Abstract
Three- and four-membered rings, widespread motifs in nature and medicinal chemistry, have fascinated chemists ever since their discovery. However, due to energetic considerations, small rings are often difficult to assemble. In this regard, homogeneous gold catalysis has emerged as a powerful tool to construct these highly strained carbocycles. This review aims to provide a comprehensive summary of all the major advances and discoveries made in the gold-catalyzed synthesis of cyclopropanes, cyclopropenes, cyclobutanes, cyclobutenes, and their corresponding heterocyclic or heterosubstituted analogs.
1. Introduction
Cyclopropane was first synthesized by August Freund in 1882 (via Wurtz coupling of 1,3-dibromopropane), who already proposed its correct structure,1 whereas cyclobutane was first prepared by hydrogenation of cyclobutene in 1907.2 The structure and properties of these highly strained carbocycles and their derivatives have fascinated chemists ever since.3−6 As a result, huge efforts have been made over the years to gain access to diverse small ring-containing molecules and to study their special reactivity patterns.
Due to their inherent strain (60° C–C bond angles compared to 109.5° for typical Csp3–Csp3 bonds), the reactivity of cyclopropanes often resembles more closely that of alkenes than that of alkanes.7,8 Strikingly, the strain energy of cyclobutane and cyclopropane is rather similar (Scheme 1).9−13 This has been rationalized by taking both C–C and C–H bond energy components into consideration: the total C–C bond strain of cyclopropane is 10 kcal/mol higher than that for cyclobutane, but this is largely compensated by the stronger C–H bonds of cyclopropane (8 kcal/mol).14,15 Stabilizing effects increase when substituents are placed on the backbone of these rings.16,17
Scheme 1. Estimated Ring Strain Energy Ranges for Selected Cyclic Hydrocarbons.
As a consequence of the uphill thermodynamics, methods for the assembly of highly strained small rings differ significantly from those employed to build medium or large rings. For example, many classical methods to obtain cyclopropanes rely on passing through highly energetic intermediates, such as carbenes or carbenoids.18,19 General strategies based on direct ring-closing cyclizations are rare for the construction of small rings.
1.1. The Importance of Small Rings
The importance of small rings to the scientific community goes far beyond purely academic reasons. The cyclopropane unit is considered a privileged motif in medicinal chemistry.20 Thus, more than 10 cyclopropane rings can be found among the 220 drugs approved by the U.S. FDA between 2015 and 2019.21−24 This does not come as a surprise, since 3-membered rings appear in a wide range of naturally occurring, biologically active molecules (Scheme 2A).25−27 Although less widespread, cyclobutanes and cyclobutenes are also present in small molecules isolated from natural sources28−30 and have been explored as drug candidates.31−33 Biosynthetically, both cyclopropanes and cyclobutanes are commonly proposed to form by ring closure of homoallyl cation intermediates, among other pathways.34−36 Furthermore, countless applications of 3-membered37−42 and 4-membered carbocycles43−46 as reactive intermediates have been developed. These strategies have been widely applied in the context of synthetic methodology development and natural product synthesis.47−50
Scheme 2. Structures of Natural or Bioactive Compounds Containing Small Rings (A) and Selected Classical Approaches for the Assembly of Cyclopropanes (B) and Cyclobutanes (C).

1.2. An Overview of the Synthetic Methods to Assemble Small Rings
The synthesis of cyclopropanes has been traditionally approached by methodologies such as the Simmons–Smith reaction (through metal carbenoids)51−56 or the Johnson–Corey–Chaykovsky reaction (also developed for the assembly of epoxides or aziridines)57−60 or via metal carbenes through decomposition of diazo compounds (Scheme 2B).61−63 Some alternative methods for the synthesis of cyclopropanes involve the use of different types of ylides,64−67 free dihalocarbenes,68 direct cyclizations,69,70 ring contractions,71−73 radical pathways,74−77 or the Kulinkovich reaction,78−80 among others.81−84
The most classical approach for the synthesis of cyclobutanes is the photoexcitation of alkenes, which can engage in concerted [2 + 2] cycloaddition pathways, thermally forbidden by symmetry (Scheme 2C).85−87 These transformations have found a broad range of applications in organic synthesis over the years.88−91 Apart from direct photoexcitation pathways,92 cyclobutanes have also been assembled via formal [2 + 2] cycloadditions with the aid of photoredox93,94 or Lewis acid catalysis.95−97 The use of highly polarized cycloaddition partners is a general theme in this context, but metal-catalyzed [2 + 2] cycloadditions of unbiased alkenes and alkynes have also been achieved.98,99 Non-cycloaddition strategies for the synthesis of 4-membered rings include ring expansion,100−103 ring contraction,104,105 strain release opening,106,107 hydroalkylation,108 or hydroacylation reactions109 among others.110,111
1.3. Gold Catalysis in the Construction of Small Rings
The origins of homogeneous gold catalysis date back to 1986, when Ito et al. described an asymmetric aldol reaction catalyzed by a chiral ferrocenylphosphine–gold(I) complex.112 In 1987, the first homogeneous gold-catalyzed addition of nucleophiles to alkynes was realized by the group of Utimoto using sodium tetrachloroaurate dihydrate.113,114 One decade later, Teles et al.115 and Tanaka et al.116 demonstrated the possibility of activating alkynes using gold(I) complexes. From that point, the field of gold(I) catalysis started to gain momentum year after year and still today remains one of the most active areas of research in organometallic chemistry.117,118 This comes as a consequence of the ability of gold(I) complexes to activate π bonds in a very selective manner.119−135 Its potential, attributed partially to relativistic effects,136 is illustrated by the wide molecular complexity137−184 that can be built through the gold(I)-catalyzed cycloisomerization of enynes (Scheme 3).185−206
Scheme 3. Fate of Cyclopropyl Gold(I) Carbenes Generated by 1,6-Enyne Cyclization.
Arising from complex mechanistic scenarios, the generated cyclopropyl gold carbenes can oftentimes evolve or be trapped to form isolable products containing 3- or 4-membered carbocycles.185−206
The discovery of novel chemical transformations catalyzed by gold grows together with the development of new and more active catalysts, such as highly electrophilic cationic gold(I) complexes.207−210 These species can be either generated in situ by chloride abstraction (Scheme 4, left)211−213 or synthesized and handled as bench-stable solids (Scheme 4, right).214,215 Design and selection of the main ligand (L) allows tuning the reactivity of the complex216−222 or achieving highly enantioselective transformations.223−234
Scheme 4. Activation of Gold(I) Chloride Complexes (Left) and Stable Cationic Gold(I) Complexes (Right).
Hand in hand with this explosive development, gold catalysis has emerged a powerful and versatile tool to assemble 3- and 4-membered rings.
1.4. Scope of the Review
In this review, we cover all the major developments in the field of small ring assembly using homogeneous gold catalysis. We include under the denomination of “small rings” all 3- and 4-membered carbocycles (cyclopropanes, cyclopropenes, cyclobutanes, and cyclobutenes) and their corresponding heterocyclic or heterosubstituted analogs.
The review is divided in two major parts: the first one discusses the synthesis of 3-membered rings, and the second one covers the preparation of 4-membered rings. The different gold-catalyzed transformations are organized based on the nature of the reaction substrates employed or the reaction products obtained in each of them. For most of the discussed reactions, a brief selection of the scope of the methodology is presented, as well as an overview of its mechanistic rationale when appropriate.
For the sake of simplicity in some mechanistic schemes throughout this review, “AuL+” is used as a surrogate of [AuLL′]+ complexes, where L′ is a relatively weak ligand such as a bound substrate (alkyne or alkene), product, or donor solvent molecule. However, it is important to stress that the existence of “naked” gold(I) species “AuL+” in solution has not yet been demonstrated.
2. Construction of 3-Membered Rings Catalyzed by Gold
Most of the gold(I)-catalyzed transformations that give rise to 3-membered carbocycles are proposed to involve the intermediacy of gold(I) carbenes.235,236 Apart from the classical decomposition of diazo compounds,237,238 different precursors of gold(I) carbenes or carbenoids have been developed for the assembly of 3-membered rings.239,240 A few previous reviews have covered the gold-catalyzed synthesis of 3-membered rings until 2016.237−240
2.1. Decomposition of Diazo Compounds
The most common application of diazo compounds241 is their decomposition to generate either free carbenes or metal carbenes, through downhill release of nitrogen (Scheme 5).242,243
Scheme 5. Mechanistic Rationale for the Generation of Metal Carbenes from Diazo-Compounds.
The resulting intermediates can take part in a wide range of transformations, among which the assembly of 3-membered rings, through either concerted or stepwise (2 + 1) cycloaddition pathways, is probably the most studied one.61−63,237,238 More recently, the use of hydrazones as precursors for the in situ generation and decomposition of diazo compounds has allowed the development of safer procedures involving these potentially hazardous reagents.244,245
2.1.1. Gold-Catalyzed Cyclopropanation with Diazo Compounds
The metal-catalyzed carbene transfer reaction from diazo compounds is the best-established method for the assembly of acceptor or donor–acceptor cyclopropanes. Gold(I) catalysts are one type of several metal complexes that are able to promote this transformation.246 Some of the most often used metals in this type of cyclopropanation of alkenes are rhodium,247,248 ruthenium,249−252 copper,253,254 cobalt,255−257 and palladium.258−260 Over the past 15 years, several gold(I) complexes have been employed to promote carbene transfer reactions to different nucleophiles.261
Pérez and co-workers disclosed the first example of the use of a gold(I) catalyst for a carbene transfer reaction from ethyl diazoacetate (EDA).262 Reaction of EDA with a large excess of cyclooctene in the presence of [(IPr)AuCl] activated with NaBArF4 led to quantitative formation of a mixture of the corresponding endo/exo cyclopropanes (Scheme 6). The same reaction with styrene gave a mixture of products of cyclopropanation (cis/trans) and formal aryl C–H insertion (ortho-, meta-, and para-). The generated acceptor gold(I) carbenes also undergo C–H, N–H, or O–H insertion reactions in the presence of alkanes,263 primary amines, or alcohols, respectively.
Scheme 6. Gold(I)-Catalyzed Cyclopropanation of Cyclooctene and Styrene with Ethyl Diazoacetate.
The same group reported the synthesis and characterization of cationic NHC–Au(I) complexes, which in the original report were generated in situ by chloride abstraction.262 Such catalysts (e.g., [(IPr)Au(MeCN)]PF6) proved to be active also in the cyclopropanation of styrene with EDA, giving quantitative conversion after 3 days to the products of cyclopropanation, 3b, exclusively. These cationic complexes did not require activation but were slower at promoting the carbene transfer process.264
The groups of Echavarren and Pérez studied this carbene transfer cyclopropanation with other cationic gold(I) catalysts, different alkenes, and donor–acceptor diazo compounds (Scheme 7).265
Scheme 7. Cyclopropanation Catalyzed by a Cationic Phosphine Gold(I) Complex.
In this work, a range of cationic gold(I) complexes bearing phosphine, phosphite, and NHC ligands were tested, among which [(JohnPhos)Au(MeCN)]SbF6 performed best. In contrast to the original reports with EDA,262 these reactions were remarkably efficient, allowing the use 1.1 equiv of the alkene partner while still maintaining significant yields. The products of cyclopropanation with these carbenes were obtained as single (trans- or exo-) diastereoisomers, following the general trend for donor–acceptor metal carbenes.266 In 2019, Pérez reported the cyclopropanation of ethylene, the simplest alkene, using ethyl diazoacetate in the presence of an NHC–gold(I) catalyst.267
A carbene transfer reaction from EDA was also carried out using gold(I) complexes bearing a polyhalogenated triazapentadienyl group and ethylene as ligands (7, Scheme 8). The reaction gives good yield of cyclopropane 3b, with only 2% C–H insertion side products.268
Scheme 8. Gold(I) Ethylene Complexes with Triazapentadienyl Ligands in Cyclopropanation.
Besides gold(I) complexes, bulk gold metal has also been used to carry out similar transformations, with moderate yields and diastereoselectivities. The size of the gold particles, together with the morphology and composition of the metal surface, influenced the outcome of the carbene transfer reactions. For instance, the authors found that a freshly prepared urchin morphology (with incorporated carbon) is less active than smoother gold powder that is formed during the catalytic diazo-transfer processes themselves (Scheme 9).269
Scheme 9. Diazo–Carbene Transfer Cyclopropanation Promoted by Metallic Gold Particles.
Major diastereoisomer (cis/trans) depicted for each cyclopropane.
Corma and co-workers demonstrated the possibility of using simple salts such as NaAuCl4 or KAu(CN)2 in combination with ionic liquids for the same transformations.270 These salts rapidly decompose and afford Au(0) nanoparticles, which remain stabilized in the ionic liquid and can be recovered after the reaction. Furthermore, the same group showed that gold-containing metal–organic frameworks (MOFs) can be employed to promote diazo-carbene transfer cyclopropanations.271 This allowed the assembly of different acceptor cyclopropanes in moderate to good yields and moderate to excellent diastereoselectivities.
The field moved one step further when Zhou and co-workers reported the first general gold(I)-catalyzed enantioselective cyclopropanation via carbene transfer using diazooxindoles 11 (Scheme 10).272 This method proved to be superior to the ones previously reported based on rhodium(II) (up to 74% ee)273 or mercury(II) catalysis (scope limited to simple styrene).274 In this work, a monoactivated digold complex derived from a spiroketal biphosphine (13) generated in situ was used to prepare a range of cyclopropanes in good yields, short reaction times, and excellent diastereo- and enantioselectivity with styrene-type alkenes. However, the yield and enantioselectivity dropped significantly when nonactivated alkenes such as 1-hexene (14d) were used. More recently, the system was expanded to the highly enantioselective cyclopropanation of interesting mono- and difluoromethylated styrenes, affording products such as 14e.275
Scheme 10. Enantioselective Gold(I)-Catalyzed Cyclopropanation with Diazooxindoles.
A gold(I)-catalyzed enantioselective cyclopropanation of enamides using donor–acceptor diazo compounds was reported by the group of Zhang (Scheme 11).276 This transformation used Carreira ligand27717 for the synthesis of a range of densely substituted donor–acceptor cyclopropanes with high diastereo- and enantioselectivity. The presence of an additional β-methyl on the starting vinyl-phthalimide further improved the stereoselectivity of the transformation (18c,d). Remarkably, the reaction could be carried out with a moderate excess of the diazo-compound (1.5 equiv) on gram scale.
Scheme 11. Enantioselective Gold(I)-Catalyzed Cyclopropanation of Enamides.
R = 2,6-i-Pr2C6H3. Products obtained as the depicted trans single diastereoisomer.
2.1.2. Cyclopropanation of Aromatic Rings: The Buchner Ring Expansion
The Buchner ring expansion reaction is used to assemble 1,3,5-cycloheptatrienes.278−280 It involves the cyclopropanation of an aromatic ring by a carbene (generated from a diazo compound with the aid of light, heat, or a metal catalyst), giving rise to a norcaradiene, which is in equilibrium with the corresponding cycloheptatriene through a 6-electron disrotatory electrocyclic process (valence tautomerism, Scheme 12).281
Scheme 12. Buchner Reaction.
The first example of gold(I)-catalyzed cyclopropanation–ring expansion of benzene was described by the group of Pérez using EDA and an NHC–Au(I) complex as catalyst (Scheme 13, top). However, the main product of the reaction was 19b, due to formal insertion of the carbene into the C–H aromatic bonds (1:3 cyclopropanation/insertion ratio). The cyclopropanation/insertion ratio was moderately higher (2:3) when toluene was used instead of benzene.262 Complementary to this reactivity, He and co-workers reported that gold(I) complexes bearing a terpyridine ligand (t-Bu3tpy) promote selectively the Buchner ring expansion of benzene, without formal insertion, giving an equimolar mixture of diethyl maleate/fumarate (19c) as the only side products (Scheme 13, bottom).282
Scheme 13. Gold(I)-Catalyzed Buchner Ring Expansion versus C–H Insertion.
t-Bu3tpy = 4,4′,4″-tri-tert-butyl-2,2′:6′,2″-terpyridine.
The reactivity of acceptor metal carbenes generated from EDA was studied using gold(I) complexes bearing several NHC ligands in addition to IPr.283 This, together with later reports,284−286 proved the ability of these coinage metal carbenes to engage not only in cyclopropanation/Buchner reactions but also in the C–H functionalization of saturated hydrocarbons. Thorough mechanistic investigations on the Buchner ring expansion versus Csp2–H insertion of acceptor metal carbenes catalyzed by Au(I), Ag(I), and Cu(I) NHC complexes were disclosed showing a clear correlation between the electronics of the aromatic ring and the ratio of Buchner/insertion products (Scheme 14).287
Scheme 14. Buchner versus Insertion Reaction of Acceptor Gold(I) Carbenes.
Combined amount of all possible regioisomers. In all cases, the total yield for the carbene transfer from EDA was at least 94%.
Finally, the cyclopropanation of a more extended aromatic system, naphthalene, was also achieved by the use of EDA in the presence of either Au(I) or Cu(I) NHC/chloride complexes (activated with NaBArF4), the latter being the most effective and selective (Scheme 15).288
Scheme 15. Cyclopropanation of Naphthalene.
Obtained as a mixture of the two possible regioisomers.
2.1.3. Cyclopropenation with Diazo Compounds
The most direct approach for the synthesis of cyclopropenes is the reaction of alkynes with metal carbenes generated from diazo compounds, which has been developed using complexes of different metals.289−294
Cyclopropenes are known to react in the presence of electrophilic gold(I) complexes, generating carbene intermediates (see section 2.3).295 In spite of this fact, the group of Davies successfully developed a highly enantioselective cyclopropenation of alkynes based on the use of donor–acceptor diazo compounds via gold-catalyzed carbene transfer (Scheme 16).296 This is the asymmetric version of a transformation previously developed by the same group using silver(I) catalysis.297 Similar reactivity was previously achieved for less challenging terminal alkynes by chiral rhodium(II) catalysis.298 In this work, chiral digold phosphine complexes such as 24 were employed in combination with AgSbF6. Internal alkynes with a broad range of substituents (alkyl, aryl, alkenyl, and alkynyl) were cyclopropenated in good yields and excellent enantioselectivities.
Scheme 16. Gold(I)-Catalyzed Enantioselective Cyclopropenation of Internal Alkynes.
All the examples covered in this first section illustrate how diazo-carbene transfer reactions catalyzed by gold, along with other metals,247−260 are one of the most straightforward ways of assembling acceptor or donor–acceptor three-membered carbocycles. However, when it comes to assembling nonacceptor cyclopropanes via carbene intermediates, diazo compounds are usually not the reagents of choice for several reasons. Even though it is possible to prepare nonstabilized diazo compounds with only H (such as poisonous299 and hazardous diazomethane), aryl, vinyl, or alkyl substituents,63 these are usually challenging to make, inherently unstable, explosive in pure form,300 difficult to store for long periods of time,244,245 and prone to diazo dimerization. For these reasons, great attention has been given recently to the development of alternative methods for the generation of donor metal carbenes.301
2.2. Retro-Cyclopropanation or Decarbenation Reactions
A general method for accessing nonacceptor carbenes relies on retro-cyclopropanation or decarbenation reactions, in which the driving force is the release of an unstrained unsaturated organic fragment, usually aromatic, instead of gaseous nitrogen.302 Volger reported that tricycle 26, which is in equilibrium with its norcaradiene tautomer 26′, reacts in the presence of [Rh(CO)2Cl]2 to give hexamethylbenzene quantitatively (Scheme 17A).303 Later, Gassman described the possibility of using highly electrophilic PhWCl3–EtAlCl2 to carry out a cyclopropane–alkene metathesis via retro-cyclopropanation of simple ethylcyclopropane (Scheme 17B).304,305 Furthermore, several groups disclosed the photolytic decarbenation reaction of phenanthrene derivatives 27 (Scheme 17C).306−309
Scheme 17. Retro-Cyclopropanation Reactions: Historical Perspective.
Despite these and a few other observations,310−312 the possibility of using carbenes released by retro-cyclopropanation in a synthetically useful manner was not clear at the time. It was not until gold(I) catalysis was brought into play that decarbenation reactions emerged as a general and safe alternative to assemble nonacceptor cyclopropanes.302
2.2.1. Decarbenation or Retro-Buchner Reaction of Cycloheptatrienes
The group of Echavarren disclosed the formation of free aryl gold(I) carbenes in the context of cycloisomerization of enynes such as 28 (Scheme 18). Cyclization results in the formation of electron-rich benzo-fused norcaradienes 31, which undergo a retro-cyclopropanation or decarbenation process to give naphthalene derivatives 33, while releasing free aryl carbenes 32. The presence of these intermediates was confirmed by the formation of 34 via cyclopropanation of another molecule of 31 (Scheme 18).313
Scheme 18. Retro-Buchner or Decarbenation Reaction of Cycloheptatrienes.
The group of Hashmi later proposed a retro-Buchner pathway for the formation of a naphthalene by a gold(I)-catalyzed diyne cycloisomerization (Scheme 19).314 Extrusion of a carbene unit from benzo-fused norcaradiene 36, which results in the release of ethylene (detected by MS), would be the driving force of the process.
Scheme 19. Formation of Naphthalenes by Retro-Cyclopropanation of a Benzo-fused Norcaradiene.
Mechanistically, these reactions proceed analogously to the formation of metal carbenes by diazo decomposition (see Scheme 5 and Scheme 20).315 Breaking the first C–C bond of the norcaradiene tautomer leads to a carbenoid-like Wheland intermediate, which evolves by release of the aromatic fragment (e.g., benzene), generating the corresponding metal carbene.
Scheme 20. Retro-Buchner or Decarbenation Reaction of Cycloheptatrienes.
This concept, discovered though the retro-cyclopropanation of benzo-fused norcaradienes such as 31, led to the development of 7-aryl-1,3,5-cycloheptatrienes 38 as precursors of aryl gold(I) carbenes, which were used to cyclopropanate a range of styrenes and stilbenes with moderate to high diastereoselectivities (Scheme 21).316 This allows the easy assembly of trisubstituted nonacceptor cyclopropanes (40a,b), which would be challenging synthetic targets by other methods involving the use of potentially dangerous aryl diazo compounds.244,245 In this case, 7-aryl cycloheptatrienes are used, which can be prepared in one step by reaction of aryl lithium or Grignard reagents with commercially available tropylium tetrafluoroborate.
Scheme 21. Aryl Cyclopropanation by Decarbenation or Retro-Buchner Reaction.
Yield and cis/trans ratio between parentheses.
This method was extended to a broad-scope gold(I)-catalyzed cis-vinylcyclopropanation of alkenes using 7-vinyl and 7-styryl-1,3,5-cycloheptatrienes 41 as carbene precursors, which are more reactive than their corresponding 7-aryl analogs (requiring 75 °C instead of the 120 °C from the original work) (Scheme 22).317
Scheme 22. Synthesis of cis-Vinylcyclopropanes.
A new design, based on the hypothesis that electron-donating groups in the cycloheptatriene ring would lower the energy required to reach the cationic Wheland-type intermediate involved in the rate-limiting step of the decarbenation reaction, led to the development of a second generation of more reactive carbene precursors318 (Scheme 23, top). Thus, 7-substituted 1,3,5-trimethyl-1,3,5-cycloheptatrienes 44 undergo the gold(I)-catalyzed retro-Buchner reaction at 25 °C. Importantly, these reagents allowed researchers to carry out, for the first time, efficient decarbenation reactions catalyzed by zinc(II) salts (such as inexpensive ZnBr2)318 or rhodium(II) complexes (unlocking new reactivity).319
Scheme 23. Second Generation of Cycloheptatrienes as General Metal Carbene Precursors.
[Au] = [(JohnPhos)Au(MeCN)]SbF6 (5 mol %) in EtOAc at 25 °C. [Rh]= Rh2TFA4 (3 mol %) in 1,2-DCE at 25–60 °C. [Zn] = ZnBr2 (10 mol %) in 1,2-DCE at 65 °C.
This kind of vinyl gold(I) or rhodium(II) carbenes can also be trapped efficiently by enol ethers (Scheme 24), assembling densely substituted cyclopropyl ethers.320 Under rhodium(II) catalysis, nonacceptor cyclopropanes 49 can be opened to give all-E trienes, upon release of methanol.
Scheme 24. Au(I)- or Rh(II)-Catalyzed Cyclopropanation of Enol Ethers.
[Au] = [(JohnPhos)Au(MeCN)]SbF6 (5 mol %) in 1,2-DCE at the specified temperature.
[Rh]= Rh2TFA4 (5 mol %) in 1,2-DCE at the specified temperature.
Based on the same concept of release of an aromatic molecule, benzo-fused norcaradienes derived from naphthalene (51) and phenanthrene (52) were also developed.321 These persistent cyclopropanes can be retro-cyclopropanated under gold(I) catalysis, and the corresponding carbenes can be trapped with alkenes to afford cyclopropanes (Scheme 25).
Scheme 25. Gold(I)-Catalyzed Decarbenation of Persistent Cyclopropanes.
Besides cyclopropanation reactions, metal carbenes generated by retro-Buchner reaction of cycloheptatrienes have been employed to develop new formal cycloadditions322,323 and C–H/X–H insertion reactions319,324 and in the total synthesis of natural products.318,319,323
2.2.2. Gold Carbenes in Gas Phase by Retro-Cyclopropanation
The group of Chen reported the first the gas-phase generation of an aryl gold(I) carbene complex. This was accomplished by collision-induced dissociation (CID) of phosphonium ylide gold complexes such as 55 (Scheme 26).325−329 These highly energetic intermediates show the expected reactivity for gold(I) carbenes, cyclopropanating alkenes (Scheme 26A). This process is reversible (Scheme 26B), and the same cyclopropanes can give back the original metal carbene and alkene (pathway B1). Alternatively, a different alkene (62) and carbene units (61) can be released (pathway B2), resulting in an overall cross-metathesis-type process.
Scheme 26. Gold(I)-Carbenes by Retro-Cyclopropanation in Gas Phase.
2.3. Cyclopropene Opening Followed by Cyclopropanation
Cyclopropenes have also been established over the past decade as a safe and reliable source of metal carbenes.295 Long after cyclopropenes were discovered to be reactive under metal catalysis,40,330 it was reported that in the presence of cationic gold(I) complexes cyclopropenes can be opened, generating vinyl carbenes 64 (or gold-stabilized carbocations), which can be characterized spectroscopically (Scheme 27A)331 or trapped by organic nucleophiles, such as alkenes, alcohols, arenes, or carbonyl groups, among others (Scheme 27B).295,332−338
Scheme 27. Characterization and Reactivity of Gold(I) Carbenes via Cyclopropenes.
Analogously to other gold(I) carbene sources, cyclopropenes react with alkenes to give cyclopropanes, as first revealed by the groups of Lee,334 and Toste332 (Scheme 27B,C). The opening process promoted by gold has also been studied computationally.339,340
The group of Cossy reported an intramolecular approach for the synthesis of fused cyclopropanes by gold(I)-catalyzed disassembly of cyclopropenes. This allowed access to densely functionalized 3-oxa- and 3-azabicyclo[4.1.0]heptanes 71 in excellent yields and diastereoselectivities (Scheme 28, top).341
Scheme 28. Cyclopropene Opening and Intramolecular Vinylcyclopropanation of O- and N-Tethered 1,6-Cyclopropene-enes.
R = TBDPS.
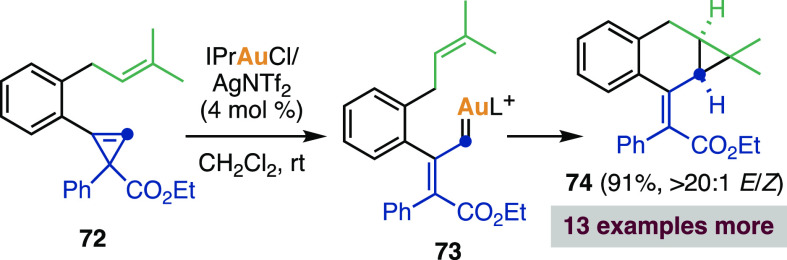
This method was extended to the synthesis of a diverse range of bicyclic structures (Scheme 28, bottom), which could be elaborated to assemble synthetically complex cyclopropanes.342,343 Following a similar strategy, Liu and co-workers reported the cyclization of cyclopropene-enes such as 72 under gold catalysis to assemble cyclopropanes 74 in good yields and high E/Z ratios. The starting cyclopropenes were prepared by rhodium(II)-catalyzed cyclopropenation of the corresponding alkynes. Further treatment with a gold(I) complex triggers the cyclopropene opening, generating a vinyl carbene that undergoes intramolecular cyclopropanation (Scheme 29).344
Scheme 29. Cyclopropane Opening and Cyclopropanation of Benzene-Tethered 1,6-Cyclopropene-enes.
Two different scenarios were explored by Tang and co-workers using propagyl esters as the nucleophilic component (after isomerization to the corresponding allene) (Scheme 30).345 When 1,3-disubstituted cyclopropenes 75 were employed (R = H), the cyclopropene ring opens under gold(I) catalysis generating vinyl carbene 79, which cyclopropanates the proximal double bond of the allene, giving 3-azabicyclo[4.1.0]heptanes such as 76. On the other hand, 1,2,3-trisubstituted cyclopropenes 75 give spirocyclic cyclopropanes 77, presumably through cationic intermediate 80.
Scheme 30. Cyclopropene Opening and Substrate-Dependent Divergent Cyclopropanation.
Using [(tBuXPhos)Au(OTf)] (5 mol %) in 1,2-DCE at 100 °C, with 4 Å molecular sieves.
The vinylcyclopropanation of furans through gold(I)-catalyzed opening of cyclopropenes results in the direct disassembly of the corresponding intermediates to afford conjugated trienyl-ketones.346 In a complementary manner, the cyclopropane intermediates can be isolated when performing the same reaction under Zn(II) catalysis.347 In heterogeneous phase, gold metal particles were also employed for the opening of 3,3-diphenylcyclopropene and subsequent trapping with styrene.269 Beyond cyclopropanations, many other methods have been recently developed based on the use cyclopropenes as carbene sources under catalysis of different metals,348−353 but they fall out of the scope of this review.
2.4. Enynes, Allenynes and Diynes Cycloisomerization Terminated with Cyclopropanation
Reactions forming cyclopropyl-containing scaffolds are presented in this section divided by substrate type: enynes (1,6-enynes, 1,5-enynes, and other kinds of enynes), allenynes and diynes.
2.4.1. Cycloisomerization of Enynes
2.4.1.1. 1,6-Enynes
Gold complexes are selective carbophilic π acids for the activation of multiple C–C bonds,119−135 such as those of alkynes, alkenes, and allenes. Gold-catalyzed cycloisomerizations of enynes have been studied extensively since the beginning of the XXI century.185−206,354 When interacting with an enyne, Au(I) can form η2-complexes with either the triple or the double bond.355,356 The higher affinity for alkynes in front of alkenes (termed alkynophilicity) is a kinetic effect driven by the lower LUMO of the η2-alkyne gold complex, hence its higher propensity to undergo nucleophilic attack.357
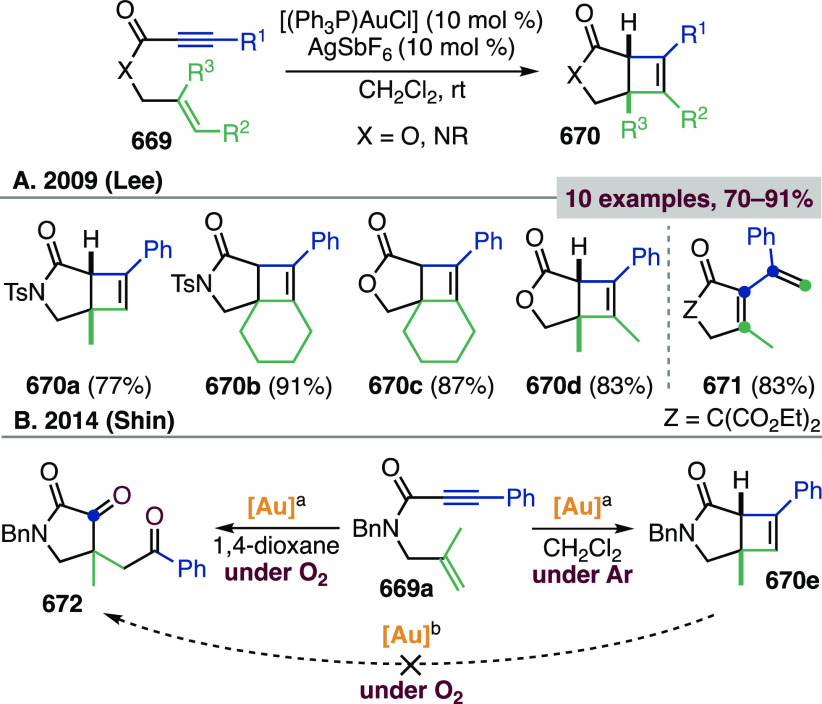
The cycloisomerizations of Au(I)-activated 1,6-enynes 81 lead to cyclopropyl gold carbenes 82 and 89 by 5-exo-dig and 6-endo-dig cyclizations, respectively (Scheme 31). The pathway followed by a given enyne depends mostly on its substitution pattern; thus 5-exo-dig cyclization is kinetically favored for terminal alkynes, while enynes with internal alkynes or heteroatoms at the tether generally undergo 6-endo-dig cyclizations.358,359 Cyclopropyl gold carbenes have highly delocalized structures, to which the resonance forms of gold(I)-stabilized cyclopropylmethyl, cyclobutyl, and homoallyl carbocations contribute to a greater or lesser extent depending on substitution and the ancillary ligand on gold.235,236,331−333,360−362 They can be trapped by alkenes, C-nucleophiles, or oxidants affording bicyclo[3.1.0]hexanes 83, 84, and 85, whose preparation is described in this section (compounds 85 are covered in section 2.6 on α-oxo gold carbenes, as they can be obtained also via alkyne oxidation followed by cyclopropanation). Alternatively, cyclopropyl gold carbenes 82 can evolve to dienes 87 and 88 through single cleavage and double cleavage rearrangements, respectively. Gold carbenes 89 lead to bicyclo[4.1.0]heptenes 90 after 1,2-H shift and deauration. On the other hand, they could also undergo single-cleavage rearrangements to dienes 91 or isomerize with ring expansion to (η2-cyclobutene)Au(I) complexes 92. The latter evolve to cyclobutenes 93 by gold decoordination (see section 3.2) or to dienes 87 by ring opening. 1,6-Enynes with an ester or amide tether (i.e., with a carbonyl rather than a CH2 unit between the alkyne and the heteroatom linker Z) proceed via a different mechanism to fused cyclobutene products (see section 3.2).363
Scheme 31. Main Au(I)-Catalyzed Cycloisomerization Pathways for 1,6-Enynes.
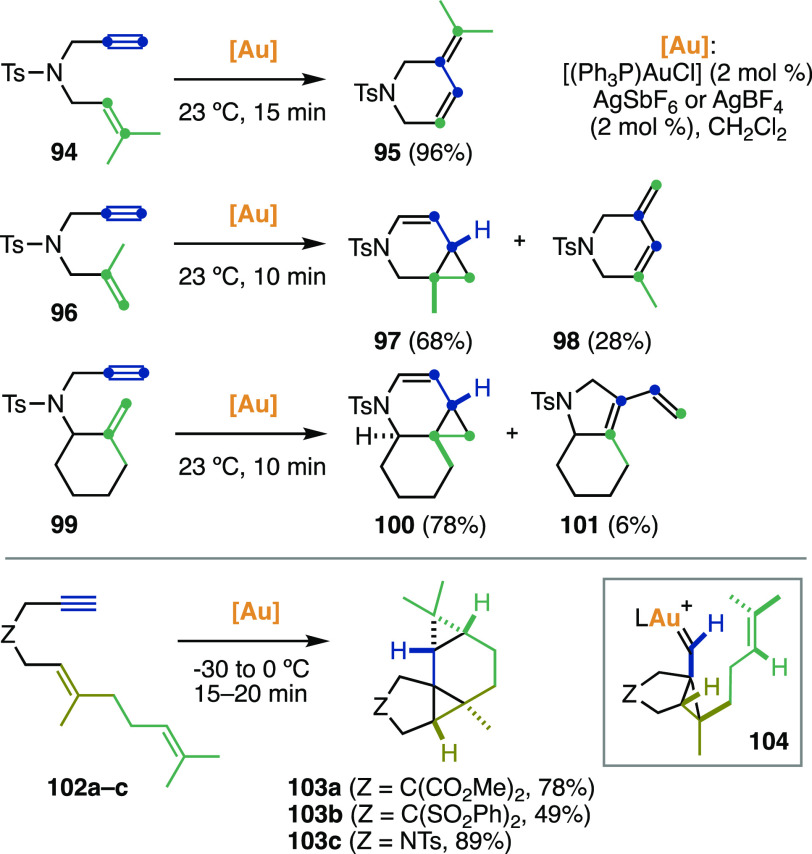
The first study on gold(I)-catalyzed rearrangements and alkoxycyclizations of a variety of 1,6-enynes was reported by the group of Echavarren (Scheme 32).364,365 Compared to the other metal catalysts known at the time for enyne activation,185−206,366−379 Au(I) complexes stood out for their high activity even at low temperatures and for their ability to trigger different mechanisms. The alkoxycyclizations of 1,6-enynes proceed more readily under Au(I) than Pt(II) catalysis,358,359 and indeed lower energy barriers were computed for the 5-exo-dig and 6-endo-dig cyclizations of model enyne (E)-6-octen-1-yne catalyzed by the [AuPH3]+ fragment with respect to trans-[Pt(H2O)Cl2].380 The high substrate dependence of the cycloisomerization pathways for N-tethered 1,6-enynes is evident from the selected examples reported in Scheme 32: substrates 94, 96, 99 preferentially underwent 6-endo-dig cyclizations,381 whereas dienynes 102a–c cyclized in a 5-exo-dig mode. Cyclopropyl gold carbene 104, with antiperiplanar arrangement of the cyclopropane and metal carbene, was then trapped by the pendant alkene affording tetracycles 103a–c as single diastereomers. The scope of the intramolecular bis-cyclopropanation of dienynes was expanded, and DFT computations indicated that the second cyclopropanation proceeding through anti, rather than syn, gold carbene intermediates such as 104 is kinetically and thermodynamically more favorable.382
Scheme 32. Au(I)-Catalyzed Cycloisomerizations of 1,6-Enynes and Intramolecular Cyclopropanation of Dienynes.
Chung and co-workers reported the bis-cyclopropanation of dienynes 105, possessing a 1,4-diene moiety, to give compounds 106 with a tetracyclo[3.3.0.0.2,8.04,6]octane skeleton (Scheme 33).383 A tentative mechanism, depicted as proposed by the authors, accounts also for the formation of products 109 and 111, obtained using other Au(I) complexes. The same group later described the formal [4 + 2] cycloaddition of N-tethered enynes with a 1,3-diene unit384 and prepared related 4-oxa-6-azatricyclo[3.3.0.02,8]octanes by cycloisomerization of N-tethered internal 1,6-enynes bearing an allylic alcohol.385O-Tethered 1,6-enynes with an enol ether as the alkene component delivered similarly complex cage structures.386
Scheme 33. Au(I)-Catalyzed Intramolecular Cyclopropanation of Cyclohexadienyl Alkynes.
The intermolecular version of these reactions was developed trapping with external alkenes two different cyclopropyl gold carbene intermediates (116 and 117) generated from 1,6-enynes (Scheme 34).387,388 Cyclopropyl gold carbene 116 can convert to intermediate 117 in a stepwise or concerted manner, namely via a 1,2-shift of the carbene C atom of 116 with concomitant cleavage of the cyclopropane distal C–C bond and double bond formation.214 1,3-Metallotropic shift of the Au(I) carbene along a conjugated alkyne, followed by intramolecular cyclopropanation by the transposed carbene, was also observed.
Scheme 34. Au(I)-Catalyzed Intermolecular Cyclopropanation of 1,6-Enynes with Alkenes.
Additional experimental and computational investigations revealed that the cyclopropanation of alkenes with 1,6-enynes is a process proceeding via electrophilic cyclopropyl gold carbenes, which can occur both in a concerted (for aliphatic, electronically unbiased alkenes) and in a stepwise fashion (for polarized alkenes such as styrenes or enol ethers).320,388,327 Regardless, the reaction remains stereospecific with respect to the alkene geometry because the second C–C bond formation has a very low barrier.
Cyclopropyl gold carbenes generated from 1,5- and 1,6-enynes were trapped also by other nucleophiles, such as electron-rich arenes and dicarbonyl compounds (Scheme 35A).389,390 With some exceptions, phosphite ligands directed nucleophilic attack at the cyclopropyl carbon a in intermediate 120 yielding predominantly exocyclic alkenes 119a, whereas more electron-donating ligands favored attack at the carbene carbon b delivering products 119b. Later, Liu and co-workers reported that cyclopropyl gold carbenes generated in a 5-exo-dig fashion from 1,6-enynes can be trapped by aryl diazo ketones (Scheme 35B).391
Scheme 35. Trapping of Cyclopropyl Gold Carbenes Generated from 1,6-Enynes.
(ArO)3P = (2,4-(t-Bu)2C6H3O)3P
Wei and Shi reported that the cycloisomerizations of N- and O-tethered alkylidenecyclopropanes 123 deliver tricyclic compounds 124 in high yields via the expected 6-endo-dig pathway, as long as the alkyne is internal (Scheme 36A).392 An enyne with a terminal triple bond proceeded instead to diene 125 via 5-exo-dig cyclization. This reactivity contrasts with the rare 6-exo-dig cyclization previously observed by the group of Toste for a C-tethered 1,6-enyne (Scheme 36B),393 highlighting once again the substrate dependence of gold(I)-catalyzed enyne cycloisomerizations. Indeed, from O- and C-tethered 1,6-enynes, allene products have also been prepared using gold catalysis.394
Scheme 36. Au(I)-Catalyzed Cycloisomerization of Alkylidenecyclopropanes.
Fensterbank and co-workers combined the cycloisomerizations of 1,6-enynes with ring expansion, preparing a range of enol ethers 130 (Scheme 37A).395 The key step involves a Wagner–Meerwein transposition onto the carbene center of intermediate 131. The group of Michelet recently exploited the cycloisomerization of O-tethered 1,6-enynes for the efficient preparation of relatively volatile enol ethers with distinctive olfactive properties (Scheme 37B).396
Scheme 37. Cycloisomerization of O-Tethered 1,6-Enynes.
All in all, the 6-endo-dig cycloisomerizations of O- and N-tethered 1,6-enynes to products 134a, 134b, and 134c have become standard reactions to test the performance of new gold complexes, such as 135,397136,398137,399 and 138(400) (Scheme 38). Enantioselective variants have been achieved employing chiral mono- or dinuclear Au(I) complexes 139,401402140,403,404141,405142,406143,407144,408145,409146,410 and 147(411) (Scheme 39). Phosphoramidite gold complex 140 was used for the asymmetric synthesis of antidepressant candidate 150,403 performing significantly better than commercially available chiral phosphines (Scheme 39, bottom).412
Scheme 38. Gold Complexes for Non-asymmetric Cycloisomerizations of O- and N-Tethered 1,6-Enynes.
Scheme 39. Gold Complexes for Enantioselective Cycloisomerizations of O- and N-Tethered 1,6-Enynes.
Pd black (2.5 mol %), H2 (1 atm), Na2CO3, EtOAc/MeOH (1:1).
Under gold(I) catalysis, C-tethered 1,6-enynes deliver cyclopropane-containing products in some particular instances: (i) upon trapping the gold carbene intermediates with alkenes intra-364,365,382 or intermolecularly387,388 (as discussed above); (ii) upon reaction with carbonyl compounds,413,414 and (iii) upon 1,5-OR migration of propargylic groups.415 The group of Helmchen disclosed that the addition of aldehydes and ketones to terminal 1,6-enynes yields 2-oxabicyclo[3.1.0]hexanes 153 stereoselectively (Scheme 40).413,414 The mechanism, supported by DFT calculations, begins with the formation of cyclopropyl gold carbene 154 by 5-exo-dig cyclization, followed by a double-cleavage rearrangement to intermediate 155. Attack by the carbonyl O atom, ring closure, and finally cyclopropane formation deliver the product and regenerate the active species.
Scheme 40. Synthesis of 2-Oxabicyclo[3.1.0]hexanes.
Under Au(I) catalysis, dienynes carrying a propargylic alcohol, ether, silyl ether, or ester react by tandem 5-exo-dig cylization/1,5-OR migration/intramolecular cyclopropanation (Scheme 41).415 Cyclopropyl gold carbene 161 was found to undergo 1,5-OR migration via nearly barrierless transition state 162, delivering gold-substituted allylic carbocation/α,β-unsaturated gold carbene 163.416 The 1,5-OR migration was faster than the intramolecular cyclopropanation and even of 1,2- and 1,3-acetoxy shift (see section 2.5), thus avoiding racemization of propargylic stereocenters.
Scheme 41. Tandem Cyclization/1,5-OR Migration/Intramolecular Cyclopropanation of Dienynes.
This cascade reaction was performed on enantioenriched dienyne 164 as the key step in the synthesis of three aromadendrane sesquiterpenes, prepared in 12–17% overall yields from (E,E)-farnesol (Scheme 42).417 Compounds 165 and 166 with epimeric stereocenters were obtained either by 1,5-OBn migration or intermolecular nucleophilic attack on cyclopropyl Au(I)-carbene-like intermediate 167.
Scheme 42. Gold-Catalyzed Formation of the Tricyclic Cores of Aromadendranes.
1,6-Enynes 168 followed the same reactivity pattern. In this case, after 1,5-OR migration, intermolecular cyclopropanation occurred (Scheme 43).415−418 From compounds 170b and 170c, first-418 and second-generation416 syntheses of sesquiterpene (+)-schisanwilsonene A were accomplished.
Scheme 43. Tandem Cyclization/1,5-OR Migration/Intermolecular Cyclopropanation of 1,6-Enynes.
A special class of 1,6-enynes with an ynamide tether and a propargylic alcohol follows yet another pattern when treated with gold. Thus, the group of Cossy described the AuCl-catalyzed formation of azabicycles 173 in moderate yields, through 5-exo-dig cyclization and 1,2-H shift onto gold carbene 174 (Scheme 44).419,420
Scheme 44. Cycloisomerization of 1,6-Ene-Ynamides.
2.4.1.2. 1.5-Enynes
Gold-activated 1,5-enynes typically react by 5-endo-dig cyclization to give bicyclo[3.1.0]hexane systems, as 4-exo-dig cyclization would afford strained bicyclo[2.1.0]pentanes.
The groups of Fürstner212 and Malacria421 independently reported that 3-hydroxylated 1,5-enynes such as 175 cycloisomerized to bicyclo[3.1.0]hexanones 176 using Au(I) complexes at room temperature or PtCl2 upon heating (Scheme 45). As already noted for the alkoxycyclizations of 1,6-enynes,364,365 the use of Au(I) rather than Pt(II) catalysts allowed the reaction to proceed under milder reaction conditions (lower temperature and reduced catalyst loading). The initially formed cyclopropyl Au(I) carbene 177 collapses by 1,2-hydride shift and loss of gold(I). Further studies by Gagosz probed the generality of the transformation on 3-hydroxy, 3-benzyloxy, and 3-acetoxy 1,5-enynes, highlighting the substrate dependence of the reaction manifold.422 Various gold(I) precatalysts combined with chloride scavengers are effective in this transformation.423 However, only one asymmetric gold-catalyzed version has been developed to date using a planar chiral ferrocenylphosphine gold(I) complex to induce moderate enantioselectivity.424,425
Scheme 45. Gold(I)- or Pt(II)-Catalyzed Cycloisomerization of 3-Hydroxy-1,5-enynes.
The group of Toste disclosed that Au(I) and Au(III) complexes greatly outperformed Pt(II), Pd(II), and Ag(I) catalysts in the cycloisomerization of 1,5-enynes 178 to bicyclo[3.1.0]hexanes 179 (Scheme 46).426 Cyclopropanation, which was found to be stereospecific, occurs by attack of the alkene to the gold-activated alkyne 182 via half-chair transition states, affording cyclopropyl gold-carbene like intermediate 183. Then, 1,2-H shift (or alkyl shift in the case of 181a and 181b) onto the carbene center takes place.427,428 Similarly, Zhang and Kozmin disclosed that the AuCl-catalyzed cycloisomerizations of 1-siloxy-1,5-enynes afforded instead cyclohexadienes.429−431
Scheme 46. Gold(I)-Catalyzed Cycloisomerization of 1,5-Enynes.
Transfer of chirality has been observed with certain enantioenriched substrates.213,432 Toste and co-workers employed a well-defined square-planar chiral Au(III) catalyst to realize a nondynamic, yet enantioconvergent, kinetic resolution of 1,5-enynes with moderate s factors (Scheme 47).433,434 In a related work, an achiral NHC–gold(III) complex, 187, anchored into a rigid metal–organic framework to geometrically prevent undesired decomposition via reductive elimination of biphenylene, was active in the cycloisomerization of a model 1,5-enyne after chloride abstraction by TlPF6.435
Scheme 47. Gold(III)-Catalyzed Enantioconvergent Kinetic Resolution of 1,5-Enynes.
The group of Toste also described a sequential cycloisomerization/sp3 C–H bond functionalization of 1,5-enynes 188 and 1,4-enallenes 190, providing all-carbon tetracycles 189 and 191 through cyclopropyl gold(I) carbene-like intermediates 192 (Scheme 48).436,437 The observed inverse primary KIEs were tentatively explained by the formation of a σ complex between the hydrogen atom and the gold(I) center before the hydrogen transfer event.
Scheme 48. Gold(I)-Catalyzed Cycloisomerization/C–H insertion of 1,5-Enynes and 1,4-Enallenes.
Gagosz, Skrydstrup and co-workers observed a mechanistically related gold-catalyzed dimerization of ynamides, leading to tricyclic compounds 194 by a final intramolecular C–H insertion of gold(I) carbene 195 (Scheme 49).438
Scheme 49. Gold(I)-Catalyzed Dimerization of Ynamides.
Davies and co-workers reported the Au(III)-catalyzed formal intramolecular insertions of cyclopropyl gold carbenes into activated C(sp3)–H bonds, affording compounds 197 (Scheme 50).439 The mechanism can be rationalized invoking as the first step the 1,5-H migration on gold keteniminium complex 198, followed by cyclization and cyclopropanation. The alternative initial cyclopropanation to afford 200 and subsequent C–H insertion was considered less likely.
Scheme 50. Au(III)-Catalyzed C–H Insertion/Cyclization Cascade of Ynamides.
The initially formed gold carbenes in cyclizations of 1,5-enynes could be trapped by external C-nucleophiles389,390,440 as well as by pendant alkenes in a concerted reaction (Scheme 51, top).441 When trapped with aldehydes, in certain cases tricyclic compounds formed by C–H insertion of the carbene into neighboring C–H bonds (Scheme 51, bottom).442
Scheme 51. Evolution of Cyclopropyl Au(I) Carbenes Generated from 1,5-Enynes.
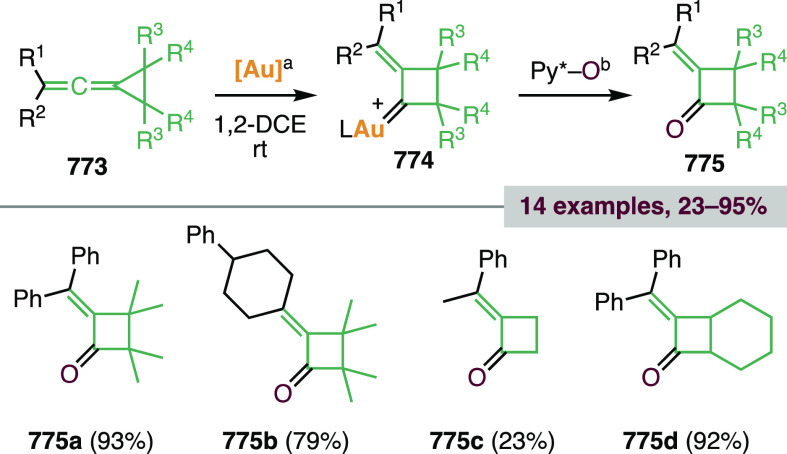
Finally, Wei, Tang, Shi, and co-workers observed the formation of polycyclic products containing both 3- and 4-membered rings in the Au(I)-catalyzed cycloisomerization of 1,5-enynes containing a 3-cyclopropyl group (see Scheme 119D in section 3.1.1.1).443
Scheme 119. Gold(I)-Catalyzed Cycloisomerization of Cyclopropyl 1,5-Enynes.
2.4.1.3. Other Enynes
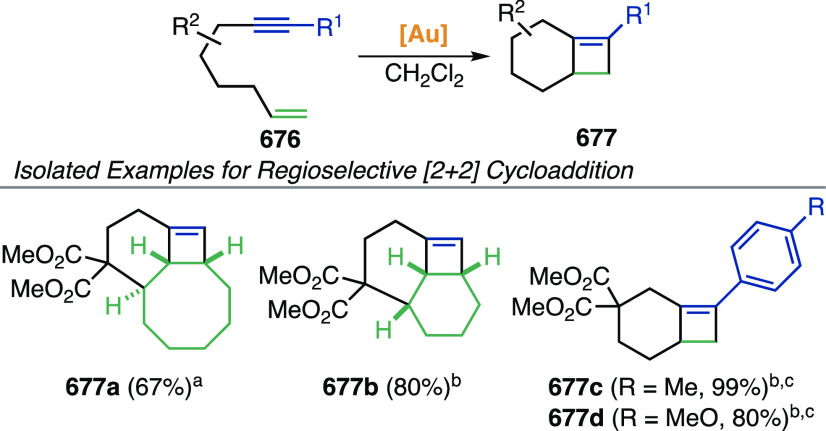
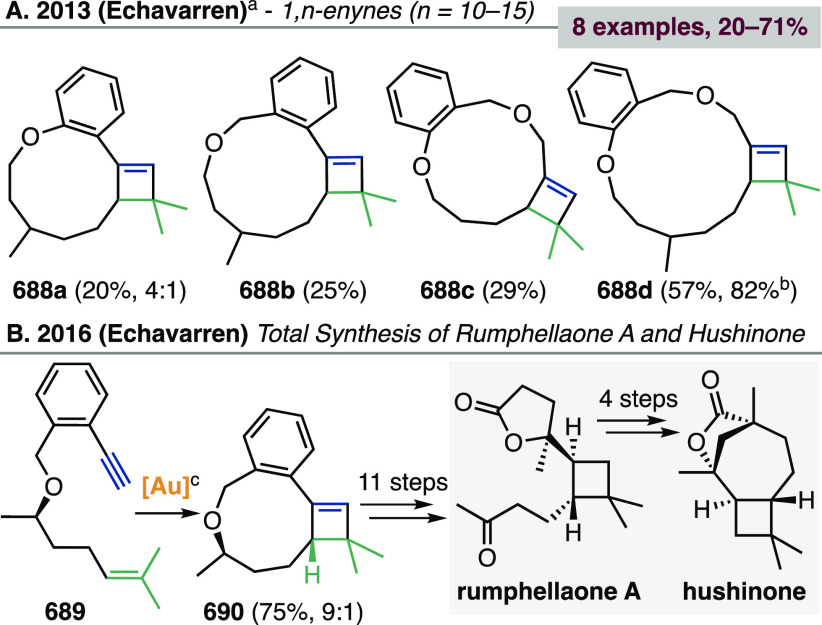
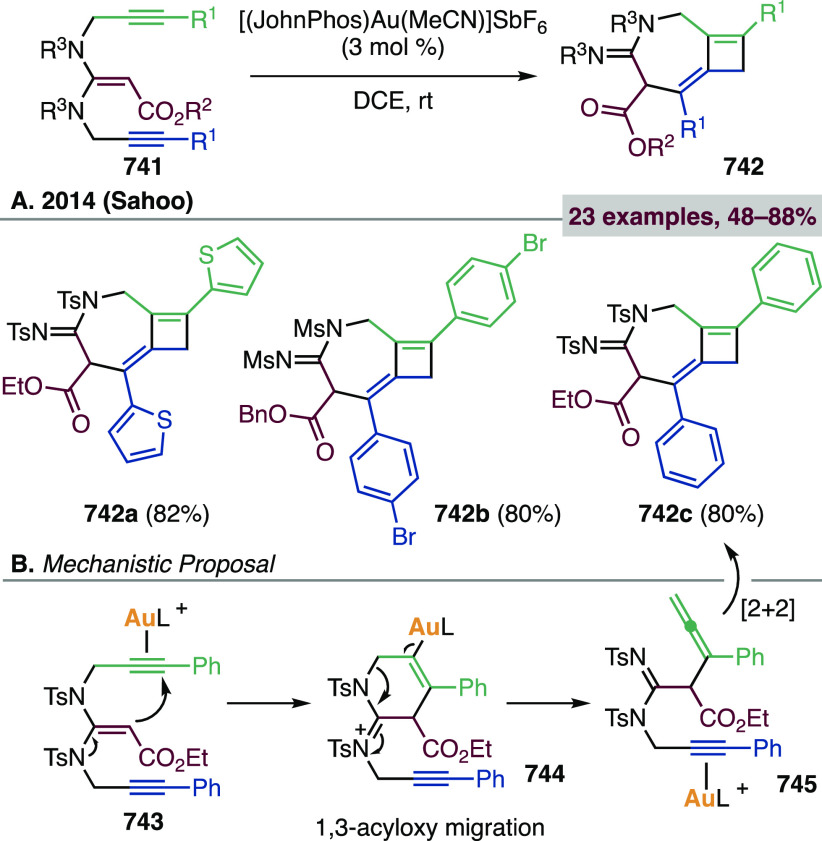
Gold-catalyzed cycloisomerizations of 1,7- and higher enynes very rarely lead to cyclopropane-containing products, because the initially formed cyclopropyl gold carbene rearranges. Treatment of 1,7-enynes with Au(I) catalysts can afford linear or cyclic 1,3-dienes,444,445 1,4-dienes,446 or hydroacenes,447 depending on enyne tether and substitution at the termini. Kumar and Waldmann observed the formation of cyclopropyl-fused compounds 207 in very low yields during the 8-endo-dig cyclization of 1,7-enynes 205 (Scheme 52).448 From 1,7- and higher enynes, cyclobutenes can be obtained via intramolecular [2 + 2] cycloadditions (see section 3.2.1.2),449,450 and occasionally other macrocycles.451
Scheme 52. Au(I)-Catalyzed 8-endo-dig Cyclization of 1,7-Enynes.
Special 1,3-enynes such as ynenals and ynenones can be used to access 2-furyl gold carbenes 210 by 5-exo-dig cyclization under gold(I) or gold(III) catalysis, as outlined in Scheme 53.168,452 These species can participate in several reactions, including intramolecular cyclopropanations as described by Oh (Scheme 53).453,454
Scheme 53. Intramolecular Furanylation–Cyclopropanation Using Ynenals and Ynenones.
A first, single example of intermolecular cyclopropanantion using styrene for the synthesis of 214a was disclosed by Zhang and co-workers.455 The generality and the efficiency of the transformation were later demonstrated by the group of Zhu, employing a combination of [(IPr)AuCl] and Selectfluor (Scheme 54).456 A putative cationic Au(III) species [(IPr)AuClF]+ was deemed responsible for the high catalytic activity.
Scheme 54. Intermolecular Furanylation–Cyclopropanation Using Ynenones.
2.4.2. Cycloisomerization of Allenynes
Using gold catalysis, allenes have proven to be valuable building blocks for the synthesis of complex molecules.130−132 Allenenes undergo various types of carbocyclizations such as [2 + 2] cycloadditions (see section 3.1) and other annulations.133−135 Acetoxyallenes can be generated from propargyl carboxylates via 1,3-acyloxy shift and take part in several reactions (see section 2.5).457,458
Gold-catalyzed carbocyclization of allenynes can proceed either with the allene attacking a gold-activated alkyne or vice versa, with the triple bond attacking the activated allene and delivering a vinyl cation. Fensterbank, Malacria, and co-workers studied the alkyne versus allene activation in platinum- and gold-catalyzed cycloisomerizations of hydroxylated 1,5-allenynes, which proved to be both substrate and catalyst dependent (Scheme 55).459 Chloride salts of Pt(II), Pt(IV), Au(I), and Au(III) converted allenyne 215 to 6-methylenebicyclo [3.1.0]hexan-3-one 217 by attack of the allene onto a metal-activated alkyne, while PPh3AuCl/AgSbF6 yielded exclusively 216 by intramolecular allene hydroalkoxylation.
Scheme 55. Pt- or Au-Catalyzed Cyclization of 1,5-Allenynes.
The group of Ohno reported that benzene-tethered 5-allenynes react with benzoheterocycles to give 1-naphthyl cyclopropane derivatives 219 (Scheme 56).460 In this transformation, the initially formed vinyl cation 221 is attacked by the 2-position (or 3-position) of benzofuran, and (E)-222 or (Z)-222, with the heterocycle in the less hindered axial position, undergoes ring closure/aromatization/protodeauration to afford regioselectively product 219a.
Scheme 56. Au(I)-Catalyzed Cyclization of 1,5-Allenynes.
2.4.3. Cycloisomerization of Diynes
Diyne substrates have been used in gold-catalyzed methodologies especially to prepare 5-membered or larger fused carbo- and heterocycles.127−129 The few gold-catalyzed examples where diynes give rise to cyclopropanes are mostly initiated by 1,2-acyloxy migration on propargylic esters (see section 2.5). As an exception, Zhu and co-workers reported the intermolecular cyclopropanation of aryl gold(I) carbenes such as 228, generated from 1,6-diynes 223 by gold-catalyzed tandem cyclizations and aromatization (Scheme 57).461
Scheme 57. Au(I)-Catalyzed Intermolecular Cyclopropanation of 1,6-Diynes.
2.5. 1,2- and 1,3-Acyloxy Migration
2.5.1. General Mechanistic Considerations
Propargylic carboxylates are prone to metal-catalyzed rearrangements.462−464 Seminal works by Ohloff465 and Rautenstrauch466 showed that propargyl acetates underwent 1,2-acyloxy migration using ZnCl2 or [PdCl2(CH3CN)2], respectively. Later studies focused on the use of Ru,467−469 Cu,470,471 Ag,470,471 Pt,472 Au,473−476,457 and Rh.477 In the presence of gold, propargylic carboxylates can undergo either a 1,2-acyloxy migration473−476 to deliver vinyl gold(I) carbenes 231 in a process known as Rautenstrauch–Ohloff rearrangement or a 1,3-acyloxy migration457,458 via a stepwise478−480 3,3-sigmatropic rearrangement to afford gold-activated allenyl esters 233 (Scheme 58).
Scheme 58. 1,2- vs 1,3-Acyloxy Migration of Au-Activated Propargylic Carboxylates.
Computations481,482 and experimental observations483−485 indicate that intermediates 231 and 233 are in equilibrium, because the steps are reversible, so 1,2- and 1,3-acyloxy migration pathways are in competition. In some cases, it was suggested that a net 1,3-OAc migration could result from 2 consecutive 1,2-OAc shifts.481,483 However, studies by Toste and co-workers using isotopically labeled substrate 234 ruled out this possibility in their specific case, as no scrambling of the 18O atom was detected (Scheme 58, bottom).479 The 1,2-shift takes place preferentially for alkynes where R1 is a H or electron-withdrawing group, and for substrates with a substituted propargylic C atom. The nature of the gold ligand, the presence of other functional groups on the substrates and reaction partners (hence downstream reactivity from 231 or 233), and the reaction conditions also influence the preferred pathway.473−485
In this section, the focus will be exclusively on Au-catalyzed rearrangements of propargylic carboxylates resulting in cyclopropanation.475 When the cyclopropanation occurs intramolecularly, that is, for enynes, three mechanisms are possible (Scheme 59): (a) complete 1,2-acyloxy migration, followed by alkene cyclopropanation by vinyl carbene intermediate 238; (b) cycloisomerization, followed by 1,2-acyloxy shift onto the α-cyclopropyl carbene 241; (c) an intermediate case where cyclopropanation occurs on a nonplanarized vinyl gold species 240.
Scheme 59. Reaction Mechanisms for Enynes Bearing a Propargylic Carboxylate.
Computations with PtCl2 show the different mechanistic routes to be close in energy, with pathway c slightly favored.486 Based on these studies and experimental investigations, the same scenario has been proposed for gold catalysis.487−489 Partial transfer of chirality, often observed under both Pt and Au catalysis with certain substrates carrying a propargylic stereocenter,432 rules out the exclusive intermediacy of mechanism a, since the stereochemical information at the propargylic position would be entirely lost, and supports instead pathways b and c.490−492
2.5.2. Intramolecular Cyclopropanations via 1,2-Acyloxy Migration
During their studies on Pt(II)- and Au(I)-catalyzed rearrangements of 3-hydroxylated 1,5-enynes, Fürstner and co-workers reported that propargylic acetate 243 underwent smooth transformation into compound 244 under Au(I) catalysis (Scheme 60).212 Methanolysis of 244 to [3.1.0]bicyclic ketone 245 highlighted the role of propargylic acetates as convenient precursors of α-oxo gold carbenes upon 1,2-acetoxy migration. The group of Gagosz found that the air-stable gold catalyst [(Ph3P)Au(NTf2)] was particularly active in this type of cycloisomerization too.493
Scheme 60. Au(I)-Catalyzed Intramolecular Cyclopropanations of 3-Acetoxy versus 3-Hydroxy 1,5-Enyne.
The group of Fürstner exploited the AuCl3-catalyzed cycloisomerizations of various propargylic acetates for the diazo-free synthesis of several carene terpenoids.490,494 The synthetic studies established that (i) the cyclopropanation step proceeded stereospecifically, with the alkene configuration dictating the configuration of the cyclopropane, (ii) the closer alkene reacted preferentially (Scheme 61, top), and (iii) under PtCl2 catalysis pathways b and c were operative (see Scheme 59), since a propargylic stereocenter influenced the reaction outcome (Scheme 61, bottom).
Scheme 61. AuCl3- and PtCl2-Catalyzed Cycloisomerizations in the Synthesis of Terpenoids.
Similar stereochemical observations on chirality transfer were made by Fehr and co-workers when performing Au-, Pt-, and Cu-catalyzed enyne cycloisomerizations for the synthesis of (−)-cubebol.491 Similarly, the Fürstner group realized the asymmetric total synthesis of sesquisabinene terpenes based on gold(III)-catalyzed Ohloff–Rautenstrauch-type cycloisomerizations of enantiopure enynes.492 A propargylic p-nitrobenzoate group allowed a more efficient transfer of the stereochemical information than an acetate ester (≥15:1 versus 5:1 dr, Scheme 62).
Scheme 62. Au(III)-Catalyzed Cycloisomerizations in the Synthesis of Terpenoids.
Nolan and co-workers reported the Au(I)-catalyzed rearrangement of more complex dienynes 253 bearing an acetate group at the fully substituted propargylic position (Scheme 63, top).495 Mixtures of cyclopropane-containing products were obtained, stemming from both possible cyclopropanation of the two alkenes and competitive 1,2- and 1,3-acetate migration. As evidenced also by later works, the product ratio was highly ligand-, counterion-, metal-, and solvent-dependent, with bulky NHC ligands necessary to obtain high ratios of 256c.496,497 Products arising from both 1,2 and 1,3-OAc migration were observed also in the gold-catalyzed cyclization of cyclohexyl-tethered 3-acetoxy 1,6-enynes.498
Scheme 63. Au(I)-Catalyzed Rearrangements of Dienynes.
[(IPr)AuCl] (2 mol %), AgBF4(2 mol %), CH2Cl2, rt, 10 min
Thorough DFT calculations painted a complex landscape with numerous reaction pathways of similar energy (“golden carousel”).496,497 In the most likely one, initial 1,2-acetate shift delivers vinyl gold carbene 254, which can be attacked by the distal alkene affording 256a or undergo a second 1,2-OAc shift delivering gold-activated allene 255 (Scheme 63, top). From this intermediate, allene-ene cyclization and retro 1,2-OAc shift produced 256c. Indeed, cycloisomerizations of allenyl ester 259 provided 258 in better yield than when starting from dienyne 257 (Scheme 63, bottom). Cycloisomerization of other allenyl esters, delivering a mixture of cyclopropane-containing products, were also studied.496,497 Synthetic studies by the group of Fehr again highlighted that 1,2-OAc and 1,3-OAc cycloisomerization pathways often coexist and are catalyst-dependent (Scheme 64).499 Indeed, cyclobutane product 262 likely arises by [2 + 2] cycloaddition of an allenene formed in situ.500
Scheme 64. Catalyst-Dependent 1,2- and 1,3-OAc Migrations on 1,6-Enynes.
The group of Gagosz described the Au(I)-catalyzed tandem cycloisomerization/1,2-OAc shift of trienynes bearing a propargylic ester to give divinyl cyclopropanes, which underwent subsequent thermal Cope rearrangement.501
The Ohloff–Rautenstrauch rearrangement of 1,7- and higher enynes was used to prepare medium-size rings. Hanna and co-workers synthesized tricyclic compounds 264 and used them in the synthesis of an allocolchinoid (Scheme 65A).502 The group of Toste later studied the substrate scope extensively (selected examples in Scheme 65B).503 Fensterbank and Malacria reported similar cycloisomerizations catalyzed by PtCl2, AuCl3, or Au(I) affording tricyclic compounds 268 as single diastereomers (Scheme 65C).504 The higher activity of AuCl3 and Au(I) catalysts in front of PtCl2 in enyne rearrangements was observed once again, with comparable yields for products 268 obtained either in 2 h at 80 °C when employing 5 mol % PtCl2 or in 5–10 min if using 2 mol % gold catalysts at room temperature. The latter reaction was carried out successfully on a trans-cyclopentyl-tethered 1,7-enyne as the key step to access neomeran skeletons.500
Scheme 65. Cycloisomerizations of 1,7- and Higher Enynes for the Formation of Medium-Size Rings.
Toste and co-workers also developed an enantioselective version of these reactions using dinuclear gold(I) complexes with chiral diphosphine ligands (Scheme 66).503 Mechanistic experiments discarded a cyclopropanation-first pathway (i.e., path b in Scheme 59) and highlighted the fluxional (E)/(Z)-alkene geometry of the vinyl gold(I) carbene intermediate.
Scheme 66. Asymmetric Synthesis of Medium-Size Rings by Intramolecular Cyclopropanation.
2.5.3. Intermolecular Cyclopropanations via 1,2-Acyloxy Migration
Uemura and co-workers reported the first intermolecular cyclopropanations effected by vinyl carbene intermediates generated from propargylic acetates using Ru, Pt, or Au catalysis (Scheme 67).467,468 Vinyl cyclopropane 273 formed via 1,2-OAc migration followed by cyclopropanation, while allenyl ester 274 resulted from competitive 1,3-OAc migration. AuCl3 showed the highest activity for both cyclopropanation and allene formation but with a lower product selectivity.
Scheme 67. First Gold-Catalyzed Intermolecular Cyclopropanation via 1,2-OAc Migration.
Toste and co-workers developed a general Au(I)-catalyzed cyclopropanation of a broad range of alkenes with propargyl esters (Scheme 68).505 The reaction was stereospecific with respect to the alkene configuration and yielded cyclopropanes 277 with moderate to excellent cis selectivity when using styrenes, as rationalized by stereochemical models (Scheme 68, bottom).
Scheme 68. General Au-Catalyzed Alkene Cyclopropanation Using Propargylic Esters.
The intermediacy of a planar vinyl gold carbene such as 278 was supported by the complete loss of stereochemical information when using enantioenriched propargyl acetates as substrates. This observation, which stands in contrast with the transfer of chirality observed in intramolecular cyclopropanations (see section 2.5.2), derives from the entropic preference for a complete intramolecular acyloxy migration before cyclopropanation of a nontethered alkene.506 Replacing PPh3 with axially chiral bisphosphine 282 allowed the enantioselective synthesis of vinyl cyclopropanes 281 with high cis selectivity (Scheme 69).
Scheme 69. Enantioselective Au(I)-Catalyzed Styrene Cyclopropanation Using Propargylic Esters.
This transformation has since become a benchmark reaction to test new chiral and achiral gold complexes, such as Au(I) complexes 283,507284,508 and 285.399 Notably, catalytically active Au(III) complexes 286,509287,510 and 288(511) did not induce any enantioselectivity, despite their chiral backbone. Complexes 286 were effective catalysts for the intramolecular cyclopropanation and subsequent in situ cis to trans isomerization of vinyl cyclopropanes at room temperature.509 Recently, trans bis(pyridine)gold(III) complexes 289 have been found to exhibit a strain-modulated reactivity in this transformation.512
Toste and co-workers investigated the effect of structural variations of the two substrates, appending an additional triple bond to either the vinyl carbene precursor or the alkene. Using 1,3-diynes 290, they developed a styrene cyclopropanation–hydroarylation cascade for the synthesis of benzonorcaradienes 291 (Scheme 70).513 The proposed mechanism involves cyclopropanation by gold(I) carbene 294, delivering cyclopropane intermediate 295, which can be isolated as 291′. The latter then undergoes a gold-catalyzed intramolecular hydroarylation.
Scheme 70. Synthesis of Benzonorcaradienes by Cyclopropanation–Hydroarylation Cascade.
When the triple bond was attached to the alkene, the annulation between 1,3-enynes 298 and propargylic pivalates afforded cis-vinyl-alkynyl-cyclopropanes, together with styrene or fluorene products derived from their rearrangement (illustrative examples in Scheme 71).514 Few additional examples of intramolecular cyclopropanations were reported in related studies.503
Scheme 71. Synthesis of cis-Vinyl-alkynyl-cyclopropanes, Styrenes, and Fluorenes.
Nevado and co-workers described the synthesis of cyclopentenes 307 or cycloheptadienes 308 by net (3 + 2) and (4 + 3) annulations, respectively, via ring-opening of vinyl cyclopropane intermediates 309 (Scheme 72A).515 The preferred outcome (cyclopropanation vs cyclopentane formation) of these reactions is very sensitive to the sterics and electronics of the substrates, particularly of the alkene partner.516−519 In this regard, the group of Fiksdahl investigated various substrate combinations in the intermolecular cyclopropanation reaction. It was established that vinyl esters and vinyl sulfonamides,516 as well as protected indoles and certain vinyl ethers,517,518 furnish vinyl cyclopropanes when used in combination with propargylic esters as gold carbene precursors (Scheme 72B). When propargylic acetals were used instead, (3 + 2) cycloaddition products were obtained.517 Reacting propargylic acetals with vinyl esters resulted instead in sequential (2 + 1) and (3 + 2) cycloadditions, affording cyclopropyl-substituted cyclopentenes.519
Scheme 72. (2 + 1), (3 + 2), and (4 + 3) Cycloadditions Using Propargylic Esters as Gold Carbene Precursors.
Barbazanges, Fensterbank, Goddard, Stoffelbach, and co-workers employed vinyl gold carbenes generated from bifunctional monomers 315 under Au(I) or Au(III) catalysts to prepare oligomers (Scheme 73).520
Scheme 73. Au(III)-Catalyzed Polymerization Using Styrene–Propargylic Ester Monomers.
Gold(I) carbenes 318a generated by 1,2-acyloxy shift of a propargylic ester group can be transferred over a tethered alkyne, generating homologated gold carbenes 318b (Scheme 74A). These intermediates, which were described by the groups of Chan,521,522 Oh,523 and Hashmi524 in intramolecular reactions involving the tethered group R2, can also be trapped intermolecularly with alkenes. The first example of this kind was disclosed by the group of Chan as a mechanistic proof for the formation of 321 (Scheme 74B).521 Following work by Hashmi and co-workers showed that removal of the R2 group at the alkyne terminus reduced side reactions available to the carbene intermediate, thus allowing intermolecular cyclopropanations to be carried out with only one equivalent of alkene to obtain 1-naphthyl cyclopropanes 323 in good yields (Scheme 74C).525
Scheme 74. Intermolecular Cyclopropanations Using 1,6-Diynes with a Propargylic Ester Group.
2.5.4. Cyclopropanations via 1,3-Acyloxy Migration
1,3-Acyloxy shifts via stepwise [3,3]-sigmatropic rearrangement are favored for propargyl acetates possessing alkyl-substituted triple bonds and deliver Au-activated allene intermediates (see section 2.5.1),457,458 which can evolve in a variety of ways.130−135 The presence of a suitably positioned tethered alkene allows stepwise intramolecular cyclopropanation, as demonstrated by Buzas and Gagosz.526 His group reported the formation of bicyclo[3.1.0]hexenes 325 by Au(I)-catalyzed cycloisomerization of 1,4-enynes 324 bearing a propargylic acetate group (Scheme 75). Chirality transfer from a propargylic stereocenter was observed (product 326, absolute configuration not determined), and the products could be further transformed in various 2-cycloalken-1-ones. The proposed mechanism involves formation of gold allene complex 328, which is attacked intramolecularly by the alkene, affording the cationic vinyl gold species 329. Attack to the carbocation assisted by electron donation from gold gives cyclopropyl gold carbene 330, which undergoes 1,2-hydride shift and protodeauration delivering product 325.
Scheme 75. Cycloisomerization of 5-En-2-yn-1-yl Acetates.
Rao, Chan, and co-workers built upon the reactivity of 1,4-enynes bearing a propargylic ester to access several tetracyclic compounds 332 (Scheme 76)527 and phenol derivatives.528
Scheme 76. Gold-Catalyzed Tandem 1,3-Migration/Double Cyclopropanation of 1-Ene-4,n-diyne Esters.
Malacria, Fensterbank, and Gandon and their co-workers studied experimentally and computationally the Au-catalyzed cycloisomerizations of related substrate classes, namely, 1,3-enynes and allenynes carrying a propargylic ester group.529,530 They developed an efficient preparation of polycyclic compounds 334 (Scheme 77, top), which was applied in total synthesis.530,531 In order to suppress the formation of cyclopentadiene side products, the internal position of the 1,3-enyne framework had to be substituted (group R in Scheme 77, bottom). Several mechanistic pathways were computed, and the lowest energy one was found to proceed through [3,3]-sigmatropic rearrangement of propargyl acetates 333 into allenyl acetates 335, followed by metalla-Nazarov reaction of gold complex 337. The resulting cyclopropyl gold carbene 338 then effected intramolecular cyclopropanation.
Scheme 77. Cycloisomerization of 4-En-2-yn-1-yl Acetates.
Efficient transfer of chirality was observed at low temperature and attributed to the formation of a bent-allene gold complex such as 341, which retained the stereochemical information on the allene, in turn derived from the propargylic acetate (Scheme 78).532 With a trisubstituted allene, racemization via planar allene gold complex 343 was disfavored. Conrotatory cyclization gave rise to intermediate 343′ with the expected relative and absolute configuration.
Scheme 78. Chirality Transfer via Bent-Allene Gold Complexes.
Based on the mechanisms presented above, it was hypothesized that the same reaction would proceed directly from vinyl allenenes, with chirality transfer in the case of trisubstituted allenes, which was experimentally demonstrated (Scheme 79).529,532
Scheme 79. Cycloisomerization from Vinyl Allenenes.
The mechanistic intricacies of Pt- and Au-catalyzed cycloisomerization of enynyl and allenynyl esters were also studied by Cavallo, Fensterbank, Malacria, and Nolan and their co-workers, as shown in Scheme 63.496 The two metals can catalyze divergent reaction pathways, which was also noted in the course of synthetic efforts toward lindenane and myliol cores: treatment of a trans-cyclohexyl-tethered 1,5-enyne with AuCl3 resulted exclusively in 1,3-OAc migration, while also products derived from 1,2-OAc shift were obtained using PtCl2.533,534
In summary, 1,2- and 1,3-acyloxy migrations of propargylic ester substrates are well-established platforms for the generation of vinyl gold carbenes and gold-activated allene intermediates, and as such have been fruitfully employed in the cyclopropanation arena. Intermolecular cyclopropanations via 1,2-acyloxy migration pathways can be performed stereo- and enantioselectively with styrenes. Intramolecular cyclopropanations via 1,2- and 1,3-acyloxy migration can leverage instead transfer of chirality from enantioenriched substrates. However, given the complex mechanistic scenario of this golden carousel, catalyst- and substrate-dependent reaction outcomes remain the norm.
2.6. Oxidation of Alkynes with Oxygen-Transfer Reagents
2.6.1. General Considerations on α-Oxo Gold Carbenes
The gold-catalyzed oxidation of alkynes takes place with reagents with a polar LG+–O– bond, such as sulfoxides and amine N-oxides (Scheme 80).535−544 These oxidants attack the π-alkyne gold(I) complex in an anti fashion at the C atom better able to accommodate the partial positive charge (e.g., positions that are benzylic, α to N atoms, or β to electron-withdrawing groups). The initially formed vinyl gold complex 347 eliminates the leaving group delivering transient α-oxo gold carbene 348.545 Computational and experimental gas-phase studies have shown that these intermediates react rapidly with the expelled pyridine to form more stable gold(I) carbenoids 349.546,547 Attack of the pyridine to the carbene C atom or its coordination to Au can be prevented by using acidic additives and by choosing less nucleophilic pyridines with bulky or electron-withdrawing substituents.
Scheme 80. Formation of α-Oxo Gold Carbenes by Oxidation of Gold-Activated Alkynes.
This oxidation strategy provides very electrophilic α-oxo gold carbenes from readily available alkynes,535−544 circumventing the use of dangerous and toxic α-diazocarbonyl precursors. Since the group of Zhang reported their generation by intermolecular alkyne oxidation,548 α-oxo gold carbenes have enjoyed a rich chemistry.535−544 When they are involved in cyclopropanations, enynes are used in most cases as substrates. Thus, after alkyne oxidation, cyclopropanation occurs intramolecularly with the pendant alkene, delivering bicyclo[3.1.0]hexane skeletons (Scheme 81, pathways a and b). A related approach to bicyclo[3.1.0] compounds had been described by the Toste group (Scheme 81, pathway c).549 In this case, 5-endo-dig cyclization of 1,6-enynes of type 354 precedes oxidation of the intermediate cyclopropyl gold carbene 355 (see section 2.4.1.1). In these oxidative cycloisomerizations, typically sulfoxides are more efficient oxidants than N-oxides.
Scheme 81. Mechanisms for the Formation of Bicyclo[3.1.0]hexane Skeletons via Oxidation of 1,6-Enynes.
Both 1,5-enynes and 1,6-enynes deliver [3.1.0] bicyclic compounds upon oxidative intramolecular cyclopropanation (Scheme 82). 1,6-Enynes give products 360 with exocyclic incorporation of the oxygen atom from the oxidant, as a pendant aldehyde or ketone, depending, respectively, on the presence of a terminal or internal alkyne in the substrate (Scheme 82A). Instead, 1,5-enynes afford compounds 363 or 366 with an endocyclic oxidant-derived carbonyl group (Scheme 82B,C). Regardless of the exact mechanism involved, in the majority of the cases these gold-catalyzed oxidative cyclopropanations occur stereospecifically with respect to the alkene configuration.
Scheme 82. Comparison between Oxidative Cyclopropanations of 1,5- and 1,6-Enynes.
While nearly all gold-catalyzed oxidative intramolecular cyclopropanations of enynes afford byciclo[3.1.0] compounds, there have been two illustrative cases where bicyclo[4.1.0] compounds formed instead (Scheme 83).
Scheme 83. Formation of Bicyclo[4.1.0] Skeletons.
The first example, disclosed by Liu and co-workers, consists in the cyclization of benzene-tethered 1,6-enyne 367 using excess 8-methylquinoline oxide as oxidant (Scheme 83A).550 The second one, reported by Zhang, involves the cyclization of amide-tethered 1,7-enyne 370, using the same oxidant and catalytic system ([(IPr)AuCl]/AgNTf2, Scheme 83B).551 The lower temperature and catalyst loading of the second example can be ascribed to the higher reactivity of the electron-deficient alkyne, and the higher electrophilicity of the corresponding doubly activated putative gold carbene intermediate.
2.6.2. Oxidative Intramolecular Cyclopropanations of 1,6-Enynes
Seminal work by the Toste group demonstrated the interception of gold(I) carbene intermediates by oxidation with sulfoxides, which were generated under gold(I) catalysis from a variety of precursors.549 Among these, α-diazoketones, propargylic esters, and 1,6-enynes were used. Specifically, the first oxidative cycloisomerizations of 1,6-enynes 373 using stoichiometric diphenylsulfoxide afforded cyclopropyl aldehydes 374 in good to excellent yields (Scheme 84).
Scheme 84. Oxidative Cycloisomerization of 1,6-Enynes.
An asymmetric version of this reaction was reported by Shi and co-workers employing chiral NHC–gold complex 377 (Scheme 85), providing cyclopropanes 376 with moderate enantioselectivities.552
Scheme 85. Enantioselective Oxidative Cycloisomerization of 1,6-Enynes Using a Carbene Ligand.
Qian and Zhang described the gold-catalyzed oxidative cyclization of amide- or ketone-tethered 1,6-enynes 378 to prepare hetero- and carbo[3.1.0]bicyclic ketones 379 (Scheme 86).551 Similar oxidative cyclopropanations of amide- or ester-tethered 1,6-enynes had been reported using catalytic Pd(OAc)2 and stoichiometric PhI(OAc)2.553,554 Yeom and Shin later showed that this methodology could be extended to propiolamide-derived 1,6-enynes with a terminal triple bonds, using SPhos as Au(I) ligand and diphenyl sulfoxide as oxidant.555
Scheme 86. Oxidative Cyclization of 1,6-Enynes.
The group of Zhang developed an enantioselective version of the oxidative cyclization of 1,6-enynes using BINOL-derived phosphoramidite ligand 382 (Scheme 87).556 Crucial for the high enantioselectivity were the 3,3′-groups on the binaphthol ligand backbone, the use of activated α-keto or α-amide alkynes (as they allowed for low reaction temperatures) and the presence of vinyl or electron-rich aromatic groups on the alkyne terminus. Based on control experiments and on the observed dependence of the enantioselectivity on the structure of the oxidant, the authors proposed the intermediacy of β-vinyloxyquinolinium species 383 rather than an α-oxo gold carbene.
Scheme 87. Enantioselective Oxidative Cyclization of 1,6-Enynes Using a Phosphoramidite Ligand.
1,6-Enynes with aromatic ketones,557 sulfones, and sulfoxides558 as linkers are also viable substrates for oxidative cycloisomerizations (Scheme 88). Davies and Grainger observed that the sulfoxide group did not interfere with the oxidation of the alkyne, carried out by 3,5-dichloropyridine N-oxide in the presence of bulky [(SPhos)Au(NTf2)]. Bicyclo[3.1.0] ketones 385 with the cyclopropyl ring on the same side of the sulfoxide were obtained as the major or only diastereomers.
Scheme 88. Oxidative Cyclization of Alkynyl Sulfoxides.
2.6.3. Oxidative Intramolecular Cyclopropanations of 1,5-Enynes
The group of Liu reported the first oxidative cyclization of 1,5-enynes, featuring the proposed intermediacy of α-oxo gold carbenes.550 Under gold catalysis and in the presence of 8-methylquinoline N-oxide, 1,5-enynes 386 with an aromatic or alkenyl linker provided cyclopropyl ketones 387 in good yields (Scheme 89). While 5 mol % catalyst loading and short reaction times sufficed for most enynes, slightly more forcing conditions were required for a substrate bearing an internal alkyne and for a 1,6-enyne to afford 387d.
Scheme 89. Oxidative Cyclization of 1,5-Enynes.
The exact structure of the N-oxide was shown to influence the chemoselectivity for the reaction of 1,3-dien-5-yne substrates,559 delivering either products like 387c or cyclopentadienyl aldehydes.560 This methodology was applied by the Echavarren group to enantioenriched 1,5-enyne 388 for the total synthesis of (−)-nardoaristolone B (391) (Scheme 90).561
Scheme 90. Oxidative Cyclization of a 1,5-Enyne in the Synthesis of (−)-Nardoaristolone B.
Zhang and co-workers disclosed an enantioselective version of the oxidative cyclization of 1,5-enynes (Scheme 91).562 The design of hemilabile P,N-ligand563,564394 endowed with a C2-symmetric piperidine ring was key to achieve good enantiocontrol. It was proposed that coordination of the N atom to the metal center and steric shielding temper the high reactivity of the very electrophilic α-oxo gold carbene intermediate 395,565,566 while the piperidine provides a suitable chiral environment. Previous attempts to perform this reaction using axially chiral diphosphine ligands resulted in very low levels of enantioselectivity.567
Scheme 91. Enantioselective Oxidative Cyclization of 1,5-Enynes Using a P,N-Ligand.
The groups of Zhang568 and Nakada569 reported the formation of complex tricyclic products starting from specific enynes. The Zhang group reported the synthesis of bi- and tricyclic cyclopropyl ketones 397 in 3 steps from cyclic enones and linear enals, respectively (Scheme 92A; major diastereomers shown).568 This work highlighted once again the importance of a conformationally rigid P,N-bidentate ligand such as 398 to tame the reactivity of α-oxo gold carbenes. Nakada described the synthesis of similar tricyclic compounds 400 as single diastereomers starting from dienynes 399 (Scheme 92B).569
Scheme 92. Preparation of Bi- and Tricyclic Cyclopropyl Ketones via Oxidative Cyclizations.
2.6.4. Oxidative Intramolecular Cyclopropanations of Ynamides
Ynamides570−575 are easy-to-make576−580 electron-rich alkynes, that after activation by Lewis-acidic transition metals (or by Brønsted acids)581 undergo facile addition of nucleophiles at the Cα.582 Li and co-workers described the first gold-catalyzed oxidative cyclopropanation of N-allyl ynamides leading to various 3-aza-bicyclo[3.1.0]hexan-2-one derivatives 402 using [(IMes)AuCl]/AgBF4 as the catalyst and pyridine N-oxide as the oxidant (Scheme 93).583 Mechanistic studies excluded the intermediacy of an α-oxo gold carbene, pointing to attack of the alkene onto the electrophilic β-oxypyridinium vinyl gold(I) species 403.
Scheme 93. Oxidative Cyclopropanation of N-Allyl Ynamides.
In this protocol, electron-rich aromatic substituents at the alkyne terminus were not tolerated because of overoxidation (Scheme 94, top), a problem that was later solved by the group of Davies employing different reaction conditions (Scheme 94, bottom).584
Scheme 94. Gold-Catalyzed Oxidation of Electron-rich Ynamides.
Tang and co-workers showed that products 407 could be accessed from ynamides also using a Rh(I) catalyst and 3,5-dichloropyridine N-oxide, likely via a concerted cyclopropanation of the pendant alkene by an α-oxo Rh(I) carbene.585 The group of Hashmi recently reported one case of Au(I)-catalyzed yne-ynamide cyclization/oxidation/intramolecular cyclopropanation cascade using diphenyl sulfoxide.586
2.6.5. Oxidative Intramolecular Cyclopropanation of Other Substrates
Gold-catalyzed oxidative cyclizations were applied by the group of Echavarren for the synthesis of highly fluxional molecules such as bullvalene (410) and analogues (Scheme 95).587,588 Several barbaralones 409 were prepared by oxidative gold-catalyzed cycloisomerization of 7-ethynyl-1,3,5-cycloheptatrienes 408. From barbaralone 409a, bullvalene 410 could be accessed in only 3 steps and overall 16% yield, comparing favorably with previous syntheses. Most likely the reaction takes place via oxidation of barbaralyl gold(I) intermediates 412, rather than by alkyne oxidation, as otherwise the opposite regioselectivity should be observed with aryl alkynes. Moreover, the optimal reaction conditions using diphenylsulfoxide suggest oxidation of a cyclopropyl-gold carbene-like intermediate, in line with the seminal report by Toste.549 The foundations of this work were laid by a previous study on the gold-catalyzed generation of fluxional barbaralyl cations (Scheme 95, bottom).589 Barbaralyl-gold complex 412 formed from activated alkyne 411 through a formal 6-endo-dig cyclization, which, according to calculations, actually involved the norcaradiene tautomer. The carbene center of 412, which was oxidized for the synthesis of barbaralone, could be trapped by intramolecular cyclopropanation.
Scheme 95. Oxidative Cyclization of 7-Ethynyl-1,3,5-cycloheptatrienes.
While all examples presented so far involve the reaction of an α-oxo gold carbene intermediate with a pendant alkene, there are also examples of cyclopropanation of a tethered aromatic ring. The group of Davies reported the oxidative Buchner-type reaction of N-benzyl ynamides 414 to afford [5.3.0] azabicycles 415′ (Scheme 96).584 A dynamic cyloheptatriene–norcaradiene equilibrium was evident by NMR. Norcaradiene tautomer 415b was trapped in a Diels–Alder reaction affording polycycle 416, while 415a was obtained exclusively as the norcaradiene product.
Scheme 96. Oxidative Intramolecular Cyclopropanation of Benzene Rings in N-Benzyl Ynamides.
Similarly, Zhang and co-workers reported the intramolecular cyclopropanation of benzene rings by oxidatively generated α-oxo gold carbenes starting from benzyl propargyl ethers 417 (Scheme 97).590 In two instances, products with an intact cyclopropane ring were obtained: whereas generally norcaradienes 418 underwent ring expansion to products 419 or 421 under basic or acidic conditions, respectively, vicinal benzo-fused norcaradiene 422 and propellane-type norcaradiene 423 were obtained as final products.
Scheme 97. Oxidative Intramolecular Cyclopropanation of Benzene Rings in Benzyl Propargyl Ethers.
2.6.6. Oxidative Intermolecular Cyclopropanations
Intermolecular cyclopropanations have proven much harder to develop. Liu and co-workers reported the first case of trapping with styrene an α-oxo gold carbene generated by intramolecular alkyne oxidation (Scheme 98).591 An epoxide functionality in the substrate acted as sacrificial alkyne oxidant to deliver gold carbene 429, which then effected the cyclopropanation of styrene in 81% yield. When using electron-rich alkenes such as 4-methoxystyrene and ethyl vinyl ether, the major products were (3 + 2)-adducts 426a and 426b, respectively.
Scheme 98. Trapping of an α-Oxo Gold Carbene Intermediate by Styrene.
A more general approach was described by the group of Zhang, who generated the putative α-oxo gold carbene using an external oxidant, 2-(tert-butyl)-6-chloropyridine N-oxide592 (Scheme 99).593 A plausible reaction mechanism involves at first the formation of carbene 432 with the expected regioselectivity, that is, oxidation at the alkyne C atom β to the electron-withdrawing group. Friedel–Crafts-type attack onto the very electrophilic carbene center then would afford episulfone 433, which would fragment with SO2 extrusion to α-oxo gold carbene 434. It is worth noting that the latter carbene would be accessible only as the minor regioisomer by the oxidation of an internal aryl alkyne.
Scheme 99. Oxidative Intermolecular Cyclopropanation of Styrenes.
The alkene scope was limited to electronically unbiased styrenes, which were used in 10-fold excess. Electron-rich alkenes such as 4-methoxystyrene or ethyl vinyl ether were not suitable reaction partners. When using α-methylstyrene as a solvent, instead of the cyclopropane, a (3 + 2) cycloaddition product was obtained. Overall, whereas intramolecular oxidative cyclopropanation of various enynes are well developed (see sections 2.6.2–2.6.5), there is still room for improving the more challenging intermolecular cyclopropanation of alkenes by oxidatively generated α-oxo gold carbenes.
2.7. Oxidation of Alkynes with Nitrogen-Transfer Reagents
2.7.1. General Considerations on α-Imino Gold Carbenes
In the same way as α-oxo gold carbenes can be accessed from gold-activated alkynes by oxygen transfer using N-oxides and sulfoxides (see section 2.6), α-imino gold carbenes 439 can be obtained employing nitrogen-based nucleophiles 437 that bear a leaving group on the N atom (Scheme 100).594−600 These reagents are also termed nucleophilic nitrenoids,594 because they perform a net nitrene transfer to the alkyne. Their anti addition to a gold(I) or gold(III) activated alkyne delivers vinyl gold species 438. The regioselectivity of addition depends on the polarization of the triple bond, that is, the nucleophile attacks the C atom where a higher build-up of partial positive charge occurs upon gold coordination. In the case of highly polarized ynamides570−575 such as 435, the addition of the nucleophile is thus generally directed at Cα.582 Subsequent gold retrodonation triggers the expulsion of the leaving group, affording α-imino gold carbene complex 439.
Scheme 100. Formation of α-Imino Gold Carbenes from Gold-Activated Alkynes.
As in the case of α-oxo gold carbenes, there is still mechanistic ambiguity on whether the competent catalytic intermediates are vinyl gold species 438 or α-imino gold carbenes 439, particularly because the latter have not yet been isolated nor characterized spectroscopically.595 In fact, since both species are electrophilic, reaction with a nucleophilic partner can be envisaged either alongside or after elimination of the leaving group.594
All cyclopropanations reported so far using α-imino gold carbenes 439 (or equivalent electrophilic species such as 438) are intramolecular reactions, performed on substrates possessing a pendant alkene in the group R1 (Scheme 100, top). Among the several nitrogen-based nucleophiles used for the generation of α-imino gold carbenes (Scheme 100, bottom), azides 440,612 iminopyridinium ylides 441,601 sulfilimines 442,613 anthranils 443 (benzo[c]isoxazoles or 2,1-benzoisoxazoles),614 and isoxazoles602444 have been employed to deliver cyclopropanes. The first three reagents take advantage of the presence of dinitrogen, pyridine, and thioethers as leaving groups, respectively. Anthranils and isoxazoles capitalize instead on the lability of the N–O bond.
As a final comparison between α-imino and α-oxo gold carbenes, it can be noted that α-imino gold carbenes are less electrophilic than their oxygen counterparts, due to the lower electronegativity of the N atom. An obvious structural difference is that α-imino gold carbenes allow the preparation of molecules incorporating N rather than O atoms (most notably, N-heterocycles).594 Moreover, an additional group (R) is necessarily transferred together with the N atom (Scheme 100). This substituent, apart from modulating the reactivity of the intermediate complexes, can participate in intramolecular reactions.
2.7.2. Cyclopropanations Involving α-Imino Gold Carbenes
The first example of intermolecular nitrene transfer to gold-activated alkynes, reported by Zhang,603,604 popularized the use of iminopyridinium ylides such as 452 as nucleophilic nitrenoids. These reagents are the nitrogen analogues of pyridine N-oxides, common oxidants in the field of α-oxo gold carbenes.535−544 Combining the use of iminopyridinium ylides and their previously reported oxidative cyclopropanation of 1,5-enynes (see Scheme 89),550 Liu and co-workers described the first cyclopropanation involving α-imino gold carbene intermediates, yielding cyclopropane-fused indanimines 453 (Scheme 101).601
Scheme 101. Intramolecular Cyclopropanation of 1,5-Enynes Using Iminopyridinium Ylides.
Ohno and co-workers described the reaction of (azido)ynamides 455 with tethered alkenes to deliver cyclopropane-fused indoloquinolines 456 (Scheme 102).612 Intramolecular attack of the azide onto the ynamide, followed by nitrogen loss, delivered the key α-imino gold carbene 457, which then effected cyclopropanation of the pendant alkene. The use of azides as intramolecular nitrogen-transfer reagents for gold-activated alkynes became commonplace605,606 after the first report by the group of Toste for the synthesis of pyrroles.607
Scheme 102. Intramolecular Cyclopropanation of (Azido)ynamides.
Due to the very specific ynamide substrates used, however, the generality of this approach was rather limited. Hashmi and co-workers improved the applicability of α-imino gold carbenes in intramolecular cyclopropanations of ynamides by using external nitrene transfer reagents. They employed N-arylsulfilimines 459 for the formation of α-imino gold carbenes 461 through intermolecular attack on gold-activated ynamides followed by N–S cleavage (Scheme 103).613 The intermediates could be trapped in a number of ways,599 including by a tethered alkene to deliver 3-azabicyclo[3.1.0]hexan-2-imines 460 in good to excellent yields. In order for the reaction to proceed efficiently, more Lewis acidic gold(III) rather than gold(I) catalysts were beneficial, a temperature of 80 °C was required, and the sulfilimines had to carry a strong electron-withdrawing group on the arene. Sulfilimines599 are the nitrogen counterpart of sulfonium ylides, previously used by Maulide and co-workers in gold-catalyzed cyclopropanations (see section 2.9.3).608−611
Scheme 103. Intramolecular Cyclopropanation of Ynamides Using Sulfilimines.
The group of Hashmi later showed the possibility of obtaining 3-azabicyclo[3.1.0]hexan-2-imines 464 from ynamides under milder conditions using alternative nucleophilic nitrenoids, namely anthranils (Scheme 104).614 The reaction in this case could proceed with catalytic amount of NaAuCl4 dihydrate at room temperature.
Scheme 104. Intramolecular Cyclopropanation of Ynamides Using Anthranils.
It is worth mentioning a related work involving α-imino gold carbenes, which in special cases allowed the generation of cyclopropane-fused bicyclic compounds 468′ (Scheme 105).602 Liu and co-workers studied the gold(I)-catalyzed formation of azepine derivatives 468 starting from 3-en-1-ynamides 466 and isoxazoles595467 through a formal (4 + 3) cycloaddition process. In the proposed mechanism, α-imino gold carbene 470, whose resonance form can be visualized as a gold-stabilized 3-azaheptatrienyl cation, underwent a 6π electrocyclization to deliver compound 471, which after proton loss and protodeauration afforded 4H-azepine 468. Since these products contain a triene conjugated with a ketone, their 6π electrocyclization to deliver 3-azanorcaradienes 468′ is thought to be disfavored due to loss of conjugation. When the ynamides bore an n-butyl group in the internal alkene position, Liu and co-workers observed the presence of the 3-azanorcaradiene 468a′ as minor isomer.
Scheme 105. (4 + 3) Annulation of 3-En-1-ynamides with Isoxazoles.
Despite this recent progress, there is still ample room to further develop the use of α-imino gold carbenes in the realm of cyclopropanations, as all examples reported to date are intramolecular, non-enantioselective reactions and, with only one exception, use activated alkynes (ynamides)612−614 as substrates.
2.8. Assembly of Heteroatom-Containing 3-Membered Rings
Gold(I) catalysis was also employed in different mechanistic scenarios to prepare heterocyclic 3-membered rings, namely, aziridines and epoxides.
2.8.1. Gold-Catalyzed Synthesis of Aziridines
Metal-catalyzed nitrene transfer reactions are analogous to carbene transfer processes. These reactions are a typical procedure to assemble aziridines from alkenes and are often carried out under rhodium, copper, or ruthenium catalysis, among others.615 The first report on gold(I)-catalyzed nitrene transfer reaction for the synthesis of aziridines was reported by the group of He (Scheme 106).282 This reaction is proposed to proceed through PhI=NNs-type nitrenoid intermediates, which are generated in situ by combining commercially available sulfonamides with iodine(III) oxidant PhI(OAc)2. This is a stereoconvergent reaction, in which both E or Z disubstituted alkenes 474 lead to the same trans-aziridine 475, which strongly suggests a stepwise nature for the nitrene aziridination process.
Scheme 106. Gold(I)-Catalyzed Nitrene Transfer for Aziridine Synthesis.
Starting from E-β-methylstyrene.
Starting from Z-β-methylstyrene.
The same group showed how a similar gold-catalyzed transfer process promoted by an iodine(III) reagent could be applied to carry out nitrene insertions into aromatic and benzylic C–H bonds.616 Aziridines 478 have been also assembled by addition of 3-hydroxybenzofurans 476 to 2H-azirines 477 catalyzed by gold(I) complexes (Scheme 107).617
Scheme 107. Aziridines from 2H-Azirines.
Heterogeneous gold catalysis has also been successfully employed in the assembly of aziridines. For example, gold nanoparticles were reported to transfer nitrogen from ammonia to styrene, giving 2-phenylaziridine.618
2.8.2. Gold-Catalyzed Synthesis of Epoxides
Under gold catalysis, epoxides have been often used as reactive units that can act both as electrophiles (upon Lewis-acid activation) and as nucleophiles (attacking other functional groups activated by gold, such as alkynes) taking part in complex tandem processes.619−622,591 However, a few cascade cyclizations that result in the formation of epoxides based on gold catalysis have been recently reported.
Sahani and Liu reported for the first time a gold-catalyzed intermolecular (4 + 2) cycloaddition of electron-poor alkynes with benzisoxazoles that results in the assembly of highly oxygenated tetrahydroquinolines (481a,b), containing an epoxide group (Scheme 108A).623 Some of these products undergo an epoxide rearrangement affording 4-quinolones.
Scheme 108. Formal (4 + 2) Cycloaddition of Alkynes with Benzisoxazoles.
Using AgSbF6 as chloride scavenger.
The group of Hashmi employed a similar strategy, using electron-rich alkynyl amines.624 This resulted in the development of a new switchable methodology, in which by choosing the appropriate gold catalyst, either 6-acylindoles (with a gold(I) complex) or quinoline epoxides such as 481c,d (with a gold(III) complex) could be obtained from the same two substrates (Scheme 108B).
Besides homogeneous catalysis, supported gold nanoparticles have been reported to be active in the oxidation of alkenes by oxygen to yield epoxides.625,626
2.9. Miscellaneous Precursors
2.9.1. Stable Gold Carbenes via Transmetalation
Seidel and Fürstner reported the solid-state characterization of a reactive gold(I) carbene, 485, which was prepared by formal chromium-to-gold transmetalation of chromium carbonyl 484. Besides being a historical landmark in the understanding of the bonding model of gold(I) carbene complexes, this species was found to react readily with styrenes to afford cyclopropanes (Scheme 109A).627 The same group disclosed the synthesis and first X-ray characterization of analogous rhodium(II) carbenes (488) that led to the development of a versatile method for the preparation of gold(I) carbenes by rhodium-to-gold transmetalation (Scheme 109B).628
Scheme 109. Synthesis, Characterization, and Reactivity of Metal Carbenes.
Ar = p-Methoxyphenyl.
Through careful ligand design, Borissou and co-workers reported the isolation and solid-state characterization of a tricoordinated α-oxo gold(I) carbene complex generated from ethyl diazo(phenyl)acetate at −40 °C, which was competent in the cyclopropanation of styrene when generated in situ from the diazo-compound.566 Other groups have also contributed to the characterization of gold(I) carbenes, using different strategies to stabilize the otherwise reactive species.629−633
2.9.2. Gold Carbenoids as Precursors of Gold Carbenes in Solution
Metal carbenoids are organometallic species of the type (X)(M)C(R1)(R2), where an sp3 carbon atom C (for which R1 and R2 can be H or organic groups) is bound to both a metal M and a leaving group X. These species can evolve or be in equilibrium with the corresponding carbenes (M)CR1R2 upon release of the leaving group X and display analogous reactivity patterns, such as cyclopropanation of alkenes. The most studied type of metal carbenoid is the proposed intermediate for metal-catalyzed diazo decomposition reactions, in which X = N2 (see section 2.1).236,634 Alternative types of gold(I) carbenoids have been prepared and employed as carbene equivalents in gas phase and solution.
2.9.2.1. Sulfone-Imidazolium Salt as Leaving Group in Gold(I) Carbenoids
The group of Chen reported the synthesis of carbenoid-type gold(I) complexes such as 489, based on their previous work on gas phase generation of carbenes (see section 2.2.2). These complexes release SO2 and a free NHC (Scheme 110, in gray) upon heating, generating free gold carbenes that can be trapped by electron-rich p-methoxystyrene to afford efficiently cis-cyclopropane 490a. A catalytic version of this transformation was developed adding stoichiometric amounts of an external imidazolium salt.635,636
Scheme 110. High Temperature Cyclopropanation Using Sulfone–Imidazolium Gold(I) Carbenoids.
PMP = p-Methoxyphenyl.
2.9.2.2. Chloride as Leaving Group in Gold(I) Carbenoids
The group of Echavarren reported an alternative type of gold(I) carbenoid 491 bearing chloride as leaving group, which can act as methylene carbene equivalent. These carbenoids, upon activation with TMSOTf or AgOTf, display the typical reactivity patterns of metal carbenes: cyclopropanation of alkenes, dimerization, and Buchner reaction (Scheme 111A).637
Scheme 111. Gold Carbenes from Chloromethyl Gold(I) Carbenoids.
The [(JohnPhos)AuCH2]+ species has only been detected by ESI-MS under very energetic conditions. In contrast, further study of these complexes revealed that chloro(aryl)methylgold(I) carbenoids such as 493 allowed the controlled generation of gold(I) carbenes in solution upon treatment with GaCl3, even at cryogenic conditions. This allowed the NMR characterization of the corresponding carbenes, which are lacking heteroatom stabilization. These carbenes undergo cyclopropanation, C–H insertion, oxidation, and dimerization reactions under very mild conditions (Scheme 111B).638 Similarly, Fürstner and co-workers reported the preparation of gold(I) difluorocarbenoid complexes, which also react with alkenes to assemble difluorocyclopropanes.639
2.9.3. Sulfonium Ylides
The group of Maulide disclosed for the first time the use of sulfonium ylides as gold(I) carbene equivalents that react with alkenes to form cyclopropanes. Rather than by metal carbene formation, these cyclopropanations occur via alkene activation by gold, an unusual pathway in gold(I) catalysis.611 Using this strategy, a highly stereoselective intramolecular cyclopropanation was developed, allowing the preparation of a wide range of cyclopropane-fused lactones 498 in high yields (Scheme 112).608
Scheme 112. Intramolecular Cyclopropanation through Catalytic Alkene Activation with Sulfonium Ylides.
An asymmetric version of this reaction was reported by the same group.610 In this work, the use of a digold complex with dimeric TADDOL phosphoramidite ligand 501 was key to the overall enantioselectivity, in a process featuring not only a diastereo- and enatioselective cyclopropanation but also an initial dynamic deracemization for chiral racemic substrates (where R2 differs from H). This reaction was also found to be regio- and stereoconvergent in terms of the position of the alkene substituents (Scheme 113, colored in green): regardless of the position of R2 in 499, the substituent ends up in the same position in products 500.
Scheme 113. Asymmetric Intramolecular Cyclopropanation with Sulfonium Ylides.
Maulide and co-workers also developed an intermolecular version of this gold(I)-catalyzed cyclopropanation reaction to afford methylenecyclopropanes 504 (Scheme 114).609 Mechanistic studies supported the hypothesis of allene activation by gold instead of the classical “carbene generation” pathway.
Scheme 114. Cyclopropanation of Allenamides with Sulfonium Ylides.
2.9.4. Acetylene as Dicarbene Precursor
Recently, Echavarren and co-workers reported the gold(I)-catalyzed activation of acetylene, as the simplest dicarbene equivalent (Scheme 115).640 By careful selection of the gold(I) complex, good yields of several biscyclopropanes 508 derived from stilbenes can be obtained. The same reaction can be carried out in the presence of cyclooctene, giving biscyclopropane 507. Alternatively, if the reaction is carried out in the presence of a mixture of alkenes, a moderate amount of cross-biscyclopropanation product 509 can be obtained (Scheme 115, above). This biscyclopropanation strategy performs best by reacting acetylene with dienes. This is clearly illustrated by the intramolecular biscyclopropanation of geranyl acetone with acetylene, which affords directly natural product waitziacuminone (511) in one step, as a single diastereoisomer (Scheme 115, below).
Scheme 115. Acetylene as Dicarbene Precursor.
Using [(tBuXPhos)AuCl]/NaBArF4 (5 mol %) as catalyst.
3. Construction of 4-Membered Rings Catalyzed by Gold
3.1. Synthesis of Cyclobutanes
The first synthesis of 4-membered rings using gold(I) catalysis was discovered in the context of the preparation of indoline-fused cyclobutanes.641 Since then, the implementation of an impressive number of gold(I)-catalyzed protocols, mostly based on ring expansions and stepwise [2 + 2] cycloadditions, has rapidly expanded the field.41,119−126,207−210,449,450,642−645 These novel methods enable construction of compounds ranging from simple rings to complex skeletons of natural products in an atom-economical manner.
This section covers all the advances on the gold-catalyzed synthesis of 4-membered cycles from the outset of the field. The section will be divided by the type of ring formed and each subsection will be structured based on mechanistic considerations.
3.1.1. Gold(I)-Catalyzed Cyclopropane Ring Expansions
The strain release derived from cyclopropane expansion has been extensively used to thermodynamically favor the construction of 4-membered cycles.7,8,41,646 Under gold(I) catalysis, a wide range of cyclopropane-embedded frameworks can undergo ring expansion to efficiently assemble substituted cyclobutanes.41,644,645,647,648
3.1.1.1. Gold(I)-Catalyzed Cycloisomerization of Cyclopropyl Enynes
The group of Echavarren disclosed the cascade gold(I)-catalyzed transformation involving the ring expansion of a cyclopropyl moiety to build a cyclobutane by the tandem enyne cyclization/ring expansion/Prins cyclization sequence of cyclopropyl 1,6-enynes 512 to form tricyclic compounds 513, as mixtures of diastereoisomers (Scheme 116).649
Scheme 116. Gold(I)-Catalyzed Cycloisomerization of Cyclopropyl 1,6-Enynes.
[(JohnPhos)Au(MeCN)]SbF6.
E/Z = 1:1.
Reaction without H2O.
Interestingly, the diastereoselectivity varied according to the gold(I) catalyst employed. Thus, using AuCl, cyclopropyl gold(I) carbene 515, generated via 5-exo-dig cyclization, undergoes ring expansion to form alkenyl gold(I) complex 516. Subsequent 5-exo-dig Prins cyclization and deauration steps release tricycles 513 (Scheme 117). For cationic gold(I) catalysts, the cyclopropyl cation (518) resonance form is favored, which evolves through a non-stereospecific ring expansion to give mixtures of diastereoisomers 513′ and 513 after Prins cyclization (Scheme 117).
Scheme 117. Mechanistic Proposal for Gold(I)-Cycloisomerization of Cyclopropyl 1,6-Enynes.
The synthetic utility of this gold(I)-cyclization cascade was demonstrated with the diastereoselective one-pot construction of the tricyclic skeleton 522 of repraesentin F, which was further converted into the natural product in 6 steps (Scheme 118A).650 A related gold(I)-catalyzed allene–vinylcyclopropane cycloisomerization gave access to the tricyclic core of the protoilludanes family in a single step (Scheme 118B).651
Scheme 118. Access to Repraesentin F and the Protoilludane Family Core.
[(t-BuXPhos)Au(MeCN)]BArF4.
[(JohnPhos)Au(MeCN)]SbF6.
Shi reported the divergent gold(I)-catalyzed cycloisomerization of cyclopropyl-tethered 1,5-enynes 525 (Scheme 119).443 When the aryl group was not ortho-substituted, cyclobutane-fused 1,4-cyclohexadienes 527 were obtained (Scheme 119A). The ortho-substitution of the aryl group resulted in the development of a switchable protocol, in which, by choice of the appropriate gold catalyst, three different polycyclic scaffolds were obtained under temperature control, namely, spiro compound 529, fused-cyclobutane conjugated cyclohexadiene 528, and tricyclic cyclobutene 530 (Scheme 119B,C,D, respectively). Computational and labeling experiments pointed to the initial formation of common biscyclopropane gold(I) carbene intermediate 526, whose evolution toward the different products was controlled by the ortho-substituent effect.
3.1.1.2. Gold(I)-Catalyzed Cycloisomerization of Cyclopropylidene Enynes
Toste and co-workers developed a methodology to construct diastereomerically pure fused cyclobutanes 532 via Wagner–Meerwein skeletal rearrangement of cyclopropylcarbinyl cations 533. These intermediates were generated in situ from the gold(I)-catalyzed 6-exo-dig cyclization of cyclopropylidene 1,6-enynes 531 (Scheme 120).393 Enantioenriched tetracycle 532b could be obtained in 82% ee using [((R)-Xyl-SDP)(AuCl)2] as gold(I) precatalyst (Scheme 120).
Scheme 120. Gold(I)-Catalyzed Cycloisomerization of Cyclopropylidene 1,6-Enynes.
Reaction using [((R)-Xyl-SDP)(AuCl)2] as precatalyst.
This work by Toste inspired several subsequent studies in which the ring strain relief of cyclopropylidene fragments was key for the assembly of cyclobutanes under gold(I) catalysis. Thus, the group of Gagné reported a gold(I)-catalyzed Cope rearrangement and cyclopropane ring enlargement of cyclic 1,5-dienes 535 leading to tricyclic compounds 536 (Scheme 121A).652 The same group disclosed the gold(I)-catalyzed enantioselective cycloisomerization/ring-expansion sequence of cyclopropylidene-bearing 1,5-enynes 538 that led to enantioenriched bicyclo[4.2.0]octanes 541 in up to 70% ee (Scheme 121B, right).653 Mechanistically, the ring-expanding cycloisomerization begins with a gold(I)-catalyzed 6-endo-dig cyclization and subsequent cyclopropane ring expansion toward the more stable allylic carbocation 539, which undergoes 1,2-hydrogen shift to yield fused cyclobutane product 541. In a follow-up report by the same group, gold(I)-stabilized allyl cation intermediates 539 were trapped by aldehydes, leading to oxo-carbenium cations, which suffer Friedel–Crafts annulation to form polycyclic structures 540 in a highly diastereoselective cascade reaction (Scheme 121B, left).654
Scheme 121. Gold(I)-Catalyzed Cycloisomerization of Cyclopropylidene-Tethered Frameworks.
Inspired by the protocol developed by the group of Gagné,653 Hönig and Carreira recently designed the key step of the first total synthesis of the unnamed harziane diterpenoid 544 (Scheme 121C).655 The target cyclobutane (543) was prepared with high diastereocontrol via gold(I)-catalyzed cycloisomerization of cyclic cyclopropylidene 1,5-enynes 542 bearing a terminal alkyne. The key cyclobutane was further transformed into harziane diterpenoid 544 in 16 steps.
Methylenecyclopropanes are known to undergo isomerization to form cyclobutenes in the presence of Pt(II),656 Pd(II),657 and Au(I)322 catalysts. The group of Shi found that treating “(propargyloxy)arenemethylenecyclopropanes 545 with an electrophilic gold(I) catalyst, [(p-CF3C6H4)3PAuCl], produced chemodivergent results, depending on the nature of the ortho-substituents on the phenyl group (R1) (Scheme 122A).658 Specifically, when bulky substituents, such as t-Bu and t-Am are used, the methylenecyclopropane group migrates, rather than expands, leading to the formation of bicyclic products 548 (Scheme 122A, left). Nonbulky substituents (Me, MeO, or halogens) favor the gold(I)-catalyzed ring expansion and subsequent migration of the propargyl group to form 2,3-dihydrobenzofuran cyclobutane fused allene derivatives 547 (Scheme 122A, right). The asymmetric version of this transformation using DTBM-SegPhos ligand was also reported with a good level of enantioinduction, but a narrow substrate scope (Scheme 122A, right).
Scheme 122. Regiodivergent Gold(I)-Catalyzed Cycloisomerizations of Cyclopropylidene 1,7-Enynes.
[(p-CF3C6H4)3PAuCl] (2.5 mol %) and AgSbF6 (2.5 mol %).
[(DTBM-SegPhos)(AuCl)2] (10 mol %), NaBArF4 (20 mol %), toluene, rt, 96 h.
Shi also developed a regiodivergent gold(I)-catalyzed cyclization/ring opening sequence from alkynylamide-tethered alkylidenecyclopropanes 550 (Scheme 122B). In this case, two different types of fused 4-membered rings, cyclobutanes 553 and cyclobutenes 552, were obtained depending on the gold(I) catalysts employed.659 Both the ligand and the counteranion of the cationic gold(I) catalyst were found to play an important role in the product distribution. When [(Ph3P)AuCl] was used in combination with AgOTf, cyclobutenes 552 were exclusively obtained (Scheme 122B, left). Instead, the use of JohnPhos as ligand with BArF4– counteranion led to cyclobutanes 553 as the only product (Scheme 122B, right). Using [(JohnPhos)Au(OTf)] as catalyst, a mixture of both carbocycles was obtained. The regiodivergent outcome was rationalized based on the mechanistic proposal. After ring expansion, the gold(I)-stabilized carbocation 551 can undergo Friedel–Crafts annulation leading to spiropolycyclic cyclobutanes 553. Alternatively, intermediate 551 can be deprotonated affording cyclobutenes 552.
Initial attempts to develop a gold(I)-catalyzed asymmetric synthesis of cyclobutanes via cyclopropylmethyl cation ring expansion were met with either only moderate levels of enantioinduction or limited substrate scope.653,658 In particular, in the context of the cycloisomerization of cyclopropylidene-tethered 1,6-enynes, the first asymmetric variant was disclosed by Toste (Scheme 120, 82% ee).393
Building upon this work, the groups of Li and Yu developed a general asymmetric synthesis of azepine-fused cyclobutanes 555 by means of a gold(I)-catalyzed cyclization/C–C cleavage/Wagner–Meerwein rearrangement sequence from cyclopropylidene 1,6-enynes 554 (Scheme 123).660 Here, the authors found that both solvent and counterion played an important role in enhancing enantioinduction. Notably, when [((R)-4-MeO-3,5-(t-Bu)2-MeOBIPHEP)(AuCl)2], AgSbF6, and toluene were used, the enantioselectivity for cyclobutane products 555 could be boosted up to 99% ee.
Scheme 123. Enantioselective Gold(I)-Catalyzed Cycloisomerization of Cyclopropylidene 1,6-Enynes.
Interestingly, when electron-poor aryl groups were attached to the cyclopropylidene moiety, the reaction delivered spirocyclic compound 555′ with complete chemoselectivity. The same outcome had been previously reported by Shi using 1,6-enynes bearing monosubstituted cyclopropylidene fragments (Scheme 36A, section 2.4.1.1).392 Using DFT calculations, the authors proposed a chemodivergent mechanism to explain this dichotomy (Scheme 124). Here, upon 6-endo-dig cyclization, cyclopropyl gold(I) carbene 560 can undergo either 1,2-hydrogen shift to spirocyclic compounds 559 or C–C cleavage, followed by ring expansion and 1,2-hydrogen shift, to azepine-fused cyclobutanes 558. The latter pathway proceeds through carbocation 561, which therefore will be stabilized by adjacent electron-rich aryl groups. The authors define intermediate 561 as a “carbocation with memory of chirality”, through which the chirality of the initially formed cyclopropane is transferred to the final product.
Scheme 124. Mechanistic Proposal for the Gold(I)-Catalyzed Cycloisomerization of Cyclopropylidene 1,6-Enynes.
3.1.1.3. Gold(I)-Catalyzed Cycloisomerization of Vinylidenecyclopropanes
Shi and co-workers disclosed a new mode for gold(I) carbene generation based on the ambiphilic nature of vinylidenecyclopropanes. Upon gold(I) activation, a vinylidenecyclopropane such as 563 generates cyclopropyl gold(I) intermediate 564, which leads to gold(I) carbene 565 by ring expansion (Scheme 125A).661
Scheme 125. Gold(I)-Catalyzed Cycloisomerization of Vinylidenecyclopropanes.
[((R)-Xyl-BINAP)Au2(MeCN)2](SbF6)2 (10 mol %), toluene, rt.
[(Me4-tBuXPhos)Au(MeCN)]NTf2 (10 mol %).
When vinylidenecyclopropane-enes of type 566 were submitted to this procedure, the transient gold(I) carbenes 568 cyclopropanated the pendant alkene moiety, building 3-, 4-, and 8-membered fused rings in a formal single step (Scheme 125B).661 Only vinylidenecyclopropane-enes tethered to non-ortho-substituted aromatic rings were suitable substrates. By carefully modifying the pendant chain of vinylidenecyclopropanes, they developed a novel approach in which the in situ generated gold(I) carbene was involved in an intramolecular C(sp3)–H insertion (Scheme 125C).662 Here, only scaffolds bearing p-CF3 substituted aryl (569) gave the desired fused cyclobutane products such as 570 with high diastereocontrol. Enantioselective variants of these transformations were also developed using bidentate chiral phosphine-supported gold(I) catalysts (Scheme 125B,C).661,662
3.1.1.4. Gold(I)-Catalyzed Oxo-Cyclization/Ring Expansion/Cycloaddition Sequences
Zhang disclosed the gold(I)-catalyzed intermolecular [4 + 2] annulation of alkynyl cyclopropyl ketones 572 with dipolarophiles, such as aldehydes, ketones, indoles, imines, and silyl ethers, to easily access polycyclic furans.663 In the course of their investigation, they found that when ethyl vinyl ether was employed as dipolarophile the reaction outcome switched to deliver a mixture of strained bicyclic cyclobutanes (Scheme 126).664 The authors proposed that enone 573 was initially formed by means of oxo-cyclization and 1,3-dipolar cycloaddition. However, this acid sensitive compound decomposed during purification, thus preventing isolation (Scheme 126). Varying the conditions, they developed a series of chemoselective one-pot three-component protocols in which unstable intermediates such as 573 were treated before purification with different nucleophiles, such as H2O, MeOH, hydride, and allyl groups, to furnish substituted bicyclic enones 574–577 in high yield.
Scheme 126. Three-Component Reactions for the Synthesis of Bicyclo[3.2.0]heptanes.
Liu reported the gold(III)-catalyzed cyclization of 1-epoxy-1-alkynylcyclopropanes 579, in which the epoxide acts as a nucleophile attacking intramolecularly the alkyne fragment (Scheme 127A).665 When the gold(III)-catalyzed electrocyclization of 579 was performed in the presence of NBS or NIS, the replacement of the gold(III) fragment by Br+ or I+ led to halogenated products 580 in good yields and complete diastereoselectivity (Scheme 127A, top left).666 In addition, the oxocyclic alcohol products 581 could engage in a [4 + 2] cycloaddition with enones and dienes to deliver polycyclic compounds 583 and 582, respectively, as single diastereoisomers (Scheme 127A, bottom).665 Originally, it was proposed that the gold(III)-catalyzed oxo-cyclization occurs simultaneously to a cyclopropane ring expansion, in a concerted electrocyclization to form exocyclic alcohols 581 in a diastereoselective manner (Scheme 127B). However, experiments using enantioenriched epoxyalkynes 579 demonstrated that the chiral information was mostly lost during the gold(III)-catalyzed cyclization and, thus, a stepwise oxo-cyclization/ring expansion sequence could not be discarded (Scheme 127B).
Scheme 127. Gold(I)-Catalyzed Cyclization of 1-Epoxy-1-alkynylcyclopropanes.
SiO2 filtration, [(Ph3P)AuCl] (10 mol %), AgSbF6 (10 mol %), CH2Cl2, rt.
3.1.1.5. Gold(I)-Catalyzed Synthesis of Cyclobutanamines
Ye and Yu disclosed the gold(I)-catalyzed synthesis of 2-alkylidenecyclobutanamines 589 with complete E-selectivity, taking place via ring enlargement of nonactivated alkynylcyclopropanes 587 and subsequent intermolecular sulfonamide trapping (Scheme 128).667 Other common Lewis Acids such as PtCl2 and Sc(OTf)3 led to low reactivity. Aryl- and alkyl-alkynylcyclopropanes 587 were suitable substrates, whereas terminal alkynes underwent ring expansion instead. A DFT study of this reactions was also carried out.668
Scheme 128. Gold(I)-Catalyzed Synthesis of Cyclobutanamines.
A heterogeneous gold(I)-catalyzed version of this transformation was developed using Fe3O4@SiO2–PPh2–AuOTf supported on magnetic nanoparticles that could be recycled up to 10 times, maintaining a catalytic activity comparable to the previous homogeneous version.669
3.1.2. Gold(I)-Catalyzed 1,3-Acyloxy Migration of Propargylic Carboxylates
Zhang disclosed a tandem gold(I)-catalyzed 1,3-acyloxy migration/[2 + 2] cycloaddition sequence from indole-tethered propargylic carboxylates to furnish indole-fused cyclobutanes 592 with complete diastereoselectivity (Scheme 129A).641 This work is not only significant for being the first example of gold(I)-catalyzed synthesis of cyclobutanes, but also because it laid the foundations for the, nowadays well-explored, field of gold(I)-catalyzed [2 + 2] cycloadditions of allenes and alkenes, which will be further detailed in section 3.1.3 of this review. The author proposed that the initial gold(I)-catalyzed 1,3-acyloxy shift proceeds through a 6-endo-dig oxo-cyclization to form oxocarbenium intermediate 594, which undergoes ring opening to carboxyallene 595 in an overall [3,3]-sigmatropic rearrangement (Scheme 129B, gray square).479,641 The in situ generated allene can be subsequently activated by gold to participate in further transformations. In this particular case, the indole moiety present on the original scaffold 591 engages in a formal [2 + 2] cycloaddition via two consecutive intramolecular trapping events. Interestingly, the carboxyallene-indole product 592c′ could be isolated, supporting their mechanistic proposal (Scheme 129A). Calculations by Cavallo et al. suggested an alternative pathway in which a gold(I) vinylidene could be formed via two sequential 1,2-acyloxy migrations (see also section 2.5 on 1,2- and 1,3-acyloxy shift).481
Scheme 129. Intramolecular Gold(I)-Catalyzed 1,3-Acyloxy Migration/[2 + 2] Cycloaddition Sequence.
AuCl3 (10 mol %), CH2Cl2, rt.
The group of Chan reported another gold(I)-catalyzed 1,3-acyloxy shift/[2 + 2] cycloaddition sequence from 1,7-enyne benzoates 598 (Scheme 130).670 This transformation gave access to azabicyclo[4.2.0]oct-5-enes 599 in a regioselective and stereoconvergent manner (Scheme 130A). In addition, the chirality could be transferred from enantiopure substrates to the products. Interestingly, the authors proposed a different activation mode, in which gold coordinates preferentially to the alkene in 1,7-allenene 602, which can be formed in situ via 1,3-acyloxy shift (Scheme 130B). This η2-(alkene)gold(I) species 602 undergoes a subsequent stepwise [2 + 2] cycloaddition leading to the product. The solvent and the optimal catalyst were essential to promote the last step and prevent the exclusive formation of the 1,7-allenene intermediate, as occurred for 599a′ (Scheme 130A).
Scheme 130. Intermolecular Gold(I)-Catalyzed [3,3]-Rearrangement/[2 + 2] Cycloaddition Sequence of 1,7-Enynes.
Reaction performed in acetonitrile.
The gold(I)-catalyzed 1,3-acyloxy migration of 1,3-diarylpropargyl carboxylates has been considered challenging due to the formation of uncharacterized mixtures. While investigating the nature of the multiple products, Shi and co-workers developed a chemo- and regioselective silver-free protocol for the intermolecular gold(I)-catalyzed synthesis of cyclobutanes 605b from highly reactive scaffolds 604 (Scheme 131).671 Here, the in situ generated allenes undergo intermolecular gold(I)-catalyzed [2 + 2] cycloaddition between themselves. In the presence of silver, 1,4-enyne 605a was formed exclusively (Scheme 131). In contrast, under silver-free conditions, the cyclobutane isomer 605b could be obtained as the major product depending on catalyst choice and substitution pattern of the carboxylate moiety.
Scheme 131. Intermolecular Gold(I)-Catalyzed 1,3-Acyloxy Shift/[2 + 2] Cycloaddition Sequence.
[(Ph3P)Au(N-methyl benzotriazole)]OTf (1 mol %), CH2Cl2, rt.
AgOTf (2 mol %).
An isolated example of this reactivity was recently reported by Fiksdahl in the context of gold(I)-catalyzed [2 + 2 + 2] cyclotrimerizations.672
3.1.3. Gold(I)-Catalyzed [2 + 2]-Cycloadditions of Allenes and Alkenes
A seminal publication by Toste and co-workers disclosed the formation fused cyclobutanes 608 via diastereoselective [2 + 2] cycloaddition of 1,6-allenenes 607 (Scheme 132A).673 In the same work, the original racemic protocol was expanded using [((R)-DTBM-SEGPHOS)(AuCl)2] 609 as precatalyst to obtain cis-cyclobutanes with excellent enantioselectivity (Scheme 132B).
Scheme 132. Gold(I)-Catalyzed [2 + 2] Cycloaddition of 1,6-Allenenes.
The gold(I)-catalyzed [2 + 2] cycloaddition of 1,6-allenenes 607 became a benchmark reaction to explore the utility of new chiral ligands in asymmetric gold(I) catalysis. For instance, it was employed by Fürstner and co-workers to study the performance of a library of chiral phosphoramidite ligands in gold(I) asymmetric catalysis.404,507 Complex 610, with a ligand based on an acyclic TADDOL, gave even better results than the bidentate phosphine SEGPHOS used in the original work by Toste (Scheme 132B, 609). In another publication by the group of Toste, spirobiindane-derived phosphoramidite gold(I) complex 611 was reported to mediate the asymmetric [2 + 2] cycloaddition efficiently in terms of both catalytic activity and enantioselectivity (Scheme 132B, 611).674 Likewise, the group of Marinetti developed gold(I) complex 612, bearing a phosphahelicene ligand, which was applied to the synthesis of enantioenriched cyclobutanes 608 (Scheme 132B, 612).675
In the original work by Toste,673 the cis-cyclobutane 614 was obtained from the gold(I)-catalyzed [2 + 2] cycloaddition of both E- and Z-1,6-allenene 613 (Scheme 133A). Interestingly, when E-1,6-allenene 613 was exposed to a gold(I) catalyst in the presence of methanol, trans-cyclopentane 615 was formed (Scheme 133A).673 These preliminary mechanistic studies pointed toward the stepwise formation of carbocationic intermediates.
Scheme 133. Experimental and Computational Mechanistic Studies on the Gold(I)-Catalyzed [2 + 2] Cycloaddition of 1,6-Allenenes.
[(Ph3P)AuCl] (5 mol %), AgBF4 (5 mol %).
[(Ph3P)Au]BF4 (5 mol %).
[((PhO)3P)Au]BF4.
[(NHC)AuCl] (5 mol %), AgSbF6 (5 mol %), CH2Cl2, −5 °C, NHC = imidazopyridine-2-ylidene derivative.
A mixture of 616 (56% ee) and 616′ (52% ee) was obtained from enantioenriched 613 (>99% ee).
To clarify the mechanistic scenario, DFT studies were performed on the gold(I)-catalyzed [2 + 2] cycloaddition of a 1,6-allenene using a model phosphoramidite–gold(I) catalyst (Scheme 133B).674 This study showed that the reaction starts by intramolecular alkene addition to the gold(I)-coordinated allenene 617, which can occur through two competitive pathways leading to cis- and trans-vinyl gold(I) complexes 618. In the presence of methanol, the kinetically favored carbocation trans-618 is intermolecularly trapped by MeOH to furnish cyclopentane trans-615 (Scheme 133B, left). In the absence of nucleophiles, carbocations trans-618 and cis-618 are in equilibrium, and the later undergoes intramolecular carbocation trapping to form the thermodynamically more stable cis-cyclobutane 614 (Scheme 133B, right). The reverse ring-opening from cis-614 to generate carbocation cis-618 was not energetically feasible, as proven by experimental data (Scheme 133A). However, in an independent work by Fürstner, this pathway was found to be effective during the gold(I)-catalyzed rearrangement of cis-614 to the ring-expanded scaffolds 616/616′ (Scheme 133A).676 Remarkably, this reaction only takes place using NHC ligands with the appropriate electronic properties.
Under gold(I) catalysis, larger 1,7-allenenes 619 gave variable mixtures of 5- and 4-membered carbocycles, 620a and 620b, respectively (Scheme 134).677−682 The product ratio was dependent on the ligand, although the formation of cyclopentene scaffolds 620a was always favored. The ligand effect on this transformation was investigated using multivariate analysis, concluding that the selectivity-determining step, which was proposed to be a concerted cyclization to form both rings, was significantly affected by the steric properties of the ligand. Based on a detailed computational study, Liu et al. suggested that the two fused rings were formed via a stepwise cyclization.683
Scheme 134. Gold(I)-Catalyzed Cycloadditions of 1,7-Allenenes.
A similar regiodivergent scenario was found independently by the groups of Toste and Mascareñas in the gold(I)-catalyzed intermolecular cycloadditions of allenedienes 621.677−681 These substrates can undergo either (4 + 2) or (4 + 3) cycloadditions to form 6- or 7-membered carbocycles, respectively, via a ligand-controlled bifurcated mechanism from intermediate 623 (Scheme 135A).678,682 Interestingly, two specific allenedienes evolve by means of gold(I)-catalyzed [2 + 2] cycloaddition to release fused cyclobutanes 622 and 622′ instead of forming larger rings (Scheme 135B).678
Scheme 135. Gold(I)-Catalyzed Cycloadditions of Allenedienes.
[((2,4-(t-Bu)2-C6H3O)3P)AuCl] (10 mol %), AgSbF6 (10 mol %), 0 °C.
622a was obtained from trans-621.
622b and 622b′ were obtained from cis-621.
Contributions by the groups of Chen,684 González,685 and López and Mascareñas686 set the foundations for the development of the intermolecular gold(I)-catalyzed [2 + 2] cycloaddition of alkenes and allenes (Scheme 136). The catalytic methods developed by these groups gave access to densely substituted cyclobutanes bearing an exocyclic Z-double bond as single regio- and diastereoisomers. In the course of their investigations, they found a competitive dimerization of N-allenylsulfonamides that could be optimized to selectively obtain cyclobutanes 626f (Scheme 136A).684,685
Scheme 136. Gold(I)-Catalyzed [2 + 2] Cycloaddition of Alkenes and Allenamides.
[(JohnPhos)AuCl] (2 mol %), AgSbF6 (2 mol %), CH2Cl2, 4 Å MS, 25 °C.
[((2,4-(t-Bu)2-C6H3O)3P)Au(NTf2)] (0.5 mol %), CH2Cl2, rt.
[(Ph3P)AuCl] (5 mol %), AgSbF6 (2 mol %), CH2Cl2, 4 Å MS, 25 °C.
Norbornene (15 mol %).
Reaction without alkene partner.
[((2,4-(t-Bu)2-C6H3O)3P)AuCl] (2 mol %), AgSbF6 (2 mol %), CH2Cl2, 4 Å MS, −15 °C.
[(JohnPhos)Au(MeCN)]SbF6 (5 mol %), DCE, rt to 85 °C.
N-allenylsulfonamide and N-allenyloxazolidinone as allene partners and electron-rich alkene counterparts, such as enol ethers, cyclic enamines, styrene derivatives, and enamides participated in the reactions (Scheme 136A,B).684−686 The use of bisalkenes led to a mixture of [2 + 2] product and N-allenyloxazolidinone dimer.687 Mascareñas and López and co-workers reported a follow-up work in which the alkene scope was expanded to include N,N-dialkyl hydrazones (Scheme 136C). Platinum complexes were found to be poorer catalysts for this transformation.688
Mechanistically, these gold(I)-catalyzed [2 + 2] cycloadditions start by the regioselective attack of the alkene to the γ-carbon of a σ-type allenamide gold(I) complex (627) to generate carbocation intermediate 628, which undergoes C–C bond formation to complete the formal [2 + 2] cycloaddition (Scheme 136D).689
The group of González disclosed the asymmetric version of the intermolecular gold(I)-catalyzed [2 + 2] cycloaddition of vinyl arenes and N-allenylsulfonamides (Scheme 137A),690 which also represents one of the leading enantioselective methods using allene scaffolds in gold(I) catalysis.691 Here, a family of chiral phosphoramidite gold(I) complexes gave excellent results in the construction of enantioenriched cyclobutanes. Recently, Chen and co-workers found that chiral bis-1,2,3-triazol-5-ylidene digold(I) complex 632 was also an ideal catalyst for the enantioselective synthesis of cyclobutanes by [2 + 2] cycloaddition.692 The use of this ligand allowed extension of the scope to several alkene and allenamide partners (Scheme 137B).
Scheme 137. Asymmetric Gold(I)-Catalyzed [2 + 2] Cycloaddition of Alkenes and Allenamides.
Zhang and co-workers reported the asymmetric gold(I)-catalyzed cycloadditions of 3-styrylindoles 633 and N-allenylsulfonamides 634 (Scheme 138).693 Here, it was found that the cycloaddition mode was switched by the electronic properties of the N-substituents. Specifically, electron-donating substituents favored the formation of 3-cyclobutylindole 636 via [2 + 2] cycloaddition (Scheme 138, right), whereas electron-poor substituents facilitated the [4 + 2] cycloaddition to form tetrahydrocarbazole scaffolds 635 (Scheme 138, left). Remarkably, both pathways were found to be diastereo- and highly enantioselective when using the same chiral phosphoramidite gold(I) catalyst. Later, a new protocol was developed using a monophosphine Xiang-Phos chiral gold(I) catalyst, 640, which allowed the use of N-allenyl oxazolidinone as electrophilic partner in the [2 + 2] cycloaddition.694
Scheme 138. Asymmetric Gold(I)-Catalyzed [2 + 2] and [4 + 2] Cycloaddition of 3-Styrylindoles and Allenamides.
The group of Bandini developed an asymmetric protocol for the gold(I)-catalyzed dearomative [2 + 2] cycloaddition of 1,2-substituted indoles 641 and allenamides 642 (Scheme 139).689,695 The use of electron-rich phosphine-based gold catalyst 644 was key to furnish indolincyclobutanes 643 with high chemo-, diastereo-, and enantioselectivity. The same group had previously reported that treatment of similar scaffolds with a highly electrophilic gold(I) catalyst led to selective C3-functionalization of indoles.696
Scheme 139. Asymmetric Gold(I)-Catalyzed [2 + 2] Cycloaddition of Indoles and Allenes.
3.1.4. Other Strategies for the Gold(I)-Catalyzed Synthesis of Cyclobutanes
3.1.4.1. Gold(I)-Catalyzed Cycloisomerization of Bisallenes
Chung and co-workers reported the gold(I)-catalyzed cycloisomerization of 1,5-bisallenes 645 to form azabicyclo[3.1.1]heptanes 646, in which the cyclobutane bears two exocyclic double bonds (Scheme 140).697 Only substituents in the proximal carbon atoms of the allenes were tolerated (Scheme 140A). DFT studies were performed to clarify the preference for the twisted head-to-head cycloaddition over other plausible mechanistic scenarios, which showed that the most favorable pathway begins with an initial attack from the middle carbon in the nonactivated allene to the internal carbon of the gold(I)-coordinated allene to generate six-membered cyclic intermediate 648. From the latter, the formation of the second C–C bond is facilitated by the stabilizing interaction of the gold atom with both carbons.
Scheme 140. Gold(I)-Catalyzed Cycloisomerization of Bisallenes.
3.1.4.2. Gold(I)-Catalyzed Formal (3 + 2)/(2 + 2)-Annulation of Allylsilane with 4-Methoxybut-2-yn-1-ols
An alternative protocol to construct strained bicyclo[3.2.0]heptane scaffolds under gold(I) catalysis was reported by Liu et al.698 Here, the formal intermolecular (3 + 2)/(2 + 2)-annulation of allylsilane with 4-methoxybut-2-yn-1-ols 650 gave access to fused cyclobutanes 651 in high yield and diastereoselectivity (Scheme 141). Their mechanistic proposal suggests the formation of 1,5-enynes 652. These intermediates can be further activated by gold to furnish cyclobutanes by annulation with allylsilane.
Scheme 141. Gold(I)-Catalyzed Formal (3 + 2)/(2 + 2)-Annulation.
3.2. Synthesis of Cyclobutenes
3.2.1. Gold(I)-Catalyzed Cycloisomerization of Enynes
3.2.1.1. Gold(I)-Catalyzed [2 + 2] Cycloaddition of 1,6-Enynes
Echavarren and co-workers found that fused cyclobutenes 654a could be obtained via gold(I)-catalyzed [2 + 2] cycloaddition only when 7-phenyl-1,6-enynes possess terminal or disubstituted alkenes, leading to 654aa and 654ab, respectively (Scheme 142A).211 Remarkably, bicyclo[3.2.0]hept-5-enes of type 654ac was formed from an alkyl-1,6-enyne bearing a trisubstituted alkene.699 However, these were isolated examples, and in most of the cases, the gold(I)-catalyzed [2 + 2] cycloaddition of 1,6-enynes competes with other skeletal rearrangements, which prevent the selective formation of cyclobutenes (Scheme 142B).643,699,700 The ligand employed was found to affect the regioselectivity of these transformations.699
Scheme 142. Gold(I)-Catalyzed Cycloisomerization of 1,6-Enynes.
[(CyJohnPhos)AuCl] (2 mol %), AgSbF6 (2 mol %).
[(Ph3P)Au(NTf2)] (2 mol %).
[(IPr)Au(PhCN)]SbF6 (5 mol %).
[(JonhPhos)Au(MeCN)]SbF6 (5 mol %), CH2Cl2, 80 °C.
Regarding the mechanism for the formation of cyclobutenes, computational studies revealed that the most favorable pathway begins with a 6-endo-dig cyclization to form cyclopropyl gold(I) carbene 657 (Scheme 143A).699 This species evolves via ring expansion toward η2-(cyclobutene)gold(I) intermediate 658. The final cyclobutene product was proposed to be formed through double bond isomerization, although the transition state for this step was not located. Interestingly, η2-(cyclobutene)gold(I) intermediates 658 can also open up to form 1,3-dienes via single-cleavage rearrangement.
Scheme 143. Mechanistic Proposal for the Gold(I)-Catalyzed [2 + 2] Cycloaddition of 7-Aryl-1,6-enynes.
In several consecutive reports, Widenhoefer and co-workers studied in detail the double bond isomerization step in the gold(I)-catalyzed [2 + 2] cycloaddition of 7-aryl-1,6-enynes 656.701−703 In this work, it was found that cyclobutene 662 evolves to the final product by means of a Brønsted acid-catalyzed formal 1,3-hydrogen migration that does not involve the intermediacy of the gold(I) catalyst (Scheme 143B).702 Thus, after deauration, cyclobutene 662 can be rapidly protonated leading to benzylic carbocation 663, which undergoes a rate-determining deprotonation to give rise to the final product. Further studies showed that the counterion, from which the Brønsted acid is generated in situ, can influence the skeletal rearrangement pattern, modifying the final outcome of the reaction.703
Inspired by their mechanistic findings, the group of Widenhoefer developed a protocol in which bicyclo[3.2.0]hept-1-ene scaffolds 667 were trapped by electron-rich arenes, to furnish cyclobutanes of type 666 (Scheme 144).704 Mechanistic investigations suggest that an initial gold(I)-catalyzed [2 + 2] cycloaddition of 7-aryl-1,6-enynes is followed by a silver-catalyzed hydroarylation of cyclobutenes 667 (Scheme 144).701−703
Scheme 144. Gold/Silver-Catalyzed [2 + 2] Cycloaddition/Hydroarylation Sequence of 7-Aryl-1,6-enynes.
Chung and co-workers developed a general synthesis of cyclobutenes 670 from 1,6-enynes bearing a carbonyl group frame (Scheme 145).363 While amide- and ester-tethered 7-aryl-1,6-enynes gave the corresponding cyclobutenes in excellent yield, C-tethered 1,6-enyne led to 1,3-diene 671 (Scheme 145A). The group of Shin found that when the reaction was performed under oxygen, a tricarbonyl compound of type 672 was exclusively obtained via cycloisomerization/oxidation sequence in a formal C–C triple bond oxygenative cleavage outcome705 (Scheme 145B, left).
Scheme 145. Gold(I)-Catalyzed [2 + 2] Cycloaddition of Carbonyl-Tethered 1,6-Enynes.
[(SPhos)AuCl] (5 mol %), AgSbF6 (5 mol %), rt.
[(SPhos)AuCl] (5 mol %), AgSbF6 (5 mol %), CF3CH2OH, rt.
The group of Yeh found that bicyclic lactams of type 675 could be prepared by a tandem gold(I)-catalyzed [2 + 2] cycloaddition/oxidation sequence of 1,6-enynes 673 (Scheme 146). In some specific cases, the highly constrained cyclobutene intermediates 674 could be isolated, and the bicyclic lactams 675 could be obtained by treatment with NMO/OsO4.706
Scheme 146. Gold(I)-Catalyzed [2 + 2] Cycloaddition of 1,6-Enynes and Subsequent Oxidation.
3.2.1.2. Gold(I)-Catalyzed [2 + 2] Cycloaddition of Large 1,n-Enynes (n ≥ 7)
As for 1,6-enynes, the gold(I)-catalyzed cycloisomerization of larger 1,7-enynes can lead to a diverse range of products via divergent mechanistic pathways. Among them, cyclobutene-fused 6-membered carbocycles can be obtained by means of a formal [2 + 2] cycloaddition from very specific substrates with high regioselectivity (Scheme 147).700
Scheme 147. Gold(I)-Catalyzed [2 + 2] Cycloaddition of 1,7-Enynes.
[(CyJohnPhos-OMe)AuCl] (2 mol %), AgSbF6 (2 mol %), CH2Cl2, rt.
[(JohnPhos)Au(NCMe)]SbF6 (2 mol %), CH2Cl2, rt.
80 °C, MW.
Another example of cyclobutene synthesis from 1,7-enynes was reported by the group of Liu.707 Here, cyclobutenes were initially obtained as side products in the gold(I)-catalyzed [2 + 2+2] cycloaddition of O-tethered 1,7-enynes with aldehydes (Scheme 148, left). Performing the reaction in the absence of aldehyde led to the exclusive formation of four-membered carbocycle 680 (Scheme 148, right).
Scheme 148. Gold(I)-Catalyzed [2 + 2] Cycloaddition of 1,7-Enynes.
[(JohnPhos)AuCl] (5 mol %).
Odabachian and Gagosz developed a general [2 + 2] cycloaddition reaction of 1,8-enynes 681 leading to carbocycles 682 bearing a bicyclo[5.2.0]nonane framework (Scheme 149A).708 When these 1,8-enynes were treated with gold(I) catalysts at higher temperatures, products of fragmentation, ene reaction, and isomerization were obtained. Inagaki found that gold(I) complexes with uncommon Z-type ligand also catalyze this transformation (Scheme 149A).709 The regioselective gold(I)-catalyzed [2 + 2] cycloaddition of 1,9-enynes 685 has been enabled by a gold complex bearing hollow-shaped triethynylphosphine ancillary ligand 687 (Scheme 149B).710
Scheme 149. Gold(I)-Catalyzed [2 + 2] Cycloaddition of 1,8- and 1,9-Enynes.
Echavarren and co-workers developed the macrocyclization of larger 1,n-enynes (n > 9) that leads to cyclobutene-fused macrocycles of type 688 (Scheme 150A).711 Besides the essential role played by sterically hindered phosphines on regioselectivity, the use of BArF4– counterion significantly improved the yield.712 Other metals, such as Pt and Ag, did not catalyze the macrocyclization.711 The same group applied this reaction for the construction of the cyclobutane moiety present in the skeleton of rumphellaone A and (+)-hushinone from 1,10-enyne 689 (Scheme 150B).713
Scheme 150. Gold(I)-Catalyzed [2 + 2] Cycloaddition of Large 1,n-Enynes (n ≥ 10).
[(t-BuXPhos)Au(MeCN)]SbF6 (3 mol %), CH2Cl2, 23 °C.
[(t-BuXPhos)Au(MeCN)]BArF4 (3 mol %), CH2Cl2, 23 °C.
[(IPr)Au(PhCN)]BArF4 (3 mol %), CH2Cl2, 25 °C.
3.2.2. Gold(I)-Catalyzed [2 + 2]-Cycloaddition of Alkynes with Alkenes
The group of Echavarren disclosed the first intermolecular gold(I)-catalyzed [2 + 2] cycloaddition of terminal alkynes 691 with alkenes 692 to furnish nonfused cyclobutenes 693 in a regioselective manner (Scheme 151).714 The use of a cationic gold(I) complex that bears a sterically crowded phosphine ligand was essential for this reaction.715 It was later found that the replacement of counterion SbF6– by BAr4F– improved the yield of the cycloaddition (Scheme 151A).712 Regarding the scope, aryl alkynes and electron-rich di- and trisubstituted alkenes were successful reaction partners (Scheme 151A).
Scheme 151. Gold(I)-Catalyzed [2 + 2] Cycloaddition between Alkynes and Alkenes.
Interestingly, under the same reaction conditions, ortho-substituted aryl alkynes led also to a variable mixture of cyclobutenes 693 and 1,3-dienes 694 (Scheme 151B).716 Deuteration experiments confirmed an insertion process of the alkyne moiety between the alkene C atoms in the formation of 1,3-dienes 694.
For the competitive formation of cyclobutenes 701 and 1,3-dienes 704, computational investigations revealed a complex divergent scenario, highly dependent on the alkyne substituents (Scheme 152A).716 Initially, a turnover-limiting alkene–alkyne associative ligand exchange leads to η2-(alkyne)gold(I) species 696. From this intermediate, cyclopropyl gold(I) carbenes 697 and 699, which are in equilibrium through ring opening, can be generated via electrophilic addition to the alkene. In particular, gold(I) carbene 699, generated by anti attack of the alkene, can evolve through cyclopropane ring expansion to furnish 4-membered carbocycles 701. In the case of ortho-substituted aryl alkynes, gold(I) carbene 699 is in equilibrium via carbon migration with carbocationic intermediate 702, which undergoes ring opening to liberate 1,3-dienes 704 in a formal single-cleavage skeletal rearrangement. The divergent evolution pathways of the key cyclopropyl gold(I) carbene 699 have similar activation energies, thus explaining the marked ortho-effect. Kinetic and NMR investigations supported the existence of an off-cycle pathway, in which η2-(alkyne)gold(I) species 696 is in equilibrium, via counteranion-assisted deprotonation, with alkynylgold(I) complexes 705, which can further react with a cationic gold(I) complex to form σ,π-digold(I) alkyne 706 (Scheme 152B).712 The latter was found to be an unproductive resting state of the catalytic cycle and its formation was less favored with BArF4– than with SbF6–.
Scheme 152. Mechanistic Proposal for the Formation of Cyclobutenes and 1,3-Dienes.
Alkenes bearing carbonyl groups react with alkynes to form 8-oxabicyclo[3.2.1]oct-3-enes by a [2 + 2 + 2] cycloaddition process in which two C–C bonds and one C–O bond are formed.717 On the other hand, 1,3-butadiynes (Scheme 153A) and 1,3-enynes (Scheme 153B) react with alkenes to form cyclobutenes selectively.718 With respect to 1,3-dienes, the reaction proceeds selectively at the most electron-rich double bond.
Scheme 153. Gold(I)-Catalyzed [2 + 2] Cycloaddition of 1,3-Diynes, 1,3-Enynes, and 1,n-Dienes (n = 3,5).
[(IPr)Au(PhCN)]BArF4 (5 mol %), CH2Cl2, 23 °C.
[(t-BuXPhos)Au(MeCN)]BArF4 (5 mol %), CH2Cl2, 23 °C.
The asymmetric intermolecular gold(I)-catalyzed [2 + 2] cycloaddition between allenes and alkenes is a versatile method to build highly enantioenriched saturated 4-membered carbocycles (section 3.1.3).690,692,695 This becomes particularly challenging when alkynes719 are used in place of allenes.720,721 This approach has been developed using Co,722 Zn,723 Ni,724 Cu,725−729 Ru,730−733 Rh,734 Pd,735 and Ir736,737 catalysts using either cyclic alkenes or alkenes substituted with electron-withdrawing groups. In this context, Echavarren and co-workers reported the enanatioselective gold(I)-catalyzed synthesis of cyclobutenes by [2 + 2] cycloaddition between alkynes and alkenes using non-C2 symmetric Josiphos chiral ligands (Scheme 154).738 With these ligands, cyclobutenes 709 could be obtained from terminal aryl alkynes and di- or trisubstituted alkenes in good enantioselectivities (Scheme 154A).
Scheme 154. Asymmetric Gold(I)-Catalyzed [2 + 2] Cycloaddition of Alkenes with Alkynes.

C6H5Cl, −20 °C.
(S,RP)-710 (2.5 mol %), NaBAr4F (2.5 mol %).
(R,SP)-711 (5 mol %), NaBAr4F (5 mol %).
Kinetic investigations suggested a complex mechanistic scenario in which the turnover-limiting step switches depending on the electronic properties of the alkene. In addition, DFT calculations revealed that the enantio-discriminating step consisted in the electrophilic addition of the η2-(alkyne)gold(I) species to the alkene. The enantiomeric discrimination arises from unfavorable steric clashes in TSS and stabilizing face-to-face π-stacking interactions in TSR, which decrease the free energy of this transition state (Scheme 154B).
This methodology was applied to a concise, second-generation713 asymmetric synthesis of rumphellaone A (Scheme 155).738 In this new approach, the skeleton of this natural product was obtained via enantioselective intermolecular [2 + 2] cycloaddition between phenylacetylene and trisubstituted alkene 712.
Scheme 155. Enantioselective Total Synthesis of Rumphellaone A.
The groups of Gao and Zhang contributed to the field of intermolecular gold(I)-catalyzed [2 + 2] cycloadditions using chloroalkynes 714 instead of terminal aryl alkynes (Scheme 156).739 This new protocol allowed the use of unactivated mono- and disubstituted alkenes to build chlorocyclobutenes 716 in high yield with excellent levels of regioselectivity. The authors also reported that bromoalkynes can undergo [2 + 2] cycloaddition with cyclopentene (Scheme 156, 717). In contrast, the group of Echavarren found that the gold(I)-catalyzed reaction of bromoalkynes with allylsilanes led to 1,4-enynes by formal cross-coupling via a rearrangement process.740
Scheme 156. Gold(I)-Catalyzed [2 + 2] Cycloaddition between Haloalkynes and Alkenes.
3.2.3. Gold(I)-Catalyzed Cyclopropyl Ring Expansions
The group of Liu reported a novel approach to construct cyclobutenylketones 719 by gold(I)-catalyzed oxidative ring expansion of cyclopropylalkynes 718 (Scheme 157).741 Here, the ketone functionality was introduced by diphenylsulfoxide via regioselective intermolecular oxidation. The protocol was expanded to unactivated cyclopropylalkynes with different substitution patterns. However, under the same conditions, donor–acceptor cyclopropylalkynes undergo oxidative ring cleavage. Mechanistically, the reaction begins with a regioselective O-attack to the β-carbon of the coordinated alkyne, forming cyclopropyl gold(I) carbene 721 (Scheme 157). As proposed for [2 + 2] cycloadditions,699,700,716 this intermediate evolves by means of ring expansion leading to cyclobutene products 719. The same scenario was suggested for cyclopropyl-alkynes embedded into diyne scaffolds.742 Alternatively, the carbene can suffer a second oxidation to furnish a diketone. This side reaction was prevented by careful optimization of the reaction conditions.743
Scheme 157. Gold(I)-Catalyzed Oxidative Ring Expansion.
As depicted in Scheme 119 (section 3.1.1.1), the gold(I)-catalyzed cycloisomerization of cyclopropyl-tethered 1,5-enynes is a versatile transformation.443 The product distribution is controlled by both the reaction conditions and the ortho-effect. In particular, when the reaction took place at −30 °C using ortho-substituted aryl-tethered 1,5-enynes, fused cyclobutenes were selectively obtained via a cyclization/ring expansion sequence (Scheme 119D and 158, right). The construction of fused cyclobutenes was also reported in the context of the oxidative (2 + 2 + 1) cycloaddition of similar cyclopropyl-tethered 1,5-enynes and nitrosobenzenes (Scheme 158, left).744
Scheme 158. Gold(I)-Catalyzed Cycloisomerization of Cyclopropyl 1,5-Enynes.
The group of Shi reported a temperature-controlled divergent gold(I)-catalyzed cycloisomerization of cyclopropylidene 1,7-enynes 725 (Scheme 159A). In contrast, no reactivity was found in the presence of Pt(cod)Cl2.745 Here, 2H-chromene derivatives 726 were obtained at room temperature via intramolecular hydroarylation. At 90 °C, the hydroarylation was followed by a ring expansion leading to the corresponding cyclobutenes 727. Interestingly, scaffolds with the same skeleton and ortho-substituted aryl groups evolve through a different pathway, affording cyclobutanes instead, as depicted in Scheme 122A (section 3.1.1.2).658 On the other hand, 1,2-dihydroquinolines containing cyclobutenes 729 were obtained as the major product when aniline-tethered cyclopropylidene 1,7-enynes 728 were exposed to gold(I) catalysts (Scheme 159B).746 The reaction was proposed to take place through two consecutive cyclopropyl gold(I) carbene intermediates, but only one expands to form the 4-membered carbocycle. In this protocol, different amounts of adduct 730, in whose formation the ring expansion does not take place, were also detected. The product distribution was highly dependent on the aryl substituents.
Scheme 159. Gold(I)-Catalyzed Cycloisomerization of Cyclopropylidene 1,7-Enynes.
Similar alkynylamide-tethered cyclopropylidene 1,7-enynes gave direct access to cyclobutanes and cyclobutenes in a ligand-dependent regiodivergent process, as shown in Scheme 122B (section 3.1.1.2).659 In this case, the mechanistic bifurcation occurs just after the ring expansion step.
3.2.4. Gold(I)-Catalyzed 1,3-Acyloxy Migration/[2 + 2] Cycloaddition of Diynes
The gold(I)-catalyzed 1,3-acyloxy shift of propargylic carboxylates is a widespread approach to generate allenes in situ that can be engaged in further transformations, among which the construction of cyclobutenes by subsequent [2 + 2] cycloaddition will be outlined here.
In this context, the group of Kim disclosed the gold(I)-catalyzed cycloisomerization of 1,7-diyne pivalates 731, which gives access to cyclobutenylketones 732 (Scheme 160). The reaction did not proceed under Pt catalysis.747 The authors proposed that the propargylic pivalate fragment undergoes 1,3-acyloxy shift followed by a [2 + 2] cycloaddition between the alkyne and the in situ formed allene. The obtained products were isolated upon hydrolysis. Under the same conditions, when two 1,7-diyne pivalate scaffolds were linked by a phenyl moiety, dimeric bicycles of type 732c were obtained by a 2-fold 1,3-acyloxy shift/[2 + 2] cycloaddition sequence. The same group broadened the scope of dimeric bicycles by using 1,7-diyne pivalates linked by a variety of other aryl rings.748
Scheme 160. Gold(I)-Catalyzed Cycloisomerization of 1,7-Diyne Pivalates.
The group of Chan developed a gold(I)-catalyzed cycloisomerization of N-tethered 1,7-diyne benzoates 733. Here, substrates bearing terminal alkynes afforded N-heterocycle-containing cyclobutenes 734 in a regio- and diastereoselective manner (Scheme 161A).749 In addition, the chiral information was effectively transferred from the starting diynes to the cyclobutene products. In this protocol, internal alkynes were essential to promote the 1,3-acyloxy migration that generates the corrresponding allenyne intermediates (Scheme 161C). These species undergo a stepwise [2 + 2] cycloaddition via 6-exo-dig cyclization, followed by subsequent Prins cyclization to liberate the cyclobutene product.
Scheme 161. Gold(I)-Catalyzed Cycloisomerization of 1,n-Diyne Benzoates.
As part of their investigations on the reactivity of diyne carboxylates, the group of Chan also developed a method for the 1,3-acyloxy migration/[2 + 2] cycloaddition sequence of 1,6-diyne carboxylates 735 (Scheme 161B).750 Accordingly, under gold(I) catalysis, 1,6-diynes bearing a vinyl substituent led to cyclopentene-fused cyclobutenes 736 preferentially. In this method, variable amounts of diketones 737 were also detected. For cyclobutenes, the authors proposed a similar mechanistic scenario as for 1,7-diynes,750 in which the tandem [3,3]-rearrangement/5-exo-dig/Prins-type cyclization sequence was the most plausible pathway (Scheme 161C).
The group of Liu reported the gold(I)-catalyzed cycloisomerization of 1,7-diyne biscarboxylates 738 that gave access to naphthocyclobutenes 739 with cis-stereoselectivity (Scheme 162).751 The authors proposed that bis(allenyl carbonates) 740 were generated via double 1,3-acyloxy shift of the propargyl carbonates.
Scheme 162. Gold(I)-Catalyzed Cycloisomerization of Diynes.
[(Ph3P)Au(N-methyl benzotriazole)]OTf (1 mol %), CH2Cl2, rt.
AgOTf (2 mol %).
Sahoo and co-workers designed stable alkyne-tethered ketene N,N-acetals 741, which, under gold(I) catalysis, underwent 1,3-acyloxy migration to form allenes 745 in situ (Scheme 163).752 Cyclobutene fused azepine heterocycles 742 were obtained selectively from symmetric ketene N,N-acetals 741 (Scheme 163A). Based on NMR studies, the authors proposed that the terminal carbon of the enamine attacks the gold(I)-activated alkyne via 6-endo-dig cyclization to form 6-membered iminium-gold-vinyl species 744. (Scheme 163B). The 6-membered ring intermediate rearranges leading to an allene functionality that further engages in a formal [2 + 2] cycloaddition with the second activated alkyne.
Scheme 163. Gold(I)-Catalyzed Cycloisomerization of Alkyne-Ketene N,N-Acetals.
3.2.5. Dual Gold(I)-Catalyzed Synthesis of Cyclobutenes
Substrates bearing a terminal alkyne can occasionally experience dual activation gold catalysis through the formation of gold acetylides.753−755 Although this field has been studied extensively in the last decade, only few particular substrates have led to cyclobutene products. This is the case for 1,6-allenyne 746, which was selectively converted into bicyclo[4.2.0]octadiene 747 using complex 752 via dual gold(I)-catalyzed [2 + 2] cycloaddition (Scheme 164A).756 For this transformation, the intermediacy of digold species 748 was supported both by deuteration experiments and computational studies. In addition, the catalyst used was previously reported to foster this activation mode.757,758
Scheme 164. Dual Gold(I)-Catalyzed Synthesis of Cyclobutenes.
In another example, cyclobutenes 750 were obtained by means of dual gold(I)-catalyzed cyclization of thiophene-tethered diynes 749 (Scheme 164 B).759 However, this reaction could be applied only to two substrates, and with very low efficiency. Other similar diynes evolved through alternative pathways forming norbornene or fulvene derivatives. Mechanistically, a 6-endo-dig cyclization was proposed to lead to intermediate 751, which subsequently undergoes an intramolecular C(sp3)–H insertion to furnish cyclobutene products 750.
3.3. Synthesis of Cyclobutanones
3.3.1. Ring Expansion of Cyclopropanols
The best-explored strategy for the synthesis of cyclobutanones using gold(I) catalysis is the ring expansion of cyclopropanols. This area was pioneered by the group of Toste, who disclosed a gold(I)-catalyzed ring expansion of 1,1-alkynylcyclopropanols 754 (Scheme 165).760 The reaction proceeds by selective migration of the more substituted carbon of the cyclopropane giving alkylidene cyclobutanones 755 in excellent yields and perfect stereoselectivity. Theoretical mechanistic studies on this ring-expansion process were performed afterward by a different group, and the results were in agreement with the experimental observations.761 It is worth highlighting that this reaction also proceeds satisfactorily when using silyl-protected cyclopropanols. Allyl ethers derived from cyclopropanol react by the intramolecular transfer of the allyl through a [3,3]-sigmatropic rearrangement followed by a [1,2]-allyl shift.762
Scheme 165. Ring Expansion of 1,1-Alkynylcyclopropanols.
Hashmi and co-workers reported that the treatment of a 1,1-alkynylcyclopropanol with pyridine N-oxide in the presence of a gold(I) catalyst leads to the formation of an α-ketocyclobutanone.763 On the other hand, Trost and co-workers explored the use of ruthenium catalysts to promote similar processes.764
Kleinbeck and Toste reported an asymmetric variant of this transformation using 1,1-allenylcyclopropanols to furnish 2-allyl-cyclobutanones 757 in high yields and enantioselectivities (Scheme 166).765
Scheme 166. Enantioselective Ring Expansion of 1,1-Allenylcyclopropanols.
The same group combined these last two approaches with photoredox catalysis to develop a gold-catalyzed arylative ring expansion.766 This method relies on the use of aryl diazonium salts as a source of aryl radicals under photoredox conditions, which leads to the generation of aryl-gold(III) intermediates (Scheme 167). The latter promote the ring expansion of cyclopropanols 759 or 760, giving rise to alkyl-aryl-gold(III) species that afford, after reductive elimination, benzyl- (761) or vinyl-cyclobutanones (762) in moderate to good yields. This arylative ring expansion was also used to transform cyclobutanols into cyclopentanones.
Scheme 167. Photoredox and Gold-Catalyzed Arylative Ring Expansion.
When studying 1,1-alkenylcycloalkanols, Chen and co-workers observed a divergent reactivity under gold(I) and zinc(II) catalysis.767 When treating 768 with [(Ph3P)AuCl]/AgSbF6, alkenyl cyclobutanone 769 was obtained (Scheme 168). Larger cycloalkanols also undergo this gold(I)-catalyzed ring-expansion process. In contrast, ZnBr2 catalyzes the transformation of substrates 768 into 2,5-dihydrofurans.
Scheme 168. Ring Expansion of a 1,1-Alkenylcyclopropanol.
Gandon, Marinetti, Voituriez, and co-workers reported an enantioselective gold(I)-catalyzed rearrangement of cyclopropyl-substituted 1,6-enynes 770 into 2-oxocyclobutylcyclopentanes 771 (Scheme 169),768 reminiscent of that previously developed by the group of Echavarren for the synthesis of tricyclic systems (see section 3.1.1.1.).649,650 This cycloisomerization proceeds in high yields, moderate to good syn/anti selectivity, and very high enantioselectivity for both isomers. This method was applied for the construction of the tricyclic skeleton of natural product russujaponol D.
Scheme 169. Asymmetric Cycloisomerization of Cyclopropyl-Substituted 1,6-Enynes.
Vinilydene cyclopropanes are precursors of alkylidene cyclobutanones.769 Thus, reaction of 773 with 2 equiv of a pyridine N-oxide in the presence of [(Ph3P)AuCl]/AgSbF6 afforded cyclobutanones 775 by stepwise ring expansion and oxidative trapping of the corresponding gold(I) carbene 774 (Scheme 170).770 Cyclobutanones were also found as side products in a gold(I)-catalyzed oxidative rearrangement of homopropargylic ethers with pyridine N-oxides to give α,β-unsaturated carbonyl compounds.771
Scheme 170. Oxidative Ring-Expansion of Vinylidene Cyclopropanes.
[Au] = [(Ph3P)AuCl] (5 mol %)/AgSbF6 (10 mol %).
Py*–O = 3,5-dibromopyridine N-oxide.
Shi and co-workers developed an intramolecular approach, in which a tethered methoxy group acts as oxygen transfer agent to the vinylidene cyclopropane. This concept was used to develop a catalyst-controlled stereodivergent synthesis of alkylidene cyclobutanones: [(IPr)AuCl] gave exclusively E-777, whereas [(BrettPhos)AuCl] gave diastereoisomer Z-777 (Scheme 171).772
Scheme 171. Stereodivergent Synthesis of Alkylidene Cyclobutanones.
3.3.2. Synthesis of Cyclobutanones by Other Methods
Cossy and co-workers reported that the gold-catalyzed cycloisomerizations of 1,6-ene-ynamides 778 possessing a terminal or trimethylsilyl-substituted alkyne provided cyclobutanones 779 in moderate to good yields (Scheme 172).419,420 A mechanism involving gold-catalyzed cycloisomerization to cyclobutenes 780 followed by hydrolysis during the workup was proposed. The high diastereoselectivity observed with substrates bearing a stereocenter at the position α or β to the N atom (779b–d) was rationalized with chair-like transition states for the formation of the cyclopropyl gold carbene intemediates, in analogy with cationic cylizations of N-acyliminium ions.
Scheme 172. AuCl-Catalyzed Cycloisomerization of 1,6-Ene-ynamides.
Li and co-workers disclosed that homopropargylic ethers 781 bearing electron-rich aromatic or alkenyl R1 groups afforded cis-cyclobutanones 782 in the presence of a gold(I) catalyst and 8-ethylquinoline N-oxide (Scheme 173).773,774 In the proposed mechanism, α-oxo gold carbene 783 is trapped by the tethered ether group to afford oxonium ylide 784, which rearranges in a concerted process to cis product 782. Under acidic conditions, the latter converts into the trans isomer.
Scheme 173. Au(I)-Catalyzed Oxidative Rearrangement of Homopropargylic Ethers to cis-Cyclobutanones.
The group of Zhang developed methods for the selective formation of cyclobutanones 786 and cyclopentanones 787 depending on the ligand and substrate structures through the intramolecular insertion into C(sp3)–H bonds by oxidatively generated β-diketone-α-gold carbenes 788 (Scheme 174).775,776
Scheme 174. Au(I)-Catalyzed Oxidative Synthesis of Cyclobutanones.
3.4. Synthesis of Heteroatom-Containing 4-Membered Rings
Zhang and co-workers devised the synthesis of O- and N-containing 4-membered rings based on the propensity of α-oxo gold carbenes, generated from alkynes by Au(I)-catalyzed intermolecular oxidation (see section 2.6),537 to effect intramolecular insertion into N–H and O–H bonds. A practical synthesis of oxetan-3-ones777790 and 792 was developed from readily available propargylic alcohols,778 and carried out under air using pyridine N-oxides as oxidants and catalytic amounts of gold(I) complexes (Scheme 175).779 With tertiary propargylic alcohol substrates 791, an electron-withdrawing group had to be installed at the alkyne terminus to avoid side reactions, arising presumably from the formation of propargylic cations under acidic conditions.
Scheme 175. Au(I)-Catalyzed Oxidative Synthesis of Oxetan-3-ones.
This method was employed for the synthesis of a drug discovery library of 419 spirocyclic oxetane–piperidine scaffolds (Scheme 176),780 which are relevant motifs in medicinal chemistry.781,782
Scheme 176. Large-Scale Au(I)-Catalyzed Synthesis of an Oxetane–Piperidine Building Block.
Zhang and co-workers applied the same strategy to prepare azetidine-3-ones 799 from N-tert-butylsulfonyl propargylamines (Scheme 177).783 The use of a bulky Buchwald ligand and of an electron-poor, hindered pyridine N-oxide obviated the need for acidic additives with secondary propargyl amine substrates, improving functional group compatibility (products 799a, 799b). By carefully tuning the reaction conditions, azetidin-3-ones with different substitution patterns and protecting groups could be obtained. It was later shown that several other sulfonyl-protecting groups were tolerated in this reaction.784 Notably, the tert-butanesulfinamide group allowed preparation of enantioenriched products by employing the chemistry of Ellman.785
Scheme 177. Au(I)-Catalyzed Oxidative Synthesis of Azetidin-3-ones.
Although the formation of oxetan-3-ones and thietane-3-ones under oxidative gold catalysis has been sporadically reported,786,787 the only gold-catalyzed preparative methods for the synthesis of heteroatom-containing 4-membered rings remain the two protocols developed by the group of Zhang.
4. Outlook and Final Remarks
In less than two decades, gold(I) catalysis has emerged as one of the most powerful tools to build molecular complexity in a single reaction step. Many of the transformations that take place through gold(I) π-bond activation proceed via gold(I) carbene intermediates, which can often evolve, inter- or intramolecularly, to assemble three- or four-membered carbocycles. This strategy has been widely applied to develop a plethora of methodologies that allow the assembly of small rings efficiently and selectively. One of the main general challenges of this field is developing versatile methods that do not rely on the use of excessively complex or specific starting materials, which may introduce potentially undesired functional groups in the reaction products. In many cases, due to the complex mechanistic scenarios of these reactions, simple variations on the substitution pattern of the substrates or on the reaction conditions lead to completely different outcomes. Furthermore, even though the field of asymmetric gold(I) catalysis has experienced significant growth over the past ten years, the development of enantioselective versions of certain transformations remains an important challenge. In particular, gold(I)-catalyzed intermolecular reactions that afford enantioenriched three- or four-membered rings are still scarce and often highly dependent on the nature of the substrates employed.
Acknowledgments
We thank the Agencia Estatal de Investigación (AEI)/FEDER, UE (CTQ2016-75960-P), Severo Ochoa Excellence Accreditation 2014–2018 (SEV-2013-0319 and FPI predoctoral fellowship to M.M.), the European Research Council (Advanced Grant No. 835080), the European Union (Horizon 2020 Marie Skłodowska-Curie COFUND postdoctoral fellowship to A.F., grant agreement No. 754510), the AGAUR (2017 SGR 1257), and CERCA Program/Generalitat de Catalunya for financial support.
Biographies
Mauro Mato completed his B.Sc. in Chemistry at the University of A Coruña (Spain) in 2016 and his M.Sc. in Synthesis and Catalysis at the Rovira i Virgili University (Tarragona, Spain) in 2017, receiving for both degrees the Extraordinary Award. Then he started his Ph.D. studies under the supervision of Prof. Antonio M. Echavarren at the Institute of Chemical Research of Catalonia (ICIQ), where he works on the generation and reactivity of metal carbenes. During this period, he carried out a short project at Scripps Research (San Diego, CA) under the supervision of Prof. Phil S. Baran and received a RSEQ–Lilly Award for Ph.D. students in 2020.
Allegra Franchino studied for her B.Sc. and M.Sc. degrees in Chemistry at the University of Milan (2008–2013), including an Erasmus semester at RWTH Aachen and research work in the group of Prof. Cesare Gennari. She continued her training in homogeneous asymmetric catalysis, particularly using transition metals, during her Marie Skłodowska-Curie Ph.D. program at the University of Oxford (2013–2017, Prof. Darren Dixon group). After a brief stint in an international API chemical company, in September 2018 she joined the laboratories of Prof. Antonio M. Echavarren at ICIQ as a Marie Skłodowska-Curie COFUND postdoctoral fellow.
Cristina García-Morales received her B.Sc. in chemistry from Universidad de Huelva (Spain), including an Erasmus year at the University of Strathclyde (U.K.), and her M.Sc. from Universitat Rovira i Virgili (Spain). She earned her Ph.D. in 2019 under the guidance of Prof. Antonio M. Echavarren and joined the group shortly after for a postdoctoral stint. During her doctoral and postdoctoral studies, she worked on enantioselective gold(I) catalysis and the characterization of highly reactive catalytic intermediates. Alongside experimental efforts, she gained expertise in DFT analyses, which she employed in deciphering Au- and Rh-catalyzed transformations.
Antonio M. Echavarren got his Ph.D. at the Universidad Autónoma de Madrid (UAM, 1982). After postdoctoral stays at Boston College and Colorado State University, he joined the Institute of Organic Chemistry (CSIC) in Madrid. In 1992, he moved to the UAM as a Professor, and in 2004, he was appointed Group Leader at the Institute of Chemical Research of Catalonia (ICIQ) in Tarragona. In 2012, he got an ERC Adv grant to develop gold catalysis and, in 2019, another ERC Adv grant on biomimetic catalysis. Prof. Echavarren is a member of the Advisory Boards of Organic & Biomolecular Chemistry, Chemical Society Reviews, Advanced Synthesis and Catalysis, and Chemical Communications, a member of the Editorial Board of Chemistry—European Journal, and a Fellow of the Royal Society of Chemistry. He received the 2004 Janssen-Cylag Award in Organic Chemistry and the 2010 Gold Medal of the Spanish Royal Society of Chemistry and, in 2015, the Arthur C. Cope Scholar Award from the ACS. Since January 2018, he has been the President of the Spanish Royal Society of Chemistry.
Author Contributions
§ M.M., A.F., and C.G.-M. contributed equally.
The authors declare no competing financial interest.
References
- Freund A. Ueber Trimethylen. J. Prakt. Chem. 1882, 26, 367–377. 10.1002/prac.18820260125. [DOI] [Google Scholar]
- Willstätter R.; Bruce J. Zur Kenntnis der Cyclobutanreihe. Ber. Dtsch. Chem. Ges. 1907, 40, 3979–3999. 10.1002/cber.19070400407. [DOI] [Google Scholar]
- Strained Organic Molecules; Greenberg A.; Liebman J. F., Eds.; Academic Press: New York, 1978. [Google Scholar]
- de Meijere A.; Kozhushkov S. I. The Chemistry of Highly Strained Oligospirocyclopropane Systems. Chem. Rev. 2000, 100, 93–142. 10.1021/cr960153y. [DOI] [PubMed] [Google Scholar]
- Faust R. Fascinating Natural and Artificial Cyclopropane Architectures. Angew. Chem., Int. Ed. 2001, 40, 2251–2253. . [DOI] [PubMed] [Google Scholar]
- Chen Z.; Mercer J. A. M.; Zhu X.; Romaniuk J. A. H.; Pfattner R.; Cegelski L.; Martinez T. J.; Burns N. Z.; Xia Y. Mechanochemical Unzipping of Insulating Polyladderene to Semiconducting Polyacetylene. Science 2017, 357, 475–479. 10.1126/science.aan2797. [DOI] [PubMed] [Google Scholar]
- Pauling L. The Nature of the Chemical Bond. J. Am. Chem. Soc. 1931, 53, 1367–1400. 10.1021/ja01355a027. [DOI] [Google Scholar]
- de Meijere A. Bonding Properties of Cyclopropane and Their Chemical Consequences. Angew. Chem., Int. Ed. Engl. 1979, 18, 809–826. 10.1002/anie.197908093. [DOI] [Google Scholar]
- For a computational study and comparison, see:; Khoury P. R.; Goddard J. D.; Tam W. Ring Strain Energies: Substituted Rings, Norbornanes, Norbornenes and Norbornadienes. Tetrahedron 2004, 60, 8103–8112. 10.1016/j.tet.2004.06.100. [DOI] [Google Scholar]
- For previous experimental and computational studies, see:; Satchell D. P. N.; Satchell R. S. Quantitative Aspects of the Lewis Acidity of Covalent Metal Halides and Their Organo Derivatives. Chem. Rev. 1969, 69, 251–278. 10.1021/cr60259a001. [DOI] [Google Scholar]
- Schleyer P. v. R.; Williams J. E.; Blanchard K. R. The Evaluation of Strain in Hydrocarbons. The Strain in Adamantane and Its Origin. J. Am. Chem. Soc. 1970, 92, 2377–2386. 10.1021/ja00711a030. [DOI] [Google Scholar]
- Wiberg K. The Concept of Strain in Organic Chemistry. Angew. Chem., Int. Ed. Engl. 1986, 25, 312–322. 10.1002/anie.198603121. [DOI] [Google Scholar]
- Bach R. D.; Dmitrenko O. The Effect of Substituents on the Strain Energies of Small Ring Compounds. J. Org. Chem. 2002, 67, 2588–2599. 10.1021/jo016241m. [DOI] [PubMed] [Google Scholar]
- Exner K.; Schleyer P. v. R. Theoretical Bond Energies: A Critical Evaluation. J. Phys. Chem. A 2001, 105, 3407–3416. 10.1021/jp004193o. [DOI] [Google Scholar]
- Bach R. D.; Dmitrenko O. Strain Energy of Small Ring Hydrocarbons. Influence of C−H Bond Dissociation Energies. J. Am. Chem. Soc. 2004, 126, 4444–4452. 10.1021/ja036309a. [DOI] [PubMed] [Google Scholar]
- Beesley R. M.; Ingold C. K.; Thorpe J. F. The Formation and Stability of spiro-Compounds. Part I. spiro-Compounds from cyclo-Hexane. J. Chem. Soc., Trans. 1915, 107, 1080–1106. 10.1039/CT9150701080. [DOI] [Google Scholar]
- Ringer A. L.; Magers D. H. Conventional Strain Energy in Dimethyl-Substituted Cyclobutane and the gem-Dimethyl Effect. J. Org. Chem. 2007, 72, 2533–2537. 10.1021/jo0624647. [DOI] [PubMed] [Google Scholar]
- Lebel H.; Marcoux J.-F.; Molinaro C.; Charette A. B. Stereoselective Cyclopropanation Reactions. Chem. Rev. 2003, 103, 977–1050. 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- Roy M.-N.; Lindsay V. N. G.; Charette A. B.. Stereoselective Synthesis: Reactions of Carbon-Carbon Double Bonds (Science of Synthesis); de Vries J. G., Ed.; Thieme: Stuttgart, 2011; Vol 1, pp 731–817. [Google Scholar]
- Talele T. T. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem. 2016, 59, 8712–8756. 10.1021/acs.jmedchem.6b00472. [DOI] [PubMed] [Google Scholar]
- Taylor R. D.; MacCoss M.; Lawson A. D. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- Wishart D. S.; Knox C.; Guo A. C.; Shrivastava S.; Hassanali M.; Stothard P.; Chang Z.; Woolsey J. DrugBank: A Comprehensive Resource for in Silico Drug Discovery and Exploration. Nucleic Acids Res. 2006, 34, D668–D672. 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A. 2019 FDA Drug Approvals. Nat. Rev. Drug Discovery 2020, 19, 79–84. 10.1038/d41573-020-00001-7. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration . FDA-Approved Drugs. Retrieved from: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed February 26, 2020).
- Donaldson W. A. Synthesis of Cyclopropane Containing Natural Products. Tetrahedron 2001, 57, 8589–8627. 10.1016/S0040-4020(01)00777-3. [DOI] [Google Scholar]
- Chen D. Y.-K.; Pouwer R. H.; Richard J.-A. Recent Advances in the Total Synthesis of Cyclopropane-Containing Natural Products. Chem. Soc. Rev. 2012, 41, 4631–4642. 10.1039/c2cs35067j. [DOI] [PubMed] [Google Scholar]
- Fan Y.-Y.; Gao X.-H.; Yue J.-M. Attractive Natural Products with Strained Cyclopropane and/or Cyclobutane Ring Systems. Sci. China: Chem. 2016, 59, 1126–1141. 10.1007/s11426-016-0233-1. [DOI] [Google Scholar]
- Collado I. G.; Hanson J. R.; Macías-Sánchez A. J. Recent Advances in the Chemistry of Caryophyllene. Nat. Prod. Rep. 1998, 15, 187–204. 10.1039/a815187y. [DOI] [Google Scholar]
- Dembitsky V. M. Bioactive Cyclobutane-Containing Alkaloids. J. Nat. Med. 2007, 62, 1–33. 10.1007/s11418-007-0166-3. [DOI] [PubMed] [Google Scholar]
- Dembitsky V. M. Naturally Occurring Bioactive Cyclobutane-Containing (CBC) Alkaloids in Fungi, Fungal Endophytes, and Plants. Phytomedicine 2014, 21, 1559–1581. 10.1016/j.phymed.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Li N.; Yuan Y.; Lu H.; Wu X.; Zhou C.; He M.; Su H.; Zhang M.; Wang M.-W.; et al. Cyclobutane Derivatives As Novel Nonpeptidic Small Molecule Agonists of Glucagon-Like Peptide-1 Receptor. J. Med. Chem. 2012, 55, 250–267. 10.1021/jm201150j. [DOI] [PubMed] [Google Scholar]
- Grongsaard P.; Bulger P. G.; Wallace D. J.; Tan L.; Chen Q.; Dolman S. J.; Nyrop J.; Hoerrner R. S.; Shultz C. S.; et al. Convergent, Kilogram Scale Synthesis of an Akt Kinase Inhibitor. Org. Process Res. Dev. 2012, 16, 1069–1081. 10.1021/op300031r. [DOI] [Google Scholar]
- Lukin K.; Kishore V.; Gordon T. Development of a Scalable Synthesis of Oxadiazole Based S1P1 Receptor Agonists. Org. Process Res. Dev. 2013, 17, 666–671. 10.1021/op300345v. [DOI] [Google Scholar]
- Liu H.-W.; Walsh C. T.. The Chemistry of the Cyclopropyl Group; Rappoport Z., Ed.; John Wiley & Sons Ltd.: New York, 1987; p 959. [Google Scholar]
- Wessjohann L. A.; Brandt W.; Thiemann T. Biosynthesis and Metabolism of Cyclopropane Rings in Natural Compounds. Chem. Rev. 2003, 103, 1625–1648. 10.1021/cr0100188. [DOI] [PubMed] [Google Scholar]
- Thibodeaux C. J.; Chang W.-c.; Liu H.-w. Enzymatic Chemistry of Cyclopropane, Epoxide, and Aziridine Biosynthesis. Chem. Rev. 2012, 112, 1681–1709. 10.1021/cr200073d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. N. C.; Hon M.-Y.; Tse C. W.; Yip Y.-C.; et al. Use of Cyclopropanes and Their Derivatives in Organic Synthesis. Chem. Rev. 1989, 89, 165–198. 10.1021/cr00091a005. [DOI] [Google Scholar]
- Reissig H.-U.; Zimmer R. Donor-Acceptor-Substituted Cyclopropane Derivatives and Their Application in Organic Synthesis. Chem. Rev. 2003, 103, 1151–1196. 10.1021/cr010016n. [DOI] [PubMed] [Google Scholar]
- Gnad F.; Reiser O. Synthesis and Applications of β-Aminocarboxylic Acids Containing a Cyclopropane Ring. Chem. Rev. 2003, 103, 1603–1624. 10.1021/cr010015v. [DOI] [PubMed] [Google Scholar]
- Rubin M.; Rubina M.; Gevorgyan V. Transition Metal Chemistry of Cyclopropenes and Cyclopropanes. Chem. Rev. 2007, 107, 3117–3179. 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]
- Lu B.-L.; Dai L.; Shi M. Strained Small Rings in Gold-Catalyzed Rapid Chemical Transformations. Chem. Soc. Rev. 2012, 41, 3318–3339. 10.1039/C2CS15295A. [DOI] [PubMed] [Google Scholar]
- Schneider T. F.; Kaschel J.; Werz D. B. A New Golden Age for Donor-Acceptor Cyclopropanes. Angew. Chem., Int. Ed. 2014, 53, 5504–5523. 10.1002/anie.201309886. [DOI] [PubMed] [Google Scholar]
- Crimmins M. T. Synthetic Applications of Intramolecular Enone-Olefin Photocycloadditions. Chem. Rev. 1988, 88, 1453–1473. 10.1021/cr00090a002. [DOI] [Google Scholar]
- Lee-Ruff E.; Mladenova G. Enantiomerically Pure Cyclobutane Derivatives and Their Use in Organic Synthesis. Chem. Rev. 2003, 103, 1449–1484. 10.1021/cr010013a. [DOI] [PubMed] [Google Scholar]
- Namyslo J. C.; Kaufmann D. E. The Application of Cyclobutane Derivatives in Organic Synthesis. Chem. Rev. 2003, 103, 1485–1538. 10.1021/cr010010y. [DOI] [PubMed] [Google Scholar]
- Seiser T.; Saget T.; Tran D. N.; Cramer N. Cyclobutanes in Catalysis. Angew. Chem., Int. Ed. 2011, 50, 7740–7752. 10.1002/anie.201101053. [DOI] [PubMed] [Google Scholar]
- Iriondo-Alberdi J.; Greaney M. F. Photocycloaddition in Natural Product Synthesis. Eur. J. Org. Chem. 2007, 2007, 4801–4815. 10.1002/ejoc.200700239. [DOI] [Google Scholar]
- Carson C. A.; Kerr M. A. Heterocycles from Cyclopropanes: Applications in Natural Product Synthesis. Chem. Soc. Rev. 2009, 38, 3051–3060. 10.1039/b901245c. [DOI] [PubMed] [Google Scholar]
- Bach T.; Hehn J. P. Photochemical Reactions as Key Steps in Natural Product Synthesis. Angew. Chem., Int. Ed. 2011, 50, 1000–1045. 10.1002/anie.201002845. [DOI] [PubMed] [Google Scholar]
- Ebner C.; Carreira E. M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679. 10.1021/acs.chemrev.6b00798. [DOI] [PubMed] [Google Scholar]
- For the original report, see:; Simmons H. E.; Smith R. D. A New Synthesis of Cyclopropanes from Olefins. J. Am. Chem. Soc. 1958, 80, 5323–5324. 10.1021/ja01552a080. [DOI] [Google Scholar]
- Simmons H. E.; Cairns T. L.; Vladuchick S. A.; Hoiness C. M. Cyclopropanes from Unsaturated Compounds, Methylene Iodide, and Zinc-Copper Couple. Org. React. 2011, 20, 1–131. 10.1002/0471264180.or020.01. [DOI] [Google Scholar]
- Charette A. B.; Beauchemin A. Simmons-Smith Cyclopropanation Reaction. Organic Reactions 2001, 1–415. 10.1002/0471264180.or058.01. [DOI] [Google Scholar]
- Charette A. B.; Cote B.; Marcoux J. F. Carbohydrates as Chiral Auxiliaries: Asymmetric Cyclopropanation Reaction of Acyclic Olefins. J. Am. Chem. Soc. 1991, 113, 8166–8167. 10.1021/ja00021a052. [DOI] [Google Scholar]
- Charette A. B.; Juteau H.; Lebel H.; Molinaro C. Enantioselective Cyclopropanation of Allylic Alcohols with Dioxaborolane Ligands: Scope and Synthetic Applications. J. Am. Chem. Soc. 1998, 120, 11943–11952. 10.1021/ja982055v. [DOI] [Google Scholar]
- Shitama H.; Katsuki T. Asymmetric Simmons-Smith Reaction of Allylic Alcohols with Al Lewis Acid/N Lewis Base Bifunctional Al(Salalen) Catalyst. Angew. Chem., Int. Ed. 2008, 47, 2450–2453. 10.1002/anie.200705641. [DOI] [PubMed] [Google Scholar]
- Johnson A. W.; LaCount R. B. The Chemistry of Ylids. VI. Dimethylsulfonium Fluorenylide—A Synthesis of Epoxides. J. Am. Chem. Soc. 1961, 83, 417–423. 10.1021/ja01463a040. [DOI] [Google Scholar]
- Corey E. J.; Chaykovsky M. Dimethyloxosulfonium Methylide ((CH3)2SOCH2) and Dimethylsulfonium Methylide ((CH3)2SCH2). Formation and Application to Organic Synthesis. J. Am. Chem. Soc. 1965, 87, 1353–1364. 10.1021/ja01084a034. [DOI] [Google Scholar]
- Johnson C. R.; Schroeck C. W. Chemistry of Sulfoxides and Related Compounds. XV. Synthesis of Optically Active Cyclopropanes and Oxiranes Using an Optically Active Oxosulfonium Methylide. J. Am. Chem. Soc. 1968, 90, 6852–6854. 10.1021/ja01026a056. [DOI] [Google Scholar]
- Li A.-H.; Dai L.-X.; Aggarwal V. K. Asymmetric Ylide Reactions: Epoxidation, Cyclopropanation, Aziridination, Olefination, and Rearrangement. Chem. Rev. 1997, 97, 2341–2372. 10.1021/cr960411r. [DOI] [PubMed] [Google Scholar]
- Doyle M. P.; McKervey M. A.; Ye T.. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds; John Wiley & Sons, New York, 1998. [Google Scholar]
- Davies H. M. L.; Antoulinakis E. G. Intermolecular Metal-Catalyzed Carbenoid Cyclopropanations. Organic Reactions 2001, 1–326. 10.1002/0471264180.or057.01. [DOI] [Google Scholar]
- Davies H. M. L.; Beckwith R. E. J. Catalytic Enantioselective C−H Activation by Means of Metal−Carbenoid-Induced C−H Insertion. Chem. Rev. 2003, 103, 2861–2904. 10.1021/cr0200217. [DOI] [PubMed] [Google Scholar]
- Aggarwal V. K.; Alonso E.; Fang G.; Ferrara M.; Hynd G.; Porcelloni M. Catalytic Asymmetric Synthesis of Epoxides from Aldehydes Using Sulfur Ylides with In Situ Generation of Diazocompounds. Angew. Chem., Int. Ed. 2001, 40, 1433–1436. . [DOI] [PubMed] [Google Scholar]
- Liao W.-W.; Li K.; Tang Y. Controllable Diastereoselective Cyclopropanation. Enantioselective Synthesis of Vinylcyclopropanes via Chiral Telluronium Ylides. J. Am. Chem. Soc. 2003, 125, 13030–13031. 10.1021/ja036254c. [DOI] [PubMed] [Google Scholar]
- Papageorgiou C. D.; Cubillo de Dios M. A.; Ley S. V.; Gaunt M. J. Enantioselective Organocatalytic Cyclopropanation via Ammonium Ylides. Angew. Chem., Int. Ed. 2004, 43, 4641–4644. 10.1002/anie.200460234. [DOI] [PubMed] [Google Scholar]
- Kunz R. K.; MacMillan D. W. C. Enantioselective Organocatalytic Cyclopropanations. The Identification of a New Class of Iminium Catalyst Based upon Directed Electrostatic Activation. J. Am. Chem. Soc. 2005, 127, 3240–3241. 10.1021/ja042774b. [DOI] [PubMed] [Google Scholar]
- Fedoryński M. Syntheses of gem-Dihalocyclopropanes and Their Use in Organic Synthesis. Chem. Rev. 2003, 103, 1099–1132. 10.1021/cr0100087. [DOI] [PubMed] [Google Scholar]
- Fleming I.; Urch C. J. Stereospecific Cyclopropane Synthesis from γ-Stannyl Alcohols. Tetrahedron Lett. 1983, 24, 4591–4594. 10.1016/S0040-4039(00)85964-X. [DOI] [Google Scholar]
- den Hartog T.; Rudolph A.; Maciá B.; Minnaard A. J.; Feringa B. L. Copper-Catalyzed Enantioselective Synthesis of trans-1-Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Addition−Intramolecular Enolate Trapping. J. Am. Chem. Soc. 2010, 132, 14349–14351. 10.1021/ja105704m. [DOI] [PubMed] [Google Scholar]
- Conia J. M.; Salaun J. R. Cyclobutane Ring Contractions not Involving Carbonium Ions. Acc. Chem. Res. 1972, 5, 33–40. 10.1021/ar50049a005. [DOI] [Google Scholar]
- Ross A. G.; Townsend S. D.; Danishefsky S. J. Halocycloalkenones as Diels−Alder Dienophiles. Applications to Generating Useful Structural Patterns. J. Org. Chem. 2013, 78, 204–210. 10.1021/jo302230m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A. N.; Schüppel F.; Eisold M.; Kreppel A.; de Vivie-Riedle R.; Didier D. Oxidative Ring Contraction of Cyclobutenes: General Approach to Cyclopropylketones including Mechanistic Insights. J. Org. Chem. 2018, 83, 4905–4921. 10.1021/acs.joc.8b00297. [DOI] [PubMed] [Google Scholar]
- del Hoyo A. M.; Herraiz A. G.; Suero M. G. A Stereoconvergent Cyclopropanation Reaction of Styrenes. Angew. Chem., Int. Ed. 2017, 56, 1610–1613. 10.1002/anie.201610924. [DOI] [PubMed] [Google Scholar]
- Li P.; Zhao J.; Shi L.; Wang J.; Shi X.; Li F. Iodine-Catalyzed Diazo Activation to Access Radical Reactivity. Nat. Commun. 2018, 9, 1972. 10.1038/s41467-018-04331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. A.; Phelan J. P.; Polites V. C.; Kelly C. B.; Molander G. A. Radical/Polar Annulation Reactions (RPARs) Enable the Modular Construction of Cyclopropanes. Org. Lett. 2018, 20, 6840–6844. 10.1021/acs.orglett.8b02968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu C.; Mega R. S.; Andreassen B. J.; Noble A.; Aggarwal V. K. Synthesis of Functionalized Cyclopropanes from Carboxylic Acids by a Radical Addition−Polar Cyclization Cascade. Angew. Chem., Int. Ed. 2018, 57, 15430–15434. 10.1002/anie.201808598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For the original report, see:; Kulinkovich O. G.; Sviridov S. V.; Vasilevskii D. A.; Pritytskaya T. S. Reaction of Ethylmagnesium Bromide with Carboxylic Acid Esters in the Presence of Tetraisopropoxytitanium. Zh. Org. Khim 1989, 25, 2244. [Google Scholar]
- Kulinkovich O. G.; de Meijere A. 1,n-Dicarbanionic Titanium Intermediates from Monocarbanionic Organometallics and Their Application in Organic Synthesis. Chem. Rev. 2000, 100, 2789–2834. 10.1021/cr980046z. [DOI] [PubMed] [Google Scholar]
- de Meijere A.; Kozhushkov S. I.; Savchenko A. I. Titanium-Mediated Syntheses of Cyclopropylamines. J. Organomet. Chem. 2004, 689, 2033–2055. 10.1016/j.jorganchem.2004.03.012. [DOI] [Google Scholar]
- Lautens M.; Klute W.; Tam W. Transition Metal-Mediated Cycloaddition Reactions. Chem. Rev. 1996, 96, 49–92. 10.1021/cr950016l. [DOI] [PubMed] [Google Scholar]
- Wu W.; Lin Z.; Jiang H. Recent Advances in the Synthesis of Cyclopropanes. Org. Biomol. Chem. 2018, 16, 7315–7329. 10.1039/C8OB01187G. [DOI] [PubMed] [Google Scholar]
- Piou T.; Romanov-Michailidis F.; Ashley M. A.; Romanova-Michaelides M.; Rovis T. Stereodivergent Rhodium(III)-Catalyzed cis-Cyclopropanation Enabled by Multivariate Optimization. J. Am. Chem. Soc. 2018, 140, 9587–9593. 10.1021/jacs.8b04243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.; Noble A.; Bedford R. B.; Aggarwal V. K. Methylenespiro[2.3]hexanes via Nickel-Catalyzed Cyclopropanations with [1.1.1]Propellane. J. Am. Chem. Soc. 2019, 141, 20325–20334. 10.1021/jacs.9b10689. [DOI] [PubMed] [Google Scholar]
- Bach T. Stereoselective Intermolecular [2 + 2]-Photocycloaddition Reactions and Their Application in Synthesis. Synthesis 1998, 1998, 683–703. 10.1055/s-1998-2054. [DOI] [Google Scholar]
- Hoffmann N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]
- Poplata S.; Tröster A.; Zou Y.-Q.; Bach T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. 10.1021/acs.chemrev.5b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppolzer W.; Godel T. A New and Efficient Total Synthesis of (±)-Longifolene. J. Am. Chem. Soc. 1978, 100, 2583–2584. 10.1021/ja00476a071. [DOI] [Google Scholar]
- Mangion I. K.; MacMillan D. W. C. Total Synthesis of Brasoside and Littoralisone. J. Am. Chem. Soc. 2005, 127, 3696–3697. 10.1021/ja050064f. [DOI] [PubMed] [Google Scholar]
- Mascitti V.; Corey E. J. Enantioselective Synthesis of Pentacycloanammoxic Acid. J. Am. Chem. Soc. 2006, 128, 3118–3119. 10.1021/ja058370g. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C.; Sarlah D.; Shaw D. M. Total Synthesis and Revised Structure of Biyouyanagin A. Angew. Chem., Int. Ed. 2007, 46, 4708–4711. 10.1002/anie.200701552. [DOI] [PubMed] [Google Scholar]
- Du J.; Skubi K. L.; Schultz D. M.; Yoon T. P. A Dual-Catalysis Approach to Enantioselective [2 + 2] Photocycloadditions Using Visible Light. Science 2014, 344, 392–396. 10.1126/science.1251511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischay M. A.; Lu Z.; Yoon T. P. [2 + 2] Cycloadditions by Oxidative Visible Light Photocatalysis. J. Am. Chem. Soc. 2010, 132, 8572–8574. 10.1021/ja103934y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu C.; Noble A.; Aggarwal V. K. Photoredox-Catalyzed Cyclobutane Synthesis by a Deboronative Radical Addition−Polar Cyclization Cascade. Angew. Chem., Int. Ed. 2019, 58, 3870–3874. 10.1002/anie.201813917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y.; Narasaka K. Asymmetric [2 + 2] Cycloaddition Reaction Catalyzed by a Chiral Titanium Reagent. Chem. Lett. 1989, 18, 793–796. 10.1246/cl.1989.793. [DOI] [Google Scholar]
- Canales E.; Corey E. J. Highly Enantioselective [2 + 2]-Cycloaddition Reactions Catalyzed by a Chiral Aluminum Bromide Complex. J. Am. Chem. Soc. 2007, 129, 12686–12687. 10.1021/ja0765262. [DOI] [PubMed] [Google Scholar]
- Boxer M. B.; Yamamoto H. Remarkable Tris(trimethylsilyl)silyl Group for Diastereoselective [2 + 2] Cyclizations. Org. Lett. 2005, 7, 3127–3129. 10.1021/ol0512334. [DOI] [PubMed] [Google Scholar]
- Russell S. K.; Lobkovsky E.; Chirik P. J. Iron-Catalyzed Intermolecular [2π + 2π] Cycloaddition. J. Am. Chem. Soc. 2011, 133, 8858–8861. 10.1021/ja202992p. [DOI] [PubMed] [Google Scholar]
- Pagar V. V.; RajanBabu T. V. Tandem Catalysis for Asymmetric Coupling of Ethylene and Enynes to Functionalized Cyclobutanes. Science 2018, 361, 68–72. 10.1126/science.aat6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. M.; Li H.; Hussain N.; Urena M.; Carroll P. J.; Walsh P. J. Applications of 1-Alkenyl-1,1-Heterobimetallics in the Stereoselective Synthesis of Cyclopropylboronate Esters, Trisubstituted Cyclopropanols and 2,3-Disubstituted Cyclobutanones. J. Am. Chem. Soc. 2009, 131, 6516–6524. 10.1021/ja900147s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbeck F.; Toste F. D. Gold(I)-Catalyzed Enantioselective Ring Expansion of Allenylcyclopropanols. J. Am. Chem. Soc. 2009, 131, 9178–9179. 10.1021/ja904055z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barluenga J.; Riesgo L.; López L. A.; Rubio E.; Tomás M. Discrimination of Diazo Compounds Toward Carbenoids: Copper(I)-Catalyzed Synthesis of Substituted Cyclobutenes. Angew. Chem., Int. Ed. 2009, 48, 7569–7572. 10.1002/anie.200903902. [DOI] [PubMed] [Google Scholar]
- Guérot C.; Tchitchanov B. H.; Knust H.; Carreira E. M. Synthesis of Novel Angular Spirocyclic Azetidines. Org. Lett. 2011, 13, 780–783. 10.1021/ol103050c. [DOI] [PubMed] [Google Scholar]
- Dong S.; Parker G. D.; Tei T.; Paquette L. A. In Pursuit of Pestalotiopsin A via Zirconocene-Mediated Ring Contraction. Org. Lett. 2006, 8, 2429–2431. 10.1021/ol060827j. [DOI] [PubMed] [Google Scholar]
- Davenport R.; Silvi M.; Noble A.; Hosni Z.; Fey N.; Aggarwal V. K. Visible-Light-Driven Strain-Increase Ring Contraction Allows the Synthesis of Cyclobutyl Boronic Esters. Angew. Chem., Int. Ed. 2020, 59, 6525–6528. 10.1002/anie.201915409. [DOI] [PubMed] [Google Scholar]
- Gianatassio R.; Lopchuk J. M.; Wang J.; Pan C.-M.; Malins L. R.; Prieto L.; Brandt T. A.; Collins M. R.; Gallego G. M.; Sach N. W.; Spangler J. E.; Zhu H.; Zhu J.; Baran P. S. Strain-Release Amination. Science 2016, 351, 241–246. 10.1126/science.aad6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett A.; Biberger T.; Aggarwal V. K. Carbopalladation of C−C σ-Bonds Enabled by Strained Boronate Complexes. Nat. Chem. 2019, 11, 117–122. 10.1038/s41557-018-0181-x. [DOI] [PubMed] [Google Scholar]
- Wang Y.-M.; Bruno N. C.; Placeres A. L.; Zhu S.; Buchwald S. L. Enantioselective Synthesis of Carbo- and Heterocycles through a CuH-Catalyzed Hydroalkylation Approach. J. Am. Chem. Soc. 2015, 137, 10524–10527. 10.1021/jacs.5b07061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. K.; Riedel J.; Kim R. S.; Dong V. M. Cobalt Catalysis for Enantioselective Cyclobutanone Construction. J. Am. Chem. Soc. 2017, 139, 10208–10211. 10.1021/jacs.7b05327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K.; Haraguchi H.; Okamoto Y. A New Strategy for Construction of Eight-Membered Carbocycles by Brook Rearrangement Mediated [6 + 2] Annulation. Org. Lett. 2003, 5, 3705–3707. 10.1021/ol0353767. [DOI] [PubMed] [Google Scholar]
- Ito H.; Toyoda T.; Sawamura M. Stereospecific Synthesis of Cyclobutylboronates through Copper(I)-Catalyzed Reaction of Homoallylic Sulfonates and a Diboron Derivative. J. Am. Chem. Soc. 2010, 132, 5990–5992. 10.1021/ja101793a. [DOI] [PubMed] [Google Scholar]
- Ito Y.; Sawamura M.; Hayashi T. Catalytic Asymmetric Aldol Reaction: Reaction of Aldehydes with Isocyanoacetate Catalyzed by a Chiral Ferrocenylphosphine-Gold(I) Complex. J. Am. Chem. Soc. 1986, 108, 6405–6406. 10.1021/ja00280a056. [DOI] [Google Scholar]
- Uchimoto K.; Fukuda Y.; Utimoto K.; Nozaki H. Preparation of 2,3,4,5-Tetrahydropyridines from 5-Alkylamines under the Catalytic Action of Au(III). Heterocycles 1987, 25, 297–300. 10.3987/S-1987-01-0297. [DOI] [Google Scholar]
- Fukuda Y.; Utimoto K. Effective Transformation of Unactivated Alkynes into Ketones or Acetals by means of Au(III) Catalyst. J. Org. Chem. 1991, 56, 3729–3731. 10.1021/jo00011a058. [DOI] [Google Scholar]
- Teles J. H.; Brode S.; Chabanas M. Cationic Gold(I) Complexes: Highly Efficient Catalysts for the Addition of Alcohols to Alkynes. Angew. Chem., Int. Ed. 1998, 37, 1415–1418. . [DOI] [PubMed] [Google Scholar]
- Mizushima E.; Sato K.; Hayashi T.; Tanaka M. Highly Efficient AuI-Catalyzed Hydration of Alkynes. Angew. Chem., Int. Ed. 2002, 41, 4563–4565. . [DOI] [PubMed] [Google Scholar]
- Schmidbaur H. Is Gold Chemistry a Topical Field of Study?. Angew. Chem., Int. Ed. Engl. 1976, 15, 728–740. 10.1002/anie.197607281. [DOI] [Google Scholar]
- Echavarren A. M.; Hashmi A. S. K.; Toste F. D. Gold Catalysis - Steadily Increasing in Importance. Adv. Synth. Catal. 2016, 358, 1347. 10.1002/adsc.201600381. [DOI] [Google Scholar]
- Fürstner A.; Davies P. W. Cationic Carbophilic Activation: Catalysis by Platinum and Gold π Acids. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]
- Jiménez-Núñez E.; Echavarren A. M. Molecular Diversity through Gold Catalysis with Alkynes. Chem. Commun. 2007, 333–346. 10.1039/B612008C. [DOI] [PubMed] [Google Scholar]
- Kirsch S. F. Construction of Heterocycles by the Strategic Use of Alkyne π-Activation in Catalyzed Cascade Reactions. Synthesis 2008, 2008, 3183–3204. 10.1055/s-0028-1083164. [DOI] [Google Scholar]
- Fürstner A. Gold and Platinum Catalysis—A Convenient Tool for Generating Molecular Complexity. Chem. Soc. Rev. 2009, 38, 3208–3221. 10.1039/b816696j. [DOI] [PubMed] [Google Scholar]
- Pérez A. G.; Álvarez R.; de Lera Á. R. Characterization of Pericyclic Steps in the Mechanisms of Gold(I) Catalyzed Rearrangement of Alkynes. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 211–225. 10.1002/wcms.1126. [DOI] [Google Scholar]
- Ohno H. Gold-Catalyzed Cascade Reactions of Alkynes for Construction of Polycyclic Compounds. Isr. J. Chem. 2013, 53, 869–882. 10.1002/ijch.201300054. [DOI] [Google Scholar]
- Dorel R.; Echavarren A. M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. 10.1021/cr500691k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürstner A. Gold Catalysis for Heterocyclic Chemistry: A Representative Case Study on Pyrone Natural Products. Angew. Chem., Int. Ed. 2018, 57, 4215–4233. 10.1002/anie.201707260. [DOI] [PubMed] [Google Scholar]
- Braun I.; Asiri A. M.; Hashmi A. S. K. Gold Catalysis 2.0. ACS Catal. 2013, 3, 1902–1907. 10.1021/cs400437s. [DOI] [Google Scholar]
- Asiri A. M.; Hashmi A. S. K. Gold-Catalysed Reactions of Diynes. Chem. Soc. Rev. 2016, 45, 4471–4503. 10.1039/C6CS00023A. [DOI] [PubMed] [Google Scholar]
- Day D. P.; Chan P. W. H. Gold-Catalyzed Cycloisomerizations of 1,n-Diyne Carbonates and Esters. Adv. Synth. Catal. 2016, 358, 1368–1384. 10.1002/adsc.201600005. [DOI] [Google Scholar]
- Krause N.; Winter C. Gold-Catalyzed Nucleophilic Cyclization of Functionalized Allenes: A Powerful Access to Carbo- and Heterocycles. Chem. Rev. 2011, 111, 1994–2009. 10.1021/cr1004088. [DOI] [PubMed] [Google Scholar]
- Yang W.; Hashmi A. S. K. Mechanistic Insights into the Gold Chemistry of Allenes. Chem. Soc. Rev. 2014, 43, 2941–2955. 10.1039/c3cs60441a. [DOI] [PubMed] [Google Scholar]
- Soriano E.; Fernández I. Allenes and Computational Chemistry: from Bonding Situation to Reaction Mechanisms. Chem. Soc. Rev. 2014, 43, 3041–3105. 10.1039/c3cs60457h. [DOI] [PubMed] [Google Scholar]
- Aubert C.; Fensterbank L.; Garcia P.; Malacria M.; Simonneau A. Transition Metal Catalyzed Cycloisomerizations of 1,n-Allenynes and -Allenenes. Chem. Rev. 2011, 111, 1954–1993. 10.1021/cr100376w. [DOI] [PubMed] [Google Scholar]
- Cañeque T.; Truscott F. M.; Rodriguez R.; Maestri G.; Malacria M. Electrophilic Activation of Allenenes and Allenynes: Analogies and Differences between Brønsted and Lewis Acid Activation. Chem. Soc. Rev. 2014, 43, 2916–2926. 10.1039/C4CS00023D. [DOI] [PubMed] [Google Scholar]
- Mascareñas J. L.; Varela I.; López F. Allenes and Derivatives in Gold(I)- and Platinum(II)-Catalyzed Formal Cycloadditions. Acc. Chem. Res. 2019, 52, 465–479. 10.1021/acs.accounts.8b00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin D. J.; Toste F. D. Relativistic Effects in Homogeneous Gold Catalysis. Nature 2007, 446, 395–403. 10.1038/nature05592. [DOI] [PubMed] [Google Scholar]
- Dyker G. An Eldorado for Homogeneous Catalysis?. Angew. Chem., Int. Ed. 2000, 39, 4237–4239. . [DOI] [PubMed] [Google Scholar]
- Stephen A.; Hashmi K. Homogeneous Catalysis by Gold. Gold Bull. 2004, 37, 51–65. 10.1007/BF03215517. [DOI] [Google Scholar]
- Hoffmann-Röder A.; Krause N. The Golden Gate to Catalysis. Org. Biomol. Chem. 2005, 3, 387–391. 10.1039/B416516K. [DOI] [PubMed] [Google Scholar]
- Hashmi A. S. K. The Catalysis Gold Rush: New Claims. Angew. Chem., Int. Ed. 2005, 44, 6990–6993. 10.1002/anie.200502735. [DOI] [PubMed] [Google Scholar]
- Hashmi A. S. K.; Hutchings G. J. Gold Catalysis. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. 10.1002/anie.200602454. [DOI] [PubMed] [Google Scholar]
- Hashmi A. S. K. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007, 107, 3180–3211. 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]
- Skouta R.; Li J.-C. Gold-Catalyzed Reactions of C−H Bonds. Tetrahedron 2008, 64, 4917–4938. 10.1016/j.tet.2008.03.083. [DOI] [Google Scholar]
- Muzart J. Gold-Catalysed Reactions of Alcohols: Isomerisation, Inter- and Intramolecular Reactions Leading to C−C and C−Heteroatom Bonds. Tetrahedron 2008, 64, 5815–5849. 10.1016/j.tet.2008.04.018. [DOI] [Google Scholar]
- Li Z.; Brouwer C.; He C. Gold-Catalyzed Organic Transformations. Chem. Rev. 2008, 108, 3239–3265. 10.1021/cr068434l. [DOI] [PubMed] [Google Scholar]
- Arcadi A. Alternative Synthetic Methods through New Developments in Catalysis by Gold. Chem. Rev. 2008, 108, 3266–3325. 10.1021/cr068435d. [DOI] [PubMed] [Google Scholar]
- Shapiro N. D.; Toste F. D. A Reactivity-Driven Approach to the Discovery and Development of Gold-Catalyzed Organic Reactions. Synlett 2010, 2010, 675–691. 10.1055/s-0029-1219369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi A. S. K. Homogeneous Gold Catalysis Beyond Assumptions and Proposals—Characterized Intermediates. Angew. Chem., Int. Ed. 2010, 49, 5232–5241. 10.1002/anie.200907078. [DOI] [PubMed] [Google Scholar]
- Boorman T. C.; Larrosa I. Gold-Mediated C−H Bond Functionalisation. Chem. Soc. Rev. 2011, 40, 1910–1925. 10.1039/C0CS00098A. [DOI] [PubMed] [Google Scholar]
- Bandini M. Gold-Catalyzed Decorations of Arenes and Heteroarenes with C−C Multiple Bonds. Chem. Soc. Rev. 2011, 40, 1358–1367. 10.1039/C0CS00041H. [DOI] [PubMed] [Google Scholar]
- Huang H.; Zhou Y.; Liu H. Recent Advances in the Gold-Catalyzed Additions to C−C Multiple Bonds. Beilstein J. Org. Chem. 2011, 7, 897–936. 10.3762/bjoc.7.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López F.; Mascareñas J. L. Recent Developments in Gold-Catalyzed Cycloaddition Reactions. Beilstein J. Org. Chem. 2011, 7, 1075–1094. 10.3762/bjoc.7.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.-P.; Hammond G. B. Recent Advances in the Isolation and Reactivity of Organogold Complexes. Chem. Soc. Rev. 2012, 41, 3129–3139. 10.1039/c2cs15318a. [DOI] [PubMed] [Google Scholar]
- Abbiati G.; Marinelli F.; Rossi E.; Arcadi A. Synthesis of Indole Derivatives from 2-Alkynylanilines by Means of Gold Catalysis. Isr. J. Chem. 2013, 53, 856–868. 10.1002/ijch.201300040. [DOI] [Google Scholar]
- Brand J. P.; Li Y.; Waser J. Gold-catalyzed Alkynylation: Acetylene-Transfer instead of Functionalization. Isr. J. Chem. 2013, 53, 901–910. 10.1002/ijch.201300044. [DOI] [Google Scholar]
- Xie J.; Pan C.; Abdukader A.; Zhu C. Gold-catalyzed C(sp3)−H Bond Functionalization. Chem. Soc. Rev. 2014, 43, 5245–5256. 10.1039/C4CS00004H. [DOI] [PubMed] [Google Scholar]
- Lo V. K.-Y.; Chan A. O.-Y.; Che C.-M. Gold and Silver Catalysis: From Organic Transformation to Bioconjugation. Org. Biomol. Chem. 2015, 13, 6667–6680. 10.1039/C5OB00407A. [DOI] [PubMed] [Google Scholar]
- Bihelovic F.; Vulovic B.; Saicic R. N. Gold(I)-Catalyzed C−O/C−C Bond-Forming Domino Reactions and Their Synthetic Applications. Isr. J. Chem. 2018, 58, 521–530. 10.1002/ijch.201700033. [DOI] [Google Scholar]
- Gagosz F. Gold Vinylidenes as Useful Intermediates in Synthetic Organic Chemistry. Synthesis 2019, 51, 1087–1099. 10.1055/s-0037-1611647. [DOI] [Google Scholar]
- Nijamudheen A.; Datta A. Gold-Catalyzed Cross-Coupling Reactions: An Overview of Design Strategies, Mechanistic Studies, and Applications. Chem. - Eur. J. 2020, 26, 1442–1487. 10.1002/chem.201903377. [DOI] [PubMed] [Google Scholar]
- Banerjee S.; Bhoyare V. W.; Patil N. T. Gold and Hypervalent Iodine(III): Liaisons over a Decade for Electrophilic Functional Group Transfer Reactions. Chem. Commun. 2020, 56, 2677–2690. 10.1039/D0CC00106F. [DOI] [PubMed] [Google Scholar]
- Meera G.; Rohit K. R.; Treesa G. S. S.; Anilkumar G. Advances and Prospects in Gold-Catalyzed C−H Activation. Asian J. Org. Chem. 2020, 9, 144–161. 10.1002/ajoc.202000020. [DOI] [Google Scholar]
- Shen H. C. Recent Advances in Syntheses of Heterocycles and Carbocycles via Homogeneous Gold Catalysis. Part 1: Heteroatom Addition and Hydroarylation Reactions of Alkynes, Allenes, and Alkenes. Tetrahedron 2008, 64, 3885–3903. 10.1016/j.tet.2008.01.081. [DOI] [Google Scholar]
- Shen H. C. Recent Advances in Syntheses of Carbocycles and Heterocycles via Homogeneous Gold Catalysis. Part 2: Cyclizations and Cycloadditions. Tetrahedron 2008, 64, 7847–7870. 10.1016/j.tet.2008.05.082. [DOI] [Google Scholar]
- Patil N. T.; Yamamoto Y. Coinage Metal-Assisted Synthesis of Heterocycles. Chem. Rev. 2008, 108, 3395–3442. 10.1021/cr050041j. [DOI] [PubMed] [Google Scholar]
- Corma A.; Leyva-Pérez A.; Sabater M. J. Gold-Catalyzed Carbon-Heteroatom Bond-Forming Reactions. Chem. Rev. 2011, 111, 1657–1712. 10.1021/cr100414u. [DOI] [PubMed] [Google Scholar]
- Rudolph M.; Hashmi A. S. K. Heterocycles from Gold Catalysis. Chem. Commun. 2011, 47, 6536–6544. 10.1039/c1cc10780a. [DOI] [PubMed] [Google Scholar]
- Qian D.; Zhang J. Gold-Catalyzed Cascade Reactions for Synthesis of Carbo- and Heterocycles: Selectivity and Diversity. Chem. Rec. 2014, 14, 280–302. 10.1002/tcr.201300039. [DOI] [PubMed] [Google Scholar]
- Hashmi A. S. K.; Rudolph M. Gold Catalysis in Total Synthesis. Chem. Soc. Rev. 2008, 37, 1766–1775. 10.1039/b615629k. [DOI] [PubMed] [Google Scholar]
- Rudolph M.; Hashmi A. S. K. Gold Catalysis in Total Synthesis—An Update. Chem. Soc. Rev. 2012, 41, 2448–2462. 10.1039/C1CS15279C. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Luo T.; Yang Z. Strategic Innovation in the Total Synthesis of Complex Natural Products Using Gold Catalysis. Nat. Prod. Rep. 2014, 31, 489–503. 10.1039/C3NP70075E. [DOI] [PubMed] [Google Scholar]
- Pflästerer D.; Hashmi A. S. K. Gold Catalysis in Total Synthesis - Recent Achievements. Chem. Soc. Rev. 2016, 45, 1331–1367. 10.1039/C5CS00721F. [DOI] [PubMed] [Google Scholar]
- Stathakis C. I.; Gkizis P. L.; Zografos A. L. Metal-Catalyzed Cycloisomerization as a Powerful Tool in the Synthesis of Complex Sesquiterpenoids. Nat. Prod. Rep. 2016, 33, 1093–1117. 10.1039/C6NP00026F. [DOI] [PubMed] [Google Scholar]
- Sugimoto K.; Matsuya Y. Recent Applications of Gold-Catalyzed Cascade Reactions in Total Synthesis of Natural Product. Tetrahedron Lett. 2017, 58, 4420–4426. 10.1016/j.tetlet.2017.10.029. [DOI] [Google Scholar]
- Hu Y.; Bai M.; Yang Y.; Zhou Q. Metal-Catalyzed Enyne Cycloisomerization in Natural Product Total Synthesis. Org. Chem. Front. 2017, 4, 2256–2275. 10.1039/C7QO00702G. [DOI] [Google Scholar]
- McGee P.; Brousseau J.; Barriault L. Development of New Gold(I)-Catalyzed Carbocyclizations and their Applications in the Synthesis of Natural Products. Isr. J. Chem. 2018, 58, 511–520. 10.1002/ijch.201700054. [DOI] [Google Scholar]
- Wang S. Alkaloid Synthesis via Gold-Catalyzed Carbon-Carbon Bond Formation Using Enamine-type Nucleophile. Isr. J. Chem. 2018, 58, 557–567. 10.1002/ijch.201700045. [DOI] [Google Scholar]
- Czekelius C. Total Synthesis of Mesembrine - The Construction of Quaternary Stereocenters by Gold-Catalyzed Diyne Desymmetrization. Isr. J. Chem. 2018, 58, 568–577. 10.1002/ijch.201700060. [DOI] [Google Scholar]
- Toullec P. Y.; Michelet V. Gold-Catalyzed Polycyclization Toward Natural Product Synthesis. Isr. J. Chem. 2018, 58, 578–585. 10.1002/ijch.201800002. [DOI] [Google Scholar]
- Mouriès-Mansuy V.; Fensterbank L. Gold-Catalyzed Migration of Propargyl Acetate as an Entry into the Total Synthesis of Natural Products. Isr. J. Chem. 2018, 58, 586–595. 10.1002/ijch.201700074. [DOI] [Google Scholar]
- Holzschneider K.; Kirsch S. F. [1, 2]-Migration Reactions Catalyzed by Gold Complexes and their Applications in Total Synthesis. Isr. J. Chem. 2018, 58, 596–607. 10.1002/ijch.201700081. [DOI] [Google Scholar]
- Zhang X.; Ma S. Transition Metal-Catalyzed Benzannulation towards Naturally Occurring Carbazole Alkaloids. Isr. J. Chem. 2018, 58, 608–621. 10.1002/ijch.201700034. [DOI] [Google Scholar]
- Pflästerer D.; Rudolph M.; Hashmi A. S. K. Gold-Catalyzed Hydrofunctionalizations and Spiroketalizations of Alkynes as Key Steps in Total Synthesis. Isr. J. Chem. 2018, 58, 622–638. 10.1002/ijch.201700056. [DOI] [Google Scholar]
- Mayans J. G.; Armengol-Relats H.; Calleja P.; Echavarren A. M. Gold(I)-Catalysis for the Synthesis of Terpenoids: From Intramolecular Cascades to Intermolecular Cycloadditions. Isr. J. Chem. 2018, 58, 639–658. 10.1002/ijch.201800006. [DOI] [Google Scholar]
- Aubert C.; Buisine O.; Malacria M. The Behavior of 1,n-Enynes in the Presence of Transition Metals. Chem. Rev. 2002, 102, 813–834. 10.1021/cr980054f. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones G. C. Mechanistic Aspects of Transition Metal Catalysed 1,6-Diene and 1,6-Enyne Cycloisomerisation Reactions. Org. Biomol. Chem. 2003, 1, 215–236. 10.1039/b209175p. [DOI] [PubMed] [Google Scholar]
- Diver S. T.; Giessert A. J. Enyne Metathesis (Enyne Bond Reorganization). Chem. Rev. 2004, 104, 1317–1382. 10.1021/cr020009e. [DOI] [PubMed] [Google Scholar]
- Echavarren A. M.; Nevado C. Non-Stabilized Transition Metal Carbenes as Intermediates in Intramolecular Reactions of Alkynes with Alkenes. Chem. Soc. Rev. 2004, 33, 431–436. 10.1039/b308768a. [DOI] [PubMed] [Google Scholar]
- Bruneau C. Electrophilic Activation and Cycloisomerization of Enynes: A New Route to Functional Cyclopropanes. Angew. Chem., Int. Ed. 2005, 44, 2328–2334. 10.1002/anie.200462568. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Sun J.; Kozmin S. A. Gold and Platinum Catalysis of Enyne Cycloisomerization. Adv. Synth. Catal. 2006, 348, 2271–2296. 10.1002/adsc.200600368. [DOI] [Google Scholar]
- Ma S.; Yu S.; Gu Z. Gold-Catalyzed Cyclization of Enynes. Angew. Chem., Int. Ed. 2006, 45, 200–203. 10.1002/anie.200502999. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; López S.; Jiménez-Núñez E.; Echavarren A. M. The Mechanistic Puzzle of Transition-Metal-Catalyzed Skeletal Rearrangements of Enynes. Chem. - Eur. J. 2006, 12, 5916–5923. 10.1002/chem.200600174. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Zhu G.; Tong X.; Wang F.; Xie X.; Wang J.; Jiang L. Transition Metal-Catalyzed Intramolecular Enyne Cyclization Reaction. Curr. Org. Chem. 2006, 10, 1457–1478. 10.2174/138527206778018203. [DOI] [Google Scholar]
- Michelet V.; Toullec P. Y.; Genêt J.-P. Cycloisomerization of 1,n-Enynes: Challenging Metal-Catalyzed Rearrangements and Mechanistic Insights. Angew. Chem., Int. Ed. 2008, 47, 4268–4315. 10.1002/anie.200701589. [DOI] [PubMed] [Google Scholar]
- Jiménez-Núñez E.; Echavarren A. M. Gold-Catalyzed Cycloisomerizations of Enynes: A Mechanistic Perspective. Chem. Rev. 2008, 108, 3326–3350. 10.1021/cr0684319. [DOI] [PubMed] [Google Scholar]
- Belmont P.; Parker E. Silver and Gold Catalysis for Cycloisomerization Reactions. Eur. J. Org. Chem. 2009, 2009, 6075–6089. 10.1002/ejoc.200900790. [DOI] [Google Scholar]
- Abu Sohel S. M.; Liu R.-S. Carbocyclisation of Alkynes with External Nucleophiles Catalysed by Gold, Platinum and Other Electrophilic Metals. Chem. Soc. Rev. 2009, 38, 2269–2281. 10.1039/b807499m. [DOI] [PubMed] [Google Scholar]
- Echavarren A. M.; Jiménez-Núñez E. Complexity via Gold-Catalyzed Molecular Gymnastics. Top. Catal. 2010, 53, 924–930. 10.1007/s11244-010-9524-6. [DOI] [Google Scholar]
- Garayalde D.; Nevado C. Gold-Containing and Gold-Generated 1,n-Dipoles as Useful Platforms toward Cycloadditions and Cyclizations. ACS Catal. 2012, 2, 1462–1479. 10.1021/cs300043w. [DOI] [Google Scholar]
- Nunes dos Santos Comprido L.; Hashmi A. S. K. Catalytic Oxidative Cyclisation Reactions of 1,6-Enynes: A Critical Comparison Between Gold and Palladium. Isr. J. Chem. 2013, 53, 883–891. 10.1002/ijch.201300072. [DOI] [Google Scholar]
- Michelet V.Noble Metal-Catalyzed Enyne Cycloisomerizations and Related Reactions. Comprehensive Organic Synthesis, 2nd ed.; Elsevier, 2014; Vol. 5, pp 1483–1536. [Google Scholar]
- Obradors C.; Echavarren A. M. Gold-Catalyzed Rearrangements and Beyond. Acc. Chem. Res. 2014, 47, 902–912. 10.1021/ar400174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradors C.; Echavarren A. M. Intriguing Mechanistic Labyrinths in Gold(I) Catalysis. Chem. Commun. 2014, 50, 16–28. 10.1039/C3CC45518A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorel R.; Echavarren A. M. Gold-Catalyzed Reactions via Cyclopropyl Gold Carbene-like Intermediates. J. Org. Chem. 2015, 80, 7321–7332. 10.1021/acs.joc.5b01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-C.; Kumar K. Gold(I) Catalyzed Enyne Cycloisomerization - A Roadmap to Privileged Heterocyclic Scaffolds. Isr. J. Chem. 2018, 58, 531–556. 10.1002/ijch.201700067. [DOI] [Google Scholar]
- Marín-Luna M.; Nieto Faza O.; Silva López C. Gold-Catalyzed Homogeneous (Cyclo)Isomerization Reactions. Front. Chem. 2019, 7, 296. 10.3389/fchem.2019.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooner R. E. M.; Widenhoefer R. A. Cationic, Two-Coordinate Gold π Complexes. Angew. Chem., Int. Ed. 2013, 52, 11714–11724. 10.1002/anie.201303468. [DOI] [PubMed] [Google Scholar]
- Ranieri B.; Escofet I.; Echavarren A. M. Anatomy of Gold Catalysts: Facts and Myths. Org. Biomol. Chem. 2015, 13, 7103–7118. 10.1039/C5OB00736D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno M. C.; Laguna A. Three- and Four-Coordinate Gold(I) Complexes. Chem. Rev. 1997, 97, 511–522. 10.1021/cr960361q. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Nevado C. Cyclometalated Gold(III) Complexes: Synthesis, Reactivity, and Physicochemical Properties. Angew. Chem., Int. Ed. 2017, 56, 1994–2015. 10.1002/anie.201607225. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; López S.; Echavarren A. M. Intramolecular [4 + 2] Cycloadditions of 1,3-Enynes or Arylalkynes with Alkenes with Highly Reactive Cationic Phosphine Au(I) Complexes. J. Am. Chem. Soc. 2005, 127, 6178–6179. 10.1021/ja042257t. [DOI] [PubMed] [Google Scholar]
- Mamane V.; Gress T.; Krause H.; Fürstner A. Platinum- and Gold-Catalyzed Cycloisomerization Reactions of Hydroxylated Enynes. J. Am. Chem. Soc. 2004, 126, 8654–8655. 10.1021/ja048094q. [DOI] [PubMed] [Google Scholar]
- Luzung M. R.; Markham J. P.; Toste F. D. Catalytic Isomerization of 1,5-Enynes to Bicyclo[3.1.0]hexenes. J. Am. Chem. Soc. 2004, 126, 10858–10859. 10.1021/ja046248w. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; López S.; Muñoz M. P.; Cárdenas D. J.; Buñuel E.; Nevado C.; Echavarren A. M. Divergent Mechanisms for the Skeletal Rearrangement and [2 + 2] Cycloaddition of Enynes Catalyzed by Gold. Angew. Chem., Int. Ed. 2005, 44, 6146–6148. 10.1002/anie.200501937. [DOI] [PubMed] [Google Scholar]
- Herrero-Gómez E.; Nieto-Oberhuber C.; López S.; Benet-Buchholz J.; Echavarren A. M. Cationic η1/η2-Gold(I) Complexes of Simple Arenes. Angew. Chem., Int. Ed. 2006, 45, 5455–5459. 10.1002/anie.200601688. [DOI] [PubMed] [Google Scholar]
- Marion N.; Nolan S. P. N-Heterocyclic Carbenes in Gold Catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. 10.1039/b711132k. [DOI] [PubMed] [Google Scholar]
- Raubenheimer H. G.; Cronje S. Carbene Complexes of Gold: Preparation, Medical Application and Bonding. Chem. Soc. Rev. 2008, 37, 1998–2011. 10.1039/b708636a. [DOI] [PubMed] [Google Scholar]
- Gorin D. J.; Sherry B. D.; Toste F. D. Ligand Effects in Homogeneous Au Catalysis. Chem. Rev. 2008, 108, 3351–3378. 10.1021/cr068430g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C. Y.; Huang R. T. W.; Lee C. S.; Bhattacharyya A.; Hwang W. S.; Lin I. J. B. Coinage Metal−N−Heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3561–3598. 10.1021/cr8005153. [DOI] [PubMed] [Google Scholar]
- Nolan S. P. The Development and Catalytic Use of N-Heterocyclic Carbene Gold Complexes. Acc. Chem. Res. 2011, 44, 91–100. 10.1021/ar1000764. [DOI] [PubMed] [Google Scholar]
- Gatineau D.; Goddard J.-P.; Mouriès-Mansuy V.; Fensterbank L. When NHC Ligands Make a Difference in Gold Catalysis. Isr. J. Chem. 2013, 53, 892–900. 10.1002/ijch.201300059. [DOI] [Google Scholar]
- Zuccarello G.; Zanini M.; Echavarren A. M. Buchwald-Type Ligands on Gold(I) Catalysis. Isr. J. Chem. 2020, 60, 360–372. 10.1002/ijch.201900179. [DOI] [Google Scholar]
- Bongers N.; Krause N. Golden Opportunities for Stereoselective Catalysis. Angew. Chem., Int. Ed. 2008, 47, 2178–2181. 10.1002/anie.200704729. [DOI] [PubMed] [Google Scholar]
- Widenhoefer R. A. Recent Developments in Enantioselective Gold(I) Catalysis. Chem. - Eur. J. 2008, 14, 5382–5391. 10.1002/chem.200800219. [DOI] [PubMed] [Google Scholar]
- Sengupta S.; Shi X. Recent Advances in Asymmetric Gold Catalysis. ChemCatChem 2010, 2, 609–619. 10.1002/cctc.201000070. [DOI] [Google Scholar]
- Pradal A.; Toullec P. Y.; Michelet V. Recent Developments in Asymmetric Catalysis in the Presence of Chiral Gold Complexes. Synthesis 2011, 2011, 1501–1514. 10.1055/s-0030-1258465. [DOI] [Google Scholar]
- Marinetti A.; Jullien H.; Voituriez A. Enantioselective, Transition Metal catalyzed Cycloisomerizations. Chem. Soc. Rev. 2012, 41, 4884–4908. 10.1039/c2cs35020c. [DOI] [PubMed] [Google Scholar]
- Watson I. D. G.; Toste F. D. Catalytic Enantioselective Carbon-Carbon Bond Formation Using Cycloisomerization Reactions. Chem. Sci. 2012, 3, 2899–2919. 10.1039/c2sc20542d. [DOI] [Google Scholar]
- López F.; Mascareñas J. L. Gold(I)-Catalyzed Enantioselective Cycloaddition Reactions. Beilstein J. Org. Chem. 2013, 9, 2250–2264. 10.3762/bjoc.9.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cera G.; Bandini M. Enantioselective Gold(I) Catalysis with Chiral Monodentate Ligands. Isr. J. Chem. 2013, 53, 848–855. 10.1002/ijch.201300029. [DOI] [Google Scholar]
- Wang Y.-M.; Lackner A. D.; Toste F. D. Development of Catalysts and Ligands for Enantioselective Gold Catalysis. Acc. Chem. Res. 2014, 47, 889–901. 10.1021/ar400188g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi W.; Toste F. D. Recent Advances in Enantioselective Gold Catalysis. Chem. Soc. Rev. 2016, 45, 4567–4589. 10.1039/C5CS00929D. [DOI] [PubMed] [Google Scholar]
- Li Y.; Li W.; Zhang J. Gold-Catalyzed Enantioselective Annulations. Chem. - Eur. J. 2017, 23, 467–512. 10.1002/chem.201602822. [DOI] [PubMed] [Google Scholar]
- Michalak M.; Kośnik W. Chiral N-heterocyclic Carbene Gold Complexes: Synthesis and Applications in Catalysis. Catalysts 2019, 9, 890. 10.3390/catal9110890. [DOI] [Google Scholar]
- Harris R. J.; Widenhoefer R. A. Gold Carbenes, Gold-Stabilized Carbocations, and Cationic Intermediates Relevant to Gold-Catalysed Enyne Cycloaddition. Chem. Soc. Rev. 2016, 45, 4533–4551. 10.1039/C6CS00171H. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Muratore M. E.; Echavarren A. M. Gold Carbene or Carbenoid: Is There a Difference?. Chem. - Eur. J. 2015, 21, 7332–7339. 10.1002/chem.201406318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F.; Song C.; Ma Y.; Zhou L.; Tung C.-H.; Xu Z. Gold Carbene Chemistry from Diazo Compounds. Sci. Bull. 2015, 60, 1479–1492. 10.1007/s11434-015-0874-0. [DOI] [Google Scholar]
- Liu L.; Zhang J. Gold-Catalyzed Transformations of α-Diazocarbonyl Compounds: Selectivity and Diversity. Chem. Soc. Rev. 2016, 45, 506–516. 10.1039/C5CS00821B. [DOI] [PubMed] [Google Scholar]
- Qian D.; Zhang J. Gold-Catalyzed Cyclopropanation Reactions Using a Carbenoid Precursor Toolbox. Chem. Soc. Rev. 2015, 44, 677–698. 10.1039/C4CS00304G. [DOI] [PubMed] [Google Scholar]
- Jia M.; Ma S. New Approaches to the Synthesis of Metal Carbenes. Angew. Chem., Int. Ed. 2016, 55, 9134–9166. 10.1002/anie.201508119. [DOI] [PubMed] [Google Scholar]
- Maas G. New Syntheses of Diazo Compounds. Angew. Chem., Int. Ed. 2009, 48, 8186–8195. 10.1002/anie.200902785. [DOI] [PubMed] [Google Scholar]
- Ye T.; McKervey M. A. Organic Synthesis with α-Diazo Carbonyl Compounds. Chem. Rev. 1994, 94, 1091–1160. 10.1021/cr00028a010. [DOI] [Google Scholar]
- Doyle M. P.; Forbes D. C. Recent Advances in Asymmetric Catalytic Metal Carbene Transformations. Chem. Rev. 1998, 98, 911–935. 10.1021/cr940066a. [DOI] [PubMed] [Google Scholar]
- Fulton J. R.; Aggarwal V. K.; de Vicente J. The Use of Tosylhydrazone Salts as a Safe Alternative for Handling Diazo Compounds and Their Applications in Organic Synthesis. Eur. J. Org. Chem. 2005, 2005, 1479–1492. 10.1002/ejoc.200400700. [DOI] [Google Scholar]
- Xia Y.; Wang J. N-Tosylhydrazones: Versatile Synthons in the Construction of Cyclic Compounds. Chem. Soc. Rev. 2017, 46, 2306–2362. 10.1039/C6CS00737F. [DOI] [PubMed] [Google Scholar]
- Coelho P. S.; Brustad E. M.; Kannan A.; Arnold F. H. Olefin Cyclopropanation via Carbene Transfer Catalyzed by Engineered Cytochrome P450 Enzymes. Science 2013, 339, 307–310. 10.1126/science.1231434. [DOI] [PubMed] [Google Scholar]
- Nishimura T.; Maeda Y.; Hayashi T. Asymmetric Cyclopropanation of Alkenes with Dimethyl Diazomalonate Catalyzed by Chiral Diene-Rhodium Complexes. Angew. Chem., Int. Ed. 2010, 49, 7324–7327. 10.1002/anie.201003775. [DOI] [PubMed] [Google Scholar]
- Kornecki K. P.; Briones J. F.; Boyarskikh V.; Fullilove F.; Autschbach J.; Schrote K. E.; Lancaster K. M.; Davies H. M. L.; Berry J. F. Direct Spectroscopic Characterization of a Transitory Dirhodium Donor-Acceptor Carbene Complex. Science 2013, 342, 351–354. 10.1126/science.1243200. [DOI] [PubMed] [Google Scholar]
- Nishiyama H.; Itoh Y.; Matsumoto H.; Park S.-B.; Itoh K. New Chiral Ruthenium Bis(oxazolinyl)pyridine Catalyst. Efficient Asymmetric Cyclopropanation of Olefins with Diazoacetates. J. Am. Chem. Soc. 1994, 116, 2223–2224. 10.1021/ja00084a104. [DOI] [Google Scholar]
- Maas G. Ruthenium-Catalysed Carbenoid Cyclopropanation Reactions with Diazo Compounds. Chem. Soc. Rev. 2004, 33, 183–190. 10.1039/b309046a. [DOI] [PubMed] [Google Scholar]
- Kim B. G.; Snapper M. L. Preparation of Alkenyl Cyclopropanes through a Ruthenium-Catalyzed Tandem Enyne Metathesis-Cyclopropanation Sequence. J. Am. Chem. Soc. 2006, 128, 52–53. 10.1021/ja055993l. [DOI] [PubMed] [Google Scholar]
- Montesinos-Magraner M.; Costantini M.; Ramírez-Contreras R.; Muratore M. E.; Johansson M. J.; Mendoza A. General Cyclopropane Assembly by Enantioselective Transfer of a Redox-Active Carbene to Aliphatic Olefins. Angew. Chem., Int. Ed. 2019, 58, 5930–5935. 10.1002/anie.201814123. [DOI] [PubMed] [Google Scholar]
- Dai X.; Warren T. H. Discrete Bridging and Terminal Copper Carbenes in Copper-Catalyzed Cyclopropanation. J. Am. Chem. Soc. 2004, 126, 10085–10094. 10.1021/ja047935q. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Zhang Y.; Wang J. Recent Developments in Copper-Catalyzed Reactions of Diazo Compounds. Chem. Commun. 2012, 48, 10162–10173. 10.1039/c2cc34406h. [DOI] [PubMed] [Google Scholar]
- Zhu S.; Perman J. A.; Zhang X. P. Acceptor/Acceptor-Substituted Diazo Reagents for Carbene Transfers: Cobalt-Catalyzed Asymmetric Z-Cyclopropanation of Alkenes with α-Nitrodiazoacetates. Angew. Chem., Int. Ed. 2008, 47, 8460–8463. 10.1002/anie.200803857. [DOI] [PubMed] [Google Scholar]
- Marquard S. L.; Bezpalko M. W.; Foxman B. M.; Thomas C. M. Stoichiometric C=O Bond Oxidative Addition of Benzophenone by a Discrete Radical Intermediate To Form a Cobalt(I) Carbene. J. Am. Chem. Soc. 2013, 135, 6018–6021. 10.1021/ja4022683. [DOI] [PubMed] [Google Scholar]
- Otte M.; Kuijpers P. F.; Troeppner O.; Ivanović-Burmazović I.; Reek J. N. H.; de Bruin B. Encapsulated Cobalt−Porphyrin as a Catalyst for Size-Selective Radical-type Cyclopropanation Reactions. Chem. - Eur. J. 2014, 20, 4880–4884. 10.1002/chem.201400055. [DOI] [PubMed] [Google Scholar]
- Straub B. F. Pd(0) Mechanism of Palladium-Catalyzed Cyclopropanation of Alkenes by CH2N2: A DFT Study. J. Am. Chem. Soc. 2002, 124, 14195. 10.1021/ja027762+. [DOI] [PubMed] [Google Scholar]
- Chen S.; Ma J.; Wang J. Palladium-Catalyzed Cyclopropanation of Electron-Deficient Olefins with Aryldiazocarbonyl Compounds. Tetrahedron Lett. 2008, 49, 6781–6783. 10.1016/j.tetlet.2008.09.024. [DOI] [Google Scholar]
- Rull S. G.; Álvarez E.; Fructos M. R.; Belderrain T. R.; Pérez P. J. The Elusive Palladium-Diazo Adduct Captured: Synthesis, Isolation and Structural Characterization of [(ArNHC-PPh2)Pd(η2-N2C(Ph)CO2Et)]. Chem. - Eur. J. 2017, 23, 7667–7671. 10.1002/chem.201701362. [DOI] [PubMed] [Google Scholar]
- Fructos M. R.; Díaz-Requejo M. M.; Pérez P. J. Gold and Diazo Reagents: a Fruitful Tool for Developing Molecular Complexity. Chem. Commun. 2016, 52, 7326–7335. 10.1039/C6CC01958G. [DOI] [PubMed] [Google Scholar]
- Fructos M. R.; Belderrain T. R.; de Frémont P.; Scott N. M.; Nolan S. P.; Díaz-Requejo M. M.; Pérez P. J. A Gold Catalyst for Carbene-Transfer Reactions from Ethyl Diazoacetate. Angew. Chem., Int. Ed. 2005, 44, 5284–5288. 10.1002/anie.200501056. [DOI] [PubMed] [Google Scholar]
- Fructos M. R.; de Frémont P.; Nolan S. P.; Díaz-Requejo N. M.; Pérez P. J. Alkane Carbon−Hydrogen Bond Functionalization with (NHC)MCl Precatalysts (M = Cu, Au; NHC = N-Heterocyclic Carbene). Organometallics 2006, 25, 2237–2241. 10.1021/om0507474. [DOI] [Google Scholar]
- de Frémont P.; Stevens E. D.; Fructos M. R.; Díaz-Requejo M. M.; Pérez P. J.; Nolan S. P. Synthesis, Isolation and Characterization of Cationic Gold(I) N-Heterocyclic Carbene (NHC) Complexes. Chem. Commun. 2006, 2045–2047. 10.1039/B601547F. [DOI] [PubMed] [Google Scholar]
- Prieto A.; Fructos M. R.; Díaz-Requejo M. M.; Pérez P. J.; Pérez-Galán P.; Delpont N.; Echavarren A. M. Gold-Catalyzed Olefin Cyclopropanation. Tetrahedron 2009, 65, 1790–1793. 10.1016/j.tet.2008.10.114. [DOI] [Google Scholar]
- Davies H. M. L.; Bruzinski P. R.; Lake D. H.; Kong N.; Fall M. J. Asymmetric Cyclopropanations by Rhodium(II)N-(Arylsulfonyl)prolinate Catalyzed Decomposition of Vinyldiazomethanes in the Presence of Alkenes. Practical Enantioselective Synthesis of the Four Stereoisomers of 2-Phenylcyclopropan-1-amino Acid. J. Am. Chem. Soc. 1996, 118, 6897–6907. 10.1021/ja9604931. [DOI] [Google Scholar]
- Rull S. G.; Olmos A.; Pérez P. J. Gold-Catalyzed Ethylene Cyclopropanation. Beilstein J. Org. Chem. 2019, 15, 67–71. 10.3762/bjoc.15.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J. A.; Dias H. V. R. Gold(I) Ethylene and Copper(I) Ethylene Complexes Supported by a Polyhalogenated Triazapentadienyl Ligand. Inorg. Chem. 2008, 47, 4448–4450. 10.1021/ic800373u. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Trewyn B. G.; Angelici R. J.; Woo L. K. Catalytic Reactions of Carbene Precursors on Bulk Gold Metal. J. Am. Chem. Soc. 2009, 131, 11734–11743. 10.1021/ja900653s. [DOI] [PubMed] [Google Scholar]
- Corma A.; Domínguez I.; Ródenas T.; Sabater M. J. Stabilization and Recovery of Gold Catalysts in the Cyclopropanation of Alkenes within Ionic Liquids. J. Catal. 2008, 259, 26–35. 10.1016/j.jcat.2008.07.009. [DOI] [Google Scholar]
- Corma A.; Iglesias M.; Llabrés i Xamena F. X.; Sánchez F. Cu and Au Metal−Organic Frameworks Bridge the Gap between Homogeneous and Heterogeneous Catalysts for Alkene Cyclopropanation Reactions. Chem. - Eur. J. 2010, 16, 9789–9795. 10.1002/chem.201000278. [DOI] [PubMed] [Google Scholar]
- Cao Z.-Y.; Wang X.; Tan C.; Zhao X.-L.; Zhou J.; Ding K. Highly Stereoselective Olefin Cyclopropanation of Diazooxindoles Catalyzed by a C2-Symmetric Spiroketal Bisphosphine/Au(I) Complex. J. Am. Chem. Soc. 2013, 135, 8197–8200. 10.1021/ja4040895. [DOI] [PubMed] [Google Scholar]
- Awata A.; Arai T. Catalytic Asymmetric Cyclopropanation with Diazooxindole. Synlett 2012, 24, 29–32. 10.1055/s-0032-1317746. [DOI] [Google Scholar]
- Cao Z.-Y.; Zhou F.; Yu Y.-H.; Zhou J. A Highly Diastereo- and Enantioselective Hg(II)-Catalyzed Cyclopropanation of Diazooxindoles and Alkenes. Org. Lett. 2013, 15, 42–45. 10.1021/ol302998m. [DOI] [PubMed] [Google Scholar]
- Cao Z.-Y.; Wang W.; Liao K.; Wang X.; Zhou J.; Ma J. Catalytic Enantioselective Synthesis of Cyclopropanes Featuring Vicinal All-Carbon Quaternary Stereocenters with a CH2F Group; Study of the Influence of C−F···H−N interactions on reactivity. Org. Chem. Front. 2018, 5, 2960–2968. 10.1039/C8QO00842F. [DOI] [Google Scholar]
- Chu Z.; Tang Z.; Zhang K.; Wang L.; Li W.; Wu H.-H.; Zhang J. Gold(I)-Catalyzed Enantioselective Cyclopropanation of α-Aryl Diazoacetates with Enamides. Organometallics 2019, 38, 4036–4042. 10.1021/acs.organomet.9b00415. [DOI] [Google Scholar]
- Rössler S. L.; Petrone D. A.; Carreira E. M. Iridium-Catalyzed Asymmetric Synthesis of Functionally Rich Molecules Enabled by (Phopshoramidite, Olefin) Ligands. Acc. Chem. Res. 2019, 52, 2657–2672. 10.1021/acs.accounts.9b00209. [DOI] [PubMed] [Google Scholar]
- Buchner E.; Curtius T. Ueber die Einwirkung von Diazoessigäther auf aromatische Kohlenwasserstoffe. Ber. Dtsch. Chem. Ges. 1885, 18, 2377–2379. 10.1002/cber.188501802119. [DOI] [Google Scholar]
- Dave V.; Warnhoff E. W. The Reactions of Diazoacetic Esters with Alkenes, Alkynes, Heterocyclic and Aromatic Compounds. Org. React. 2011, 18, 217–401. 10.1002/0471264180.or018.03. [DOI] [Google Scholar]
- Reisman S. E.; Nani R. R.; Levin S. Buchner and Beyond: Arene Cyclopropanation as Applied to Natural Product Total Synthesis. Synlett 2011, 2011, 2437–2442. 10.1055/s-0031-1289520. [DOI] [Google Scholar]
- McNamara O. A.; Maguire A. R. The Norcaradiene−Cycloheptatriene Equilibrium. Tetrahedron 2011, 67, 9–40. 10.1016/j.tet.2010.10.030. [DOI] [Google Scholar]
- Li Z.; Ding X.; He C. Nitrene Transfer Reactions Catalyzed by Gold Complexes. J. Org. Chem. 2006, 71, 5876–5880. 10.1021/jo060016t. [DOI] [PubMed] [Google Scholar]
- Delgado-Rebollo M.; Beltrán A.; Prieto A.; Díaz-Requejo M. M.; Echavarren A. M.; Pérez P. J. Catalytic Hydrocarbon Functionalization with Gold Complexes Containing N-Heterocyclic Carbene Ligands with Pendant Donor Groups. Eur. J. Inorg. Chem. 2012, 2012, 1380–1386. 10.1002/ejic.201101158. [DOI] [Google Scholar]
- Fructos M. R.; Belderrain T. R.; Nicasio M. C.; Nolan S. P.; Kaur H.; Díaz-Requejo M. M.; Pérez P. J. Complete Control of the Chemoselectivity in Catalytic Carbene Transfer Reactions from Ethyl Diazoacetate: An N-Heterocyclic Carbene-Cu System That Suppresses Diazo Coupling. J. Am. Chem. Soc. 2004, 126, 10846–10847. 10.1021/ja047284y. [DOI] [PubMed] [Google Scholar]
- Caballero A.; Despagnet-Ayoub D.; Díaz-Requejo M. M.; Díaz-Rodríguez A.; González-Núñez M. E.; Mello R.; Muñoz B. K.; Ojo W.-S.; Asensio G.; Etienne M.; Pérez P. J. Silver-Catalyzed C−C Bond Formation Between Methane and Ethyl Diazoacetate in Supercritical CO2. Science 2011, 332, 835–838. 10.1126/science.1204131. [DOI] [PubMed] [Google Scholar]
- Besora M.; Olmos A.; Gava R.; Noverges B.; Asensio G.; Caballero A.; Maseras F.; Pérez P. J. A Quantitative Model for Alkane Nucleophilicity Based on C−H Bond Structural/Topological Descriptors. Angew. Chem., Int. Ed. 2020, 59, 3112–3116. 10.1002/anie.201914386. [DOI] [PubMed] [Google Scholar]
- Fructos M. R.; Besora M.; Braga A. A. C.; Díaz-Requejo M. M.; Maseras F.; Pérez P. J. Mechanistic Studies on Gold-Catalyzed Direct Arene C−H Bond Functionalization by Carbene Insertion: The Coinage-Metal Effect. Organometallics 2017, 36, 172–179. 10.1021/acs.organomet.6b00604. [DOI] [Google Scholar]
- Pérez P. J.; Díaz-Requejo M. M.; Rivilla I. Gold-Catalyzed Naphthalene Functionalization. Beilstein J. Org. Chem. 2011, 7, 653–657. 10.3762/bjoc.7.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopova M. N.; Doyle M. P.; Mueller P.; Ene D. High Enantioselectivity for Intermolecular Cyclopropenation of Alkynes by Diazo Esters Catalyzed by Chiral Dirhodium(II) Carboxamides. J. Am. Chem. Soc. 1992, 114, 2755–2757. 10.1021/ja00033a081. [DOI] [Google Scholar]
- Lou Y.; Horikawa M.; Kloster R. A.; Hawryluk N. A.; Corey E. J. A New Chiral Rh(II) Catalyst for Enantioselective [2 + 1]-Cycloaddition. Mechanistic Implications and Applications. J. Am. Chem. Soc. 2004, 126, 8916–8918. 10.1021/ja047064k. [DOI] [PubMed] [Google Scholar]
- Briones J. F.; Hansen J.; Hardcastle K. I.; Autschbach J.; Davies H. M. L. Highly Enantioselective Rh2(S-DOSP)4-Catalyzed Cyclopropenation of Alkynes with Styryldiazoacetates. J. Am. Chem. Soc. 2010, 132, 17211–17215. 10.1021/ja106509b. [DOI] [PubMed] [Google Scholar]
- Cui X.; Xu X.; Lu H.; Zhu S.; Wojtas L.; Zhang X. P. Enantioselective Cyclopropenation of Alkynes with Acceptor/Acceptor-Substituted Diazo Reagents via Co(II)-Based Metalloradical Catalysis. J. Am. Chem. Soc. 2011, 133, 3304–3307. 10.1021/ja111334j. [DOI] [PubMed] [Google Scholar]
- Thomas T. J.; Merritt B. A.; Lemma B. E.; McKoy A. M.; Nguyen T.; Swenson A. K.; Mills J. L.; Coleman M. G. Cyclopropenation of Internal Alkynylsilanes and Diazoacetates Catalyzed by Copper(I) N-Heterocyclic Carbene Complexes. Org. Biomol. Chem. 2016, 14, 1742–1747. 10.1039/C5OB02259B. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Li Q.; Liao P.; Bi X. Silver-Catalyzed [2 + 1] Cyclopropenation of Alkynes with Unstable Diazoalkanes: N-Nosylhydrazones as Room-Temperature Decomposable Diazo Surrogates. Chem. - Eur. J. 2017, 23, 4756–4760. 10.1002/chem.201605335. [DOI] [PubMed] [Google Scholar]
- Miege F.; Meyer C.; Cossy J. When Cyclopropenes Meet Gold Catalysts. Beilstein J. Org. Chem. 2011, 7, 717–734. 10.3762/bjoc.7.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones J. F.; Davies H. M. L. Gold(I)-Catalyzed Asymmetric Cyclopropenation of Internal Alkynes. J. Am. Chem. Soc. 2012, 134, 11916–11919. 10.1021/ja304506g. [DOI] [PubMed] [Google Scholar]
- Briones J. F.; Davies H. M. L. Silver Triflate-Catalyzed Cyclopropenation of Internal Alkynes with Donor-/Acceptor-Substituted Diazo Compounds. Org. Lett. 2011, 13, 3984–3987. 10.1021/ol201503j. [DOI] [PubMed] [Google Scholar]
- Davies H. M. L.; Lee G. H. Dirhodium(II) Tetra(N-(dodecylbenzenesulfonyl)prolinate) Catalyzed Enantioselective Cyclopropenation of Alkynes. Org. Lett. 2004, 6, 1233–1236. 10.1021/ol049928c. [DOI] [PubMed] [Google Scholar]
- LeWinn E. B. Diazomethane Poisoning: Report of a Fatal Case with Autopsy. Am. J. Med. Sci. 1949, 218, 556–562. 10.1097/00000441-194911000-00011. [DOI] [PubMed] [Google Scholar]
- Green S. P.; Wheelhouse K. M.; Payne A. D.; Hallett J. P.; Miller P. W.; Bull J. A. Thermal Stability and Explosive Hazard Assessment of Diazo Compounds and Diazo Transfer Reagents. Org. Process Res. Dev. 2020, 24, 67–84. 10.1021/acs.oprd.9b00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D.; Chen L.; Fan H.; Yao Q.; Zhu S. Recent Progress on Donor and Donor−Donor Carbenes. Chem. Soc. Rev. 2020, 49, 908–950. 10.1039/C9CS00542K. [DOI] [PubMed] [Google Scholar]
- Mato M.; García-Morales C.; Echavarren A. M. Generation of Gold(I) Carbenes by Retro-Buchner Reaction: From Cyclopropanes to Natural Products Synthesis. ChemCatChem 2019, 11, 53–72. 10.1002/cctc.201801201. [DOI] [Google Scholar]
- Volger H. C.; Hogeveen H.; Roobeek C. F. Transition-Metal-Catalysed Cheletropic Elimination of Carbene and Oxygen from Seven-Membered Ring Systems. Recl. Trav. Chim. Pays-Bas 1973, 92, 1223–1231. 10.1002/recl.19730921106. [DOI] [Google Scholar]
- Gassman P. G.; Johnson T. H. Retrocarbene Additions. Dissection of Alkyl-Substituted Cyclopropanes under Metathesis Conditions. J. Am. Chem. Soc. 1976, 98, 6057–6058. 10.1021/ja00435a057. [DOI] [Google Scholar]
- Gassman P. G.; Johnson T. H. Cyclopropane-Olefin Cross Metathesis. J. Am. Chem. Soc. 1976, 98, 6058–6059. 10.1021/ja00435a058. [DOI] [Google Scholar]
- Richardson D. B.; Durrett L. R.; Martin J. M.; Putnam W. E.; Slaymaker S. C.; Dvoretzky I. Generation of Methylene by Photolysis of Hydrocarbons. J. Am. Chem. Soc. 1965, 87, 2763–2765. 10.1021/ja01090a047. [DOI] [Google Scholar]
- Glick H. C.; Likhotvorik I. R.; Jones M. A Photochemical Source of Real Alkylcarbenes. Tetrahedron Lett. 1995, 36, 5715–5718. 10.1016/00404-0399(50)1113V-. [DOI] [Google Scholar]
- Nigam M.; Platz M. S.; Showalter B. M.; Toscano J. P.; Johnson R.; Abbot S. C.; Kirchhoff M. M. Generation and Study of Benzylchlorocarbene from a Phenanthrene Precursor. J. Am. Chem. Soc. 1998, 120, 8055–8059. 10.1021/ja980251w. [DOI] [Google Scholar]
- Moore K. A.; Vidaurri-Martinez J. S.; Thamattoor D. M. The Benzylidenecarbene−Phenylacetylene Rearrangement: An Experimental and Computational Study. J. Am. Chem. Soc. 2012, 134, 20037–20040. 10.1021/ja310440e. [DOI] [PubMed] [Google Scholar]
- Saito K.; Kozaki M.; Takahashi K. Aromatization and Hydrogen-Shift of 7-Substituted 1, 3, 5-Cycloheptatrienes in the Presence of Palladium(II) Acetate. Chem. Pharm. Bull. 1993, 41, 2187–2189. 10.1248/cpb.41.2187. [DOI] [Google Scholar]
- Takaya H.; Suzuki T.; Kumagai Y.; Hosoya M.; Kawauchi H.; Noyori R. Nickel-Catalyzed Reactions Involving Strained Bonds. 17. Nickel(0)-Catalyzed Reactions of Bicyclo[1.1.0]butanes. Geminal Two-Bond Cleavage Reaction and the Stereospecific Olefin Trapping of the Carbenoid Intermediate. J. Org. Chem. 1981, 46, 2854–2861. 10.1021/jo00327a004. [DOI] [Google Scholar]
- Walczak M. A. A.; Wipf P. Rhodium(I)-Catalyzed Cycloisomerizations of Bicyclobutanes. J. Am. Chem. Soc. 2008, 130, 6924–6925. 10.1021/ja802906k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorio-Alvarado C. R.; Echavarren A. M. Gold-Catalyzed Annulation/Fragmentation: Formation of Free Gold Carbenes by Retro-Cyclopropanation. J. Am. Chem. Soc. 2010, 132, 11881–11883. 10.1021/ja104743k. [DOI] [PubMed] [Google Scholar]
- Lauterbach T.; Higuchi T.; Hussong M. W.; Rudolph M.; Rominger F.; Mashima K.; Hashmi A. S. K. Gold-Catalyzed Carbenoid Transfer Reactions of Diynes−Pinacol Rearrangement versus Retro-Buchner Reaction. Adv. Synth. Catal. 2015, 357, 775–781. 10.1002/adsc.201400849. [DOI] [Google Scholar]
- Wang Y.; McGonigal P. R.; Herlé B.; Besora M.; Echavarren A. M. Gold(I) Carbenes by Retro-Buchner Reaction: Generation and Fate. J. Am. Chem. Soc. 2014, 136, 801–809. 10.1021/ja411626v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorio-Alvarado C. R.; Wang Y.; Echavarren A. M. Cyclopropanation with Gold(I) Carbenes by Retro-Buchner Reaction from Cycloheptatrienes. J. Am. Chem. Soc. 2011, 133, 11952–11955. 10.1021/ja205046h. [DOI] [PubMed] [Google Scholar]
- Herlé B.; Holstein P. M.; Echavarren A. M. Stereoselective cis-Vinylcyclopropanation via a Gold(I)-Catalyzed Retro-Buchner Reaction under Mild Conditions. ACS Catal. 2017, 7, 3668–3675. 10.1021/acscatal.7b00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato M.; Herlé B.; Echavarren A. M. Cyclopropanation by Gold- or Zinc-Catalyzed Retro-Buchner Reaction at Room Temperature. Org. Lett. 2018, 20, 4341–4345. 10.1021/acs.orglett.8b01791. [DOI] [PubMed] [Google Scholar]
- Mato M.; Echavarren A. M. Donor Rhodium Carbenes by Retro-Buchner Reaction. Angew. Chem., Int. Ed. 2019, 58, 2088–2092. 10.1002/anie.201813512. [DOI] [PubMed] [Google Scholar]
- Mato M.; García-Morales C.; Echavarren A. M. Synthesis of Trienes by Rhodium-Catalyzed Assembly and Disassembly of Non-Acceptor Cyclopropanes. ACS Catal. 2020, 10, 3564–3570. 10.1021/acscatal.0c00006. [DOI] [Google Scholar]
- Mato M.; Martín-Torres I.; Herlé B.; Echavarren A. M. Cyclopropane-Alkene Metathesis by Gold(I)-Catalyzed Decarbenation of Persistent Cyclopropanes. Org. Biomol. Chem. 2019, 17, 4216–4219. 10.1039/C9OB00359B. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Muratore M. E.; Rong Z.; Echavarren A. M. Formal (4 + 1) Cycloaddition of Methylenecyclopropanes with 7-Aryl-1,3,5-cycloheptatrienes by Triple Gold(I) Catalysis. Angew. Chem., Int. Ed. 2014, 53, 14022–14026. 10.1002/anie.201404029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X.; Mato M.; Echavarren A. M. Gold(I)-Catalyzed Synthesis of Indenes and Cyclopentadienes: Access to (±)-Laurokamurene B and the Skeletons of the Cycloaurenones and Dysiherbols. Angew. Chem., Int. Ed. 2017, 56, 14591–14595. 10.1002/anie.201708947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X.; Zuccarello G.; García-Morales C.; Echavarren A. M. Gold(I)-Catalyzed Intramolecular C(sp3)−H Insertion by Decarbenation of Cycloheptatrienes. Chem. - Eur. J. 2019, 25, 9485–9490. 10.1002/chem.201900919. [DOI] [PubMed] [Google Scholar]
- Fedorov A.; Moret M.-E.; Chen P. Gas-Phase Synthesis and Reactivity of a Gold Carbene Complex. J. Am. Chem. Soc. 2008, 130, 8880–8881. 10.1021/ja802060t. [DOI] [PubMed] [Google Scholar]
- Fedorov A.; Chen P. Electronic Effects in the Reactions of Olefin-Coordinated Gold Carbene Complexes. Organometallics 2009, 28, 1278–1281. 10.1021/om800795v. [DOI] [Google Scholar]
- Batiste L.; Fedorov A.; Chen P. Gold Carbenes via 1,2-Dialkoxycyclopropane Ring-Opening: A Mass Spectrometric and DFT Study of the Reaction Pathways. Chem. Commun. 2010, 46, 3899–3901. 10.1039/c0cc00086h. [DOI] [PubMed] [Google Scholar]
- Fedorov A.; Chen P. Mechanistic Insights from the Gas-Phase Reactivity of Phosphorus-Ylid-Supported Benzylidene Gold Complexes. Organometallics 2010, 29, 2994–3000. 10.1021/om100224h. [DOI] [Google Scholar]
- Fedorov A.; Batiste L.; Bach A.; Birney D. M.; Chen P. Potential Energy Surface for (Retro-)Cyclopropanation: Metathesis with a Cationic Gold Complex. J. Am. Chem. Soc. 2011, 133, 12162–12171. 10.1021/ja2041699. [DOI] [PubMed] [Google Scholar]
- Zhu M.-B.; Wei Y.; Shi M. Recent Developments of Cyclopropene Chemistry. Chem. Soc. Rev. 2011, 40, 5534–5563. 10.1039/c1cs15074j. [DOI] [PubMed] [Google Scholar]
- Seidel G.; Mynott R.; Fürstner A. Elementary Steps of Gold Catalysis: NMR Spectroscopy Reveals the Highly Cationic Character of a “Gold Carbenoid”. Angew. Chem., Int. Ed. 2009, 48, 2510–2513. 10.1002/anie.200806059. [DOI] [PubMed] [Google Scholar]
- Benitez D.; Shapiro N. D.; Tkatchouk E.; Wang Y.; Goddard W. A. III; Toste F. D. A Bonding Model for Gold(I) Carbene Complexes. Nat. Chem. 2009, 1, 482–486. 10.1038/nchem.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echavarren A. M. Carbene or Cation?. Nat. Chem. 2009, 1, 431–433. 10.1038/nchem.344. [DOI] [PubMed] [Google Scholar]
- Bauer J. T.; Hadfield M. S.; Lee A.-L. Gold Catalysed Reactions with Cyclopropenes. Chem. Commun. 2008, 6405–6407. 10.1039/b815891f. [DOI] [PubMed] [Google Scholar]
- Zhu Z.-B.; Shi M. Gold(I)-Catalyzed Cycloisomerization of Arylvinylcyclopropenes: An Efficient Synthetic Protocol for the Construction of Indene Skeletons. Chem. - Eur. J. 2008, 14, 10219–10222. 10.1002/chem.200801370. [DOI] [PubMed] [Google Scholar]
- Li C.; Zeng Y.; Wang J. Au-Catalyzed Isomerization of Cyclopropenes: a Novel Approach to Indene Derivatives. Tetrahedron Lett. 2009, 50, 2956. 10.1016/j.tetlet.2009.03.203. [DOI] [Google Scholar]
- Hadfield M. S.; Bauer J. T.; Glen P. E.; Lee A.-L. Gold(I)-Catalysed Alcohol Additions to Cyclopropenes. Org. Biomol. Chem. 2010, 8, 4090–4095. 10.1039/c0ob00085j. [DOI] [PubMed] [Google Scholar]
- Seraya E.; Slack E.; Ariafard A.; Yates B. F.; Hyland C. J. T. Experimental and Theoretical Investigation into the Gold-Catalyzed Reactivity of Cyclopropenylmethyl Acetates. Org. Lett. 2010, 12, 4768–4771. 10.1021/ol101862u. [DOI] [PubMed] [Google Scholar]
- Hadfield M. S.; Häller L. J. L.; Lee A. L.; Macgregor S. A.; O’Neill J. A. T.; Watson A. M. Computational Studies on the Mechanism of the Gold(I)-Catalysed Rearrangement of Cyclopropenes. Org. Biomol. Chem. 2012, 10, 4433–4440. 10.1039/c2ob25183c. [DOI] [PubMed] [Google Scholar]
- Rajabi N. A.; Atashgah M. J.; BabaAhmadi R.; Hyland C.; Ariafard A. Theoretical Study on the Ring-Opening Reactions of Cyclopropenes Mediated by a AuI Complex. J. Org. Chem. 2013, 78, 9553–9559. 10.1021/jo401544e. [DOI] [PubMed] [Google Scholar]
- Miege F.; Meyer C.; Cossy J. Synthesis of 3-Oxa- and 3-Azabicyclo[4.1.0]heptanes by Gold-Catalyzed Cycloisomerization of Cyclopropenes. Org. Lett. 2010, 12, 4144–4147. 10.1021/ol101741f. [DOI] [PubMed] [Google Scholar]
- Miege F.; Meyer C.; Cossy J. Gold(I)-Catalysed Cycloisomerisation of 1,6-Cyclopropene-enes. Chem. - Eur. J. 2012, 18, 7810–7822. 10.1002/chem.201200566. [DOI] [PubMed] [Google Scholar]
- López-Rodríguez A.; Domínguez G.; Pérez-Castells J. Ruthenium Catalyzed Rearrangement of Ene-cyclopropenes. Divergent Reaction Pathways. J. Org. Chem. 2019, 84, 924–933. 10.1021/acs.joc.8b02849. [DOI] [PubMed] [Google Scholar]
- Wagh S. B.; Hsu Y.-C.; Liu R.-S. Two Distinct Cyclizations of 2-Propenyl-1-ethynyl Benzenes with Aryldiazo Esters Using Au and Rh/Au Catalysts Respectively. ACS Catal. 2016, 6, 7160–7166. 10.1021/acscatal.6b02330. [DOI] [Google Scholar]
- Zhu P.-L.; Tang X.-Y.; Shi M. Gold-Catalyzed Intramolecular Cyclizations of Cyclopropenes with Propargylic Esters. ChemistryOpen 2016, 5, 33–37. 10.1002/open.201500181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield M. S.; Lee A.-L. Gold(I)-Catalysed Synthesis of Conjugated Trienes. Chem. Commun. 2011, 47, 1333–1335. 10.1039/C0CC04217J. [DOI] [PubMed] [Google Scholar]
- González J.; de la Fuente A.; González M. J.; Díez de Tejada L.; López L. A.; Vicente R. Synthesis of 1,2-Divinylcyclopropanes by Metal-Catalyzed Cyclopropanation of 1,3-Dienes with Cyclopropenes as Vinyl Carbene Precursors. Beilstein J. Org. Chem. 2019, 15, 285–290. 10.3762/bjoc.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. C.; Hadfield M. S.; Arrowsmith L.; Macleod K. M.; Mudd R. J.; Jordan-Hore J. A.; Lee A.-L. Divergent Outcomes of Gold(I)-Catalyzed Indole Additions to 3,3-Disubstituted Cyclopropenes. Org. Lett. 2012, 14, 898–901. 10.1021/ol203418u. [DOI] [PubMed] [Google Scholar]
- Mudd R. J.; Young P. C.; Jordan-Hore J. A.; Rosair G. M.; Lee A.-L. Gold(I)-Catalyzed Addition of Thiols and Thioacids to 3,3- Disubstituted Cyclopropenes. J. Org. Chem. 2012, 77, 7633–7639. 10.1021/jo300930c. [DOI] [PubMed] [Google Scholar]
- González M. J.; González J.; López L. A.; Vicente R. Zinc-Catalyzed Alkene Cyclopropanation through Zinc Vinyl Carbenoids Generated from Cyclopropenes. Angew. Chem., Int. Ed. 2015, 54, 12139–12143. 10.1002/anie.201505954. [DOI] [PubMed] [Google Scholar]
- Archambeau A.; Miege F.; Meyer C.; Cossy J. Intramolecular Cyclopropanation and C−H Insertion Reactions with Metal Carbenoids Generated from Cyclopropenes. Acc. Chem. Res. 2015, 48, 1021–1031. 10.1021/acs.accounts.5b00016. [DOI] [PubMed] [Google Scholar]
- Mulks F. F.; Antoni P. W.; Rominger F.; Hashmi A. S. K. Cyclopropenylgold(I) Complexes as Aurated Carbenoids or Quasi-Carbenes. Adv. Synth. Catal. 2018, 360, 1810–1821. 10.1002/adsc.201701526. [DOI] [Google Scholar]
- Dong K.; Marichev K. O.; Doyle M. P. Role of Donor-Acceptor Cyclopropenes in Metal Carbene Reactions. Conversion of E-Substituted Enoldiazoacetates to Z-Substituted Metallo-Enolcarbenes. Organometallics 2019, 38, 4043–4050. 10.1021/acs.organomet.9b00427. [DOI] [Google Scholar]
- Echavarren A. M.; Muratore M. E.; López-Carrillo V.; Escribano-Cuesta A.; Huguet N.; Obradors C.. Gold-Catalyzed Cyclizations of Alkynes with Alkenes and Arenes. Organic Reactions; John Wiley & Sons, Inc., 2017; Vol. 92, pp 1–411. [Google Scholar]
- Schmidbaur H.; Schier A. Gold η2-Coordination to Unsaturated and Aromatic Hydrocarbons: The Key Step in Gold-Catalyzed Organic Transformations. Organometallics 2010, 29, 2–23. 10.1021/om900900u. [DOI] [Google Scholar]
- Zhdanko A.; Ströbele M.; Maier M. E. Coordination Chemistry of Gold Catalysts in Solution: A Detailed NMR Study. Chem. - Eur. J. 2012, 18, 14732–14744. 10.1002/chem.201201215. [DOI] [PubMed] [Google Scholar]
- García-Mota M.; Cabello N.; Maseras F.; Echavarren A. M.; Pérez-Ramírez J.; Lopez N. Selective Homogeneous and Heterogeneous Gold Catalysis with Alkynes and Alkenes: Similar Behavior, Different Origin. ChemPhysChem 2008, 9, 1624–1629. 10.1002/cphc.200800246. [DOI] [PubMed] [Google Scholar]
- Nevado C.; Cárdenas D. J.; Echavarren A. M. Reaction of Enol Ethers with Alkynes Catalyzed by Transition Metals: 5-exo-dig versus 6-endo-dig Cyclizations via Cyclopropyl Platinum or Gold Carbene Complexes. Chem. - Eur. J. 2003, 9, 2627–2635. 10.1002/chem.200204646. [DOI] [PubMed] [Google Scholar]
- Mattalia J.-M.; Nava P. Gold-Catalyzed Cycloisomerizations of 1,6-Enynes. A Computational Study. J. Organomet. Chem. 2014, 749, 335–342. 10.1016/j.jorganchem.2013.09.041. [DOI] [Google Scholar]
- Fürstner A.; Morency L. On the Nature of the Reactive Intermediates in Gold-Catalyzed Cycloisomerization Reactions. Angew. Chem., Int. Ed. 2008, 47, 5030–5033. 10.1002/anie.200800934. [DOI] [PubMed] [Google Scholar]
- Hashmi A. S. K. “High Noon” in Gold Catalysis: Carbene versus Carbocation Intermediates. Angew. Chem., Int. Ed. 2008, 47, 6754–6756. 10.1002/anie.200802517. [DOI] [PubMed] [Google Scholar]
- Jiménez-Núñez E.; Claverie C. K.; Bour C.; Cárdenas D. J.; Echavarren A. M. cis-Selective Single-Cleavage Skeletal Rearrangement of 1,6-Enynes Reveals the Multifaceted Character of the Intermediates in Metal-Catalyzed Cycloisomerizations. Angew. Chem., Int. Ed. 2008, 47, 7892–7895. 10.1002/anie.200803269. [DOI] [PubMed] [Google Scholar]
- Lee Y. T.; Kang Y. K.; Chung Y. K. Au(I)-Catalyzed Cycloisomerization Reaction of Amide- or Ester-Tethered 1,6-Enynes to Bicyclo[3.2.0]hept-6-en-2-ones. J. Org. Chem. 2009, 74, 7922–7934. 10.1021/jo901771p. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; Muñoz M. P.; Buñuel E.; Nevado C.; Cárdenas D. J.; Echavarren A. M. Cationic Gold(I) Complexes: Highly Alkynophilic Catalysts for the exo- and endo-Cyclization of Enynes. Angew. Chem., Int. Ed. 2004, 43, 2402–2406. 10.1002/anie.200353207. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; Muñoz M. P.; López S.; Jiménez-Núñez E.; Nevado C.; Herrero-Gómez E.; Raducan M.; Echavarren A. M. Gold(I)-Catalyzed Cyclizations of 1,6-Enynes: Alkoxycyclizations and exo/endo Skeletal Rearrangements. Chem. - Eur. J. 2006, 12, 1677–1693. 10.1002/chem.200501088. [DOI] [PubMed] [Google Scholar]; Correction:; Chem. - Eur. J. 2008, 14, 5096 - 5096.10.1002/chem.200890064
- Trost B. M. Palladium-Catalyzed Cycloisomerizations of Enynes and Related Reactions. Acc. Chem. Res. 1990, 23, 34–42. 10.1021/ar00170a004. [DOI] [Google Scholar]
- Blum J.; Beer-Kraft H.; Badrieh Y. A Novel Platinum Tetrachloride-Catalyzed Cyclorearrangement of Allyl Propynyl Ethers to 3-Oxabicyclo[4.1.0]heptenes. J. Org. Chem. 1995, 60, 5567–5569. 10.1021/jo00122a043. [DOI] [Google Scholar]
- Chatani N.; Furukawa N.; Sakurai H.; Murai S. PtCl2-Catalyzed Conversion of 1,6- and 1,7-Enynes to 1-Vinylcycloalkenes. Anomalous Bond Connection in Skeletal Reorganization of Enynes. Organometallics 1996, 15, 901–903. 10.1021/om950832j. [DOI] [Google Scholar]
- Fürstner A.; Szillat H.; Gabor B.; Mynott R. Platinum- and Acid-Catalyzed Enyne Metathesis Reactions: Mechanistic Studies and Applications to the Syntheses of Streptorubin B and Metacycloprodigiosin. J. Am. Chem. Soc. 1998, 120, 8305–8314. 10.1021/ja981183g. [DOI] [Google Scholar]
- Oi S.; Tsukamoto I.; Miyano S.; Inoue Y. Cationic Platinum-Complex-Catalyzed Skeletal Reorganization of Enynes. Organometallics 2001, 20, 3704–3709. 10.1021/om010316v. [DOI] [Google Scholar]
- Fürstner A.; Stelzer F.; Szillat H. Platinum-Catalyzed Cycloisomerization Reactions of Enynes. J. Am. Chem. Soc. 2001, 123, 11863–11869. 10.1021/ja0109343. [DOI] [PubMed] [Google Scholar]
- Chatani N.; Morimoto T.; Muto T.; Murai S. Highly Selective Skeletal Reorganization of 1,6- and 1,7-Enynes to 1-Vinylcycloalkenes Catalyzed by [RuCl2(CO)3]2. J. Am. Chem. Soc. 1994, 116, 6049–6050. 10.1021/ja00092a098. [DOI] [Google Scholar]
- Cao P.; Wang B.; Zhang X. Rh-Catalyzed Enyne Cyclosiomerization. J. Am. Chem. Soc. 2000, 122, 6490–6491. 10.1021/ja994220s. [DOI] [Google Scholar]
- Sturla S. J.; Kablaoui N. M.; Buchwald S. L. A Titanocene-Catalyzed Intramolecular Ene Reaction: Cycloisomerization of Enynes and Dienynes. J. Am. Chem. Soc. 1999, 121, 1976–1977. 10.1021/ja9839567. [DOI] [Google Scholar]
- Ojima I.; Tzamarioudaki M.; Li Z.; Donovan R. J. Transition Metal-Catalyzed Carbocyclizations in Organic Synthesis. Chem. Rev. 1996, 96, 635. 10.1021/cr950065y. [DOI] [PubMed] [Google Scholar]
- Inglesby P. A.; Evans P. A. Stereoselective Transition Metal-Catalysed Higher-Order Carbocyclisation Reactions. Chem. Soc. Rev. 2010, 39, 2791–2805. 10.1039/b913110h. [DOI] [PubMed] [Google Scholar]
- Méndez M.; Muñoz M. P.; Echavarren A. M. Platinum-Catalyzed Alkoxy- and Hydroxycyclization of Enynes. J. Am. Chem. Soc. 2000, 122, 11549–11550. 10.1021/ja002577m. [DOI] [Google Scholar]
- Pérez Sestelo J.; Sarandeses L. A.; Martínez M. M.; Alonso-Marañón L. Indium(iii) as π-Acid Catalyst for the Electrophilic Activation of Carbon−Carbon Unsaturated Systems. Org. Biomol. Chem. 2018, 16, 5733–5747. 10.1039/C8OB01426D. [DOI] [PubMed] [Google Scholar]
- Frontier A. J.; Raghavan S.; Danishefsky S. J. A Highly Stereoselective Total Synthesis of Hispidospermidin: Derivation of a Pharmacophore Model. J. Am. Chem. Soc. 2000, 122, 6151–6159. 10.1021/ja9944960. [DOI] [Google Scholar]
- Méndez M.; Muñoz M. P.; Nevado C.; Cárdenas D. J.; Echavarren A. M. Cyclizations of Enynes Catalyzed by PtCl2 or Other Transition Metal Chlorides: Divergent Reaction Pathways. J. Am. Chem. Soc. 2001, 123, 10511–10520. 10.1021/ja0112184. [DOI] [PubMed] [Google Scholar]
- Lee S. I.; Kim S. M.; Kim S. Y.; Chung Y. K. The Gold(I)-Catalyzed Cycloisomerization of 1,6-Enynes to 1,4-Dienes. Synlett 2006, 2006, 2256–2260. 10.1055/s-2006-949629. [DOI] [Google Scholar]; Correction:; Synlett 2009, 2009, 1355 - 1356.10.1055/s-0029-1216749
- Nieto-Oberhuber C.; López S.; Muñoz M. P.; Jiménez-Núñez E.; Buñuel E.; Cárdenas D. J.; Echavarren A. M. Gold(I)-Catalyzed Intramolecular Cyclopropanation of Dienynes. Chem. - Eur. J. 2006, 12, 1694–1702. 10.1002/chem.200501089. [DOI] [PubMed] [Google Scholar]
- Kim S. M.; Park J. H.; Choi S. Y.; Chung Y. K. (N-Heterocyclic Carbene)Gold(I)-Catalyzed Cycloisomerization of Cyclohexadienyl Alkynes to Tetracyclo[3.3.0.02,8.04,6]octanes. Angew. Chem., Int. Ed. 2007, 46, 6172–6175. 10.1002/anie.200702028. [DOI] [PubMed] [Google Scholar]
- Kim S. M.; Park J. H.; Chung Y. K. Au(PPh3)OPOF2-Catalyzed Intramolecular [4 + 2] Cycloaddition Reaction of Dienynes. Chem. Commun. 2011, 47, 6719–6721. 10.1039/c1cc11127b. [DOI] [PubMed] [Google Scholar]
- Park Y.; Kim S. Y.; Park J. H.; Cho J.; Kang Y. K.; Chung Y. K. Gold(I)-Catalyzed Cycloisomerization of Alkynyl Hydroxyallyl Tosylamides to 4-Oxa-6-azatricyclo[3.3.0.02,8]octanes. Chem. Commun. 2011, 47, 5190–5192. 10.1039/c1cc10907c. [DOI] [PubMed] [Google Scholar]
- Ferrer C.; Raducan M.; Nevado C.; Claverie C. K.; Echavarren A. M. Missing Cyclization Pathways and New Rearrangements Unveiled in the Gold(I) and Platinum(II)-Catalyzed Cyclization of 1,6-Enynes. Tetrahedron 2007, 63, 6306–6316. 10.1016/j.tet.2007.02.122. [DOI] [Google Scholar]
- López S.; Herrero-Gómez E.; Pérez-Galán P.; Nieto-Oberhuber C.; Echavarren A. M. Gold(I)-Catalyzed Intermolecular Cyclopropanation of Enynes with Alkenes: Trapping of Two Different Gold Carbenes. Angew. Chem., Int. Ed. 2006, 45, 6029–6032. 10.1002/anie.200602448. [DOI] [PubMed] [Google Scholar]
- Pérez-Galán P.; Herrero-Gómez E.; Hog D. T.; Martin N. J. A.; Maseras F.; Echavarren A. M. Mechanism of the Gold-Catalyzed Cyclopropanation of Alkenes with 1,6-Enynes. Chem. Sci. 2011, 2, 141–149. 10.1039/C0SC00335B. [DOI] [Google Scholar]
- Amijs C. H. M.; Ferrer C.; Echavarren A. M. Gold(I)-Catalysed Arylation of 1,6-Enynes: Different Site Reactivity of Cyclopropyl Gold Carbenes. Chem. Commun. 2007, 698–700. 10.1039/B615335F. [DOI] [PubMed] [Google Scholar]
- Amijs C. H. M.; López-Carrillo V.; Raducan M.; Pérez-Galán P.; Ferrer C.; Echavarren A. M. Gold(I)-Catalyzed Intermolecular Addition of Carbon Nucleophiles to 1,5- and 1,6-Enynes. J. Org. Chem. 2008, 73, 7721–7730. 10.1021/jo8014769. [DOI] [PubMed] [Google Scholar]
- Kale B. S.; Lee H.-F.; Liu R.-S. A Sequential Route to Cyclopentenes from 1,6-Enynes and Diazo Ketones through Gold and Rhodium Catalysis. Adv. Synth. Catal. 2017, 359, 402–409. 10.1002/adsc.201600980. [DOI] [Google Scholar]
- Zhang D.-H.; Wei Y.; Shi M. Gold(I)-Catalyzed Cycloisomerization of Nitrogen- and Oxygen-Tethered Alkylidenecyclopropanes to Tricyclic Compounds. Chem. - Eur. J. 2012, 18, 7026–7029. 10.1002/chem.201200978. [DOI] [PubMed] [Google Scholar]
- Sethofer S. G.; Staben S. T.; Hung O. Y.; Toste F. D. Au(I)-Catalyzed Ring Expanding Cycloisomerizations: Total Synthesis of Ventricosene. Org. Lett. 2008, 10, 4315–4318. 10.1021/ol801760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrak Y.; Simonneau A.; Malacria M.; Gandon V.; Fensterbank L. Gold(I)-Catalysed Cycloisomerisation of 1,6-Enynes into Functionalised Allenes. Chem. Commun. 2010, 46, 865–867. 10.1039/b919240a. [DOI] [PubMed] [Google Scholar]
- Simonneau A.; Harrak Y.; Jeanne-Julien L.; Lemière G.; Mouries-Mansuy V.; Goddard J.-P.; Malacria M.; Fensterbank L. Ring Expansions Within the Gold-Catalyzed Cycloisomerization of O-Tethered 1,6-Enynes. Application to the Synthesis of Natural-Product-like Macrocycles. ChemCatChem 2013, 5, 1096–1099. 10.1002/cctc.201200484. [DOI] [Google Scholar]
- Laher R.; Marin C.; Michelet V. When Gold Meets Perfumes: Synthesis of Olfactive Compounds via Gold-Catalyzed Cycloiosmerization Reactions. Org. Lett. 2020, 22, 4058–4062. 10.1021/acs.orglett.0c00843. [DOI] [PubMed] [Google Scholar]
- Ito S.; Zhai L.; Mikami K. Combination of sp2- and sp3-Type Phosphorus Atoms for Gold Chemistry: Preparation, Structure, and Catalytic Activity of Gold Complexes That Bear Ligated 2-Silyl-1,3-Diphosphapropenes. Chem. - Asian J. 2011, 6, 3077–3083. 10.1002/asia.201100310. [DOI] [PubMed] [Google Scholar]
- Dubarle-Offner J.; Barbazanges M.; Augé M.; Desmarets C.; Moussa J.; Axet M. R.; Ollivier C.; Aubert C.; Fensterbank L.; Gandon V.; Malacria M.; Gontard G.; Amouri H. Gold Compounds Anchored to a Metalated Arene Scaffold: Synthesis, X-ray Molecular Structures, and Cycloisomerization of Enyne. Organometallics 2013, 32, 1665–1673. 10.1021/om301101z. [DOI] [Google Scholar]
- Fourmy K.; Mallet-Ladeira S.; Dechy-Cabaret O.; Gouygou M. Gold Phosphole Complexes as Efficient Catalysts for Alkyne Activation. Organometallics 2013, 32, 1571–1574. 10.1021/om301244m. [DOI] [Google Scholar]
- Medena C.; Calogero F.; Lemoine Q.; Aubert C.; Derat E.; Fensterbank L.; Gontard G.; Khaled O.; Vanthuyne N.; Moussa J.; Ollivier C.; Petit M.; Barbazanges M. A HELIXOL-Derived Bisphosphinite Ligand: Synthesis and Application in Gold-Catalyzed Enynes Cycloisomerization. Eur. J. Org. Chem. 2019, 2019, 2129–2137. 10.1002/ejoc.201801722. [DOI] [Google Scholar]
- Chao C.-M.; Beltrami D.; Toullec P. Y.; Michelet V. Asymmetric Au(I)-Catalyzed Synthesis of Bicyclo[4.1.0]heptene Derivatives via a Cycloisomerization Process of 1,6-Enynes. Chem. Commun. 2009, 6988–6990. 10.1039/b913554e. [DOI] [PubMed] [Google Scholar]
- Pradal A.; Chao C.-M.; Toullec P. Y.; Michelet V. Asymmetric Au-Catalyzed Cycloisomerization of 1,6-Enynes: An Entry to Bicyclo[4.1.0]heptene. Beilstein J. Org. Chem. 2011, 7, 1021–1029. 10.3762/bjoc.7.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller H.; Fürstner A. Concise Synthesis of the Antidepressive Drug Candidate GSK1360707 by a Highly Enantioselective Gold-Catalyzed Enyne Cycloisomerization Reaction. Chem. - Eur. J. 2011, 17, 7764–7767. 10.1002/chem.201101346. [DOI] [PubMed] [Google Scholar]
- Teller H.; Corbet M.; Mantilli L.; Gopakumar G.; Goddard R.; Thiel W.; Fürstner A. One-Point Binding Ligands for Asymmetric Gold Catalysis: Phosphoramidites with a TADDOL-Related but Acyclic Backbone. J. Am. Chem. Soc. 2012, 134, 15331–15342. 10.1021/ja303641p. [DOI] [PubMed] [Google Scholar]
- Guitet M.; Zhang P.; Marcelo F.; Tugny C.; Jiménez-Barbero J.; Buriez O.; Amatore C.; Mouriès-Mansuy V.; Goddard J.-P.; Fensterbank L.; Zhang Y.; Roland S.; Ménand M.; Sollogoub M. NHC−Capped Cyclodextrins (ICyDs): Insulated Metal Complexes, Commutable Multicoordination Sphere, and Cavity-Dependent Catalysis. Angew. Chem., Int. Ed. 2013, 52, 7213–7218. 10.1002/anie.201301225. [DOI] [PubMed] [Google Scholar]
- Yavari K.; Aillard P.; Zhang Y.; Nuter F.; Retailleau P.; Voituriez A.; Marinetti A. Helicenes with Embedded Phosphole Units in Enantioselective Gold Catalysis. Angew. Chem., Int. Ed. 2014, 53, 861–865. 10.1002/anie.201308377. [DOI] [PubMed] [Google Scholar]
- Aillard P.; Voituriez A.; Dova D.; Cauteruccio S.; Licandro E.; Marinetti A. Phosphathiahelicenes: Synthesis and Uses in Enantioselective Gold Catalysis. Chem. - Eur. J. 2014, 20, 12373–12376. 10.1002/chem.201402822. [DOI] [PubMed] [Google Scholar]
- Barreiro E. M.; Boltukhina E. V.; White A. J. P.; Hii K. K. M. Atropisomeric [(diphosphine)Au2Cl2] Complexes and their Catalytic Activity Towards Asymmetric Cycloisomerisation of 1,6-Enynes. Chem. - Eur. J. 2015, 21, 2686–2690. 10.1002/chem.201404496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Isaac K.; Retailleau P.; Betzer J.-F.; Voituriez A.; Marinetti A. Planar Chiral Phosphoramidites with a Paracyclophane Scaffold: Synthesis, Gold(I) Complexes, and Enantioselective Cycloisomerization of Dienynes. Chem. - Eur. J. 2016, 22, 3278–3281. 10.1002/chem.201504658. [DOI] [PubMed] [Google Scholar]
- Zhang P.-C.; Wang Y.; Zhang Z.-M.; Zhang J. Gold(I)/Xiang-Phos-Catalyzed Asymmetric Intramolecular Cyclopropanation of Indenes and Trisubstituted Alkenes. Org. Lett. 2018, 20, 7049–7052. 10.1021/acs.orglett.8b02999. [DOI] [PubMed] [Google Scholar]
- Zuccarello G.; Mayans J. G.; Escofet I.; Scharnagel D.; Kirillova M. S.; Pérez-Jimeno A. H.; Calleja P.; Boothe J. R.; Echavarren A. M. Enantioselective Folding of Enynes by Gold(I) Catalysts with a Remote C2-Chiral Element. J. Am. Chem. Soc. 2019, 141, 11858–11863. 10.1021/jacs.9b06326. [DOI] [PubMed] [Google Scholar]
- Deschamps N. M.; Elitzin V. I.; Liu B.; Mitchell M. B.; Sharp M. J.; Tabet E. A. An Enyne Cycloisomerization Approach to the Triple Reuptake Inhibitor GSK1360707F. J. Org. Chem. 2011, 76, 712–715. 10.1021/jo102098y. [DOI] [PubMed] [Google Scholar]
- Schelwies M.; Dempwolff A. L.; Rominger F.; Helmchen G. Gold-Catalyzed Intermolecular Addition of Carbonyl Compounds to 1,6-Enynes. Angew. Chem., Int. Ed. 2007, 46, 5598–5601. 10.1002/anie.200701378. [DOI] [PubMed] [Google Scholar]
- Schelwies M.; Moser R.; Dempwolff A. L.; Rominger F.; Helmchen G. Gold-Catalyzed Intermolecular Addition of Carbonyl Compounds to 1,6-Enynes: Reactivity, Scope, and Mechanistic Aspects. Chem. - Eur. J. 2009, 15, 10888–10900. 10.1002/chem.200901614. [DOI] [PubMed] [Google Scholar]
- Jiménez-Núñez E.; Raducan M.; Lauterbach T.; Molawi K.; Solorio C. R.; Echavarren A. M. Evolution of Propargyl Ethers into Allylgold Cations in the Cyclization of Enynes. Angew. Chem., Int. Ed. 2009, 48, 6152–6155. 10.1002/anie.200902248. [DOI] [PubMed] [Google Scholar]
- Calleja P.; Pablo Ó.; Ranieri B.; Gaydou M.; Pitaval A.; Moreno M.; Raducan M.; Echavarren A. M. α,β-Unsaturated Gold(I) Carbenes by Tandem Cyclization and 1,5-Alkoxy Migration of 1,6-Enynes: Mechanisms and Applications. Chem. - Eur. J. 2016, 22, 13613–13618. 10.1002/chem.201602347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras J.; Livendahl M.; McGonigal P. R.; Echavarren A. M. Gold(I) as an Artificial Cyclase: Short Stereodivergent Syntheses of (g)-Epiglobulol and (−)-4β,7α- and (−)-4α,7α-Aromadendranediols. Angew. Chem., Int. Ed. 2014, 53, 4896–4899. 10.1002/anie.201402044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaydou M.; Miller R. E.; Delpont N.; Ceccon J.; Echavarren A. M. Synthesis of (+)-Schisanwilsonene A by Tandem Gold-Catalyzed Cyclization/1,5-Migration/Cyclopropanation. Angew. Chem., Int. Ed. 2013, 52, 6396–6399. 10.1002/anie.201302411. [DOI] [PubMed] [Google Scholar]
- Couty S.; Meyer C.; Cossy J. Diastereoselective Gold-Catalyzed Cycloisomerizations of Ene-Ynamides. Angew. Chem., Int. Ed. 2006, 45, 6726–6730. 10.1002/anie.200602270. [DOI] [PubMed] [Google Scholar]
- Couty S.; Meyer C.; Cossy J. Gold-Catalyzed Cycloisomerizations of Ene-Ynamides. Tetrahedron 2009, 65, 1809–1832. 10.1016/j.tet.2008.10.108. [DOI] [PubMed] [Google Scholar]
- Harrak Y.; Blaszykowski C.; Bernard M.; Cariou K.; Mainetti E.; Mouriès V.; Dhimane A.-L.; Fensterbank L.; Malacria M. PtCl2-Catalyzed Cycloisomerizations of 5-En-1-yn-3-ol Systems. J. Am. Chem. Soc. 2004, 126, 8656–8657. 10.1021/ja0474695. [DOI] [PubMed] [Google Scholar]
- Gagosz F. Unusual Gold(I)-Catalyzed Isomerization of 3-Hydroxylated 1,5-Enynes: Highly Substrate-Dependent Reaction Manifolds. Org. Lett. 2005, 7, 4129–4132. 10.1021/ol051397k. [DOI] [PubMed] [Google Scholar]
- Wegener M.; Huber F.; Bolli C.; Jenne C.; Kirsch S. F. Silver-Free Activation of Ligated Gold(I) Chlorides: The Use of [Me3NB12Cl11]− as a Weakly Coordinating Anion in Homogeneous Gold Catalysis. Chem. - Eur. J. 2015, 21, 1328–1336. 10.1002/chem.201404487. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Retailleau P.; Gandon V.; Voituriez A.; Marinetti A. Use of Planar Chiral Ferrocenylphosphine-Gold(I) Complexes in the Asymmetric Cycloisomerization of 3-Hydroxylated 1,5-Enynes. Eur. J. Org. Chem. 2016, 2016, 70–75. 10.1002/ejoc.201501121. [DOI] [Google Scholar]
- Zhang Y.; Jullien H.; Brissy D.; Retailleau P.; Voituriez A.; Marinetti A. Platinum(II) Catalyzed Enantioselective Cycloisomerizations of 3-Hydroxylated 1,5-Enynes. ChemCatChem 2013, 5, 2051–2057. 10.1002/cctc.201200743. [DOI] [Google Scholar]
- Luzung M. R.; Markham J. P.; Toste F. D. Catalytic Isomerization of 1,5-Enynes to Bicyclo[3.1.0]hexenes. J. Am. Chem. Soc. 2004, 126, 10858–10859. 10.1021/ja046248w. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhang D.; Bi S. Theoretical Investigation on the Isomerization Reaction of 4-Phenyl-hexa-1,5-enyne Catalyzed by Homogeneous Au Catalysts. J. Phys. Chem. A 2010, 114, 12893–12899. 10.1021/jp105292s. [DOI] [PubMed] [Google Scholar]
- Fan T.; Chen X.; Sun J.; Lin Z. DFT Studies on Gold-Catalyzed Cycloisomerization of 1,5-Enynes. Organometallics 2012, 31, 4221–4227. 10.1021/om300167u. [DOI] [Google Scholar]
- Zhang L.; Kozmin S. A. Gold-Catalyzed Cycloisomerization of Siloxy Enynes to Cyclohexadienes. J. Am. Chem. Soc. 2004, 126, 11806–11807. 10.1021/ja046112y. [DOI] [PubMed] [Google Scholar]
- Sun J.; Conley M. P.; Zhang L.; Kozmin S. A. Au- And Pt-Catalyzed Cycloisomerizations of 1,5-Enynes to Cyclohexadienes with a Broad Alkyne Scope. J. Am. Chem. Soc. 2006, 128, 9705–9710. 10.1021/ja063384n. [DOI] [PubMed] [Google Scholar]
- Soriano E.; Marco-Contelles J. A DFT-Based Analysis of the Gold-Catalyzed Cycloisomerization of 1-Siloxy 1,5-Enynes to Cyclohexadienes. J. Org. Chem. 2012, 77, 6231–6238. 10.1021/jo301057j. [DOI] [PubMed] [Google Scholar]
- Patil N. T. Chirality Transfer and Memory of Chirality in Gold-Catalyzed Reactions. Chem. - Asian J. 2012, 7, 2186–2194. 10.1002/asia.201200194. [DOI] [PubMed] [Google Scholar]
- Bohan P. T.; Toste F. D. Well-Defined Chiral Gold(III) Complex Catalyzed Direct Enantioconvergent Kinetic Resolution of 1,5-Enynes. J. Am. Chem. Soc. 2017, 139, 11016–11019. 10.1021/jacs.7b06025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J.; Bourissou D. Well-Defined Chiral Gold(III) Complexes: New Opportunities in Asymmetric Catalysis. Angew. Chem., Int. Ed. 2018, 57, 386–388. 10.1002/anie.201710105. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Kapustin E. A.; Pei X.; Llopis S.; Yaghi O. M.; Toste F. D. Architectural Stabilization of a Gold(III) Catalyst in Metal-Organic Frameworks. Chem. 2020, 6, 142–152. 10.1016/j.chempr.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horino Y.; Yamamoto T.; Ueda K.; Kuroda S.; Toste F. D. Au(I)-Catalyzed Cycloisomerizations Terminated by sp3 C−H Bond Insertion. J. Am. Chem. Soc. 2009, 131, 2809–2811. 10.1021/ja808780r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Zhang D.; Zhou J.; Liu C. Theoretical Elucidation of Au(I)-Catalyzed Cycloisomerizations of Cycloalkyl-substituted 1,5-Enynes: 1,2-alkyl Shift versus C−H Bond Insertion Products. J. Phys. Chem. A 2010, 114, 6164–6170. 10.1021/jp102542r. [DOI] [PubMed] [Google Scholar]
- Kramer S.; Odabachian Y.; Overgaard J.; Rottländer M.; Gagosz F.; Skrydstrup T. Taking Advantage of the Ambivalent Reactivity of Ynamides in Gold Catalysis: A Rare Case of Alkyne Dimerization. Angew. Chem., Int. Ed. 2011, 50, 5090–5094. 10.1002/anie.201100327. [DOI] [PubMed] [Google Scholar]
- Adcock H. V.; Chatzopoulou E.; Davies P. W. Divergent C—H Insertion−Cyclization Cascades of N-Allyl Ynamides. Angew. Chem., Int. Ed. 2015, 54, 15525–15529. 10.1002/anie.201507167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradal A.; Chen Q.; Faudot dit Bel P.; Toullec P. Y.; Michelet V. Gold-Catalyzed Cycloisomerization of Functionalized 1,5-Enynes - An Entry to Polycyclic Framework. Synlett 2012, 2012, 74–79. 10.1055/s-0031-1289867. [DOI] [Google Scholar]
- López-Carrillo V.; Huguet N.; Mosquera Á.; Echavarren A. M. Nature of the Intermediates in Gold(I)-Catalyzed Cyclizations of 1,5-Enynes. Chem. - Eur. J. 2011, 17, 10972–10978. 10.1002/chem.201101749. [DOI] [PubMed] [Google Scholar]
- Escribano-Cuesta A.; López-Carrillo V.; Janssen D.; Echavarren A. M. Gold-Catalyzed Reactions of 1,5- and 1,6-Enynes with Carbonyl Compounds: Cycloaddition vs. Metathesis. Chem. - Eur. J. 2009, 15, 5646–5650. 10.1002/chem.200900668. [DOI] [PubMed] [Google Scholar]
- Chen G.-Q.; Fang W.; Wei Y.; Tang X.-Y.; Shi M. Divergent Reaction Pathways in Gold-Catalyzed Cycloisomerization of 1,5-Enynes Containing a Cyclopropane Ring: Dramatic ortho Substituent and Temperature Effects. Chem. Sci. 2016, 7, 4318–4328. 10.1039/C6SC00058D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello N.; Rodríguez C.; Echavarren A. M. Gold-Catalyzed Cyclizations of 1,7-Enynes. Synlett 2007, 2007, 1753–1758. 10.1055/s-2007-984502. [DOI] [Google Scholar]
- Huang C.; Kothandaraman P.; Koh B. Q.; Chan P. W. H. Study of Substrate Dependence on the Chemoselectivity of the Gold-Catalyzes Cycloisomerisation of Aryl Substituted 1,7-Enynes. Org. Biomol. Chem. 2012, 10, 9067–9078. 10.1039/c2ob26458g. [DOI] [PubMed] [Google Scholar]
- Matsuda T.; Kadowaki S.; Yamaguchi Y.; Murakami M. Gold-Catalysed Intramolecular trans-Allylsilylation of Alkynes Forming 3-Allyl-1-silaindenes. Chem. Commun. 2008, 2744–2746. 10.1039/b804721a. [DOI] [PubMed] [Google Scholar]
- Dorel R.; Echavarren A. M. From Palladium to Gold Catalysis for the Synthesis of Crushed Fullerenes and Acenes. Acc. Chem. Res. 2019, 52, 1812–1823. 10.1021/acs.accounts.9b00227. [DOI] [PubMed] [Google Scholar]
- Wittstein K.; Kumar K.; Waldmann H. Gold(I)-Catalyzed Synthesis of Benzoxocines by an 8-endo-dig Cyclization. Angew. Chem., Int. Ed. 2011, 50, 9076–9080. 10.1002/anie.201103832. [DOI] [PubMed] [Google Scholar]
- Muratore M. E.; Homs A.; Obradors C.; Echavarren A. M. Meeting the Challenge of Intermolecular Gold(I)-Catalyzed Cycloadditions of Alkynes and Allenes. Chem. - Asian J. 2014, 9, 3066–3082. 10.1002/asia.201402395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Morales C.; Echavarren A. M. From Straightforward Gold(I)-Catalyzed Enyne Cyclizations to more Demanding Intermolecular Reactions of Alkynes with Alkenes. Synlett 2018, 29, 2225–2237. 10.1055/s-0037-1610203. [DOI] [Google Scholar]
- Comer E.; Rohan E.; Deng L.; Porco J. A. An Approach to Skeletal Diversity Using Functional Group Pairing of Multifunctional Scaffolds. Org. Lett. 2007, 9, 2123–2126. 10.1021/ol070606t. [DOI] [PubMed] [Google Scholar]
- For the first disclosure of this strategy for the formation of 2-furylcarbenes, using a variety of transition-metal catalysts, see:; Miki K.; Nishino F.; Ohe K.; Uemura S. Novel Approach for Catalytic Cyclopropanation of Alkenes via (2-Furyl)carbene Complexes from 1-Benzoyl-cis-buten-3-yne. J. Am. Chem. Soc. 2002, 124, 5260–5261. 10.1021/ja025776+. [DOI] [PubMed] [Google Scholar]
- Oh C. H.; Lee S. J.; Lee J. H.; Na Y. J. Regioselectivities in Alkyne Activation: Synthesis of 2-(Bicyclo[3.1.0]hexan-1-yl)furan Derivatives by Au-Catalyzed Cyclization and Cyclopropanation. Chem. Commun. 2008, 5794–5796. 10.1039/b812077c. [DOI] [PubMed] [Google Scholar]
- Oh C. H.; Piao L.; Kim J. H. Gold(III)-Catalyzed Intramolecular Furanylation and Cyclopropanation of Acyclic Conjugated Enynones. Synthesis 2013, 45, 174–182. 10.1055/s-0032-1317926. [DOI] [Google Scholar]
- Wang T.; Zhang J. Synthesis of 2-Acylfurans from 3-(1-Alkynyl)-2-alken-1-ones via the Oxidation of Gold-Carbene Intermediates by H2O2. Dalton Trans. 2010, 39, 4270–4273. 10.1039/c0dt00024h. [DOI] [PubMed] [Google Scholar]
- Ma J.; Jiang H.; Zhu S. NHC-AuCl/Selectfluor: A Highly Efficient Catalytic System for Carbene-Transfer Reactions. Org. Lett. 2014, 16, 4472–4475. 10.1021/ol502018d. [DOI] [PubMed] [Google Scholar]
- Wang S.; Zhang G.; Zhang L. Gold-Catalyzed Reaction of Propargylic Carboxylates via an Initial 3,3-Rearrangement. Synlett 2010, 2010, 692–706. 10.1055/s-0029-1219527. [DOI] [Google Scholar]
- Fensterbank L.; Malacria M. Molecular Complexity from Polyunsaturated Substrates: The Gold Catalysis Approach. Acc. Chem. Res. 2014, 47, 953–965. 10.1021/ar4002334. [DOI] [PubMed] [Google Scholar]
- Zriba R.; Gandon V.; Aubert C.; Fensterbank L.; Malacria M. Alkyne versus Allene Activation in Platinum- and Gold-Catalyzed Cycloisomerization of Hydroxylated 1,5-Allenynes. Chem. - Eur. J. 2008, 14, 1482–1491. 10.1002/chem.200701522. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T.; Inuki S.; Oishi S.; Ohno H. Gold(I)-Catalyzed Cascade Cyclization Reactions of Allenynes for the Synthesis of Fused Cyclopropanes and Acenaphthenes. Angew. Chem., Int. Ed. 2019, 58, 7792–7796. 10.1002/anie.201903384. [DOI] [PubMed] [Google Scholar]
- Cao T.; Chen K.; Zhu S. An Efficient Approach to Generate Aryl Carbenes: Gold-Catalyzed Sequential Activation of 1,6-Diynes. Org. Chem. Front. 2017, 4, 450–454. 10.1039/C6QO00769D. [DOI] [Google Scholar]
- Tsuji J.; Mandai T. Palladium-Catalyzed Reactions of Propargylic Compounds in Organic Synthesis. Angew. Chem., Int. Ed. Engl. 1996, 34, 2589–2612. 10.1002/anie.199525891. [DOI] [Google Scholar]
- Mauleón P.; Toste F. D.. Gold-Catalyzed Reactions of Propargyl Esters, Propargyl Alcohols, and Related Compounds. Modern Gold Catalyzed Synthesis; Wiley-VCH, 2012; pp 75–134. [Google Scholar]
- Ayers B.; Chan P. Harnessing the Versatile Reactivity of Propargyl Alcohols and their Derivatives for Sustainable Complex Molecule Synthesis. Synlett 2015, 26, 1305–1339. 10.1055/s-0034-1380402. [DOI] [Google Scholar]
- Strickler H.; Davis J. B.; Ohloff G. Zur Cyclisierung von Dehydrolinalylacetat in Gegenwart von Zinkchlorid. Helv. Chim. Acta 1976, 59, 1328–1332. 10.1002/hlca.19760590435. [DOI] [Google Scholar]
- Rautenstrauch V. 2-Cyclopentenones from 1-Ethynyl-2-propenyl Acetates. J. Org. Chem. 1984, 49, 950–952. 10.1021/jo00179a044. [DOI] [Google Scholar]
- Miki K.; Ohe K.; Uemura S. Ruthenium-Catalyzed Cyclopropanation of Alkenes Using Propargylic Carboxylates as Precursors of Vinylcarbenoids. J. Org. Chem. 2003, 68, 8505–8513. 10.1021/jo034841a. [DOI] [PubMed] [Google Scholar]
- Miki K.; Ohe K.; Uemura S. A New Ruthenium-Catalyzed Cyclopropanation of Alkenes using Propargylic Acetates as a Precursor of Vinylcarbenoids. Tetrahedron Lett. 2003, 44, 2019–2022. 10.1016/S0040-4039(03)00219-3. [DOI] [PubMed] [Google Scholar]
- Miki K.; Fujita M.; Uemura S.; Ohe K. Ru-Catalyzed Ring-Opening and Substitution Reactions of Heteroaromatic Compounds Using Propargylic Carboxylates as Precursors of Vinyl Carbenoids. Org. Lett. 2006, 8, 1741–1743. 10.1021/ol0604769. [DOI] [PubMed] [Google Scholar]
- Sromek A. W.; Kel’in A. V.; Gevorgyan V. A Novel 1,2-Migration of Acyloxy, Phosphatyloxy, and Sulfonyloxy Groups in Allenes: Efficient Synthesis of Tri- and Tetrasubstituted Furans. Angew. Chem., Int. Ed. 2004, 43, 2280–2282. 10.1002/anie.200353535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroodi R. K.; Gevorgyan V. Metal−Catalyzed Double Migratory Cascade Reactions of Propargylic Esters and Phosphates. Chem. Soc. Rev. 2013, 42, 4991–5001. 10.1039/c3cs35514d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainetti E.; Mouriès V.; Fensterbank L.; Malacria M.; Marco-Contelles J. The Effect of a Hydroxy Protecting Group on the PtCl2-Catalyzed Cyclization of Dienynes—A Novel, Efficient, and Selective Synthesis of Carbocycles. Angew. Chem., Int. Ed. 2002, 41, 2132.. [DOI] [PubMed] [Google Scholar]
- Marco-Contelles J.; Soriano E. Recent Developments in the Metal-Catalyzed Reactions of Metallocarbenoids from Propargylic Esters. Chem. - Eur. J. 2007, 13, 1350–1357. 10.1002/chem.200601522. [DOI] [PubMed] [Google Scholar]
- Marion N.; Nolan S. P. Propargylic Esters in Gold Catalysis: Access to Diversity. Angew. Chem., Int. Ed. 2007, 46, 2750–2752. 10.1002/anie.200604773. [DOI] [PubMed] [Google Scholar]
- Qian D.; Zhang J. Gold-Catalyzed Cyclopropanation Reactions Using a Carbenoid Precursor Toolbox. Chem. Soc. Rev. 2015, 44, 677–698. 10.1039/C4CS00304G. [DOI] [PubMed] [Google Scholar]
- Wang S. Transition-Metal Catalyzed Reactions of Propargylic Carboxylates via an Initial 1,2-Acyloxy Migration. Tetrahedron Lett. 2018, 59, 1317–1327. 10.1016/j.tetlet.2018.02.054. [DOI] [Google Scholar]
- Shu X.-Z.; Shu D.; Schienebeck C. M.; Tang W. Rhodium-Catalyzed Acyloxy Migration of Propargylic Esters in Cycloadditions, Inspiration from the Recent ‘’Gold Rush’’. Chem. Soc. Rev. 2012, 41, 7698–7711. 10.1039/c2cs35235d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F.-Q.; Li X.; Xia Y.; Zhang L.; Yu Z.-X. DFT Study of the Mechanisms of In Water Au(I)-Catalyzed Tandem [3,3]-Rearrangement/Nazarov Reaction/[1,2]-Hydrogen Shift of Enynyl Acetates: A Proton-Transport Catalysis Strategy in the Water-Catalyzed [1,2]-Hydrogen Shift. J. Am. Chem. Soc. 2007, 129, 15503–15512. 10.1021/ja071070+. [DOI] [PubMed] [Google Scholar]
- Mauleón P.; Krinsky J. L.; Toste F. D. Mechanistic Studies on Au(I)-Catalyzed [3,3]-Sigmatropic Rearrangements using Cyclopropane Probes. J. Am. Chem. Soc. 2009, 131, 4513–4520. 10.1021/ja900456m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freindorf M.; Cremer D.; Kraka E. Gold(I)-Assisted Catalysis - A Comprehensive View on the [3,3]-Sigmatropic Rearrangement of Allyl Acetate. Mol. Phys. 2018, 116, 611–630. 10.1080/00268976.2017.1382735. [DOI] [Google Scholar]
- Correa A.; Marion N.; Fensterbank L.; Malacria M.; Nolan S. P.; Cavallo L. Golden Carousel in Catalysis: The Cationic Gold/Propargylic Ester Cycle. Angew. Chem., Int. Ed. 2008, 47, 718–721. 10.1002/anie.200703769. [DOI] [PubMed] [Google Scholar]
- Garayalde D.; Gómez-Bengoa E.; Huang X.; Goeke A.; Nevado C. Mechanistic Insights in Gold-Stabilized Nonclassical Carbocations: Gold-Catalyzed Rearrangement of 3-Cyclopropyl Propargylic Acetates. J. Am. Chem. Soc. 2010, 132, 4720–4730. 10.1021/ja909013j. [DOI] [PubMed] [Google Scholar]
- Marion N.; Lemière G.; Correa A.; Costabile C.; Ramón R. S.; Moreau X.; de Frémont P.; Dahmane R.; Hours A.; Lesage D.; Tabet J.-C.; Goddard J.-P.; Gandon V.; Cavallo L.; Fensterbank L.; Malacria M.; Nolan S. P. Gold- and Platinum-Catalyzed Cycloisomerization of Enynyl Esters versus Allenenyl Esters: An Experimental and Theoretical Study. Chem. - Eur. J. 2009, 15, 3243–3260. 10.1002/chem.200801387. [DOI] [PubMed] [Google Scholar]
- Cho E. J. Factors Controlling the Equilibrium of Pt-Catalyzed [1,2]-versus [1,3]-Acyloxy Migration. Chem. - Eur. J. 2012, 18, 4495–4498. 10.1002/chem.201103799. [DOI] [PubMed] [Google Scholar]
- Rao W.; Berry S. N.; Chan P. W. H.; Sally Gold-Catalyzed Cycloisomerization of 1,6,8-Dienyne Carbonates and Esters to cis-Cyclohepta-4,8-diene-Fused Pyrrolidines. Chem. - Eur. J. 2014, 20, 13174–13180. 10.1002/chem.201402500. [DOI] [PubMed] [Google Scholar]
- Soriano E.; Ballesteros P.; Marco-Contelles J. Theoretical Investigation on the Mechanisms of the PtCl2-Mediated Cycloisomerization of Polyfunctionalized 1,6-Enynes. 2. Propargylic Carboxylates. Organometallics 2005, 24, 3182–3191. 10.1021/om050134r. [DOI] [Google Scholar]
- Soriano E.; Marco-Contelles J. On Accounting for Stereoselective Control of the Metal-Catalyzed Rautenstrauch Cyclopropanation by Computational Methods. J. Org. Chem. 2007, 72, 2651–2654. 10.1021/jo062594f. [DOI] [PubMed] [Google Scholar]
- Soriano E.; Marco-Contelles J. Mechanistic Insights on the Cycloisomerization of Polyunsaturated Precursors Catalyzed by Platinum and Gold Complexes. Acc. Chem. Res. 2009, 42, 1026–1036. 10.1021/ar800200m. [DOI] [PubMed] [Google Scholar]
- Computational Mechanisms of Au and Pt Catalyzed Reactions; Soriano E., Marco-Contelles J., Eds.; Topics in Current Chemistry 302; Springer-Verlag: Berlin Heidelberg, 2011. [Google Scholar]
- Fürstner A.; Hannen P. Platinum- and Gold-Catalyzed Rearrangement Reactions of Propargyl Acetates: Total Syntheses of (−)-α-Cubebene, (−)-Cubebol, Sesquicarene and Related Terpenes. Chem. - Eur. J. 2006, 12, 3006–3019. 10.1002/chem.200501299. [DOI] [PubMed] [Google Scholar]
- Fehr C.; Galindo J. Synthesis of (−)-Cubebol by Face-Selective Platinum-, Gold-, or Copper-Catalyzed Cycloisomerization: Evidence for Chirality Transfer. Angew. Chem., Int. Ed. 2006, 45, 2901–2904. 10.1002/anie.200504543. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Schlecker A. A Gold-Catalyzed Entry into the Sesquisabinene and Sesquithujene Families of Terpenoids and Formal Total Syntheses of Cedrene and Cedrol. Chem. - Eur. J. 2008, 14, 9181–9191. 10.1002/chem.200801382. [DOI] [PubMed] [Google Scholar]
- Mézailles N.; Ricard L.; Gagosz F. Phosphine Gold(I) Bis-(Trifluoromethanesulfonyl)imidate Complexes as New Highly Efficient and Air-Stable Catalysts for the Cycloisomerization of Enynes. Org. Lett. 2005, 7, 4133–4136. 10.1021/ol0515917. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Hannen P. Carene Terpenoids by Gold-Catalyzed Cycloisomerization Reactions. Chem. Commun. 2004, 2546–2547. 10.1039/B412354A. [DOI] [PubMed] [Google Scholar]
- Marion N.; de Frémont P.; Lemière G.; Stevens E. D.; Fensterbank L.; Malacria M.; Nolan S. P. AuI-Catalyzed Cycloisomerization of 1,5-Enynes Bearing a Propargylic Acetate: Formation of Unexpected Bicyclo[3.1.0]hexene. Chem. Commun. 2006, 2048–2050. 10.1039/B602839J. [DOI] [PubMed] [Google Scholar]
- Marion N.; Lemière G.; Correa A.; Costabile C.; Ramón R. S.; Moreau X.; de Frémont P.; Dahmane R.; Hours A.; Lesage D.; Tabet J.-C.; Goddard J.-P.; Gandon V.; Cavallo L.; Fensterbank L.; Malacria M.; Nolan S. P. Gold- and Platinum-Catalyzed Cycloisomerization of Enynyl Esters versus Allenenyl Esters: An Experimental and Theoretical Study. Chem. - Eur. J. 2009, 15, 3243–3260. 10.1002/chem.200801387. [DOI] [PubMed] [Google Scholar]
- Moreau X.; Hours A.; Fensterbank L.; Goddard J.-P.; Malacria M.; Thorimbert S. Use of Ionic Liquids in the Platinum- and Gold-Catalyzed Cycloisomerization of Enyne Systems. J. Organomet. Chem. 2009, 694, 561–565. 10.1016/j.jorganchem.2008.10.054. [DOI] [Google Scholar]
- Harrak Y.; Makhlouf M.; Azzaro S.; Mainetti E.; Lopez Romero J. M.; Cariou K.; Gandon V.; Goddard J.-P.; Malacria M.; Fensterbank L. New Elements in the Gold(I)-Catalyzed Cycloisomerization of Enynyl Esters Derivatives Embedding a Cyclohexane Template. J. Organomet. Chem. 2011, 696, 388–399. 10.1016/j.jorganchem.2010.10.016. [DOI] [Google Scholar]
- Fehr C.; Vuagnoux M.; Buzas A.; Arpagaus J.; Sommer H. Gold- and Copper-Catalyzed Cycloisomerizations towards the Synthesis of Thujopsanone-Like Compounds. Chem. - Eur. J. 2011, 17, 6214–6220. 10.1002/chem.201002797. [DOI] [PubMed] [Google Scholar]
- Tugny C.; Khaled O.; Derat E.; Goddard J.-P.; Mouriès-Mansuy V.; Fensterbank L. Gold(I)-Catalyzed Access to Neomerane Skeletons. Org. Chem. Front. 2017, 4, 1906–1916. 10.1039/C7QO00360A. [DOI] [Google Scholar]
- Cao Z.; Gagosz F. Gold-Catalyzed Tandem Cycloisomerization/Cope Rearrangement: An Efficient Access to the Hydroazulenic Motif. Angew. Chem., Int. Ed. 2013, 52, 9014–9018. 10.1002/anie.201304497. [DOI] [PubMed] [Google Scholar]
- Boyer F.-D.; Le Goff X.; Hanna I. Gold(I)-Catalyzed Cycloisomerization of 1,7- and 1,8-Enynes: Application to the Synthesis of a New Allocolchicinoid. J. Org. Chem. 2008, 73, 5163–5166. 10.1021/jo800797c. [DOI] [PubMed] [Google Scholar]
- Watson I. D. G.; Ritter S.; Toste F. D. Asymmetric Synthesis of Medium-Sized Rings by Intramolecular Au(I)-Catalyzed Cyclopropanation. J. Am. Chem. Soc. 2009, 131, 2056–2057. 10.1021/ja8085005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau X.; Goddard J.-P.; Bernard M.; Lemière G.; López-Romero J. M.; Mainetti E.; Marion N.; Mouriès V.; Thorimbert S.; Fensterbank L.; Malacria M. Gold- vs. Platinum-Catalyzed Polycyclizations by O-Acyl Migration. Solvent-Free Reactions. Adv. Synth. Catal. 2008, 350, 43–48. 10.1002/adsc.200700356. [DOI] [Google Scholar]
- Johansson M. J.; Gorin D. J.; Staben S. T.; Toste F. D. Gold(I)-Catalyzed Stereoselective Olefin Cyclopropanation. J. Am. Chem. Soc. 2005, 127, 18002–18003. 10.1021/ja0552500. [DOI] [PubMed] [Google Scholar]
- Soriano E.; Marco-Contelles J. New Insights on the Mechanism of the Transition-Metal Stereoselective Olefin Cyclopropanation. Chem. - Eur. J. 2008, 14, 6771–6779. 10.1002/chem.200800305. [DOI] [PubMed] [Google Scholar]
- Teller H.; Flügge S.; Goddard R.; Fürstner A. Enantioselective Gold Catalysis: Opportunities Provided by Monodentate Phosphoramidite Ligands with an Acyclic TADDOL Backbone. Angew. Chem., Int. Ed. 2010, 49, 1949–1953. 10.1002/anie.200906550. [DOI] [PubMed] [Google Scholar]
- Petuškova J.; Bruns H.; Alcarazo M. Cyclopropenylylidene-Stabilized Diaryl and Dialkyl Phosphenium Cations: Applications in Homogeneous Gold Catalysis. Angew. Chem., Int. Ed. 2011, 50, 3799–3802. 10.1002/anie.201100338. [DOI] [PubMed] [Google Scholar]
- Reiersølmoen A. C.; Østrem E.; Fiksdahl A. Gold(III)-Catalysed cis-to-trans Cyclopropyl Isomerization. Eur. J. Org. Chem. 2018, 2018, 3317–3325. 10.1002/ejoc.201800419. [DOI] [Google Scholar]
- Reiersølmoen A. C.; Csókas D.; Pápai I.; Fiksdahl A.; Erdélyi M. Mechanism of Au(III)-Mediated Alkoxycyclization of a 1,6-Enyne. J. Am. Chem. Soc. 2019, 141, 18221–18229. 10.1021/jacs.9b09108. [DOI] [PubMed] [Google Scholar]
- Reiersølmoen A. C.; Fiksdahl A. Pyridine- and Quinoline-Based Gold(III) Complexes: Synthesis, Characterization, and Application. Eur. J. Org. Chem. 2020, 2020, 2867–2877. 10.1002/ejoc.202000139. [DOI] [Google Scholar]
- Reiersølmoen A. C.; Csókás D.; Øien-Ødegaard S.; Vanderkooy A.; Gupta A. K.; Carlsson A.-C. C.; Orthaber A.; Fiksdahl A.; Pápai I.; Erdélyi M. Catalytic Activity of trans-Bis(pyridine)gold Complexes. J. Am. Chem. Soc. 2020, 142, 6439–6446. 10.1021/jacs.0c01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin D. J.; Dubé P.; Toste F. D. Synthesis of Benzonorcaradienes by Gold(I)-Catalyzed [4 + 3] Annulation. J. Am. Chem. Soc. 2006, 128, 14480–14481. 10.1021/ja066694e. [DOI] [PubMed] [Google Scholar]
- Gorin D. J.; Watson I. D. G.; Toste F. D. Fluorenes and Styrenes by Au(I)-Catalyzed Annulation of Enynes and Alkynes. J. Am. Chem. Soc. 2008, 130, 3736–3737. 10.1021/ja710990d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garayalde D.; Krüger K.; Nevado C. Gold-Catalyzed Cyclopenta- and Cycloheptannulation Cascades: A Stereocontrolled Approach to the Scaffold of Frondosins A and B. Angew. Chem., Int. Ed. 2011, 50, 911–915. 10.1002/anie.201006105. [DOI] [PubMed] [Google Scholar]
- Sperger C. A.; Tungen J. E.; Fiksdahl A. Gold(I)-Catalyzed Reactions of Propargyl Esters with Vinyl Derivatives. Eur. J. Org. Chem. 2011, 2011, 3719–3722. 10.1002/ejoc.201100291. [DOI] [Google Scholar]
- Iqbal N.; Sperger C. A.; Fiksdahl A. Gold(I)-Catalysed Alkene Cycloaddition Reactions of Propargyl Acetals. Eur. J. Org. Chem. 2013, 2013, 907–914. 10.1002/ejoc.201201328. [DOI] [Google Scholar]
- Conyers R. C.; Gung B. W. Gold(I)-Catalyzed Divergence in the Preparation of Bicyclic Enol Esters: From Exclusively [3C+2C]-Cycloaddition Reactions to Exclusive Formation of Vinylcyclopropanes. Chem. - Eur. J. 2013, 19, 654–664. 10.1002/chem.201202881. [DOI] [PubMed] [Google Scholar]
- Siah H.-S. M.; Kaur M.; Iqbal N.; Fiksdahl A. Gold(I)-Catalysed Tandem Cyclisation of Propargyl Acetals and Vinyl Esters. Eur. J. Org. Chem. 2014, 2014, 1727–1740. 10.1002/ejoc.201301674. [DOI] [Google Scholar]
- Nzulu F.; Bontemps A.; Robert J.; Barbazanges M.; Fensterbank L.; Goddard J.-P.; Malacria M.; Ollivier C.; Petit M.; Rieger J.; Stoffelbach F. Gold-Catalyzed Polymerization Based on Carbene Polycyclopropanation. Macromolecules 2014, 47, 6652–6656. 10.1021/ma501516s. [DOI] [Google Scholar]
- Rao W.; Koh M. J.; Li D.; Hirao H.; Chan P. W. H. Gold-Catalyzed Cycloisomerization of 1,6-Diyne Carbonates and Esters to 2,4a-Dihydro-1H-fluorenes. J. Am. Chem. Soc. 2013, 135, 7926–7932. 10.1021/ja4032727. [DOI] [PubMed] [Google Scholar]
- Rao W.; Boyle J. W.; Chan P. W. H. Gold-Catalyzed Sequential Cyclization of 1-En-3,9-Diyne Esters to Partially Hydrogenated 3H-Dicyclopenta[a, b]naphthalenes. Chem. - Eur. J. 2016, 22, 6532–6536. 10.1002/chem.201600915. [DOI] [PubMed] [Google Scholar]
- Oh C. K.; Kim J. K.; Piao L.; Yu J.; Kim S. Y. Gold Carbenes from Substituted Propargyl Acetates: Intramolecular Ene-type Reactions to 2-Acetoxynaphthalene Derivatives. Chem. - Eur. J. 2013, 19, 10501–10505. 10.1002/chem.201301384. [DOI] [PubMed] [Google Scholar]
- Lauterbach T.; Gatzweiler S.; Nösel P.; Rudolph M.; Rominger F.; Hashmi A. S. K. Carbene Transfer − A New Pathway for Propargylic Esters in Gold Catalysis. Adv. Synth. Catal. 2013, 355, 2481–2487. 10.1002/adsc.201300572. [DOI] [Google Scholar]
- Lauterbach T.; Ganschow M.; Hussong M. W.; Rudolph M.; Rominger F.; Hashmi A. S. K. Gold-Catalyzed Synthesis of 1-Naphthylcarbenoids and Their Synthetic Utilization in Cyclopropanation Reactions. Adv. Synth. Catal. 2014, 356, 680–686. 10.1002/adsc.201400104. [DOI] [Google Scholar]
- Buzas A.; Gagosz F. Gold(I) Catalyzed Isomerization of 5-en-2-yn-1-yl Acetates: An Efficient Access to Acetoxy Bicyclo[3.1.0]hexenes and 2-Cycloalken-1-ones. J. Am. Chem. Soc. 2006, 128, 12614–12615. 10.1021/ja064223m. [DOI] [PubMed] [Google Scholar]
- Chen C.; Zou Y.; Chen X.; Zhang X.; Rao W.; Chan P. W. H. Gold-Catalyzed Tandem 1,3-Migration/Double Cyclopropanation of 1-Ene-4,n-diyne Esters to Tetracyclodecene and Tetracycloundecene Derivatives. Org. Lett. 2016, 18, 4730–4733. 10.1021/acs.orglett.6b02404. [DOI] [PubMed] [Google Scholar]
- Chen C.; Chen X.; Zhang X.; Wang S.; Rao W.; Chan P. W. H. Gold-Catalyzed Dehydrogenative Cycloisomerization of 1,4-Enyne Esters to 3,5-Disubstituted Phenol Derivates. Adv. Synth. Catal. 2017, 359, 4359–4368. 10.1002/adsc.201701068. [DOI] [Google Scholar]
- Lemière G.; Gandon V.; Cariou K.; Fukuyama T.; Dhimane A.-L.; Fensterbank L.; Malacria M. Tandem Gold(I)-Catalyzed Cyclization/Electrophilic Cyclopropanation of Vinyl Allenes. Org. Lett. 2007, 9, 2207–2209. 10.1021/ol070788r. [DOI] [PubMed] [Google Scholar]
- Lemière G.; Gandon V.; Cariou K.; Hours A.; Fukuyama T.; Dhimane A.-L.; Fensterbank L.; Malacria M. Generation and Trapping of Cyclopentenylidene Gold Species: Four Pathways to Polycyclic Compounds. J. Am. Chem. Soc. 2009, 131, 2993–3006. 10.1021/ja808872u. [DOI] [PubMed] [Google Scholar]
- Mouriès-Mansuy V.; Fensterbank L. Gold-Catalyzed Migration of Propargyl Acetate as an Entry into the Total Synthesis of Natural Products. Isr. J. Chem. 2018, 58, 586–595. 10.1002/ijch.201700074. [DOI] [Google Scholar]
- Gandon V.; Lemière G.; Hours A.; Fensterbank L.; Malacria M. The Role of Bent Acyclic Allene Gold Complexes in Axis-to-Center Chirality Transfers. Angew. Chem., Int. Ed. 2008, 47, 7534–2209. 10.1002/anie.200802332. [DOI] [PubMed] [Google Scholar]
- Demertzidou V. P.; Zografos A. L. Metal−Catalyzed Platinum-Catalyzed Cycloisomerizations of a Common Enyne: A Divergent Entry to Cyclopropane Sesquiterpenoids. Formal Synthesis of Sarcandralactone A. Org. Biomol. Chem. 2016, 14, 6942–6946. 10.1039/C6OB01226D. [DOI] [PubMed] [Google Scholar]
- Sotorríos L.; Demertzidou V. P.; Zografos A. L.; Gómez-Bengoa E. DFT Studies on Metal-Catalyzed Cycloisomerization of trans-1,5-Enynes to Cyclopropane Sesquiterpenoids. Org. Biomol. Chem. 2019, 17, 5112–5120. 10.1039/C9OB00890J. [DOI] [PubMed] [Google Scholar]
- Davies P. W. Alkynes as “Masked” Ylides under Noble-Metal Catalysis. Pure Appl. Chem. 2010, 82, 1537–1544. 10.1351/PAC-CON-09-10-18. [DOI] [Google Scholar]
- Xiao J.; Li X. Gold α-Oxo Carbenoids in Catalysis: Catalytic Oxygen-Atom Transfer to Alkynes. Angew. Chem., Int. Ed. 2011, 50, 7226–7236. 10.1002/anie.201100148. [DOI] [PubMed] [Google Scholar]
- Zhang L. A Non-Diazo Approach to α-Oxo Gold Carbenes via Gold-Catalyzed Alkyne Oxidation. Acc. Chem. Res. 2014, 47, 877–888. 10.1021/ar400181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeom H.-S.; Shin S. Catalytic Access to α-Oxo Gold Carbenes by N−O Bond Oxidants. Acc. Chem. Res. 2014, 47, 966–977. 10.1021/ar4001839. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang L. Recent Developments in the Chemistry of Heteroaromatic N-Oxides. Synthesis 2015, 47, 289–305. 10.1055/s-0034-1379884. [DOI] [Google Scholar]
- Qian D.; Zhang J. Gold-Catalyzed Cyclopropanation Reactions Using a Carbenoid Precursor Toolbox. Chem. Soc. Rev. 2015, 44, 677–698. 10.1039/C4CS00304G. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Wang Z.; Wang Y.; Zhang L. Au-Catalysed Oxidative Cyclisation. Chem. Soc. Rev. 2016, 45, 4448–4458. 10.1039/C5CS00887E. [DOI] [PubMed] [Google Scholar]
- Huple D. B.; Ghorpade S.; Liu R.-S. Recent Advances in Gold-Catalyzed N- and O-Functionalizations of Alkynes with Nitrones, Nitroso, Nitro and Nitroxy Species. Adv. Synth. Catal. 2016, 358, 1348–1367. 10.1002/adsc.201600018. [DOI] [Google Scholar]
- Calleja P.; Dorel R.; Echavarren A. M.. Gold-Catalyzed Oxidation of Alkynes. Science of Synthesis. Catalytic Oxidation in Organic Synthesis; Thieme, 2018; pp 479–528. [Google Scholar]
- Shen W.-B.; Tang X.-T. Recent Advances in Catalytic Asymmetric Intermolecular Oxidation of Alkynes. Org. Biomol. Chem. 2019, 17, 7106–7113. 10.1039/C9OB01096C. [DOI] [PubMed] [Google Scholar]
- Henrion G.; Chavas T. E. J.; Le Goff X.; Gagosz F. Biarylphosphonite Gold(I) Complexes as Superior Catalysts for Oxidative Cyclization of Propynyl Arenes into Indan-2-ones. Angew. Chem., Int. Ed. 2013, 52, 6277–6282. 10.1002/anie.201301015. [DOI] [PubMed] [Google Scholar]
- Schulz J.; Jašíková L.; Škríba A.; Roithová J. Role of Gold(I) α-Oxo Carbenes in the Oxidation Reactions of Alkynes Catalyzed by Gold(I) Complexes. J. Am. Chem. Soc. 2014, 136, 11513–11523. 10.1021/ja505945d. [DOI] [PubMed] [Google Scholar]
- Schulz J.; Jašík J.; Gray A.; Roithová J. Formation of Oxazoles from Elusive Gold(I) α-Oxo Carbenes: A Mechanistic Study. Chem. - Eur. J. 2016, 22, 9827–9834. 10.1002/chem.201601634. [DOI] [PubMed] [Google Scholar]
- Ye L.; Cui L.; Zhang G.; Zhang L. Alkynes as Equivalents of α-Diazo Ketones in Generating α-Oxo Metal Carbenes: A Gold-Catalyzed Expedient Synthesis of Dihydrofuran-3-ones. J. Am. Chem. Soc. 2010, 132, 3258–3259. 10.1021/ja100041e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham C. A.; Mauleón P.; Shapiro N. D.; Sherry B. D.; Toste F. D. Gold(I)-Catalyzed Oxidative Rearrangements. J. Am. Chem. Soc. 2007, 129, 5838–5839. 10.1021/ja071231+. [DOI] [PubMed] [Google Scholar]
- Vasu D.; Hung H.-H.; Bhunia S.; Gawade S. A.; Das A.; Liu R.-S. Gold-Catalyzed Oxidative Cyclization of 1,5-Enynes Using External Oxidants. Angew. Chem., Int. Ed. 2011, 50, 6911–6914. 10.1002/anie.201102581. [DOI] [PubMed] [Google Scholar]
- Qian D.; Zhang J. A Gold(I)-Catalyzed Intramolecular Oxidation-Cyclopropanation Sequence of 1,6-Enynes: A Convenient Access to [n.1.0]Bicycloalkanes. Chem. Commun. 2011, 47, 11152–11154. 10.1039/c1cc14788a. [DOI] [PubMed] [Google Scholar]
- Wang W.; Yang J.; Wang F.; Shi M. Axially Chiral N-Heterocyclic Carbene Gold(I) Complex Catalyzed Asymmetric Cycloisomerization of 1,6-Enynes. Organometallics 2011, 30, 3859–3869. 10.1021/om2004404. [DOI] [Google Scholar]
- Tong X.; Beller M.; Tse M. K. A Palladium-Catalyzed Cyclization-Oxidation Sequence: Synthesis of Bicyclo[3.1.0]hexanes and Evidence for SN2 C−O Bond Formation. J. Am. Chem. Soc. 2007, 129, 4906–4907. 10.1021/ja070919j. [DOI] [PubMed] [Google Scholar]
- Welbes L. L.; Lyons T. W.; Cychosz K. A.; Sanford M. S. Synthesis of Cyclopropanes via Pd(II/IV)-Catalyzed Reactions of Enynes. J. Am. Chem. Soc. 2007, 129, 5836–5837. 10.1021/ja071204j. [DOI] [PubMed] [Google Scholar]
- Yeom H.-S.; Shin S. Au(I)-Catalyzed Intramolecular Oxidative Cyclopropanation of 1,6-Enynes Derived from Propiolamides with Diphenyl Sulfoxide. Org. Biomol. Chem. 2013, 11, 1089–1092. 10.1039/c2ob27394b. [DOI] [PubMed] [Google Scholar]
- Qian D.; Hu H.; Liu F.; Tang B.; Ye W.; Wang Y.; Zhang J. Gold(I)-Catalyzed Highly Diastereo- and Enantioselective Alkyne Oxidation/Cyclopropanation of 1,6-Enynes. Angew. Chem., Int. Ed. 2014, 53, 13751–13755. 10.1002/anie.201407717. [DOI] [PubMed] [Google Scholar]
- Ji K.; Yang F.; Gao S.; Tang J.; Gao J. Gold-Catalyzed Oxidation/C−H Functionalization of Ynones: Efficient and Rapid Access to Functionalized Polycyclic Salicyl Ketones. Chem. - Eur. J. 2016, 22, 10225–10229. 10.1002/chem.201600736. [DOI] [PubMed] [Google Scholar]
- Barrett M. J.; Khan G. F.; Davies P. W.; Grainger R. S. Alkynyl Sulfoxides as α-Sulfinyl Carbene Equivalents: Gold-Catalysed Oxidative Cyclopropanation. Chem. Commun. 2017, 53, 5733–5736. 10.1039/C7CC02244A. [DOI] [PubMed] [Google Scholar]
- Aguilar E.; Sanz R.; Fernández-Rodríguez M. A.; García-García P. 1,3-Dien-5-ynes: Versatile Building Blocks for the Synthesis of Carbo- and Heterocycles. Chem. Rev. 2016, 116, 8256–8311. 10.1021/acs.chemrev.6b00181. [DOI] [PubMed] [Google Scholar]
- Hung H.-H.; Liao Y.-C.; Liu R.-S. Oxidant-Dependent Chemoselectivity in the Gold-Catalyzed Oxidative Cyclizations of 3,4,6,6-Tetrasubstituted 3,5-Dien-1-ynes. J. Org. Chem. 2013, 78, 7970–7976. 10.1021/jo401161h. [DOI] [PubMed] [Google Scholar]
- Homs A.; Muratore M. E.; Echavarren A. M. Enantioselective Total Synthesis of (−)-Nardoaristolone B via a Gold(I)-Catalyzed Oxidative Cyclization. Org. Lett. 2015, 17, 461–463. 10.1021/ol503531n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K.; Zheng Z.; Wang Z.; Zhang L. Enantioselective Oxidative Gold Catalysis Enabled by a Designed Chiral P,N-Bidentate Ligand. Angew. Chem., Int. Ed. 2015, 54, 1245–1249. 10.1002/anie.201409300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren R. J.; Sappong-Kumankumah A.; Stradiotto M. A Highly Versatile Catalyst System for the Cross-Coupling of Aryl Chlorides and Amines. Chem. - Eur. J. 2010, 16, 1983–1991. 10.1002/chem.200902316. [DOI] [PubMed] [Google Scholar]
- Lundgren R. J.; Peters B. D.; Alsabeh P. G.; Stradiotto M. A P,N-Ligand for Palladium-Catalyzed Ammonia Arylation: Coupling of Deactivated Aryl Chloride, Chemoselective Arylations, and Room Temperature Reactions. Angew. Chem., Int. Ed. 2010, 49, 4071–4074. 10.1002/anie.201000526. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Ji K.; Li Y.; Zhang L. Tempering the Reactivities of Postulated α-Oxo Gold Carbenes Using Bidentate Ligands: Implication of Tricoordinated Gold Intermediates and the Development of an Expedient Bimolecular Assembly of 2,4-Disubstituted Oxazoles. J. Am. Chem. Soc. 2012, 134, 17412–17415. 10.1021/ja307948m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineddine A.; Rekhroukh F.; Sosa Carrizo E. D.; Mallet-Ladeira S.; Miqueu K.; Amgoune A.; Bourissou D. Isolation of a Reactive Tricoordinate α-Oxo Gold Carbene Complex. Angew. Chem., Int. Ed. 2018, 57, 1306–1310. 10.1002/anie.201711647. [DOI] [PubMed] [Google Scholar]
- Huple D. B.; Ghorpade S.; Liu R.-S. Gold-Catalyzed Oxidative Cycloadditions to Activate a Quinoline Framework. Chem. - Eur. J. 2013, 19, 12965–12969. 10.1002/chem.201302533. [DOI] [PubMed] [Google Scholar]
- Ji K.; Zhang L. A Non-Diazo Strategy to Cyclopropanation via Oxidatively Generated Gold Carbene: The Benefit of a Conformationally Rigid P,N-Bidentate Ligand. Org. Chem. Front. 2014, 1, 34–38. 10.1039/C3QO00080J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake Y.; Niwa T.; Nakada M. Synthesis of Cycloalkanone-Fused Cyclopropanes by Au(I)-Catalyzed Oxidative Ene-Yne Cyclizations. Tetrahedron Lett. 2014, 55, 6847–6850. 10.1016/j.tetlet.2014.10.084. [DOI] [Google Scholar]
- DeKorver K. A.; Li H.; Lohse A. G.; Hayashi R.; Lu Z.; Zhang Y.; Hsung R. P. Ynamides: A Modern Functional Group for the New Millennium. Chem. Rev. 2010, 110, 5064–5106. 10.1021/cr100003s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evano G.; Coste A.; Jouvin K. Ynamides: Versatile Tools in Organic Synthesis. Angew. Chem., Int. Ed. 2010, 49, 2840–2859. 10.1002/anie.200905817. [DOI] [PubMed] [Google Scholar]
- Evano G.; Jouvin K.; Coste A. General Amination Reactions for the Synthesis of Ynamides. Synthesis 2012, 45, 17–26. 10.1055/s-0032-1317880. [DOI] [Google Scholar]
- Wang X.-N.; Yeom H.-S.; Fang L.-C.; He S.; Ma Z.-X.; Kedrowski B. L.; Hsung R. P. Ynamides in Ring Forming Transformations. Acc. Chem. Res. 2014, 47, 560–578. 10.1021/ar400193g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F.; Shu C.; Ye L.-W. Recent Progress Towards Gold-Catalyzed Synthesis of N-Containing Tricyclic Compounds Based on Ynamides. Org. Biomol. Chem. 2016, 14, 9456–9465. 10.1039/C6OB01774F. [DOI] [PubMed] [Google Scholar]
- Prabagar B.; Ghosh N.; Sahoo A. K. Cyclization and Cycloisomerization of π-Tethered Ynamides: An Expedient Synthetic Method to Construct Carbo- and Heterocycles. Synlett 2017, 28, 2539–2555. 10.1055/s-0036-1590877. [DOI] [Google Scholar]
- Tu Y.; Zeng X.; Wang H.; Zhao J. A Robust One-Step Approach to Ynamides. Org. Lett. 2018, 20, 280–283. 10.1021/acs.orglett.7b03665. [DOI] [PubMed] [Google Scholar]
- Mansfield S. J.; Campbell C. D.; Jones M. W.; Anderson E. A. A Robust and Modular Synthesis of Ynamides. Chem. Commun. 2015, 51, 3316–3319. 10.1039/C4CC07876D. [DOI] [PubMed] [Google Scholar]
- Cook A. M.; Wolf C. Terminal Ynamides: Synthesis, Coupling Reactions, and Additions to Common Electrophiles. Tetrahedron Lett. 2015, 56, 2377–2392. 10.1016/j.tetlet.2015.03.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste A.; Karthikeyan G.; Couty F.; Evano G. Copper-Mediated Coupling of 1,1-Dibromo-1-alkenes with Nitrogen Nucleophiles: A General Method for the Synthesis of Ynamides. Angew. Chem., Int. Ed. 2009, 48, 4381–4385. 10.1002/anie.200901099. [DOI] [PubMed] [Google Scholar]
- Hamada T.; Ye X.; Stahl S. S. Copper-Catalyzed Aerobic Oxidative Amidation of Terminal Alkynes: Efficient Synthesis of Ynamides. J. Am. Chem. Soc. 2008, 130, 833–835. 10.1021/ja077406x. [DOI] [PubMed] [Google Scholar]
- Patil D. V.; Shin S. Organocatalytic Oxidative Functionalizations of Alkynes. Asian J. Org. Chem. 2019, 8, 63–73. 10.1002/ajoc.201800632. [DOI] [Google Scholar]
- Zhou B.; Tan T.-D.; Zhu X.-Q.; Shang M.; Ye L.-W. Reversal of Regioselectivity in Ynamide Chemistry. ACS Catal. 2019, 9, 6393–6406. 10.1021/acscatal.9b01851. [DOI] [Google Scholar]
- Wang K.-B.; Ran R.-Q.; Xiu S.-D.; Li C.-Y. Synthesis of 3-Aza-bicyclo[3.1.0]hexan-2-one Derivatives via Gold-Catalyzed Oxidative Cyclopropanation of N-Allylynamides. Org. Lett. 2013, 15, 2374–2377. 10.1021/ol4007629. [DOI] [PubMed] [Google Scholar]
- Sánchez-Cantalejo F.; Priest J. D.; Davies P. W. A Gold Carbene Manifold to Prepare Fused γ-Lactams by Oxidative Cyclisation of Ynamides. Chem. - Eur. J. 2018, 24, 17215–17219. 10.1002/chem.201804378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.; Winston-McPherson G. N.; Yang Z.-Y.; Zhou X.; Song W.; Guzei I. A.; Xu X.; Tang W. Generation of Rhodium(I) Carbenes from Ynamides and Their Reactions with Alkynes and Alkenes. J. Am. Chem. Soc. 2013, 135, 8201–8204. 10.1021/ja4047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus V.; Molinari L.; Büllmann S.; Thusek J.; Rudolph M.; Rominger F.; Hashmi A. S. K. Gold-Catalyzed Cyclisation by 1,4-Dioxidation. Chem. - Eur. J. 2019, 25, 9385–9389. 10.1002/chem.201900996. [DOI] [PubMed] [Google Scholar]
- Ferrer S.; Echavarren A. M. Synthesis of Barbaralones and Bullvalenes Made Easy by Gold Catalysis. Angew. Chem., Int. Ed. 2016, 55, 11178–11182. 10.1002/anie.201606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer S.; Echavarren A. M. Synthesis of Bullvalenes: Classical Approaches and Recent Developments. Synthesis 2019, 51, 1037–1048. 10.1055/s-0037-1611637. [DOI] [Google Scholar]
- McGonigal P. R.; de León C.; Wang Y.; Homs A.; Solorio-Alvarado C. R.; Echavarren A. M. Gold for the Generation and Control of Fluxional Barbaralyl Cations. Angew. Chem., Int. Ed. 2012, 51, 13093–13096. 10.1002/anie.201207682. [DOI] [PubMed] [Google Scholar]
- Ji K.; Zhang L. Cyclopropanation of Benzene Rings by Oxidatively Generated α-Oxo Gold Carbene: One-Pot Access to Tetrahydropyranone-Fused Cycloheptatrienes from Propargyl Benzyl Ethers. Adv. Synth. Catal. 2018, 360, 647–651. 10.1002/adsc.201701322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G.-Y.; Li C.-W.; Hung S.-H.; Liu R.-S. Diversity in Gold- and Silver-Catalyzed Cycloisomerization of Epoxide-Alkyne Functionalities. Org. Lett. 2008, 10, 5059–5062. 10.1021/ol802047g. [DOI] [PubMed] [Google Scholar]
- Henrion G.; Chavas T. E. J.; Le Goff X.; Gagosz F. Biarylphosphonite Gold(I) Complexes as Superior Catalysts for Oxidative Cyclization of Propynyl Arenes into Indan-2-ones. Angew. Chem., Int. Ed. 2013, 52, 6277–6282. 10.1002/anie.201301015. [DOI] [PubMed] [Google Scholar]
- Chen H.; Zhang L. A Desulfonylative Approach in Oxidative Gold Catalysis: Regiospecific Access to Donor-Substituted Acyl Gold Carbenes. Angew. Chem., Int. Ed. 2015, 54, 11775–11779. 10.1002/anie.201504511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. W.; Garzón M. Nucleophilic Nitrenoids Through π-Acid Catalysis: Providing a Common Basis for Rapid Access into Diverse Nitrogen Heterocycles. Asian J. Org. Chem. 2015, 4, 694–708. 10.1002/ajoc.201500170. [DOI] [Google Scholar]
- Li L.; Tan T.-D.; Zhang Y.-Q.; Liu X.; Ye L.-W. Recent Advances in Transition-Metal-Catalyzed Reactions of Alkynes with Isoxazoles. Org. Biomol. Chem. 2017, 15, 8483–8492. 10.1039/C7OB01895A. [DOI] [PubMed] [Google Scholar]
- Liao Y.; Zhu L.; Yu Y.; Chen G.; Huang X. N-Heterocycle Synthesis via Gold-Catalyzed Intermolecular Nitrene Transfer Reactions of Alkynes. Youji Huaxue 2017, 37, 2785–2799. 10.6023/cjoc201708021. [DOI] [Google Scholar]
- Aguilar E.; Santamaría J. Gold-Catalyzed Heterocyclic Syntheses Through α-Imino Gold Carbene Complexes as Intermediates. Org. Chem. Front. 2019, 6, 1513–1540. 10.1039/C9QO00243J. [DOI] [Google Scholar]
- Zhao X.; Rudolph M.; Asiri A. M.; Hashmi A. S. K. Easy Access to Pharmaceutically Relevant Heterocycles by Catalytic Reactions Involving α-Imino Gold Carbene Intermediates. Front. Chem. Sci. Eng. 2020, 14, 317–349. 10.1007/s11705-019-1874-4. [DOI] [Google Scholar]
- Tian X.; Song L.; Hashmi A. S. K. α-Imino Gold Carbene Intermediates from Readily Accessible Sulfilimines: Intermolecular Access to Structural Diversity. Chem. - Eur. J. 2020, 26, 3197–3204. 10.1002/chem.201904869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahani R. L.; Ye L.-W.; Liu R.-S. Synthesis of Nitrogen-Containing Molecules via Transition Metal-Catalyzed Reactions on Isoxazoles, Anthranils and Benzoisoxazoles. Adv. Organomet. Chem. 2020, 73, 195–251. 10.1016/bs.adomc.2019.12.001. [DOI] [Google Scholar]
- Hung H.-H.; Liao Y.-C.; Liu R.-S. Silver-Catalyzed Stereoselective [3 + 2] Cycloadditions of Cyclopropyl-Indanimines with Carbonyl Compounds. Adv. Synth. Catal. 2013, 355, 1545–1552. 10.1002/adsc.201300090. [DOI] [Google Scholar]
- Giri S. S.; Liu R.-S. Gold-Catalyzed [4 + 3]- and [4 + 2]-Annulations of 3-En-1-ynamides with Isoxazoles via Novel 6π-Electrocyclizations of 3-Azahepta Trienyl Cations. Chem. Sci. 2018, 9, 2991–2995. 10.1039/C8SC00232K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Zhang L. Gold-Catalyzed Nitrene Transfer to Activated Alkynes: Formation of α,β-Unsaturated Amidines. Org. Lett. 2011, 13, 1738–1741. 10.1021/ol2002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a DFT study of this reaction:; Costa I.; Marín-Luna M.; González Comesaña M.; Nieto Faza O.; Silva López C. The Outer-Sphere Mechanism of Nitrene Transfer onto Gold(I) Alkyne Complexes. ChemCatChem 2016, 8, 2387–2392. 10.1002/cctc.201600354. [DOI] [Google Scholar]
- Wetzel A.; Gagosz F. Gold-Catalyzed Transformation of 2-Alkynyl Arylazides: Efficient Access to the Valuable Pseudoindoxyl and Indolyl Frameworks. Angew. Chem., Int. Ed. 2011, 50, 7354–7358. 10.1002/anie.201102707. [DOI] [PubMed] [Google Scholar]
- Lu B.; Luo Y.; Liu L.; Ye L.; Wang Y.; Zhang L. Umpolung Reactivity of Indoles through Gold Catalysis. Angew. Chem., Int. Ed. 2011, 50, 8358–8362. 10.1002/anie.201103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin D. J.; Davis N. R.; Toste F. D. Gold(I)-Catalyzed Intramolecular Acetylenic Schmidt reaction. J. Am. Chem. Soc. 2005, 127, 11260–11261. 10.1021/ja053804t. [DOI] [PubMed] [Google Scholar]
- Huang X.; Klimczyk S.; Veiros L. F.; Maulide N. Stereoselective Intramolecular Cyclopropanation Through Catalytic Olefin Activation. Chem. Sci. 2013, 4, 1105–1110. 10.1039/c2sc21914j. [DOI] [Google Scholar]
- Sabbatani J.; Huang X.; Veiros L. F.; Maulide N. Gold-Catalyzed Intermolecular Synthesis of Alkylidenecyclopropanes through Catalytic Allene Activation. Chem. - Eur. J. 2014, 20, 10636–10639. 10.1002/chem.201402935. [DOI] [PubMed] [Google Scholar]
- Klimczyk S.; Misale A.; Huang X.; Maulide N. Dimeric TADDOL Phosphoramidites in Asymmetric Catalysis: Domino Deracemization and Cyclopropanation of Sulfonium Ylides. Angew. Chem., Int. Ed. 2015, 54, 10365–10369. 10.1002/anie.201503851. [DOI] [PubMed] [Google Scholar]
- Klimczyk S.; Huang X.; Kählig H.; Veiros L. F.; Maulide N. Stereoselective Gold(I) Domino Catalysis of Allylic Isomerization and Olefin Cyclopropanation: Mechanistic Studies. J. Org. Chem. 2015, 80, 5719–5729. 10.1021/acs.joc.5b00666. [DOI] [PubMed] [Google Scholar]
- Tokimizu Y.; Oishi S.; Fujii N.; Ohno H. Gold-Catalyzed Cascade Cyclization of (Azido)ynamides: An Efficient Strategy for the Construction of Indoloquinolines. Org. Lett. 2014, 16, 3138–3141. 10.1021/ol5012604. [DOI] [PubMed] [Google Scholar]
- Tian X.; Song L.; Rudolph M.; Rominger F.; Oeser T.; Hashmi A. S. K. Sulfilimines as Versatile Nitrene Transfer Reagents: Facile Access to Diverse Aza-Heterocycles. Angew. Chem., Int. Ed. 2019, 58, 3589–3593. 10.1002/anie.201812002. [DOI] [PubMed] [Google Scholar]
- Song L.; Tian X.; Rudolph M.; Rominger F.; Hashmi A. S. K. Gold(III)-Catalyzed Chemoselective Annulations of Anthranils with N-Allylynamides for the Synthesis of 3-Azabicyclo[3.1.0]hexan-2-imines. Chem. Commun. 2019, 55, 9007–9010. 10.1039/C9CC04027G. [DOI] [PubMed] [Google Scholar]
- Pellissier H.; Lattanzi A.; Dalpozzo R.. Asymmetric Aziridination in Asymmetric Synthesis of Three-Membered Rings; Wiley-VCH, 2017. [Google Scholar]
- Li Z.; Capretto D. A.; Rahaman R. O.; He C. Gold(III)-Catalyzed Nitrene Insertion into Aromatic and Benzylic C−H Groups. J. Am. Chem. Soc. 2007, 129, 12058–12059. 10.1021/ja0724137. [DOI] [PubMed] [Google Scholar]
- Sakharov P. A.; Rostovskii N. V.; Khlebnikov A. F.; Khoroshilova O. V.; Novikov M. S. Transition Metal-Catalyzed Synthesis of 3-Coumaranone-Containing NH-Aziridines from 2H-Azirines: Nickel(II) versus Gold(I). Adv. Synth. Catal. 2019, 361, 3359–3372. 10.1002/adsc.201900366. [DOI] [Google Scholar]
- Deng X.; Baker T. A.; Friend C. M. A Pathway for NH Addition to Styrene Promoted by Gold. Angew. Chem., Int. Ed. 2006, 45, 7075–7078. 10.1002/anie.200602876. [DOI] [PubMed] [Google Scholar]
- Raptis C.; Garcia H.; Stratakis M. Selective Isomerization of Epoxides to Allylic Alcohols Catalyzed by TiO2-Supported Nanoparticles. Angew. Chem., Int. Ed. 2009, 48, 3133–3136. 10.1002/anie.200805838. [DOI] [PubMed] [Google Scholar]
- Liu L.; Xu B.; Hammond G. B. Construction of Cyclic Enones via Gold-Catalyzed Oxygen Transfer Reactions. Beilstein J. Org. Chem. 2011, 7, 606–614. 10.3762/bjoc.7.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karad S. N.; Bhunia S.; Liu R.-S. Retention of Stereochemistry in Gold-Catalyzed Formal [4 + 3] Cycloaddition of Epoxides with Arenynamides. Angew. Chem., Int. Ed. 2012, 51, 8722–8726. 10.1002/anie.201203723. [DOI] [PubMed] [Google Scholar]
- Thota G. K.; Tarigopula C.; Balamurugan R. Tandem Activation by Gold: Synthesis of Dioxolanes by Intermolecular Reaction of Epoxides and Alkynes in Acetone. Tetrahedron 2015, 71, 2280–2289. 10.1016/j.tet.2015.02.040. [DOI] [Google Scholar]
- Sahani R. L.; Liu R.-S. Gold-Catalyzed [4 + 2] Annulation/Cyclization Cascades of Benzisoxazoles with Propiolate Derivatives to Access Highly Oxygenated Tetrahydroquinolines. Angew. Chem., Int. Ed. 2017, 56, 12736–12740. 10.1002/anie.201707423. [DOI] [PubMed] [Google Scholar]
- Tian X.; Song L.; Farshadfar K.; Rudolph M.; Rominger F.; Oeser T.; Ariafard A.; Hashmi A. S. K. Acyl Migration versus Epoxidation in Gold Catalysis: Facile, Switchable, and Atom-Economic Synthesis of Acylindoles and Quinoline Derivatives. Angew. Chem., Int. Ed. 2020, 59, 471–478. 10.1002/anie.201912334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X.; Friend C. M. Selective Oxidation of Styrene on an Oxygen-Covered Au(111). J. Am. Chem. Soc. 2005, 127, 17178–17179. 10.1021/ja0557031. [DOI] [PubMed] [Google Scholar]
- Guillois K.; Mangematin S.; Tuel A.; Caps V. Gold-Catalyzed Aerobic Epoxidation of trans-Stilbene in Methylcyclohexane. Part II: Identification and Quantification of a Key Reaction Intermediate. Catal. Today 2013, 203, 111–115. 10.1016/j.cattod.2012.03.031. [DOI] [Google Scholar]
- Seidel G.; Fürstner A. Structure of a Reactive Gold Carbenoid. Angew. Chem., Int. Ed. 2014, 53, 4807–4811. 10.1002/anie.201402080. [DOI] [PubMed] [Google Scholar]
- Werlé C.; Goddard R.; Fürstner A. The First Crystal Structure of a Reactive Dirhodium Carbene Complex and a Versatile Method for the Preparation of Gold Carbenes by Rhodium-to-Gold Transmetalation. Angew. Chem., Int. Ed. 2015, 54, 15452–15456. 10.1002/anie.201506902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fañanás-Mastral M.; Aznar F. Carbene Transfer Reactions from Chromium(0) to Gold(I): Synthesis and Reactivity of New Fischer-Type Gold(I) Alkenyl Carbene Complexes. Organometallics 2009, 28, 666–668. 10.1021/om801146z. [DOI] [Google Scholar]
- Brooner R. E. M.; Widenhoefer R. A. Experimental Evaluation of the Electron Donor Ability of a Gold Phosphine Fragment in a Gold Carbene Complex. Chem. Commun. 2014, 50, 2420–2423. 10.1039/C3CC48869A. [DOI] [PubMed] [Google Scholar]
- Harris R. J.; Widenhoefer R. A. Synthesis, Structure, and Reactivity of a Gold Carbenoid Complex That Lacks Heteroatom Stabilization. Angew. Chem., Int. Ed. 2014, 53, 9369–9371. 10.1002/anie.201404882. [DOI] [PubMed] [Google Scholar]
- Hussong M. W.; Rominger F.; Krämer P.; Straub B. F. Isolation of a Non-Heteroatom-Stabilized Gold-Carbene Complex. Angew. Chem., Int. Ed. 2014, 53, 9372–9375. 10.1002/anie.201404032. [DOI] [PubMed] [Google Scholar]
- Joost M.; Estévez L.; Mallet-Ladeira S.; Miqueu K.; Amgoune A.; Bourissou D. Enhanced π-Backdonation from Gold(I): Isolation of Original Carbonyl and Carbene Complexes. Angew. Chem., Int. Ed. 2014, 53, 14512–14516. 10.1002/anie.201407684. [DOI] [PubMed] [Google Scholar]
- Caballero A.; Pérez P. J. Dimensioning the Term Carbenoid. Chem. - Eur. J. 2017, 23, 14389–14393. 10.1002/chem.201702392. [DOI] [PubMed] [Google Scholar]
- Ringger D. H.; Chen P. Rational Design of a Gold Carbene Precursor Complex for a Catalytic Cyclopropanation Reaction. Angew. Chem., Int. Ed. 2013, 52, 4686–4689. 10.1002/anie.201209569. [DOI] [PubMed] [Google Scholar]
- Ringger D. H.; Kobylianskii I. J.; Serra D.; Chen P. Quantitative Description of Structural Effects on the Stability of Gold(I) Carbenes. Chem. - Eur. J. 2014, 20, 14270–14281. 10.1002/chem.201403988. [DOI] [PubMed] [Google Scholar]
- Sarria Toro J. M.; García-Morales C.; Raducan M.; Smirnova E. S.; Echavarren A. M. Gold(I) Carbenoids: On-Demand Access to Gold(I) Carbenes in Solution. Angew. Chem., Int. Ed. 2017, 56, 1859–1863. 10.1002/anie.201611705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Morales C.; Pei X.-L.; Sarria Toro J. M.; Echavarren A. M. Direct Observation of Aryl Gold(I) Carbenes that Undergo Cyclopropanation, C−H Insertion, and Dimerization Reactions. Angew. Chem., Int. Ed. 2019, 58, 3957–3961. 10.1002/anie.201814577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tskhovrebov A. G.; Lingnau J. B.; Fürstner A. Gold Difluorocarbenoid Complexes: Spectroscopic and Chemical Profiling. Angew. Chem., Int. Ed. 2019, 58, 8834–8838. 10.1002/anie.201903957. [DOI] [PubMed] [Google Scholar]
- Scharnagel D.; Escofet I.; Armengol-Relats H.; de Orbe M. E.; Korber J. N.; Echavarren A. M. Acetylene as a Dicarbene Equivalent for Gold(I) Catalysis: Total Synthesis of Waitziacuminone in One Step. Angew. Chem., Int. Ed. 2020, 59, 4888–4891. 10.1002/anie.201915895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Tandem Au-Catalyzed 3,3-Rearrangement-[2 + 2] Cycloadditions of Propargylic Esters: Expeditious Access to Highly Functionalized 2,3-Indoline-Fused Cyclobutanes. J. Am. Chem. Soc. 2005, 127, 16804–16805. 10.1021/ja056419c. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Shi M. Synthesis of Cyclic and Heterocyclic Compounds via Gold-Catalyzed Reactions. Synlett 2017, 28, 2230–2240. 10.1055/s-0036-1590827. [DOI] [Google Scholar]
- Fang W.; Shi M. Recent Advances in the Cycloisomerizations of Methylenecyclopropanes using Gold Catalysis. Chem. - Eur. J. 2018, 24, 9998–10005. 10.1002/chem.201705788. [DOI] [PubMed] [Google Scholar]
- Garayalde D.; Nevado C. Synthetic Applications of Gold-Catalyzed Ring Expansions. Beilstein J. Org. Chem. 2011, 7, 767–780. 10.3762/bjoc.7.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino E.; Fernández L.; Nevado C.. Gold-Catalyzed Migrations and Ring Expansions. Organogold Compounds; Patai’s Chemistry of Functional Groups; John Wiley & Sons, Ltd, 2014. [Google Scholar]
- Mack D. J.; Njardarson J. T. Recent Advances in the Metal-Catalyzed Ring Expansions of Three- and Four-Membered Rings. ACS Catal. 2013, 3, 272–286. 10.1021/cs300771d. [DOI] [Google Scholar]
- Brandi A.; Cicchi S.; Cordero F. M.; Goti A. Progress in the Synthesis and Transformations of Alkylidenecyclopropanes and Alkylidenecyclobutanes. Chem. Rev. 2014, 114, 7317–7420. 10.1021/cr400686j. [DOI] [PubMed] [Google Scholar]
- Cao H.; Chen F.; Su C.; Yu L. Construction of Carbocycles from Methylenecyclopropanes. Adv. Synth. Catal. 2020, 362, 438–461. 10.1002/adsc.201900615. [DOI] [Google Scholar]
- Jiménez-Núñez E.; Claverie C. K.; Nieto-Oberhuber C.; Echavarren A. M. Prins Cyclizations in Au-Catalyzed Reactions of Enynes. Angew. Chem., Int. Ed. 2006, 45, 5452–5455. 10.1002/anie.200601575. [DOI] [PubMed] [Google Scholar]
- Ferrer S.; Echavarren A. M. Total Synthesis of Repraesentin F and Configuration Reassignment by a Gold(I)-Catalyzed Cyclization Cascade. Org. Lett. 2018, 20, 5784–5788. 10.1021/acs.orglett.8b02478. [DOI] [PubMed] [Google Scholar]
- Pitaval A.; Leboeuf D.; Ceccon J.; Echavarren A. M. Access to the Protoilludane Core by Gold-Catalyzed Allene-vinylcyclopropane Cycloisomerization. Org. Lett. 2013, 15, 4580–4583. 10.1021/ol402188b. [DOI] [PMC free article] [PubMed] [Google Scholar]; Correction:; Org. Lett. 2013, 15, 5146. 10.1021/ol402688f
- Felix R. J.; Gutierrez O.; Tantillo D. J.; Gagné M. R. Gold(I)-Catalyzed Formation of Bicyclo[4.2.0]oct-1-enes. J. Org. Chem. 2013, 78, 5685–5690. 10.1021/jo400139g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.; Felix R. J.; Gagné M. R. Gold-Catalyzed Enantioselective Ring-Expanding Cycloisomerization of Cyclopropylidene Bearing 1,5-Enynes. Org. Lett. 2014, 16, 2272–2275. 10.1021/ol5007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli C. A.; Gagné M. R. Gold(I)-Catalyzed Addition of Aldehydes to Cyclopropylidene Bearing 6-Aryl-1,5-Enynes. Org. Biomol. Chem. 2016, 14, 11261–11265. 10.1039/C6OB02128J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönig M.; Carreira E. M. Total Synthesis and Structural Revision of a Harziane Diterpenoid. Angew. Chem., Int. Ed. 2020, 59, 1192–1196. 10.1002/anie.201912982. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Aïssa C. PtCl2-Catalyzed Rearrangement of Methylenecyclopropanes. J. Am. Chem. Soc. 2006, 128, 6306–6307. 10.1021/ja061392y. [DOI] [PubMed] [Google Scholar]
- Shi M.; Liu L.-P.; Tang J. Palladium-Catalyzed Ring Enlargement of Aryl-Substituted Methylenecyclopropanes to Cyclobutenes. J. Am. Chem. Soc. 2006, 128, 7430–7431. 10.1021/ja061749y. [DOI] [PubMed] [Google Scholar]
- Fang W.; Wei Y.; Tang X.-Y.; Shi M. Gold(I)-Catalyzed Cycloisomerization of ortho-(Propargyloxy)arenemethylenecyclopropanes Controlled by Adjacent Substituents at the Aromatic Rings. Chem. - Eur. J. 2017, 23, 6845–6852. 10.1002/chem.201700600. [DOI] [PubMed] [Google Scholar]
- Zhang J.-H.; Wei Y.; Shi M. Gold-Catalyzed Ring Enlargement and Cycloisomerization of Alkynylamide tethered Alkylidenecyclopropanes. Org. Chem. Front. 2018, 5, 2980–2985. 10.1039/C8QO00907D. [DOI] [Google Scholar]
- Li C.-L.; Yu Z.-X. Asymmetric Synthesis of Azepine-Fused Cyclobutanes from Yne- Methylenecyclopropanes Involving Cyclopropanation/C−C Cleavage/Wagner−Meerwein Rearrangement and Reaction Mechanism. J. Org. Chem. 2019, 84, 9913–9928. 10.1021/acs.joc.9b01071. [DOI] [PubMed] [Google Scholar]
- Li D.-Y.; Wei Y.; Marek I.; Tang X.-Y.; Shi M. Gold(I)-Catalyzed Cycloisomerization of Vinylidenecyclopropane-enes via Carbene or Non-Carbene Processes. Chem. Sci. 2015, 6, 5519–5525. 10.1039/C5SC01806D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.-Y.; Fang W.; Wei Y.; Shi M. C(sp3)−H Functionalizations Promoted by the Gold Carbene Generated from Vinylidenecyclopropanes. Chem. - Eur. J. 2016, 22, 18080–18084. 10.1002/chem.201604200. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Huang X.; Li G.; Zhang L. Au-Containing All-Carbon 1,4-Dipoles: Generation and [4 + 2] Annulation in the Formation of Carbo-/Heterocycles. J. Am. Chem. Soc. 2008, 130, 1814–1815. 10.1021/ja077948e. [DOI] [PubMed] [Google Scholar]
- Li G.; Huang X.; Zhang L. Au(I)-Catalyzed Efficient Synthesis of Functionalized Bicyclo[3.2.0]heptanes. J. Am. Chem. Soc. 2008, 130, 6944–6945. 10.1021/ja802294t. [DOI] [PubMed] [Google Scholar]
- Yang C.-Y.; Lin M.-S.; Liao H.-H.; Liu R.-S. Diversity of Products in the Gold-Catalyzed Cyclization of 1-Epoxy-1-alkynylcyclopropanes by Using 1-Oxyallyl Cations. Chem. - Eur. J. 2010, 16, 2696–2699. 10.1002/chem.200903419. [DOI] [PubMed] [Google Scholar]
- Liao H.-H.; Liu R.-S. Effects of Haloniums on Gold-Catalyzed Ring Expansion of 1-Oxiranyl-1-alkynylcycloprpropanes. Chem. Commun. 2011, 47, 1339–1341. 10.1039/C0CC03309J. [DOI] [PubMed] [Google Scholar]
- Ye S.; Yu Z.-X. Gold(I)-Catalyzed Ring Expansions of Unactivated Alkynylcyclopropanes to (E)-2-Alkylidenecyclobutanamines in the Presence of Sulfonamides. Org. Lett. 2010, 12, 804–807. 10.1021/ol9028786. [DOI] [PubMed] [Google Scholar]
- Kuo T.-N.; Liao H.-Y. Computational Study of the Substitution Effect on the Mechanism for the Gold(I)-Catalyzed Ring Expansions of Unactivated Alkynylcyclopropanes. J. Chin. Chem. Soc. 2013, 60, 473–480. 10.1002/jccs.201200517. [DOI] [Google Scholar]
- Yi F.; Huang B.; Nie Q.; Cai M. A Heterogeneous Gold(I)-Catalyzed Ring Expansion of Unactivated Alkynylcyclopropanes with Sulfonamides Leading to (E)-2-Alkylidenecyclobutanamines. Tetrahedron Lett. 2016, 57, 4405–4410. 10.1016/j.tetlet.2016.08.062. [DOI] [Google Scholar]
- Rao W.; Susanti D.; Chan P. W. H. Gold-Catalyzed Tandem 1,3-Migration/[2 + 2] Cycloaddition of 1,7-Enyne Benzoates to Azabicyclo[4.2.0]oct-5-enes. J. Am. Chem. Soc. 2011, 133, 15248–15251. 10.1021/ja2052304. [DOI] [PubMed] [Google Scholar]
- Su Y.; Zhang Y.; Akhmedov N. G.; Petersen J. L.; Shi X. Ambient Intermolecular [2 + 2] Cycloaddition: An Example of Carbophilicity and Oxophilicity Competition in Au/Ag Catalysis. Org. Lett. 2014, 16, 2478–2481. 10.1021/ol500854m. [DOI] [PubMed] [Google Scholar]
- Jońsson H. F.; Evjen S.; Fiksdahl A. Gold(I)-Catalyzed [2 + 2 + 2] Cyclotrimerization of 1,3-Diarylpropargyl Acetals. Org. Lett. 2017, 19, 2202–2205. 10.1021/acs.orglett.7b00411. [DOI] [PubMed] [Google Scholar]
- Luzung M. R.; Mauleón P.; Toste F. D. Gold(I)-Catalyzed [2 + 2]-Cycloaddition of Allenenes. J. Am. Chem. Soc. 2007, 129, 12402–12403. 10.1021/ja075412n. [DOI] [PubMed] [Google Scholar]
- González A. Z.; Benitez D.; Tkatchouk E.; Goddard W. A. III; Toste F. D. Phosphoramidite Gold(I)-Catalyzed Diastereo- and Enantioselective Synthesis of 3,4-Substituted Pyrrolidines. J. Am. Chem. Soc. 2011, 133, 5500–5507. 10.1021/ja200084a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aillard P.; Retailleau P.; Voituriez A.; Marinetti A. Synthesis of New Phosphahelicene Scaffolds and Development of Gold(I)-Catalyzed Enantioselective Allenene Cyclizations. Chem. - Eur. J. 2015, 21, 11989–11993. 10.1002/chem.201501697. [DOI] [PubMed] [Google Scholar]
- Alcarazo M.; Stork T.; Anoop A.; Thiel W.; Fürstner A. Steering the Surprisingly Modular π-Acceptor Properties of N-Heterocyclic Carbenes: Implications for Gold Catalysis. Angew. Chem., Int. Ed. 2010, 49, 2542–2546. 10.1002/anie.200907194. [DOI] [PubMed] [Google Scholar]
- Mauleón P.; Zeldin R. M.; González A. Z.; Toste F. D. Ligand-Controlled Access to [4 + 2] and [4 + 3] Cycloadditions in Gold-Catalyzed Reactions of Allene-Dienes. J. Am. Chem. Soc. 2009, 131, 6348–6349. 10.1021/ja901649s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso I.; Trillo B.; López F.; Montserrat S.; Ujaque G.; Castedo L.; Lledós A.; Mascareñas J. L. Gold-Catalyzed [4C+2C] Cycloadditions of Allenedienes, Including an Enantioselective Version with New Phosphoramidite-Based Catalysts: Mechanistic Aspects of the Divergence between [4C+3C] and [4C+2C] Pathways. J. Am. Chem. Soc. 2009, 131, 13020–13030. 10.1021/ja905415r. [DOI] [PubMed] [Google Scholar]
- Trillo B.; López F.; Montserrat S.; Ujaque G.; Castedo L.; Lledós A.; Mascareñas J. L. Gold-Catalyzed [4C+3C] Intramolecular Cycloaddition of Allenedienes: Synthetic Potential and Mechanistic Implications. Chem. - Eur. J. 2009, 15, 3336–3339. 10.1002/chem.200900164. [DOI] [PubMed] [Google Scholar]
- González A. Z.; Toste F. D. Gold(I)-Catalyzed Enantioselective [4 + 2]-Cycloaddition of Allene-dienes. Org. Lett. 2010, 12, 200–203. 10.1021/ol902622b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso I.; Faustino H.; López F.; Mascareñas J. L. Enantioselective Gold(I)-Catalyzed Intramolecular (4 + 3) Cycloadditions of Allenedienes. Angew. Chem., Int. Ed. 2011, 50, 11496–11500. 10.1002/anie.201105815. [DOI] [PubMed] [Google Scholar]
- Christian A. H.; Niemeyer Z. L.; Sigman M. S.; Toste F. D. Uncovering Subtle Ligand Effects of Phosphines Using Gold(I) Catalysis. ACS Catal. 2017, 7, 3973–3978. 10.1021/acscatal.7b00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F.; Lv K.; Liu T. Computational Study on the mechanisms of [2 + 3] and [2 + 2] Cycloisomerization Reaction Catalyzed by Gold Complex. J. Organomet. Chem. 2018, 874, 63–69. 10.1016/j.jorganchem.2018.08.016. [DOI] [Google Scholar]
- Li X.-X.; Zhu L.-L.; Zhou W.; Chen Z. Formal Intermolecular [2 + 2] Cycloaddition Reaction of Alleneamides with Alkenes via Gold Catalysis. Org. Lett. 2012, 14, 436–439. 10.1021/ol202703a. [DOI] [PubMed] [Google Scholar]
- Suárez-Pantiga S.; Hernández-Díaz C.; Piedrafita M.; Rubio E.; González J. M. Phosphite-Gold(I)-Catalyzed [2 + 2] Intermolecular Cycloaddition of Enol Ethers with N-Allenylsulfonamides. Adv. Synth. Catal. 2012, 354, 1651–1657. 10.1002/adsc.201200043. [DOI] [Google Scholar]
- Faustino H.; Bernal P.; Castedo L.; López F.; Mascareñas J. L. Gold(I)-Catalyzed Intermolecular [2 + 2] Cycloadditions between Allenamides and Alkenes. Adv. Synth. Catal. 2012, 354, 1658–1664. 10.1002/adsc.201200047. [DOI] [Google Scholar]
- Faustino H.; Alonso I.; Mascareñas J. L.; López F. Gold(I)-Catalyzed Cascade Cycloadditions between Allenamides and Carbonyl-Tethered Alkenes: An Enantioselective Approach to Oxa-Bridged Medium-Sized Carbocycles. Angew. Chem., Int. Ed. 2013, 52, 6526–6530. 10.1002/anie.201302713. [DOI] [PubMed] [Google Scholar]
- Bernal-Albert P.; Faustino H.; Gimeno A.; Asensio G.; Mascareñas J. L.; Lopez F. Gold(I)-Catalyzed Intermolecular Cycloaddition of Allenamides with α,β-Unsaturated Hydrazones: Efficient Access to Highly Substituted Cyclobutanes. Org. Lett. 2014, 16, 6196–6199. 10.1021/ol503121q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocello R.; De Nisi A.; Jia M.; Yang Q.-Q.; Monari M.; Giacinto P.; Bottoni A.; Miscione G. P.; Bandini M. Gold(I)-Catalyzed Dearomative [2 + 2]-Cycloaddition of Indoles with Activated Allenes: A Combined Experimental-Computational Study. Chem. - Eur. J. 2015, 21, 18445–18453. 10.1002/chem.201503598. [DOI] [PubMed] [Google Scholar]
- Suárez-Pantiga S.; Hernández-Díaz C.; Rubio E.; González J. M. Intermolecular [2 + 2] Reaction of N-Allenylsulfonamides with Vinylarenes: Enantioselective Gold(I)-Catalyzed Synthesis of Cyclobutane Derivatives. Angew. Chem., Int. Ed. 2012, 51, 11552–11555. 10.1002/anie.201206461. [DOI] [PubMed] [Google Scholar]
- Francos J.; Grande-Carmona F.; Faustino H.; Iglesias-Sigüenza J.; Díez E.; Alonso I.; Fernandez R.; Lassaletta J. M.; Lopez F.; Mascareñas J. L. Axially Chiral Triazoloisoquinolin-3-ylidene Ligands in Gold(I)-Catalyzed Asymmetric Intermolecular (4 + 2) Cycloadditions of Allenamides and Dienes. J. Am. Chem. Soc. 2012, 134, 14322–14325. 10.1021/ja3065446. [DOI] [PubMed] [Google Scholar]
- Huang W.; Zhang Y.-C.; Jin R.; Chen B.-L.; Chen Z. Synthesis of Axially Chiral 1,2,3-Triazol-5-ylidene-Au(I) Complex and Its Application in Enantioselective [2 + 2] Cycloaddition of Alleneamides with Alkenes. Organometallics 2018, 37, 3196–3209. 10.1021/acs.organomet.8b00524. [DOI] [Google Scholar]
- Wang Y.; Zhang P.; Liu Y.; Xia F.; Zhang J. Enantioselective Gold-Catalyzed Intermolecular [2 + 2] versus [4 + 2]-Cycloadditions of 3-Styrylindoles with N-Allenamides: Observation of Interesting Substituent Effects. Chem. Sci. 2015, 6, 5564–5570. 10.1039/C5SC01827G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.; Wang Y.; Qian D.; Zhang Z.-M.; Liu L.; Zhang J. Enantioselective Gold-Catalyzed Intermolecular [2 + 2]-Cycloadditions of 3-Styrylindoles with N-Allenyl Oxazolidinone. Org. Chem. Front. 2016, 3, 759–763. 10.1039/C6QO00087H. [DOI] [Google Scholar]
- Jia M.; Monari M.; Yang Q.-Q.; Bandini M. Enantioselective Gold Catalyzed Dearomative [2 + 2]-Cycloaddition between Indoles and Allenamides. Chem. Commun. 2015, 51, 2320–2323. 10.1039/C4CC08736D. [DOI] [PubMed] [Google Scholar]
- Jia M.; Cera G.; Perrotta D.; Monari M.; Bandini M. Taming Gold(I)-Counterion Interplay in the De-aromatization of Indoles with Allenamides. Chem. - Eur. J. 2014, 20, 9875–9878. 10.1002/chem.201403155. [DOI] [PubMed] [Google Scholar]
- Kim S. M.; Park J. H.; Kang Y. K.; Chung Y. K. N-Heterocyclic Carbene Gold(I) Catalyzed Transformation of N-Tethered 1,5-Bisallenes to 6,7-Dimethylene-3-azabicyclo[3.1.1]heptanes. Angew. Chem., Int. Ed. 2009, 48, 4532–4535. 10.1002/anie.200806394. [DOI] [PubMed] [Google Scholar]
- Yang C.-Y.; Wang C.-D.; Tian S.-T.; Liu R.-S. Gold-Catalyzed Synthesis of Bicyclo[3.2.0]heptenes via a Formal [3 + 2]/[2 + 2]-Annulation of Allylsilane with 4-Methoxybut-2-yn-1-ols. Adv. Synth. Catal. 2010, 352, 1605–1609. 10.1002/adsc.201000201. [DOI] [Google Scholar]
- Escribano-Cuesta A.; Pérez-Galán P.; Herrero-Gómez E.; Sekine M.; Braga A. A. C; Maseras F.; Echavarren A. M. The Role of Cyclobutenes in Gold(I)-Catalysed Skeletal Rearrangement of 1,6-Enynes. Org. Biomol. Chem. 2012, 10, 6105–6111. 10.1039/c2ob25419k. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; Pérez-Galán P.; Herrero-Gómez E.; Lauterbach T.; Rodríguez C.; López S.; Bour C.; Rosellón A.; Cárdenas D. J.; Echavarren A. M. Gold(I)-Catalyzed Intramolecular [4 + 2] Cycloadditions of Arylalkynes or 1,3-Enynes with Alkenes: Scope and Mechanism. J. Am. Chem. Soc. 2008, 130, 269–279. 10.1021/ja075794x. [DOI] [PubMed] [Google Scholar]
- Brooner R. E. M.; Brown T. J.; Widenhoefer R. A. Direct Observation of a Cationic Gold(I)-Bicyclo[3.2.0]hept-1(7)-ene Complex Generated in the Cycloisomerization of a 7-Phenyl-1,6-Enyne. Angew. Chem., Int. Ed. 2013, 52, 6259–6261. 10.1002/anie.201301640. [DOI] [PubMed] [Google Scholar]
- Brooner R. E. M.; Robertson B. D.; Widenhoefer R. A. Mechanism of 1,3-Hydrogen Migration in a Gold Bicyclo[3.2.0]heptene Complex: The Role of Brønsted Acid in the Gold-Catalyzed Cycloisomerization of 7-Aryl-1,6-Enynes. Organometallics 2014, 33, 6466–6473. 10.1021/om500815k. [DOI] [Google Scholar]
- Kim N.; Brooner R. E. M.; Widenhoefer R. A. Unexpected Skeletal Rearrangement in the Gold(I)/Silver(I)-Catalyzed Conversion of 7-Aryl-1,6-Enynes to Bicyclo[3.2.0]hept-6-enes via Hidden Brønsted Acid Catalysis. Organometallics 2017, 36, 673–678. 10.1021/acs.organomet.6b00868. [DOI] [Google Scholar]
- Robertson B. D.; Brooner R. E. M.; Widenhoefer R. A. Tandem Gold/Silver-Catalyzed Cycloaddition/Hydroarylation of 7-Aryl-1,6-enynes to Form 6,6-Diarylbicyclo[3.2.0]heptanes. Chem. - Eur. J. 2015, 21, 5714–5717. 10.1002/chem.201500371. [DOI] [PubMed] [Google Scholar]
- Patil D. V.; Park H.-S.; Koo J.; Han J. K.; Shin S. Aerobic Oxygenative Cleavage of Electron Deficient C−C Triple bonds in the Gold-Catalyzed Cyclization of 1,6-Enynes. Chem. Commun. 2014, 50, 12722–12725. 10.1039/C4CC04153D. [DOI] [PubMed] [Google Scholar]
- Zhong C.-Z.; Tung P.-T.; Chao T.-H.; Yeh M.-C. P. Gold-Catalyzed Stereoselective Synthesis of Bicyclic Lactams and Ketones from N-Tosylynamidomethyl-Tethered Cyclohexenes. J. Org. Chem. 2017, 82, 481–501. 10.1021/acs.joc.6b02479. [DOI] [PubMed] [Google Scholar]
- Huple D. B.; Liu R.-S. Gold-Catalyzed Diastereoselective [2 + 2+2]-Cycloaddition of 1,7-Enynes with Carbonyl Compounds. Chem. Commun. 2012, 48, 10975–10977. 10.1039/c2cc36219h. [DOI] [PubMed] [Google Scholar]
- Odabachian Y.; Gagosz F. Cyclobutenes as Isolable Intermediates in the Gold(I)-Catalysed Cycloisomerisation of 1,8-Enynes. Adv. Synth. Catal. 2009, 351, 379–386. 10.1002/adsc.200900056. [DOI] [Google Scholar]
- Inagaki F.; Matsumoto C.; Okada Y.; Maruyama N.; Mukai C. Air-Stable Cationic Gold(I) Catalyst Featuring a Z-Type Ligand: Promoting Enyne Cyclizations. Angew. Chem., Int. Ed. 2015, 54, 818–822. 10.1002/anie.201408037. [DOI] [PubMed] [Google Scholar]
- Iwai T.; Ueno M.; Okochi H.; Sawamura M. Synthesis of Cyclobutene-Fused Eight-Membered Carbocycles through Gold-Catalyzed Intramolecular Enyne [2 + 2] Cycloaddition. Adv. Synth. Catal. 2018, 360, 670–675. 10.1002/adsc.201701193. [DOI] [Google Scholar]
- Obradors C.; Leboeuf D.; Aydin J.; Echavarren A. M. Gold(I)-Catalyzed Macrocyclization of 1,n-Enynes. Org. Lett. 2013, 15, 1576–1579. 10.1021/ol400358f. [DOI] [PubMed] [Google Scholar]
- Homs A.; Obradors C.; Lebœuf D.; Echavarren A. M. Dissecting Anion Effects in Gold(I)-Catalyzed Intermolecular Cycloadditions. Adv. Synth. Catal. 2014, 356, 221–228. 10.1002/adsc.201300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri B.; Obradors C.; Mato M.; Echavarren A. M. Synthesis of Rumphellaone A and Hushinone by a Gold-Catalyzed [2 + 2] Cycloaddition. Org. Lett. 2016, 18, 1614–1617. 10.1021/acs.orglett.6b00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Carrillo V.; Echavarren A. M. Gold(I)-Catalyzed Intermolecular [2 + 2] Cycloaddition of Alkynes with Alkenes. J. Am. Chem. Soc. 2010, 132, 9292–9294. 10.1021/ja104177w. [DOI] [PubMed] [Google Scholar]
- For the use of other gold(I) catalysts in the [2 + 2] cycloaddition of alkynes and alkenes, see ref (423)
- de Orbe M. E.; Amenós L.; Kirillova M. S.; Wang Y.; López-Carrillo V.; Maseras F.; Echavarren A. M. Cyclobutene vs 1,3-Diene Formation in the Gold-Catalyzed Reaction of Alkynes with Alkenes: The Complete Mechanistic Picture. J. Am. Chem. Soc. 2017, 139, 10302–10311. 10.1021/jacs.7b03005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradors C.; Echavarren A. M. Intermolecular Gold-Catalyzed Cycloaddition of Alkynes with Oxoalkenes. Chem. - Eur. J. 2013, 19, 3547–3551. 10.1002/chem.201300131. [DOI] [PubMed] [Google Scholar]
- de Orbe M. E.; Echavarren A. M. Broadening the Scope of the Gold-Catalyzed [2 + 2] Cycloaddition Reaction: Synthesis of Vinylcyclobutenes and Further Transformations. Eur. J. Org. Chem. 2018, 2018, 2740–2752. 10.1002/ejoc.201800170. [DOI] [Google Scholar]
- For an example on the use of alkynes in intermolecular asymmetric gold(I) catalysis:; Kim H.; Choi S. Y.; Shin S. Asymmetric Synthesis of Dihydropyranones via Gold(I)-Catalyzed Intermolecular [4 + 2] Annulation of Propiolates and Alkenes. Angew. Chem., Int. Ed. 2018, 57, 13130–13134. 10.1002/anie.201807514. [DOI] [PubMed] [Google Scholar]
- Grande-Carmona F.; Iglesias-Sigüenza J.; Álvarez E.; Díez E.; Fernańdez R.; Lassaletta J. M. Synthesis and Characterization of Axially Chiral Imidazoisoquinolin-2-ylidene Silver and Gold Complexes. Organometallics 2015, 34, 5073–5080. 10.1021/acs.organomet.5b00681. [DOI] [Google Scholar]
- Zhang J.-Q.; Liu Y.; Wang X.-W.; Zhang L. Synthesis of Chiral Bifunctional NHC Ligands and Survey of Their Utilities in Asymmetric Gold Catalysis. Organometallics 2019, 38, 3931–3938. 10.1021/acs.organomet.9b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsutkar M. M.; Pagar V. V.; RajanBabu T. V. Catalytic Enantioselective Synthesis of Cyclobutenes from Alkynes and Alkenyl Derivatives. J. Am. Chem. Soc. 2019, 141, 15367–15377. 10.1021/jacs.9b07885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T.; Ge S.; Lin L.; Lu Y.; Liu X.; Feng X. A Chiral N, N′−Dioxide−ZnII Complex Catalyzes the Enantioselective [2 + 2] Cycloaddition of Alkynones with Cyclic Enol Silyl Ethers. Angew. Chem., Int. Ed. 2016, 55, 5541–5544. 10.1002/anie.201600801. [DOI] [PubMed] [Google Scholar]
- Qin H.; Chen J.; Li K.; He Z.; Zhou Y.; Fan B. Nickel-Catalyzed Asymmetric [2 + 2] Cycloaddition Reaction of Hetero-Bicyclic Alkenes with Internal Alkynes. Chem. - Asian J. 2018, 13, 2431–2434. 10.1002/asia.201800492. [DOI] [PubMed] [Google Scholar]
- Ito H.; Hasegawa M.; Takenaka Y.; Kobayashi T.; Iguchi K. Enantioselective Total Synthesis of (+)-Tricycloclavulone. J. Am. Chem. Soc. 2004, 126, 4520–4521. 10.1021/ja049750p. [DOI] [PubMed] [Google Scholar]
- Takenaka Y.; Ito H.; Iguchi K. Enantioselective Formal Synthesis of (D)-Precapnelladiene by Chiral Copper-Catalyzed Asymmetric [2 + 2]-Cycloaddition Reaction. Tetrahedron 2007, 63, 510–513. 10.1016/j.tet.2006.10.072. [DOI] [Google Scholar]
- Takenaka Y.; Ito H.; Hasegawa M.; Iguchi K. Catalytic Enantioselective [2 + 2]-Cycloaddition Reaction of 2-Methoxycarbonyl-2-cyclopenten-1-one by Chiral Copper Catalyst. Tetrahedron 2006, 62, 3380–3388. 10.1016/j.tet.2006.01.050. [DOI] [Google Scholar]
- Ishihara K.; Fushimi M. Catalytic Enantioselective [4 + 2] and [2 + 2] Cycloaddition Reactions with Propiolamides. J. Am. Chem. Soc. 2008, 130, 7532–7533. 10.1021/ja8015318. [DOI] [PubMed] [Google Scholar]
- Enomoto K.; Oyama H.; Nakada M. Highly Enantioselective Catalytic Asymmetric [2 + 2] Cycloadditions of Cyclic α-Alkylidene β-Oxo Imides with Ynamides. Chem. - Eur. J. 2015, 21, 2798–2802. 10.1002/chem.201406189. [DOI] [PubMed] [Google Scholar]
- Schotes C.; Mezzetti A. Enantioselective Ficini Reaction: Ruthenium/PNNP-Catalyzed [2 + 2] Cycloaddition of Ynamides with Cyclic Enones. Angew. Chem., Int. Ed. 2011, 50, 3072–3074. 10.1002/anie.201007753. [DOI] [PubMed] [Google Scholar]
- Schotes C.; Bigler R.; Mezzetti A. Bicyclo[3.2.0]heptane-Based Enamides by Ru/PNNP-Catalyzed Enantioselective Ficini Reactions: Scope and Application in Ligand Design. Synthesis 2012, 2012, 513–526. 10.1055/s-0031-1289679. [DOI] [Google Scholar]
- Kossler D.; Cramer N. Neutral Chiral Cyclopentadienyl Ru(II)Cl Catalysts Enable Enantioselective [2 + 2]-Cycloadditions. Chem. Sci. 2017, 8, 1862–1866. 10.1039/C6SC05092A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossler D.; Perrin F. G.; Suleymanov A. A.; Kiefer G.; Scopelliti R.; Severin K.; Cramer N. Divergent Asymmetric Synthesis of Polycyclic Compounds via Vinyl Triazenes. Angew. Chem., Int. Ed. 2017, 56, 11490–11493. 10.1002/anie.201706013. [DOI] [PubMed] [Google Scholar]
- Shibata T.; Takami K.; Kawachi A. Rh-Catalyzed Enantioselective [2 + 2] Cycloaddition of Alkynyl Esters and Norbornene Derivatives. Org. Lett. 2006, 8, 1343–1345. 10.1021/ol060055r. [DOI] [PubMed] [Google Scholar]
- Jiao Z.; Shi Q.; Zhou J. S. Asymmetric Intermolecular Heck Reaction of Propargylic Acetates and Cycloalkenes to Access Fused Cyclobutenes. Angew. Chem., Int. Ed. 2017, 56, 14567–14571. 10.1002/anie.201708435. [DOI] [PubMed] [Google Scholar]
- Fan B.-M.; Li X.-J.; Peng F.-Z.; Zhang H.-B.; Chan A. S. C.; Shao Z.-H. Ligand-Controlled Enantioselective [2 + 2] Cycloaddition of Oxabicyclic Alkenes with Terminal Alkynes Using Chiral Iridium Catalysts. Org. Lett. 2010, 12, 304–306. 10.1021/ol902574c. [DOI] [PubMed] [Google Scholar]
- Hu J.; Yang Q.; Yu L.; Xu J.; Liu S.; Huang C.; Wang L.; Zhou Y.; Fan B. A study on the substituent effects of norbornadiene derivatives in iridium-catalyzed asymmetric [2 + 2] cycloaddition reactions. Org. Biomol. Chem. 2013, 11, 2294–2301. 10.1039/c3ob27382b. [DOI] [PubMed] [Google Scholar]
- García-Morales C.; Ranieri B.; Escofet I.; Lopez-Suarez L.; Obradors C.; Konovalov A. I.; Echavarren A. M. Enantioselective Synthesis of Cyclobutenes by Intermolecular [2 + 2] Cycloaddition with Non-C2 Symmetric Digold Catalysts. J. Am. Chem. Soc. 2017, 139, 13628–13631. 10.1021/jacs.7b07651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y.-B.; Luo Z.; Wang Y.; Gao J.-M.; Zhang L. Au-Catalyzed Intermolecular [2 + 2] Cycloadditions between Chloroalkynes and Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 5860–5865. 10.1021/jacs.8b01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Orbe M. E.; Zanini M.; Quinonero O.; Echavarren A. M. Gold- or Indium-Catalyzed Cross-Coupling of Bromoalkynes with Allylsilanes through a Concealed Rearrangement. ACS Catal. 2019, 9, 7817–7822. 10.1021/acscatal.9b02314. [DOI] [Google Scholar]
- Li C.-W.; Pati K.; Lin G.-Y.; Sohel S. Md. A.; Hung H.-H.; Liu R.-S. Gold-Catalyzed Oxidative Ring Expansions and Ring Cleavages of Alkynylcyclopropanes by Intermolecular Reactions Oxidized by Diphenylsulfoxide. Angew. Chem., Int. Ed. 2010, 49, 9891–9894. 10.1002/anie.201004647. [DOI] [PubMed] [Google Scholar]
- Xu W.; Chen M.; Sun N.; Liu Y. Gold-Catalyzed Cyclization of 1,6-Diynyl Dithioacetals via 1,7-Carbene Transfer and Aromatic C−H Functionalization. Chem. Commun. 2016, 52, 11000–11003. 10.1039/C6CC05302E. [DOI] [PubMed] [Google Scholar]
- Xu C.-F.; Xu M.; Jia Y.-X.; Li C.-Y. Gold-Catalyzed Synthesis of Benzil Derivatives and α-Keto Imides via Oxidation of Alkynes. Org. Lett. 2011, 13, 1556–1559. 10.1021/ol200270t. [DOI] [PubMed] [Google Scholar]
- Chen C.-H.; Tsai Y.-C.; Liu R.-S. Gold-Catalyzed Cyclization/Oxidative [3 + 2] Cycloadditions of 1,5-Enynes with Nitrosobenzenes without Additional Oxidants. Angew. Chem., Int. Ed. 2013, 52, 4599–4603. 10.1002/anie.201209850. [DOI] [PubMed] [Google Scholar]
- Fang W.; Tang X.-Y.; Shi M. Gold(I)-Catalyzed Intramolecular Hydroarylation and the Subsequent Ring Enlargement of Methylenecyclopropanes to Cyclobutenes. RSC Adv. 2016, 6, 40474–40479. 10.1039/C6RA02549H. [DOI] [Google Scholar]
- Jiang B.; Wei Y.; Shi M. Gold- and Silver-Catalyzed Intramolecular Annulation and Rearrangement of Aniline-linked 1,6-Enynes Containing Methylenecyclopropanes. Org. Chem. Front. 2018, 5, 2091–2097. 10.1039/C8QO00358K. [DOI] [Google Scholar]
- Oh H.; Kim A. Gold-Catalyzed [2 + 2] Cyclization of Alkyne-Propargylic Pivaloates to Fused Bicyclic Compounds. Synlett 2008, 2008, 777–781. 10.1055/s-2008-1032110. [DOI] [Google Scholar]
- Lee K. H.; Jillella R.; Kim J.; Oh C. H. Synthesis of Primitive Dendrimer Systems Bearing Bicyclo[3,2,0]Hept-6-en-6-yl Groups via Unique Au-catalyzed [2 + 2] Cyclization. Bull. Korean Chem. Soc. 2018, 39, 651–656. 10.1002/bkcs.11445. [DOI] [Google Scholar]
- Rao W.; Koh M. J.; Kothandaraman P.; Chan P. W. H. Gold-Catalyzed Cycloisomerization of 1,7-Diyne Benzoates to Indeno[1,2-c]azepines and Azabicyclo[4.2.0]octa-1(8),5-dines. J. Am. Chem. Soc. 2012, 134, 10811–10814. 10.1021/ja304964s. [DOI] [PubMed] [Google Scholar]
- Li D.; Rao W.; Tay G. L.; Ayers B. J.; Chan P. W. H. Gold-Catalyzed Cycloisomerization of 1,6-Diyne Esters to 1H-Cyclopenta[b]naphthalenes, cis-Cyclopenten-2-yl δ-Diketones, and Bicyclo[3.2.0]hepta-1,5-dienes. J. Org. Chem. 2014, 79, 11301–11315. 10.1021/jo5020195. [DOI] [PubMed] [Google Scholar]
- Chen M.; Liu J.; Wang L.; Zhou X.; Liu Y. Gold-Catalyzed Cascade Cycloisomerization of 1,7-Diyn-3,6-bis(propargyl carbonate)s: Stereoselective Synthesis of Naphtho[b]cyclobutenes. Chem. Commun. 2013, 49, 8650–8652. 10.1039/c3cc44757j. [DOI] [PubMed] [Google Scholar]
- Nayak S.; Ghosh N.; Sahoo A. K. Access to Cyclobutene-Fused Azepines through Au-Catalyzed Cycloisomerization of Stable Alkyne Tethered Ketene N,N-Acetals. Org. Lett. 2014, 16, 2996–2999. 10.1021/ol501125r. [DOI] [PubMed] [Google Scholar]
- Gomez-Suárez A.; Nolan S. P. Dinuclear Gold Catalysis: Are Two Gold Centers Better than One?. Angew. Chem., Int. Ed. 2012, 51, 8156–8159. 10.1002/anie.201203587. [DOI] [PubMed] [Google Scholar]
- Braun I.; Asiri A. M.; Hashmi A. S. K. Gold Catalysis 2.0. ACS Catal. 2013, 3, 1902–1907. 10.1021/cs400437s. [DOI] [Google Scholar]
- Hashmi A. S. K. Dual Gold Catalysis. Acc. Chem. Res. 2014, 47, 864–876. 10.1021/ar500015k. [DOI] [PubMed] [Google Scholar]
- Jaroschik F.; Simonneau A.; Lemière G.; Cariou K.; Agenet N.; Amouri H.; Aubert C.; Goddard J.-P.; Lesage D.; Malacria M.; Gimbert Y.; Gandon V.; Fensterbank L. Assessing Ligand and Counterion Effects in the Noble Metal Catalyzed Cycloisomerization Reactions of 1,6-Allenynes: a Combined Experimental and Theoretical Approach. ACS Catal. 2016, 6, 5146–5160. 10.1021/acscatal.5b02696. [DOI] [Google Scholar]
- Cheong P. H.-Y.; Morganelli P.; Luzung M. R.; Houk K. N.; Toste F. D. Gold-Catalyzed Cycloisomerization of 1,5-Allenynes via Dual Activation of an Ene Reaction. J. Am. Chem. Soc. 2008, 130, 4517–4526. 10.1021/ja711058f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau A.; Jaroschik F.; Lesage D.; Karanik M.; Guillot R.; Malacria M.; Tabet J.-C.; Goddard J.-P.; Fensterbank L.; Gandon V.; Gimbert Y. Tracking Gold Acetylides in Gold(I)-Catalyzed Cycloisomerization Reactions of Enynes. Chem. Sci. 2011, 2, 2417–2422. 10.1039/c1sc00478f. [DOI] [Google Scholar]
- Tsupova S.; Hansmann M. M.; Rudolph M.; Rominger F.; Hashmi A S. K. New Pathways for the Dual Gold-Catalyzed Cyclization of Diynes. Chem. - Eur. J. 2016, 22, 16286–16291. 10.1002/chem.201602873. [DOI] [PubMed] [Google Scholar]
- Markham J. P.; Staben S. T.; Toste F. D. Gold(I)-Catalyzed Ring Expansion of Cyclopropanols and Cyclobutanols. J. Am. Chem. Soc. 2005, 127, 9708–9709. 10.1021/ja052831g. [DOI] [PubMed] [Google Scholar]
- Sordo T. L.; Ardura D. On the Mechanism of Gold(I)-Catalyzed Ring Expansion of Cyclopropanols: Theoretical Calculations Uncover a Bottle-Neck 1,4-H Shift and Suggest Adequate Reaction Conditions. Eur. J. Org. Chem. 2008, 2008, 3004–3013. 10.1002/ejoc.200800043. [DOI] [Google Scholar]
- Zang W.; Wang L.; Wei Y.; Shi M.; Guo Y. Gold(I)-Catalyzed Ring Expansion of Alkynylcyclopropyl Allyl Ethers to Construct Tetrasubstituted Methylenecyclobutanones: A Mechanistic Investigation about the Character of Catalytic Amount of Water. Adv. Synth. Catal. 2019, 361, 2321–2328. 10.1002/adsc.201900053. [DOI] [Google Scholar]
- Hashmi A. S. K.; Wang T.; Shi S.; Rudolph M. Regioselectivity Switch: Gold(I)-Catalyzed Oxidative Rearrangement of Propargyl Alcohols to 1,3-Diketones. J. Org. Chem. 2012, 77, 7761–7767. 10.1021/jo301381z. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Xie J.; Maulide N. Stereoselective, Dual-Mode Ruthenium-Catalyzed Ring Expansion of Alkynylcyclopropanols. J. Am. Chem. Soc. 2008, 130, 17258–17259. 10.1021/ja807894t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbeck F.; Toste D. F. Gold(I)-Catalyzed Enantioselective Ring Expansion of Allenylcyclopropanols. J. Am. Chem. Soc. 2009, 131, 9178–9179. 10.1021/ja904055z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X.-Z.; Zhang M.; He Y.; Frei H.; Toste F. D. Dual Visible Light Photoredox and Gold-Catalyzed Arylative Ring Expansion. J. Am. Chem. Soc. 2014, 136, 5844–5847. 10.1021/ja500716j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.-L.; Li X.-X.; Zhou W.; Li X.; Chen Z. Divergent Synthetic Routes for Ring Expansion or Cyclization from 1,4-Allylic Diol Derivatives via Gold(I) Catalysis or Zinc(II) Mediation. J. Org. Chem. 2011, 76, 8814–8823. 10.1021/jo2015517. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Lebœuf D.; Retailleau P.; Gandon V.; Marinetti A.; Voituriez A. Enantioselective Gold(I)-Catalyzed Rearrangement of Cyclopropyl-Substituted 1,6-Enynes into 2-Oxocyclobutyl-Cyclopentanes. Chem. Commun. 2017, 53, 7026–7029. 10.1039/C7CC03234J. [DOI] [PubMed] [Google Scholar]
- Zhang D.-H.; Tang X.-Y.; Shi M. Gold-Catalyzed Tandem Reactions of Methylenecyclopropanes and Vinylidenecyclopropanes. Acc. Chem. Res. 2014, 47, 913–924. 10.1021/ar400159r. [DOI] [PubMed] [Google Scholar]
- Yuan W.; Dong X.; Wei Y.; Shi M. An Efficient Method for the Synthesis of Alkylidenecyclobutanones by Gold-Catalyzed Oxidative Ring Enlargement of Vinylidenecyclopropanes. Chem. - Eur. J. 2012, 18, 10501–10505. 10.1002/chem.201201161. [DOI] [PubMed] [Google Scholar]
- Xu M.; Ren T.-T.; Li C.-Y. Gold-Catalyzed Oxidative Rearrangement of Homopropargylic Ether via Oxonium Ylide. Org. Lett. 2012, 14, 4902–4905. 10.1021/ol302238t. [DOI] [PubMed] [Google Scholar]
- Li D.-Y.; Wei Y.; Shi M. Gold(I)-Catalyzed Intramolecular Carbon-Oxygen Bond Cleavage Reaction via Gold Carbenes Derived from Vinylidenecyclopropanes. Adv. Synth. Catal. 2016, 358, 3002–3009. 10.1002/adsc.201600483. [DOI] [Google Scholar]
- Xu M.; Ren T.-T.; Li C.-Y. Gold-Catalyzed Oxidative Rearrangement of Homopropargylic Ether via Oxonium Ylide. Org. Lett. 2012, 14, 4902–4905. 10.1021/ol302238t. [DOI] [PubMed] [Google Scholar]
- Xu M.; Ren T.-T.; Wang K.-B.; Li C.-Y. Synthesis of Cyclobutanones via Gold-Catalyzed Oxidative Rearrangement of Homopropargylic Ethers. Adv. Synth. Catal. 2013, 355, 2488–2494. 10.1002/adsc.201300227. [DOI] [Google Scholar]
- Wang Y.; Zheng Z.; Zhang L. Intramolecular Insertions into Unactivated C(sp3)−H Bonds by Oxidatively Generated β-Diketone-α-Gold Carbenes: Synthesis of Cyclopentanones. J. Am. Chem. Soc. 2015, 137, 5316–5319. 10.1021/jacs.5b02280. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Wang Y.; Ma X.; Li Y.; Zhang L. Non-Diazo C−H Insertion Approach to Cyclobutanones through Oxidative Gold Catalysis. Angew. Chem., Int. Ed. 2020, 59, 17398–17402. 10.1002/anie.202003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull J. A.; Croft R. A.; Davis O. A.; Doran R.; Morgan K. F. Oxetanes: Recent Advances in Synthesis, Reactivity, and Medicinal Chemistry. Chem. Rev. 2016, 116, 12150–12233. 10.1021/acs.chemrev.6b00274. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Wang T. Gold-Catalyzed Transformations of Propargyl Alcohols and Propargyl Amines. Asian J. Org. Chem. 2018, 7, 1758–1783. 10.1002/ajoc.201800324. [DOI] [Google Scholar]
- Ye L.; He W.; Zhang L. Gold-Catalyzed One-Step Practical Synthesis of Oxetan-3-ones from Readily Available Propargylic Alcohols. J. Am. Chem. Soc. 2010, 132, 8550–8551. 10.1021/ja1033952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary G. C.; Nortcliffe A.; Pearce C. A.; Hamza D.; Jones G.; Moody C. J. Densely Functionalised Spirocyclic Oxetane-Piperidine Scaffolds for Drug Discovery. Bioorg. Med. Chem. 2018, 26, 791–797. 10.1016/j.bmc.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Tice C. M.; Singh S. B. The Use of Spirocyclic Scaffolds in Drug Discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. 10.1016/j.bmcl.2014.06.081. [DOI] [PubMed] [Google Scholar]
- Carreira E. M.; Fessard T. C. Four-Membered Ring-Containing Spyrocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. 10.1021/cr500127b. [DOI] [PubMed] [Google Scholar]
- Ye L.; He W.; Zhang L. A Flexible and Stereoselective Synthesis of Azetidin-3-ones through Gold-Catalyzed Intermolecular Oxidation of Alkynes. Angew. Chem., Int. Ed. 2011, 50, 3236–3239. 10.1002/anie.201007624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P.; Xiang J.-N. Excellent Preparation of Azetidin-3-ones from Propargylic Alcohols by Gold-Catalysis. Adv. Mater. Res. 2013, 634, 970–974. 10.4028/www.scientific.net/AMR.634-638.970. [DOI] [Google Scholar]
- Robak M. T.; Herbage M. A.; Ellman J. A. Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
- Sahani R. L.; Liu R.-S. Gold-Catalyzed Oxidative Arylations of 3-Butyn-1-ols and 2-Propyn-1-ols with Nitrones to Yield Distinct Fused Indoles Bearing a Heterocyclic Ring. ACS Catal. 2019, 9, 5890–5896. 10.1021/acscatal.9b01491. [DOI] [Google Scholar]
- Wagh S. B.; Singh R. R.; Sahani R. L.; Liu R.-S. Gold-Catalyzed Oxidative Hydrative Alkenylations of Propargyl Aryl Thioethers with Quinoline N-Oxides Involving a 1,3-Sulfur Migration. Org. Lett. 2019, 21, 2755–2758. 10.1021/acs.orglett.9b00705. [DOI] [PubMed] [Google Scholar]