Abstract
Background:
Whether rhythm control for post-operative atrial fibrillation after cardiac surgery (POAF) is superior to rate control in patients with heart failure or systolic dysfunction (HF) is not known.
Methods
We performed a post-hoc analysis of a trial by the Cardiothoracic Surgical Trials Network, which randomized patients with POAF after cardiac surgery to rate control or rhythm control with amiodarone/cardioversion. We assessed subgroups of trial participants defined by heart failure/cardiomyopathy history or left ventricular ejection fraction (LVEF) < 50%. We conducted a stratified analysis in patients with and without HF to explore outcomes of rhythm versus rate control strategy.
Results
Of 523 subjects with POAF after cardiac surgery, 131 (25%) had HF. 49% of HF patients were randomized to rhythm control. In HF patients, rhythm control was associated with less atrial fibrillation within the first 7 days. There were no differences in rhythm at 30- and 60-day followup. In the HF group, there were significantly more subjects with AF < 48 hours in the rhythm control group compared to rate control group- 68.8% compared to 46.3%, P=0.009. By comparison, in the non-HF stratum, 54.4% of the rate control group had AF < 48 hours compared to 63.5% of the rhythm control group (P=0.067). ), though there was no significant interaction of heart failure with cardiac rhythm at 7 days (Pinteraction 0.16).
Conclusion
Rhythm control for HF patients with POAF after cardiac surgery increases early restoration of sinus rhythm. Rate and rhythm control are both reasonable for HF patients with AF after cardiac surgery
Introduction
Post-operative atrial fibrillation (POAF) occurs in up to one third of cardiac surgical procedures[1]. An understanding of how best to treat POAF is essential. Treatment strategies include “rate control” and “rhythm control.” The Cardiothoracic Surgical Trials Network conducted a multicenter randomized trial of rate versus rhythm control in POAF [2]. There were no differences in outcome between the strategies, and 25% of study participants crossed over to the unassigned treatment. The patient characteristics predicting clinical response to rate control versus rhythm control remain unclear, representing a gap in knowledge.
Patients with heart failure are vulnerable to hemodynamic adverse effects of POAF [3]. Moreover, clinicians often pursue a rhythm control strategy inn heart failure patients with POAF. Whether a rate control or a rhythm control strategy is best for management of POAF in patients with known heart failureis not known. We hypothesize that patients with a history of heart failure would have improved outcomes with rhythm control. Therefore, we assessed whether heart failure history or systolic dysfunction is an effect measure modifier of the relationship between rate versus rhythm control and outcome in POAF after cardiac surgery.
Methods
Study population
We performed a stratified analysis of the CTSN-POAF trial [2]. Trial data was obtained from the NIH BioLINCC data repository [4]. The CTSN-POAF trial randomized 523 patients with POAF after cardiac surgery to rate or rhythm control. Subjects had CABG, valve procedure or a combined valve-CABG. Follow-up time began at time of randomization. The heart rhythm was assessed by electrocardiography at hospital discharge, study day 30 and study day 60. Patients receiving mechanical valves, those who were hemodynamically unstable, and those with history of atrial fibrillation or atrial flutter were excluded. If POAF persisted for 60 minutes or longer or had recurrent POAF within 7 days, subjects were randomized to rate control or rhythm control strategy. Those receiving rate control were prescribed nodal agents to a target heart rate of 100 beats per minute or less. Rhythm control consisted of amiodarone with electrical cardioversion at 48 hours. Subjects crossed over to the other arm at clinician discretion for intolerance of the randomized treatment strategy (hemodynamic status or symptoms). Crossover from rate to rhythm control consisted of cardioversion and amiodarone. Crossover from rhythm to rate control included discontinuation of amiodarone and prescription of nodal agents. Anticoagulation was prescribed at 48 hours if there was persistent AF for 48 hours duration or recurrent AF. Anticoagulation consisted of warfarin with target INR 2–3.
To determine if history of heart failure or systolic dysfunction was an effect measure modifier of the relationship between treatment strategy and outcome, we performed a stratified analysis determining the outcome as a function of treatment strategy in patients with and without a history of heart failure or systolic dysfunction.
We stratified by the presence or absence of a history of heart failure. We considered HF as heart failure/cardiomyopathy history as defined by trial entry criteria, or left ventricular ejection fraction (LVEF) < 50% on pre-operative echocardiogram. Within each stratum, the primary exposure variable was “initial as randomized” treatment strategy, representing an “intention to treat” analysis rather than an“ as treated” analysis.
Primary and secondary outcomes
The primary study outcome is total number of days alive and out of hospital within 60 days of surgery. Relevant secondary outcomes include death, percentage of patients in sinus rhythm at 7 days, hospital discharge, day 30 and day 60, and cross-over from assigned therapy.
Statistical analysis
The outcome of days alive out of hospital was compared using the Wilcoxon rank-sum test due to skewed distribution, and categorical variables compared using the Chi Square test. We performed survival analysis for the endpoint of conversion from atrial fibrillation within 7 days and generated Kaplan-Meier curves for the proportion of patients remaining in atrial fibrillation over 7 days time in the heart failure and no heart failure groups. Curves were compared using the log-rank test. We assessed the interaction of randomized treatment strategy and HF status in Cox Proportional Hazard models for rhythm at the 7 day time point and logistic regression models for rhythm at the 30 and 60 day time point. All analyses were performed using Stata 14.0. A P value < 0.05 was considered statistically significant.
Results
Of 523 subjects, 131 (25%) had HF. Demographics, clinical characteristics and outcomes for POAF HF subjects compared to those without HF are shown in the Table. Similar proportions of POAF HF and non-HF subjects required crossover to the alternate management strategy- 28% of HF subjects and 24% of non-HF subjects. Outcomes of subjects with POAF were similar in the HF and non-HF groups, with between 23 and 25% 30-day readmission rates, low death rates, and similar numbers of days alive and out of hospital. Rhythm outcomes were likewise similar between HF and non-HF subjects with high and similar proportions of subjects in sinus rhythm at 7, 30, and 60 days post-operatively. Within the HF group, the low EF (<50%, N=86) and preserved EF (>50%, N=45) groups were overall comparable. Low EF HF subjects were more likely to be male (80.2% v. 57.8%), more likely to have prior MI (44.2% v. 20%), and more likely to have isolated CABG (57% v. 35.6%). Otherwise, demographics, clinical characteristics, procedural conduct, cross-over rates, and outcomes were similar between EF groups.
Table:
Demographics and outcomes for subjects with and without HF
| Heart failure (N=131) | No heart failure (N=392) | |
| Age (years) | 68 (9.4) | 68.4 (9.1) |
| Male sex | 95 (72.5) | 301 (76.8) |
| Prior MI | 47 (35.9) | 51 (13.0) |
| Hypertension | 104 (79.4) | 287 (73.2) |
| Diabetes | 55 (42.0) | 106 (27.0) |
| Sleep apnea | 19 (14.5) | 44 (11.2) |
| Body-mass index | 29.1 (5.6) | 28.7 (5.1) |
| Pre-operative medical therapy | ||
| Beta blocker | 98 (74.8) | 209 (53.3) |
| ACE or ARB | 73 (55.7) | 196 (50.0) |
| Calcium channel blocker | 26 (19.9) | 84 (21.4) |
| Preoperative LVEF (%) | 45.1 (13.3) | 60.3 (5.6) |
| Procedure | ||
| CABG | 65 (49.6) | 147 (37.5) |
| Valve repair | 15 (11.5) | 67 (17.1) |
| CABG + valve repair | 6 (4.6) | 11 (2.8) |
| Valve replacement | 20 (15.2) | 106 (27.0) |
| CABG + valve replacement | 25 (19.1) | 61 (15.6) |
| CABG only (%) | 65 (49.6) | 147 (37.5) |
| Cardiopulmonary bypass time | 122.2 (50.0) | 99.3 (40.0) |
| Rhythm control strategy | 64 (48.9) | 197 (50.3) |
| Cross-over to alternate strategy (%) | 37 (28.2) | 95 (24.2) |
| ICU length of stay (days) | 3.9 (5.5) | 2.6 (3.8) |
| Readmission | 30 (22.9) | 96 (24.5) |
| Death | 2 (1.5) | 3 (0.8) |
| Days alive and out of hospital (days) | 51.0 (12.0) | 52.1 (13.2) |
| Sinus rhythm at 7 days | 104 (82.5) | 318 (83.9) |
| Sinus rhythm at 30 days | 114 (93.4) | 348 (94.2) |
| Sinus rhythm at 60 days | 112 (94.1) | 348 (96.9) |
| AF for less than 48 hours | 75 (57.3) | 231 (58.9) |
|
| ||
| Heart failure (N=131) | Rate control | Rhythm control |
| Age (years) | 68.0 (11.05) | 67.9 (7.3) |
| Male sex | 52 (77.6) | 43 (67.2) |
| Prior MI | 26 (38.8) | 21 (32.8) |
| Hypertension | 53 (79.1) | 51 (79.7) |
| Diabetes | 28 (41.8) | 27 (42.2) |
| Sleep apnea | 10 (14.9) | 9 (14.1) |
| Body-mass index | 29.2 (5.3) | 29.1 (5.9) |
| Pre-operative medical therapy | ||
| Beta blocker | 52 (77.6) | 46 (71.9) |
| ACE or ARB | 37 (55.2) | 36 (56.3) |
| Calcium channel blocker | 10 (14.9) | 16 (25.0) |
| Preoperative LVEF (%) | 44.3 (13.9) | 46.0 (12.6) |
| Procedure | ||
| CABG | 36 (53.7) | 29 (45.3) |
| Valve repair | 8 (11.9) | 7 (10.9) |
| CABG + valve repair | 5 (7.5) | 1 (1.6) |
| Valve replacement | 9 (13.4) | 11 (17.2) |
| CABG + valve replacement | 9 (13.4) | 16 (25.0) |
| CABG only (%) | 36 (53.7) | 29 (45.3) |
| Cardiopulmonary bypass time | 122.5 (50.4) | 121.9 (50.0) |
| Off-pump procedure | 3 (4.5) | 4 (6.3) |
| Cross-over to alternate strategy (%) | 17 (25.4) | 20 (31.3) |
| Readmission | 16 (23.9) | 14 (21.9) |
| Death | 1 (1.5) | 1 (1.6) |
| Days alive and out of hospital (days) | 51.1 (11.4) | 50.8 (12.7) |
| Sinus rhythm at 7 days | 50 (76.9) | 54 (88.5) |
| Sinus rhythm at 30 days | 57 (91.9) | 57 (95.0) |
| Sinus rhythm at 60 days | 56 (90.3) | 56 (98.3) |
| AF for less than 48 hours | 31 (46.3) | 44 (68.8) |
|
| ||
| No heart failure (N=392) | Rate control | Rhythm control |
| Age (years) | 68.9 (9.4) | 67.9 (8.8) |
| Male sex | 145 (74.4) | 156 (79.2) |
| Prior MI | 24 (12.3) | 27 (13.7) |
| Hypertension | 140 (71.8) | 147 (74.6) |
| Diabetes | 54 (27.7) | 52 (26.4) |
| Sleep apnea | 23 (11.8) | 21 (10.7) |
| Body-mass index | 28.1 (4.8) | 29.2 (5.4) |
| Pre-operative medical therapy | ||
| Beta blocker | 110 (56.4) | 99 (50.3) |
| ACE or ARB | 102 (52.3) | 94 (47.7) |
| Calcium channel blocker | 42 (21.5) | 42 (21.3) |
| Preoperative LVEF (%) | 61.0 (6.04) | 59.6 (5.06) |
| Procedure | ||
| CABG | 76 (39.0) | 71 (36.0) |
| Valve repair | 31 (15.9) | 36 (18.3) |
| CABG + valve repair | 5 (2.6) | 6 (3.1) |
| Valve replacement | 51 (26.2) | 55 (27.9) |
| CABG + valve replacement | 32 (16.4) | 29 (14.7) |
| CABG only (%) | 76 (39.0) | 71 (36.0) |
| Cardiopulmonary bypass time | 97.6 (39.9) | 101.04 (40.1) |
| Off-pump procedure | 3 (1.5) | 8 (4.1) |
| Cross-over to alternate strategy (%) | 53 (27.2) | 42 (21.3) |
| Readmission | 46 (23.6) | 50 (25.4) |
| Death | 2 (1.03) | 1 (0.5) |
| Days alive and out of hospital (days) | 51.3 (13.0) | 52.8 (13.4) |
| Sinus rhythm at 7 days | 153 (80.5) | 165 (87.3) |
| Sinus rhythm at 30 days | 170 (94.4) | 178 (95.2) |
| Sinus rhythm at 60 days | 170 (94.4) | 178 (95.2) |
| AF for less than 48 hours | 106 (54.4) | 125 (63.5) |
Clinical characteristics and outcomes for an initial as-randomized rate versus rhythm control strategies are shown in the Table, stratified by HF status. In the HF group, 25% of those randomized initially to rate control crossed over to rhythm control and 31% of those randomized initially to rhythm control crossed over time rate control. In subjects without HF, 27% of those randomized initially to rate control crossed over to rhythm control and 21% of those randomized initially to rhythm control crossed over to rate control. Rates of readmission and death, and the number of days alive and out of hospital were similar in as-randomized rate and rhythm control groups, in both the HF and non-HF strata. In the HF group, there was a trend towards lower rates of sinus rhythm at 7 and 60 days with initial rate control strategy compared to rhythm control, which was less evident in the non-HF group (Table).
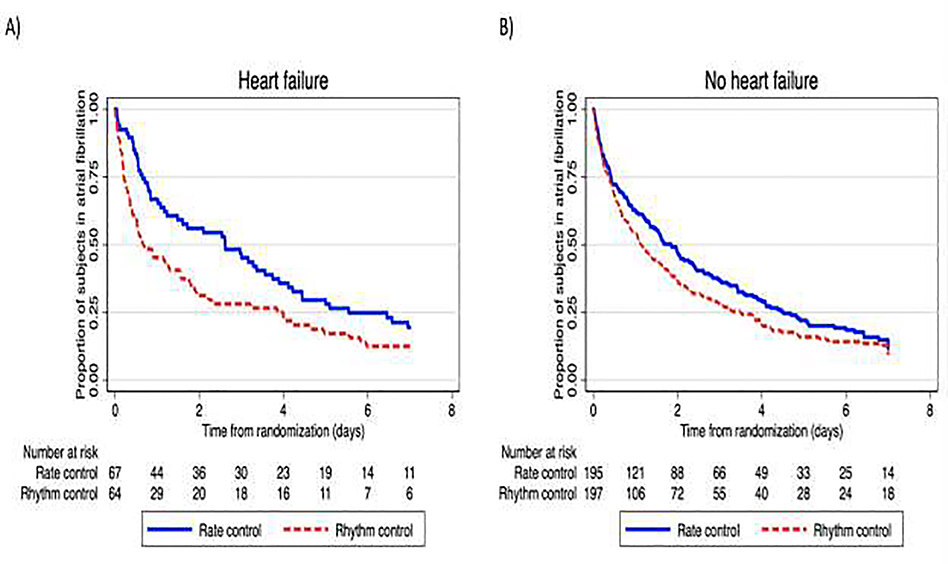
In the HF group, there were significantly more subjects with AF < 48 hours in the randomized rhythm control group compared to rate control group- 68.8% compared to 46.3%, P=0.009. The time-to-event curves confirm a lower proportion of patients remaining in atrial fibrillation early with initial rhythm control in heart failure patients (Figure 1, A). There was a non-significant trend towards shorter time to sinus rhythm in those without HF (Figure 1, B), though initial rate control resulted in a slightly greater percentage of those without HF achieving sinus rhythm. Rates of sinus rhythm were high at 30 and 60 days irrespective of treatment strategy, in both the HF and non-HF subgroups (Table). There was no significant interaction of heart failure with treatment effect at 7 days (Pinteraction 0.16), 30 days (Pinteraction 0.67) or 60 days (Pinteraction 0.55).
Figure 1:
Kaplan-Meier curves demonstrating proportion of subjects in atrial fibrillation by treatment strategy, in cardiac surgical patients with post-operative atrial fibrillation and heart failure (A; P=0.015 by log-rank test) and without heart failure (B; P=0.075 by log-rank test)
Discussion
In this stratified analysis of a randomized trial of rate versus rhythm control to treat post-operative atrial fibrillation after cardiac surgery, we report several major findings. A substantial percentage of HF patients with POAF treated with either initial rate or rhythm control will require crossover to the other treatment strategy for efficacy or intolerance, supporting that clinicians must continually re-evaluate their initial treatment strategy. In addition, an initial rhythm control strategy may shorten time to sinus rhythm in HF patients compared to rate control leading to significantly fewer patients requiring discharge on anticoagulation.
HF and AF are synergistic conditions- patients with HF are predisposed to AF via a variety of mechanisms including elevated left atrial pressure and atriopathy, mitral valve disease, shared risk factors, as well as the neurohormonal milieu of HF [3]. Moreover, patients with HF are uniquely predisposed to adverse hemodynamic consequences of atrial fibrillation including worsening HF [5] and diastolic dysfunction [6]. Our results suggest that HF patients with POAF initially selected for rate or rhythm control have high rates of crossovers into the other treatment strategy for intolerance or lack of efficacy. HF patients manifest a varied dependence on atrial contribution to left ventricular filling and hence are expected to have a varied hemodynamic response to AF. For example, those with a dominant atrial filling wave, the “impaired relaxation” pattern, are greatly dependent on atrial kick while patients with restrictive diastolic filling less so [6–8]. The heart rate and rhythm irregularity also contribute to reduced cardiac output in AF [9, 10]. Given this varied hemodynamic response and the high rate of crossovers we report, clinicians should use the entire armamentarium of treatments available for POAF in HF and tailor the strategy to the individual patient.
We report high rates of sinus rhythm at discharge, 30 and 60 days among HF patients with POAF, however it is not known whether these points in time reflect overall arrhythmic burden. We report a shorter time to sinus rhythm in POAF patients with HF - 64% of the initial rhythm control HF group had AF < 48 hours and hence would not require anticoagulation at discharge. This finding should be considered hypothesis generating, given the nature of the subgroup analysis. The optimal anticoagulation strategy for POAF merits further study; our results suggest a hypothesis that if a patient is at high risk for bleeding complications with anticoagulation at discharge, rhythm control may result in lower likelihood of requiring anticoagulation at discharge. This strategy should be assessed in additional studies.
Limitations of our study include that it is a retrospective subgroup analysis of a previously performed randomized trial and hence is hypothesis generating; subgroup analysis is subject to type I and type II error and thus can be considered exploratory only. This subgroup analysis was conducted in a clinically relevant patient population defined on the basis of a scientific hypothesis of purported differential treatment effect. Although this subgroup was not prespecified by the trial, the analytic plan was fully prespecified prior to receipt of trial data. The small overall sample size is underpowered for clinical events such as mortality and stroke which are rare. Long-term arrhythmia monitoring was not performed, and as such, total arrhythmia burden is underestimated in both arms. Finally, it is possible that patients enrolled in clinical trials may not reflect patients seen in clinical practice[11] which has implications for generalizability and external validity.
In conclusion, a substantial percentage of HF patients with POAF will require tailored and individualized AF treatment strategy. An initial rhythm control strategy may shorten time to sinus rhythm in HF patients compared to rate control. Our findings suggest taking an individualized approach to the management of POAF in patients with HF and highlight the need for dedicated prospective studies of treatment of POAF in the HF population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–9. [DOI] [PubMed] [Google Scholar]
- [2].Gillinov AM, Bagiella E, Moskowitz AJ, Raiten JM, Groh MA, Bowdish ME, et al. Rate Control versus Rhythm Control for Atrial Fibrillation after Cardiac Surgery. N Engl J Med. 2016;374:1911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carlisle MA, Fudim M, DeVore AD, Piccini JP. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019;7:447–56. [DOI] [PubMed] [Google Scholar]
- [4].Giffen CA, Wagner EL, Adams JT, Hitchcock DM, Welniak LA, Brennan SP, et al. Providing researchers with online access to NHLBI biospecimen collections: The results of the first six years of the NHLBI BioLINCC program. PLoS One. 2017;12:e0178141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gorenek B, Halvorsen S, Kudaiberdieva G, Bueno H, Van Gelder IC, Lettino M, et al. Atrial fibrillation in acute heart failure: A position statement from the Acute Cardiovascular Care Association and European Heart Rhythm Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2020:2048872619894255. [DOI] [PubMed] [Google Scholar]
- [6].Metkus TS, Suarez-Pierre A, Crawford TC, Lawton JS, Goeddel L, Dodd OJ, et al. Diastolic dysfunction is common and predicts outcome after cardiac surgery. J Cardiothorac Surg. 2018;13:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Caldwell JC, Mamas MA. Heart failure, diastolic dysfunction and atrial fibrillation; mechanistic insight of a complex inter-relationship. Heart Fail Rev. 2012;17:27–33. [DOI] [PubMed] [Google Scholar]
- [8].Rosenberg MA, Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: a mechanistic appraisal. Circulation. 2012;126:2353–62. [DOI] [PubMed] [Google Scholar]
- [9].Daoud EG, Weiss R, Bahu M, Knight BP, Bogun F, Goyal R, et al. Effect of an irregular ventricular rhythm on cardiac output. Am J Cardiol. 1996;78:1433–6. [DOI] [PubMed] [Google Scholar]
- [10].Muntinga HJ, Gosselink AT, Blanksma PK, De Kam PJ, Van Der Wall EE, Crijns HJ. Left ventricular beat to beat performance in atrial fibrillation: dependence on contractility, preload, and afterload. Heart. 1999;82:575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gaudino M, Kappetein AP, Di Franco A, Bagiella E, Bhatt DL, Boening A, et al. Randomized Trials in Cardiac Surgery: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;75:1593–604. [DOI] [PubMed] [Google Scholar]