Abstract
The use of many psychotropic drugs (PDs) is associated with increased caloric intake, significant weight gain, and metabolic disorders. The nematode Caenorhabditis elegans (C. elegans) has been used to study the effects of PDs on food intake. However, little is known about PDs effects on the body fat of C. elegans. In C. elegans, feeding behavior and fat metabolism are regulated through independent mechanisms. This study aims to evaluate the body fat and food intake of C. elegans in response to treatment olanzapine and fluoxetine. Here we report that, with careful consideration to the dosage used, administration of fluoxetine and olanzapine increases body fat and food intake in C. elegans.
Keywords: C. elegans, Obesity, Olanzapine, Fluoxetine, Body fat, Food intake
1. Introduction
Psychotropic drugs (PDs) are a class of drugs used to treat and manage the symptoms of mental disorders(WHO, 2009). The prescription rate of these psychotropic drugs has rapidly increased in the last few decades(Czarny et al., 2011, Ilyas and Moncrieff, 2012, Karanges et al., 2014, Olfson and Marcus, 2009); however, the use of PDs has been linked to a number of severe extrapyramidal and metabolic side effects (Citrome et al., 2011). Notably, recent generations of PDs have been linked with lowering rates of extrapyramidal symptoms; however, they induce metabolic disorders (Citrome et al., 2011, Gentile, 2006). These disorders include impaired glucose metabolism, weight gain, diabetes mellitus, and increased appetite (Bak et al., 2014, Citrome et al., 2011, De Hert et al., 2011). Patients treated with recent generations of PDs typically gain substantial weight after treatment initiation(Kahn et al., 2008) and are four times more likely to develop diabetes compared to the general population(McCreadie et al., 1998, Verhaegen and Van Gaal, 2017).
Among these PDs, olanzapine has been noted to be linked to the most significant changes in body weight in humans(Citrome et al., 2011). The use of olanzapine has been suggested to reduce metabolism, increase body fat, and increase appetite(Citrome et al., 2011). It has been suggested that olanzapine stimulates weight gain and affects appetite by regulating the levels of certain neurotransmitters, such as serotonin (Lord et al., 2017, Nihalani et al., 2011, Shrivastava and Johnston, 2010). It has also been suggested that several PDs, such as olanzapine, block 5-HT 2C receptors, leading to increased food intake and obesity in rodents (Davey et al., 2012, Lord et al., 2017, Nihalani et al., 2011). Furthermore, accumulating evidence suggests that both first and second generations of PDs induce oxidative stress (Güneş et al., 2016, Sadowska-Bartosz et al., 2016, Zhang et al., 2012). The increase in both oxidative stress and reactive oxygen species (ROS) production has been linked to fat accumulation in humans and rodents (Furukawa et al., 2004, Vincent et al., 2007). It has also been suggested that oxidative stress may play an important role in the weight gain induced by the use of PDs (An et al., 2018).
Because the general use of PDs promotes weight gain in humans, scientists have been using different models to explore the underlying mechanisms by which PDs promotes weight gain (Davey et al., 2012, Lord et al., 2017, Nihalani et al., 2011). The model organism Caenorhabditis elegans (C. elegans) has gained increasing importance in pharmacological and toxicological research (Hunt, 2017, Leung et al., 2008). Interestingly, the C. elegans model has been used to study the effects of PDs on food intake as well as on food-seeking behaviors (Dwyer et al., 2015, Gubert et al., 2013, Perez-Gomez et al., 2018). A study by Gomez et al. suggested that C. elegans treated with PDs, such as olanzapine, displayed hyperphagia that was similar to that displayed by humans (Perez-Gomez et al., 2018). In this study, only food intake was determined as the driving force by which PDs induce weight gain; however, little is known about the effects of the PDs on the body fat of C. elegans. The mechanisms of food intake regulation in C. elegans are different than the mechanisms of fat regulation (Srinivasan et al., 2008). A study by S. Srinivasan et al. showed that treatment with exogenous 5-HT in C. elegans resulted in a significant increase in food intake that was accompanied by a decrease in fat content. They also suggest that that the serotonergic regulation of feeding in C. elegans is independent of fat regulation. Therefore, we wanted to examine the body fat of the nematode C. elegans in response to treatment with PDs. The aim of the present study was to examine the effects of olanzapine, a commonly prescribed antipsychotic, and of fluoxetine, a popular antidepressant, on the body fat of C. elegans. We also examined the effects of the drugs on food intake, food-seeking behavior, and the mitochondrial function of C. elegans.
2. Methods:
-
-
Strains and media:
Wild-type Bristol N2 was the reference strain used throughout the study. Additional strains included daf-2(e1370) (#CB1370), prdx-2(gk169) (#VC289), ser-1(ok345) (#DA1814), and mgIs42 [tph-1] (#GR1366). Worms were obtained from the Caenorhabditis Genetics Center (CGC), incubated at 20 °C, and maintained on standard Nematode Growth Medium (NGM) plates as previously described by Brenner (1974). In addition, antibiotics (100 µL of 100 mg/mL Ampicillin) and antifungals (0.5 ml of Nystatin suspension 10,000U/ml) were added to 100 ml of NGM to prevent any contamination. Worms were cultured by feeding on a typical diet that contains concentrated Escherichia coli (E. coli) OP50 lawn as previously described (Brenner, 1974).
-
-
Drugs used in this experiment:
Treatments used in this study included different exposure concentrations of olanzapine (zyprexa 10 mg) and fluoxetine (Lovan 20 mg). All drugs were obtained from La Trobe University Medical Center. Stock solutions of each drug were freshly prepared for each replicate by dissolving drugs in Milli-Q water containing (2%) DMSO. Stock solutions were diluted to the desired concentrations using Milli-Q water containing (2%) DMSO. Treatments were introduced to worms by spotting desired concentrations onto the surface of the NGM plate and on top of OP50 lawn at a ratio of 1:3 (OP50:desired treatment). The control group received only Milli-Q water containing (2%) DMSO.
-
-
Fat staining: With slight modifications, the staining of fat, using [0.5 % Oil-Red O (ORO)], was conducted following a previous protocol (Escorcia, Ruter, Nhan, & Curran, 2018). ORO stock solution was prepared by dissolving 500 mg of ORO powder in 100 ml of 100% isopropanol. Prior to the experiment, ORO working solution was prepared by diluting ORO stock solution in water (3:2) to 60% isopropanol. The working solution was filtered using a 0.2 µm syringe filter. The body fat of worms treated with PDs was examined by taking images of stained worms. Images were analyzed for color density using Image J software. Treatments included different concentrations of olanzapine and fluoxetine dissolved in 2% DMSO. Treatments were introduced to worms from larval stage 1 (L1), and worms were stained to measure their body fat at the L4 stage (48 hrs after the L1 stage). For each condition, experiments were run in duplicate with suitable control and repeated three times with an average of 10 animals per replicate.
-
-
Body size: Worms were maintained with OP50 and desired treatments from the L1 stage until the L4 stage. For each condition, images were taken of worms at the L4 stage at 5X magnification. Nikon stereo microscope equipped with with a Nikon DS-Fi2 camera was used to obtain images. Measurements of body length and width were obtained using ImageJ software.
-
-
Motility: Worms were maintained with OP50 and desired treatments from the L1 stage until the L4 stage. Worms were then starved by transferring them to plain agar plates with no food source and leaving them for 40 min. Movements were recorded at 20 °C with no lid at 5X magnification. Nikon stereo microscope equipped with a Nikon DS-Fi2 camera was used to obtain videos. Recordings of movements were analyzed using WormLab 3.1 software (MBF Bioscience). Analysis included speed/peristaltic speed, max amplitude, straight-line distance, and wavelength. The recording period was 30 s at a rate of 30 frames per s. This experiment was repeated at least twice with an average of 20 animals per replicate.
- Food intake: Pharyngeal pumping rates of L4 worms were measured at room temperature as an indicator of food intake following a previous protocol (Raizen et al., 2005). Worms were maintained on OP50 as a source of nutrient and desired treatments from the L1 stage. Life Science technologies EVOS FL Auto microscope was used to count pharyngeal contractions at 10X magnification. Pharyngeal contractions were measured for each treatment under two different conditions. First, worms were deprived of food for 60 min, and pharyngeal pumping was counted for 30 s using a hand counter. The number of contractions was multiplied to obtain the number of pharyngeal pumps per minute (pumps/min). Second, starved worms were transferred onto food, and pharyngeal pumping was counted for 30 s using a hand counter. The number of contractions was multiplied to obtain the number of pharyngeal pumps per minute (pumps/min). Food intake was calculated by subtracting the basal rate of pumping (off-food) from the pump rate (on food). This experiment was repeated two times for each treatment with an average of 10 animals per replicate.
-
-
Reactive oxygen species: L1 worms were maintained for 24 hr with OP50 and desired treatments for 24 hr after the L1 stage. Following a previous protocol (Yoon, Lee, & Cha, 2018), 25 μM of 2′,7′-dichlorofluorescein diacetate (H2DCFDA) was added to worms suspended in a m9 buffer. Readings of the fluorescence signal were performed using a fluorophotometer. This experiment was repeated three times with an average of 20 animals per replicate
-
-
Mitochondrial function: L1 worms were maintained with OP50 as a source of nutrient and desired treatments until they reached the L4 stage. With slight modifications, the experiment was conducted following the protocol prescribed by Koopman and colleagues(Koopman et al., 2016). The mitochondrial respiration was evaluated using a Seahorse XF 24 Extracellular Flux Analyzer in the presence and absence of 20 mM of sodium azide (an inhibitor of mitochondrial respiration). Sodium azide was added to 500uL of m9 containing ~ 70 worms and all readings were normalized based on the number of worms per each well. Readings included 8 cycles in the absence of sodium azide (basal respiration) and 8 cycles in the presence of sodium azide (non-mitochondrial respiration), then the mitochondrial respiration was calculated.
-
-
Statistical analysis:
All statistical analyses of the data presented in this paper were performed using IBM SPSS® statistics software (version 24). Data were presented as (mean ± SEM). Significant differences between means were assessed by a one-way ANOVA and the General Linear Model (multivariate test). P ≤ 0.05 was considered significant.
3. Results
3.1. Fat staining results of wild-type N2 worms treated with psychotropic drugs (PDs)
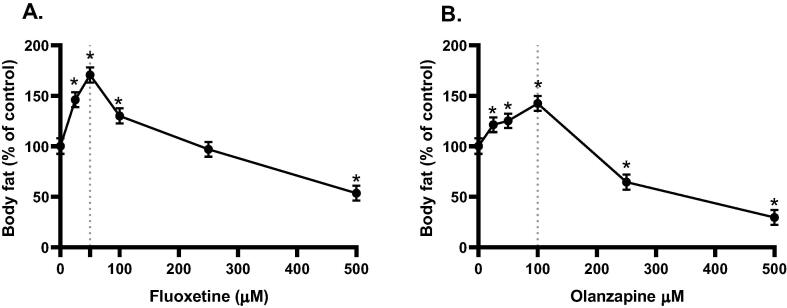
To investigate the effects of fluoxetine and olanzapine on body fat, we first exposed worms to different concentrations of each drug to determine the concentrations at which these medications may affect the body fat of C. elegans (Fig. 1). In these experiments, wild-type N2 worms were treated with various concentrations of either fluoxetine or olanzapine. All treatments were introduced to worms from the L1 stage, and body fat was examined at the L4 stage. The response of worms to treatments was dependent on the concentration of the drug. As shown in (Fig. 1A), the antidepressant fluoxetine showed a concentration-dependent increase (p ≤ 0.05) in body fat up to a 50 µM concentration. A reduction in body fat was observed in response to fluoxetine at higher concentrations. Similar results of body fat were found in N2 worms treated with the antipsychotic olanzapine (Fig. 1B). In response to treatment with olanzapine, worms showed a steady increase (p ≤ 0.05) in body fat up to a 100 µM concentration, and a reduction in body fat was observed at higher concentrations.
Fig. 1.
Effect of various concentrations of fluoxetine and olanzapine on the body fat of N2 worms. Body fat was measured at the L4 stage (48hrs after L1), and treatments were introduced to worms from the L1 stage. Panel (A) shows a concentration-dependent effect of the antidepressant fluoxetine on body fat. Panel (B) shows a concentration-dependent effect of the antipsychotic olanzapine on body fat. (*p ≤ 0.05 statistically different when compared to the respective control, n = 30).
3.2. Effects of psychotropic drugs (PDs) on the food intake of wild-type N2 worms
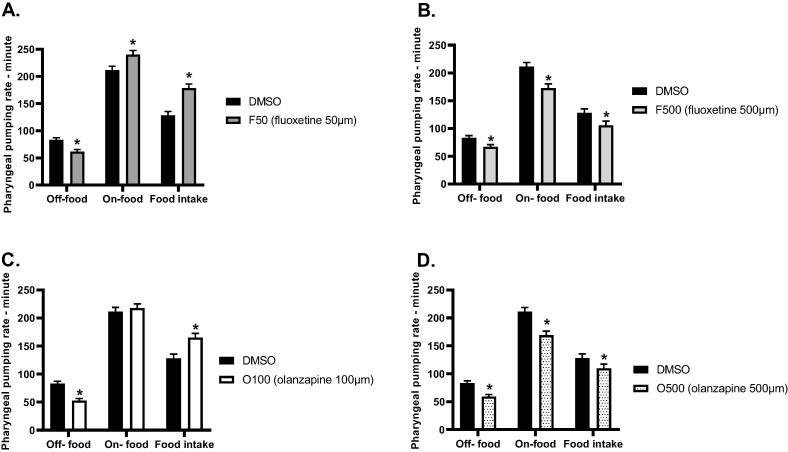
Fat storage in living organisms is affected by energy intake and consumption. Therefore, it is possible that the observed effects of PDs on the body fat of C. elegans originate from changes in their food intake. Therefore, the aim was to determine the effect of PDs on the food intake of wild-type N2 worms. To investigate this effect, the food intake of wild-type N2 worms was evaluated at the L4 stage after exposure to treatments from the L1 stage. Treatments included multiple exposure concentrations of olanzapine (100 µM,500 µM) and fluoxetine (50 µM,500 µM). Food intake was affected differently in response to treatment with different concentrations of fluoxetine (Fig. 2 A,B). Worms treated with 50 µM of fluoxetine (F50) showed a significant increase (*p ≤ 0.05) in food intake compared to the control (DMSO), whereas treatment with 500 µM of fluoxetine (F500) showed an insignificant decrease in food intake. Interestingly, worms showed similar patterns in response to treatment with olanzapine. The effect of olanzapine on food intake was dependent on the concentrations of the drug (Fig. 2 C,D). Worms treated with 100 µM of olanzapine (O100) showed a significant increase (*p ≤ 0.05) in food intake compared to the control (DMSO), whereas treatment with 500 µM of olanzapine (O500) showed an insignificant decrease in food intake. These results suggest that the increase in body fat observed in the exposure experiments appear to be associated with changes in food intake.
Fig. 2.
Effect of several concentrations of fluoxetine and olanzapine on the food intake of N2 worms. Food intake was determined at the L4 stage, and worms were exposed to treatments from the L1 stage. Panel (A) Food intake of worms treated with (50 µM) fluoxetine. Panel (B) Food intake of worms treated with (500 µM) fluoxetine. Panel (C) Food intake of worms treated with (100 µM) olanzapine. Panel (D) Food intake of worms treated with (500 µM) olanzapine. (*p ≤ 0.05, statistically different when compared to the untreated control, n = 10).
3.3. Effects of olanzapine (O100) and fluoxetine (F50) on C. Elegans body size
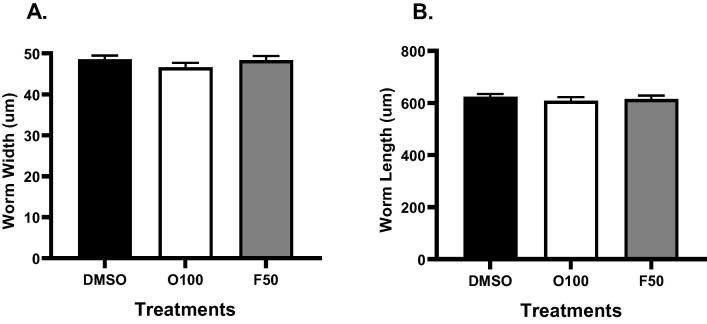
Because the treatment with 100 µM olanzapine (O100) and 50 µM fluoxetine (F50) had shown an increase in the body fat of worms, the aim was to determine whether the development and body size of treated worms were affected. Thus, we first examined the effect of (O100) and (F50) on the width of N2 worms. Evaluation of body width was done at the L4 stage, and treatments were introduced to worms from the L1 stage. As shown in (Fig. 3A), worms treated with (O100) or (F50) had a mean body width of (46.6 µm) and (48.3 µm), respectively. Worms in the control group (DMSO) scored a mean body width of (48.6 µm). No statistically significant difference was found between (DMSO) and the treatment groups. No statistical difference was found between the groups when worms were analyzed for their body length. As shown in (Fig. 3B), the body length in worms treated with (O100) (609.3 µm) or (F50) (616.01 µm) was similar to the body length in worms in the (DMSO) group (623.6 µm).
Fig. 3.
C. elegans wild-type body size after treatment with 100 µM olanzapine (O100) and 50 µM fluoxetine (F50). Treatments were introduced from the L1 stage, and size parameters were evaluated at the L4 stage. A. Representative results of the effects of (O100) and (F50) on width. B. Representative results of the effects of (O100) and (F50) on length.
3.4. Effects of olanzapine (O100) and fluoxetine (F50) on C. Elegans movement behavior:
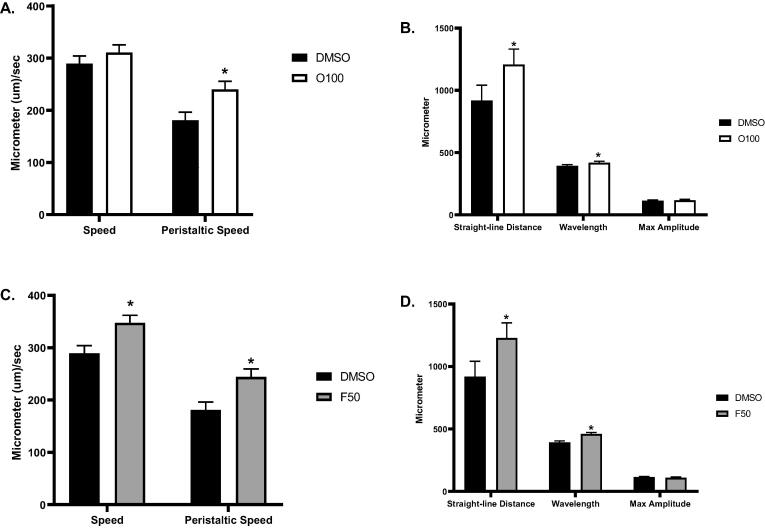
To better understand the observed effects of 100 µM olanzapine (O100) and 50 µM fluoxetine (F50) on the body fat and food intake of N2 worms, we examined the food-seeking strategy of starved worms after exposure to treatments. It has been suggested that specific movement parameters of C. elegans, such as speed and straight-line distance, are increased in the absence of food (Shtonda and Avery, 2006). To evaluate this, we analyzed several movement parameters in N2 worms after exposure to treatments. The evaluation of these parameters was conducted at the L4 stage and included the assessment of speed, peristaltic speed, straight-line distance, wavelength, and max amplitude. As illustrated in (Fig. 4A,B) and (Table 1), the mean speed of olanzapine-treated worms (O100) 310.77 µm/s was slightly higher than the control (DMSO) 289.31 µm/s. The peristaltic speed of olanzapine-treated worms (O100) 240.08 µm/s was significantly (*p ≤ 0.05) higher than the control (DMSO) 180.85 µm/s. Moreover, we did not detect any significant changes in max amplitude in worms treated with (O100) 118.238 µm/s compared to (DMSO) 113.985 µm/s. We also found that there is a significant increase (*p ≤ 0.05) in the straight-line distance covered by worms treated with (O100) 1208 µm/s compared to (DMSO) 917.91 µm/s. In addition, the wavelength of the worms treated with (O100) 419.5 µm/s was significantly (*p ≤ 0.05) higher than the control (DMSO) 392.9 µm/s.
Fig. 4.
N2 wild-type worms’ movement behavior after treatment with 100 µM olanzapine (O100) and 50 µM fluoxetine (F50). Treatments were introduced from the L1 stage, and movement was evaluated at the L4 stage. A. Results of speed and peristaltic speed of N2 worms treated with (O100). B. Results of movement behavior of N2 worms treated with (O100). C. Results of speed and peristaltic speed of N2 worms treated with (F50). D. Results of movement behavior of N2 worms treated with (F50). (*p ≤ 0.05, statistically different when compared to the respective control, n = 20).
Table 1.
Means of C. elegans movement parameters after treatments with 100 µM olanzapine (O100) and 50 µM fluoxetine (F50).
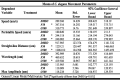 |
Similar results of movement parameters were found in worms in response to treatment with fluoxetine (F50). As shown in (Fig. 4C,D) and (table 1), the mean speed of worms treated with (F50) 347.52 µm/s was significantly (*p ≤ 0.05) higher than the control (DMSO) 289.31 µm/s. A significant increase (*p ≤ 0.05) was also observed in the mean peristaltic speed of worms treated with (F50) 244.12 µm/s as opposed to worms in the control group (DMSO) 180.85 µm/s. Moreover, we did not observe a significant difference in max amplitude in worms treated with (F50) 110.009 µm/s compared to (DMSO) 113.985 µm/s. We did observe a significant increase (*p ≤ 0.05) in the straight-line distance covered by worms treated with (F50) 1229.21 µm/s compared to (DMSO) 917.91 µm/s. We also found that the wavelength of the worms treated with (O100) 460.652 µm/s was significantly (*p ≤ 0.05) higher than the control (DMSO) 392.9 µm/s. These results suggest that the food-seeking behavior of worms is affected by treatment with (O100) and (F50) compared to the control group.
3.5. Candidate genes linked to the effect of olanzapine (O100) and fluoxetine (F50) on fat content in worms:
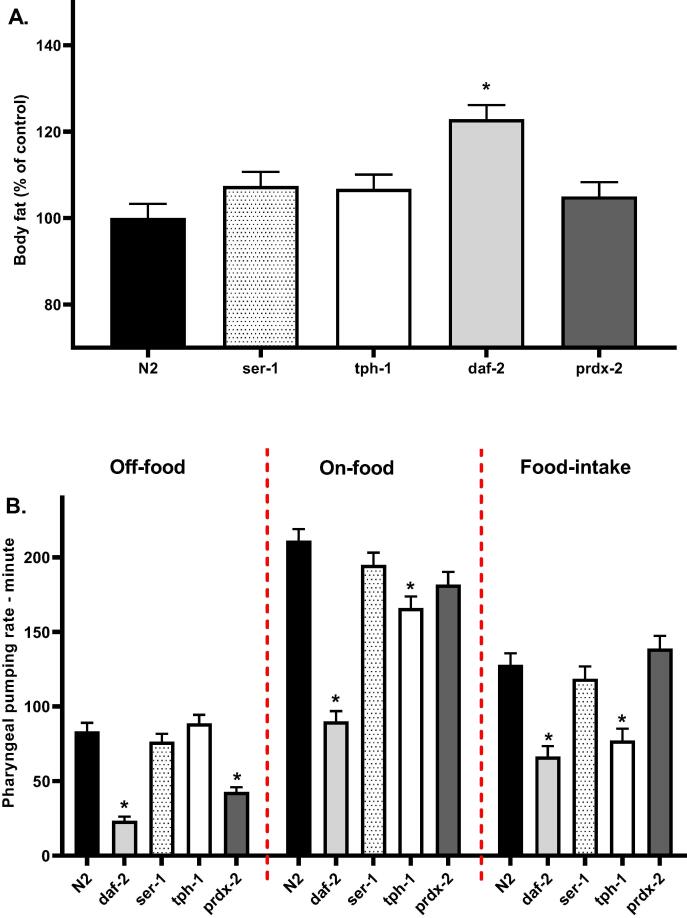
Due to the established role of the insulin signaling pathway(Loh et al., 2017, Nelson and Padgett, 2003), serotonin(Srinivasan et al., 2008, Voigt and Fink, 2015), and oxidative stress(Furukawa et al., 2004, Vincent et al., 2007) in the regulation of body fat and food intake, we used C. elegans strains with mutations in the insulin signaling pathway (daf-2), serotonergic pathway(ser-1, tph-1), and peroxiredoxin-2 gene (prdx-2) to determine the role of these signaling pathways in the observed effect of body fat and food intake in response to treatment with 100 µM olanzapine (O100) and 50 µM fluoxetine (F50). To use these strains to investigate the effects of (O100) and (F50), we initially compared the food intake and body fat of these strains to N2 wild-type worms without treatments. The evaluation of body fat and food intake was done at the L4 stage. As shown in (Fig. 5A), compared to N2 worms, ser-1, tph-1, and prdx-2 mutants exhibited an insignificant increase in body fat, whereas a significant increase (*p ≤ 0.05) in body fat was observed for daf-2 mutants. Furthermore, the food intake of untreated mutants was evaluated in comparison to N2 worms. We found that daf-2 and tph-1mutants showed a significant reduction (*p ≤ 0.05) in food intake, whereas prdx-2 and ser-1 did not show significant differences in their food intake when compared to N2 worms.
Fig. 5.
Results of the body fat and feeding of untreated mutants at the L4 stage of lifespan. A. Representative results of the body fat of untreated mutants. B. Results of the food intake of untreated mutants. (*p ≤ 0.05, statistically different when compared to the N2 worms, n = 10).
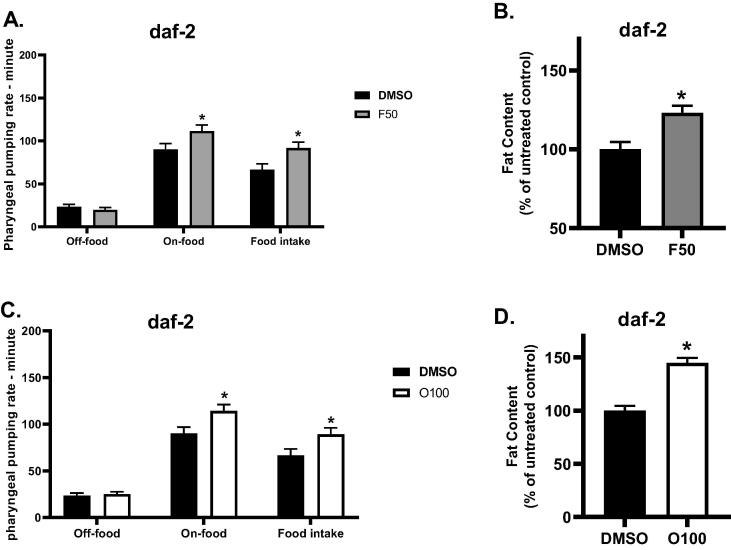
Next, using daf-2 mutants, we examined the involvement of the insulin signaling pathway in the effects of (O100) and (F50) on the body fat and food intake. Treatments were introduced to mutants from the L1 stage, and body fat and food intake were evaluated at the L4 stage. As illustrated in (Fig. 6A,B), treatment with (F50) led to significantly increased body fat and food intake (*p ≤ 0.05) in treated daf-2 mutants as opposed to mutants in the control group (DMSO). A similar result was found in daf-2 mutants treated with (O100). As shown in (Fig. 6 C,D), daf-2 mutants treated with (O100) showed a significant increase (*p ≤ 0.05) in body fat and food intake compared to mutants in the control group (DMSO). These results indicate that both fluoxetine (F50) and olanzapine (O100) do not require daf-2 activity for generating their effects on body fat and feeding.
Fig. 6.
Results of the effect of 100 µM olanzapine (O100) and 50 µM fluoxetine (F50) on the fat content of daf-2 mutants at the L4 stage of lifespan. A. Results of the effects of (F50) on the food intake of daf-2 mutants. B. Results of the effects of (F50) on the body fat of daf-2 mutants. C. Results of the effects of (O100) on the food intake of daf-2 mutants. D. Results of the effects of (O100) on the body fat of daf-2 mutants. (*p ≤ 0.05, statistically different when compared to the respective control, n = 10).
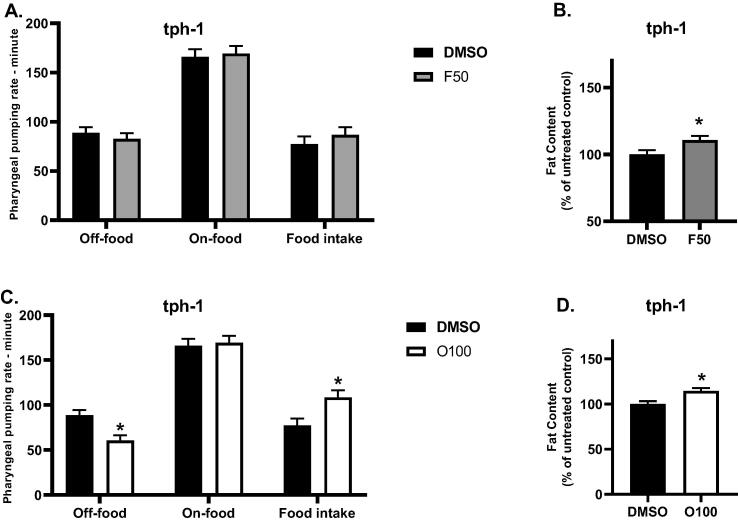
We also investigated the role of the serotonergic signaling pathways in the effects of (O100) and (F50) on the body fat and food intake by using tph-1 mutants. An evaluation of these parameters was done at the L4 stage after exposure to the treatment from the L1 stage. As illustrated in (Fig. 7A,B), treatment with (F50) led to significantly increased body fat (*p ≤ 0.05) in tph-1 mutants as opposed to the untreated control group (DMSO). Remarkably, treatment with (F50) did not result in a significant difference in the food intake of tph-1 mutants compared to (DMSO). We also evaluated the body fat and food intake of tph-1 mutants in response to treatment with (O100). As shown in (Fig. 7 C,D), tph-1 mutants treated with (O100) showed a significant increase (*p ≤ 0.05) in body fat and food intake compared to mutants in the control group (DMSO).
Fig. 7.
Results of the effect of 100 µM olanzapine (O100) and 50 µM fluoxetine (F50) on the fat content of tph-1 mutants at the L4 stage of the lifespan. A. Results of the effects of (F50) on the food intake of tph-1 mutants. B. Results of the effects of (F50) on the body fat of tph-1 mutants. C. Results of the effects of (O100) on the food intake of tph-1 mutants. D. Results of the effects of (O100) on the body fat of tph-1 mutants. (*p ≤ 0.05, statistically different when compared to the respective control, n = 10).
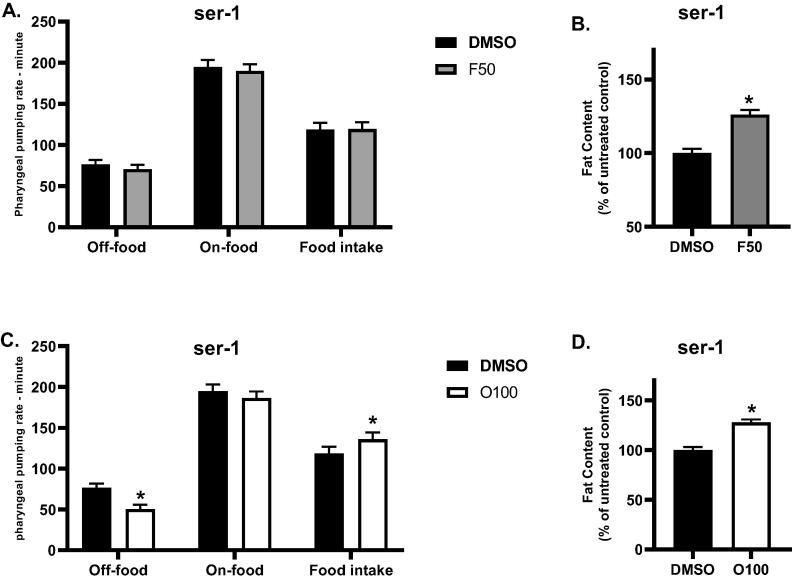
We also decided to further evaluate the role of serotonin in the effects of (O100) and (F50) on body fat and food intake by using mutants that carry a defect in the serotoninergic ser-1 receptor. An evaluation of the food intake and body fat of se1-1 mutants was done at the L4 stage after exposure to the treatment from the L1 stage. As shown in (Fig. 8A,B), a significant increase (*p ≤ 0.05) in the body fat was observed in ser-1 mutants in response to treatment with (F50) as opposed to the untreated control (DMSO); however, treatment with (F50) did not affect the food intake of ser-1 mutants. We also evaluated the body fat and food intake of ser-1 mutants in response to treatment with (O100). As shown in (Fig. 8 C,D), compared to the untreated control (DMSO), ser-1 mutants treated with (O100) showed a significant increase (*p ≤ 0.05) in body fat and food intake. These results suggest that the functions of ser-1 and tph-1 in the serotoninergic pathway are required for the observed effects of fluoxetine (F50) on food intake only.
Fig. 8.
Results of the effect of 100 µM olanzapine (O100) and 50 µM fluoxetine (F50) on the fat content of ser-1 mutants at the L4 stage of the lifespan. A. Results of the effects of (F50) on the food intake of ser-1 mutants. B. Results of the effects of (F50) on the body fat of ser-1 mutants. C. Results of the effects of (O100) on the food intake of ser-1 mutants. D. Results of the effects of (O100) on the body fat of ser-1 mutants. (*p ≤ 0.05, statistically different when compared to the respective control, n = 10).
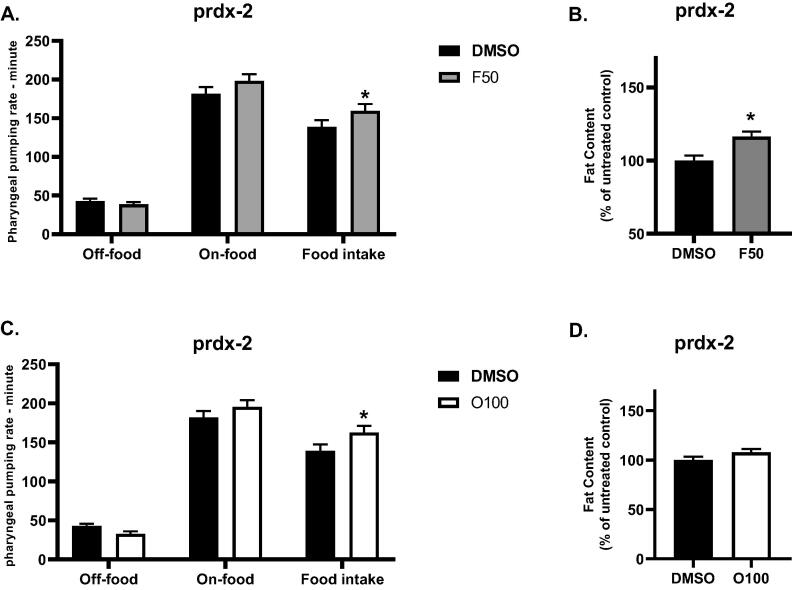
Next, we decided to examine the role of oxidative stress in the effect of (O100) and (F50) on the body fat and food intake. In this experiment, we used prdx-2 mutants that are known to have protection against oxidative stress(Oláhová et al., 2008, Oláhová and Veal, 2015). Treatments were introduced to prdx-2 mutants from the L1 stage, and the body fat and food intake were evaluated at the L4 stage. Compared to the control, mutants treated with (F50) showed a significant increase (*p ≤ 0.05) in body fat as well as food intake (Fig. 9A,B). We also found that treatment with (O100) resulted in a significant increase in the food intake of prdx-2 mutants as opposed to the untreated control (DMSO). Interestingly, treatment with olanzapine (O100) did not affect the body fat of prdx-2 mutants. (Fig. 9C,D). These results suggest that oxidative stress is a possible cause for the observed increase in body fat in N2 worms in response to treatment with olanzapine (O100).
Fig. 9.
Results of the effect of 100 µM olanzapine (O100) and 50 µM fluoxetine (F50) on the fat content of prdx-2 mutants at the L4 stage of lifespan. A. Results of the effects of (F50) on the food intake of prdx-2 mutants. B. Results of the effects of (F50) on the body fat of prdx-2 mutants. C. Results of the effects of (O100) on the food intake of prdx-2 mutants. D. Results of the effects of (O100) on the body fat of prdx-2 mutants. (*p ≤ 0.05, statistically different when compared to the respective control, n = 10).
3.6. Effects of olanzapine (O100) and fluoxetine (F50) on the formation of the intracellular reactive oxygen species (ROS) and on basal mitochondrial respiration:
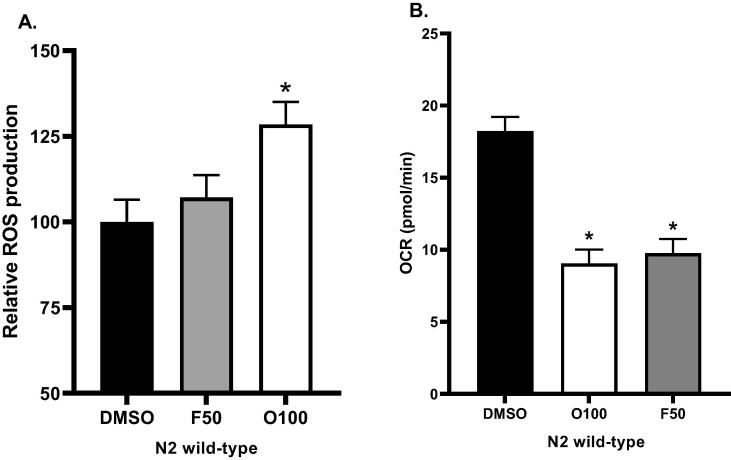
Psychotropic drugs PDs have been reported to affect the formation of intracellular reactive oxygen species (ROS) formation in humans and other models(Heiser et al., 2010, Vucicevic et al., 2014). In addition, our previous results with prdx-2 mutants indicated that oxidative stress might be causing the increase in body fat in response to treatment with olanzapine (O100). To investigate this further, we examined the effects of 100 µM olanzapine (O100) and 50 µM fluoxetine (F50) on the formation of the intracellular reactive oxygen species (ROS) using fluorescent probe 2′,7′(H2DCFDA). N2 worms were exposed to treatments from the L1 stage, and the detection of intracellular (ROS) was performed 24 h after exposure. We found that compared to the control, the treatment with (O100) significantly increased (*p ≤ 0.05) the value of DCF fluorescence signals in N2 worms, whereas the treatment with (F50) showed an insignificant increase (Fig. 10A). The increase of (ROS) production in response to treatment with (O100) is consistent with our finding with prdx-2 mutants. Both results indicate that oxidative stress plays an important role in the effect of olanzapine (O100) on the body fat of C. elegans.
Fig. 10.
A. Results of the percentages of the mean value of DCF fluorescence signals associated with ROS production and induced by 2′,7′(H2DCFDA) prob. N2 worms were treated with 100 μM olanzapine (O100) 50 μM fluoxetine (F50) from the L1 stage. B. Mitochondrial respiration of N2 worms treated with 100 µM olanzapine (O100) or 50 µM fluoxetine (F50). Treatments were introduced to worms from the L1 stage, and measurements were taken at the L4 stage. (*p ≤ 0.05, statistically different when compared to the respective control).
We next investigated the effects of 100 µM olanzapine (O100) and 50 µM fluoxetine (F50) on the mitochondrial respiration of N2 worms. Worms were introduced to treatments at the L1 stage, and measurements of the oxygen consumption rate (OCR) were taken at the L4 stage. As shown in (Fig. 10B), the mitochondrial respiration of worms in the control group was significantly higher than worms treated with (O100) or (F50) (*p ≤ 0.05).
4. Discussion
The aim of the present study was to investigate the effects of several psychotropic drugs (PDs) on the body fat of C. elegans and other related parameters. The C. elegans model was chosen to investigate the effect of PDs on body fat as it has been an established model to study the mechanisms that regulate fat accumulation in tissues (Zheng & Greenway, 2012). Compared to other models, C. elegans has a relatively short lifespan, a transparent body, and a fully sequenced genome, which allow researchers to study a wide range of metabolic genes and pathways. Moreover, the C. elegans model has been widely used to screen drugs and to evaluate their efficacy and toxicity (Hunt, 2017, Leung et al., 2008).
Here, we report that the effect of the antipsychotic olanzapine as well as the effect of the antidepressant fluoxetine on the body fat of C. elegans is dependent on the concentrations of the drugs presented in the media. This study demonstrates that young worms (L4) exhibit an increase in body fat in response to treatment with lower concentrations of olanzapine, up to 100 µM (O100), and a reduction in body fat in response to treatment with higher concentrations of this medication. Similarly, young worms (L4) show a significant increase in food intake in response to treatment with lower concentrations of olanzapine (O100) and a slight decrease in food intake in response to treatment with higher concentrations of this medication. These results suggest that hyperphagia is a possible cause for the observed increase in body fat in response to treatment with olanzapine (O100). These results also suggest that careful consideration should be taken in regard to the exposure concentration of olanzapine when using this model to study the metabolic effects of this medication. It is noteworthy that the food intake in this study was evaluated by measuring the pharyngeal pumping rates. The measurement of pharyngeal pumping rates in C. elegans has previously been used for estimating food intake (Avery, 1993, Avery, 2012), but is not a direct measure of food intake. Alternative methods that offer the advantage of directly measuring the food intake of worms have been developed recently (Boyd et al., 2003, Gomez-Amaro et al., 2015, Rodríguez-Palero et al., 2018). Therefore, further research, using a direct technique to measure food intake, needs to be conducted to confirm the feeding results reported in our study.
Our results of the increased food intake and body fat of C. elegans in response to treatment with olanzapine are consistent with previous findings (Perez-Gomez et al., 2018, Zheng and Greenway, 2012, Zheng et al., 2016). Treatment with olanzapine has been previously shown to increase the body fat (Zheng and Greenway, 2012, Zheng et al., 2016) and food intake (Perez-Gomez et al., 2018) of adult C. elegans. Nevertheless, a previous study by Karmacharya et al. reported a reduction in food intake in L1 worms in response to treatment with olanzapine(Karmacharya et al., 2009). In addition to our findings, these results suggest that the effects of olanzapine may be influenced by the age of the worms. In clinical settings, the use of olanzapine is known to be associated with a general increase in caloric intake and substantial weight gain in humans(Citrome et al., 2011, Gothelf et al., 2002, Solmi et al., 2017). Moreover, changes in body weight is significantly associated with noncompliance among patients receiving treatment with olanzapine(Citrome et al., 2011). In addition, olanzapine is known to induce significant body weight gain and increased food intake and to lead to an increased deposition of fat in rodents(Davey et al., 2012, Lord et al., 2017). Our results of the increased body fat and food intake of young worms (L4s) in response to treatment with olanzapine (O100) are consistent with previous findings reported in humans, rodents, and C. elegans.
Similar to the results found with olanzapine, our findings demonstrate that young worms (L4) show a steady increase in body fat after treatment with lower concentrations of fluoxetine, up to 50 µM (F50), and a reduction in body fat in response to treatment with higher concentrations of this medication. Similarly, L4 worms show a significant increase in food intake in response to treatment with lower concentrations of fluoxetine (F50) and a slight decrease in food intake in response to treatment with higher concentrations of this medication. The results of the increased food intake and body fat in response to treatment with (F50) suggest that the increase in food intake may be a possible cause for the observed increase in body fat. In animal models, the general use of fluoxetine has been associated with weight loss and reduction in the food intake in rodents (Aggarwal et al., 2016, Fuller and Wong, 1989); however, it has been suggested that exposure to fluoxetine in rats is a long-term risk factor for weight gain even after discontinuation of treatment (Mastronardi et al., 2011). In clinical settings, the evidence is varied regarding the effect of fluoxetine on body fat and appetite in humans. Most of the evidence in the literature suggests that the use of fluoxetine is linked to a reduction in body weight and caloric intake (Foltin et al., 1996, McGuirk and Silverstone, 1990, Michelson et al., 1999); however, several clinical reports have indicated that fluoxetine may cause an increase in food intake and body weight among its users (Fichtner and Braun, 1994, Fogelson, 1991).
To better understand the observed effect of olanzapine (O100) and fluoxetine (F50) on the body fat of C. elegans, we tested the effects of treatment with (O100) or (F50) on body size by measuring the length and width of (L4) worms. Although treatments with (O100) or (F50) result in a significant increase in the body fat of L4 worms, this increase in body fat is not associated with any changes in the body size of treated worms. In addition, we investigated the food-seeking strategy of young worms (L4) treated with olanzapine (O100) or fluoxetine (F50). Generally, the movement of C. elegans is known to alternate between two phases, called dwelling (slow movement) and roaming (rapid straight movement). It has been estimated that in the presence of food, worms spend up to 80% of their time in dwelling and 20% in roaming (Fujiwara, Sengupta, & McIntire, 2002). Remarkably, in the absence of food, it has been suggested that starved worms seek food by allocating their entire time to roaming (Shtonda and Avery, 2006). We report that certain movement parameters, such as speed and straight-line distance, of (L4) worms treated with olanzapine (O100) increase in the absence of food compared to untreated (L4) worms. Similar patterns of movements were detected in L4 worms after treatment with fluoxetine (F50). Our results do not only confirm an increase in the food intake of L4 worms in response to treatment with olanzapine (O100) or fluoxetine (F50) but more interestingly also indicate that the roaming behavior is increased in treated worms.
Due to the high degree of homology between the C. elegans genes and the human genes (Lai et al., 2000), the C. elegans model can be effectively used to uncover the molecular mechanisms involved in the adverse effects of olanzapine and fluoxetine in humans. To identify the molecular mechanisms and pathways underlying the effect of olanzapine (O100) and fluoxetine (F50) on the body fat and food intake of C. elegans, we used mutants that carry defects in the insulin signaling pathway, serotoninergic pathway, and peroxiredoxin-2 gene. First, we examined the effect of olanzapine (O100) and fluoxetine (F50) using daf-2 mutants. Daf-2 is a homolog of the mammalian insulin/IGF receptor family (Pierce et al., 2001). The insulin signaling pathway has been shown to be involved in the control of satiety and energy balance (Loh et al., 2017, Zhang and Liu, 2014). In C. elegans, daf-2 receptors have been shown to play an important role in the regulation of body fat (Perez and Van Gilst, 2008, Watts, 2009). Our findings show that the body fat of untreated daf-2 mutants is higher than that of N2 wild-type worms. We also report that untreated daf-2 mutants show a reduction in food intake when compared to N2 worms. These results are consistent with previously published studies (Dillon et al., 2016, Kimura et al., 1997). Our results reveal that daf-2 loss of function did not alter the effects of olanzapine (O100) or the effect of fluoxetine (F50) on body fat and food intake. Thus, the insulin signaling pathway may not have a significant role in the reported effect of these drugs on body fat and food intake, and further research needs to be conducted to evaluate the role of this pathway.
We also investigated the effect of olanzapine (O100) and fluoxetine (F50) on the body fat and food intake of (ser-1, tph-1) mutants that carry defects in the serotoninergic pathway. The serotonin signaling pathway plays a vital role in the regulation of food intake and energy balance in C. elegans and other models (Donovan and Tecott, 2013, Srinivasan et al., 2008). Furthermore, many PDs, including olanzapine and fluoxetine, release their actions by regulating serotonin receptors (Molla, 2020, Saadabadi, Jan 2020). Our results revealed that untreated ser-1 and tph-1 mutants have a slightly higher body fat than N2 worms. This result is in line with the findings from previous studies (Gubert et al., 2013, Sze et al., 2000). In addition, we report that tph-1 mutants have lower food intake rates than N2 worms, whereas ser-1 mutants display similar food intake rates as that displayed in N2 worms. This result is also in line with the findings from previous studies (Srinivasan et al., 2008, Sze et al., 2000). Our results show that ser-1 and tph-1 loss of function do not alter the effects of olanzapine (O100) on body fat or food intake. On the other hand, we show that the treatment with fluoxetine (F50) results in an increase in the body fat of ser-1 and tph-1 mutants and does not have any effect on their food intake. These findings suggest that the functions of ser-1 and tph-1 in the serotoninergic pathway are required for the observed effects of fluoxetine (F50) on food intake.
Although (PDs) are known to possess antioxidant properties (Sadowska-Bartosz et al., 2016), it has been suggested that both first and second generations of PDs induce oxidative stress(Güneş et al., 2016, Sadowska-Bartosz et al., 2016, Zhang et al., 2012). It has also been suggested that some PDs, such as olanzapine, affect neurons by inducing the production of reactive oxygen species (ROS), mitochondrial depolarization, and mitochondrial damage (Vucicevic et al., 2014). Interestingly, oxidative stress has been linked to obesity in humans as well as fat accumulation in other models (Furukawa et al., 2004, Manna and Jain, 2015, Vincent et al., 2007). Here, we used mutants that carry a defect in the peroxiredoxin-2 gene to investigate the effects of olanzapine (O100) and fluoxetine (F50). The thioredoxin peroxidase activity of prdx-2 protects C. elegans against oxidative damage by ROS (Oláhová et al., 2008). Our results show that a loss of prdx-2 function does not alter the observed effect of fluoxetine (F50) on body fat and food intake; however, our results show that the prdx-2 function is required for the observed effect of olanzapine (O100) on body fat. In addition, we report an increase in the production reactive oxygen species (ROS) in N2 worms treated with (O100). Taken together, these results suggest that the observed effects of olanzapine on body fat may be caused by the increase in oxidative stress.
5. Conclusion
In conclusion, our results show that careful consideration must be given to the dosage used for the studies on the effects of fluoxetine or olanzapine in C. elegans. The results also show that fluoxetine and olanzapine increase body fat in C. elegans by different mechanisms. The results reported in this study emphasize the importance of the C. elegans model, as an initial screening tool, for understanding the mechanisms involved in the adverse effects of PDs in humans. Although the results reported in this study need confirmation in higher organisms, the use of C. elegans allows those future studies to incur lower costs and take less time.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and material
The dataset presented in this paper was generated in our lab, and is available from the corresponding author upon request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aggarwal A., Jethani S.L., Rohatgi R.K., Kalra J. Selective Serotonin Re-uptake Inhibitors (SSRIs) Induced Weight Changes: A Dose and Duration Dependent Study on Albino Rats. Journal of clinical and diagnostic research : JCDR. 2016;10(3):AF01-AF03. doi: 10.7860/JCDR/2016/16482.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An H., Du X., Huang X., Qi L., Jia Q., Yin G., Zhang X.Y. Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl. Psychiatry. 2018;8(1):258. doi: 10.1038/s41398-018-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133(4):897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, L. a. Y., Y.J. (2012). C. elegans feeding. In WormBook. [DOI] [PMC free article] [PubMed]
- Bak M., Fransen A., Janssen J., van Os J., Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0094112. e94112 e94112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd W.A., Cole R.D., Anderson G.L., Williams P.L. The effects of metals and food availability on the behavior of Caenorhabditis elegans. Environ Toxicol Chem. 2003;22(12):3049–3055. doi: 10.1897/02-565. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citrome L., Holt R.I., Walker D.J., Hoffmann V.P. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455–482. doi: 10.2165/11589060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Czarny M.J., Arthurs E., Coffie D.-F., Smith C., Steele R.J., Ziegelstein R.C., Thombs B.D. Prevalence of Antidepressant Prescription or Use in Patients with Acute Coronary Syndrome: A Systematic Review. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey K.J., O'Mahony S.M., Schellekens H., O'Sullivan O., Bienenstock J., Cotter P.D., Cryan J.F. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. 2012;221(1):155–169. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- Raizen D., Trojanowski N., You Y.-J. WormBook. 2005. Methods for measuring pharyngeal behaviors. WormBook: The Online Review of C. elegans Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M., Detraux J., van Winkel R., Yu W., Correll C.U. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- Dillon J., Holden-Dye L., O'Connor V., Hopper N.A. Context-dependent regulation of feeding behaviour by the insulin receptor, DAF-2. Caenorhabditis elegans. Invertebrate neuroscience : IN. 2016;16(2):4. doi: 10.1007/s10158-016-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan M.H., Tecott L.H. Serotonin and the regulation of mammalian energy balance. Front. Neurosci. 2013;7:36. doi: 10.3389/fnins.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D.S., Awatramani P., Thakur R., Seeni R., Aamodt E.J. Social feeding in Caenorhabditis elegans is modulated by antipsychotic drugs and calmodulin and may serve as a protophenotype for asociality. Neuropharmacology. 2015;92:56–62. doi: 10.1016/j.neuropharm.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Escorcia W., Ruter D.L., Nhan J., Curran S.P. Quantification of Lipid Abundance and Evaluation of Lipid Distribution in Caenorhabditis elegans by Nile Red and Oil Red O Staining. Journal of visualized experiments. 2018;JoVE(133):57352. doi: 10.3791/57352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner C.G., Braun B.G. Hyperphagia and weight loss during fluoxetine treatment. Ann Pharmacother. 1994;28(12):1350–1352. doi: 10.1177/106002809402801205. [DOI] [PubMed] [Google Scholar]
- Fogelson, D. L. (1991). Weight Gain During Fluoxetine Treatment. Journal of Clinical Psychopharmacology, 11(3), 220. Retrieved from https://journals.lww.com/psychopharmacology/Fulltext/1991/06000/Weight_Gain_During_Fluoxetine_Treatment.20.aspx [PubMed]
- Foltin R.W., Haney M., Comer S.D., Fischman M.W. Effect of fluoxetine on food intake of humans living in a residential laboratory. Appetite. 1996;27(2):165–181. doi: 10.1006/appe.1996.0043. [DOI] [PubMed] [Google Scholar]
- Fujiwara M., Sengupta P., McIntire S.L. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron. 2002;36(6):1091–1102. doi: 10.1016/s0896-6273(02)01093-0. [DOI] [PubMed] [Google Scholar]
- Fuller R.W., Wong D.T. Fluoxetine: A serotonergic appetite suppressant drug. Drug Dev. Res. 1989;17(1):1–15. doi: 10.1002/ddr.430170102. [DOI] [Google Scholar]
- Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile S. Long-term treatment with atypical antipsychotics and the risk of weight gain : a literature analysis. Drug Saf. 2006;29(4):303–319. doi: 10.2165/00002018-200629040-00002. [DOI] [PubMed] [Google Scholar]
- Gomez-Amaro R.L., Valentine E.R., Carretero M., LeBoeuf S.E., Rangaraju S., Broaddus C.D., Petrascheck M. Measuring Food Intake and Nutrient Absorption in Caenorhabditis elegans. Genetics. 2015;200(2):443–454. doi: 10.1534/genetics.115.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D., Falk B., Singer P., Kairi M., Phillip M., Zigel L., Apter A. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. The American journal of psychiatry. 2002;159(6):1055–1057. doi: 10.1176/appi.ajp.159.6.1055. [DOI] [PubMed] [Google Scholar]
- Gubert P., Aguiar G.C., Mourão T., Bridi J.C., Barros A.G., Soares F.A., Romano-Silva M.A. Behavioral and metabolic effects of the atypical antipsychotic ziprasidone on the nematode Caenorhabditis elegans. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0074780. e74780 e74780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güneş M., Camkurt M.A., Bulut M., Demir S., İbiloğlu A.O., Kaya M.C., Sir A. Evaluation of Paraoxonase, Arylesterase and Malondialdehyde Levels in Schizophrenia Patients Taking Typical, Atypical and Combined Antipsychotic Treatment. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology. 2016;14(4):345–350. doi: 10.9758/cpn.2016.14.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser P., Sommer O., Schmidt A.J., Clement H.W., Hoinkes A., Hopt U.T., Dobschütz E. Effects of antipsychotics and vitamin C on the formation of reactive oxygen species. Journal of psychopharmacology (Oxford, England) 2010;24(10):1499–1504. doi: 10.1177/0269881109102538. [DOI] [PubMed] [Google Scholar]
- Hunt P.R. The C. elegans model in toxicity testing. Journal of applied toxicology : JAT. 2017;37(1):50–59. doi: 10.1002/jat.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas S., Moncrieff J. Trends in prescriptions and costs of drugs for mental disorders in England, 1998–2010. Br J Psychiatry. 2012;200(5):393–398. doi: 10.1192/bjp.bp.111.104257. [DOI] [PubMed] [Google Scholar]
- Kahn R.S., Fleischhacker W.W., Boter H., Davidson M., Vergouwe Y., Keet I.P., Grobbee D.E. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085–1097. doi: 10.1016/s0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- Karanges E.A., Stephenson C.P., McGregor I.S. Longitudinal trends in the dispensing of psychotropic medications in Australia from 2009–2012: focus on children, adolescents and prescriber specialty. Aust N Z J Psychiatry. 2014;48(10):917–931. doi: 10.1177/0004867414538675. [DOI] [PubMed] [Google Scholar]
- Karmacharya R., Sliwoski G.R., Lundy M.Y., Suckow R.F., Cohen B.M., Buttner E.A. Clozapine Interaction with Phosphatidyl Inositol 3-Kinase (PI3K)/Insulin-Signaling Pathway in Caenorhabditis elegans. Neuropsychopharmacology. 2009;34(8):1968–1978. doi: 10.1038/npp.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K.D., Tissenbaum H.A., Liu Y., Ruvkun G. daf-2, an Insulin Receptor-Like Gene That Regulates Longevity and Diapause in Caenorhabditis elegans. Science. 1997;277(5328):942. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Koopman M., Michels H., Dancy B.M., Kamble R., Mouchiroud L., Auwerx J., Houtkooper R.H. A screening-based platform for the assessment of cellular respiration in Caenorhabditis elegans. Nat. Protoc. 2016;11(10):1798–1816. doi: 10.1038/nprot.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.H., Chou C.Y., Ch'ang L.Y., Liu C.S., Lin W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000;10(5):703–713. doi: 10.1101/gr.10.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung M.C., Williams P.L., Benedetto A., Au C., Helmcke K.J., Aschner M., Meyer J.N. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106(1):5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K., Zhang L., Brandon A., Wang Q., Begg D., Qi Y., Herzog H. Insulin controls food intake and energy balance via NPY neurons. Molecular metabolism. 2017;6(6):574–584. doi: 10.1016/j.molmet.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C.C., Wyler S.C., Wan R., Castorena C.M., Ahmed N., Mathew D., Elmquist J.K. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J. Clin. Investig. 2017;127(9):3402–3406. doi: 10.1172/JCI93362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna P., Jain S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metabolic syndrome and related disorders. 2015;13(10):423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronardi C., Paz-Filho G.J., Valdez E., Maestre-Mesa J., Licinio J., Wong M.L. Long-term body weight outcomes of antidepressant-environment interactions. Mol. Psychiatry. 2011;16(3):265–272. doi: 10.1038/mp.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreadie R., Macdonald E., Blacklock C., Tilak-Singh D., Wiles D., Halliday J., Paterson J. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case-control study. BMJ (Clinical research ed.) 1998;317(7161):784–785. doi: 10.1136/bmj.317.7161.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirk J., Silverstone T. The effect of the 5-HT re-uptake inhibitor fluoxetine on food intake and body weight in healthy male subjects. Int J Obes. 1990;14(4):361–372. [PubMed] [Google Scholar]
- Michelson D., Amsterdam J.D., Quitkin F.M., Reimherr F.W., Rosenbaum J.F., Zajecka J., Beasley C.M., Jr. Changes in weight during a 1-year trial of fluoxetine. The American journal of psychiatry. 1999;156(8):1170–1176. doi: 10.1176/ajp.156.8.1170. [DOI] [PubMed] [Google Scholar]
- Molla, A. J. S. M. C. S. M. (2020 Jan). Fluoxetine. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK459223/
- Nelson D.W., Padgett R.W. Insulin worms its way into the spotlight. Genes Dev. 2003;17(7):813–818. doi: 10.1101/gad.1090203. [DOI] [PubMed] [Google Scholar]
- Nihalani N., Schwartz T.L., Siddiqui U.A., Megna J.L. Weight gain, obesity, and psychotropic prescribing. Journal of obesity. 2011;2011 doi: 10.1155/2011/893629. 893629 893629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláhová M., Taylor S.R., Khazaipoul S., Wang J., Morgan B.A., Matsumoto K., Veal E.A. A redox-sensitive peroxiredoxin that is important for longevity has tissue- and stress-specific roles in stress resistance. Proc. Natl. Acad. Sci. 2008;105(50):19839–19844. doi: 10.1073/pnas.0805507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláhová M., Veal E.A. A peroxiredoxin, PRDX-2, is required for insulin secretion and insulin/IIS-dependent regulation of stress resistance and longevity. Aging Cell. 2015;14(4):558–568. doi: 10.1111/acel.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M., Marcus S.C. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- Perez-Gomez A., Carretero M., Weber N., Peterka V., To A., Titova V., Petrascheck M. A phenotypic Caenorhabditis elegans screen identifies a selective suppressor of antipsychotic-induced hyperphagia. Nat Commun. 2018;9(1):5272. doi: 10.1038/s41467-018-07684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C.L., Van Gilst M.R. A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. Cell Metab. 2008;8(3):266–274. doi: 10.1016/j.cmet.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Pierce S.B., Costa M., Wisotzkey R., Devadhar S., Homburger S.A., Buchman A.R., Ruvkun G. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15(6):672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Palero M.J., López-Díaz A., Marsac R., Gomes J.-E., Olmedo M., Artal-Sanz M. An automated method for the analysis of food intake behaviour in Caenorhabditis elegans. Sci. Rep. 2018;8(1):3633. doi: 10.1038/s41598-018-21964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadabadi K.T.A. StatPearls Publishing; Retrieved from: Jan 2020. Olanzapine. https://www.ncbi.nlm.nih.gov/books/NBK532903/ [Google Scholar]
- Sadowska-Bartosz I., Galiniak S., Bartosz G., Zuberek M., Grzelak A., Dietrich-Muszalska A. Antioxidant properties of atypical antipsychotic drugs used in the treatment of schizophrenia. Schizophr Res. 2016;176(2–3):245–251. doi: 10.1016/j.schres.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Shrivastava A., Johnston M.E. Weight-gain in psychiatric treatment: risks, implications, and strategies for prevention and management. Mens Sana Monogr. 2010;8(1):53–68. doi: 10.4103/0973-1229.58819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtonda B.B., Avery L. Dietary choice behavior in Caenorhabditis elegans. The Journal of experimental biology. 2006;209(Pt 1):89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi M., Murru A., Pacchiarotti I., Undurraga J., Veronese N., Fornaro M., Carvalho A.F. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther. Clin. Risk Manag. 2017;13:757–777. doi: 10.2147/TCRM.S117321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Sadegh L., Elle I.C., Christensen A.G.L., Faergeman N.J., Ashrafi K. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell Metab. 2008;7(6):533–544. doi: 10.1016/j.cmet.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze J.Y., Victor M., Loer C., Shi Y., Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403(6769):560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Verhaegen A.A., Van Gaal L.F. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J. Endocrinol. Invest. 2017;40(11):1165–1174. doi: 10.1007/s40618-017-0719-6. [DOI] [PubMed] [Google Scholar]
- Vincent H.K., Innes K.E., Vincent K.R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9(6):813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- Voigt J.P., Fink H. Serotonin controlling feeding and satiety. Behav Brain Res. 2015;277:14–31. doi: 10.1016/j.bbr.2014.08.065. [DOI] [PubMed] [Google Scholar]
- Vucicevic L., Misirkic-Marjanovic M., Paunovic V., Kravic-Stevovic T., Martinovic T., Ciric D., Trajkovic V. Autophagy inhibition uncovers the neurotoxic action of the antipsychotic drug olanzapine. Autophagy. 2014;10(12):2362–2378. doi: 10.4161/15548627.2014.984270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J.L. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends in endocrinology and metabolism: TEM. 2009;20(2):58–65. doi: 10.1016/j.tem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Pharmacological treatment of mental disorders in primary health care. 2009. https://www.who.int/mental_health/management/psychotropic/en/ Retrieved from. [PubMed]
- Yoon D.S., Lee M.-H., Cha D.S. Measurement of Intracellular ROS in Caenorhabditis elegans Using 2',7'-Dichlorodihydrofluorescein Diacetate. Bio-protocol. 2018;8(6) doi: 10.21769/BioProtoc.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu F. Tissue-specific insulin signaling in the regulation of metabolism and aging. IUBMB Life. 2014;66(7):485–495. doi: 10.1002/iub.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Y., Zhou D.F., Shen Y.C., Zhang P.Y., Zhang W.F., Liang J., Kosten T.R. Effects of risperidone and haloperidol on superoxide dismutase and nitric oxide in schizophrenia. Neuropharmacology. 2012;62(5–6):1928–1934. doi: 10.1016/j.neuropharm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Zheng J., Greenway F.L. Caenorhabditis elegans as a model for obesity research. International Journal of Obesity. 2012;36(2):186–194. doi: 10.1038/ijo.2011.93. [DOI] [PubMed] [Google Scholar]
- Zheng J., Vasselli J.R., King J.F., King M.L., We W., Fitzpatrick Z., Greenway F.L. Using Caenorhabditis elegans as a Model for Obesity Pharmacology Development. Am. J .Ther. 2016;23(6):e1363–e1370. doi: 10.1097/MJT.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]