Abstract
Introduction:
Patients with relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL) require further treatment options, especially in cases that cannot tolerate stem cell transplant or cytotoxic chemotherapy. CD19 has emerged as an attractive target in B-cell malignancy and is the subject of several therapeutic strategies. The anti-CD19, humanized, monoclonal antibody tafasitamab (MOR208) has an engineered, modified Fc region with increased affinity for Fcγ receptors, leading to increased cytotoxicity via natural killer cells and macrophages (antibody-dependent cellular cytotoxicity and antibody-dependent cell-mediated phagocytosis) in a promising approach.
Areas covered:
The development of tafasitamab is reviewed, together with the pharmacokinetics and clinical experience of tafasitamab in R/R DLBCL; clinical data have led to FDA approval and inclusion in NCCN treatment guidelines for tafasitamab in combination with lenalidomide in this indication.
Expert opinion:
Patients with R/R DLBCL who have failed rituximab-containing regimens may be resistant to CD20-directed therapies; therefore, therapies with an alternative mode of action are of great interest in this setting. Tafasitamab, an anti-CD19 monoclonal antibody, in combination with lenalidomide has demonstrated promising efficacy for patients with R/R DLBCL who are ineligible for autologous stem cell transplantation. This could provide an alternative approach to classical chemotherapy-based regimens in the relapsed setting.
Keywords: CD19, diffuse large B-cell lymphoma/DLBCL, immunotherapy, lenalidomide, MOR208, non-Hodgkin’s lymphoma/NHL, tafasitamab
1. Introduction
Worldwide, non-Hodgkin’s lymphoma (NHL) was responsible for ~509,590 new cases and 248,724 deaths in 2018 [1]. The most common aggressive subtype is the diffuse large B-cell lymphoma (DLBCL) (30–35% [2]) [3]. Most DLBCL cases are diagnosed in patients ≥65 years old, and 35–40% of patients relapse after initial therapy [3,4]. In relapsing/refractory (R/R) patients, 12-month survival has been estimated at <30% [4].
R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone), remains the first-line standard of care (SOC) for DLBCL [5,6]. Less-aggressive chemotherapy may be required in frail patients or those with comorbidities [5,6]. In R/R disease, salvage chemotherapy followed – in responding cases – by autologous stem cell transplantation (ASCT) consolidation is preferred if patients can tolerate it. Other second-line options are usually palliative. ASCT-ineligible patients receive platinum-and/or gemcitabine-based regimens, bendamustine, palliative care or entry into a clinical trial [6,7]. Without a generally approved SOC, development of additional regimens is urgently required. Options are dependent on geographic location and may include alternative chemotherapy regimens (± rituximab), polatuzumab vedotin (plus bendamustine-rituximab) or brentuximab vedotin (in CD30+ cases), and ibrutinib or lenalidomide ± rituximab in untreated non-germinal center B-cell (non-GCB) DLBCL. A further option approved in the third-line is anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy, which is potentially curative, and for which patients may be eligible even if ASCT is not an option for them [6].
2. Overview of the treatment landscape
R/R DLBCL patients failing first-line regimens have limited options. Intensive or repetitive chemotherapy, or ASCT are usually not possible in frail patients and, given that most patients are ≥65 years old at diagnosis, tolerability is a prominent concern.
CAR-T therapy is an area of great interest. Two second-generation CD19-targeted CAR-T therapies (axicabtagene ciloleucel and tisagenlecleucel) are FDA approved in R/R DLBCL, with 6-month overall response rates (ORRs) of ~50%, and evaluation is ongoing in earlier settings [5,8–11]. A Biologics License Application has been submitted for a third CD19-targeted CAR-T therapy, lisocabtagene maraleucel in R/R large B-cell lymphoma, including DLBCL [12]. Although CAR-T therapies offer durable responses, various barriers prevent their widespread uptake, including: 1) neurologic toxicity and the risk of cytokine release syndrome; intensive management of these events necessitates administration at a specialized or large academic center; 2) manufacturing time and delay, precluding their use for rapidly growing tumors; and 3) high cost [8,9,13]. Hence, it is possible that only selected patients can benefit from CAR-T therapy. Various approaches to mitigate toxicity or enhance effectiveness are in development, including redesigned CAR-T cells, bispecific CAR-T cells, and armored CAR-T cells [9,13].
A few small-molecule targeted therapies are available in DLBCL. The Bruton’s tyrosine kinase inhibitor, ibrutinib, and lenalidomide are under evaluation alone or in combination in non-GCB DLBCL [6,14–16], and inhibitors of other targets, such as PI3Kδ and bromodomain, are in early development [9]. Selinexor is a selective inhibitor of exportin 1, a nuclear export protein for tumor-suppressor proteins, under investigation in various hematologic cancers. The FDA has recently approved selinexor for R/R DLBCL after ≥2 prior therapies [17].
Bispecific CD20-targeted agents, which link and activate T cells to tumor targets, are also in development. Antibody–drug conjugates comprise a cytotoxic component attached to the targeting antibody, and the CD79b-targeted polatuzumab vedotin is FDA approved [18]. The CD30-targeting brentuximab vedotin is recommended in ≥2nd-line for transplant-ineligible CD30+ R/R DLBCL [19] and polatuzumab vedotin plus bendamustine and rituximab is indicated for transplant-ineligible R/R DLBCL [6].
CD19 is a key target for novel antibody-based approaches, including bispecific T-cell antibodies. Blinatumomab (anti-CD19/anti-CD3) is being investigated in combination with other immunomodulatory agents, although neurologic toxicity was apparent in Phase I/II monotherapy studies [9,20,21]. Loncastuximab tesirine is another antibody–drug conjugate under development [22].
We will focus on tafasitamab (MOR208/XmAb5574) as a treatment option in R/R DLBCL (Box 1).
Box 1. Drug summary box.
| Drug name | Tafasitamab |
| Phase | II |
| Indication | Relapsed/refractory DLBCL |
| Pharmacology/mechanism of action | Humanized anti-CD19 monoclonal antibody with modified Fc for enhanced ADCC and ADCP |
| Route of administration | Intravenous |
| Pivotal trial | L-MIND (NCT02399085): ongoing open-label Phase II single-arm multicenter study of tafasitamab (12 mg/kg i.v. once weekly) and lenalidomide (25 mg/day orally, Days 1–21) for up to twelve 28-day cycles, followed by tafasitamab monotherapy in patients with DLBCL and 1–3 prior systemic regimens who are not candidates for high-dose chemotherapy and ASCT |
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cell-mediated phagocytosis; ASCT, autologous stem cell transplant; DLBCL, diffuse large B-cell lymphoma; i.v., intravenously.
3. Introduction to the compound
With its multi-faceted mode of action, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and apoptosis induction, alone and in combination with chemotherapy, coupled with a relatively benign toxicity profile, rituximab quickly became a standard component of NHL regimens [23,24]. Research into other anti-CD20 antibodies followed, but the development of resistance to CD20-directed therapies necessitates the use of alternative targets [25].
CD19 is a glycoprotein B-cell surface molecule essential for B-cell signaling and balancing humoral, antigen-induced responses and tolerance induction [26]. It is expressed throughout B-cell development in most B-cell lymphomas, including DLBCL [27,28]. Antibody-mediated cytotoxicity via ADCC and antibody-dependent cell-mediated phagocytosis (ADCP) are commonly regulated via interaction between the Fc antibody chain and Fcγ receptors (FcγR) on immune effector cells: natural killer (NK) cells, macrophages and γδ T-cells [29–31]. Modification of human Fc to enhance FcγRIIIa-mediated ADCC and ADCP [31] led to the development of tafasitamab for a range of hematologic malignancies, with the initial clinical study R/R chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) [32].
4. Structure of the compound
Tafasitamab is a humanized anti-CD19 monoclonal antibody with an affinity-matured murine Fv region and a human Fc domain containing S239D/I332E substitutions [30]. These modifications increase FcγRIIIa binding compared with the unmodified parent immunoglobulin (Ig)G1 CD19 antibody, demonstrated in vitro and in vivo in leukemia and lymphoma models [30,33]. Tafasitamab was designed via computational structure-based protein design methods and high-throughput protein screening [31]. Human Fc with S239D and I332E mutations has 70–254-fold enhanced affinity for V158 FcγRIIIa compared with wild-type Fc (IC50 –9.44M and –8.83M by alemtuzumab AlphaScreen and trastuzumab AlphaScreen, respectively; KD 2 nM by trastuzumab surface plasmon resonance), and 31–63-fold enhancement (IC50 –8.70M and –8.10M by alemtuzumab AlphaScreen and trastuzumab AlphaScreen, respectively) for F158 FcγRIIIa [31]. Similarly, tafasitamab displayed increased FcγR binding compared with an anti-CD19 IgG1 analog with the same Fv domain and wild-type Fc (47-fold increased affinity for V158 FcγRIIIa, and 136-fold for F158 FcγRIIIa); enhanced FcγRIIIa binding was associated with increased caspase-induced antiproliferative apoptosis in target cells, possibly as a result of increased cell-surface CD19 cross-linking. Importantly, CD19 binding by tafasitamab was associated with minimal receptor internalization, which may otherwise affect the antibody effector function [30].
5. Pharmacodynamics
Tafasitamab’s mode of action comprises direct cytotoxicity with enhanced ADCC and ADCP, mediated through modification of the antibody Fc portion (Figure 1) [30].
Figure 1.
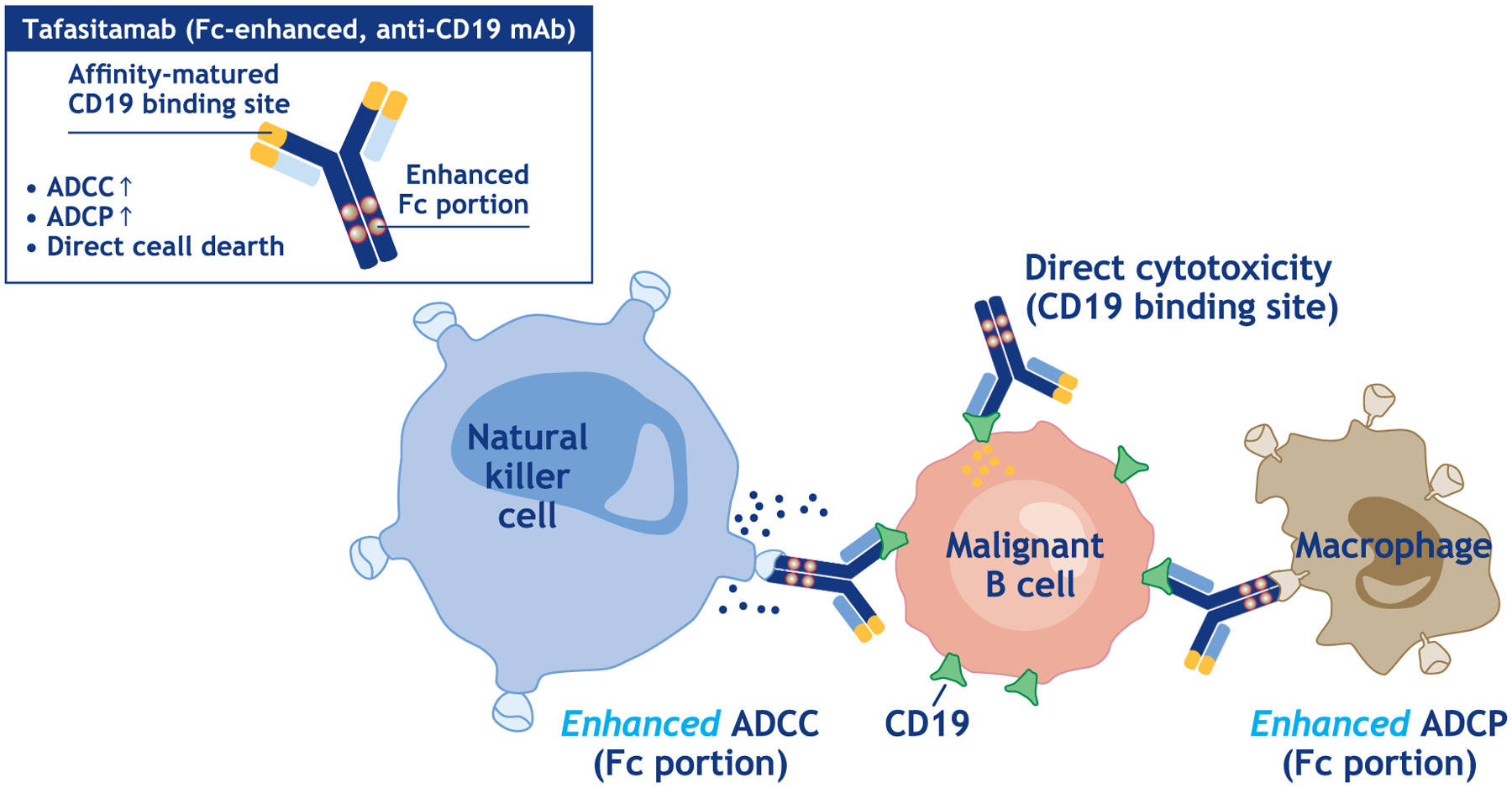
Mode of action of tafasitamab [38].
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cell-mediated phagocytosis; mAb, monoclonal antibody.
Tafasitamab displayed 100–1000-fold increased in vitro ADCC (EC50 0.1–1.0 ng/mL) compared with an anti-CD19 IgG1 analog against a range of B-lymphoma and leukemia cell lines; ADCC was not correlated with the level of cell-surface CD19 expression [30]. Both the humanized Fv region and substituted Fc antibody regions were necessary to induce ADCC. Enhanced ADCC was also shown in acute lymphoblastic leukemia (ALL) and mantle cell lymphoma tumor cells compared with the IgG1 analog, which showed no detectable ADCC [30]. Tafasitamab also demonstrated a 10-fold increased in vitro ADCP compared with the IgG1 analog in monocyte-derived macrophages and two ALL cell lines [30].
Data from 14/27 patients with R/R CLL/SLL enrolled in the Phase I study of tafasitamab indicate that tafasitamab does not induce the loss of CD19 expression on CLL cells [34]. After a median of 84 days following the last tafasitamab dose, the median CD19 expression was 109% (range, 71–166%), relative to baseline [34].
Pre-treatment of macrophages with lenalidomide enhanced tafasitamab-associated cytotoxicity by 3–5 fold in lymphoma cell lines and autologous lymphoma cells, which could not be attributed to lenalidomide alone or target-cell modification of CD19 expression. The recruitment of lymphoma-associated macrophages as effector cells via a combination of tafasitamab with the immunomodulatory agent lenalidomide is therefore an attractive strategy [35].
6. Pharmacokinetics and immunogenicity
In the Phase II MOR208C201 study (N = 92, R/R NHL), the mean terminal half-life of tafasitamab was ~16 days, and mean steady-state Cmax and trough levels were observed at ~500 μg/mL and ~250 μg/mL, respectively, at the end of the (initially) weekly dosing of 12 mg/kg [36]. A pharmacokinetic model based on these data showed that a trough level of ~150 μg/mL would be maintained with two weekly maintenance dosing (Figure 2) [37].
Figure 2.
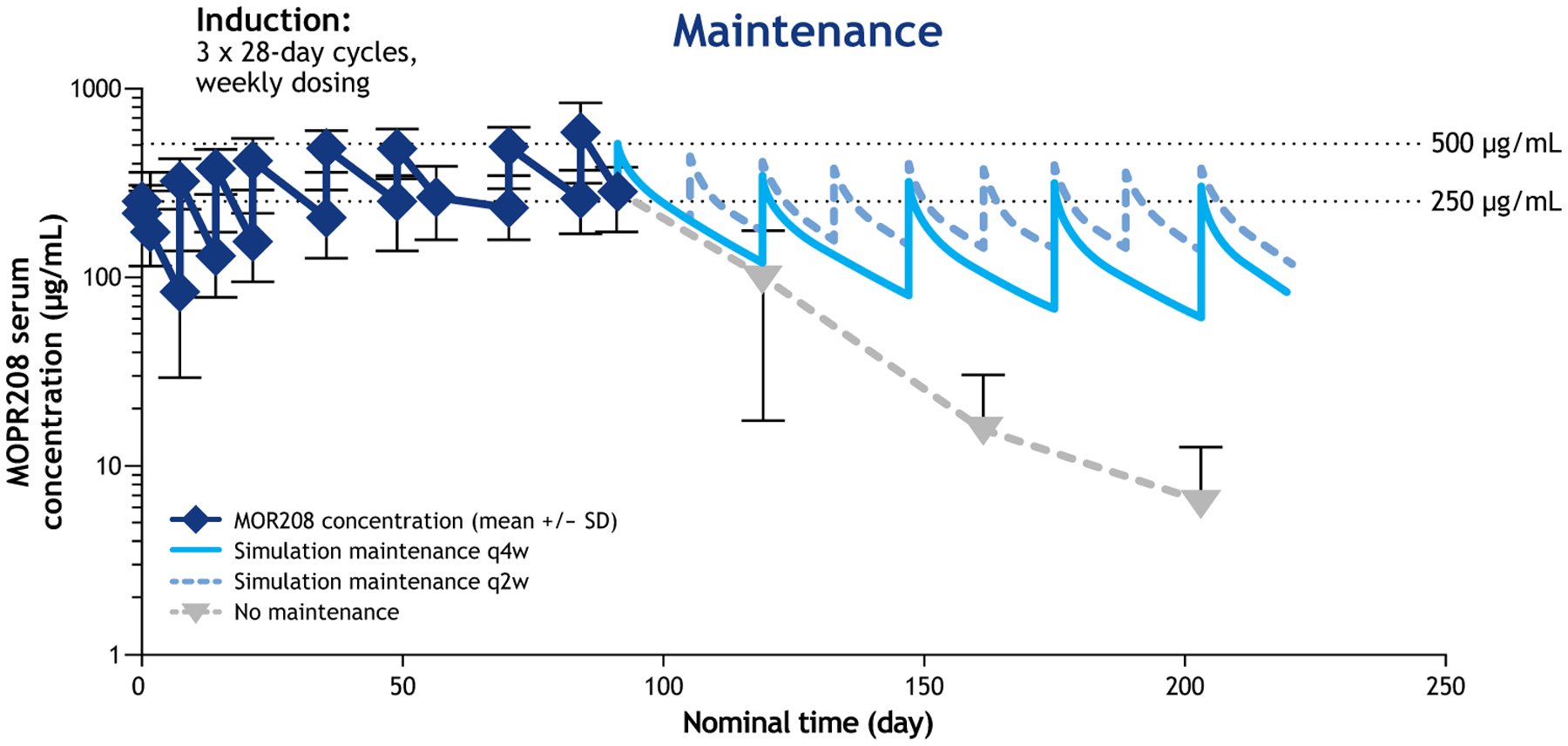
Pharmacokinetics of tafasitamab in patients with R/R NHL [37].
Notes: Mean values +/− SD are shown for the observed MOR208 concentrations (lower error bars not shown for 161 and 203 days).
Abbreviations: q2w, every 2 weeks; q4w, every 4 weeks; SD, standard deviation.
No treatment-emergent or treatment-boosted anti-drug antibodies were observed in trial MOR208C201 or L-MIND [38,39].
7. Clinical efficacy
7.1. Phase I studies
Dose-finding, dose-limiting toxicity, and preliminary efficacy of tafasitamab were investigated in a Phase I study in R/R CLL/SLL (N = 27) [32]. Tafasitamab was well tolerated; no maximum tolerated dose was reached across the investigated range of 3–12 mg/kg, 12 mg/kg weekly was established as the recommended dose in further studies. Infusion reactions were common but manageable, and generally did not recur during repeated dosing. Tafasitamab demonstrated preliminary efficacy with 66.7% of patients achieving partial response (PR) and the remaining 33.3% achieving stable disease.
Dosing at 12 mg/kg weekly has been used in Phase II studies in R/R CLL and later protocols in DLBCL (Table 1) [36,39,40].
Table 1.
Tafasitamab clinical trial overview.
| Trial name | NCT reference number | Phase | Setting/indication | Regimen | Status (May 2020) |
|---|---|---|---|---|---|
| First-MIND | NCT04134936 | Ib | 1 L DLBCL | 6 × 21-day cycles of tafasitamab plus R-CHOP ± lenalidomide (25 mg/day, Days 1–10) [47] | Recruiting |
| MOR208C201 | NCT01685008 | IIa | R/R NHL | Tafasitamab (12 weeks); extended treatment available for responding patients [36] [38] | Active, not recruiting |
| L-MIND | NCT02399085 | II | R/R DLBCL | 12 × 28-day cycles of tafasitamab + lenalidomide (25 mg/day, Days 1–21), then tafasitamab monotherapy [39] [41] | Active, not recruiting |
| B-MIND | NCT02763319 | II/III | R/R DLBCL | 6 cycles of tafasitamab + bendamustine (90 mg/m2 i.v.) vs rituximab (375 mg/m2 i.v.) + bendamustine, followed by tafasitamab or rituximab monotherapy for up to 18 additional cycles in responding patients [43] | Recruiting |
| COSMOS | NCT02639910 | II | R/R CLL/SLL | Tafasitamab (28-day cycles: Cycles 1–3, weekly; Cycles 4–6, every 2 weeks; then from Cycle 7, monthly) plus idelalisib (150 mg twice daily) or venetoclax (400 mg once daily) [40] | Active, not recruiting |
Notes: Tafasitamab dose: 12 mg/kg weekly i.v (for L-MIND first 3 cycles weekly and then every 2 weeks thereafter).
Abbreviations: 1 L, first-line; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; i.v., intravenously; NHL, non-Hodgkin’s lymphoma; R/R, relapsed or refractory; R-CHOP, rituximab-cyclophosphamide-doxorubicin-vincristine-prednisolone; SLL, small lymphocytic lymphoma.
7.2. Phase II studies
7.2.1. MOR208C201
The ongoing MOR208C201 Phase IIa study (NCT01685008) enrolled 92 patients with R/R NHL (35 with DLBCL) who had progressed after ≥1 prior rituximab-containing regimen (Table 1). Of the 35 enrolled patients with DLBCL, 74% had experienced a response of ≤12 months to their last therapy, 69% were rituximab-refractory and 60% had been off rituximab for >6 months. Patients received tafasitamab 12 mg/kg (intravenously [i.v.]) weekly for 8 weeks, with an additional 4 weeks available for patients with stable disease and extended treatment available for responding patients. The response rate in DLBCL patients was 26% (21% in 24 patients with rituximab-refractory DLBCL), with 14% experiencing stable disease (Figure 3). Responses were seen across all subgroups defined by FCGR2A codon 131 genotypes and FCGR3A codon 158 genotypes. Median duration of response was 20.1 months (range 1.1–26.5), and responses lasted ≥12 months in 5/9 responding patients, three of whom had rituximab-refractory disease [36]. At long-term follow-up (≤4 years), median progression-free survival (PFS) was 2.7 months (95% confidence interval [CI] 2.1–13.2) in DLBCL patients (Figure 4); 12-month PFS was 34% and the median duration of response remained at 20.1 months [38].
Figure 3.
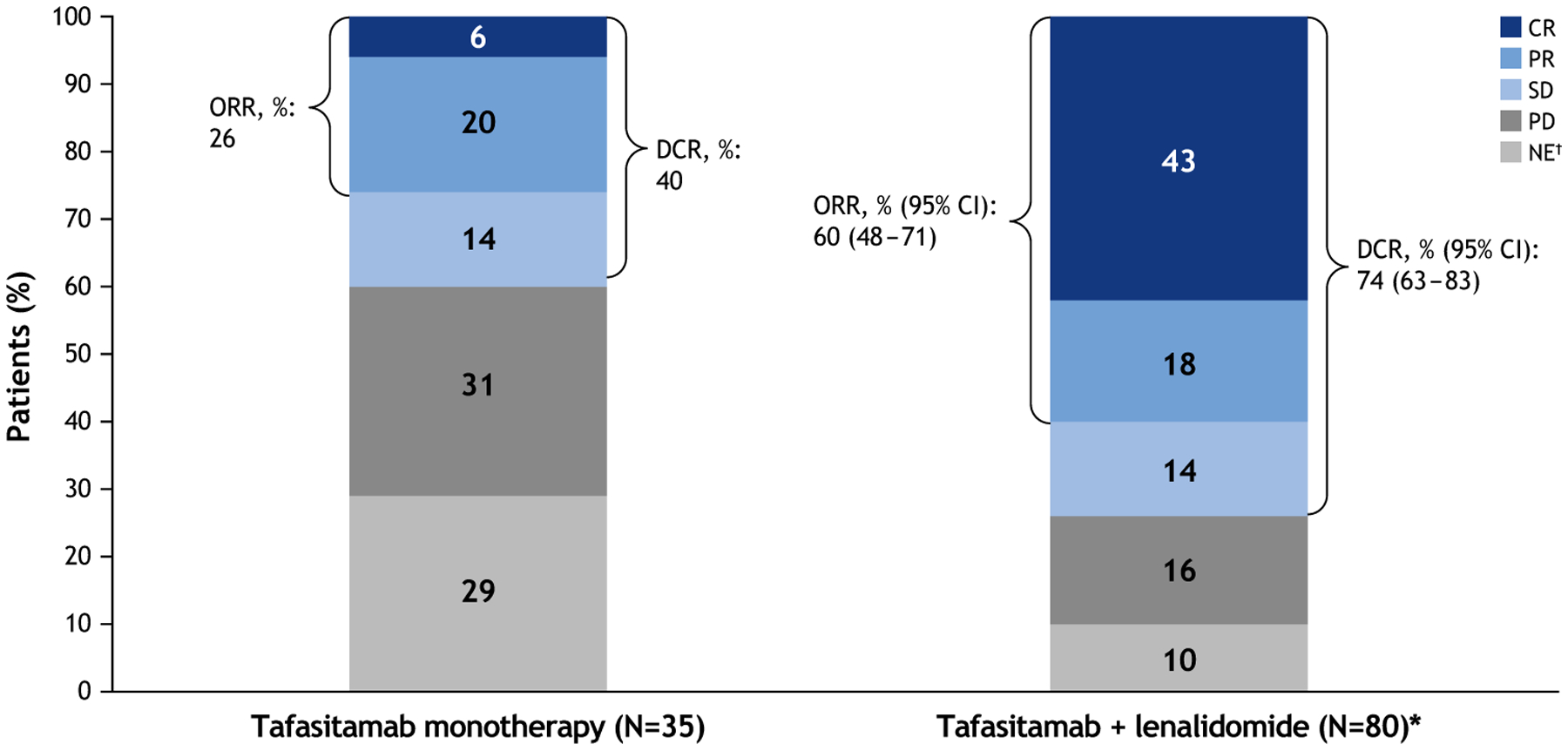
Best objective response for tafasitamab monotherapy (MOR208C201) [38] and tafasitamab plus lenalidomide (L-MIND study) in patients with R/R DLBCL [39].
Notes: Assessments by the independent radiology/clinical review committee. *One patient received tafasitamab only. †NE patients had no valid post-baseline response assessments.
Abbreviations: CI, confidence interval; CR, complete response; DCR, disease control rate (CR + PR + SD); NE, not evaluable; ORR, objective response rate (CR+ PR); PD, progressive disease; PR, partial response; SD, stable disease.
Figure 4.

PFS in patients with DLBCL who received tafasitamab monotherapy [36].
PFS was similar in rituximab-refractory and non-refractory patients. An exploratory post-hoc analysis at the final analysis found that patients with a baseline peripheral NK cell count above a threshold of 100 cells/μL had longer PFS (8.8 months) than those below the threshold (2.3 months; hazard ratio [HR] 0.17; 95% CI 0.06–0.45; p = 0.0004) [36].
7.2.2. L-MIND
L-MIND (NCT02399085) is an ongoing open-label Phase II single-arm multicenter study including 81 patients >18 years with DLBCL and 1–3 prior systemic regimens (including ≥1 anti-CD20 therapy), ineligible for high-dose chemotherapy and ASCT. Patients received tafasitamab (12 mg/kg i.v. once weekly) and lenalidomide (25 mg/day orally, Days 1–21) for up to twelve 28-day cycles, followed by tafasitamab monotherapy (in patients with stable disease or better) until disease progression. Patients with known double- or triple-hit DLBCL were excluded. Approximately half of patients received one prior therapy (49%; n = 40/81), 19% had primary refractory disease (progression within 6 months after completing their first line of therapy; n = 15/81) and 44% (n = 36/81) were refractory to their last line of therapy [39].
The median follow-up was 13.2 months at primary analysis (data cut-off: 30 November 2018). The independently reviewed ORR was 60% (95% CI 48–71), with 43% of patients (n = 34/80) experiencing a complete response (CR) (Figure 3), and was consistent across patient subgroups [39]. Response was achieved a median of 2 months after therapy initiation. The ORR was 70% in patients with one prior line of therapy, 50% in patients with ≥2 prior lines, 60% in patients with primary refractory disease and 60% in patients refractory to their last line of therapy. Median duration of response was 21.7 months (95% CI 21.7–not reached [NR] Figure 5A); overall, 4.4 months in partial responders, and was NR in complete responders. Median PFS was 12.1 months (95% CI 5.7–NR) at a median follow-up of 17.3 months (interquartile range [IQR] 11.5–21.2 Figure 5B). Median PFS after discontinuation of lenalidomide was 12.7 months (95% CI 2.3–NR). Median overall survival (OS) was NR, and 64% of patients were alive at 18 months [39]. After an additional year’s follow-up (data cut-off: 30 November 2019), the ORR was 59% (41% CR, 18% PR), consistent with the primary analysis [41]. The median duration of response was 34.6 months (and NR in CR patients), the median PFS was 16.2 months and median OS was 31.6 months [41]. Notably, all seven patients that had DLBCL transformed from pre-existing indolent lymphoma responded to treatment (29% CR, 71% PR) [39]. Only a minority of patients were refractory to their first-line regimen and two patients were, respectively, found to have double- or triple-hit high-grade B-cell lymphoma (after central review). Although combination therapy was active (and even with prolonged responses) in some of these patients, low numbers of patients with these characteristics preclude definitive conclusion [39]. Anecdotal experience of the use of CD19 CAR-T cell therapy was reported in a patient that failed the L-MIND regimen, but further studies are needed to verify the persistence of CD19 antigen expression in DLBCL patients exposed to tafasitamab [42].
Figure 5.
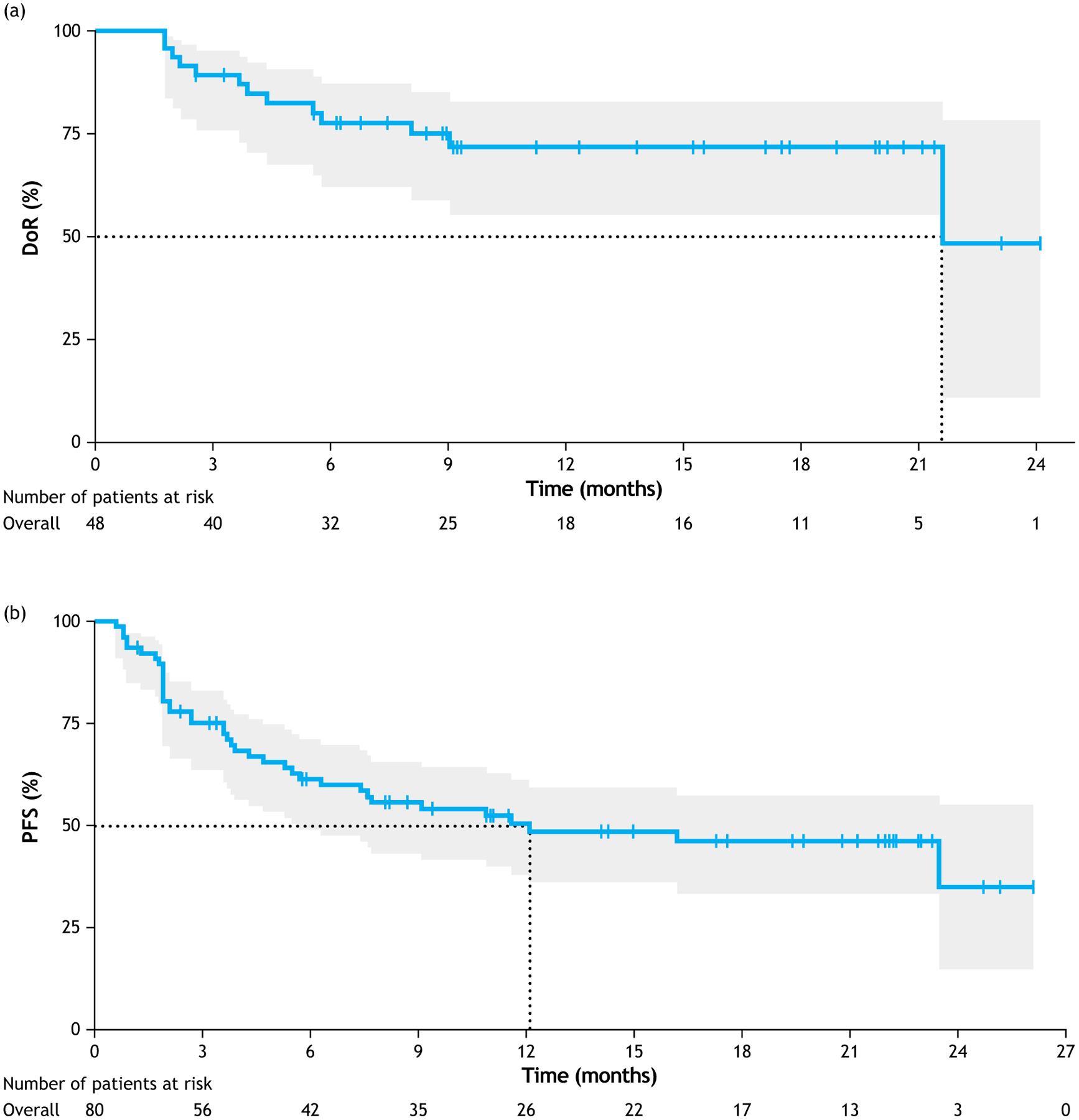
(a) Duration of response, and (b) progression-free survival with tafasitamab plus lenalidomide in patients with R/R DLBCL in the L-MIND study. Reproduced from [39], with permission from Elsevier.
Abbreviations: CI, confidence interval; doR, duration of response; PFS, progression-free survival.
7.3. Phase III studies
B-MIND (NCT02763319) is an ongoing Phase II/III randomized study of tafasitamab or rituximab + bendamustine in patients with R/R DLBCL who are ineligible for high-dose chemotherapy and ASCT. The primary endpoint is PFS and target enrollment is 450; recruitment is underway [43].
7.4. Real-world data
RE-MIND (NCT04150328) is an observational, real-world, retrospective cohort study of lenalidomide monotherapy in R/R DLBCL, designed to characterize the effectiveness of lenalidomide monotherapy for patients with R/R DLBCL ineligible for ASCT and to provide a matched comparator cohort for the L-MIND study. Of the 490 patients who received lenalidomide monotherapy for R/R DLBCL, 140 fulfilled the key L-MIND inclusion/exclusion criteria, had a 25 mg/day starting dose of lenalidomide (as in L-MIND), fulfilled the 6-month follow-up rule, and had information on the nine prespecified baseline covariates and, therefore, qualified for matching. Patients were matched with the L-MIND population using an estimated propensity score-based Nearest Neighbor 1:1 Matching methodology, resulting in 76 patients from each cohort included in the primary analysis. Baseline characteristics were well balanced across cohorts. ORR was significantly improved with combination therapy (67.1%) versus lenalidomide monotherapy (34.2%; p < 0.0001), with CR rates of 39.5% and 13.2%, respectively. PFS and OS were also significantly improved with combination therapy (PFS: HR 0.463 [95% CI 0.307–0.698], p = 0.0002; OS: HR 0.499 [95% CI 0.317–0.785], p = 0.0026) [44]. Outcomes with lenalidomide monotherapy were similar to literature values in R/R DLBCL [15,45,46]. RE-MIND demonstrated significantly improved outcomes with the tafasitamab–lenalidomide combination compared with lenalidomide monotherapy in a closely matched patient population [44].
7.5. Ongoing studies in earlier treatment lines
First-MIND (NCT04134936) is an ongoing Phase Ib, open-label, randomized study of tafasitamab + R-CHOP ± lenalidomide, in patients with newly diagnosed DLBCL (Table 1). In each arm, patients will receive up to six 21-day cycles of R-CHOP, with tafasitamab 12 mg/kg i.v. on days 1, 8 and 15 each cycle, and lenalidomide 25 mg orally on days 1–10 each cycle (in the tafasitamab + RR-CHOP + lenalidomide arm). Target enrollment is 30 patients per arm, and the primary endpoint is safety [47].
7.6. Safety and tolerability
7.6.1. Clinical safety profile
Tafasitamab monotherapy was well tolerated in patients with R/R NHL in the Phase II MOR208C201 study, with 19/ 35 patients with DLBCL (54%) experiencing grade ≥3 treatment-emergent adverse events (TEAEs) [38]. The most common hematologic events were neutropenia (17.1%), anemia (8.6%), and thrombocytopenia (5.7%); and the most common non-hematologic events included pneumonia (8.6%), dyspnea, fatigue and hypokalemia (each 2.9%). Overall, grade ≥3 events were slightly more frequent in patients with DLBCL (19/35 patients; 54.3%) than the overall population (40.2%; N = 92 with various NHL types). No treatment-emergent or treatment-boosted anti-tafasitamab antibodies were observed [38].
In the Phase II L-MIND study [39], the median duration of exposure to combination treatment or lenalidomide was 6.2 months, and to tafasitamab monotherapy was 4.1 months (9.3 months median exposure to study treatment overall; N = 81). The most common grade 3 and 4 TEAEs were neutropenia (27% and 21%, respectively; 10% and 2% were febrile), thrombocytopenia (12% and 5%), leukopenia (7% and 1%), anemia (7% and 0%) and pneumonia (6% and 0%). Non-hematologic adverse events (AEs) were predominantly grade 1–2, with the most common being diarrhea (32%), with a median duration of 8 days. Rash was experienced by 36% of patients (>76% of rashes were grade 1–2; no serious events). Serious AEs occurred in 51% of patients, most frequently pneumonia (6%), febrile neutropenia (6%), pulmonary embolism (4%), bronchitis, atrial fibrillation and congestive cardiac failure (all 2%). Treatment-related serious AEs included pulmonary embolism (2%), and agranulocytosis, chronic obstructive pulmonary disease, fatigue, pyrexia, atrial fibrillation and tumor flare (all 1%). Discontinuation of one or both study drugs due to AEs occurred in 25% of patients, with around half discontinuing tafasitamab. No clinically relevant treatment-emergent immunogenicity was observed [39]. No additional safety signals were observed during the long-term follow-up (data cut-off: 30 November 2019) [41].
7.7. Regulatory affairs
Tafasitamab was approved by the FDA in combination with lenalidomide for the treatment of adults with R/R DLBCL who are ineligible for ASCT [48]. The European Medicines Agency has validated a Marketing Authorization Application for tafasitamab + lenalidomide, followed by tafasitamab monotherapy, for adult patients with R/R DLBCL who are not candidates for ASCT [49].
8. Conclusion
Patients with R/R DLBCL, especially those ineligible for ASCT, urgently need additional alternative options to further chemotherapy. Tafasitamab + lenalidomide has demonstrated favorable efficacy in R/R DLBCL, with a predictable safety profile, and is an important consideration following recent FDA approval and inclusion in NCCN treatment guidelines [6,48]. Given the role of CD19 in several malignancies, tafasitamab has the potential to be incorporated into the treatment backbone across a range of hematologic indications.
9. Expert opinion
Tafasitamab is the first ‘naked’ anti-CD19 monoclonal antibody (mAb) approved for DLBCL patients with relapsed/refractory disease who are ineligible for ASCT, used in combination with lenalidomide. An anti-CD20 immunochemotherapy is an undisputable first-line SOC in all B-cell lymphoma patients. However, in patients failing rituximab-containing regimens, an anti-CD19 mAb is a potentially very interesting compound, especially with regard to the decreased density of CD20 antigen expression sometimes observed in the R/R setting.
An acceptable toxicity profile and long-lasting responses to tafasitamab monotherapy in a quarter of R/R DLBCL patients provides encouragement for combination with other compounds. The L-MIND study demonstrated efficacy for a ‘non-cytotoxic’ regimen. Tafasitamab with lenalidomide was well tolerated, and represents a promising alternative approach to classical chemotherapy-based regimens in this setting. The sustained response duration in patients who achieved a complete response is very promising. This immunomodulatory-based regimen is also a good starting point for future combinations with other molecules having an immune-based mechanism of action.
Tafasitamab–lenalidomide activity requires further evaluation in patients that are primary refractory to R-CHOP. Currently, available CAR-T therapies also target CD19, and since the loss of CD19 expression has been reported in about one third of patients failing CAR-T, further studies are needed to evaluate CD19 cell-surface expression levels prior to and following anti-CD19-based therapy. Preclinical data indicate that tafasitamab exposure does not impair subsequent CAR-T CD19 binding [50]. Until this observation can be confirmed with clinical data, and given that the transplant-ineligible patient populations in which tafasitamab–lenalidomide and CAR-T therapy are approved are not mutually exclusive, optimal treatment sequencing strategies remain to be determined.
With the recent approval of tafasitamab-lenalidomide, future pharmacoeconomic studies of approved treatment options within the DLBCL therapeutic landscape will be of interest to better understand the implications of long-term therapy in the context of rapid availability and treatment administration within community-based oncology practice.
Future development may include combination with additional agents, including cytostatics, such as gemcitabine. In a planned Phase III protocol, tafasitamab and lenalidomide are added to R-CHOP as first-line therapy for high-risk DLBCL patients. This first-in-class approved anti-CD19 monoclonal antibody is likely to be frequently used in a variety of regimens.
Acknowledgments
Medical writing assistance was provided by Claire Stoker, PhD, of Syneos Health and funded by MorphoSys AG.
Funding
This article is funded by MorphoSys AG.
Footnotes
Declaration of interest
Wojciech Jurczak: Research Grants – Roche, Sandoz-Novartis, Celltrion, Takeda, NovoNordisk, Celgene. Monika Długosz Danecka: Research Grants – Roche, Sandoz-Novartis, Celltrion, Takeda, NovoNordisk, Celgene. Gilles Salles: Advisory board, Consultancy or participation in Educational events – Abbvie, Amgen, Autolus, BMS/Celgene, Debiopharm, Genmab, Kite/Gilead, Epizyme, Janssen, Karyopharm, MorphoSys, Novartis, Roche, Takeda. Hervé Ghesquières: Consultancy – Gilead Sciences, Celgene, Roche; honoraria – Gilead Sciences, Janssen, Celgene; travel, accommodations, or expenses – Roche, Gilead Sciences, Celgene, Takeda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
- 1.Miranda-Filho A, Piñeros M, Znaor A, et al. Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes Control. 2019;30(5):489–499. [DOI] [PubMed] [Google Scholar]
- 2.Armitage JO. My treatment approach to patients with diffuse large B-cell lymphoma. Mayo Clin Proc. 2012;87(2):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020 [Google Scholar]
- 4.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94 (5):604–616. [DOI] [PubMed] [Google Scholar]
- 6.NCCN. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: b-cell lymphomas [Internet]. 2020. [cited 2020 Jul 7]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf.
- 7.Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26Suppl 5:116–v125. [DOI] [PubMed] [Google Scholar]
- 8.Chow VA, Shadman M, Gopal AK. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood 2018;132(8):777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayyappan S, Maddocks K. Novel and emerging therapies for B cell lymphoma. J Hematol Oncol. 2019;12(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novartis Pharmaceuticals Corporation. KYMRIAHTM (tisagenlecleucel) suspension for intravenous infusion. Prescribing information. [Internet] [cited 2020 Jul 27]. Available from: https://www.fda.gov/media/107296/download. [Google Scholar]
- 11.Kite Pharma Inc. YESCARTA® (axicabtagene ciloleucel) suspension for intravenous infusion. Prescribing information. [Internet] Available from: https://www.fda.gov/media/108377/download. [Google Scholar]
- 12.Bristol Myers Squibb. Bristol-Myers Squibb Announces Submission of Biologics License Application for CAR T-Cell Therapy Lisocabtagene Maraleucel (liso-cel) to FDA [Internet]. 2019. Available from: https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-submission-biologics-license-ap.Access date18.12.2019.
- 13.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czuczman MS, Trněný M, Davies A, et al. A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2017;23 (15):4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goy A, Ramchandren R, Ghosh N, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non–germinal center B-cell–like DLBCL. Blood. 2019;134 (13):1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karyopharm Therapeutics Inc. U.S. Food and Drug Administration Accepts Karyopharm’s Supplemental New Drug Application for XPOVIO® (Selinexor) as a Treatment for Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma [Internet] 2020; [cited2020 Jun 22]. Available from: https://investors.karyopharm.com/node/12646/pdf.
- 18.Genentech I HIGHLIGHTS OF PRESCRIBING INFORMATION FULL PRESCRIBING INFORMATION: POLIVYTM (polatuzumab vedotin-piiq) [Internet]. 2019. [cited 2019 Jul 16]. Available from: www.fda.gov/medwatch.
- 19.Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/ refractory DLBCL with variable CD30 expression. Blood. 2015;125 (9):1394–1402. [DOI] [PubMed] [Google Scholar]
- 20.Goebeler M-E, Knop S, Viardot A, et al. Bispecific T-Cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34(10):1104–1111. [DOI] [PubMed] [Google Scholar]
- 21.Viardot A, Goebeler ME, Hess G, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127(11):1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahl BS, Hamadani M, Radford J, et al. A phase I study of ADCT-402 (loncastuximab tesirine), a novel pyrrolobenzodiazepine-based antibody–drug conjugate, in relapsed/refractory B-cell non-hodgkin lymphoma. Clin Cancer Res. 2019;25(23):6986–6994. [DOI] [PubMed] [Google Scholar]
- 23.Avivi I, Robinson S, Goldstone A. Clinical use of rituximab in haematological malignancies. Br J Cancer. 2003;89(8):1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boye J, Elter T, Engert A. An overview of the current clinical use of the anti-CD20 monoclonal antibody rituximab. Ann Oncol. 2003;14 (4):520–535. [DOI] [PubMed] [Google Scholar]
- 25.Marshall MJE, Stopforth RJ, Cragg MS. Therapeutic antibodies: what have we learnt from targeting CD20 and where are we going? Front Immunol. 2017;8:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang W, Agrawal N, Patel J, et al. Diminished expression of CD19 in B-cell lymphomas. Cytometry B Clin Cytom. 2005;63 (1):28–35. [DOI] [PubMed] [Google Scholar]
- 28.Olejniczak SH, Stewart CC, Donohue K, et al. A quantitative exploration of surface antigen expression in common B-cell malignancies using flow cytometry. Immunol Invest. 2006;35(1):93–114. [DOI] [PubMed] [Google Scholar]
- 29.Her JH, Pretscher D, Cho S, et al. Functional characterization of gamma delta T Cells and allogeneic activated NK cells as effector cells for tafasitamab (MOR208). Blood. 2019;134(Supplement_1):3801. [Google Scholar]
- 30.Horton HM, Bernett MJ, Pong E, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68(19):8049–8057. [DOI] [PubMed] [Google Scholar]
- 31.Lazar GA, Dang W, Karki S, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103 (11):4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woyach JA, Awan F, Flinn IW, et al. A phase 1 trial of the Fc-engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and 7 preliminary efficacy in relapsed CLL. Blood. 2014;124(24):3553–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Awan FT, Lapalombella R, Trotta R, et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood. 2010;115(6):1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boxhammer R, Striebel F, Baumgartner R, et al. Expression of CD19 antigen on chronic lymphocytic cells after tafasatamab (anti-CD19) treatment: phase 1 trial data (Abstract release date: 05/14/20) EHA Library. Boxhammer R. 06/12/20; 294589; EP671. [Google Scholar]
- 35.Mougiakakos D, Voelkl S, Bach C, et al. Mechanistic characterization of tafasitamab-mediated antibody-dependent cellular phagocytosis alone or in combination with lenalidomide. Blood. 2019;134 (Supplement_1):4064. [Google Scholar]
- 36.Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2018;29(5):1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurczak W, Zinzani P, Gaidano G. et al. Phase IIa study of single-agent MOR208 in patients with relapsed or refractory B-Cell non-Hodgkin’s lymphoma. Blood. 2015; 126(23):1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jurczak W, Zinzani PL, Hess G, et al. A Phase IIa, open-label, multicenter study of single-agent tafasitamab (MOR208), an Fc-optimized anti-CD19 antibody, in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma: long-term follow-up, final analysis. Blood. 2019;134(Supplement_1):4078. [Google Scholar]
- 39.Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–988. [DOI] [PubMed] [Google Scholar]
- 40.Staber PB, Jurczak W, Brugger W, et al. Primary analysis of anti-CD19 tafasitamab (MOR208) treatment in combination with idelalisib or venetoclax in R/R CLL patients who failed prior BTK inhibitor therapy (COSMOS Trial). Blood. 2019;134(Supplement_1):1754. [Google Scholar]
- 41.Salles G, Duell J, Gonzalez-Barca E. et al. Long-term outcomes from the Phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. (Abstract release date: 05/14/20) EHA Library. Salles G. 06/12/20; 293691; EP1201. [Google Scholar]
- 42.Oliai C, de Vos S. Case report: sustained remission achieved from anti-CD19 CAR T cell therapy despite prior treatment with anti-CD19 antibody tafasitamab (MOR208) in a patient with relapsed and refractory diffuse large B-cell lymphoma. Blood. 2019;134 (Supplement_1):5360. [Google Scholar]
- 43.Nowakowski GS, Belada D, Molina L, et al. B-MIND: MOR208 plus bendamustine (BEN) versus rituximab (RTX) plus BEN in patients with relapsed or refractory (R-R) diffuse large B-cell lymphoma (DLBCL): an open-label, randomized phase II/III trial. J Clin Oncol. 2017;35(Supplement_15):TPS7571. [Google Scholar]
- 44.Nowakowski G, Rodgers TD, Marino D, et al. RE-MIND study: a propensity score-based 1:1-matched comparison of tafasitamab + lenalidomide (L-MIND) versus lenalidomide monotherapy (real-world data) in transplant-ineligible patients with relapsed/ refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38 (Supplement_15): 8020 and Poster 353. [Google Scholar]
- 45.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(30):4952–4957. [DOI] [PubMed] [Google Scholar]
- 46.Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011;22(7):1622–1627. [DOI] [PubMed] [Google Scholar]
- 47.Burke JM, André M, Cheson BD, et al. A phase Ib, open-label, randomized study to assess safety and preliminary efficacy of tafasitamab (MOR208) or tafasitamab + lenalidomide in addition to R-CHOP in patients with newly diagnosed diffuse large B-cell lymphoma: the First-Mind trial. Blood. 2019;134(Supplement_1):2877. [Google Scholar]
- 48.MONJUVI. Prescribing Information. Boston, MA: Morphosys. 2020July. [Google Scholar]
- 49.MorphoSys AG. MorphoSys and Incyte Announce the Validation of the European Marketing Authorization Application for Tafasitamab. Media Release Planegg/Munich, Germany [Internet]. May20, 2020Available from: https://www.morphosys.com/media-investors/media-center/morphosys-and-incyte-announce-the-validation-of-the-european-marketing [Google Scholar]
- 50.Horvei P, Sakemura R, Cox MJ, et al. Targeting of CD19 by tafasitamab does not impair CD19 directed chimeric antigen receptor T cell activity in vitro. Blood. 2019;134(Supplement_1):2859. [Google Scholar]


