Abstract
Anti-tumour efficacy of doxorubicin is hindered by the cumulative dose-dependent cardiotoxicity induced by reactive oxygen species during its metabolism. As Cinnamomum zeylanicum has proven antioxidant potential, objective of this study was to investigate the cardioprotective activity of Cinnamomum bark extract against doxorubicin induced cardiotoxicity in Wistar rats. Physicochemical and phytochemical analysis was carried out and dose response effect and the cardioprotective activity of Cinnamomum were determined in vivo. 180 mg/kg dexrazoxane was used as the positive control. Plant extracts were free of heavy metals and toxic phytoconstituents. In vivo study carried out in Wistar rats revealed a significant increase (p < 0.05) in cardiac troponin I, NT-pro brain natriuretic peptide, AST and LDH concentrations in the doxorubicin control group (18 mg/kg) compared to the normal control. Rats pre-treated with the optimum dosage of Cinnmamomum (2.0 g/kg) showed a significant reduction (p < 0.05) in all above parameters compared to the doxorubicin control. A significant reduction was observed in the total antioxidant capacity, reduced glutathione, glutathione peroxidase, glutathione reductase, superoxide dismutase and catalase activity while the lipid peroxidation and myeloperoxidase activity were significantly increased in the doxorubicin control group compared to the normal control (p < 0.05). Pre-treatment with Cinnamomum bark showed a significant decrease in lipid peroxidation, myeloperoxidase activity and significant increase in rest of the parameters compared to the doxorubicin control (p < 0.05). Histopathological analysis revealed a preserved appearance of the myocardium and lesser degree of cellular changes of necrosis in rats pre-treated with Cinnamomum extract. In conclusion, Cinnamomum bark extract has the potential to significantly reduce doxorubicin induced oxidative stress and inflammation in Wistar rats.
Abbreviations: WHO, World Health Organization; DNA, Deoxyribonucleic acid; NADPH, Nicotinamide adenine dinucleotide phosphate hydrogen; SOD, Superoxide dismutase; ROS, Reactive oxygen species; DPPH, 2,2-diphenyl-1-picrylhydrazyl; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); ABEC, Aqueous bark extract of Cinnamomum zeylanicum; FRAP, Ferric reducing antioxidant power; NO, Nitric oxide; IP, Intraperitoneal; ELISA, Enzyme-linked immunosorbent assay; cTnI, Cardiac troponin I; NT-pro BNP, N terminal- pro brain natriuretic peptide; AST, Aspartate aminotransferase; LDH, Lactate dehydrogenase; PBS, Phosphate buffered saline; GSH, Reduced glutathione; GPx, Glutathione peroxidase; GR, Glutathione reductase; MPO, Myeloperoxidase; USA, United States of America; H & E, Haematoxylin and eosin; MDA, Malondialdehyde
Keywords: Doxorubicin, Cardiotoxicity, Oxidative-stress, Cinnamomum zeylanicum bark extract, Antioxidant effect, Myeloperoxidase
1. Introduction
Anthracyclines are a group of antibiotics which play a key role in the treatment of cancer in the modern era and they are among the essential medicine listed by the World Health Organization (WHO) (McGowan et al., 2017). These anti-neoplastic agents are used in the treatment of a wide range of malignancies including haematologic malignancies such as leukemia, lymphoma and solid organ tumors such as sarcoma, breast cancer, multiple myeloma and lung cancer. Among the members of the anthracycline family, doxorubicin is the most effective and commonly used antineoplastic drug (Shaker et al., 2018).
The clinical usefulness of doxorubicin is hampered by its dose related cardiotoxicity (Khattry et al., 2009, Minotti et al., 2004). Although the underlying mechanism of doxorubicin induced cardiotoxicity is not fully understood, reactive oxygen species induced oxidative stress is considered as the most accepted phenomenon (Chatterjee et al., 2010, Mobaraki et al., 2017). Some of the other proposed mechanisms are alterations in protein synthesis, deoxyribonucleic acid (DNA) damage, lipid peroxidation, cell membrane lesions, induction of immunogenic reactions and dysregulation of calcium homeostasis. As a result of doxorubicin metabolism, a semiquinone form is produced which is mediated by reduced flavoenzymes such as nicotinamide adenine dinucleotide phosphate hydrogen (NADPH)-cytochrome P450 reductase (Mitry and Edwards, 2016, Mobaraki et al., 2017). This semiquinone is able to complex with iron (Fe2+) and the free radical complex which reduce molecular oxygen to superoxide. In addition to this, enzymatic pathway produces free oxygen radicals by accepting electrons from nicotinamide adenine dinucleotide (NADH) or NADPH when doxorubicin gets reduced at Complex I of the electron transport chain. This reaction sequence is known as the redox cycling and it may be very harmful as even a low amount of doxorubicin is capable of producing many superoxide radicals. As doxorubicin has high affinity for cardiac-specific phospholipid named cardiolipin found in the internal membrane of mitochondria, it enters the mitochondria easily and suppresses the respiratory chain (Goormaghtigh et al., 1990, Aryal and Rao, 2016). Doxorubicin also diminishes the activity of cardiac enzymes such as catalase, superoxide dismutase (SOD) and glutathione S-
transferase. Furthermore, the heart tissues are more susceptible to damage by doxorubicin as the antioxidant storage is low in heart tissues compared to other organs in the body (Halestrap, 2006).
Therapeutic strategies which have the ability to supplement the cellular endogenous defence systems have been recognized as hopeful tactics to combat the oxidative stress conditions and in this respect, natural products which enhance endogenous antioxidants, have been found to offer protection in preventing doxorubicin induced cardiotoxicity (Mukherjee et al., 2003). Cinnamomum zeylanicum Blume (Ceylon Cinnamon) is a commonly found medicinal plant in Sri Lanka with proven antioxidant activity. It belongs to the family Lauraceae and has been reported to have many beneficial activities such as being an antioxidant, antimicrobial, anticancer, anti-inflammatory, antidiabetic, anti-mutagenic and as an anti-tyrosinase agent (Rao and Gan, 2014). It was previously reported that the bark extract of Ceylon cinnamon has numerous antioxidant compounds, which can effectively counteract with reactive oxygen species (ROS) such as hydroxyl radicals, superoxide anions as well as other free radicals. Many in vitro studies reported the antioxidant effect of Cinnamomum zeylanicum Blume in the recent past (Rao and Gan, 2014, Ghosh et al., 2015, Ranasinghe and Galappaththy, 2016, Premakumara and Abeysekera, 2020). The essential oils obtained from the bark of Cinnamomum zeylanicum Blume and eugenol have shown very powerful antioxidant activities (Chericoni et al., 2005) and in vitro studies revealed that Cinnamomum bark extracts effectively scavenged 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cations (Ranasinghe et al., 2013). Although it has already been proven that Cinnamomum zeylanicum Blume has a significant antioxidant activity, effect of its bark extract has never been investigated against doxorubicin induced cardiotoxicity in an animal model. Hence, the objective of this study was to investigate the ameliorative effects of Cinnamomum zeylanicum Blume bark against doxorubicin induced cardiac injury by the attenuation of oxidative stress and structural cardiomyocyte changes in rats.
2. Material and methods
2.1. Collection of Cinnamomum zeylanicum Blume bark
The cultivated Cinnamomum zeylanicum Blume bark was collected and identified according to the descriptions given by Jayaweera (1982). The species identification was confirmed by the curator of the National Herbarium, Royal Botanical Gardens, Peradeniya, Sri Lanka. A voucher specimen (2015/PG/VS/02) was deposited at the Department of Biochemistry, Faculty of Medicine, University of Ruhuna, Sri Lanka.
2.2. Standardization of plant material
2.2.1. Physicochemical analysis
The bark parts (cut into small pieces) of Cinnamomum were dried at 40 °C until a constant weight was reached and finely grounded. The powdered plant material was taken for the physicochemical analysis. Tests for moisture content, extractable matter and heavy metal analysis were followed according to the WHO standards (1996). Microscopic analysis of the plant was carried out according to the WHO (2011) guidelines on quality control and standardization of plant materials.
2.2.2. Phytochemical analysis
Phytochemical screening of Cinnamomum zeylanicum bark was followed to identify medicinally active substances found in the plant. Plant material was dried at 40 °C for three days, ground coarsely, and extracted in distilled water or organic solvents according to the method used. The relevant extracts were subjected to qualitative phytochemical screening assays for the detection of anthracene glycosides, cyanogenic glycosides, cardenoloid glycosides, saponins, polyphenols, alkaloids, flavonoids, tannins, reducing sugars and proteins (Trease and Evans, 2009, Mushtaq et al., 2014, Yusuf et al., 2014).
2.2.3. Total polyphenol content and in vitro antioxidant activity of aqueous bark extract of Cinnamomum zeylanicum (ABEC)
Constant weight of the Cinnamomum zeylanicum bark was ensured by drying the plant material at 40 °C and coarsely ground. Then the plant material (2.50 g) was mixed with distilled water (60 mL) and extracted using a reflux system for the preparation of the aqueous extract. After cooling, the mixture was filtered using a cheese cloth and the final volume was concentrated to 50 mL. Then final concentration of the refluxed ABEC was 0.05 g/mL. A concentration series (1–500 µg/mL) of plant extract was prepared for the in vitro antioxidant assays.
Folin-Ciocalteau spectrophotometric method described by Singleton et al. (1999) was used to measure the total polyphenol content in ABEC. Result was expressed as milligrams of gallic acid equivalent per gram of extract dry weight (mgGAE/g dw). Ferric reducing antioxidant power (FRAP) was determined according to the method described by Galketiya et al. (2017). DPPH assay was used to determine the radical scavenging ability of the ABEC according to modified method of Rahman et al. (2015). Nitric oxide (NO) assay was performed according to the modified Griess reaction (Boora et al., 2014). Following formula was used to calculate the radical scavenging activity in terms of percentage inhibition of free radicals by the sample.
Percentage inhibition = [(Abs control – Abs test)]/ (Abs control)] × 100
IC50 value (concentration of the plant extract or standard required to inhibit DPPH radical formation by 50%) was finally calculated to measure the antioxidant activity of the bark.
L- Ascorbic acid was used as the standard for DPPH, NO radical scavenging assay and the FRAP assay.
2.3. Preparation of plant extract for in vivo studies
The bark (cut into small pieces) of Cinnamomum was dried at 40 °C until a constant weight was reached and coarsely ground. Ground plant material (24.00 g) was refluxed in distilled water for 4hrs to be compatible with the extraction method used by the traditional ayurvedic medical practitioners in Sri Lanka. The filtered mixture was freeze dried after adjusting the final volume to 500.0 mL.
2.4. Experimental animals
Healthy, Wistar albino rats in both sexes which are 6–8 weeks old weighing 175 ± 25 g were purchased from the Medical Research Institute, Colombo, Sri Lanka. They were kept in a well-ventilated animal house located in the Faculty of Medicine, University of Ruhuna, Sri Lanka. A standard laboratory diet of rat pellets was used for feeding and water ad libitum. Rats were used in experiments after they were allowed to acclimatize to the settings of the new animal house such as the temperature (23 ± 2 °C), relative humidity (50 ± 5%), and 12hr light–dark cycle) for one week prior to the experiments. Approval was obtained from the Ethical Review Committee of the Faculty of Medicine, University of Ruhuna, Sri Lanka (23.10.2014:3.10).
2.5. Dose response effect of ABEC for cardioprotective effect in doxorubicin induced cardiotoxicity in vivo
Healthy Wistar albino rats (male and female) were divided into seven groups as ten animals in each group (Beery, 2018). Group I was the control group which was given distilled water orally for 14 days and on the 11th day a single intraperitoneal (IP) injection (10 mL/kg) of saline was injected after a 16hr fast. Group 2 was considered as the doxorubicin control group and they were administered distilled water orally for 14 days. On the 11th day, a single injection of 18 mg/kg of doxorubicin was administered intraperitonially after a 16hr fast. Group 3–7 received freeze dried ABEC (0.125, 0.25, 0.5, 1.0 & 2.0 g/kg) via oral administration for 14 days. Then, on the 11th day, a single dose of doxorubicin was injected intraperitoneally after a 16hr fast. All animals were sacrificed on the 15th day, blood was collected for the estimation of serum concentration of cardiac troponin I (cTnI), aspartate aminotransferase (AST, EC 2.6.1.1) and lactate dehydrogenase (LDH, EC 1.1.1.27) and heart tissues were collected in to 10% formal saline to be processed for the histological assessment of myocardial damage.
2.6. Experimental procedure for screening of ABEC for cardioprotective effect against doxorubicin induced cardiotoxicity in vivo
Healthy male and female Wistar albino rats were randomly allocated to five groups of ten animals in each group. Following test protocol was followed (Beery, 2018, Sandamali et al., 2020).
Group I (normal control); distilled water administered orally for 14 days, single IP injection of normal saline (10 mL/kg) on the day11 after 16hr fast
Group II (plant extract control); freeze dried ABEC (2.0 g/kg) administered orally for 14 days, single IP injection of normal saline (10 mL/kg) on the day 11 after 16hr fast
Group III (doxorubicin control); first distilled water administered orally for 14 days, then, a single dose of doxorubicin (18 mg/kg) intraperitoneally on the day 11 after 16hr fast
Group IV (plant + doxorubicin); freeze dried ABEC at 2.0 g/kg administered orally for 14 days, a single dose of doxorubicin at 8 mg/kg administered intraperitoneally on the day 11 after 16hr fast
Group V (positive control group); Distilled water was administered orally for 14 days, then a single injection of dexrazoxane (180 mg/kg, IP) was administered 30 min before the single dose of doxorubicin (18 mg/kg) was administered intraperitoneally on the day 11
On day15, all Wistar rats were sacrificed and blood was drawn by cardiac puncture for the estimation of AST activity, LDH activity, N terminal- pro brain natriuretic peptide (NT-pro BNP) cTnI concentration, concentration and myeloperoxidase (MPO, EC 1.11.2.2) activity. A portion of heart tissue was collected into phosphate buffered saline (PBS) to prepare the homogenate for the estimation of anti-oxidant parameters such as total antioxidant level, reduced glutathione (GSH), glutathione peroxidase (GPx, EC 1.11.1.9), glutathione reductase (GR, EC 1.8.1.7), catalase (EC 1.11.1.6) activity, SOD (EC 1.15.1.1) activity, and the lipid peroxidation. Remaining portion of heart tissues was stored in 10% formal saline for the histological assessment myocardial damage.
2.7. Assessment of blood parameters
The separated serum was used for the estimation of cardiac biomarkers and MPO activity. NT-pro BNP and cTnI concentrations were estimated based on sandwich-Enzyme-linked immunosorbent assay (ELISA) method using the test kits purchased from Elabscience Biotechnology Co., Ltd, China. AST activity and LDH activity were measured using spectrophotometric enzyme assay kit purchased from Biorex Diagnostic, United Kingdom. MPO activity was estimated using the ELISA kit purchased from DRG International Inc., (USA).
2.8. Assessment of antioxidant parameters and lipid peroxidation in the homogenate of heart tissues
Homogenate of the heart tissues was prepared by using ice-cold PBS buffer (tissue weight to homogenization buffer; 1:10). The supernatant of the homogenate was collected to assess the total antioxidant activity, GSH concentration, GR and GPx activities using commercially available spectrophotometric assay kits purchased from Biorex Diagnostic (UK). Activity of catalase was measured by an assay kit purchased from antibodies-online.com (USA). SOD activity and lipid peroxidation were assessed by the colourimetric assay kits purchased from Sigma Aldrich (USA).
2.9. Histological assessment of myocardial damage
The left half of the heart tissues fixed in 10% formal saline was processed and sections with 3 µm thickness were stained with routine histological stain, haematoxylin and eosin (H & E). The sections cut from each group were examined under the light microscope and necrotic changes were scored. The scoring system mentioned below was developed by the authors by observing the myocardium of rats (tissue section with 5 mm diameter).
Cells without necrotic changes: 0; Up to 10 cells with necrotic changes: 1; 10–50 cells with necrotic changes: 2; 50–100 cells with necrotic changes: 3; >100 cells with necrotic changes: 4
Cardiomyocytes with early necrotic changes including hyper eosinophilic cytoplasm with no striations and nuclear changes such as pyknosis, karrheorhexis or karyolysis were identified as necrotic cells. Density of necrotic myocytes was assessed in the peripheral and sub- endocardial regions of the myocardium separately.
2.10. Statistical analysis
Results are expressed as mean ± SD. The significance of inter-group differences was evaluated by one-way analysis of variance using SPSS 22.0 software. Differences between groups were considered statistically significant at P < 0.05.
3. Results
3.1. Physicochemical and phytochemical analysis
The physicochemical properties of Cinnamomum zeylanicum bark are shown in Table 1 (Supplementary data). When consider the extractable mater in water and methanol, hot extraction resulted in a higher yield of the plant. None of the heavy metals including lead (Pb), cadmium (Cd), arsenic (As) and mercury (Hg) were detected in the plant extract. Microscopic observations are also shown in Table 1 (Supplementary data).
In phytochemical analysis, Cinnamomum bark was positive for saponins, polyphenols, alkaloids, tannins, proteins and reducing sugars as shown in Table 2 (Supplementary data). The Cinnamomum plant extract was negative for toxic phytochemicals including anthracene, cyanogenic and cardenoloid glycosides.
3.2. Total polyphenol content and in vitro antioxidant activity of ABEC
Total polyphenol content and the in vitro antioxidant activity of ABEC are shown in Table 3 (Supplementary data). The correlation between the polyphenol content and the antioxidant activities of ABEC was determined to evaluate the appropriateness and consistency of the in vitro antioxidant assay methods. The linear regression analysis results are shown in Fig. 1 (Supplementary data). A substantial positive correlation (0.95 to 0.98) was detected between the polyphenolic content and antioxidant activities. Therefore, it can be assumed that there is a significant influence of phenolic substances to the recognized antioxidant activity of ABEC.
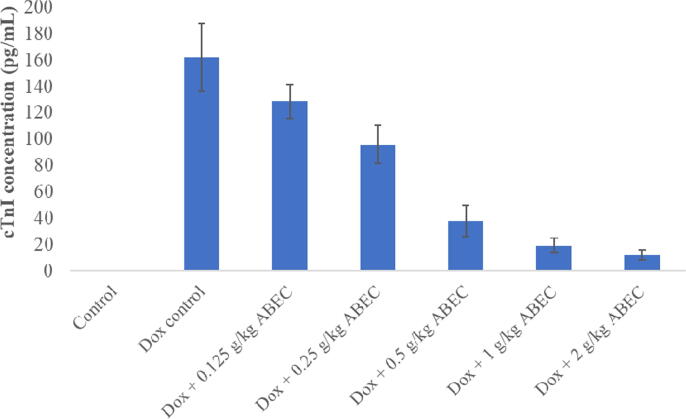
Fig. 1.
Serum cTnI concentration of rats treated with different concentrations of ABEC cTnI; cardiac troponinI, Dox; Doxorubicin, ABEC; Aqueous bark extract of Cinnamomum zeylanicum.
3.3. Dose response effect of ABEC
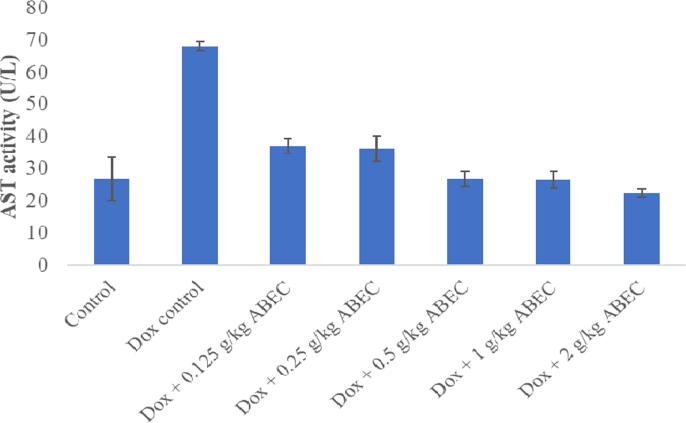
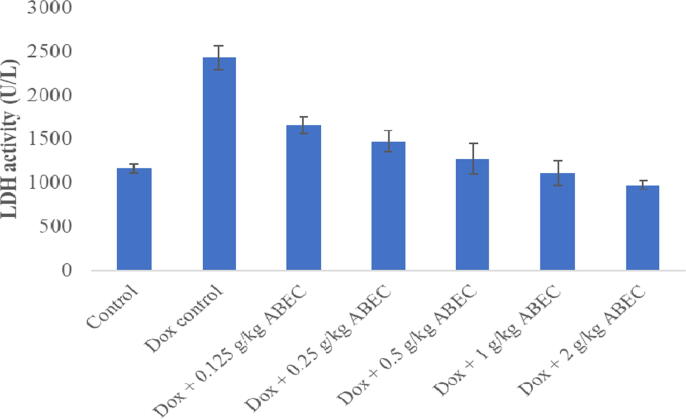
Doxorubicin control group showed significant increase (p < 0.001) in serum cTnI concentration (161.9 ± 25.7 pg/mL) compared to the control group (Fig. 1). When the dosage of ABEC was increased gradually, a gradual decrease in serum cTnI concentration was observed in rat groups treated with 0.25, 0.5, 1.0 and 2.0 g/kg dosages of plant extract compared to the doxorubicin control group (P < 0.05). When consider the measurement of serum AST and LDH activity, rat groups treated with doxorubicin alone showed a significant increase (p < 0.001) in enzyme activities compared to the control (Fig. 2, Fig. 3). However, all groups of rats treated with ABEC showed a significant decrease (p < 0.05) in AST and LDH activities compared to the doxorubicin control group.
Fig. 2.
Serum AST activity of rats treated with different concentrations of ABEC AST; Aspartate amino transferase, Dox; Doxorubicin, ABEC; Aqueous bark extract of Cinnamomum zeylanicum.
Fig. 3.
Serum LDH activity of rats treated with different concentrations of ABEC LDH; Lactate dehydrogenase, Dox; Doxorubicin, ABEC; Aqueous bark extract of Cinnamomum zeylanicum.
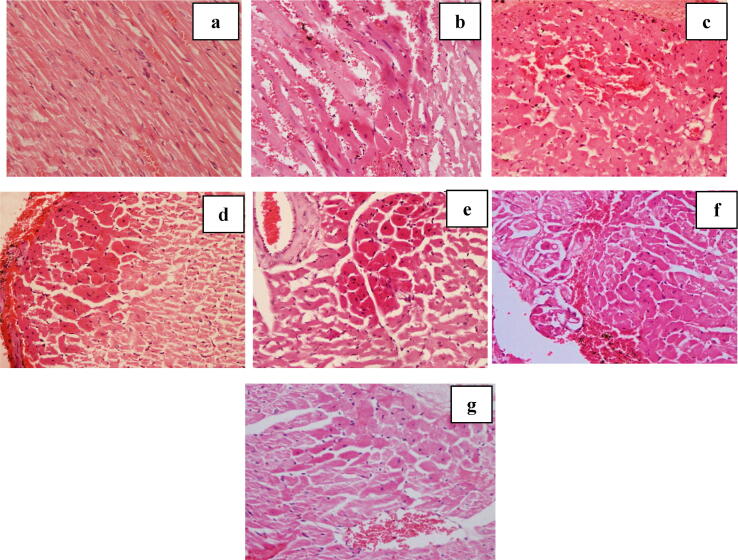
A normal morphology was observed in the cross section of the myocardial tissues of normal control group (Fig. 4, Fig. 5). However, group of rats that were treated with doxorubicin alone showed extensive early changes of necrosis in both peripheral and subendocardium of the myocardial tissue giving the highest score of 7.9 (Supplementary data Table 4, Fig. 4, Fig. 5). All rat groups treated with the ABEC (Group 3–7) showed a gradual reduction of the score for the early changes of necrosis with increasing dosage of the plant extracts (Supplementary data Table 4). Reversible histological changes such as inflammatory infiltrations, haemorrhages, interstitial oedema, congestion of blood vessels, intracellular vacuoles and wavy myocardial fibers were observed in doxorubicin control group (Supplementary data Table 5, Fig. 6). Groups of rats treated with the ABEC showed different degree of reversible histological changes while some changes were completely absent in the groups treated with higher doses of the ABEC.
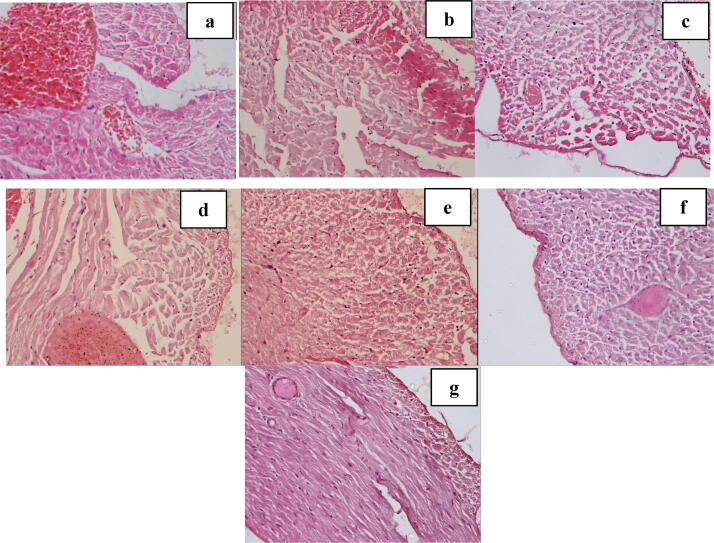
Fig. 4.
Cells with early necrotic changes in subendocardial region of rat heart treated with different doses of ABEC (Light micrograph, H & E, ×400). a – Control, b - Doxorubicin control, c - Dox + 0.125 g/kg of ABEC, d – Dox + 0.25 g/kg of ABEC, e – Dox + 0.5 g/kg of ABEC, f – Dox + 1.0 g/kg of ABEC, g – Dox + 2.0 g/kg of ABEC. ABEC; Aqueous bark extract of Cinnamomum zeylanicum, Dox; doxorubicin.
Fig. 5.
Cells with early necrotic changes in peripheral region of rat heart treated with different doses of ABEC (Light micrograph, H & E, ×400). a – Control, b - Doxorubicin control, c - Dox + 0.125 g/kg of ABEC, d – Dox + 0.25 g/kg of ABEC, e – Dox + 0.5 g/kg of ABEC, f – Dox + 1.0 g/kg of ABEC, g – Dox + 2.0 g/kg of ABEC. ABEC; Aqueous bark extract of Cinnamomum zeylanicum, Dox; doxorubicin.
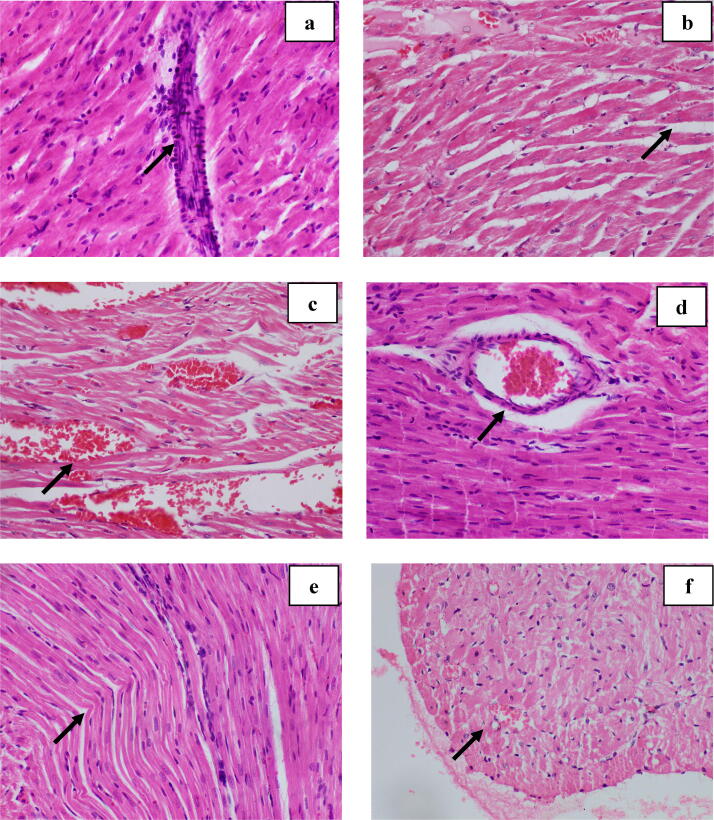
Fig. 6.
Photomicrographs of reversible cellular changes in myocardium of rats treated with doxorubicin (H & E, ×400) a- Inflammatory infiltrations, b- Interstitial oedema, c- Haemorrhages, d- Congestion of blood vessel, e- Wavy myocardial fibers, f- Intracellular vacuoles. Arrows indicate reversible cellular changes.
3.4. Screening of ABEC for cardioprotective effect
3.4.1. Effect on serum cardiac biomarkers
The concentration of cTnI as a specific marker of myocardial damage was significantly increased (p < 0.001) in doxorubicin control group exhibiting the mean value of 145.15 pg/mL compared to the normal control group (0.00 ng/mL). The treatment with ABEC alone (plant extract control) didn’t show any significant change in cTnI concentration compared to the normal control. Pre-treatment with ABEC showed a significant reduction (p < 0.001) in cTnI concentration with the mean value of 21.85 pg/mL compared to the doxorubicin control group (Table 1).
Table 1.
Effect of ABEC on serum cardiac biomarkers.
| Cardiac biomarkers | Group I (Control) |
Group II (Plant control) |
Group III (Dox Control) |
Group IV (ABEC + Dox) |
Group V (Positive control) |
|---|---|---|---|---|---|
| cTnI concentration (pg/mL) | 0.00 | 0.00 | 145.15 ± 10.77 | 21.85 ± 3.84*** | 11.46 ± 2.59*** |
| NT-pro BNP concentration (pg/mL) | 41.57 ± 7.29 | 35.86 ± 3.10 | 371.14 ± 9.69 | 198.57 ± 7.07*** | 159.43 ± 12.39*** |
| AST activity (U/L) | 25.71 ± 1.41 | 24.18 ± 1.60 | 66.10 ± 2.07 | 28.79 ± 1.98*** | 26.90 ± 1.26*** |
| LDH activity (U/L) | 1057.21 ± 38.6 | 1076.64 ± 49.8 | 1584.19 ± 83.4 | 1190.77 ± 77.2*** | 1104.97 ± 58.7*** |
ABEC; Aqueous bark extract of Cinnamomum zeylanicum, Dox; Doxorubicin, cTnI; cardiac Troponin I, NT-pro BNP; N-terminal pro brain natriuretic peptide, AST; Aspartate amino transferase, LDH; Lactate dehydrogenase. All values are expressed as mean ± SD (n = 10). p values: * < 0.05,**< 0.01, *** <0.001 compared to the doxorubicin control group (Group III).
The serum concentration of NT-pro BNP was significantly higher (371.14 ± 9.69 pg/mL) in animals treated with doxorubicin compared to the normal control group (41.57 ± 7.29 pg/mL) and there was no significant change in NT-pro BNP concentration in plant extract control (35.86 ± 3.10 pg/mL). Serum NT-pro BNP concentration was significantly decreased (p < 0.001) in animals treated with ABEC before the administration of doxorubicin showing the mean value, 198.57 pg/mL compared to the doxorubicin control group (Table 1).
The doxorubicin treated rats showed a significant increase (p < 0.001) in AST and LDH activity in the serum compared to the normal control group. Pre-treatment with ABEC significantly decreased (p < 0.001) the elevated level of AST and LDH activity in rats compared to the doxorubicin control group. Plant extract control group didn’t show any significant changes in AST and LDH activity compared to the normal control group (Table 1).
3.4.2. Effect on antioxidant parameters
The mean values of GSH level, GPx and GR enzyme activities were 2.3 nmol/mL, 277.60 U/L and 30.84 U/L respectively in heart tissues of rats treated with doxorubicin and a significant decrease (p < 0.001) was observed in these antioxidant parameters compared to the normal control group (4.7 ± 0.49 nmol/mL, 378.25 ± 3.81 U/L and 76.04 ± 3.09 U/L respectively) (Table 2). Pre-treatment with ABEC showed significant increase (p < 0.001) in GSH level, GPx and GR enzyme activities with the respective mean values, 4.01 nmol/mL, 342.79 U/L and 57.90 U/L compared to the doxorubicin control group.
Table 2.
Effect of ABEC on antioxidant parameters, lipid peroxidation and MPO activity.
| Biochemical parameters | Group I (Normal control) |
Group II (Plant control) |
Group III (Dox Control) |
Group IV (ABEC + Dox) |
Group V (Positive control) |
|---|---|---|---|---|---|
| GSH (nmol/mL) | 4.70 ± 0.49 | 4.83 ± 0.45 | 2.30 ± 0.37 | 4.01 ± 0.35*** | 4.42 ± 0.36*** |
| GPx (U/L) | 378.25 ± 3.81 | 377.78 ± 5.48 | 277.60 ± 6.03 | 342.79 ± 6.95*** | 362.94 ± 5.24*** |
| GR (U/L) | 76.04 ± 3.09 | 75.84 ± 2.33 | 30.84 ± 4.15 | 57.90 ± 3.48*** | 67.57 ± 3.88*** |
| SOD activity (%) | 87.60 ± 2.09 | 86.90 ± 2.11 | 49.42 ± 2.28 | 70.12 ± 1.59*** | 81.03 ± 3.44*** |
| Catalase activity (µmol/L) | 193.31 ± 3.46 | 190.23 ± 4.43 | 150.74 ± 6.68 | 173.46 ± 6.6*** | 178.61 ± 4.34*** |
| Total antioxidant capacity (mmol/L) | 5.52 ± 0.33 | 5.65 ± 0.25 | 2.92 ± 0.32 | 4.03 ± 0.30*** | 4.85 ± 0.35*** |
| Lipid peroxidation (nmol/µL) | 1.19 ± 0.009 | 1.21 ± 0.012 | 2.05 ± 0.023 | 1.51 ± 0.020*** | 1.28 ± 0.015*** |
| MPO activity (AAU/mL) | 157.74 ± 1.76 | 155.29 ± 2.27 | 285.32 ± 1.64 | 210.46 ± 4.6*** | 197.66 ± 1.97*** |
ABEC; Aqueous bark extract of Cinnamomum zeylanicum, Dox; Doxorubicin, GSH; Reduced glutathione, GPx; Glutathione peroxidase, GR; Glutathione reductase, SOD; Superoxide dismutase, MPO; Myeloperoxidase. All values are expressed as mean ± SD (n = 10). P values: * < 0.05, **< 0.01 *** <0.001 compared to the doxorubicin control group (Group III).
Doxorubicin treatment in Wistar rats caused significant decrease (p < 0.001) in SOD activity and catalase activity in the myocardium as compared to the normal control group (Table 2). Pre-treatment with freeze dried ABEC significantly increased (p < 0.001) the SOD and catalase activity compared to the doxorubicin treated animals. No significant changes in SOD and catalase activities were noticed in plant extract control compared to the normal control group.
3.4.3. Effect on lipid peroxidation in the cardiac tissues
There was a significant increase (p < 0.001) in the myocardial lipid peroxidation evident with the increased malondialdehyde levels (MDA) in the doxorubicin treated rat group (2.05 ± 0.023 nmol/µL) compared to the normal control (1.19 ± 0.009 nmol/µL) (Table 2). Pre-treatment with ABEC prevented the elevation of this oxidative stress marker giving the mean value of 1.51 nmol/µL. Plant extract control, however, exhibited no significant effect on the MDA level (1.21 ± 0.012 nmol/µL) when compared to the normal control group.
3.4.4. Effect on MPO enzyme activity
Any changes in the activity of MPO enzyme was not observed in the rats treated with ABEC alone. Doxorubicin control group exhibited significant (p < 0.001) elevation in the MPO activity (285.32 ± 1.64 AAU/mL) compared to the control group (157.74 ± 1.76 AAU/mL) (Table 2). Pre-treatment with ABEC in animals injected with doxorubicin showed significant reduction (p < 0.001) in MPO enzyme activity (210.46 ± 4.60 AAU/mL) compared to the doxorubicin control group.
Positive control group was administered with dexrazoxane before the injection of doxorubicin. Dexrazoxane treatment in rats showed a significant change (p < 0.001) in biochemical parameters compared to the doxorubicin control exhibiting the considerable cardio-protection. Although all biochemical parameters except catalase showed a significant difference between the positive control and the ABEC pre-treated group, pre-treatment with ABEC showed a significant difference (p < 0.001) in all parameters against the doxorubicin control group and showed a considerable protection against doxorubicin induced cardiotoxicity in Wistar rats.
3.4.5. Effect on histology of the myocardial tissues
Control group of rats showed normal morphology in both subendocardial as well as the peripheral region of the myocardium as shown in Fig. 7, Fig. 8. Necrotic changes were extensively seen in doxorubicin control group and they showed the highest score among the study groups (7.8) (Table 3). However, necrotic changes were more visible in the subendocardial region when compared to the peripheral region (Fig. 7, Fig. 8). Doxorubicin treated rats also showed many other histological changes of cell injury showed in Fig. 6 including intracellular vacuoles, wavy myocardial fibers, inflammatory infiltration, haemorrhages, interstitial oedema and congestion of blood vessel (Supplementary data Table 6). Pre-treatment with ABEC was capable to lessen the degree of damage in the myocardium showing significant reduction (p < 0.001) in the necrotic changes which was evident with decreased score (3.8) (Table 3). This group also showed much necrotic changes in subendocardial region than the peripheral region (Fig. 7, Fig. 8). Only the intracellular vacuoles and congestion of blood vessels were observed as reversible histological changes (Supplementary data Table 6). Treatment with ABEC alone did not reveal histological changes in the myocardium and showed normal morphology as in the normal control group.
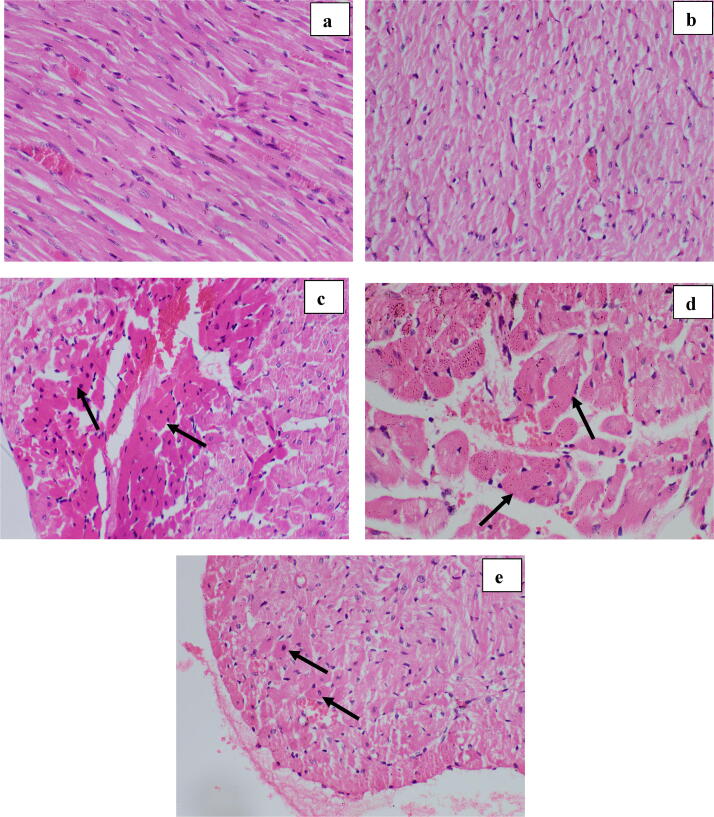
Fig. 7.
Effect of optimum dose of ABEC: Photomicrographs of subendocardial region of rat heart (H & E, ×400). a- Group I (normal control); no any histological changes, b- Group II (Plant control); shows normal architecture, c- Group III (Doxorubicin control); shows a higher degree of necrotic changes (hyper-eosinophilic cytoplasm and nuclear changes in cell death; pyknosis/ karrheorhexis/ karyolysis), d– Group IV (Dox + 2.0 g/kg of ABEC); shows lesser degree of necrosis, e– Group V (Positive control); shows occasional cells with early changes of necrosis. Arrows indicate areas of sub-endocardium with necrotic changes. ABEC; Aqueous bark extract of Cinnamomum zeylanicum, Dox; doxorubicin.
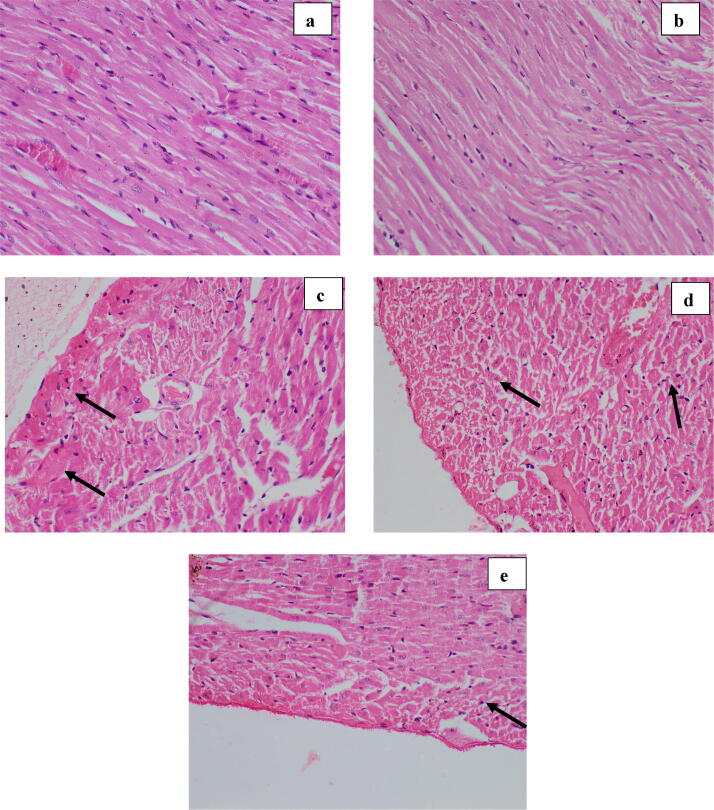
Fig. 8.
Effect of optimum dose of ABEC: Photomicrographs of peripheral region of rat heart (H & E, ×400). a- Group I (normal control); no any histological changes, b- Group II (Plant control); shows normal architecture, c- Group III (Doxorubicin control); shows a higher degree of necrotic changes (hyper-eosinophilic cytoplasm and nuclear changes in cell death; pyknosis/ karrheorhexis/ karyolysis), d– Group IV (Dox + 2.0 g/kg of ABEC); shows lesser degree of necrosis, e– Group V (Positive control); shows occasional cells with early changes of necrosis. Arrows indicate areas of peripheral region of myocardium with necrotic changes. ABEC; Aqueous bark extract of Cinnamomum zeylanicum, Dox; doxorubicin.
Table 3.
Average grading of cells with necrotic changes in myocardial tissues.
| Group I (Normal control) |
Group II (Plant control) |
Group III (Dox Control) |
Group IV (ABEC + Dox) |
Group V (Positive control) |
|
|---|---|---|---|---|---|
| Sub-endocardial region of heart tissues (score out of 4) | 0 | 0 | 4 | 2.6 | 1.9 |
| Peripheral region of heart tissues (score out of 4) | 0 | 0 | 3.8 | 1.2 | 0.8 |
| Total score (out of 8) | 0 | 0 | 7.8 | 3.8 | 2.7 |
ABEC; Aqueous bark extract of Cinnamomum zeylanicum, Dox; Doxorubicin. Grading scale; no cells with necrotic changes: 0; up to 10 cells with necrotic changes: 1; 10–50 cells with necrotic changes: 2; 50–100 cells with necrotic changes: 3; >100 cells with necrotic changes: 4.
4. Discussion
Among the strategies to attenuate doxorubicin induced cardiotoxicity, optimization of dosage, nanoencapsulation, usage of different analogues that lessen the oxidative stress have been identified as effective approaches. Although dexrazoxane is the only Food and Drug Administration (FDA) approved drug to treat anthracycline induced cardiotoxicity, it has several limitations (Bansal et al., 2019). Therefore, the present study sheds light on the ameliorative effect of Cinnamomum zeylanicum against doxorubicin induced cardiotoxicity and its potential to be developed further as a therapeutic agent.
According to the results obtained for the qualitative and quantitative analysis of antioxidants in the present study, important phytochemicals such as polyphenols, alkaloids and tannins were present in ABEC in significant quantities while toxic phytochemicals such as cyanogenic glycosides and cardenoloid glycosides were absent. These results corroborated with many previous findings where the presence of antioxidants such as polyphenols contributed significantly to the protective effect against doxorubicin induced cardiotoxicity (Kaiserová et al., 2007, Hamza et al., 2016, Ojha et al., 2016, Afsar et al., 2017, Ibrahim et al., 2017, Sergazy et al., 2020).
Previous studies have shown that proinflammatory cytokine expression, inflammatory cell infiltration and necrosis are commonly found in doxorubicin induced oxidative stress which ultimately lead to increased release of cardiac markers and natriuretic peptides to blood (Ikegami et al., 2007, Riad et al., 2009). A study done by Baniahmad et al. (2020) showed that doxorubicin treatment increases cardiac biomarkers including cTnI, AST and LDH levels in rat serum while treatment with vanillic acid, a pharmaceutical compound which belongs to phenolic acid family significantly reduce the release of cardiac biomarkers indicating its cardioprotective activity based on antioxidant effect. Similar to these results ABEC which has high polyphenolic content and in vitro antioxidant effect also showed cardio protection against doxorubicin treatment by significantly reducing the release of cardiac biomarkers. Several other studies also reported results consistent with the present study (Afsar et al., 2017, Oyagbemi et al., 2018, Li et al., 2020). In the present study, high concentrations of NT-proBNP was observed in doxorubicin treated rats indicating an increased ventricular dysfunction while the pre-treatment with ABEC lead to a significant reduction in NT-proBNP concentration. Previous studies conducted on experimental rats also showed similar results to the present study (Koh et al., 2004, Argun et al., 2016).
Doxorubicin induced cardiotoxicity was biochemically confirmed by the increase in oxidative stress as shown by the reduced antioxidant markers such as GSH, GPx, GR, SOD and catalase and total antioxidant capacity (Baniahmad et al., 2020). Several previous investigations showed that doxorubicin treatment increases oxidative stress in rats and plant extracts or some other compounds with significant antioxidant activity have the ability to attenuate free radical induced oxidative stress indicating an increased activity of antioxidant markers (Singh et al., 2008, Al-Harthi et al., 2014, Hamza et al., 2016, Afsar et al., 2017, Alam et al., 2018, Shaker et al., 2018, Li et al., 2020). In the present study, pre-treatment with ABEC also exhibited a significant increase in GSH and other antioxidant enzymes and total antioxidant capacity suggesting that pre-treatment with the plant extract may replenish the cardiomyocytes with antioxidant enzymes which exert cardioprotective effect against doxorubicin induced cardiotoxicity.
GSH is an important antioxidant compound in cellular defence system and depletion of GSH may contribute to enhanced lipid peroxidation in the cell membrane (Afsar et al., 2017). Doxorubicin treatment that increases the free radical formation in cardiomyocytes consequently enhance the lipid peroxidation indicated by an increase in MDA concentration, which is a stable end product of lipid peroxidation (Xiao, 2015). Previous investigations demonstrated that some herbal plants or related compounds with high antioxidant activity are capable of reducing MDA concentration in myocardial tissues of rats treated with doxorubicin (Hamza et al., 2008, Singh et al., 2008, Hamza et al., 2016, Kwatra et al., 2016, Afsar et al., 2017). In the present study also ABEC with high antioxidant effect was capable of significantly reducing the MDA concentration in homogenates of heart tissues suggesting that reduction of lipid peroxidation may be due to the radical scavenging ability of Cinnamomum bark.
Free radical induced oxidative stress in doxorubicin treatment may up regulate the inflammation through activation of NF-κB which stimulate the pro-inflammatory cytokine production (Hamza et al., 2016). Therefore, several investigations have shown that doxorubicin treatment increases MPO activity in rat serum which is considered as an inflammatory marker (Hamza et al., 2008, Hamza et al., 2016, Ibrahim et al., 2017; Oyagbemi et al., 2017; Bin Jardan et al., 2020). All these studies have shown that pre-treatment with compounds which have antioxidant effect are effective to reduce the MPO activity. Pre-treatment with ABEC also showed a significant reduction in MPO activity exhibiting its cardio protective activity through antioxidant effect. Further, Cinnamomum zeylanicum contains high amount of cinnamaldehyde which has anti-inflammatory effect which may contribute to down regulate the inflammatory pathway induced by the doxorubicin treatment (Han and Parker, 2017).
Histopathological assessment of myocardial damage is considered as the gold standard to diagnose acute doxorubicin induced cardiotoxicity (Octavia et al., 2012). In the present study, biochemical changes in doxorubicin induced cardiotoxicity were confirmed by histological changes in myocardial tissues including early changes of necrosis, inflammatory infiltrations, haemorrhages, interstitial oedema and wavy myocardial fibers. A previous study conducted by Zhang et al. (2017) also reported that doxorubicin produces massive changes in rat myocardium, consisting of necrosis, intracellular oedema, swollen and damaged mitochondria, and wavy degeneration of cardiac muscle fibers. In addition, El-Agamy et al. (2016) also demonstrated focal necrosis, signs of necrosis with inflammatory infiltrations and loss of muscle striations in rat heart treated with single dose of 20 mg/kg doxorubicin. Koti et al., 2013, Erboga et al., 2016 also showed similar histological changes in rats after the doxorubicin treatment. However, the pre-treatment with ABEC in doxorubicin treated rats showed better preservation of myocardium by reducing the reversible histological changes and mean score of early changes of necrosis indicating that Cinnamomum zeylanicum has a potential cardioprotective activity.
Collectively, biochemical and histopathological findings confirmed a potential cardioprotective effect of ABEC against doxorubicin induced cardiotoxicity. Therefore, the antioxidant and anti-inflammatory effect of Cinnamomum zeylanicum may be a strong contributing factor which protects the cells from degenerative changes. Thus, in this study, ABEC effectively prevented the tissue damage by decreasing the oxidative-stress and restoring the antioxidant status.
5. Conclusion
In conclusion, the present study revealed an ameliorative effect of Cinnamomum zeylanicum to counteract with doxorubicin induced cardiac injury for the first time. According to our results, ABEC exerted its protective effect against doxorubicin induced cardiotoxicity via antioxidant and anti-inflammatory activities.
Author contributions
JANS performed all experiments, analysed data and wrote the original draft of the manuscript. RPH conceptualized the study, critically reviewed and edited the manuscript, supervised & administered the project and secured funding. KAPWJ reviewed and revised the manuscript and supervised the project. LKB supervised experiments on histopathological evaluation of cardiac damage and data interpretation. All authors approved the final manuscript submitted and agreed to be accountable for all aspects of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Mr. G.H.J.M. Priyashantha and Mr. E.G. Rukman Asiri of the Department of Biochemistry, Faculty of Medicine, University of Ruhuna are acknowledged for the assistance provided in conducting animal experiments.
Funding
This work was supported by the National Research Council, Sri Lanka (Grant No: 18-050) and University Grants Commission, Sri Lanka (UGC/VC/DRIC/PG2015 (III)/RUH/01).
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2021.06.004.
Contributor Information
Jayasinghe Arachchige Nirosha Sandamali, Email: jansandamali@ahs.ruh.ac.lk.
Ruwani Punyakanthi Hewawasam, Email: rphewawasam@med.ruh.ac.lk.
Kamani Ayoma Perera Wijewardana Jayatilaka, Email: ayomaj@med.ruh.ac.lk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Afsar T., Razak S., Batoo K.M., Khan M.R. Acacia hydaspica R. Parker prevents doxorubicin-induced cardiac injury by attenuation of oxidative stress and structural cardiomyocyte alterations in rats. BMC Complement. Altern. Med. 2017;17 doi: 10.1186/s12906-017-2061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M.F., Khan G., Safhi M.M., Alshahrani S., Siddiqui R., Moni S.S., Anwer T. Thymoquinone ameliorates doxorubicin-induced cardiotoxicity in Swiss Albino mice by modulating oxidative damage and cellular inflammation. Cardiol. Res. Pract. 2018;2018 doi: 10.1155/2018/1483041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harthi S.E., Alarabi O.M., Ramadan W.S., Alaama M.N., Al-Kreathy H.M., Damanhouri Z.A., Khan L.M., Osman A.M. Amelioration of doxorubicin-induced cardiotoxicity by resveratrol. Mol. Med. Rep. 2014;10:1455–1460. doi: 10.3892/mmr.2014.2384. [DOI] [PubMed] [Google Scholar]
- Argun M., Üzüm K., Sönmez M.F., Özyurt A., Derya K., Çilenk K.T., Unalmış S., Pamukcu Ö., Baykan A., Narin F., Elmalı F., Narin N. Cardioprotective effect of metformin against doxorubicin cardiotoxicity in rats. Anatol. J. Cardiol. 2016;16:234–241. doi: 10.5152/akd.2015.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal B., Rao V.A. Deficiency in cardiolipin reduces doxorubicin-induced oxidative stress and mitochondrial damage in human B lymphocytes. PLoS ONE. 2016 doi: 10.1371/journal.pone.0158376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad B., Safaeian L., Vaseghi G., Rabbani M., Mohammadi B. Cardioprotective effect of vanillic acid against doxorubicin-induced cardiotoxicity in rat. Res. Pharm. Sci. 2020;15:87–96. doi: 10.4103/1735-5362.278718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal N., Adams M.J., Ganatra S., Colan S.D., Aggarwal S., Steiner R., Amdani S., Lipshultz E.R., Lipshultz S.E. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardio-oncol. 2019;5 doi: 10.1186/s40959-019-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery A.K. Inclusion of females does not increase variability in rodent research studies. Curr. Opin. Behav. Sci. 2018;23:143–149. doi: 10.1016/j.cobeha.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin Jardan Y.A., Ansari M.A., Raish M., Alkharfy K.M., Ahad A., Al-Jenoobi F.I., Haq N., Khan M.R., Ahmad A. Sinapic acid ameliorates oxidative Stress, Inflammation, and apoptosis in acute doxorubicin-induced cardiotoxicity via the NF-κB-mediated pathway. Biomed Res. Int. 2020;2020 doi: 10.1155/2020/3921796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boora F., Chirisa E., Mukanganyama S. Evaluation of nitrite radical scavenging properties of selected zimbabwean plant extracts and their phytoconstituents. J. Food Process. 2014;2014 doi: 10.1155/2014/918018. [DOI] [Google Scholar]
- Chatterjee K., Zhang J., Honbo N., Karliner J.S. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chericoni S., Prieto J.M., Iacopini P., Cioni P., Morelli I. In vitro activity of the essential oil of Cinnamomum zeylanicum and eugenol in peroxynitrite-induced oxidative processes. J. Agric. Food Chem. 2005;53:4762–4765. doi: 10.1021/jf050183e. [DOI] [PubMed] [Google Scholar]
- El-Agamy, D.S., Abo-Haded, H.M., Elkablawy, M.A., 2016. Cardioprotective effects of sitagliptin against doxorubicin-induced cardiotoxicity in rats. Exp. Biol. Med. (Maywood, N.J.). 241, 1577–1587. https://doi.org/10.1177/1535370216643418. [DOI] [PMC free article] [PubMed]
- Erboga, M., Donmez, Y.B., Sener, U., Erboga, Z.F., Aktas, C., Kanter, M., 2016. Effect of Urtica Dioica against doxorubicin-induced cardiotoxicity in rats through suppression of histological damage, oxidative stress and lipid peroxidation. Eur. J. Gen. Med. 13, 139-144. https://doi.org/10.15197/ejgm.1567.
- Galketiya C., Weerarathna T.S., Punchihewa J.C., Wickramaratne M.N., Wickramaratne D.B.M. Screening of edible plants in Sri Lanka for antioxidant activity. J. Med. Plants Stud. 2017;5:91–95. [Google Scholar]
- Ghosh T., Basu A., Adhikari D., Roy D., Pal A.K. Antioxidant activity and structural features of Cinnamomum zeylanicum. 3. Biotech. 2015;5:939–947. doi: 10.1007/s13205-015-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goormaghtigh E., Huart P., Praet M., Brasseur R., Ruysschaert J.M. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys. Chem. 1990;35:247–257. doi: 10.1016/0301-4622(90)80012-v. [DOI] [PubMed] [Google Scholar]
- Halestrap A.P. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- Hamza A.A., Ahmed M.M., Elwey H.M., Amin A. Melissa officinalis protects against doxorubicin-induced cardiotoxicity in rats and potentiates its anticancer activity on MCF-7 cells. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza A.A., Amin A., Daoud S. The protective effect of a purified extract of Withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol. Toxicol. 2008;24:63–73. doi: 10.1007/s10565-007-9016-z. [DOI] [PubMed] [Google Scholar]
- Han X., Parker T.L. Antiinflammatory activity of Cinnamon (Cinnamomum zeylanicum) bark essential oil in a human skin disease model. Phytother. Res. 2017;31:1034–1038. doi: 10.1002/ptr.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D.M., Radwan R.R., Fattah S.M.A. Antioxidant and antiapoptotic effects of sea cucumber and valsartan against doxorubicin-induced cardiotoxicity in rats: The role of low dose gamma irradiation. J. Photochem. Photobiol., B. 2017;170:70–78. doi: 10.1016/j.jphotobiol.2017.03.022. [DOI] [PubMed] [Google Scholar]
- Ikegami E., Fukazawa R., Kanbe M., Watanabe M., Abe M., Watanabe M., Kamisago M., Hajikano M., Katsube Y., Ogawa S. Edaravone, a potent free radical scavenger, prevents anthracycline-induced myocardial cell death. Circ. J. 2007;71:1815–1820. doi: 10.1253/circj.71.1815. [DOI] [PubMed] [Google Scholar]
- Jayaweera D.M.A. National Science Foundation in Sri Lanka; Sri Lanka: 1982. Medicinal plants (Indigenous and Exotic) used in Ceylon. [Google Scholar]
- Kaiserová H., Šimůnek T., van der Vijgh W.J.F., Bast A., Kvasničková E. Flavonoids as protectors against doxorubicin cardiotoxicity: Role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochim. Biophys. Acta, Mol. Basis Dis. 2007;1772:1065–1074. doi: 10.1016/j.bbadis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Khattry N., Malhotra P., Grover A., Sharma S.C., Varma S. Doxorubicin-induced cardiotoxicity in adult Indian patients on chemotherapy. Indian J. Med. Paediatr. Oncol. 2009;30:9–13. doi: 10.4103/0971-5851.56329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh E., Nakamura T., Takahashi H. Troponin-T and brain natriuretic peptide as predictors for adriamycin-induced cardiomyopathy in rats. Circ. J. 2004;68:163–167. doi: 10.1253/circj.68.163. [DOI] [PubMed] [Google Scholar]
- Koti B.C., Nagathan S., Vishwanathswamy A., Gadad P.C., Thippeswamy A. Cardioprotective effect of Vedic Guard against doxorubicin-induced cardiotoxicity in rats: A biochemical, electrocardiographic, and histopathological study. Pharmacogn. Mag. 2013;9:176–181. doi: 10.4103/0973-1296.111287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwatra M., Kumar V., Jangra A., Mishra M., Ahmed S., Ghosh P., Vohora D., Khanam R. Ameliorative effect of naringin against doxorubicin-induced acute cardiac toxicity in rats. Pharm. Biol. 2016;54:637–647. doi: 10.3109/13880209.2015.1070879. [DOI] [PubMed] [Google Scholar]
- Li Z., Chinnathambi A., Alharbi S.A., Yin F. Plumbagin protects the myocardial damage by modulating the cardiac biomarkers, antioxidants, and apoptosis signaling in the doxorubicin-induced cardiotoxicity in rats. Environ. Toxicol. 2020;35:1374–1385. doi: 10.1002/tox.23002. [DOI] [PubMed] [Google Scholar]
- McGowan J.V., Chung R., Maulik A., Piotrowska I., Walker J.M., Yellon D.M. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc. Drugs Ther. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minotti G., Menna P., Salvatorelli E., Cairo G., Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Mitry M.A., Edwards J.G. Doxorubicin induced heart failure: Phenotype and molecular mechanisms. IJC Heart Vasc. 2016;10:17–24. doi: 10.1016/j.ijcha.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobaraki, M., Faraji, A., Zare1, M., Dolati, P., Ataei, M., Manshadi, H.R.D., 2017. Molecular mechanisms of cardiotoxicity: A review on major side-effect of doxorubicin. Indian J. Pharm. Sci. 79, 335-344. https://doi.org/10.4172/pharmaceutical-sciences.1000235.
- Mukherjee S., Banerjee S.K., Maulik M., Dinda A.K., Talwar K.K., Maulik S.K. Protection against acute adriamycin-induced cardiotoxicity by garlic: role of endogenous antioxidants and inhibition of TNF-alpha expression. BMC Pharmacol. 2003;3 doi: 10.1186/1471-2210-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq A., Akbar S., Zargar M.A., Wali A.F., Malik A.H., Dar M.Y., Hamid R., Ganai B.A. Phytochemical screening, physicochemical properties, acute toxicity testing and screening of hypoglycaemic activity of extracts of Eremurus himalaicus baker in normoglycaemic Wistar strain albino rats. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/867547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Octavia Y., Tocchetti C.G., Gabrielson K.L., Janssens S., Crijns H.J., Moens A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Ojha S., Taee H.A., Goyal S., Mahajan U.B., Patil C.R., Arya D.S., Rajesh M. Cardioprotective potentials of plant-derived small molecules against doxorubicin associated cardiotoxicity. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/5724973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyagbemi A.A., Omobowale T.O., Olopade J.O., Farombi E.O. Kolaviron and Garcinia kola attenuate doxorubicin-induced cardiotoxicity in Wistar rats. J. Complement. Integr. Med. 2018;15 doi: 10.1515/jcim-2016-0168. [DOI] [PubMed] [Google Scholar]
- Premakumara G.A.S., Abeysekera W.P.K.M., 2020. Pharmacological properties of Ceylon cinnamon. In: Senaratne R., Pathirana R. (eds) Cinnamon. Springer, Cham. https://doi.org/10.1007/978-3-030-54426-3_12.
- Rahman M.M., Islam M.B., Biswas M., Alam A.H.M.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes. 2015;8 doi: 10.1186/s13104-015-1618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe P., Galappaththy P. Health benefits of Ceylon cinnamon (Cinnamomum zeylanicum): a summary of the current evidence. Ceylon Med. J. 2016;61:1–5. doi: 10.4038/cmj.v61i1.8251. [DOI] [PubMed] [Google Scholar]
- Ranasinghe P., Pigera S., Premakumara G.A., Galappaththy P., Constantine G.R., Katulanda P. Medicinal properties of 'true' cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement. Altern. Med. 2013;22 doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P.V., Gan S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Alternat. Med. 2014;2014 doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad A., Bien S., Westermann D., Becher P.M., Loya K., Landmesser U., Kroemer H.K., Schultheiss H.P., Tschöpe C. Pretreatment with statin attenuates the cardiotoxicity of doxorubicin in mice. Cancer Res. 2009;69:695–699. doi: 10.1158/0008-5472.CAN-08-3076. [DOI] [PubMed] [Google Scholar]
- Sandamali, J.A.N., Hewawasam, R.P., Jayatilaka, K.A.P.W., Mudduwa, L.K.B., 2020. Cardioprotective potential of Murraya koenigii (L.) Spreng. leaf extract against doxorubicin-induced cardiotoxicity in rats. Evid. Based Complement. Alternat. Med. 2020. https://doi.org/10.1155/2020/6023737. [DOI] [PMC free article] [PubMed]
- Sergazy S., Shulgau Z., Fedotovskikh G., Chulenbayeva L., Nurgozhina A., Nurgaziyev M., Krivyh E., Kamyshanskiy Y., Kushugulova A., Gulyayev A., Aljofan M. Cardioprotective effect of grape polyphenol extract against doxorubicin induced cardiotoxicity. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-71827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker R.A., Abboud S.H., Assad H.C., Hadi N. Enoxaparin attenuates doxorubicin induced cardiotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. BMC Pharmacol. Toxicol. 2018;19 doi: 10.1186/s40360-017-0184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G., Singh A.T., Abrahama A., Bhat B., Mukherjee A., Verma R., Agarwal S.K., Jha S., Mukherjee R., Burman A.C. Protective effects of Terminalia arjuna against doxorubicin-induced cardiotoxicity. J. Ethnopharmacol. 2008;117:123–129. doi: 10.1016/j.jep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Ortofer R., Lamuda-raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Trease G.E., Evans W.C. 16th ed. Bailliere Tindall Ltd; London: 2009. Pharmacognosy. [Google Scholar]
- World Health Organization . WHO Technical Report Series; World Health Organization, Geneva: 1996. Guidelines for the assessment of herbal medicines. Available at: https://www.who.int/traditional-complementary-integrative-medicine/publications/trs1010_annex2.pdf?ua=1. [Google Scholar]
- World Health Organization . WHO Press; World Health Organization, Geneva, Switzerland: 2011. Quality control methods for herbal materials. Available at: https://apps.who.int/iris/bitstream/handle/10665/44479/9789241500739_eng.pdf;jsessionid=698837D8E9627AA38A9A831738FC6F95?sequence=1. [Google Scholar]
- Xiao N.N. Effects of resveratrol supplementation on oxidative damage and lipid peroxidation induced by strenuous exercise in rats. Biomol. Ther. 2015;23:374–378. doi: 10.4062/biomolther.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf A.Z., Zakir A., Shemau Z., Abdullahi M., Halima S.A. Phytochemical analysis of the methanol leaves extract of Paullinia pinnata linn. J. Pharmacog. Phytother. 2014;6:10–16. doi: 10.5897/JPP2013.0299. [DOI] [Google Scholar]
- Zhang Y.Y., Yi M., Huang Y.P. Oxymatrine ameliorates doxorubicin-induced cardiotoxicity in rats. Cell. Physiol. Biochem. 2017;43:626–635. doi: 10.1159/000480471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.