Abstract
Tyro3, Axl and Mertk (collectively TAM receptors) are three homologous receptor tyrosine kinases (RTKs) that bind vitamin K-dependent endogenous ligands, Protein S (ProS) and Growth arrest specific factor 6 (Gas6), and act as bridging molecules to promote phosphatidylserine (PS)-mediated clearance of apoptotic cells (efferocytosis). TAM receptors are overexpressed in a vast array of tumor types, whereby the level of expression correlates with the tumor grade and the emergence of chemo- and radio-resistance to targeted therapeutics, but also have been implicated as inhibitory receptors on infiltrating myeloid-derived cells in the tumor microenvironment (TME) that can suppress host anti-tumor immunity. In the present study, we utilized TAM-IFNγR1 reporter lines and expressed TAM receptors in a variety of epithelial cell model systems to show that each TAM receptor has a unique pattern of activation by Gas6 or ProS, as well as unique dependency for PS on apoptotic cells and PS liposomes for activity. In addition, we leveraged this system to engineer epithelial cells that express WT TAM receptors, and show that while each receptor can promote PS-mediated efferocytosis, AKT-mediated chemo-resistance, as well as up-regulate the immune checkpoint molecule PD-L1 on tumor cells, Mertk is most dominant in the aforementioned pathways. Functionally, TAM receptor-mediated efferocytosis could be partially blocked by PS-targeting antibody 11.31 and Annexin V, demonstrating the existing of a PS/PS-Receptor (i.e. TAM-receptor) /PD-L1 axis that operates in epithelial cells to foster immune escape. These data provide a rationale that PS-targeting, anti-TAM receptor, and anti-PD-L1 based therapeutics will have merit as combinatorial checkpoint inhibitors.
Keywords: Phosphatidylserine, TAM RTKs, PD-L1, Efferocytosis, breast cancer, PS targeting
Introduction
Tyro3, Axl and Mertk (TAMs) are homologous type I receptor tyrosine kinases that share a conserved sequence (KW (I/L) A (I/L) ES) in their kinase domain and have similar extracellular structural organization, consisting of two immunoglobulin-like (Ig) domains and two fibronectin type III (FNIII) domains (1, 2). TAMs are expressed on a variety of cell types that function in efferocytosis (clearance of apoptotic cells) (3) that include bone marrow-derived hematopoietic cells (macrophages, DCs, NK cells), resident microglia, as well as specialized epithelial cells that participate in bystander efferocytosis including alveolar lung epithelial cells, mammary epithelial cells, and retinal pigmented epithelial (RPEs) cells (4–6). The TAM triple knockout mice have surprisingly normal phenotypes at birth, but develop age-dependent autoimmune diseases due to impaired apoptotic cell clearance (7), indicating that while TAMs are not essential kinases for development, they have more specialized functions to regulate inflammatory responses and promote tolerance. Indeed, at the molecular level, TAMs can function as “dampening” receptors that inhibit NF-κβ and TLR signaling, and subsequently suppress expression of pro-inflammatory cytokines such as IL-1β and TNF-α (8–10).
While expression of TAMs are tightly regulated under native conditions, they are overexpressed in a wide range of human cancers including leukemia, breast cancer, pancreatic cancer, and prostate cancer (4, 11), and like many RTKs, can contribute to cell invasion, migration, angiogenesis, cell survival and metastasis (4, 11, 12). Functionally, TAM receptors are activated by Gas6 or ProS, vitamin K-dependent proteins that function as indirect bridging molecules that bind externalized PS on the surface of apoptotic cells and microvesicles (13). Dysregulation of PS in the tumor microenvironment is caused by increased PS externalization on the tumor vasculature, tumor-derived exosomes, and on stressed viable tumor cells (14–16). These factors have emerged as a common theme in many solid cancers and more recently implicated in tumor progression and immunosuppression (17, 18). Recent studies have demonstrated that PS may act as an important immune checkpoint molecule and can be targeted for anti-tumor therapy (13, 19).
Expression of TAMs on professional phagocytes (macrophages and DCs) is important for homeostasis under physiological conditions, in order to clear apoptotic cells and eliminate auto-antigens that prevent autoimmune activation (20–22). However, in contrast, TAMs in the tumor microenvironment appear to contribute to immune evasion by suppressing the production of tumor antigens. In support of this idea, conditional knockout of TAMs, particularly Mertk, on infiltrating macrophages inhibit macrophage efferocytosis, reduce expression of immunosuppressive factors, repress formation of lung metastasis, and increase CD8+ T cell infiltration to the tumor site (23). Similar findings have been reported using pharmacological inhibitors of Gas6 and/or indirect TAM inhibitors (i.e. Warfarin) that result improved tumor immunity, mediated at least in part, by the impaired NK and T cell mediated tumor killing (24). However, as noted above, TAMs are also commonly overexpressed on adenocarcinomas where the level of expression is correlated with poor survival outcomes (25–27). Both native epithelial cells and adenocarcinomas can participate in efferocytosis; for example Mertk is critical for the clearance of dying mammary cells during post-lactating mammary involution (28). We have also shown that Mertk, when ectopically expressed, can act as a “stand-alone” receptor to increase epithelial efferocytosis (29), (30). However, despite the prominence of TAM over expression in a high percentage of aggressive cancers and adenocarcinomas (4, 11, 12, 29, 31–33), their role as PS receptors on epithelial cells in skewing immune responses is not well understood.
In the current study, we explored the contribution of TAM receptor as PS sensors and signaling receptors on epithelial cells to better understand the consequences of TAM overexpression in adenocarcinomas. We observed that PS differentially regulates TAMs in the presence of their native ligands Gas6 and ProS. While Mertk and Tyro3 are hyper-activated in the presence of PS-positive lipid particles, Axl is constitutively activated by its native ligand Gas6. Moreover, the hyper-activation of Mertk and Tyro3 by PS concomitantly increased TAM-mediated Akt activation, as well as the ensuing up-regulation of the T cell checkpoint molecule PD-L1. Furthermore, we show here that TAM receptor activation, TAM-induced efferocytosis and enhanced PD-L1 expression on epithelial cells could be significantly abrogated using PS-targeting therapeutics, supporting the idea that combination therapies targeting PD-L1/PD-1, TAMs, and PS may have potential to treat cancer as immunotherapeutic modalities.
Materials and Methods
Apoptotic cell induction, PS liposome preparation and efferocytosis assay
All cell lines used in this study were purchased from ATCC and grown in their respective growth medium as per the instructions with 1X Penicillin and Streptomycin solution. For apoptosis induction, Jurkat cells were washed twice with PBS, UV-irradiated for 5min (25mJ/cm2) and kept in serum-free media for 5hr. The cells were then stained with propidium iodide and FITC-conjugated Annexin V and the extent of apoptosis evaluated by flow cytometry. For PS liposome preparation, powder form of L-α-phosphatidylserine (Brain, Porcine) was purchased from Avanti Polar Lipids, and was dissolved in EtOH/ddH2O (1:1) buffer at a concentration of 20 mM by vigorous vortexing for 5 min. For efferocytosis assay, epithelial cells, used as non-professional phagocytes, were labeled green with PKH67 (Sigma-Aldrich) and apoptotic Jurkat cells were labeled red with PKH26 (Sigma-Aldrich) according to the manufacturer’s protocols. Phagocytes were maintained for 6 hr in serum-free medium and then apoptotic cells in Gas6-conditioned medium were added to phagocytes and further incubated for 5 hr at 37°C at the ratio of 1:5 (phagocytes:AC). After 5 hr, the phagocytes were washed three times with 10mM EDTA to eliminate non-specific apoptotic cell binding and the extent of efferocytosis analyzed by flow cytometry (BD Calibur). In all experiments, the concentration of Gas6 in the conditioned media was estimated to be ~250 nM as determined by standard curve against a commercial recombinant human Gas6 (R&D), and γ–carboxylation (a marker for Gas6 activity) was verified by LC-MS/MS (not shown).
To study the effects of tyrosine kinase inhibition on epithelial efferocytosis, 300nM BMS777607 was added to Gas6-conditioned medium, and added to phagocyte/apoptotic cell co-cultures at the ratio of 1:5. To investigate the effect of PS-targeting agents on efferocytosis, PKH26 stained apoptotic cells in 100μl DMEM were treated with 1 mg/ml 11.31 (a PS-targeting human IgG1 generated by Peregrine Pharmaceuticals, Inc. as described previously (34, 35) or 0.1 mg/ml Annexin V-128 dimer (D19) (generated by Advanced Proteome Therapeutics Inc.) for 30min at room temperature. Pre-incubated apoptotic cells were added to phagocytes with Gas6 at the ratio of 1:1 and incubated for 5 hr before efferocytosis evaluation by flow cytometry.
Generation of Chimeric Receptor Constructs and Reporter cell lines
Extracellular domain of TAM receptor chimeric reporter cell lines generations have been previously described (30). To generate Ig domain swaps, cDNA fragments encoding Ig-I and Ig-II domain of hAxl and hMertk were cloned into pEF2-FL plasmids, which have Fibronectin (FN-III) domains of hAXL and hMERtk with trans-membrane and intracellular domains of IFN-γR1. The cloning generated pEF2-FL hAxl Ig-I /Mertk Ig-II /MertkFNIII/IF, N-γR1, pEF2-FL hAxl Ig-I /Axl Ig-II /MertkFNIII/FN-γR1 and pEF2-FL-hMertk/IFN-γR1 plasmids, which contain Ig swaps human Axl and Mertk and intracellular domains of human IFN-γR1 (Fig. 1E). The constructs were transfected into CHO cells and single stable clones were selected using G418 and the activation by Gas6-conditioned medium and apoptotic Jurkat cells was determined by immunoblot with pSTAT1 activation as the read-out.
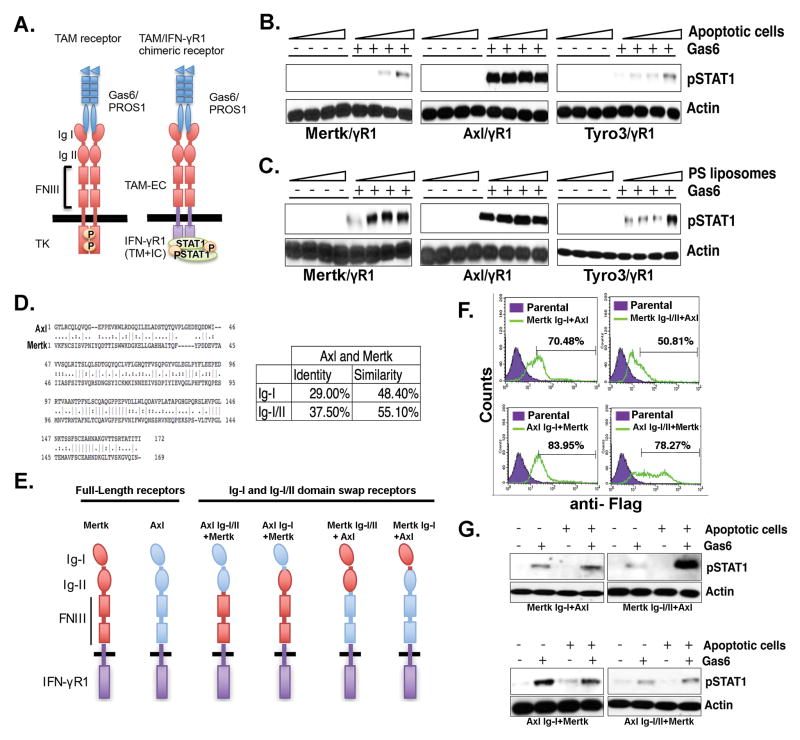
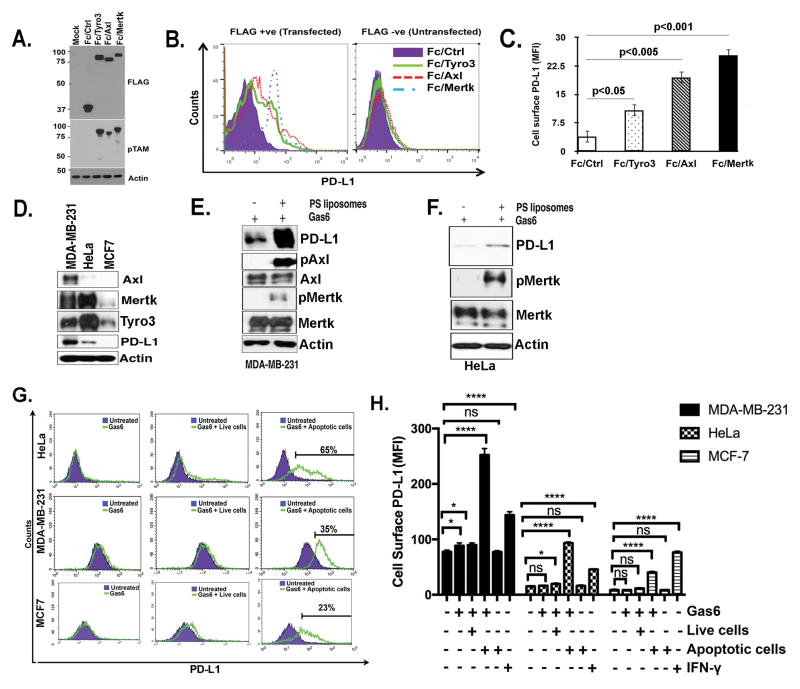
Figure 1. Differential PS sensing of chimeric TAM receptors is dependent on the TAM Ig-I/II domains.
A. Schematic representation of TAM/IFN-γR1 chimeric receptors that exhibit pSTAT1 as a “read-out” for receptor activation. Effect of PS-positive apoptotic cells (B) PS-positive liposomes (C) on Gas6-inducible activation of Tyro3, Axl, and Mertk. D. Alignment of Ig-I/II domains of Axl and Mertk receptors and summary of the conservation between their Ig-I and Ig-II domains. E. Schematic representation of Ig-I or Ig-I/II swaps of Axl and Mertk chimeric receptors. F. Expression profiling by flow cytometry for cells expressing Axl and Mertk Ig-I/II domain swaps. Numbers indicate MFI of chimeric receptor expression using FACS staining with an anti-Flag antibody that is engineered into the extracellular domain of each chimeric receptor G. Effects of Ig-I or Ig-I/II swap on apoptotic cells and Gas6 mediated Axl and Mertk activation.
Activation of chimeric TAM receptors, native TAM receptors and cancer cells
Chimeric TAM receptor cells, native TAM receptors-expressing cells and cancer cells were seeded in 35 mm plate (1.0×106 cells per plate) and serum-starved for 6h. Next, the cells were incubated with Gas6-conditioned medium or 100 nM purified ProS (Hematologic Technologies Inc.) at 37°C for 30min. For apoptotic cells and PS liposome treatment, 1.0×107 apoptotic Jurkat cells were added to MCF10A cells, 1.0×106 – 1.0×107 apoptotic Jurkat cells were added to reporter lines, 10nM – 60nM PS liposomes were added to the reporter cells, or 5×106 apoptotic Jurkat cells or 60 nM PS liposomes were added to cancer cells respectively, for 30min at 37°C. The cells were then washed three times by PBS and lysed using HNTG buffer. For TAM and AKT inhibition studies, cells were treated with 300nM BMS777607 (Selleckchem) or 50μM LY294002 (Cell Signaling) in Gas6-conditioned medium with or without 5×105 AC or 60 nM PS liposomes at 37°C for 30min. MCF-10A breast mammary cells were washed three times with PBS and lysed using HNTG buffer. For blocking the PS-enhanced activation by PS targeting agents, 4.0×105 apoptotic Jurkat cells in 100μl DMEM were pre-incubated with 0.1–1mg/ml PS targeting 11.31 antibody or with 0.01–0.1mg/ml Annexin V-128 dimer (D19) or 1mg/ml isotype control antibody for 30min at room temperature. Then apoptotic cells were added to reporter cell line with Gas6-conditioned medium and incubated for 30min at 37°C and untreated 4.0×105 apoptotic Jurkat cells were added as a control.
Flow cytometry and immunoblotting
TAM-overexpressing and vector-expressing MCF-10A cells were detached with accutase (Sigma-Aldrich) and washed with PBS and resuspended in 100μl 1% BSA/PBS. For flow cytometry, anti-human Mertk (R&D), PE-conjugated anti-human Axl (R&D) and PE-conjugated anti-human Tyro3 (R&D) were used according to manufacturer protocol. To evaluate binding of PS-targeting agents to apoptotic cells, 1.0×106 apoptotic or live Jurkat cells in 100 μl DMEM were pre-incubated 10 μg/ml of the PS-targeting 11.31 antibody followed by a second incubation of 0.5 μg FITC-conjugated anti-human IgG (Sigma-Aldrich) or 5μl FITC-Annexin V (Biolegend) for 30min at room-temperature. Immunoblotting was performed by standard protocols with the following antibodies: human Mertk (Cell Signaling), human phospho Mertk (FabGennix), human Axl (Santa Cruz), human phospho Axl (Cell Signaling), human Tyro3 (Cell Signaling), human phospho Tyro3 (Aviva Systems), human Akt (Cell Signaling), human phospho Akt (Cell Signaling), pSTAT1-Y701 (BD Bioscience), human PD-L1 (Cell Signaling), human beta-actin (Cell Signaling)
Chemo-resistance or survival assay
TAM overexpressing or empty vector expressing MCF10A-TAMS stable cells were seed to 12 well plate (0.5×106 cells) and were serum-starved for 6 hr, followed by addition of Gas6-conditioned medium containing 50μM camptothecin (Sigma-Aldrich) with or without 50 μM LY-294002 (Cell Signaling) and 60 nM PS liposome. After 16 hr, viable cells were evaluated by staining with propidium iodide and FITC-conjugated Annexin V (BioLegend) using flow cytometry.
Cell proliferation assay
Real-time cell proliferation was performed as described previously (36). MCF10A-TAMs stable cell lines were starved for 6 hr in serum-free medium. Cells were collected in serum free medium and 2.5×103 cells were suspended in 150μl Gas6-conditioned medium with or without 60 nM PS liposome with 5% horse serum (HS) and MCF10A cells supplements and added to E-Plate 16 in quadruplicate samples. Real-time proliferation was monitored every 4 hr for 72 hr by xCELLigence system (ACEA Biosciences) and media was changed every 24 hr with fresh Gas6-conditioned medium with or without 60 nM PS liposome with 5% HS and MCF10A cells supplements. Delta cell index values from xCELLigence experiments were used to draw graphs using GraphPad Prism.
Surface PD-L1 and PD-L2 Expression
293TN cells were transfected with pEF2-FL-Fc/truncated-IFN-γR1 control or pEF2-FL-Fc-Axl or pEF2-FL-Fc-Mertk or pEF2-FL-Fc-Tyro3 constructs. 48 h post-transfection, cells were collected and stained with FITC-conjugated anti-FLAG (Sigma-Aldrich) and PE-conjugated anti-PD-L1 antibody (BioLegend) according to the manufacturer’s protocol, and analyzed by flow cytometry. 1×106 MDA-MB-231 and HeLa cells were seeded in 35 mm plate and were serum-starved for 6 hr and then cells were incubated with Gas6 conditioned medium with 60 nM PS liposome. After 24 hr, cancer cells were lysed and PD-L1 expression analyzed by western blotting. 1×106 MDA-MB-231, MCF-7 and HeLa cells were seeded in 6-well plate and were serum-starved for 6 hr and incubated with Gas6 conditioned medium with or without 5.0×106 apoptotic Jurkat cells or with live Jurkat cells and 293T conditioned medium were added to cells to serve as untreated control. After 12 hr, live or apoptotic cells were washed away with DMEM twice and cancer cells were incubated in DMEM with 0.5% serum for an additional 24 hr. Cancer cells were collected and stained with PE-conjugated anti-PD-L1 (Biolegend) according to the manufacturer’s protocol, and analyzed by flow cytometry. For treatments using PS-targeting agents, 1.0×106 apoptotic cells in 100μl DMEM were pre-incubated with up to 1 mg/ml 11.31 or 0.1 mg/ml Annexin V dimers (D19) for 30min at room temperature. Then apoptotic cells were added to cancer cells with Gas6-conditioned medium and they were incubated for 12 hr at 37°C and 1.0×106 non-treated apoptotic cells were added as a control. Apoptotic cells were washed away and cancer cells were incubated in DMEM with 0.5% serum. After 24 hr, cancer cells were stained with PE-conjugated anti-PD-L1.
Site-specific formation of the homodimer of Annexin V-128 dimer (D19) with bis-Mal-PEG19
A stock solution (2–6 mM) of the bis-maleimido-PEG19 crosslinker (BroadPharm, San Diego, CA) in DMSO was dissolved in water and 0.3 eq. was added to Annexin V-128 (20–80 μM, 25 mM phosphate buffer, pH 6.7–6.8). The mixture was incubated at room temperature for 30 min, after which an additional two portions (0.2 eq and 0.15 eq) of the PEG crosslinker were added at 30 min interval. The reaction was allowed to proceed at room temperature for 2 hr. The reaction mixture was then applied to a Zenix-C SEC-150 column (3 μM, 150Å, 21.2×300 mm, Sepax Technologies, Inc.) and eluted with 150 mM phosphate buffer at pH 7.0 using a flow rate of 3mL/min. Fractions were analyzed by ESI-MS to distinguish monomeric and dimeric species, and the separated fractions containing the product dimers were combined to yield the desired conjugate at ≥95% homogeneity. Typical yields for the production of the homodimer were 30%.
Statistical Analysis
GraphPad Prism was used for statistical analysis. Descriptive statistics for quantitative variables were summarized using mean ± S.D. Differences between groups were tested by t test or one-way ANOVA followed by Tukey post-hoc test. p values by t test or Tukey post-hoc test are shown, and p < 0.05 is considered as significant.
Results
PS-mediated hyper-activation of Mertk and Tyro3; Role of Ig-I and Ig-II domains
Previously we engineered TAM reporter CHO 16.9 cell lines, in which the extracellular and trans-membrane domains of each TAM was fused in frame to the cytoplasmic domain of the human IFN-γR1 chain to access ligand-inducible activation of TAMs by Gas6 and ProS (Fig. 1A)(30). Using pStat1 as surrogate readout for TAM activation, we observed that while both Gas6 and ProS required vitamin-K dependent γ-carboxylation as a requisite post-translational modification for TAM activity, Tyro, Axl, and Mertk individually showed differential selectivity towards ligands and differential capacity to be hyper-activated by PS-containing liposomes or PS+ apoptotic cells. Indeed, as shown in Fig. 1B and 1C, while Axl was maximally activated by Gas6 (but not ProS, not shown) and not further hyper-activated in the presence of PS liposomes/PS+ apoptotic cells, both Tyro3 and Mertk showed weaker activation by Gas6 and ProS, but exhibited significant hyper-activation in the presence of PS lipids.
To ascertain whether the aforementioned differences in TAM affinities for PS/Gas6 was due to intrinsic ligand-dependent binding to the Ig-I/Ig-II domains (known to directly interact with the C-terminal Laminin-like LG domains of Gas6 or ProS that induce receptor dimerization and activation), we performed Ig domain swapping experiments to create a series of Axl/Mertk chimeric receptors for stable expression in CHO cells; that include; Axl Ig-I/Ig-II-Mertk-γR1 Axl Ig-I-Mertk-γR1, Mertk Ig-I/Ig-II-Axl-γR1, and Mertk Ig-I-Axl-γR1 (Fig 1A,E). The Ig domains of Axl and Mertk show significant sequence divergence (29% for Ig-I and 37.5% for Ig-I/Ig-II), (Fig. 1D), so it is plausible that the observed differences in PS sensing between Axl and Mertk were mediated by one or both Ig-like domains. Indeed, while all chimeric receptors were expressed on CHO cells (Fig. 1F) only those receptors that contained the Ig-I and Ig-II of Mertk showed enhanced PS sensing in the presence of Gas6 and PS+ apoptotic cells (Fig. 1G). Taken together, these results indicate that both the Ig-I and Ig-II domains of Mertk contribute to the observed phenotypic differences in TAM-mediated receptor hyper-activation by ligands in the presence of PS.
Overexpressed native TAM receptors demonstrate distinct PS-induced activity in MCF10A breast mammary epithelial cells
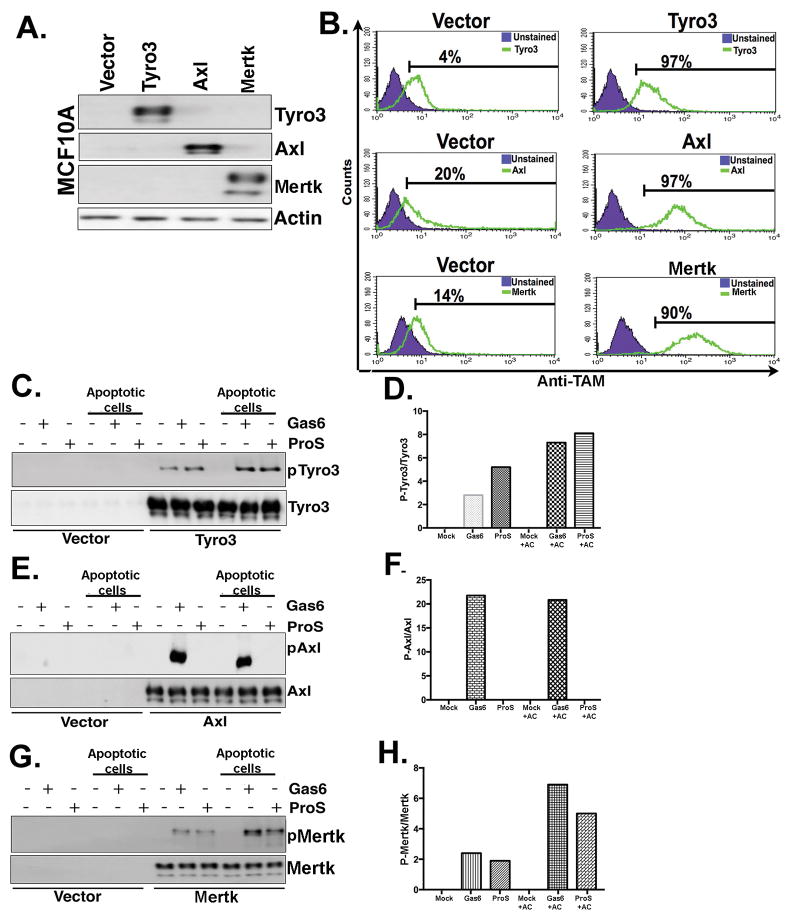
To extend the aforementioned findings from artificial chimeric receptors to native TAM receptors and query whether native Tyro3, Axl, and have distinct interaction itineraries with Gas6, ProS, and PS, we stably overexpressed native TAMs using retroviral transduction in MCF10A cells, a non-transformed mammary epithelial cell line that shows minimal surface expression of endogenous TAMs. Following TAM overexpression and selection by geometric mean intensity, surface expression of individual TAMs were verified using FACS with TAM specific antibodies that recognize the native extracellular domain (Fig. 2A, 2B). Subsequently, MCF10A TAM receptor cell lines were treated with either Gas6 or ProS, with or without apoptotic cells, as described above. Consistent with results using chimeric receptors, Gas6/ProS in the presence of apoptotic cells consistently hyper-activated Mertk and Tyro3, while Axl was maximally activated by Gas6, but not activated by ProS, and was not hyper-activated in the presence of exogenous PS lipids (Fig. 2C–H).
Figure 2. Differential PS sensing of native TAM receptors towards apoptotic cells.
A. Generation of stable native human Tyro3, Axl and Mertk expressing MCF10A cell lines. B. Flow cytometric analysis of TAM expressing cells show comparable receptor expression in stable MCF10A-expressing cells. C. TAM receptors phosphorylation levels were evaluated by Western blotting after Tyro3-MCF10A cells (C), Axl-MCF10A cells (E), or Mertk-MCF10A cells (G) were treated with Gas6 or ProS in the presence or absence of apoptotic cells. Densitometric analysis of the Western blots shown in panels C, E and G respectively (D, F & H) are indicated to show expression levels of phospho TAMs normalized to total TAMs expression.
TAMs promote epithelial cell efferocytosis in a tyrosine kinase dependent manner
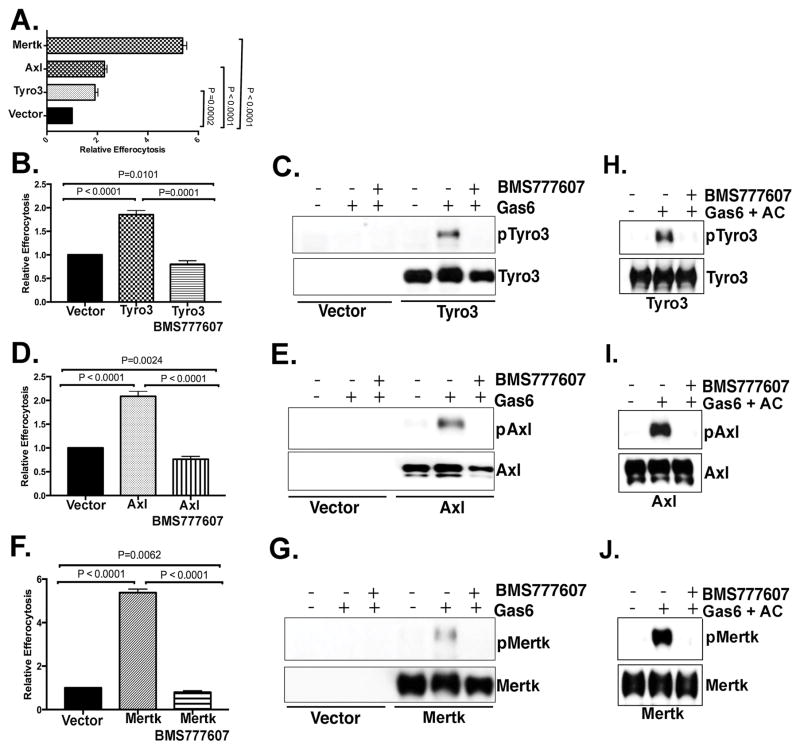
Previous studies showed have shown that deficiency of Mertk results in high level of apoptotic cells accumulation in mammary gland during mammary gland involution, while loss of Axl or Tyro3 had less severe effects (28), suggesting a preferential effect of Mertk on epithelial efferocytosis in the mammary gland. Moreover, we have previously shown that ectopic overexpression of Mertk on mammary epithelial cells increased efferocytosis as a stand-alone receptor in a gain-of-function manner (29). Given the differential capacity of each TAM to become hyper-activated by exogenous PS on apoptotic cells (Fig. 2), we explored the effects of overexpressed TAM receptors on epithelial cell efferocytosis using 2-color FACS. While each TAM was equivalently expressed on the surface (between 90 and 97% surface positive), Mertk overexpression increased efferocytosis of MCF10A cells approximately 6-fold, while overexpression of Tyro3 and Axl enhanced MCF-10A cells’ efferocytosis ~ 2-fold compared to native MCF-10A cells (Fig. 3A). Further we treated MCF10A-TAMs cells with BMS777607, an inhibitor of Met related kinases (c-Met, Ron, Axl, Tyro3, Mertk) that shows robust inhibitory activity towards all three TAMs to examine whether TAM-mediated MCF-10A efferocytosis was dependent on TAMs’ kinase activity (37–39). As indicated in Fig. 3B–J, the pan-TAM kinase inhibitor BMS777607 inhibited TAM phosphorylation (induced by Gas6 (Fig. 3C, E, G) and by Gas6 and apoptotic cells (Fig. 3H–J) and TAM-mediated efferocytosis in each of the MCF-10A TAM cell lines (Fig. 3B–J). Taken together, while all three TAMs can induce epithelial efferocytosis in a kinase-dependent manner, Mertk demonstrated higher net efferocytosis of the three TAMs.
Figure 3. TAM mediated epithelial cell efferocytosis is dependent on the tyrosine kinase activity of TAMs.
A. TAM-MCF10A cells, as shown in Fig. 2, were tested for epithelial efferocytosis of apoptotic Jurkat cells. MCF10A and apoptotic cells were co-cultured at a ratio of 1:5 (phagocyte/apoptotic cell ratio) for 5 hr. Effect of 300 nM BMS777607 (pan-TAM kinase inhibitor) on epithelial cell efferocytosis in MCF10A cells induced by Tyro3 (B), Axl (D) and Mertk (F). Effect of BMS777607 on TAM phosphorylation induced by Gas6 (C, E, G) and TAM phosphorylation induced by Gas6 in the presence of apoptotic cells (H–J).
PS/Gas6-mediated activation of TAMs promote Akt-mediated cell survival
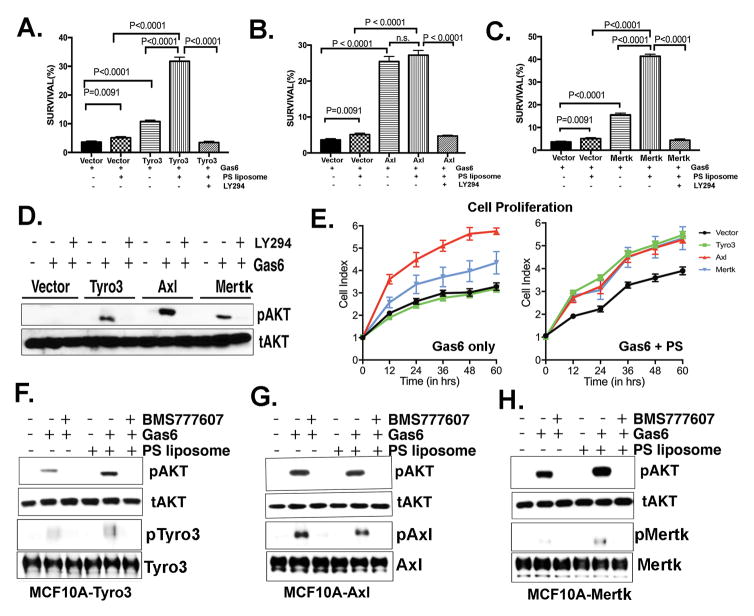
To investigate putative oncogenic events associated with TAM overexpression, as well as whether PS may have a potentiating role in signaling in TAM-overexpressing epithelial cells, we explored survival and proliferation capacity of TAM-expressing MCF10A cells following activation with Gas6 and/or activation with Gas/PS liposomes. Indeed, while Gas6 stimulation of each TAM overexpressing MCF10A cells showed enhanced survival following camptothecin treatment as compared with vector expressing MCF10A cells (Fig. 4A, B and C), this effect was highest with Axl-, while modest in Tyro3- and Mertk-expressing cells. However, when Tyro3- or Mertk-expressing MCF10A cells were pretreated with Gas6 plus PS liposomes, the survival of MCF10A cells was significantly enhanced, implicating a potential survival-inducing effect of PS, particularly in Mertk- and Tyro3-expressing tumor cells. Notably, all TAM-mediated chemo-sensitivity and phosphorylation of Akt (40) (Fig. 4D) was strongly suppressed by the PI3-kinase inhibitor LY294002, which reversed TAMs mediated survival of MCF-10A cells and increased the apoptosis induced by camptothecin (Fig. 4A, B, C). Additionally, Gas6 treatment induced highest proliferation rates in Axl-expressing MCF10A cells as compared to Mertk and Tyro3. However, addition of PS liposome with Gas6 increased the proliferation rate of Mertk- and Tyro3-expressing MCF10A cells to a rate equivalent to the Axl-expressing cells (Fig. 4E). Finally, these effects on survival and Akt were all abrogated by treatment with the pan-TAM inhibitor BMS777607, suggesting a TAM/PS->Akt-axis that impinges on chemo-sensitivity in epithelial cells (Fig. 4F, G and H).
Figure 4. TAM expression in MCF10A cells induces chemo-resistance, proliferation, and the activation of Akt; Effects of Gas6 and PS liposomes.
A. Effect of Gas6 and/or Gas6 PS liposomes on camptothecin-mediated cell death in Tyro3-MCF10A cells (A), Axl-MCF10A cells (B) and Mertk-MCF10A cells (C) (see Materials and methods). After 16 hr, cells were evaluated by PI and Annexin V-conjugated FITC to quantify apoptosis. Bar 5 represents a control in which cells were treated in the presence of 50 μM Akt inhibitor LY-294002. D. Effect of Akt inhibitor LY294002 on TAM inducible pAkt. E. Real-time Xcelligence assay to assess cell proliferation induced by Gas6 alone (left panel) and Gas6 with PS liposomes (right panel) on Tyro3-MCF10A, Axl-MCF10A, and Mertk-MCF10A cells. Effect of BMS777607 pretreatment on the Gas6 inducible phosphorylation of Akt (pAkt) and phosphorylation of TAMs; pTyro3 (F), pAxl (G) and pMertk (H) is shown.
TAM-driven epithelial cells efferocytosis promotes PD-L1 expression in cancer cell lines
Previously, we reported that ectopic expression of Mertk in HEK293 cells could induce the transcriptional up-regulation of PD-L1 (programmed death ligand-1), a known immune checkpoint molecule overexpressed in many poorly immunogenic cancers (29). To better understand the functional interplay between TAMs and PD-L1, particularly whether PS has an augmentory role in PD-L1 signaling to T cells, we examined PD-L1 surface expression induced by TAMs and PS. Upon transient transfection of constitutively active TAM receptor constructs (Fc-Tyro3, Fc-Axl and Fc-Mertk) in 293T cells (Fig. 5A), all three Fc-TAMs elevated surface expression of PD-L1 although this was most prominent with Axl and Mertk (Fig. 5B and C). To assess PD-L1 expression and regulation by TAMs in native cell lines, we first evaluated endogenous PD-L1 expression on various immortalized and transformed cell lines. Generally, native cells showed heterogeneous TAM and PD-L1 expression whereby PD-L1 was highest on MDA-MB-231 and HeLa cells and virtually low levels on MCF7 and MCF10A cells (Fig. 5D, and data not shown). In the MCF10A cells whereby TAMs were ectopically expressed (Fig. 2), overexpression of TAMs did not increase PD-L1 expression, suggesting that TAM expression, per se, was not a requisite for PD-L1 expression (not shown). However, in the PD-L1-expressing MDA-MB-231 and HeLa cells, Gas6/PS liposomes increased steady state PD-L1 protein expression and induced Axl phosphorylation in MDA-MB-231 cells and Mertk phosphorylation in HeLa and MDA-MB-231 cells (phospho AXL levels were not detectable in HeLa cells and phospho Tyro3 levels were not evaluative in both cell lines (data not shown)) (Fig. 5E–F). Furthermore, to show physiological significance, we co-incubated tumor cells with Gas6, Gas6/live cells, or Gas6/PS+ apoptotic cells. As shown in Fig. 5G–H, only the PS+ apoptotic cells together with Gas6 elevated PD-L1 surface expression on HeLa cells, MDA-MB-231 cells, and MCF7 cells. Interferonγ (IFNγ) was used as a positive control for PD-L1 expression in cancer cells (Fig. 5H). Taken together, these data suggest that PS->TAM->PD-L1 axis can be activated on breast adenocarcinoma cells and potentiated by PS+ apoptotic cells or liposomes.
Figure 5. Role of TAMs and TAM-mediated efferocytosis on PD-L1 expression in cancer cells.
A-B. Expression of constitutively active Fc-TAM receptors up-regulate surface PD-L1 expression in HEK293 cells. Data in panel B are quantified in panel C. D. Expression levels of endogenous PD-L1, Axl, Mertk and Tyro3 in MDA-MB-231, HeLa, and MCF7 cancer cell lines. Effect of PS liposomes on PD-L1 expression and phosphorylation of Mertk and Axl in MDA-MB-231 cells (E) and HeLa cells (F). G. Flow cytometric analysis for efferocytosis mediated changes in surface expression of PD-L1 on HeLa, MDA-MB-231 and MCF-7 cells by treatment with Gas6 and apoptotic cells. H. Quantification of cells surface expression of PD-L1 in multiple replicates samples (n=3). P values for * and **** are <0.01.
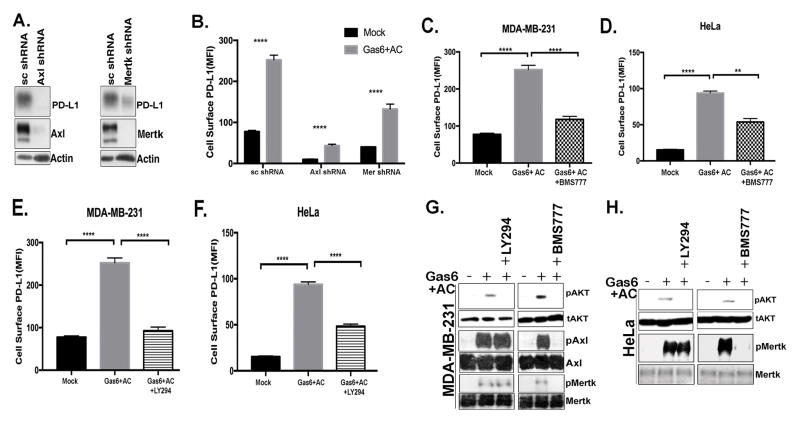
Axl and Mertk regulate PD-L1 expression in kinase dependent and Akt mediated manner
The aforementioned observations that Gas6 opsonized PS+ cells induced up-regulation of PDL1 suggested the involvement of one or more of the TAMs in regulating intracellular signaling. Interestingly, in the MDA-MB-231 cells, which offered robust PD-L1 up-regulation, stable knockdown of either Axl or Mertk reduced PD-L1 expression (Fig. 6A–B). This inhibitory effect on PD-L1 up-regulation could be partially phenol-copied in cells pretreated with either the pan-TAM kinase inhibitor BMS77607 (Fig. 6C–D) or the PI3 kinase inhibitor LY294002 (Fig. 6E–F) and these treatments affected phosphorylation of TAMs and Akt, which is downstream of TAMs, in HeLa and MDA-MB-231 cells (Fig. 6G–H) suggesting PD-L1 could be regulated directly by post-receptor TAM kinase signaling, at least in part, via PI3-kinase/Akt.
Figure 6. Axl and Mertk regulate PD-L1 expression by the activation of Akt.
A. Effect of Axl and Mertk knockdown on PD-L1 expression in MDA-MB-231 cells by Western blotting. B. Quantification of cells surface expression of PD-L1 by Axl and Mertk knockdown and Gas6 and apoptotic cell stimulation in multiple replicates (n=3). Effect of BMS777607 (pan-TAM kinase inhibitor) on Gas6 and apoptotic cell induced PD-L1 surface expression in MDA-MB-231 (C) and HeLa cells (D). Effect of LY294 (Akt inhibitor) treatment on Gas6 and apoptotic cells induced PD-L1 surface expression MDA-MB-231 (E) and HeLa cells (F). Effects of LY294 (Akt inhibitor) and BMS777607 (pan-TAM kinase inhibitor) on phosphorylation of Akt, Mertk and Axl shown by western blot in MDA-MB-231 cells (G) and HeLa cells (H). P values **** are <0.01.
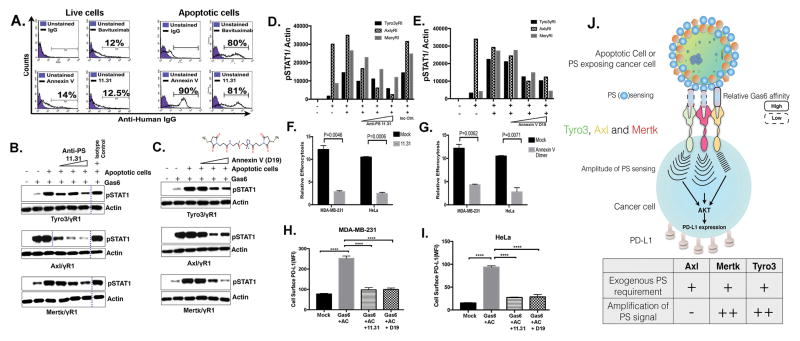
Targeting PS-induced events with PS targeting agents
Finally, to explore whether the aforementioned PS-initiated chemoresistance and immune evasion (i.e. PD-L1 up-regulation) events could be targeted, we employed two PS-targeting agents, namely PS-targeting body 11.31 and a dimer of Annexin V-128 (D19), both of which specifically bind to PS on apoptotic cells and not live cells (Fig. 7A and B). Interestingly, both PS-targeting antibody 11.31 and dimerized Annexin V-128 (D19) blocked TAM receptor activation induced by apoptotic cells in a dose-dependent manner (Fig. 7B–E). Curiously, even though apoptotic cells did not enhance Axl activity in the presence of Gas6, increasing concentration of PS-targeting antibody 11.31 or dimerized Annexin V-128 (D19) also blocked Axl activation. While this affirms that Axl activity likely remains dependent on PS, it does not become hyper-activated by PS, it also suggests that TAMs may interact differentially with exogenous PS as PS sensors. Consistent with a functional role of 11.31 and Annexin V-128 dimers, these agents also abrogated epithelial cell efferocytosis (Fig. 7F and G), as well as the efferocytosis-induced PD-L1 expression (Fig. 7H–I). These data suggest that PS-targeting agents can function, at least in part, by interfering with TAM receptor activation.
Figure 7. PS targeting agents 11.31 and Dimerized Annexin V suppress epithelial cell efferocytosis and PD-L1 expression.
A. Flow cytometry based binding studies of PS targeting agents to PS on live and apoptotic cells. UV treatment mediated apoptosis and PS exposure was measured by Annexin-V staining by flow cytometry. Anti-human PS targeting antibody 11.31 or Bavituximab were incubated with thus produced apoptotic or live cells using Annexin V as control. Percentage of PS targeting antibodies bound with apoptotic cells were measured with conjugated flow antibodies by flow cytometry. (B–E) PS targeting agents affect Gas6 and apoptotic cells induced TAM receptor activation. Effects of PS targeting 11.31 antibody (B–D) and dimerized Annexin V D19 (C–E) on Tyro3, Axl and Mertk receptor activation by apoptotic cells and Gas6 in TAM-IFN-YR1 chimeric receptor system. Effects of 11.31 PS targeting antibody (F) and Annexin V dimer D19 (G) on relative efferocytosis by MDA-MB-231 and HeLa epithelial cells. Role of 11.31 and Annexin V dimer D19 treatments on cell surface expression of PD-L1 by flow cytometric analysis in Gas6 and apoptotic cells induced MDA-MB-231 (H) and HeLa (I) cells. Western blots bands that are cropped are separated with dotted lines. (J) Schematic model representing different PS sensing and PS signal amplifications paradigms by TAM receptors.
Discussion
TAMs (Tyro3, Axl, and Mertk) comprise a family of three highly conserved RTKs that have important roles in innate immunity by regulating the clearance of apoptotic cells (efferocytosis) and by acting as dampening receptors that control the magnitude of inflammation (41–43). Additionally, TAM expressions on epithelial cells display distinct and diverse mode of post-receptor signaling and gene expression profiles (44). More recently, TAMs have also been recognized to have direct roles in cancer biology by acting as both oncogenes to promote survival and proliferation in lymphomas and carcinomas as well as acting as immune modulators on tumor-infiltrating monocytes and lymphocytes (45). For example, on many tumor cell types, TAM receptors have been shown to activate Akt and Erk signaling, promoting survival and proliferation, and in some cases, particularly Axl, TAMs can activate Src kinases that induce the epithelial–mesenchymal transition (EMT) and emergence of drug resistance (46). In addition, on infiltrating monocyte-derived cells, particularly macrophages, immature dendritic cells and NK cells, TAMs suppress host tumor immunity and block the generation and/or expression of tumor-derived antigens that lead to tumor progression. This latter notion is supported by recent findings that knockout of TAMs on monocytes improves tumor immunity (47) suggesting that these receptors act as immune checkpoints, possibly akin to PD-1/PD-L1 and CTLA-4 that, when targeted, “release brakes” on innate immune inhibitory pathways.
While the aforementioned discussion suggest that TAMs may act as checkpoint inhibitors in cancer, particularly via their expression and activation of inhibitory pathways on myeloid-derived macrophages, DCs and NK cells, the role of TAMs as efferocytosis receptors on epithelial cells is less understood, but potentially important given that one or more of the TAMs are frequently up-regulated in adenocarcinomas (25–27). Here, using a combination of experimental chimeric reporter lines and native TAM-expressing epithelial cells, we show that while each TAM can act as a PS receptor, TAMs have intrinsically different “PS sensing” capabilities in their ability to respond to ligands in the presence of PS liposomes or PS+ apoptotic cells. In this capacity, Mertk and Tyro3 have low affinities towards Gas6 and ProS but are hyper-activated in the presence of PS, while Axl has higher affinity for Gas6 and is maximally activated by PS lipids (Fig. 7J). The aforementioned differences between Axl and Mertk are mediated by intrinsic differences in the primary sequences the Ig-I and Ig-II domains, as we have shown here that domain swaps can shift the PS dependency of these receptors. Rationale mutagenesis can be used to finely map the critical differences between Axl and Mertk that should explain these intrinsic differences at the molecular level.
The present findings that Mertk and Tyro3 act preferentially as PS sensors, relative to Axl, might have implications in a cell biological context to the frequently observe dys-regulation of PS in the tumor microenvironment. Such predictions hold that tumors with high PS content (i.e. tumors with high apoptotic particles, excessive exosome secretion, or vascularized tumors with immature PS+ vascular endothelial cells) may be expected to engage Mertk and Tyro-3 receptors to a greater degree than compared Axl-expressing tumor cells. Indeed, as shown here, when each TAM was ectopically expressed to a similar level in MCF10A cells, Mertk consistently produced higher PS-dependent efferocytosis compared to Tyro3 and Axl. Such data are not inconsistent with physiological examples of epithelial efferocytosis, where it has been shown that Mertk (−/−) mice show significant phenotypic deficiencies in mammary epithelial efferocytosis (during mammary gland involution) (28) and rod outer segment clearance by retinal-pigmented epithelial cells (during diurnal cycles) (5, 48).
The above differences in TAMs as “PS sensors” and the preferential response of Mertk versus Axl may also have therapeutic implications for combinatorial checkpoint strategies, particularly in the context of PS targeting and anti-PD-1/PD-L1 modules. For example, in the recent studies by Gray et al., using a syngeneic E0771 model of triple negative breast cancer, these investigators showed that combinatorial anti-PD-1 and PS-targeting antibody mch1N11 had significant synergistic activity compared to single agent mono-therapies (49, 50). Based on the putative PS-Mertk-PD-L1 axis identified in this study, future studies may predict that Mertk, and to a lesser degree, Axl inhibition will favor synergy with anti-PD-1 or anti-PD-L1 combinations. Moreover, these studies support the idea that individual TAM inhibitors (i.e. TKIs or therapeutic Mabs) might hold unique characteristics compared to pan-TAM inhibitors. Taken together, these studies identify the existence of a PS->PS-R (TAM)->PD-L1 axis in breast cancer, and support combinations of PS targeting, anti-TAM, and anti-PD-1/PD-L1 therapeutics as rationale immune checkpoint inhibitor therapeutics. Our studies also reveal the need to understand the effect of PS from different sources such as exosomes, cancer cells and stressed tumor vasculature cells that affect the TAM activation and result in immune evasion by mechanisms that include but are not limited to M2 macrophage phenotype, IL-10 secretion, and PD-L1 expression in the tumor microenvironment.
Conclusion
These data posit a role of PS in driving PD-L1 expression via TAM receptors that may contribute to tumor immune evasion and chemo-resistance. Targeting of the aforementioned PS->TAM->PD-L1 circuit may have therapeutic value in cancer.
Implications.
Many tumor cells are known to up-regulate the immune checkpoint inhibitor PD-L1. This study demonstrates a role for PS and TAM receptors in the regulation of PD-L1 on breast cancers cells.
Acknowledgments
We thank Sukhwinder Singh of Rutgers University Flow cytometry core facility for flow cytometry technical support and cell sorting. We acknowledge Rutgers Society of Research Scholars award to Sushil Kumar.
Grant Support:
This research was supported by NIH CA 1650771 to RBB, and a Rutgers Foundation grant to RBB and SVK.
Footnotes
Disclosure of potential conflicts of interest:
Cyril Empig, Bruce Freimark, Michael Gray and Jeff Hutchins are employees of Peregrine Pharmaceuticals, Inc. and have financial interest in the company. Allen Krantz and Andrzej Wilczynski are employees of Advanced Proteome Therapeutics Corporation. Allen Krantz has a financial interest in the company. Raymond B. Birge reports receiving a commercial research grant from Peregrine Pharmaceuticals, Inc. No potential conflicts of interest were disclosed by the other authors.
Authors’ contributions:
Canan Kasikara and Sushil Kumar designed, performed and analyzed the experiments and wrote the paper. Stanley Kimani, Wen-I Tsou, Ke Geng, Viralkumar Davra and Connor Devoe performed experiments and analyzed results. Ganapathy Sriram and Khanh Nguyen and Anita Lewis-Antes provided technical assistance. Allen Krantz, Grzegorz Rymarczyk and Andrzej Wilczynski produced Annexin V-128 dimer proteins. Cyril Empig, Bruce Freimark, Michael Gray, Kyle Schlunegger, Jeff Hutchins provided PS-targeting antibodies and helped in preparation of manuscript. Sergei V. Kotenko conceived the study. Raymond B. Birge conceived and coordinated the study, helped in design of experiments and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
References
- 1.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–57. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 2.Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1994;5:647–57. [PubMed] [Google Scholar]
- 3.Kumar S, Birge RB. Efferocytosis. Current biology : CB. 2016;26:R558–r9. doi: 10.1016/j.cub.2016.01.059. [DOI] [PubMed] [Google Scholar]
- 4.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Advances in cancer research. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, et al. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14:769–85. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- 7.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RAS, et al. Delayed Apoptotic Cell Clearance and Lupus-like Autoimmunity in Mice Lacking the c-mer Membrane Tyrosine Kinase. The Journal of Experimental Medicine. 2002;196:135–40. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nature reviews Immunology. 2008;8:327–36. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tibrewal N, Wu Y, D’Mello V, Akakura R, George TC, Varnum B, et al. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. The Journal of biological chemistry. 2008;283:3618–27. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- 10.Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, et al. Apoptotic cells induce Mer tyrosine kinase–dependent blockade of NF-κB activation in dendritic cells. Blood. 2007;109:653–60. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemke G. Biology of the TAM receptors. Cold Spring Harbor perspectives in biology. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Molecular cancer therapeutics. 2011;10:1763–73. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 13.Birge RB, Boeltz S, Kumar S, Carlson J, Wanderley J, Calianese D, et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016;23:962–78. doi: 10.1038/cdd.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062–6. [PubMed] [Google Scholar]
- 15.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Seminars in immunopathology. 2011;33:441–54. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 16.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–40. [PubMed] [Google Scholar]
- 17.Huynh M-LN, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. The Journal of Clinical Investigation. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimani SG, Geng K, Kasikara C, Kumar S, Sriram G, Wu Y, et al. Contribution of Defective PS Recognition and Efferocytosis to Chronic Inflammation and Autoimmunity. Frontiers in immunology. 2014;5:566. doi: 10.3389/fimmu.2014.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber DE, Hao G, Watkins L, Stafford JH, Anderson J, Holbein B, et al. Tumor-specific targeting by Bavituximab, a phosphatidylserine-targeting monoclonal antibody with vascular targeting and immune modulating properties, in lung cancer xenografts. American journal of nuclear medicine and molecular imaging. 2015;5:493–503. [PMC free article] [PubMed] [Google Scholar]
- 20.Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, et al. Clearance deficiency and systemic lupus erythematosus (SLE) Journal of autoimmunity. 2007;28:114–21. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Lemke G, Burstyn-Cohen T. TAM receptors and the clearance of apoptotic cells. Ann N Y Acad Sci. 2010;1209:23–9. doi: 10.1111/j.1749-6632.2010.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–11. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 23.Cook RS, Jacobsen KM, Wofford AM, DeRyckere D, Stanford J, Prieto AL, et al. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. J Clin Invest. 2013;123:3231–42. doi: 10.1172/JCI67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirane A, Ludwig KF, Sorrelle N, Haaland G, Sandal T, Ranaweera R, et al. Warfarin Blocks Gas6-Mediated Axl Activation Required for Pancreatic Cancer Epithelial Plasticity and Metastasis. Cancer Res. 2015;75:3699–705. doi: 10.1158/0008-5472.CAN-14-2887-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hector A, Montgomery EA, Karikari C, Canto M, Dunbar KB, Wang JS, et al. The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer biology & therapy. 2010;10:1009–18. doi: 10.4161/cbt.10.10.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N, et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:130–8. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- 27.Koorstra JB, Karikari CA, Feldmann G, Bisht S, Rojas PL, Offerhaus GJ, et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer biology & therapy. 2009;8:618–26. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandahl M, Hunter DM, Strunk KE, Earp HS, Cook RS. Epithelial cell-directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation. BMC developmental biology. 2010;10:122. doi: 10.1186/1471-213X-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen KQ, Tsou WI, Calarese DA, Kimani SG, Singh S, Hsieh S, et al. Overexpression of MERTK receptor tyrosine kinase in epithelial cancer cells drives efferocytosis in a gain-of-function capacity. The Journal of biological chemistry. 2014;289:25737–49. doi: 10.1074/jbc.M114.570838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsou WI, Nguyen KQ, Calarese DA, Garforth SJ, Antes AL, Smirnov SV, et al. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. The Journal of biological chemistry. 2014;289:25750–63. doi: 10.1074/jbc.M114.569020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meric F, Lee W-P, Sahin A, Zhang H, Kung H-J, Hung M-C. Expression Profile of Tyrosine Kinases in Breast Cancer. American Association for Cancer Research. 2002;8:361–7. [PubMed] [Google Scholar]
- 32.Berclaz G, Altermatt HJ, Rohrbach V, Kieffer I, Dreher E, Andres AC. Estrogen dependent expression of the receptor tyrosine kinase axl in normal and malignant human breast. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2001;12:819–24. doi: 10.1023/a:1011126330233. [DOI] [PubMed] [Google Scholar]
- 33.Gjerdrum C, Tiron C, Høiby T, Stefansson I, Haugen H, Sandal T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proceedings of the National Academy of Sciences. 2010;107:1124–9. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li T, Aredo B, Zhang K, Zhong X, Pulido JS, Wang S, et al. Phosphatidylserine (PS) Is Exposed in Choroidal Neovascular Endothelium: PS-Targeting Antibodies Inhibit Choroidal Angiogenesis In Vivo and Ex Vivo. Invest Ophthalmol Vis Sci. 2015;56:7137–45. doi: 10.1167/iovs.15-17302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moody MA, Liao HX, Alam SM, Scearce RM, Plonk MK, Kozink DM, et al. Anti-phospholipid human monoclonal antibodies inhibit CCR5-tropic HIV-1 and induce beta-chemokines. The Journal of Experimental Medicine. 2010;207:763–76. doi: 10.1084/jem.20091281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Lu B, Dixit U, Hossain S, Liu Y, Li J, et al. Reciprocal regulation of Abl kinase by Crk Y251 and Abi1 controls invasive phenotypes in glioblastoma. doi: 10.18632/oncotarget.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dransfield I, Zagorska A, Lew ED, Michail K, Lemke G. Mer receptor tyrosine kinase mediates both tethering and phagocytosis of apoptotic cells. Cell death & disease. 2015;6:e1646. doi: 10.1038/cddis.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimani SG, Kumar S, Davra V, Chang YJ, Kasikara C, Geng K, et al. Normalization of TAM post-receptor signaling reveals a cell invasive signature for Axl tyrosine kinase. Cell communication and signaling : CCS. 2016;14:19. doi: 10.1186/s12964-016-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suarez RM, Chevot F, Cavagnino A, Saettel N, Radvanyi F, Piguel S, et al. Inhibitors of the TAM subfamily of tyrosine kinases: synthesis and biological evaluation. European journal of medicinal chemistry. 2013;61:2–25. doi: 10.1016/j.ejmech.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. Journal of cellular and molecular medicine. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan PY, Carrera Silva EA, De Kouchkovsky D, Joannas LD, Hao L, Hu D, et al. The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science. 2016;352:99–103. doi: 10.1126/science.aaf1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM Receptor Signaling in Immune Homeostasis. Annual review of immunology. 2015;33:355–91. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miner JJ, Daniels BP, Shrestha B, Proenca-Modena JL, Lew ED, Lazear HM, et al. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med. 2015;21:1464–72. doi: 10.1038/nm.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimani SG, Kumar S, Davra V, Chang Y-J, Kasikara C, Geng K, et al. Normalization of TAM post-receptor signaling reveals a cell invasive signature for Axl tyrosine kinase. Cell Communication and Signaling. 2016;14:1–15. doi: 10.1186/s12964-016-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosurgi L, Bernink JH, Delgado Cuevas V, Gagliani N, Joannas L, Schmid ET, et al. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13091–6. doi: 10.1073/pnas.1302507110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rankin EB, Fuh KC, Castellini L, Viswanathan K, Finger EC, Diep AN, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13373–8. doi: 10.1073/pnas.1404848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook RS, Jacobsen KM, Wofford AM, DeRyckere D, Stanford J, Prieto AL, et al. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. The Journal of Clinical Investigation. 123:3231–42. doi: 10.1172/JCI67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N, et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci. 2003;44:826–38. doi: 10.1167/iovs.02-0438. [DOI] [PubMed] [Google Scholar]
- 49.Gray M, Gong J, Nguyen V, Osada T, Hartman Z, Hutchins J, et al. Targeting of phosphatidylserine by monoclonal antibodies augments the activity of paclitaxel and anti-PD1/PD-L1 therapy in the murine breast model E0771. Journal for Immunotherapy of Cancer. 2015;3:P357-P. [Google Scholar]
- 50.Gray MJ, Gong J, Hatch MM, Nguyen V, Hughes CC, Hutchins JT, et al. Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers. Breast Cancer Res. 2016;18:50. doi: 10.1186/s13058-016-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]