Abstract
The current study focuses on the development and evaluation of nano lipidic carriers (NLCs) for codelivery of sorafenib (SRF) and ganoderic acid (GA) therapy in order to treat hepatocellular carcinoma (HCC). The dual drug-loaded NLCs were prepared by hot microemulsion technique, where SRF and GA as the drugs, Precirol ATO5, Capmul PG8 as the lipids, while Solutol HS15 and ethanol was used as surfactant and cosolvents. The optimized drug-loaded NLCs were extensively characterized through in vitro and in vivo studies. The optimized formulation had particle size 29.28 nm, entrapment efficiency 93.1%, and loading capacity 14.21%. In vitro drug release studies revealed>64% of the drug was released in the first 6 h. The enzymatic stability analysis revealed stable nature of NLCs in various gastric pH, while accelerated stability analysis at 25◦C/60% RH indicated the insignificant effect of studied condition on particle size, entrapment efficiency, and loading capacity of NLCs. The cytotoxicity performed on HepG2 cells indicated higher cytotoxicity of SRF and GA-loaded NLCs as compared to the free drugs (p < 0.05). Furthermore, the optimized formulation suppressed the development of hepatic nodules in the Wistar rats and significantly reduced the levels of hepatic enzymes and nonhepatic elements against DEN intoxication. The SRF and GA-loaded NLCs also showed a significant effect in suppressing the tumor growth and inflammatory cytokines in the experimental study. Further, histopathology study of rats treated SRF and GA-loaded NLCs and DEN showed absence of necrosis, apoptosis, and disorganized hepatic parenchyma, etc. over other treated groups of rats. Overall, the dual drug-loaded NLCs outperformed over the plain drugs in terms of chemoprotection, implying superior therapeutic action and most significantly eliminating the hepatic toxicity induced by DEN in Wistar rat model.
Keywords: Sorafenib, Nanolipdic carrier, Hepatocellular carcinoma, Antioxidants, Inflammatory markers, Nonhepatic parameters
1. Introduction
Hepatocellular carcinoma (HCC) is liver cancer and 3rd most common cause of death across the globe. 70–85% of the liver malignancy is attributed due to hepatitis infection or persistent fatty liver (Harshita, Rizwanullah, Beg, Pottoo, Siddiqui, Ahmad, 2019, Perz et al., 2006, Anwanwan et al., 2020). Annually, >6,20,000 cases are reported and the cases from South East Asia, Africa and China (Cheng et al., 2020). Eventually, the available therapy for HCC is limited. Only surgical resection and liver transplantation are the effective options, but the detection should recognize at the earlier stage of HCC (Cheng et al., 2020, Escudier et al., 2019). In all HCCs, chemotherapy and immunotherapy are very effective in >90% of the cases as the treatment of choice (Zhu et al., 2017).
Sorafenib (SRF) got approval in 2007 for the treatment of advanced HCC. In the subsequent years, SRF gained popularity and become the first-line drug recommended for advanced HCC treatment. Extensive clinical data revealed their maximizing benefit and it is also given in combination with other drugs for the treatment of HCC (Escudier et al., 2019). However, non-specific uptake induces severe side effects and elevated toxicity (Tahavi et al., 2016, Zhu et al., 2017). SRF is a multi-kinase inhibitor that impairs tumor development and shows cytostatic activity, which improves the survival rate of the patients (Tahavi et al., 2016). SRF also possess antiangiogenic effects in HCC and its combination with doxorubicin (DOX) is feasible and effective in enhancing the effects of SRF (Lee et al., 2020).
Another side, the utility of plant-based drugs has received wider attention, which possesses excellent chemotherapeutic and chemopreventive activities (Bahman et al., 2018). They are well-tolerated, non-toxic, cheap, and easy to obtain. Some biologically active natural products are considered as adjuvant therapy to provide synergistic action along with the conventional chemotherapeutic agents (Bahman et al., 2018). Earlier, the combined delivery of SRF with curcumin showed synergistic effects by arresting the growth of HCC cells and inducing apoptosis as compared to the treatment with SRF or curcumin (Bahman, et al., 2018). SRF has limitations such as poor aqueous solubility and low oral bioavailability, thus requires the administration of higher doses for proper therapeutic action (Tahavi, et al., 2016). In this regard, the simultaneous delivery of anticancer drugs with natural antioxidants in nanocarriers may provide synergistic effects and can effectively work with minimal doses (Tahavi et al., 2016, Bahman et al., 2018, Lee et al., 2020).
For decades, common medicinal plants including fungi have been used in the treatment of liver diseases. The important pharmacologically active constituent of ganoderma lucidum is ganoderic acid (GA). There are numerous literatures reported its uses for hepatoprotective, antitumor, antioxidant, hypocholesteraemia, and antihistaminic action (Rahman et al., 2019, Rahman et al., 2021). It is well known for its ability to neutralize free radicals and protect cells from mutagen-induced damage in animal studies, and also improves liver detoxification. Several literature reports on the development of NLCs of various drugs including the chemotherapeutic molecules for tumor targeting have been documented. The nanocarriers help in targeting drugs to the tumor site via leaky vasculature (Müller et al., 2002, Rahman et al., 2019, Xu et al., 2020).
In our research, SRF and GA-loaded NLCs were used as a combination therapy for achieving synergistic anticancer effect on HCC. The study revealed superior cytotoxicity on HepG2 cell line promoting greater tumor volume inhibition and vital change in additional parameters including hepatic injury markers, biochemical parameters, and antioxidant enzymes over SRF or GA solution in male albino Wistar rats.
2. Material and methods
2.1. Materials
Sorafenib (free base) was purchased from Active Biochem, Billion Centre, Hong Kong. Ganoderic Acid (GA) was received from Sigma Aldrich, USA. Capmul MCM C10 and Capmul PG8 were purchased from M/s Gattefosse, Cedex, France. Precirol® ATO5 and Solutol HS15 were purchased from Sigma-Aldrich Inc., Alabama, USA. Solvents with HPLC grades were purchased from local vendors. Span Diagnostics, Surat, India, supplied the kits for detection of the serum sickness Alkaline Phosphatase Test (APT), albumin, Aspartate Transaminase (AST) Test, Alanine transferase (ALT) and total protein.
2.2. Analytical method development
The reversed-phase high-performance liquid chromatography (HPLC) method was employed to measure the amount of SRF and GA from the in vitro samples and biological samples. The solvent system containing 0.1% v/v glacial acetic in water (pH 3.5) and acetonitrile (70:30% v/v) under isocratic flow rate at 0.2 mL/min was used. The separation was achieved on a BEH C18 (4.6 mm × 250 mm, particle size 5 μm) with the help of a photodiode array detector. The retention times (Rt) for SRF and GA were observed at 3.89 and 7.24 min, respectively, with good peak symmetry and absence of any peak tailing.
2.3. Preparation of SRF and GA-loaded NLCs
2.3.1. Pseudo ternary phase diagram
The lipid mixtures were selected in the ratio of 3:1, where Precirol® ATO5 was taken as the solid lipid and Capmul PG8 was taken as the liquid lipid. The combinations of Solutol HS15 and ethanol were taken as Smix and distilled water was used as the aqueous phase. To equalize the temperature of the surfactant and lipid phase, Smix and other components were heated at the same temperature. In the finding of microemulsion (ME) region, the temperature kept at 60–70 °C, and known quantity of Smix and lipid phase (10:0, 0:10 w/w) was added. The appearance of turbidity in the lipid and aqueous phase mixture was taken as the end-point of titration. The weight percentage of individual components at the end-point is calculated and phase diagrams are constructed for each Smix ratio (Rahman, et al., 2019).
2.3.2. Preparation of the NLCs
The SRF and GA-loaded NLCs were prepared by hot microemulsion technique (Heidolph, Silent Crusher M, Germany). To obtain the primary microemulsion, SRF and GA, Precirol® ATO5, Capmul PG8 were melted, while Solutol HS15 and ethanol were dissolved in the required amount of water. The entire operation is processed at 60–70 °C and proceeds to be revived at 3 W by a probe sonicator at a sonic duration of 15 s (Sonicator 3000, Misonix). The resulting hot microemulsion was poured into a solution of 0.5% w/v of Precirol® ATO5 with high shear by micro-syringe. Furthermore, providing shear homogenization at the 10000 rpm for 15 min and provides magnetic stirring at 500 rpm for 1–2 h. Whereas the unencapsulated drug was removed with the use of a water-to-dimethylformamide (DMF) mixture in the ratio of 3:1 with a cellulose dialysis bag (MW of 10 kDa).
2.4. Characterization of the NLCs
2.4.1. Size, particle-diameter dispersity index (Ɖ) and surface morphology
The drug-loaded NLCs were evaluated for determining the particle size and particle-diameter dispersity index (Ɖ) using Zetasizer (Nano ZS, Malvern, UK) (Kumar et al., 2016). The samples were diluted 10–20 times with the deionized water and taken in polystyrene cuvettes to analyze the aforementioned parameters. For determining the surface morphology, NLC dispersion was placed on a glass slide, mounted with a coverslip, and placed at an appropriate magnification using an optical microscope (Medilux, Kyowa opticals Co. Ltd, Hashimoto, Japan). The surface morphology was evaluated by transmission electron microscopy (TEM) after staining with the help of 1% phosphotungstic acid solution on the grid surface and observed under TEM at room temperature (JEM-2100F, Jeol, Tokyo, Japan).
2.4.2. Entrapment efficiency and loading capacity
The entrapment efficiency (EE) and loading capacity (LC) were measured by particle lysis method. EE is the percentage of a drug that is essentially incarcerated or adsorbed. LC is the total amount of drug encapsulated into the formulation in total amount particle weight. At first, the unentrapped drug-loaded formulation was subjected to lysis by adding ethanol. The lysed samples were diluted by adding mobile phase and filtered out. The drug concentration in the filtrate was analyzed by the HPLC method described in Section 2.2. EE and LC were then calculated using equations (1), (2) (Ahmad et al., 2018).
| (1) |
| (2) |
2.5. In vitro gastrointestinal stability
An aliquot (3 mL) of the optimized SRF and GA-loaded NLCs was added to 250 mL of simulated gastric fluid for 2 h and simulated intestinal fluid for 6 h time duration. After incubation, 1.5 mL of the sample was taken for determining of the particle size, Ɖ and EE, as per the experimental methods described in the previous sections.
2.6. In vitro drug release
The SRF and GA-loaded NLCs were performed out in dialysis bag (12 kDa, M/s Himedia Limited, Mumbai, India), kept in the saline phosphate buffer (pH 7.4), which were incubated at 37 ± 0.5 °C and provide agitation at 100 rpm for 24 h. The NLC dispersion containing 50 mg drugs was taken in the dialysis bag and kept in the beaker containing 50 mL dissolution medium. At periodic time intervals, aliquot 0.5 mL samples were withdrawn and immediately replaced with the fresh dissolution media. The obtained samples were suitably diluted, filtered and drug concentration was quantified by the HPLC method described in Section 2.2. The drug release data analysis was carried out with the help of ZOREL software by applying correction factors for the drug loss during sampling (Singh and Singh, 1998).
2.7. Cell culture study and cell viability
Human hepatocellular carcinoma cell line (HepG2) was obtained from NCCS, Pune, India. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM), 1 mM L-glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin with 10% (v/v) of foetal bovine serum (FBS). Furthermore, the cells (5 × 104 cells/well) were incubated at 37 °C temperature supplied with 5% CO2/95% air, and (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (MTT) assay was carried out for measuring the cellular cytotoxic potential of the various treatments and control groups. The treatment formulations were seeded with cells in 96 well-plates and incubated at temperature of 37 °C for 24 and 48 h. After that, 10 μL MTT solution prepared in phosphate buffer saline (pH 7.4) was added to the plates and stored at 37 °C for 2 h for incubation. The enzyme-linked immunosorbent assay (ELISA) plate reader (BioTek, USA) was used to calculate the optical density at 570 nm and cell viability was measured using the equation (3) given below. The graph pad prism software (GraphPad Inc., MA, USA) was used to calculate the overall inhibitory half concentration (IC50).
| (3) |
2.8. Stability studies
To evaluate the stability of the drugs in NLCs, the optimized formulations were kept in Binderfi KBF-240 climate chambers (Hashemi et al., 2020). The stability studies were performed at 25 °C/60% relative humidity (RH) and 40 °C/75% RH conditions. The formulations stored in the glass ointment jars and kept in the stability chambers. At predetermined intervals such as 1, 2, 3, 6 and 12 weeks of storage, the vials were removed and tested for the formulation quality attributes such as particle size, Ɖ, EE and LC, respectively.
2.9. Animal experiments
The male albino Wistar rats (weight 140–175 g) were used for the experimental study and were acclimatized at 25 ± 2 °C; 12/12 h with light and dark cycles and fed the normal rattan chow and water ad libitum. The study protocols were duly approved by the Institutional Animal Ethical Committee approved by the Institutional Animal Ethics committee of Patliputra University, Patna, Bihar, India (1840/PO/ReBi/S/15/CPCSEA Reg. No.).
2.10. Induction of the HCC
The rats were administered with a single intraperitoneal injection of diethyl nitrosamine (DEN; 200 mg/kg) in phosphate buffer saline (pH 7.4) to induce the HCC (Kumar et al; 2017a,b). After 10 days of administration of DEN, the animals in various treatment groups and control groups were subjected to the determination of by α-fetoprotein (AFP) level to confirm the development of HCC (Ravenzwaay and Tennekes, 2002).
2.11. Experimental procedure
The rats were divided into six groups, with six animals in each group. Group I was as control group which were administered with 0.9% w/v normal saline at the dose of 5 mL/kg/day p.o. (orally). Group II animals were administered with SRF (50 mg/kg) 5 mL/kg/day for 14 weeks. Group III animals were administered with DEN at the dose of 200 mg/kg in phosphate buffer. Group IV animals were given GA (50 mg/kg) single-dose and DEN (5 mL/kg/day p.o) for 14 weeks. Group V animals were administered with SRF and GA solution (25 mg/kg each of drug) single dose and DEN (5 mL/kg/day p.o.) for 14 weeks. Group VI animals were administered with SRF and GA-loaded NLC (25 mg/kg) and DEN (5 mL/kg/day p.o.) for 14 weeks. All the rats were euthanized using cervical dislocation method after completion of the experiment. The body weight of rats was recorded over the time to observe the changes and the relative tumor volume was calculated using equation (4). The blood sample (~0.5 mL) were withdrawn from the retro-orbital plexus of rats and stored in heparinized tubes at 4 °C (Kumar et al; 2017a). The serum was collected by centrifugation at 5000 rpm for 15 min at 37 °C, and then various biochemical parameters and oxidative stress markers were estimated for various study groups.
| (4) |
where, Tx indicates absolute tumor volume of the respective tumor on day “x”) and T0 = represents the absolute tumor volume of the on day 0 when treatment was started
2.12. Pharmacokinetic studies
The healthy male Wistar rats (weight 150–200 g) were taken for pharmacokinetic study and the animals were divided into four groups (n = 6) and kept under over night fasting. Animals were perorally administered with various treatment formulations such as SRF solution (50 mg/kg), GA solution (50 mg/kg), SRF and GA solution (25 mg/kg of each drug), SRF and GA-loaded NLC (25 mg/kg of each drug). After oral administration, the blood samples were collected from the retro-orbital plexus at periodic time intervals in heparinized tubes and subsequently centrifuged at 10,000 rpm for 15 min to separate the plasma and analysis was carried out as per the previously developed and validated HPLC method described in Section 2.2. The obtained data were analyzed through model-independent pharmacokinetic approach to calculate various drug absorption and elimination parameters.
2.13. Biodistribution studies
The rats carrying HCC was classified randomly in the biodistribution studies in the 4 groups (n = 6) and orally administered formulation entitled as SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLC at the same dose of 50 mg/kg. After administration of aforementioned formulations each group of rats was sacrificed at post 10 h. The vital organs such as kidney, lung, heart, spleen, liver and tumor were collected. These organs were homogenized separately and drugs were extracted with ethanol and water (70:30% v/v). The drug concentrations in the organ samples were estimated by HPLC method described in Section 2.2 by considering the tissue weight (µg/g tissue).
2.14. Hepatic tissue injury markers
α-fetoprotein (AFP), alanine transaminase (ALT) and aspartate transaminase (AST) were determined as hepatic tissue injury markers, as per the procedure described in literature (Kumar et al., 2017b, Harshita, Rizwanullah, Beg, Pottoo, Siddiqui, Ahmad, 2019) as according to the given instruction of kits manufacturer (Span Diagnostic, Surat, India).
2.15. Biochemical parameters
The biochemical parameters including total protein, total bilirubin and globulin were determined as per the methods described in literature (Rahman et al., 2019). Besides, the samples were also kept to evaluate the membrane enzymes such as Mg2+ATPase, Ca2+ATPase and Na+/K+-ATPase as per the reported procedures (Rahman et al., 2019).
2.16. Antioxidant parameters
The antioxidants parameters such as lipid peroxidation (LPO), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase and glutathione-S-transferase (GST) were determined as per the methods described in literature (Rahman et al., 2019).
2.17. Inflammatory mediators
The formation and development of HCC is dominated by inflamed mediators such as interleukin-1β (IL-1ß), interleukin-6 (IL-6), (TNF-α) tumor necrosis factor-α and NF- κB (Rahman et al., 2019, Harshita, Rizwanullah, Beg, Pottoo, Siddiqui, Ahmad, 2019). In addition, these inflammatory mediators have been measured using diagnostic kits which are commercially available (Span Diagnostic, Surat, India).
2.18. Histopathology examination
Liver tissues were prepared from each group of rats, embedded in the 10% formalin, dehydrated in 50–100% gradual alcohol, washed in xylene and were fixed in paraffin wax (Rahman, et al., 2019). After that, the liver tissue stained with hematoxylin and eosin (H-E) for microscopical examination.
2.19. Data analysis
The obtained results were reported as mean ± S.E.M and One-way variance analysis (ANOVA) followed by post-hoc analysis was performed using Dunnett’s multiple comparison test with 5% level of statistical significance (P < 0.05).
3. Results and discussion
3.1. Phase diagram
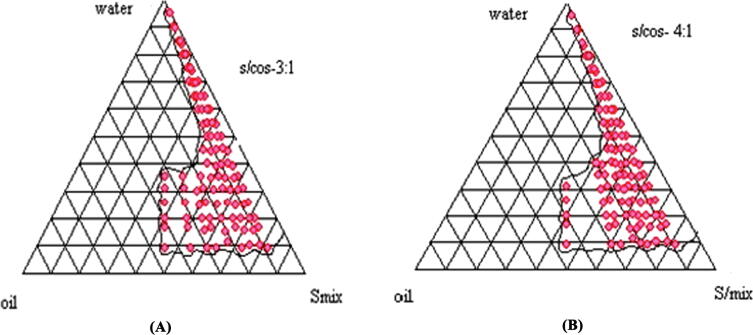
In the construction of a pseudo-phase diagram by titration process, the probable area of ME was recognised with different composition analysis. The border points were also analysed with the use of Gibbs triangle, which comprises water, lipid mixtures and Smix fractions, as shown in Fig. 1A-B. In addition, the axis of the triangle has the title aqueous-oil, aqueous-surfactant or surfactant with oil for one of the three binary mixtures. The changes made in Solutol HS15 and ethanol (Smix) ratio, the ME region was altered. From the phase diagram evaluation, Precirol® ATO5 and Capmul PG8 were taken as the solid and liquid lipids at the ratio of 4:1. The ME region was increased when Smix ratio goes up to 4:1 (as shown in Fig. 1B). Hence, the selected Smix ratio was utilized for preparing the NLCs.
Fig. 1.
Pseudo ternary phase diagrams indicating oil-in-water microemulsion (shaded area) region of Precirol® ATO5, Capmul PG8 as the lipidic phase, Solutol HS15 and ethanol (as surfactant and cosurfactant) at different Smix ratios 3:1 and 4:1.
3.2. Preparation and optimization of the NLCs
The NLCs were prepared using glyceryl palmitostearate mixture Precirol® ATO5 and Capmul PG8 as the solid and liquid lipids. Ethanol was used as a co-surfactant in the making of primary emulsion and further it is effective for easy separation during the development process. The variation in particle size and particle-diameter dispersity index (Ɖ) was observed from the NLCs prepared with different lipid concentrations, which selected from the microemulsion region. The NLCs were developed at the concentration of 12% lipid and 35% of Smix, to receive the maximum LC. In addition, at poor concentration of surfactant led to bigger particle size, lower LC and EE. In the other hand, the increased surfactant concentration (0.25–1% w/v) results in larger particle sizes. In addition, the particle size and EE decreased above 0.5% of surfactant concentration. However, at 0.5% surfactant concentration, the maximum LC, EE and smaller particle size was achieved. For optimization, sonication time and sonication energy are critical. The sonication time and probe sonicator energy>20 s and 3 W, produces unstable NLC with lower particle size, entrapment and agglomeration can result in the formation of the larger particle size (data not shown).Thus, the sonic duration of up to 20 s at 3 W results in improved entrapment ability and a smaller particle size, which may lead to produce the fine NLCs due to the increased lipid solubility of SRF and GA-loaded NLCs. Homogenization speed and time exhibited major effect on particle size and EE, which are critical parameters for development of NLCs. In the range between 7000 and 12000 rpm for a 10–25-min period, the homogenization speed was optimized for uniform size distribution (Mitri et al., 2011). The resulting NLCs were found to be with particle size ranging between 20 and 50 nm, while EE was found to be ranging between 90 and 93%. The optimization studies revealed high drug loading, smaller particle size and good EE, ostensibly owing to the rational selection of the blend of lipid mixture which helped in designing the NLC formulation with desired formulation attributes (Moraes et al., 2021).
3.3. Characterizations of the SRF and GA-loaded NLCs
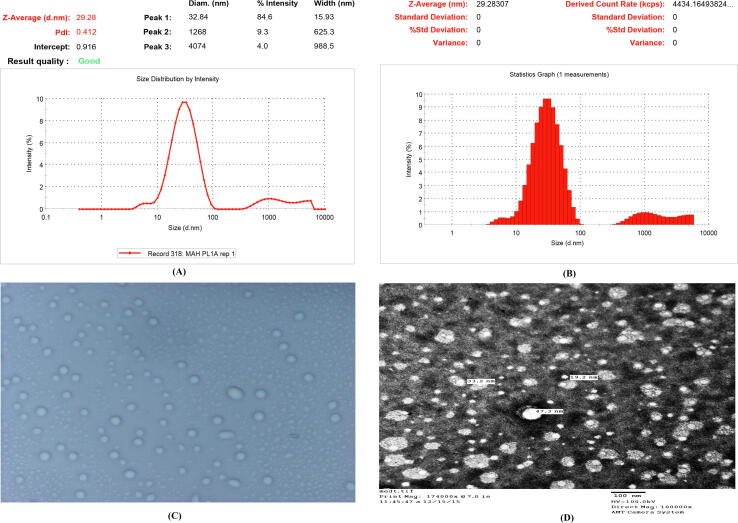
The particle size below 10 nm is extracted quickly from the renal filtration, while the particle size >300 nm is rapidly absorbed and removed from the circulation of the blood by reticuloendothelial system (Rahman et al., 2021). Additionally, NLC/nanoparticles in 10 to 200 nm from a disorderly tumor vasculature can be extravasated (Rahman et al., 2021). Thus, NLCs with size of <200 nm and a negative surface charge helps in inhibiting the protein adsorption, and encourages accumulation in the tumor. In the present research, the optimized formulation, found particle sizes of 29.28 nm with Ɖ of 0.412, it confirmed the homogeneous dispersion of NLC (as shown in Fig. 2A-B). The photo microscopy indicated the uniform morphology and poor degree of aggregation (shown in the Fig. 2C). The morphology of the same formulation also shows that the optical micrograph image is of uniform morphology and a poor degree of aggregation. In addition, TEM studies have shown spherical particles of 19. 2 nm to 47.3 nm of scale (shown in Fig. 2D). The optimized SRF and GA-loaded NLC showed EE and LC of 93.1% and 14.21%, that reflects the appropriate concentration of lipids, drug concentration, Smix, homogenization speed, sonication time and power in the development of aforementioned formulation. In addition, this also showed the method employed for preparation and other process parameters used are effective. Overall, the results observed were in agreement with the finding of other researcher and our previous published article and validate their results (Beg et al., 2018a, Beg et al., 2018b, Rahman et al., 2021).
Fig. 2.
(A-B) Particle size distribution of SRF and GA-loaded NLCs, (C) photo-microscopy of SRF and GA-loaded NLCs, D) Transmission Electron microscopy (TEM) of SRF and GA-loaded NLCs. (SRF; Sorafenib: GA; Ganoderic Acid: NLC; Nano lipid carriers).
3.4. In vitro gastrointestinal stability
Table 1 shows the particle size, Ɖ, and EE of the optimized SRF and GA-loaded NLCs employed with different GI fluids at the pH of 1.2 and 6.8. Further, insignificant differences (P > 0.005) observed in the formulation properties before and after the treatment and finally revealed the adequate stability of the aforementioned formulation in the gastrointestinal pH conditions.
Table 1.
In vitro gastrointestinal stability of optimized SRF cum GA loaded NLC in variousgastric media.
| Stability Parameters | SGF (pH 1.2) |
SIF (pH 6.8) |
||
|---|---|---|---|---|
| Before | After | Before | After | |
| Particle size (nm) | 29.28 ± 0. 2 nm | 30.32 ± 2.20 nm | 29.28 ± 0. 3 nm | 30.12 ± 1.34 nm |
| Particle-diameter dispersity index (Ɖ) | 0.41 | 0.51 | 0.41 | 0.51 |
| Entrapment Efficiency (%) | 93.1 ± 1.5 | 92.02 ± 0.10 | 93.1 ± 1.41 | 92.21 ± 1.01 |
SGF: Simulated gastric fluid; SIF: Simulated intestinal fluid.
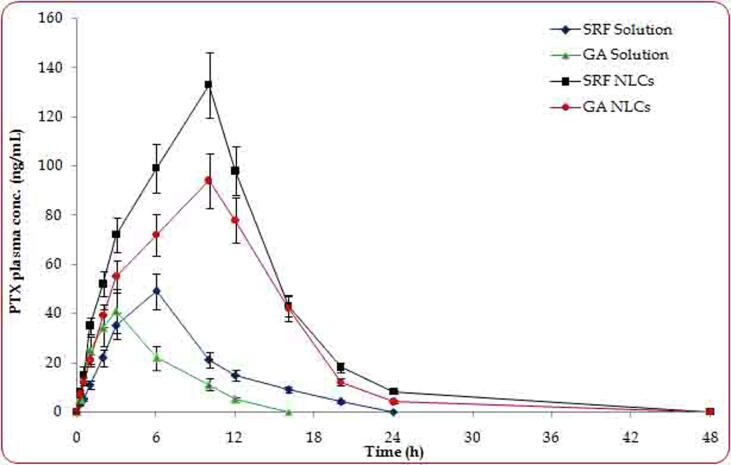
3.5. In vitro release studies
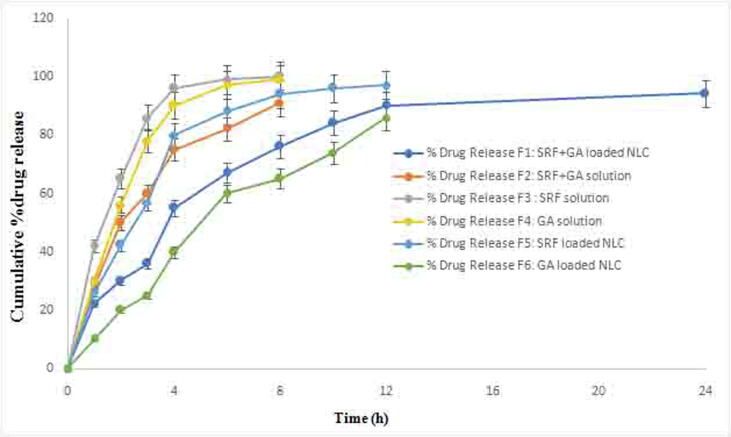
The in vitro release data of 24 h study showed the biphasic pattern at initial burst release with 64% drug release in first 6 h and then followed sustained drug release profile, as shown in Fig. 3. The release rate of SRF and GA was determined by HPLC. In the in vitro drug release studies, SRF solution showed a faster release profile than GA and a combination of both the drugs (Fig. 3). After the burst release, the NLC showed sustained release until 24 h study and provided the maximum liberation of SRF and GA-loaded NLC (84%). The observed results are inconsonance with the NLCs formulations being extensively reported in literature (Beg et al., 2018a, Beg et al., 2018b, Rahman et al., 2019).
Fig. 3.
In vitro release profiles of SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLCs up to 24 h duration using of dialysis method (SRF; Sorafenib: GA; Ganoderic Acid: NLC; Nano lipid carriers).
3.6. In vitro cytotoxic activity
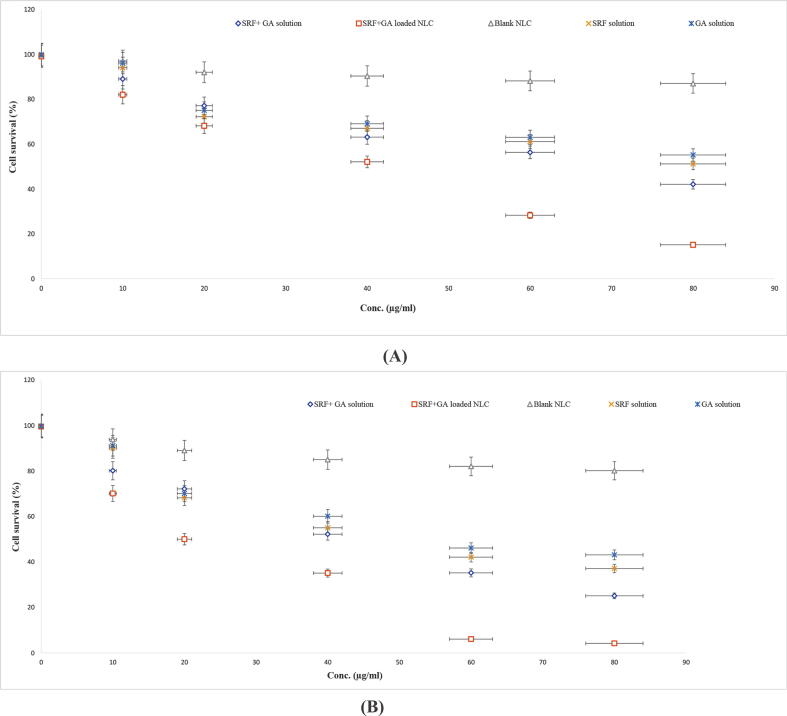
MTT testing was conducted in order to assess the in vitro cytotoxic activity of the drug in the solution and NLCs on HepG2 cancer cells. In Fig. 4A and 4B, the cell remains viable in the blank NLCs treated cells. With the increasing amount of drug in the solution (SRF, GA, or SRF and GA solution), the cell viability for 24 h duration reached to 51, 55 and 42%, respectively. On the other hand, SRF and GA-loaded NLCs showed cell viability of only 15% which revealed the highest cytotoxicity (Fig. 4A). For the 48 h study, the increasing concentration of SRF, GA solution, SRF and GA solution, the cell viability found to be 37, 43 and 25%, respectively. In case of SRF and GA-loaded NLCs, the cell viability was further reduced to 4%. Thus, SRF and GA-loaded NLCs reduced the cell viability more substantially and sustained way over the solution of the same drugs (Fig. 4B). In addition, the drug sensitivity assay (IC50) results have coherence with %cell viability. The lower number of viable cells led by GA, SRF and both drugs were found in higher concentrations (IC50) but the lowest number of viable cells was detected in 48 h of analysis at lower IC50 by SRF and GA-loaded NLCs (shown in Table 2).
Fig. 4.
A-B) Evaluation of SRF solution, GA solution, SRF + GA solution and SRF with GA-loaded NLC formulation at various levels after the incubation of HEPG2 for 24 h and 48 h; Data expressed as mean ± S.D. (n = 6) (SRF; Sorafenib: GA; Ganoderic Acid: NLC; Nano lipid carriers).
Table 2.
MTT viability assay of SRF + GA solution and optimized SRF + GA loaded NLC. The SRF + GA loaded NLC shows significant (p < 0.05) cytotoxicity compared to SRF + GA solution after 24 and 48 h. Data are expressed as mean ± SD (n = 6).
| Formulations | IC50 (µg/mL) |
|
|---|---|---|
| 24 h | 48 h | |
| SRF + GA solution | 47.31 | 41.22 |
| SRF + GA loaded NLC | 30.01 | 18.10 |
(SRF; Sorafenib GA; Ganoderic Acid: NLC; Nano lipidic carrier).
3.7. Stability studies
3.7.1. Particle size distribution
The SRF and GA-loaded NLCs stored at 25◦C/60 %RH and 40◦C/75 %RH showed particle size ranging between 29.2 nm and 30.1 nm, and 29.2 nm to 35.0 nm, respectively (shown in Supplementary Table-S1 and Supplementary Table-S2). The particle growth retained a fairly constant size with SRF and GA-loaded NLCs at 25◦C/60% RH and remained stable. However, at 40%/75 %RH, enhancement in the kinetic energy resuled into more collision among the particles, thus leading to particle aggregation. The Ɖ data ranging between 0.41 and 0.54 showed physically stability of formulation at 25◦C/60 %RH for up to 12 weeks (as shown in Supplementary Table-S1). SRF and GA-loaded NLCs stored at 40◦C/75% RH indicated significant variation in the Ɖ range of 0.41 and 0.68, which resulted into heterogeneous dispersion and formulation instability (Beg et al., 2018a).
3.7.2. EE and LC
At 25◦C/60% RH condition, the EE and LC of the SRF and GA-loaded NLCs found93.1% to92.2%, and 14.21 to 13.61 respectively, these are shown in Supplementary Table-S1. However, EE and LC of the aforementioned formulation at 40◦C/75% RH were found to be in the range of 93.1% to 90.12% and 14.21% to 12.5%, respectively, as shown in Supplementary Table-S2. Overall, the parameters, EE and LC of the aforementioned formulation revealed a significant declining value after storage at 40◦C/75% RH, which may be linked to lipid and drug release polymorphic forms (Ahmad et al., 2018).
3.8. Effect of treatment formulations on macroscopic characters
The macroscopic test showed that the normal control group have no hepatic nodules and anomalies were detected. The DEN treated rats that displayed hepatic nodules spread on the liver and hepatic cirrhosis. In DEN induced HCC rats, the white nodule on the liver and blood vessels decreased treating with SRF, GA, SRF and GA solution (50 mg/kg, 50 mg/kg, 25 mg/kg of each drug) over the DEN induced HCC rats. Furthermore, HCC rats treated with SRF and GA-loaded NLCs, found no appearance of hepatic nodules of size < 3 mm > 1 mm and ≥ 3 mm in the liver (shown in Table 3). The normal control did not express any appearance of hepatic nodules during the complete study periods. However, in the DEN rat groups, nodules were 100% expanded. Table 3, Table 4 indicating the DEN group of rats found 250 as total number of nodules with the size varying of ≤ 1 mm (1 1 5), <3mm > 1 mm (75) and ≥ 3 mm (60). Furthermore, in the treated groups the SRF, GA solution, SRF and GA solution showed 83.3, 83.3 and 66.6% of hepatic nodules with size of ≤ 1 mm (101, 110, 52), <3mm > 1 mm (61, 65, 45,) and ≥ 3 mm (52, 47 and 35) at the dose of 50 mg/kg respectively. On the contrary, the rats treated with SRF and GA-loaded NLCs treated DEN showed significant reduction (16.6%) in the number of hepatic nodules with size ≤ 1 mm (20), <3mm > 1 mm (0) and ≥ 3 mm (0), respectively.
Table 3.
Effect of SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLCs on the number of rats, number of nodules and average number of nodules bearing rats.
| S. No | Groups | Number of rats with nodules/ Number of rats | Total Number of Nodules | Relative size (% of number size) |
||
|---|---|---|---|---|---|---|
| ≤1mm | <3mm > 1 mm | ≥3mm | ||||
| 1 | Normal control | 0/6 | 0 | 0 | 0 | 0 |
| 2 | DEN control receiving SRF (50 mg/kg) 5 mL/kg/day for 14 weeks | 5/6 | 214 | 101 | 61 | 52 |
| 3 | DEN control | 6/6 | 250 | 115 | 75 | 60 |
| 4 | DEN control group of rats administered with GA (50 mg/kg) | 6/6 | 222 | 110 | 65 | 47 |
| 5 | DENcontrol rats administered withSRF and GA (25 mg/kg each of drug) | 4/6 | 132 | 52 | 45 | 35 |
| 6 | DEN control administered with SRF cum GA loaded NLC (25 mg/kg each of drug dose) single dose 5 mL/kg/day p.o. for 14 weeks | 1/6 | 20 | 20 | 0 | 0 |
Group I did not show the any sign of hepatic nodules and VI did show least sign of hepatic nodules among group (SRF; Sorafenib GA; Ganoderic Acid: DEN; Diethyl nitrosamine: NLC; Nano lipidic carrier).
Table 4.
SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLCs, and their effects on the number of rats with tumour incidence.
| S. No | Groups | Number of rats with tumour/Number of rats | Tumour incidence (%) |
|---|---|---|---|
| 1 | Normal control | 6/0 | 0 |
| 2 | SRF (50 mg/kg) 5 mL/kg/day for 14 weeks | 5/6 | 83.3% |
| 3 | DEN control | 6/6 | 100% |
| 4 | DEN control group of rats administered with GA (50 mg/kg) | 5/6 | 83.3 |
| 5 | DENcontrol rats administered withSRF and GA (25 mg/kg each of drug) | 4/6 | 66.6 |
| 6 | DEN control administered with SRF cum GA loaded NLC (25 mg/kg each of drug dose) single dose 5 mL/kg/day p.o. for 14 weeks | 1/6 | 16.6 |
Group I did not show the any sign of hepatic nodules and VI did show least sign of hepatic nodules among group (SRF; Sorafenib GA; Ganoderic Acid: DEN; Diethyl nitrosamine: NLC; Nano lipidic carrier).
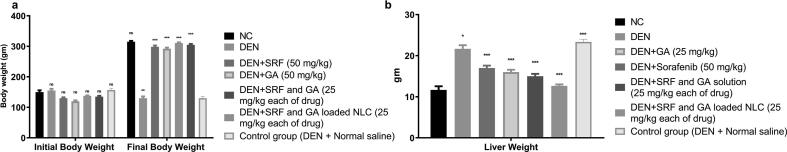
3.9. Effect of treatment formulations on animal weight and liver weight
Bodyweight effect on rat by DEN, normal control group body weight and other groups which is DEN treated with SRF, GA, SRF and GA solution, SRF and GA-loaded NLCs are presented in Fig. 5A. In the DEN-induced group of rats, bodyweight initially increased and diminished with time in contrast with the other rats. In comparison to DEN rat groups, SRF and GA-loaded NLCs group particularly increased body weight more significantly than DEN group and quite effectively approached body weight of normal control group of rats (Fig. 5A). Another parameter is liver weight determination, where DEN group showed an improvement in the liver weight in comparison to the standard control group of rats. SRF, GA, SRF and GA solution also showed only a mild reduction in the liver weight, as shown in Fig. 5B. On the contrary, SRF and GA-loaded NLCs group showed a significant reduction in the liver weight which reached quite near to the liver weight of rats in the normal control group, as shown in Fig. 5B.
Fig. 5.
Effect of various treatments such as SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLCs on body weight of various groups of rats. (A) rat's body weight initial and final, (B) liver weight (DEN = Diethylnitrosamine, All the data corresponds to mean ± SEM, P < 0.05; P < 0.01; P < 0.001, ns = non-significant; ANOVA, followed by Dunnett’s multiple comparison test) (SRF; Sorafenib: GA; Ganoderic Acid: NLC; Nano lipid carriers).
3.10. Pharmacokinetic studies
The pharmacokinetic evaluation of the SRF, GA solution, SRF and GA solution, SRF and GA-loaded NLCs showed significant difference in their drug absorption profiles. Fig. 6 summarizes the drug absorption parameters (Cmax, Tmax and AUC0-∞) observed for the treatment formulations and their statistical significance. The data indicated Tmax of the drugs from SRF and GA-loaded NLCs was observed nearly in 10 h, which could be attributed to the sustained drug release performance of the prepared nanoformulations (Beg et al., 2018a,b). The delayed Tmax (P < 0.05) from SRF and GA-loaded NLCs over the SRF, GA solution, SRF and GA solution is a typical characteristic of the NLC formulations. Furthermore, a significant difference was observed in Cmax from all the treatment formulations, where NLCs showed superior plasma concentration of both the drugs (P < 0.05). Also, the drug-loaded NLCs exhibited a significant improvement in the values of AUC0-∞ vis-à-vis other treatment groups (P < 0.01). The observed results indicated that drug-loaded NLCs showed significant improvement in the pharmacokinetic attributes of the drugs over the plain drug solutions alone or in combination, as lipids in the NLCs are particularly responsible for potentiating absorption of the drugs probably through intestinal lymphatics and transcellular pathways. The delayed Cmax and Tmax observed for the NLCs are typical characteristics attributed to the mechanistic absorption of the lymphatic pathways for drug absorption (Rahman et al., 2019).
Fig. 6.
Pharmacokinetic study performed under fasting condition after various treatment formulations in Wistar rats; Data expressed as mean ± S.D. (n = 6).
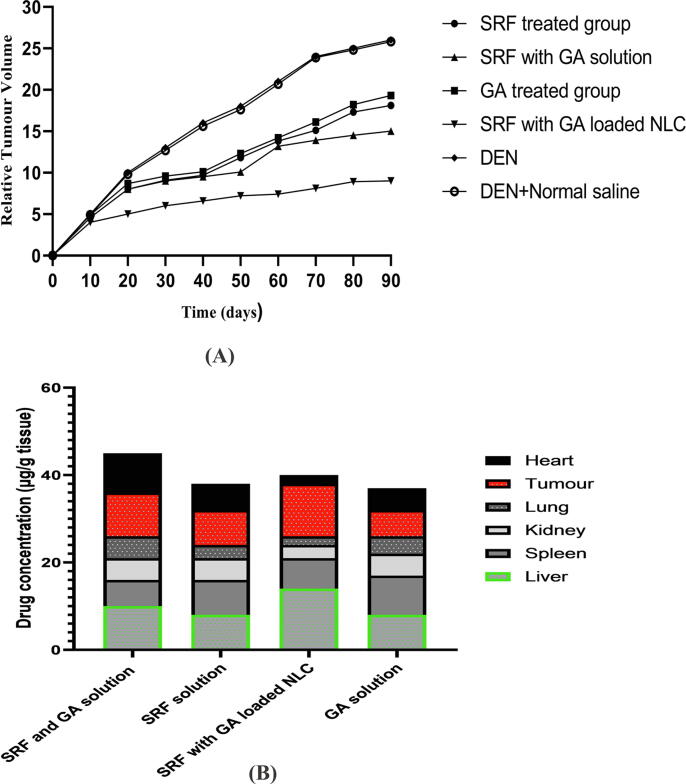
3.11. Relative tumor volume and biodistribution studies
The anticancer effect of the drugs was tested at the dose of 50 mg/kg and 25 mg/kg via oral route of administration. SRF solution (50 mg/kg), GA solution (50 mg/kg), SRF and GA solution (25 mg/kg of each drug), SRF and GA-loaded NLCs (25 mg/kg of each drug) was administered after one week of tumor development. Fig. 7A represents the relative tumor volume of aforementioned formulations. In the particular treatment groups, the SRF and GA-loaded NLCs revealed higher tumor regression with a significant difference over the SRF solution (P < 0.001), GA solution (P < 0.001), SRF and GA solution (P < 0.001) on day of 90. Furthermore, SRF and GA-loaded NLCs found the relative tumor volume at the end of 90 days was 1.5 ± 0.12 over the SRF solution (2.8 ± 0.01), GA solution (3.0 ± 0.05), SRF and GA solution (2.5 ± 0.12) (shown in Fig. 7A). The biodistribution study revealed that SRF and GA-loaded NLCs in the HCC bearing albino Wistar male rats at 10 h after oral administration. Fig. 7B revealed that SRF and GA-loaded NLCs found concentration in hepatic tumor was 2, 1.5 and 1.2 times higher over GA solution, SRF solution, and SRF and GA solution respectively at 10 h after oral administration. SRF and GA-loaded NLCs found poorly distributed over comparative formulations in the lungs, heart, kidneys and highly distributed in the liver. Thus, it concludes that oral administration of SRF and GA-loaded NLCs, a significantly higher accumulation of SRF and GA into the tumor and liver over other treatments (as shown in Fig. 7B).
Fig. 7.
A) Relative tumor development of normal control (DEN + normal saline), SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLCs on rats with HCC. A. Significance relative to both of them: p < 0.001 and p < 0.01. B) SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLCs at 10 h after formulation administration in the rats carrying HCC. Statistical significance compared with all: p < 0.01 and p < 0.01 (SRF: Sorafenib, GA: Ganoderic Acid, NLCs: Nano lipid carriers).
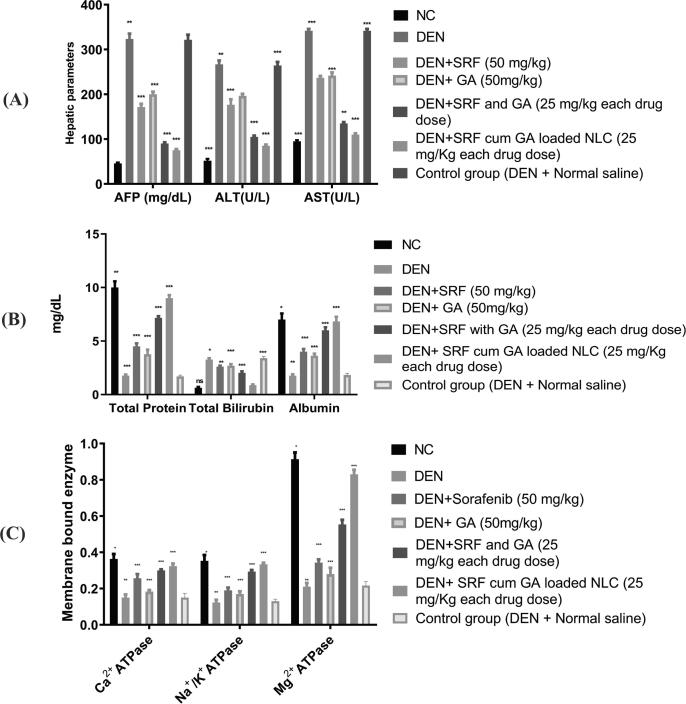
3.12. Hepatic injury markers and non-hepatic parameters
The DEN group of rats found significant elevation in the hepatic serum markers including AFP, ALT and AST over the normal control group of rats (as shown in Fig. 8A). In the DEN group of rats, the most significant upregulation of AFP observed. The treatment groups of rats decrease AFP level over the DEN control group of rats, as shown in Fig. 8A. Furthermore, the most significant reduction of AFP among the treated group of rats showed by SRF and GA-loaded NLCs (as shown in Fig. 8A). There are several literature that reported the elevation of hepatic serum enzymes such as AST and ALT which is connected to the expansion of HCC (Kim et al., 2008). During hepatotoxicity and liver cancer, these enzymes are surprisingly enhanced several times (20–40 times) over the normal control level. The plasma membrane begins the secretion of cytosolic cellular material in the external region during the development of the HCC. The treatment group especially SRF and GA-loaded NLCs most significantly downregulated the serum AST and ALT levels. Consequently, the findings revealed that SRF and GA-loaded NLCs reduce liver damage by balancing the plasma membrane integrity and reducing membrane enzyme leaks to increase hepatic function and safety. Another is nonhepatic parameters include total protein, albumin, and total bilirubin, which played a key role in HCC development and significantly reduced in the DEN group of rats. In the treatment groups, SRF and GA-loaded NLCs is the most significantly increased total protein and albumin nearly reaches the control group. The level of total bilirubin was enhanced in the DEN group of rats, while SRF and GA-loaded NLCs showed significantly reduction in the bilirubin levels over other treatment groups (as shown in Fig. 8B).
Fig. 8.
A) Effect on the hepatic parameter of different group of rats by the SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLCs, and control group (DEN + normal saline). (AFP = α-feto-Protein, AST = Aspartate Aminotransferase, ALT = ALT). All data is corresponding to mean ± SEM, p < 0.05; P < 0.01; P < 0.001, ns = non-significant; ANOVA, accompanied by multiple Dunnett comparison test. B) Effect on non-hepatic parameters in the multiple classes of rats with SRF solution, GA, SRF + GA solution and SRF with GA-loaded NLC. The results correspond to mean ± SEM P < 0.05. All data correlates to mean P < 0.01. P < 0.001. ns = non-significant; ANOVA. C) Impact on the membrane-bound behaviour by various groups of rats of SRF solution, GA solution and SRF with GA loaded NLC. DEN = Diethylnitrosamine. All the data correspond to mean ± SEM, P < 0.05; P < 0.01;P < 0.001, ns = nonsignificant; ANOVA, followed by Dunnett’s multiple comparison test. (SRF: Sorafenib, GA: Ganoderic Acid, NLCs: Nano lipid carriers).
3.13. Antioxidant markers
For various biofunctional purposes, antioxidants are essential such as reducing prostaglandin synthesis, drug metabolization enzyme induction, carcinogenic reversing, excess free radicals and ROS toxicity (Pandey et al., 2018). Researchers have confirmed the use of different natural antioxidants to reduce the formation of ROS and to reduce the toxicity causes by ROS (Pandey et al., 2018). DEN administration in rats has resulted in increased oxidative stress, which may occur during the development of HCC from over-free radical production. It also leads to elevated lipid peroxidation levels resulting in increased oxidative stress. GST and GPx elevation were observed in DEN induced HCC rats and its level are most significantly downregulates (p < 0.001) by the SRF and GA-loaded NLCs comparatively to the other treatment groups. Other antioxidant parameters like CAT and SOD are downregulated in the DEN group of rats, but the trends are just reversed with the SRF and GA-loaded NLCs and significantly much increased (P < 0.001) over other treatment groups of rats (as shown in Table 5).
Table 5.
Effect of SRF solution, GA solution, SRF with GA solution and SRF cum GA loaded NLC on antioxidant enzymes in hepatocellular carcinoma caused by DEN in rats.
| Group | LPO | CAT | SOD | GPx | GST |
|---|---|---|---|---|---|
| (µM/mg Protein) | (nmol/min/ml) | (U/ml) | (µmol) | (U/min/mg Protein) | |
| Normal control | 8.10 ± 0.41 | 1.34 ± 0.41 | 3.11 ± 0.25 | 9.10 ± 0.79 | 1.0 ± 0.03 |
| DEN + SRF solution (50 mg/kg) |
9.21 ± 1.02** | 0.78 ± 0.04** | 1.31 ± 0.50** | 6.91 ± 0.21** | 0.39 ± 0.31** |
| DEN control |
14.11 ± 1.57a | 0.08 ± 0.50a | 1.0 ± 0.71a | 5.12 ± 0.62a | 0.06 ± 0.10a |
| DEN + GA solution (50 mg/kg) |
9.11 ± 0.75** | 0.71 ± 0.14** | 1.25 ± 0.65** | 6.81 ± 0.52** | 0.38 ± 0.45** |
| DEN + SRF and GA solution |
8.31 ± 0.45** | 0.81 ± 0.21** | 1.45 ± 0.41** | 7.21 ± 0.41** | 0.43 ± 0.40** |
| DEN with SRF cum GA loaded NLC |
7.3 ± 1.35** | 0.92 ± 0.31* | 1.67 ± 0.50* | 7.94 ± 0.52* | 0. 62 ± 0.22** |
SRF; Sorafenib; GA: Ganoderic Acid;NLC; Nano lipidic carrier.
Gr 1 - Normal control, Gr II - DEN + Sorafenib (50 mg/kg), Gr III - DEN control; Gr IV - DEN + GA (50 mg/kg), Gr V - DEN + Sorafenib and GA (25 mg/kg each of drug); Gr VI DENA with Sorafenib cum GA loaded NLC (25 mg/kg each of drug dose). Values are expressed as mean ± SEM. (n = 6). Statistical significance P < 0.05. aP < 0.001 as compared to normal group *P < 0.01 as compared to DEN treated group **P < 0.001 as compared to DEN treated group.
Different cellular abnormalities closely connected with lipid peroxidation are observed. Its determinations are therefore highly necessary in order to know the redox cell status. The DEN mediated group of rats mainly increase lipid peroxidase (LPO) reflecting severe cell redox imbalances and initiating oxidative stress [22]. In the therapeutic approach group, the SRF and GA-loaded NLCs significantly (P < 0.001) lowered the level of LPO over other mentioned groups, as shown in the Table 5. The endogenous enzymes CAT and SOD are the first to inhibit the oxidative harm caused by the reduction of superoxide radicals. SOD is predominantly responsible for reducing superoxide to radical hydrogen peroxide and water (Pandey et al., 2018).
Glutathione peroxidase (GPx) is a selenium containing antioxidant that can reduce H2O2 and lipid peroxides effectively into water and lipid alcohols. Detoxification of ROS is governed by GPx. The DEN group of rats abnormally increased the oxidative stress and lowered the GPx. The administration of SRF and GA-loaded NLCs increased the GPx level to the normal control level. It possesses free radical scavenging action in the body to obstruct the carcinogenesis progress. Glutathione S transferase (glutathione s transferase, GST) is a multipurpose enzyme that is used to detoxify glutathione via catalytic conjugation with a number of toxins and carcinogens to non-toxic products (Verma et al., 2018). DEN group of rats abnormally altered or decrease this enzyme to induce HCC development. SRF and GA-loaded NLCs most significantly enhanced the GST level and destruct the HCC progress.
3.14. Effect of treatment formulation on membrane bound enzymes
Ca2+ATPase decrease in the DEN rat population in comparison with the normal control group of rats. SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLCs enhanced the Ca2+ATPase level and approaching to value of normal value in DEN treated rats (Fig. 8C). In the DEN rat group, the same trend was seen in Na+/K+ and in Mg2+ATPase. Na+/K+ and Mg2+ATPase were restored to the normal control level by the same formulation referred to earlier. The SRF and GA-loaded NLCs in DEN-treated rats demonstrated efficient Na+/K+/Mg2+ATPase improvements to the level of normal control.
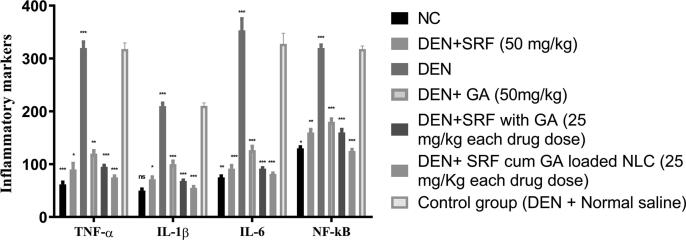
3.15. Effect on inflammatory mediators
TNF-α, IL-1β and IL-6 are the cytokines which are typically produced by the liver. IL-6 also produces by blood cells such as monocytes and lymphocytes. Apart from the liver and blood cells, some other cells such as murine, kupffer cells and human hepatocytes also produce the IL-1β, IL-6, and TNF-α. Kupffer cells secrete IL-6 via modulating the NF-κB signaling and MyD88 dependent, thus further activates IL-1α from the hepatocytes (Kumar et al., 2017a). TNF-α have a significant role in the inflammatory reaction and plays a key role in cell proliferation. The mechanism behind the hepatocarcinogenesis induced by DEN may be attributed to its action on the kupffer cells via activation of NF-κB, which ultimately regulates IL-6 and TNF-α (Kumar et al., 2017a). Furthermore, these two mediators also cause neoplasia, necrosis, and expansion of fibroblast, and tumor cells. Fig. 9 portrays the various proinflammatory cytokines such as TNF-α, IL-1β, IL-6 and NF-κβ expressed by different control and treatment groups. The experimental study revealed that TNF-α induces secretion of genotoxic molecules and accelerates the production of NF-κβ molecule, which ultimately affects the tumor growth (Rahman et al., 2020). In the DEN group of rats, TNF-α level was increased, while SRF and GA-loaded NLCs showed significant reduction (P < 0.05) in TNF-α level and inhibition in the tumor growth. However, in the case of IL-6, it displayed a similar observation as that of TNF-α, and its levels are most significantly (P < 0.01) reduced after treatment with SRF and GA-loaded NLCs over other treated groups. In the case of IL- 1β, its level was increased in the DEN group of rats, and further after administration of SRF and GA-loaded NLCs significant reduction (P < 0.001) in its level over other treated groups of rats.
Fig. 9.
Results on various inflammatory mediators in different group of rats with SRF solution, GA solution, SRF and GA solution, SRF and GA-loaded NLCs and control group (DEN + normal saline). (TNF-α = Tumor necrosis factor-α, IL-1β = Interlukin-1β, IL-6 = Interlukin-6, NF-κB, DEN = Diethylnitrosamine and RV = Resveratrol). All the data correspond to mean ± SEM P < 0.05; P < 0.01; P < 0.001, ns = non-significant; ANOVA, followed by Dunnett’s multiple comparison test. (SRF: Sorafenib, GA: Ganoderic Acid, NLCs: Nano lipid carriers).
3.16. Effect of the SRF and GA-loaded NLCs on histopathology
The evaluation of normal control group of rats and their histopathology revealed normal articulature, small uniform shape of nuclei, typical structure, average size of polyhedral shape of hepatocyte, average central vein and contained small uniform nuclei of cytoplasm. The normal control group showed almost similar histopathological features like the characters of healthy rats (Rahman et al., 2019). DEN induced group of rats and their histopathological characters revealed inflammatory cells, inflammatory blood vessels, necrosis cells, nonuniform and dark cytoplasm, pseudo acini, trabeculae (hepatic parenchyma with thick cords) and hyperchromatic nuclei. Furthermore, the observations also confirmed proliferation in hepatic stellate cells (HSCs), binucleate masses of eosinophils in vacuolation, irregular macro lipid droplets and nonuniform structure of cytoplasm. The rats treated with SRF and GA/or combination of both drugs in solution form only showed mild improvement in the histopathological characters such as poor altered hepatocytes, less inflammatory cells, absence of bile cyst, lesser disorganized hepatic parenchyma and less cell necrosis, etc (as shown in Table 6). On the contrary, SRF and GA-loaded NLCs treated group of rats revealed significant improvement in histopathological characteristics such as absence of necrosis, apoptosis, and disorganized hepatic parenchyma, etc. (Rahman et al., 2020).
Table 6.
It showed the various group of treated groups on the histopathological characteristics.
| S. No. | Histopathology Changes | Groups |
||||||
|---|---|---|---|---|---|---|---|---|
| NC | DEN + NC (DEN + normal saline) |
DEN | DEN + SRF |
DEN + GA |
DEN + SRF + GA | DEN + SRF + GA-NLC | ||
| 1. | Necrosis | – | + | + | – | + | + | – |
| 2. | Hydropic degeneration | – | + | + | + | + | + | – |
| 3. | HSCs focal proliferation | – | + | + | + | + | + | + |
| 4. | Bile cysts | – | – | + | – | – | – | – |
| 5. | Pseudo-nucleoli | – | + | + | + | + | + | – |
| 6. | Peliosis hepatis | – | + | + | + | + | – | – |
| 7. | disorganized hepatic parenchyma | – | + | + | – | – | – | – |
| 8. | Apoptosis | – | + | + | + | + | – | – |
| 9. | Hepatocelluar adenoma | – | + | + | + | + | + | – |
| 10. | Cell necrosis | – | + | + | – | – | – | – |
| 11. | Altered basophilic | – | + | + | + | + | + | + |
| 12. | small dark cytoplasm | – | + | + | – | + | – | – |
| 13. | Enlargement of karyomegali | – | + | + | + | + | – | – |
| 14. | Macro lipid droplets | – | + | + | + | + | + | – |
| 15. | Diffuse dysplasia | – | + | + | + | + | + | – |
| 16. | Hyperplastic foci | – | + | + | – | – | – | – |
NC; Normal control: DEN + NC: Diethyl nitrosamine: SRF; Sorafenib: GA; Ganoderic acid: NLC: Nanolipid carrier.
4. Conclusions
The optimized SRF and GA-loaded NLCs were successfully developed found an average particle size of 29.28 nm, with an encapsulation efficiency of 93.1%. The in vitro release data revealed fast release in the duration of 6 h followed by sustained release behaviour of NLCs, which exhibited nearly complete drug release within 24 h. The cell viability studies construed higher cytotoxicity of combined drugs in NLCs over the usage of a single drug and/or combination of the drugs in solution form. The stability studies of SRF and GA-loaded nano lipidic carrier showed good stability at 25◦C/60 %RH and unstable at 40◦C/75 %RH. The macroscopical study of liver tumor after treatment with SRF and GA-loaded NLCs showed a 16.6% reduction in the hepatic nodules. The pharmacokinetic evaluation construed significant augmentation in the drug absorption parameters and biodistribution studies revealed a higher accumulation of SRF and GA-loaded NLCs in the tumor and liver. Moreover, the SRF and GA-loaded NLCs most effectively restored hepatic parameters, non-hepatic parameters and inflammatory markers, which were approaching to the normal level close to the values of control group. From the observed findings, it can be construed that SRF and GA-loaded NLCs proved as beneficial chemopreventive tool for the treatment of HCC, while their clinical safety remains a challenge and further topic of investigation to for rational usage of SRF and GA combined drug therapy for HCC management.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: 20UQU0019DSR.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2021.06.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahmad N., Alam M.A., Ahmad R. Improvement of oral efficacy of Irinotecan through biodegradable polymeric nanoparticles through in vitro and in vivo investigations. J. Microencapsul. 2018;35(4):327–343. doi: 10.1080/02652048.2018.1485755. [DOI] [PubMed] [Google Scholar]
- Anwanwan D., Singh S.K., Singh S. Challenges in liver cancer and possible treatment approaches.Biochim Biophys Acta Rev. Cancer. 2020;1873(1) doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahman A.A., Abaza M.S.I., Khoushiash S.I., Al-Attiyah R.J. Sequence-dependent effect of sorafenib in combination with natural phenolic compounds on hepatic cancer cells and the possible mechanism of action.Int. J. Mol. Med. 2018;42(3):1695–1715. doi: 10.3892/ijmm.2018.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg S., Choudhry H., Zamzami M.A., Alharbi K.S., Rahman M., Singh B. Nanocolloidal lipidic carriers of olmesartan medoxomil surface-tailored with Concavalin-A for lectin receptor targeting. Nanomedicine (Lond). 2018;13(24):3107–3128. doi: 10.2217/nnm-2018-0188. [DOI] [PubMed] [Google Scholar]
- Beg S., Saini S., Bandopadhyay S., Katare O.P., Singh B. QbD-driven development and evaluation of nanostructured lipid carriers (NLCs) of olmesartan medoxomil employing multivariate statistical techniques. Drug Dev. Ind. Pharm. 2018;44(3):407–420. doi: 10.1080/03639045.2017.1395459. [DOI] [PubMed] [Google Scholar]
- Cheng Z., Wei-Qi J., Jin D. New insights on sorafenib resistance in liver cancer with correlation of individualized therapy.Biochim Biophys Acta Rev. Cancer. 2020;1874(1) doi: 10.1016/j.bbcan.2020.188382. [DOI] [PubMed] [Google Scholar]
- Escudier B., Worden F., Kudo M. Sorafenib: key lessons from over 10 years of experience. Expert Rev. Anticancer Ther. 2019;19(2):177–189. doi: 10.1080/14737140.2019.1559058. [DOI] [PubMed] [Google Scholar]
- Harshita, Barkat, M.A., Rizwanullah, M., Beg, S., Pottoo, F.H., Siddiqui, S., Ahmad, F.J., 2019. Paclitaxel-loaded nanolipidic carriers with improved oral bioavailability and anticancer activity against human liver carcinoma. Curr. Pharm. Design. 20(2), 1-14. [DOI] [PubMed]
- Hashemi F.S., Farzadnia F., Aghajani A., Ahmadzadeh N.F., Pezeshki A. Conjugated linoleic acid loaded nanostructured lipid carrier as a potential antioxidant nanocarrier for food applications.Food Sci Nutr. 2020;8(8):4185–4195. doi: 10.1002/fsn3.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.R., Flamm S.L., Di Bisceglie. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- Kumar A., Ahuja A., Ali J. Curcumin-loaded lipid nanocarrier for improving bioavailability, stability and cytotoxicity against malignant glioma cells. Drug Deliv. 2016;23(1):214–229. doi: 10.3109/10717544.2014.909906. [DOI] [PubMed] [Google Scholar]
- Kumar V., Bhatt P.C., Rahman M. Fabrication, optimization, and characterization of umbelliferone β-D-galactopyranoside-loaded PLGA nanoparticles in treatment of hepatocellular carcinoma: in vitro and in vivo studies. Int. J. Nanomedicine. 2017;12:6747–6758. doi: 10.2147/IJN.S136629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Bhatt P.C., Rahman M. Umbelliferon-α-d-glucopyranosyl-(2I→1II)-α-D-glucopyranoside ameliorates Diethyl-nitrosamine induced precancerous lesion development in liver via regulation of inflammation, hyperproliferation and antioxidant at pre-clinical stage. Biomed. Pharmacother. 2017;94:834–842. doi: 10.1016/j.biopha.2017.07.047. [DOI] [PubMed] [Google Scholar]
- Lee S., Kim J.H., Moon H., Lee H.J., Han J.K. Combined treatment of sorafenib and doxorubicin-loaded microbubble-albumin nanoparticle complex for hepatocellular carcinoma: A feasibility study. PLoS ONE. 2020;15(12) doi: 10.1371/journal.pone.0243815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri K., Shegokar R., Gohla S. Lipid nanocarriers for dermal delivery of lutein: preparation, characterization, stability and performance. Int. J. Pharm. 2011;414:267–275. doi: 10.1016/j.ijpharm.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Moraes S., Marinho A., Lima S. Targeted nanostructured lipid carriers for doxorubicin oral delivery. Int. J. Pharm. 2021;592 doi: 10.1016/j.ijpharm.2020.120029. [DOI] [PubMed] [Google Scholar]
- Müller R.H., Radtke M., Wissing S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002;242:121–128. doi: 10.1016/s0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- Pandey, P., Rahman, M., Bhatt, P.C., et al., 2018.Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin.Nanomedicine(Lond) 13(8), 849-870. [DOI] [PubMed]
- Perz J.F., Armstrong G.L., Farrington L.A. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Rahman M., Al-Ghamdi S.A., Alharbi K.S. Ganoderic acid loaded nano-lipidic carriers improvise treatment of hepatocellular carcinoma. Drug Deliv. 2019;26(1):782–793. doi: 10.1080/10717544.2019.1606865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Beg S., Alharbi K.S. Implications of Solid Lipid Nanoparticles of Ganoderic Acid for the Treatment and Management of Hepatocellular Carcinoma. J Pharm Innov. 2020 doi: 10.1007/s12247-020-09450-4. [DOI] [Google Scholar]
- Rahman M., Almalki W.H., Alrobaian M., Iqbal J., Alghamdi S., Alharbi K.S., Alruwaili N.K., Hafeez A., Shaharyar A., Singh T., Waris M., Kumar V., Beg S. Nanocarriers-loaded with natural actives as newer therapeutic interventions for treatment of hepatocellular carcinoma. Expert Opin Drug Deliv. 2021;20:1–25. doi: 10.1080/17425247.2021.1854223. [DOI] [PubMed] [Google Scholar]
- Ravenzwaay B., Tennekes H. A Wistar rat strain prone to spontaneous liver tumor development: implications for carcinogenic risk assessment. Regul. Toxicol. Pharm. 2002;36(1):86–95. doi: 10.1006/rtph.2002.1567. [DOI] [PubMed] [Google Scholar]
- Singh B., Singh S. A comprehensive computer program for the study of drug release kinetics from compressed matrices. Indian J. Pharm. Sci. 1998;60(6):358–362. [Google Scholar]
- Tahavi T., Lanton T., Divon M.S. Sorafenib treatment during partial hepatectomy reduces tumorgenesis in an inflammation-associated liver cancer model. Oncotarget. 2016;7(4):4860–4870. doi: 10.18632/oncotarget.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Singh D., Anwar F. Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kB pathway. Inflammopharmacology. 2018;26:133–146. doi: 10.1007/s10787-017-0350-3. [DOI] [PubMed] [Google Scholar]
- Xu M., Li G., Zhang H. Sequential delivery of dual drugs with nanostructured lipid carriers for improving synergistic tumor treatment effect. Drug Deliv. 2020;27(1):983–995. doi: 10.1080/10717544.2020.1785581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.J., Zheng B., Wang H.Y., Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol. Sin. 2017;38(5):614–622. doi: 10.1038/aps.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.