experiments with thorium labeled antibodies using freshly purified thorium or perform a separation to isolate the complexed thorium82.
Figure 6.
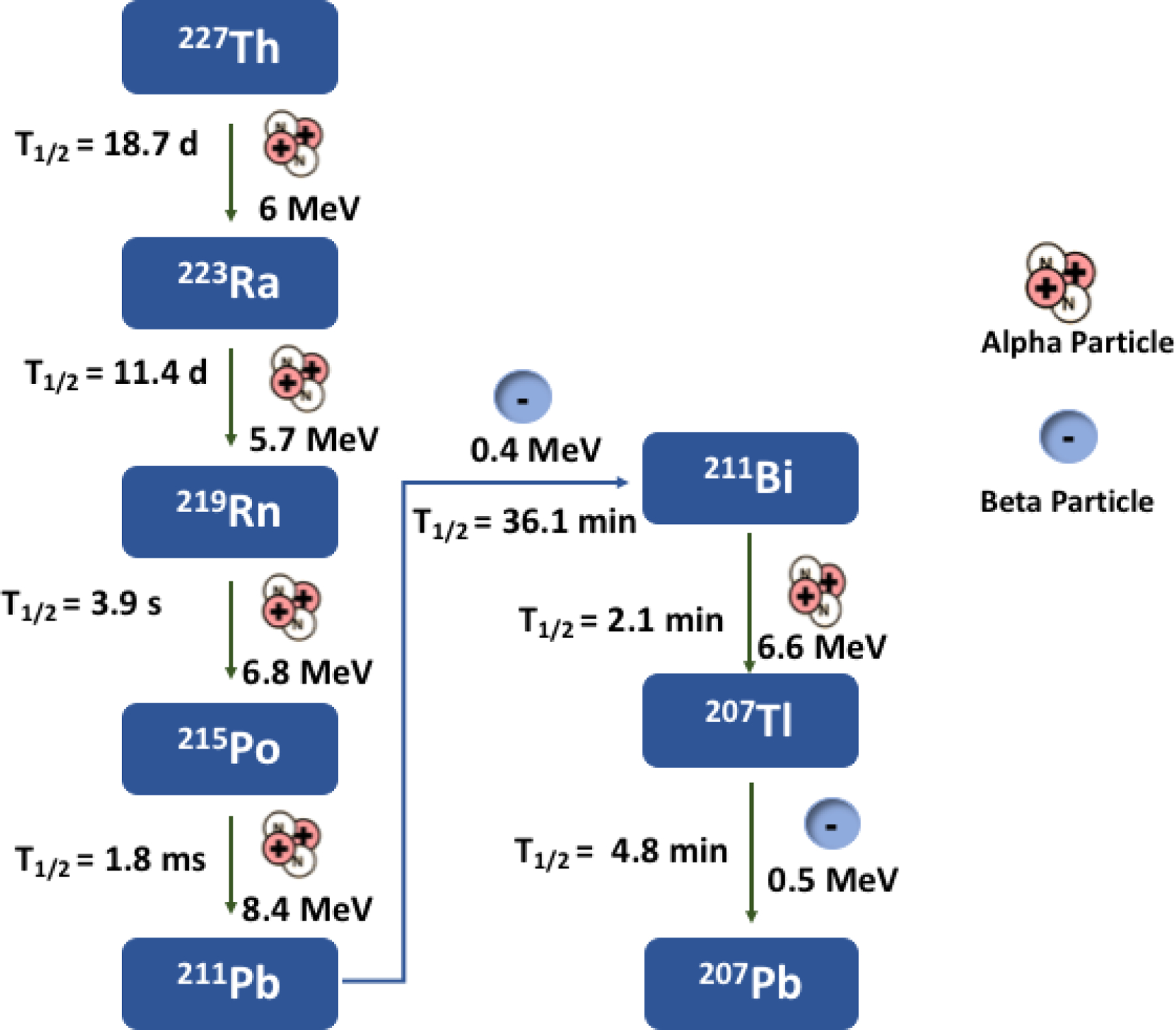
Decay Scheme of Th-227.
Thorium Labeling and Preclinical Studies
For all preclinical studies 227Th was eluted from a 227Ac generator in 12 M HCl, evaporated to dryness and the residue was suspended or dissolved in 0.01M HCl or 0.1M HNO3 and buffered for complexation. In early studies by Dahle and co-workers83, 227Th was originally conjugated to the antibody, rituximab, an anti-CD20 monoclonal antibody. This work used the bifunctional chelator, p-SCN-Bn-DOTA to coordinate 227Th to the antibody. 227Th eluted from the 227Ac generator was evaporated to dryness and the residue resuspended in 0.01M HCl. Subsequently the 227Th suspension was added to p-SCN-Bn-DOTA buffered at pH 5.5 for 40 min at 55 – 60ºC. The [227Th]Th-p-SCN-Bn-DOTA complex was then cooled to 37ºC and reacted with rituximab at pH 8–9 for 45 min. The thorium labeled antibodies demonstrated in vitro and in vivo stability with ≥75% of the labeled antibody remaining intact after 7 days84. The overall radiolabeling yield was 17% and the immunoreactivity of the [227Th]Th-DOTA-rituximab was 56–65%. The specific activity 500–1000 kBq/ug. The challenge with this work is that the authors do not say how many DOTA per antibody which may explain the low immunoreactivity.
[227Th]Th-DOTA-rituximab (200–1000 kBq/kg) was administered to mice bearing Raji lymphoma xenografts resulting in delayed tumor growth and prolonged mean survival when compared with a control group which were injected with cold rituximab. The mice did not experience serious toxicity. A significant tumor growth delay was achieved with a dose of 1000 kBq /kg (40 days) compared to the dose of 200 kBq /kg (17 days). The 227Th low-dose strategy appeared to be effective in the treatment of macroscopic tumor and single tumor cells85. A study was completed comparing the relative biological effects of low-dose and low dose-rate alpha radiation from [227Th]Th-rituximab to Zevalin ([90Y]Y-antibody) and external beam radiation. It was demonstrated that treatment with 227Th was 2.5 to 7.2 times more effective in inhibiting tumor growth than external beam radiation. Beta radiation treatment with [90Y]Y-Zevalin was 1 to 1.3 times more effective than external beam radiation86. Nasir and coworkers87 also compared the therapeutic effects of 177Lu and 227Th on HER2 expressing ovarian cancer tumors. Here, 227Th demonstrated a greater antitumor effect than 177Lu using the 4 Gy of radiation. The tumor growth was delayed further with 227Th and the mean survival of the mice was longer (129 days vs 88 days) Figure 7.
Figure 7.
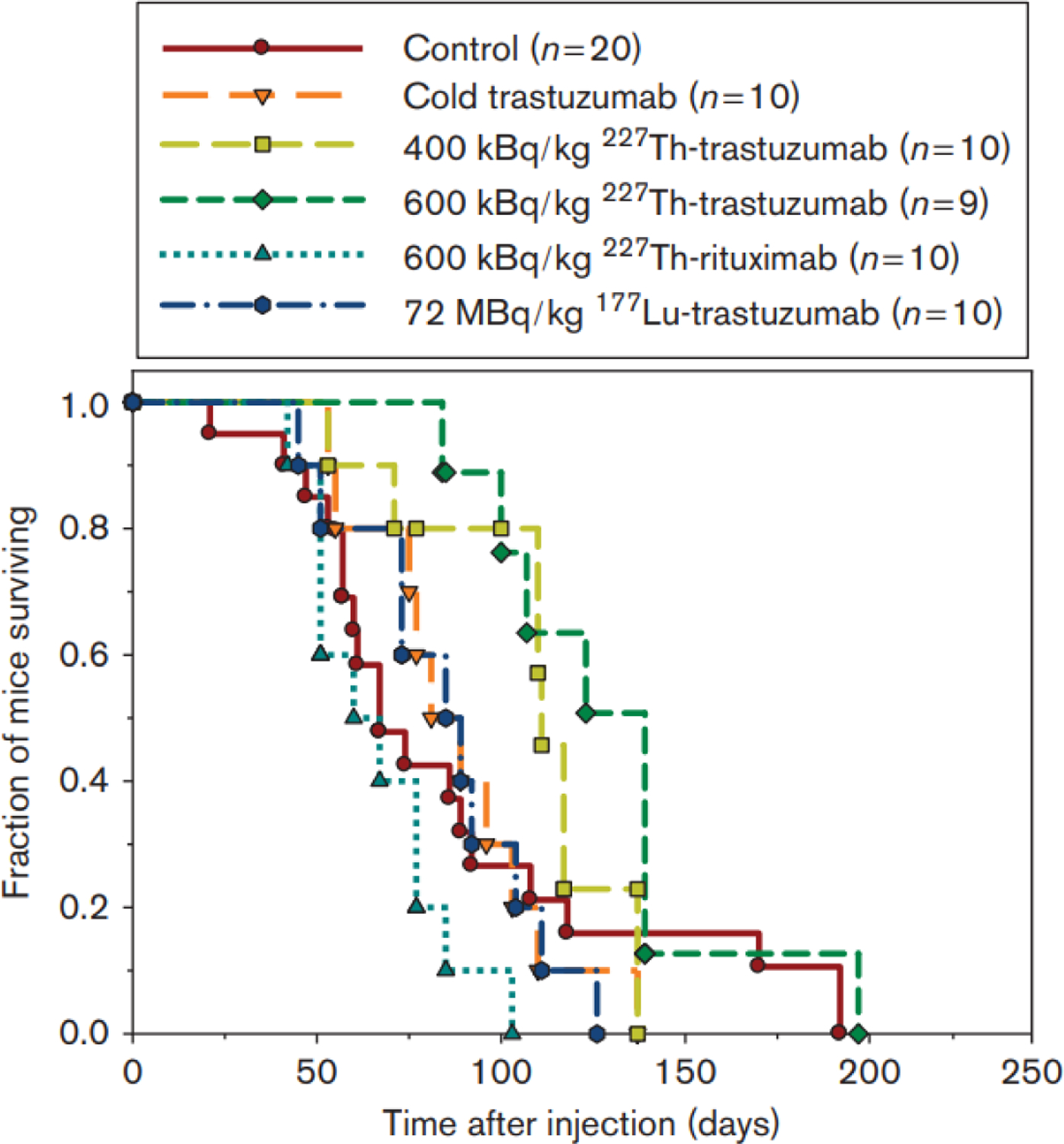
The Kaplan-Meier survival plot of mice treated with 177Lu-trastuzumab and 227Th-Trastuzumab.
Investigation of [227Th]Th-rituximab for long-term radiotoxicity involved injection of the labeled construct into tumor bearing animals at 52, 200, and 1000 kBq /kg and observation for one year. A total of 120 mice treated with 1000 kBq /kg experienced weight loss and a decrease in white blood cells and platelet count. This study determined that the “no-observed-adverse-effect” level was 200 kBq/kg and the “maximum tolerated activity” was determined to be between 592 –1000 kBq/kg.88 In these studies, [227Th]Th-DOTA-trastuzumab was evaluated in two HER-2 positive breast cancer cell lines (BT-474 and SKBR-3) and in the ovarian cell line SKOV-3. [227Th]Th-DOTA-trastuzumab inhibited cell growth and induced apoptosis in all cell lines at doses that were clinically relevant89. In fact, doses as low as 51.8 kBq/kg of 227Th antibody resulted in complete inhibition of tumor growth in mice bearing human renal cell carcinoma subcutaneous xenografts90. Myelosuppression was observed in days 44–65. However, the mice recovered by day 114 and the toxicity was considered transient. The recovery time is consistent with previously reported literature91.
DOTA is the ligand typically used for binding 227Th as it is FDA approved and commercially available. DOTA–antibody labeling has also been reported for 227Th by a 2-step method83 and by a 1-step method wherein the labeling proceeded overnight to afford high yield of the [227Th]Th-DOTA-antibody92. Both of these methods are not optimal. The “2-step” method used an isothiocyanate C-functionalized derivative of the DOTA chelator first radiolabeled with 227Th and then conjugated to the antibody at 37˚C.
Ramdahl prepared Me-2,3-HOPO, that is based on the methyl-2,3-hydroxypyridinone and is an 8-coordinate ligand93 as well as a bifunctional version of Me-2,3-HOPO Figure 8. The bifunctional Me-2,3-HOPO was conjugated to the CD33-targeting antibody lintuzumab and radiolabeled with 227Th. The 227Th construct induced in vitro cytotoxicity on CD33-positive cells, manifested by accumulated DNA double-strand breaks after exposure to the [227Th]Th-lintuzumab. In a subcutaneous xenograft mouse model using HL-60 cells, the 227Th conjugate demonstrated antitumor activity in a single dose injection of 500 kBq/kg. Dose-dependent survival was demonstrated in a mouse tumor model after single dose injection or after fractionated dose administration91.
Figure 8.
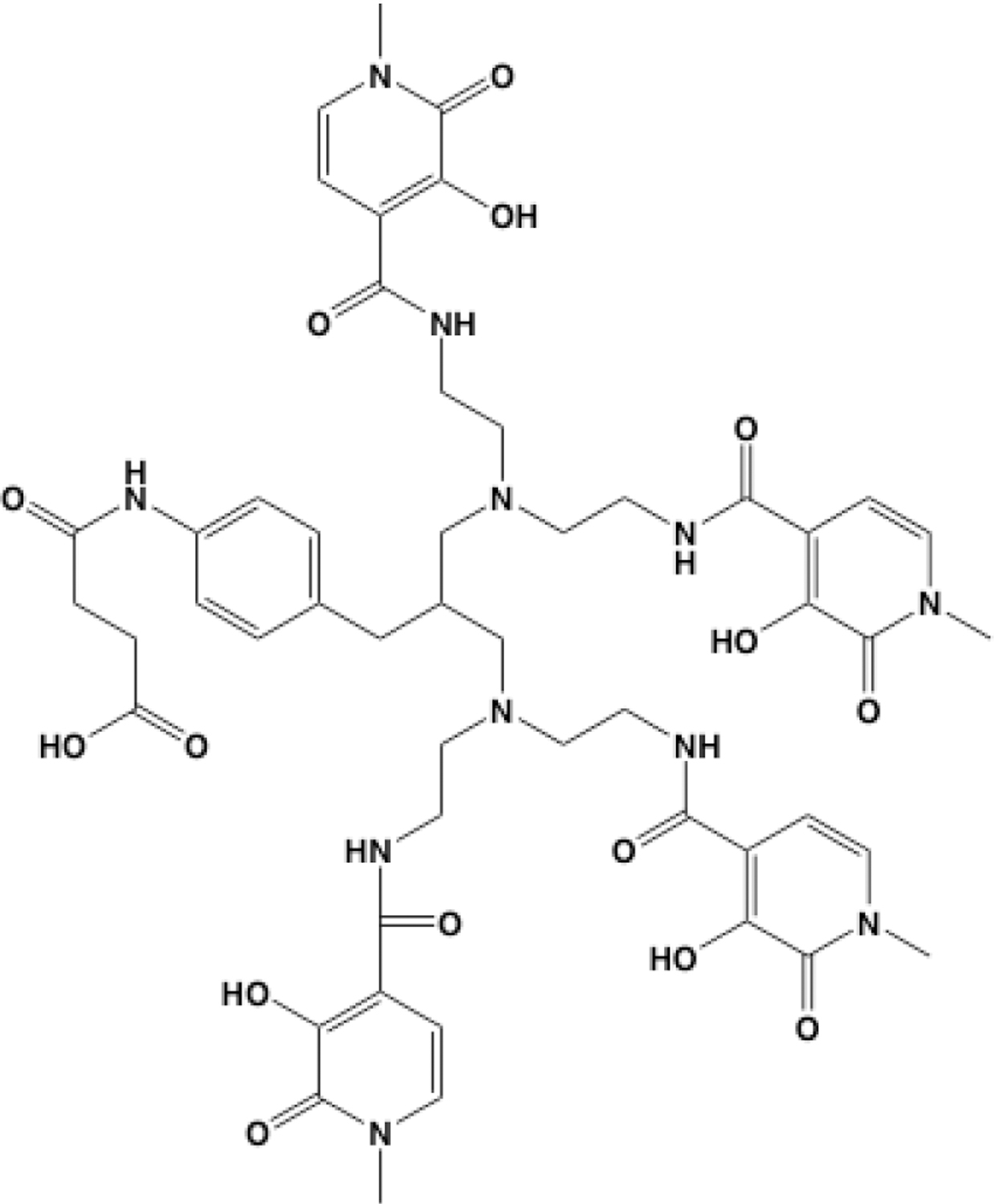
Me-2,3-HOPO
Preclinical investigation of 227Th labeled PSMA-IgG antibody provides encouragement for future clinical trials with this construct. 227Th-PMSA-IgG demonstrated tolerability and antitumor activity in several prostate cancer xenograft models. Tumor growth inhibition was observed with a single dose of 227Th-PMSA-IgG in prostate cancer xenograft models with moderate and high PMSA expression. In bone-metastatic PC model, a single dose of 227Th-PMSA-IgG reduced tumor burden, abnormal osteoblastic growth and serum PSA levels94. Dose -dependent antitumor activity was observed in hormone-sensitive patient derived PC xenograft models95.
Clinical Trials
Bayer is conducting clinical trials with 227Th however, the results of these trials have not been published as the trials are ongoing or have only recently ended. Currently, there are three clinical trials which are actively recruiting participants. The first, BAY 2315497, is a study to evaluate the safety, tolerability, pharmacokinetics and anti-tumor activity of a thorium-227 labeled antibody-chelator conjugate in patients with metastatic castration resistant prostate cancer96. Another study, BAY2701439 will be recruiting patients96. This is a first in human study to examine safety, how well the drug works in patients with advanced cancer where the protein HER2 is expressed. The study will include patients with HER2 expressing breast, gastric, or gastroesophageal cancers and any cancers that express HER2. The objective is to find the best dose for patients and to understand the pharmacokinetics of the drug. Another clinical study in the recruiting phase is the first human injection of BAY2287411, a thorium-227 labeled antibody-chelator conjugate, in patients with tumors that express mesothelin96. The purpose is to evaluate the safety, tolerability, maximum tolerated dose, anti-tumor activity, pharmacokinetics and recommended dose for further clinical development97.
Bayer has completed one study of the safety and tolerability of BAY1862864 injection in subjects with relapsed or refractory CD-2 positive Non-Hodgkins lymphoma98. This study concluded in December of 2019 and the results have not been reported at the time of this publication.
Conclusions
Recent approval of NetSpot and Lutathera has heightened interest in the use of targeted radiotherapy particularly to treat patients with metastatic disease. The first radionuclides conjugated to targeting molecules and used to treat disseminated disease have been used with the most studied beta emitting radionuclides, iodine-131 and yttrium-90. Largely due to the toxicity observed for Y-90, investigators began evaluating lutetium-177 as it could be imaged and its lower beta range resulted in lower toxicity to the kidney and normal tissues. Further it could be made in quantities sufficient to support clinical trials and drug development. However, although the use of 177Lu in clinical trials is safe it is not always effective in sterilizing disseminated disease. The shorter particle range and high LET of alpha particles offer the ability to deliver significant dose to tumor cells while reducing toxicity. A major stumbling block for using alpha emitting radionuclides was their limited availability and the need for improved chelators or labeling methods to ensure they remain stably complexed in vivo and deliver their payload to the tumor sites and minimize the dose that can arise due to dissociation. New production methods have come online such as the production of 225Ac via the high energy accelerator irradiation of 232Th which can result in 10s to 100 Curie levels of production. The DOE isotope program is now routinely supplying 225Ac produced in this manner and has demonstrated it to be similar to 225Ac obtained from the 229Th generator. This means supply is now at a level to support pre-clinical as well as clinical studies on a routine basis. Recently, the DOE submitted a drug master file to the FDA to support its clinical use. Further, other routes of production are being pursued such as the photonuclear route on 226Ra and the low energy cyclotron route on 226Ra. Thorium-227 which was not even available for pre-clinical studies is now available from multiple sites including DOE as a byproduct from 227Ac. This means supply is now at a level to support pre-clinical as well as clinical studies on a routine basis. New ligands are also being developed for both isotopes such as macropa, HEHA, and Me-2,3- HOPO which offer enhanced stability and mitigate toxicity. Additionally, some of the new liposomal or nanoparticle delivery systems could offer the ability to deliver higher doses and mitigate release of daughters to neighboring tissues. A further enhancement is the development of dosimetry methods for alpha particle emitting radionuclides that can further facilitate personalized medicine. We appear to be entering a phase where many advancements are finally allowing for the promise of targeted alpha therapy to come into existence. Although many see this as a new area, it is actually an area that was under investigation for some time and researchers are strongly recommended to go back and look at the pioneering work that was done. Due to technological advancements in targetry, production, chelation, detection, and dosimetry we can now build on this earlier work to facilitate the use of 225Ac and 227Th in both the pre-clinical and clinical setting.
Acknowledgements:
We are grateful to NSF-DGE-0965983 IGERT Program (LCF); NSF-1441401 Graduate Research Fellowship (JLHL, LCF); NIH 1R21 CA201999–01A1 (LCF); NIH/Clinical Translation Science Center, Weill Cornell University (LCF) and the DOE Nuclear Physics and Isotope Program (CSC).
References
- 1.Sgouros G; Ballangrud AM; Jurcic JG; McDevitt MR; Humm JL; Erdi YE; Mehta BM; Finn RD; Larson SM; Scheinberg DA, Pharmacokinetics and dosimetry of an a-paticle emitter antibody: 213Bi-HuM195 (anti-CD33) in patients with leukemia. J. Nucl. Mater 1999, 40, 1935–1946. [PubMed] [Google Scholar]
- 2.Thiele NA; Wilson JJ, Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother Radiopharm 2018, 33 (8), 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poty S; McDevitt Michael R; Lewis Jason S; Francesconi Lynn C; Francesconi Lynn C; McDevitt Michael R; Morris Michael J; Lewis Jason S, α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 1. Journal of Nuclear Medicine 2018, 59 (6), 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poty S; McDevitt Michael R; Lewis Jason S; Francesconi Lynn C; Francesconi Lynn C; McDevitt Michael R; Morris Michael J; Lewis Jason S, α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 2. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2018, 59 (7), 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgenstern A; Apostolidis C; Kratochwil C; Sathekge M; Krolicki L; Bruchertseifer F, An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm 2018, 11 (3), 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baidoo KE; Yong K; Brechbiel MW, Molecular Pathways: Targeted α-Particle Radiation Therapy. Clinical Cancer Research 2013, 19 (3), 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheinberg DA; McDevitt MR, Actinium-225 in targeted alpha-particle therapeutic applications. Current Radiopharmaceuticals 2011, 4 (4), 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radchenko V; Schaffer P; Knapp FF, The Evolving Clinical Role of Actinium-225 and Bismuth-213 for Targeted Alpha Therapy (TAT) - Production, Radiopharmaceutical Development and Clinical Applications. Curr. Radiopharm 2018, 11 (3), 154–155. [DOI] [PubMed] [Google Scholar]
- 9.Haberkorn U; Giesel F; Kratochwil C; Haberkorn U; Morgenstern A, The Future of Radioligand Therapy: α, β, or Both? Journal of Nuclear Medicine 2017, 58 (7), 1017–1018. [DOI] [PubMed] [Google Scholar]
- 10.Soyland C; Hassfjell SP, Survival of human lung epithelial cells following in vitro α-particle irradiation with absolute determination of the number of α-particle traversals of individual cells. International Journal of Radiation Biology 2000, 76 (10), 1315–1322. [DOI] [PubMed] [Google Scholar]
- 11.Behling K; Maguire WF; Di Gialleonardo V; Heeb LEM; Hassan IF; Veach DR; Keshari KR; Gutin PH; Scheinberg DA; McDevitt MR, Remodeling the vascular microenvironment of glioblastoma with α-particles. J. Nucl. Med 2016, 57 (11), 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behling K; Maguire WF; Puebla JCL; Sprinkle SR; Ruggiero A; O’Donoghue J; Gutin PH; Scheinberg DA; McDevitt MR, Vascular targeted radioimmunotherapy for the treatment of glioblastoma. J. Nucl. Med 2016, 57 (10), 1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kratochwil C; Giesel FL; Bruchertseifer F; Mier W; Apostolidis C; Boll R; Murphy K; Haberkorn U; Morgenstern A, 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. European Journal of Nuclear Medicine and Molecular Imaging 2014, 41 (11), 2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kratochwil C; Bruchertseifer F; Giesel FL; Weis M; Verburg FA; Mottaghy F; Kopka K; Apostolidis C; Haberkorn U; Morgenstern A, 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. Journal of Nuclear Medicine 2016, 57 (12), 1941–1944. [DOI] [PubMed] [Google Scholar]
- 15.Gorin J-B; Menager J; Gouard S; Maurel C; Guilloux Y; Faivre-Chauvet A; Morgenstern A; Bruchertseiferf F; Cherel M; Davodeau F; Gaschet J, Antitumor immunity induced after α irradiation. Neoplasia (Ann Arbor, MI, United States) 2014, 16 (4), 319–328, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wulbrand C; Seidl C; Gaertner FC; Bruchertseifer F; Morgenstern A; Essler M; Senekowitsch-Schmidtke R, Alpha-particle emitting 213Bi-anti-EGFR immunoconjugates eradicate tumor cells independent of oxygenation. PLoS One 2013, 8 (5), e64730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y-S; Brechbiel MW, An overview of targeted alpha therapy. Tumor biology 2012, 33 (3), 573–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastren T; Radchenko V; Brugh M; Engle JW; Nortier FM; Birnbaum ER; John KD; Fassbender ME; Radchenko V; Owens A; Copping R; Boll R; Griswold JR; Mirzadeh S; Wyant LE; Engle JW, Simultaneous Separation of Actinium and Radium Isotopes from a Proton Irradiated Thorium Matrix. Scientific reports 2017, 7 (1), 8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suominen MI; Fagerlund KM; Rissanen JP; Konkol YM; Morko JP; Peng ZQ; Alhoniemi EJ; Laine SK; Corey E; Mumberg D; Ziegelbauer K; Kakonen S-M; Halleen JM; Vessella RL; Scholz A, Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clinical Cancer Research 2017, 23 (15), 4335–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritter MA; Cleaver JE; Tobias CA, High-LET radiations induce a large proportion of non-rejoining DNA breaks. Nature (London, United Kingdom) 1977, 266 (5603), 653–5. [DOI] [PubMed] [Google Scholar]
- 21.Pandit-Taskar N; Larson SM; Carrasquillo JA, Bone-seeking radiopharmaceuticals for treatment of osseous metastases, part 1: α therapy with 223Ra-dichloride. J. Nucl. Med 2014, 55 (2), 268–274. [DOI] [PubMed] [Google Scholar]
- 22.Du Y; Carrio I; De Vincentis G; Fanti S; Ilhan H; Mommsen C; Nitzsche E; Sundram F; Vogel W; Oyen W; Lewington V, Practical recommendations for radium-223 treatment of metastatic castration-resistant prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging 2017, 44 (10), 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosley RB; Simpson JA, Choice of alpha-probe operating voltage to suit a wide range of conditions. Journal of radiological protection : official journal of the Society for Radiological Protection 2002, 22 (3), 293–303. [DOI] [PubMed] [Google Scholar]
- 24.Shannon RD, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica, Section A: Crystal Physics, Diffraction, Theoretical and General Crystallography 1976, A32 (5), 751–67. [Google Scholar]
- 25.Zielinska B; Bilewicz A, The hydrolysis of actinium. Journal of Radioanalytical and Nuclear Chemistry 2004, 261 (1), 195–198. [Google Scholar]
- 26.Lopez-Gonzalez H; Solache-Rios M; Jimenez-Reyes M; Ramirez-Garcia JJ; Rojas-Hernandez A, Effect of chloride ions on the hydrolysis of trivalent lanthanum, praseodymium and lutetium in aqueous solutions of 2M ionic strength. J. Solution Chemistry 2005, 34 (4), 427–441. [Google Scholar]
- 27.Ferrier MG; Batista ER; Berg JM; Birnbaum ER; Cross JN; Engle JW; La Pierre HS; Kozimor SA; Lezama Pacheco JS; Stein BW; Stieber SCE; Wilson JJ, Spectroscopic and computational investigation of actinium coordination chemistry. Nature Communications 2016, 7, 12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrier MG; Stein BW; Batista ER; Berg JM; Birnbaum ER; Engle JW; John KD; Kozimor SA; Lezama Pacheco JS; Redman LN, Synthesis and Characterization of the Actinium Aquo Ion. ACS Central Science 2017, 3 (3), 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis IA; Glowienka KA; Boll RA; Deal KA; Brechbiel MW; Stabin M; Bochsler PN; Mirzadeh S; Kennel SJ, Comparison of 225actinium chelates: tissue distribution and radiotoxicity. Nuclear Medicine and Biology 1999, 26 (5), 581–589. [DOI] [PubMed] [Google Scholar]
- 30.Boll Rose A; Malkemus D; Mirzadeh S, Production of actinium-225 for alpha particle mediated radioimmunotherapy. Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine 2005, 62 (5), 667–79. [DOI] [PubMed] [Google Scholar]
- 31.Griswold JR; Medvedev DG; Engle JW; Copping R; Fitzsimmons JM; Radchenko V; Cooley J; Fassbender M; Denton DL; Murphy KE, Large scale accelerator production of 225Ac: Effective cross sections for 78–192 MeV protons incident on 232Th targets. Applied Radiation and Isotopes 2016, 118, 366–374. [DOI] [PubMed] [Google Scholar]
- 32.Harvey J; Nolen JA; Kroc T; Gomes I; Horwitz EP; McAlister DR, Production of Actinium-225 via High Energy Proton Induced Spallation of Thorium-232. In Applications Of High Intensity Proton Accelerators, World Scientific: 2010; pp 321–326. [Google Scholar]
- 33.Weidner JW; Mashnik SG; John KD; Hemez F; Ballard B; Bach H; Birnbaum ER; Bitteker LJ; Couture A; Dry D; Fassbender ME; Gulley MS; Jackman KR; Ullmann JL; Wolfsberg LE; Nortier FM, Proton-induced cross sections relevant to production of 225Ac and 223Ra in natural thorium targets below 200 MeV. Applied Radiation and Isotopes 2012, 70 (11), 2602–2607. [DOI] [PubMed] [Google Scholar]
- 34.Mastren T; Radchenko V; Brugh M; Engle Jonathan W; Nortier Francois M; Birnbaum Eva R; John Kevin D; Fassbender Michael E; Radchenko V; Owens A; Copping R; Boll R; Griswold Justin R; Mirzadeh S; Wyant Lance E; Engle Jonathan W, Simultaneous Separation of Actinium and Radium Isotopes from a Proton Irradiated Thorium Matrix. Scientific reports 2017, 7 (1), 8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzsimmons J; Griswold J; Medvedev D; Cutler CS; Mausner L, Defining processing times for accelerator produced 225Ac and other isotopes from proton irradiated thorium. Molecules 2019, 24 (6), 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apostolidis C; Molinet R; McGinley J; Abbas K; Möllenbeck J; Morgenstern A, Cyclotron production of Ac-225 for targeted alpha therapy. Applied Radiation and Isotopes 2005, 62 (3), 383–387. [DOI] [PubMed] [Google Scholar]
- 37.Beyer G-J; Bergmann R; Schomäcker K; Rösch F; Schäfer G; Kulikov E; Novgorodov A, Comparison of the biodistribution of 225Ac and radio-lanthanides as citrate complexes. 1990.
- 38.McDevitt MR; Ma D; Simon J; Frank RK; Scheinberg DA, Design and synthesis of 225Ac radioimmunopharmaceuticals. Applied Radiation and Isotopes 2002, 57 (6), 841–847. [DOI] [PubMed] [Google Scholar]
- 39.Deal KA; Davis IA; Mirzadeh S; Kennel SJ; Brechbiel MW, Improved in vivo stability of actinium-225 macrocyclic complexes. Journal of medicinal chemistry 1999, 42 (15), 2988–2992. [DOI] [PubMed] [Google Scholar]
- 40.Henriksen G; RULAND ØS; Larsen RH, Thorium and actinium polyphosphonate compounds as bone-seeking alpha particle-emitting agents. Anticancer research 2004, 24 (1), 101–106. [PubMed] [Google Scholar]
- 41.Maguire WF; McDevitt MR; Smith-Jones PM; Scheinberg DA, Efficient 1-Step radiolabeling of monoclonal antibodies to high specific activity with 225Ac for α-particle radioimmunotherapy of cancer. J. Nucl. Med 2014, 55 (9), 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poty S; Membreno R; Glaser JM; Ragupathi A; Scholz WW; Zeglis BM; Lewis JS, The inverse electron-demand Diels-Alder reaction as a new methodology for the synthesis of (225)Ac-labelled radioimmunoconjugates. Chemical Communications (Cambridge, England) 2018, 54 (21), 2599–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu SL; Horrocks WD Jr., Direct determination of stability constants of lanthanide ion chelates by laser-excited europium(III) luminescence spectroscopy: application to cyclic and acyclic aminocarboxylate complexes. Journal of the Chemical Society, Dalton Transactions 1997, (9), 1497–1502. [Google Scholar]
- 44.Chappell LL; Deal KA; Dadachova E; Brechbiel MW, Synthesis, conjugation, and radiolabeling of a novel bifunctional chelating agent for 225Ac radioimmunotherapy applications. Bioconjugate Chemistry 2000, 11 (4), 510–519. [DOI] [PubMed] [Google Scholar]
- 45.Thiele NA; Brown V; Kelly JM; Amor‐Coarasa A; Jermilova U; MacMillan SN; Nikolopoulou A; Ponnala S; Ramogida CF; Robertson AK, An eighteen-membered macrocyclic ligand for actinium-225 targeted alpha therapy. Angewandte Chemie International Edition 2017, 56 (46), 14712–14717. [DOI] [PubMed] [Google Scholar]
- 46.McDevitt MR; Ma D; Lai LT; Simon J; Borchardt P; Frank RK; Wu K; Pellegrini V; Curcio MJ; Miederer M; Bander NH; Scheinberg DA, Tumor therapy with targeted atomic nanogenerators. Science (Washington, DC, United States) 2001, 294 (5546), 1537–1540. [DOI] [PubMed] [Google Scholar]
- 47.Jaggi JS; Henke E; Seshan SV; Kappel BJ; Chattopadhyay D; May C; McDevitt MR; Nolan D; Mittal V; Benezra R, Selective alpha-particle mediated depletion of tumor vasculature with vascular normalization. PLoS One 2007, 2 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodward J; Kennel Stephen J; Stuckey A; Osborne D; Wall J; Rondinone Adam J; Standaert Robert F; Mirzadeh S, LaPO4 nanoparticles doped with actinium-225 that partially sequester daughter radionuclides. Bioconjugate Chemistry 2011, 22 (4), 766–76. [DOI] [PubMed] [Google Scholar]
- 49.Ruggiero A; Villa CH; Holland JP; Sprinkle SR; May C; Lewis JS; Scheinberg DA; McDevitt MR, Imaging and treating tumor vasculature with targeted radiolabeled carbon nanotubes. International Journal of Nanomedicine 2010, 5, 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polkinghorn WR; Parker JS; Lee MX; Kass EM; Spratt DE; Iaquinta PJ; Arora VK; Yen W-F; Cai L; Zheng D, Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discovery 2013, 3 (11), 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodwin JF; Schiewer MJ; Dean JL; Schrecengost RS; de Leeuw R; Han S; Ma T; Den RB; Dicker AP; Feng FY, A hormone–DNA repair circuit governs the response to genotoxic insult. Cancer Discovery 2013, 3 (11), 1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDevitt MR; Thorek DLJ; Hashimoto T; Gondo T; Veach DR; Sharma SK; Kalidindi TM; Abou DS; Watson PA; Beattie BJ; Timmermand OV; Strand S-E; Lewis JS; Scardino PT; Scher HI; Lilja H; Larson SM; Ulmert D, Feed-forward alpha particle radiotherapy ablates androgen receptor-addicted prostate cancer. Nature Communications 2018, 9 (1), 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDevitt MR; Ma D; Simon J; Frank RK; Scheinberg DA, Design and synthesis of 225Ac radioimmunopharmaceuticals. Applied Radiation and Isotopes 2002, 57 (6), 841–847. [DOI] [PubMed] [Google Scholar]
- 54.Sofou S; Thomas JL; Lin H.-y.; McDevitt MR; Scheinberg DA; Sgouros G, Engineered liposomes for potential α-particle therapy of metastatic cancer. Journal of Nuclear Medicine 2004, 45 (2), 253–260. [PubMed] [Google Scholar]
- 55.Boerman OC; Storm G; Oyen WJ; van Bloois L; van der Meer JW; Claessens RA; Crommelin DJ; Corstens FH, Sterically stabilized liposomes labeled with indium-111 to image focal infection. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 1995, 36 (9), 1639–44. [PubMed] [Google Scholar]
- 56.Emfietzoglou D; Kostarelos K; Sgouros G, An analytic dosimetry study for the use of radionuclide–liposome conjugates in internal radiotherapy. Journal of Nuclear Medicine 2001, 42 (3), 499–504. [PubMed] [Google Scholar]
- 57.Rojas J; Woodward J; Chen N; Rondinone A; Castano C; Mirzadeh S, Synthesis and characterization of lanthanum phosphate nanoparticles as carriers for 223Ra and 225Ra for targeted alpha therapy. Nuclear Medicine and Biology 2015, 42 (7), 614–620. [DOI] [PubMed] [Google Scholar]
- 58.McLaughlin MF; Woodward J; Boll RA; Rondinone AJ; Mirzadeh S; Robertson JD, Gold-coated lanthanide phosphate nanoparticles for an 225Ac in vivo alpha generator. Radiochimica Acta 2013, 101 (9), 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salvanou E-A; Tsoukalas C; Paravatou-Petsotas M; Xanthopoulos S; Bouziotis P; Stellas D; Mavroidi B; Kalogeropoulos N; Denat F; Laurent G; Bazzi R; Roux S, A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics 2020, 12 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cedrowska E; Pruszynski M; Majkowska-Pilip A; Meczynska-Wielgosz S; Bruchertseifer F; Morgenstern A; Bilewicz A, Functionalized TiO2 nanoparticles labelled with 225Ac for targeted alpha radionuclide therapy. Journal of Nanoparticle Research 2018, 20 (3), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urbanska AM; Khanin R; Alidori S; Wong S; Mello BP; Almeida BA; Chen F; Ma K; Turker MZ; Korontsvit T, Scheinberg DA, Zanzonico PB, Wiesner U, Bradbury MS, Quinn TP, McDevitt MR A Genomic Profile of Local Immunity in the Melanoma Microenvironment Following Treatment with α Particle-Emitting Ultrasmall Silica Nanoparticles. Cancer Biotherapy and Radiopharmaceuticals 2020, Published Online:3 Feb 2020 10.1089/cbr.2019.3150. [DOI] [PMC free article] [PubMed]
- 62.Sattiraju A; Xiong X; Pandya DN; Wadas TJ; Xuan A; Sun Y; Jung Y; Sai KKS; Dorsey JF; Li KC, Alpha particle enhanced blood brain/tumor barrier permeabilization in glioblastomas using integrin Alpha-v Beta-3–targeted liposomes. Molecular Cancer Therapeutics 2017, 16 (10), 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kratochwil C; Haberkorn U; Giesel FL, 225Ac-PMSA-617 for therapy of prostate cancer. Seminars in Nuclear Medicine 2020, 50 (2), 133–140. [DOI] [PubMed] [Google Scholar]
- 64.Tagawa ST; Vallabhajosula S; Jhanwar Y; Ballman KV; Hackett A; Emmerich L; Babich J; Sartor AO; Harshman LC; Beltran H, Phase I dose-escalation study of 225Ac-J591 for progressive metastatic castration resistant prostate cancer (mCRPC). American Society of Clinical Oncology: 2018. [DOI] [PMC free article] [PubMed]
- 65.Jurcic JG, Targeted alpha-particle therapy for hematologic malignancies. Seminars in Nuclear Medicine 2020, 50 (2), 152–161. [DOI] [PubMed] [Google Scholar]
- 66.Jurcic JG; Rosenblat TL; McDevitt MR; Pandit-Taskar N; Carrasquillo JA; Chanel SM; Ryan C; Frattini MG; Cicic D; Larson SM; Scheinberg DA, Phase I trial of the targeted alpha-particle nano-generator actinium-225 (225Ac-lintuzumab) (anti-CD33; HuM195) in acute myeloid leukemia (AML). Journal of Clinical Oncology 2011, 29 (15_suppl), 6516–6516. [Google Scholar]
- 67.Jurcic JG; Levy MY; Park JH; Ravandi F; Perl AE; Pagel JM; Smith BD; Estey EH; Kantarjian H; Cicic D; Scheinberg DA, Phase I Trial of Targeted Alpha-Particle Therapy with Actinium-225 (225Ac)-Lintuzumab and Low-Dose Cytarabine (LDAC) in Patients Age 60 or Older with Untreated Acute Myeloid Leukemia (AML). Blood 2016, 128 (22), 4050–4050. [Google Scholar]
- 68.Finn LE; Levy M; Orozco JJ; Park JH; Atallah E; Craig M; Perl AE; Scheinberg DA; Cicic D; Bergonio GR; Berger MS; Jurcic JG, A Phase 2 Study of Actinium-225 (225Ac)-Lintuzumab in Older Patients with Previously Untreated Acute Myeloid Leukemia (AML) Unfit for Intensive Chemotherapy. Blood 2017, 130 (Supplement 1), 2638–2638. [Google Scholar]
- 69.Jaggi JS; Seshan SV; McDevitt MR; Sgouros G; Hyjek E; Scheinberg DA, Mitigation of radiation nephropathy after internal α-particle irradiation of kidneys. International Journal of Radiation Oncology* Biology* Physics 2006, 64 (5), 1503–1512. [DOI] [PubMed] [Google Scholar]
- 70.Miederer M; Henriksen G; Alke A; Mossbrugger I; Quintanilla-Martinez L; Senekowitsch-Schmidtke R; Essler M, Preclinical evaluation of the alpha particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin. Cancer Res 2008, 14 (11), 3555–3561. [DOI] [PubMed] [Google Scholar]
- 71.Kratochwil C; Bruchertseifer F; Giesel FL, EANM’15 presented at the Annual Meeting of the European Association of Nuclear Medicine and Molecular Imaging. European journal of nuclear medicine and molecular imaging 2015, 42 (1), 1–924.25319711 [Google Scholar]
- 72.Majkowska-Pilip A; Rius M; Bruchertseifer F; Apostolidis C; Weis M; Bonelli M; Laurenza M; Królicki L; Morgenstern A, In vitro evaluation of (225) Ac-DOTA-substance P for targeted alpha therapy of glioblastoma multiforme. Chemical Biology & Drug Design 2018, 92 (1), 1344–1356. [DOI] [PubMed] [Google Scholar]
- 73.Morgenstern A; Bruchertseifer F, Development of Targeted Alpha Therapy from Bench to Bedside. Journal of Medical Imaging and Radiation Sciences 2019, 50, S18–S20. [DOI] [PubMed] [Google Scholar]
- 74.Natrajan LS; Swinburne AN; Andrews MB; Randall S; Heath SL, Redox and environmentally relevant aspects of actinide(IV) coordination chemistry. Coordination Chemistry Reviews 2014, 266–267, 171–193. [Google Scholar]
- 75.Tutson CD; Gorden AEV, Thorium coordination: A comprehensive review based on coordination number. Coordination Chemistry Reviews 2017, 333, 27–43. [Google Scholar]
- 76.Kukleva E; Kozempel J; Vlk M; Mičolová P; Vopálka D, Preparation of 227Ac/223Ra by neutron irradiation of 226Ra. Journal of Radioanalytical and Nuclear Chemistry 2015, 304 (1), 263–266. [Google Scholar]
- 77.McAlister Daniel R; Horwitz EP, Chromatographic generator systems for the actinides and natural decay series elements. In Radiochimica Acta International journal for chemical aspects of nuclear science and technology, 2011; Vol. 99, p 151. [Google Scholar]
- 78.Hogle S; Boll RA; Murphy K; Denton D; Owens A; Haverlock TJ; Garland M; Mirzadeh S, Reactor production of Thorium-229. Applied Radiation and Isotopes 2016, 114, 19–27. [DOI] [PubMed] [Google Scholar]
- 79.Mirzadeh S; Copping R; Radchenko V; Fassbender M; Murphy K; Denton D; Owens a.; Boll RA; Griswold J; Fitzsimmons J; Medvedev DG; Mausner LF, Challenges in chemical separation of 225Ac produced via proton irradiation of 232Th target. Abstracts of Papers, 252nd ACS National Meeting & Exposition, Philadelphia, PA, United States, August 21–25, 20162016, NUCL-48. [Google Scholar]
- 80.Webb O; Boll R; Lucero A; DePaoli D, Purification of thorium from uranium-233 process residue. Separation Science and Technology 1999, 34 (6–7), 975–985. [Google Scholar]
- 81.Pippin CG; McMurry TJ; Brechbiel MW; McDonald M; Lambrecht R; Milenic D; Roselli M; Colcher D; Gansow OA, Lead (II) complexes of 1, 4, 7, 10-tetraazacyclododecane-N, N′, N ″, N‴-tetraacetate: solution chemistry and application to tumor localization with 203Pb labeled monoclonal antibodies. Inorganica Chimica Acta 1995, 239 (1–2), 43–51. [Google Scholar]
- 82.Frenvik JO; Dyrstad K; Kristensen S; Ryan OB, Development of separation technology for the removal of radium-223 from targeted thorium conjugate formulations. Part II: purification of targeted thorium conjugates on cation exchange columns. Drug Development and Industrial Pharmacy 2017, 43 (9), 1440–1449. [DOI] [PubMed] [Google Scholar]
- 83.Dahle J; Borrebaek J; Melhus KB; Bruland OS; Salberg G; Olsen DR; Larsen RH, Initial evaluation of 227Th-p-benzyl-DOTA-rituximab for low-dose rate α-particle radioimmunotherapy. Nucl. Med. Biol 2006, 33 (2), 271–279. [DOI] [PubMed] [Google Scholar]
- 84.Larsen RH; Borrebaek J; Dahle J; Melhus KB; Krogh C; Valan MH; Bruland OS, Preparation of Th227-labeled radioimmunoconjugates, assessment of serum stability and antigen binding ability. Cancer Biother. Radiopharm 2007, 22 (3), 431–437. [DOI] [PubMed] [Google Scholar]
- 85.Dahle J; Borrebaek J; Jonasdottir TJ; Hjelmerud AK; Melhus KB; Bruland OS; Press OW; Larsen RH, Targeted cancer therapy with a novel low-dose rate α-emitting radioimmunoconjugate. Blood 2007, 110 (6), 2049–2056. [DOI] [PubMed] [Google Scholar]
- 86.Dahle J; Bruland OS; Larsen RH, Relative Biologic Effects of Low-Dose-Rate α-Emitting 227Th-Rituximab and β-Emitting 90Y-Tiuexetan-Ibritumomab Versus External Beam X-Radiation. International Journal of Radiation Oncology, Biology, Physics 2008, 72 (1), 186–192. [DOI] [PubMed] [Google Scholar]
- 87.Abbas N; Bruland ØS; Brevik EM; Dahle J, Preclinical evaluation of 227Th-labeled and 177Lu-labeled trastuzumab in mice with HER-2-positive ovarian cancer xenografts. Nuclear Medicine Communications 2012, 33 (8), 838–847. [DOI] [PubMed] [Google Scholar]
- 88.Dahle J; Jonasdottir TJ; Heyerdahl H; Nesland JM; Borrebaek J; Hjelmerud AK; Larsen RH, Assessment of long-term radiotoxicity after treatment with the low-dose-rate alpha-particle-emitting radioimmunoconjugate 227Th-rituximab. European Journal of Nuclear Medicine and Molecular Imaging 2010, 37 (1), 93–102. [DOI] [PubMed] [Google Scholar]
- 89.Heyerdahl H; Krogh C; Borrebaek J; Larsen Ñ; Dahle J, Treatment of HER2-expressing breast cancer and ovarian cancer cells with alpha particle-emitting 227Th-trastuzumab. Int J Radiat Oncol Biol Phys 2011, 79 (2), 563–70. [DOI] [PubMed] [Google Scholar]
- 90.Hagemann Urs B; Mihaylova D; Uran Steinar R; Borrebaek J; Grant D; Bjerke Roger M; Karlsson J; Cuthbertson Alan S, Targeted alpha therapy using a novel CD70 targeted thorium-227 conjugate in in vitro and in vivo models of renal cell carcinoma. Oncotarget 2017, 8 (34), 56311–56326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hagemann UB; Wickstroem K; Wang E; Shea AO; Sponheim K; Karlsson J; Bjerke RM; Ryan OB; Cuthbertson AS, In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia. Molecular Cancer Therapeutics 2016, 15 (10), 2422–2431. [DOI] [PubMed] [Google Scholar]
- 92.Abbas N; Heyerdahl H; Bruland OS; Borrebak J; Nesland J; Dahle J, Experimental α-particle radioimmunotherapy of breast cancer using 227Th-labeled p-benzyl-DOTA-trastuzumab. EJNMMI Research 2011, 1 (1), 18/1–18/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramdahl T; Bonge-Hansen HT; Ryan OB; Larsen A; Herstad G; Sandberg M; Bjerke RM; Grant D; Brevik EM; Cuthbertson AS, An efficient chelator for complexation of thorium-227. Bioorganic & Medicinal Chemistry Letters 2016, 26 (17), 4318–4321. [DOI] [PubMed] [Google Scholar]
- 94.Hammer S; Larssen A; Ellingsen C; Geraudie S; Grant D; Indrevoll B; Ahsen O. v.; Kristian A; Hagemann UB; Karlsson J; Bjerke RM; Ryan OB; Mumberg D; Kreft B; Cuthbertson A, Abstract 5200: Preclinical pharmacology of the PSMA-targeted thorium-227 conjugate PSMA-TTC: a novel targeted alpha therapeutic for the treatment of prostate cancer. Cancer Research 2017, 77 (13 Supplement), 5200. [Google Scholar]
- 95.Hammer S; Hagemann UB; Zitzmann-Kolbe S; Larsen A; Ellingsen C; Ahsen O. v.; Karlsson J; Bjerke RM; Ryan OB; Lejeune P; Hennekes H; Cuthbertson A; Ziegelbauer K; Mumberg D, Abstract 844: Preclinical activity of PSMA-TTC, a targeted alpha therapeutic in patient-derived prostate cancer models. Cancer Research 2018, 78 (13 Supplement), 844. [Google Scholar]
- 96.clinicaltrials.gov/ct2/show/NCT03724747?term=thorium&cond=Metastatic+Castration-resistant+Prostate+Cancer&draw=2&rank=1.
- 97.Hagemann Urs B; Schatz Christoph A; Kneip C; Golfier S; Hennekes H; Mumberg D; Ziegelbauer K; Ellingsen C; Kristian A; Mobergslien A; Cruciani V; Wickstroem K; Smeets R; Uran S; Karlsson J; Bjerke Roger M; Ryan Olav B; Cuthbertson Alan S; Schuhmacher J, Mesothelin-Targeted Thorium-227 Conjugate (MSLN-TTC): Preclinical Evaluation of a New Targeted Alpha Therapy for Mesothelin-Positive Cancers. Clinical Cancer Research 2019, 25 (15), 4723–4734. [DOI] [PubMed] [Google Scholar]
- 98.Safety and Tolerability of BAY1862864 Injection in Subjects With Relapsed or Refractory CD22-positive Non-Hodgkin’s Lymphoma https://clinicaltrials.gov/ct2/show/NCT02581878?term=thorium&cond=Non+Hodgkin+Lymphoma&draw=2&rank=1).


